Abstract
The antinociceptive properties of cannabinoids in persistent pain are not fully elucidated. We investigated the effect of repeated treatment with the synthetic cannabinoid receptor agonist WIN 55,212-2 on the neuropathic pain induced in rats by chronic constriction of the sciatic nerve. WIN 55,212-2 administered daily throughout the development of neuropathy reversed the hyperalgesia, at a dose (0.1 mg kg−1, s.c.) that had no effect on the nociceptive responses of either paw contralateral to the sciatic ligation or of animals subjected to sham surgery. At 14 days after injury, the levels of mediators known to be involved in neuropathic pain, such as prostaglandin E2, NO and the neuronal NOS, were increased. Repeated treatment with WIN 55,212-2 abolished these increases. In the light of the current clinical need for neuropathic pain treatments, these findings indicate that cannabinoid agonists, at doses devoid of psychoactive effects, could constitute important compounds for the development of new analgesics.
Keywords: Endogenous cannabinoid system; neuropathic pain; WIN 55,212-2; nitric oxide system
Introduction
The chronic pain that follows peripheral nerve injury differs fundamentally from inflammatory pain and there is a need to identify an effective clinical treatment (Bridges et al., 2001b). The involvement of the endogenous cannabinoid system in pain modulation has mainly been shown in animal models of acute and inflammatory pain (Pertwee, 2001). Only a few studies have shown the effectiveness of cannabinoids in models of neuropathic pain. In these studies, a single injection of WIN 55,212-2, a synthetic cannabinoid that binds to both cannabinoid receptor 1 and 2 (CB1 and CB2), was shown to alleviate typical signs of neuropathy, such as cold and mechanical allodynia and thermal and mechanical hyperalgesia (Herzberg et al., 1997; Fox et al., 2001; Bridges et al., 2001a). The doses of WIN 55,212-2 that successfully reversed the hyperalgesia in these studies were relatively high (2–4 mg kg−1), so there was little separation between their antihyperalgesic effects and their activity in the classical tetrad of tests for cannabimimetic agents (Compton et al., 1993). In addition, the antihyperalgesia evoked by these doses disappeared within a few hours. The same authors showed antagonism of the effect of WIN 55,212-2 by the selective CB1 antagonist SR141716A, indicating the involvement of this receptor in the cannabinoid-elicited antihyperalgesia. There is also evidence to suggest that changes in cannabinoid receptors can occur in injured animals, and that the activity of the cannabinoid system is increased after injury. In fact, CB1 upregulation in the contralateral thalamus of neuropathic rats (Siegling et al., 2001) and a plasticity of spinal CB1 function following peripheral nerve injury (Chapman, 2001) have been reported. There is also evidence that the CB2 overexpression induced by peripheral nerve injury occurs in a highly restricted and specific manner within the lumbar spinal cord (Zhang et al., 2003). In the present study, we tested the hypothesis that repeated administration of WIN 55,212-2 during the development of neuropathic pain, at a dose (0.1 mg kg−1) much lower than that having psychoactive effects, normalizes the changes in nociceptive thresholds induced by the injury. In addition to monitoring behaviour, we assessed the effect of the cannabinoid agonist on mediators known to be involved in the development and maintenance of neuropathic pain, such as NO and PGE2 (Levy & Zochodne, 1998; Ma & Eisenach, 2002).
Methods
Animals and treatment
All experiments performed were in accordance with Italian State regulations governing the care and treatment of laboratory animals (permission no. 94/2000A), and conformed to the guidelines for the study of pain in awake animals established by the International Association for the Study of Pain (Zimmermann, 1983).
Painful neuropathy was induced in male Wistar rats weighing 200–220 g (Harlan, Italy) by CCI, as previously described by Bennet & Xie (1988). Briefly, animals were anaesthetized with sodium pentobarbital (65 mg kg−1 i.p.), the right sciatic nerve was exposed and four ligatures were loosely tied around the nerve just proximal to the trifurcation. Control rats underwent a sham surgery with exposure of the sciatic nerve, but no ligation or injury. WIN 55,212-2 (0.1 mg kg−1) or its vehicle was administered subcutaneously to both groups of rats once a day for 13 days, starting the day after surgery. The effect of a single dose of WIN 55,212-2 was studied on CCI rats injected with vehicle for 13 days.
Characterization of nociceptive behaviour
Behaviour was monitored before surgery, on days 7 (24 h after the last daily administration) and 14 (24 h after the last administration). The withdrawal threshold of acute WIN 55,212-2 was recorded 90 min after its administration. Latency for the withdrawal of both hindpaws from a thermal stimulus was measured by use of a radiant heat method (Hargreaves et al., 1988). The Plantar Test (Ugo Basile, Varese, Italy) that provides an infrared emission of 190 mW(cm2)−1 s−1, corresponding to a temperature of about 46°C, was used.
The withdrawal thresholds to mechanical stimulation were measured in both hind paws using the Randall–Selitto procedure (Ugo Basile, Varese, Italy).
Biochemical evaluations
After pain behaviour had been evaluated, rats were killed, blood was collected and plasma prepared; right hind limbs were cut at the level of the calcaneus bone, weighed, crushed, homogenized and centrifuged to obtain the S9 fraction; at least 1 cm of sciatic nerve proximal to the ligature was removed and immediately frozen in liquid nitrogen. Levels of nitrite/nitrate (NO2−/NO3−), the end products of NO oxidation, were assayed fluorimetrically in the S9 fraction according to Misko et al. (1993). The PGE2 level in plasma was measured with an enzyme immunoassay kit (Amersham Pharmacia Biotech, Milano, Italy). To evaluate nNOS, Western blot analysis, followed by enhanced chemiluminescence detection, was performed on the cytosolic fraction obtained from sciatic nerve homogenized in lysis buffer according to Qi et al. (2001) the specific polyclonal antibody from Cayman Chemical (Ann Arbor, MI, U.S.A.) was used.
Data analysis
Changes in nociceptive behaviour were assessed over time and analysed by two-way (group × time) analysis of variance (ANOVA) followed by Tukey's test. Differences in nitrite/nitrate and PGE2 levels between groups were compared by one-way ANOVA followed by Tukey's test. All results are presented as mean±s.e.m. A P-value <0.05 was considered significant.
Drugs
WIN 55,212-2 from Tocris (Avonmouth, U.K.) was dissolved in a 1 : 1 : 18 mixture of ethanol : cremophor : saline.
Results
Antihyperalgesic effect of WIN 55,212-2
At 7 and 14 days after injury, a significant decrease (about 50%) in both thermal withdrawal latency (Figure 1) and mechanical withdrawal threshold (Figure 2) was obtained (P<0. 01 by two-way ANOVA). WIN 55,212-2 did not modify nociceptive responses of either sham-operated animals or contralateral paws of CCI rats (data not shown). At 1 week after nerve injury, the repeated administration of WIN 55,212 significantly attenuated both mechanical hypersensitivity and thermal hyperalgesia (P<0.01 by two-way ANOVA).
Figure 1.
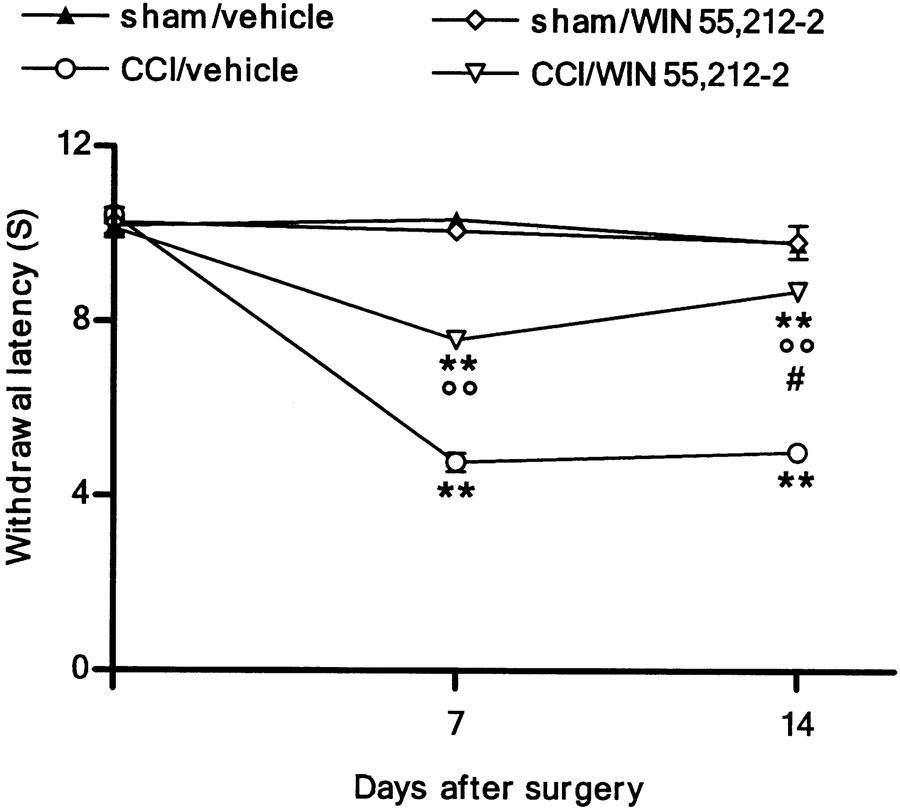
Effect of WIN 55,212-2 (0.1 mg kg−1, s.c.) daily administered in CCI rats on thermal hyperalgesia. Withdrawal latency is expressed as S. Data represent mean±s.e.m. of 8–10 rats. **P<0.01 vs presurgery; °°P<0.01 vs CCI/vehicle animals; #P<0.05 vs CCI/WIN 55,212-2 on day 7.
Figure 2.
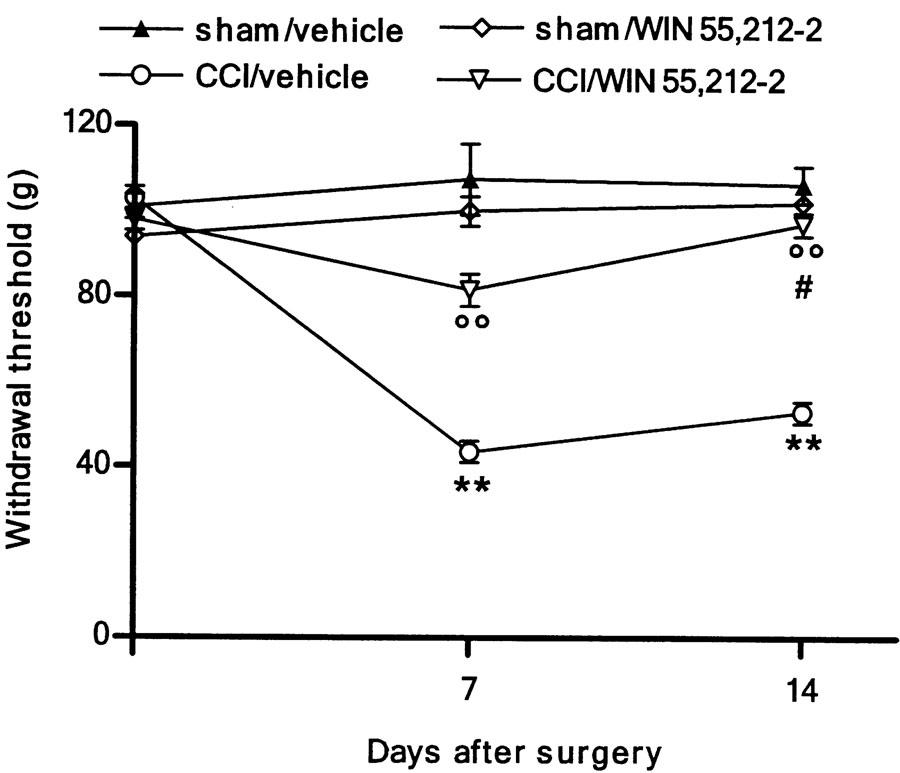
Effect of WIN 55,212-2 (0.1 mg kg−1, s.c.) daily administered in CCI rats on mechanical hypersensitivity. Withdrawal threshold is expressed as g. Data represent mean±s.e.m. of 8–10 rats. **P<0.01 vs presurgery; °°P<0.01 vs CCI/vehicle; #P<0.05 vs CCI/WIN 55,212-2 on day 7.
After 14 days, the cannabinoid abolished the mechanical allodynia (Figure 2) (P<0.01 by two-way ANOVA); the reduction of thermal hyperalgesia was always higher after 14 days compared to that after 7 days, even though the cannabinoid did not restore it to its physiological threshold (Figure 1) (P<0.05 by two-way ANOVA). The single dose of WIN 55,212-2 did not reverse neuropathic pain (data not shown).
WIN 55,212-2 restored plasma PGE2 to its physiological level
At 14 days after nerve lesion, a 100% increased plasma concentration of PGE2 occurred (Figure 3). Repeated doses of WIN 55,212-2 reduced this level to that found in sham-operated rats, but did not alter PGE2 production in sham-operated animals (Figure 3). A single dose of the cannabinoid did not modify the increased level of PGE2 in CCI rats (data not shown).
Figure 3.
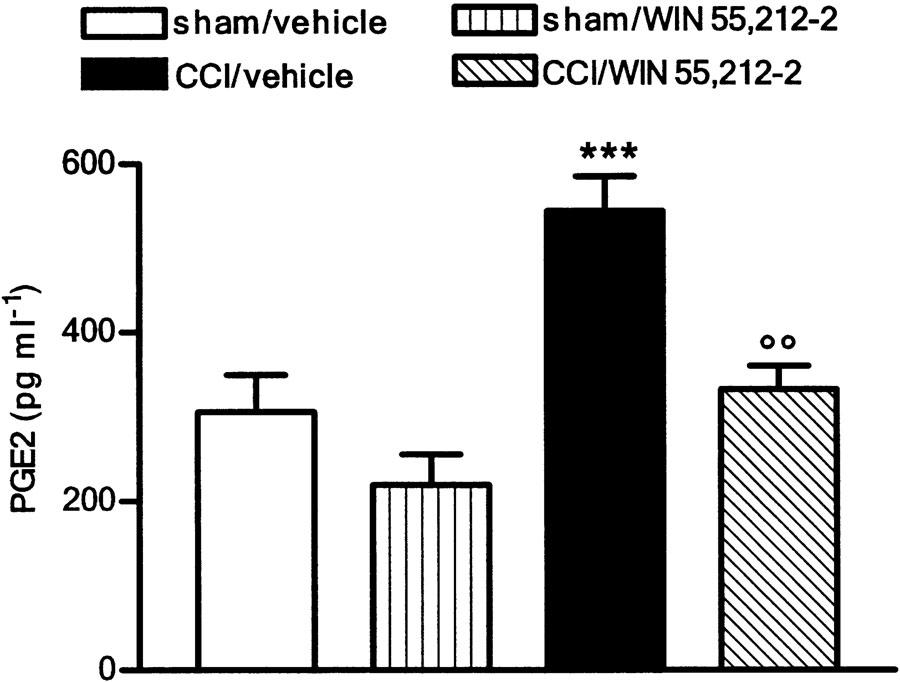
Effect of WIN 55,212-2 (0.1 mg kg−1, s.c.) daily administered in CCI rats on PGE2 plasma level. Data represent mean±s.e.m. of 8–10 rats. ***P<0.001 vs sham/vehicle animals; °°P<0.01 vs CCI/vehicle animals.
WIN 55,212-2 blocked the increased production of NO
At 14 days after the lesion, the nitrite/nitrate content in injured paws was quadrupled. This increase was not evident after repeated administration of WIN 55,212-2. Sham-operated animals had physiological levels of NO even following repeated treatment with the cannabinoid (Figure 4). The increase in the nitrite/nitrate content of CCI rats was not altered by the single dose of WIN 55,212-2 (data not shown). The enhanced production of NO found in CCI animals was associated with an increase in the concentration of a protein which corresponded immunologically to the 155 kDa neuronal isoform of NOS, detected in sciatic nerve (Figure 5). Repeated treatment with WIN 55,212-2 blocked the overexpression of nNOS so that the protein concentration did not differ from that of sham-operated rats; the same treatment did not affect nNOS content in sham animals (Figure 5).
Figure 4.
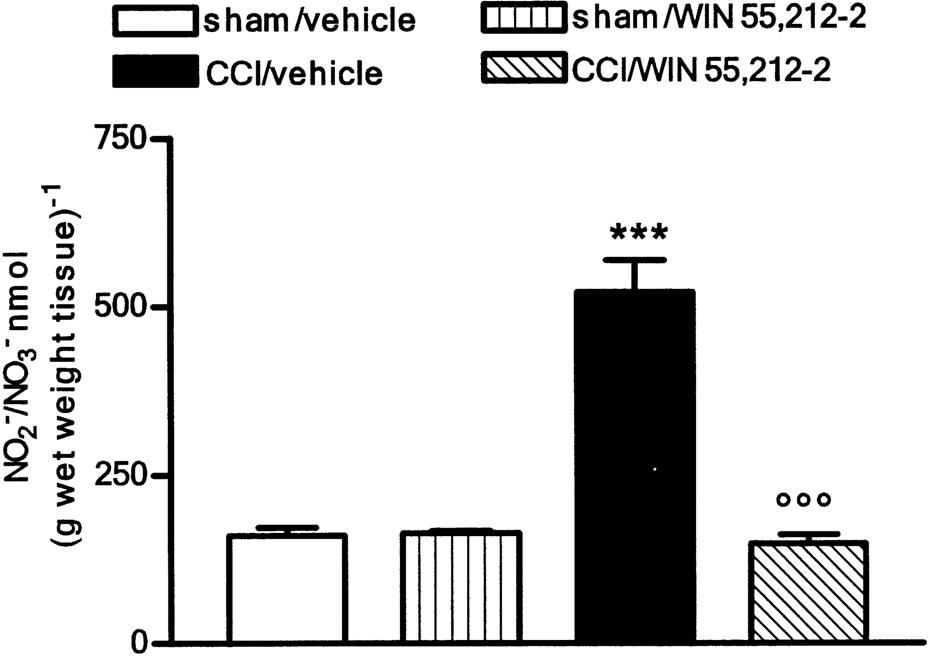
Effect of WIN 55,212-2 (0.1 mg kg−1, s.c.) daily administered in CCI rats on nitrite/nitrate content. Data represent mean±s.e.m. of 8–10 rats. ***P<0.001 vs sham/vehicle animals; °°°P<0.001 vs CCI/vehicle.
Figure 5.
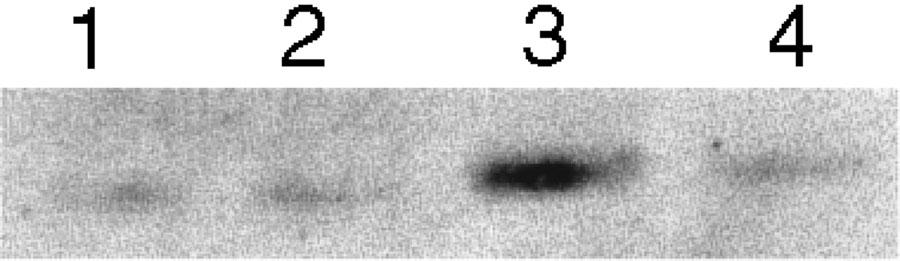
Representative immunoreactive bands of nNOS protein in cytosolic fraction of sciatic nerve homogenate. Each lane was loaded with 100 μg of proteins. Lane 1: sham/vehicle; lane 2: sham/WIN 55, 212-2; lane 3; CCI/vehicle; lane 4: CCI/WIN 55, 212-2.
Discussion
Clinically, neuropathic pain is one of the most difficult types of pain to treat and, to date, there is no effective treatment that can specifically control it once it has become established. Antidepressants and anticonvulsants have been demonstrated to provide analgesia, but are effective in less than half of the patients. Opioid treatment of neuropathic pain is unsatisfactory, because of concerns about its effectiveness, the potential for the development of tolerance, the risk of addiction and adverse side effects (Foley, 2003). Thus, the identification of novel therapeutic agents for the treatment of neuropathic pain is crucial.
There is considerable evidence supporting a role for cannabinoids in pain. Endogenous cannabinoids and cannabinoid receptors have been found to exist in key areas associated with pain pathways, from peripheral sensory nerve endings to the spinal cord and supraspinal centres, in a system that is parallel to but distinct from that involving endorphin and opiate receptors. In addition, in behavioural studies, it has been shown that a single injection of cannabinoids reduces thermal and mechanical allodynia and hyperalgesia (Herzberg et al., 1997; Bridges et al., 2001a; Fox et al., 2001).
Hence, in the present work, we tested the hypothesis that repeated administration of WIN 55,212-2 relieves the pain associated with neuropathy. The results obtained show, for the first time, that WIN 55,212-2, at a nonpsychoactive dose, which did not alter sensory threshold in either sham-operated animals or the contralateral paw of CCI rats, can inhibit thermal hyperalgesia and mechanical hypersensitivity. Animals repeatedly administered WIN 55,212-2 did not show any changes in overt behaviour, indicating that the dose used was well tolerated. The antihyperalgesic effect of WIN 55,212-2 was time-dependent; 7 days following injury, hyperalgesia was partially attenuated and, on day 14, thermal hyperalgesia was further reduced, whereas the mechanical allodynia was abolished. In contrast to the effects of repeated treatment, a single dose of the cannabinoid did not modify the pain threshold in CCI animals. The higher efficacy of WIN 55,212-2 on day 14 suggests that the daily treatment over this period did not induce tolerance to the antihyperalgesic and antiallodynic effects. This contrasts with the findings of Mao et al. (2000); they showed the development of tolerance to Δ9-tetrahydrocannabinol-induced antinociception in CCI rats. However, it should be noted that the dose of Δ9-tetrahydrocannabinol (80 μg, i.t.) used by Mao et al. was able to induce antinociception in CCI animals following a single dose, and that, 14 days after daily administration of the same dose, tolerance to Δ9-tetrahydrocannabinol antinociception developed. Whereas the concentration of WIN 55,212-2 used by us was ineffective when given as a single dose, an antihyperalgesic effect was only seen after 14 days of treatment. However, the possibility that prolonged treatment will result in tolerance development to WIN 55,212-2-induced anti-hyperalgesia cannot be excluded.
At present, the efficacy of cannabinoids in neuropathic pain behaviour has only been studied by evaluating the effect of single doses of the compounds at high concentrations, which often have psychoactive effects. It is of interest to note that, in our experiments, CCI animals treated with WIN 55,212-2 for 13 days exhibited a reversal of hyperalgesia when tested 24 h later. This result supports the hypothesis that pain relief is due to the persistent stimulation of the cannabinoid system, which would explain why the same dose of WIN 55,212-2 given acutely had no effect. The possibility that accumulation of the cannabinoid is responsible for its antihyperalgesia can be excluded, as Herzberg et al. (1997) showed that the antihyperalgesic effect of a high dose of WIN 55,212-2 (4.3 mg kg−1 i.p.) was not apparent 24 h later.
The WIN 55,212-2-induced inhibition of neuropathic pain behaviour found by us could be mediated by CB1 and/or CB2 receptors, as this cannabinoid has a high affinity for both receptors. Previous reports have provided evidence for an involvement of CB1 receptors in the antihyperalgesic effect of acute WIN 55,212-2 treatment in neuropathic animals (Herzberg et al., 1997; Bridges et al., 2001a; Fox et al., 2001). More recently, Nackley et al. (2003) showed an involvement of CB2 receptors in the suppressive effect of locally administered WIN 55,212-2 on carrageenan-evoked pain behaviour. Interestingly, in a very recent study, Ibrahim et al. (2003) demonstrated that the aminoalkylindole analogue AM1241, a selective CB2 cannabinoid receptor agonist, inhibited experimental neuropathic pain in both rats and CB1-deficient mice. This finding strongly supports the hypothesis that CB2 receptors have a role in the antihyperalgesic action of cannabinoids in neuropathic pain states. While CB1 receptors are known to modulate transmission in neuronal pain pathways, it is not clear how CB2 receptors can affect pain responses. The evidence regarding the expression of CB2 receptors of primary afferent neurons is conflicting; CB2 receptors may participate in this complex circuit by modulating the release of endogenous inflammatory agents from non-neuronal cells, such as mast cells, located in the vicinity of nociceptive neurons. These cells are recruited at the site of injury following the neurogenic inflammation evoked by chronic constriction of the sciatic nerve. Therefore, we hypothesize that a peripheral inflammatory process initiated by nerve injury contributes to the sensitization of primary afferent neurons. If this is the case, the activation of CB2 receptors by WIN 55,212-2 could result in the inhibition of the release of sensitizing molecules with consequent attenuation of pain responses. Prostaglandins are probably one of these important inflammatory mediators; they are known to sensitize the peripheral sensory nerve endings eliciting hyperalgesia. Here we present evidence that repeated treatment with WIN 55,212-2 is effective at reducing the plasma PGE2 content to that of control animals. It was also found that NO is produced locally within the injured sciatic nerve following CCI. Local NO may contribute to the development of hyperalgesia directly or indirectly by influencing the local inflammatory and repair process of a partially injured peripheral nerve (Levy & Zochodne, 1998). Changes in NOS expression, particularly nNOS, within sensory neurons following nerve injury have been documented previously (Luo et al., 1999). In accordance with these previous findings, we showed increased nitrite/nitrate levels in paw tissues and an overexpression of nNOS in sciatic nerve of CCI rats; again, these effects were abolished by repeated treatment with WIN 55,212-2. CB1 receptors located in sensory afferent neurons could mediate the effect of WIN 55,212-2 on nNOS overexpression in CCI rats. Moreover, WIN 55,212-2 could modulate the levels of NO and PGE2 by activating CB2 receptors present on inflammatory cells, such as macrophages, natural killer cells and T lymphocytes; specifically, the cannabinoid could reduce the release of those mediators known to sensitize peripheral nociceptors. Taken together, our findings highlight the effectiveness of WIN 55,212-2 at alleviating not only neuropathic pain but also peripheral inflammatory conditions. In the light of the current clinical need for neuropathic pain treatment, this study provides novel evidence for the therapeutic potential of compounds able to modulate the endogenous cannabinoid system at doses that do not cause psychotropic effects, which is the main limiting factor affecting the clinical use of cannabinoids.
This work was funded by the Italian Ministry for Education, University and Research (MIUR).
Abbreviations
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- CCI
chronic constriction injury
- nNOS
neuronal NOS
- PGE2
prostaglandin E2
References
- BENNET G.J., XIE Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- BRIDGES D., AHMAD K., RICE A.S.C. The synthetic cannabinoid WIN 55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Br. J. Pharmacol. 2001a;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGES D., THOMPSON S.W.N., RICE A.S.C. Mechanisms of neuropathic pain. Br. J. Anaesth. 2001b;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- CHAPMAN V. Functional changes in the inhibitory effect of spinal cannabinoid (CB) receptor activation in nerve injured rats. Neuropharmacology. 2001;41:870–877. doi: 10.1016/s0028-3908(01)00125-3. [DOI] [PubMed] [Google Scholar]
- COMPTON D.R., RICE K.C., DE COSTA B.R., RAZDAN R.K., MELVIN L.S., JOHNSON M.R., MARTIN B.R. Cannabinoid structure–activity relationships: correlation of receptor binding and in vivo activities. J. Pharmacol. Exp. Ther. 1993;265:218–226. [PubMed] [Google Scholar]
- FOLEY K.M. Opioids and chronic neuropathic pain. N. Engl. J. Med. 2003;348:1279–1281. doi: 10.1056/NEJMe030014. [DOI] [PubMed] [Google Scholar]
- FOX A., KESINGLAND A., GENTRY C., McNAIR K., PATEL S., URBAN L., JAMES I. The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- HARGREAVES K.M., DUBNER R., BROWN S., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HERZBERG U., ELIAV E., BENNETT G.J., KOPIN I.J. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci. Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- IBRAHIM M.M., DENG H., ZWONOK A., COCKAYNE D.A., KWAN J., MATA H.P., VANDERAH T.W., LAI J., PORRECA F., MAKRIYANNIS A., MALAN T.P., JR Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY D., ZOCHODNE D.W. Local nitric oxide synthase activity in a model of neuropathic pain. Eur. J. Neurosci. 1998;10:1846–1855. doi: 10.1046/j.1460-9568.1998.00186.x. [DOI] [PubMed] [Google Scholar]
- LUO Z.D., CHAPLAN S.R., SCOTT B.P., CIZKOVA D., CALCUTT N.A., YAKSH T.L. Neuronal nitric oxide synthase mRNA upregulation in rat sensory neurons after spinal nerve ligation: lack of a role in allodynia development. J. Neurosci. 1999;19:9201–9208. doi: 10.1523/JNEUROSCI.19-21-09201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA W., EISENACH J.C. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur. J. Neurosci. 2002;15:1037–1047. doi: 10.1046/j.1460-9568.2002.01940.x. [DOI] [PubMed] [Google Scholar]
- MAO J., PRICE D.D., LU J., KENISTON L., MAYER D.J. Two distinctive antinociceptive systems in rats with pathological pain. Neurosci. Lett. 2000;280:13–16. doi: 10.1016/s0304-3940(99)00998-2. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorimetric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- NACKLEY A.G., SUPLITA R.L., HOHMANN A.G. A peripheral cannabinoid mechanism suppresses spinal Fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;117:659–670. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Cannabinoid receptors and pain. Prog. Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- QI W.N., YAN Z.Q., WHANG P.G., ZHOU Q., CHEN L.E., SEABER A.V., STAMLER J.S., URBANIAK J.R. Gene and protein expressions of nitric oxide synthases in ischemia-reperfused peripheral nerve of the rat. Am. J. Physiol. Cell Physiol. 2001;281:C849–C856. doi: 10.1152/ajpcell.2001.281.3.C849. [DOI] [PubMed] [Google Scholar]
- SIEGLING A., HOFMANN H.A., DENZER D., MAULER F., DE VRY J. Cannabinoid CB1 receptor upregulation in a rat model of chronic neuropathic pain. Eur. J. Pharmacol. 2001;415:R5–R7. doi: 10.1016/s0014-2999(01)00798-1. [DOI] [PubMed] [Google Scholar]
- ZHANG J., HOFFERT C., VU H.K., GROBLEWSKI T., AHMAD S., O'DONNEL D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


