Abstract
N-type Ca2+ channel modulation by an endogenous P2Y receptor was investigated by the whole-cell patch-clamp method in HEK 293 cells transfected with the functional rabbit N-type calcium channel.
The current responses (ICa(N)) to depolarizing voltage steps were depressed by ATP in a concentration-dependent manner. Inclusion of either guanosine 5′-O-(3-thiodiphosphate) or pertussis toxin into the pipette solution as well as a strongly depolarizing prepulse abolished the inhibitory action of ATP.
In order to identify the P2Y receptor subtype responsible for this effect, several preferential agonists and antagonists were studied. Whereas the concentration–response curves of ADP and adenosine 5′-O-(2-thiodiphosphate) indicated a higher potency of these agonists than that of ATP, α,β-methylene ATP, UTP and UDP were considerably less active. The effect of ATP was abolished by the P2Y receptor antagonists suramin and N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene-ATP, but not by pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid, 2′deoxy-N6-methyladenosine-3′,5′-diphosphate or 2-methylthio AMP.
Using reverse transcription and polymerase chain reaction, mRNA for the P2Y1, P2Y4, P2Y6, P2Y11 and P2Y13 receptor subtypes, but not the P2Y2, and P2Y12 subtypes, was detected in HEK 293 cells.
Immunocytochemistry confirmed the presence of P2Y1, and to a minor extent that of P2Y4, but not of P2Y2 receptors.
Hence, it is tempting to speculate that P2Y13 receptors may inhibit N-type Ca2+ channels via the βγ subunits of the activated Gi protein.
Keywords: N-type calcium channel, P2Y13 receptor, G protein, HEK 293 cell, presynaptic inhibition
Introduction
ATP is not only a ubiquitous enzyme cofactor and energy source of the cell, but also a potent extracellular signaling molecule involved in the mediation of numerous physiological processes (Burnstock & Williams, 2000; Illes et al., 2000). ATP (ADP)- and/or UTP (UDP)-sensitive receptors are classified into two types belonging to the ligand-gated ionotropic family (P2X) and the metabotropic, G-protein-coupled family (P2Y) (Ralevic & Burnstock, 1998; Chizh & Illes, 2000; Nörenberg & Illes, 2000). In humans, eight different P2Y receptors (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14; Burnstock & Williams, 2000; von Kügelgen & Wetter, 2000; Barnard & Simon, 2001; Communi et al., 2001; Abbracchio et al., 2003) have been detected until now.
In the central nervous system, ATP is released as a cotransmitter with a number of classic transmitters such as noradrenaline and acetylcholine (von Kügelgen & Starke, 1991; Burnstock, 1999). At peripheral and central noradrenergic neurons, both noradrenaline and its cotransmitter ATP inhibit their own release by activating presynaptically located α2-adrenergic and P2Y autoreceptors (von Kügelgen et al., 1993; Poelchen et al., 2001).
Recent studies classified voltage-activated calcium channels (VACCs) into three groups: high voltage-activated, which included L, N, P and Q types, intermediate (R type) and low voltage-activated (T-type) (Davila, 1999; Varadi et al., 1999). VACCs consist of four distinct subunits, α1, α2δ, β and γ (Dolphin, 1998; Varadi et al., 1999). Whereas the α1 subunit forms the pore through which Ca2+ enters the cell, the other subunits may play a functional role in modifying the kinetic properties of the channel (Bleakman et al., 1995). Coexpression of the neuronal human brain α1B subunit with α2 and β subunits in HEK 293 cells produced a functional N-type Ca2+ channel, which was irreversibly blocked by ω-conotoxin GVIA (Williams et al., 1992).
During the last few years, inhibition of N-type calcium channels has received a lot of attention because of their involvement, for example, in the regulation of neurotransmitter release (Wheeler et al., 1994; Powell et al., 2000). One important form of N-type Ca2+ current (ICa(N)) modulation is the strongly voltage-dependent channel inhibition by receptor-coupled, heterotrimeric G proteins (Herlitze et al., 1996; Ikeda, 1996). The G proteins usually involved in this process belong to the pertussis toxin (PTX)-sensitive G proteins Gi and Go (Zamponi & Snutch, 1998). An inhibition of ICa(N) after activation of P2Y12 receptors has been described in brain capillary endothelial cells (Simon et al., 2002) and in a neuronally differentiated rat pheochromocytoma (PC12) cell line (Kulick & von Kügelgen, 2002; Kubista et al., 2003). A similar effect has been reported for rat sympathetic neurons after stimulation of P2Y1, P2Y2, P2Y4 or P2Y6 receptors that were transfected additionally to the physiological P2Y receptor endowment of these cells (Filippov et al., 1998,1999,2000,2003).
Native HEK 293 cells express endogenous P2Y receptors (Schachter et al., 1997; Moore et al., 2001; Fischer et al., 2003), but do not possess either P2X receptors or high VACCs (McNaughton & Randall, 1997). In the present study, a HEK 293 cell line transfected with the rabbit N-type Ca2+ channel (Fujita et al., 1993) was used to investigate the modulation by human P2Y receptors of this channel type. Thereby, for the first time, selective effects on N-type Ca2+ currents could be investigated without the need of functional isolation of the native channel from all other types of voltage-activated Ca2+ currents by means of physiological or pharmacological procedures. It is suggested that, although both P2Y1, P2Y4, P2Y6 and P2Y13 receptors are present in these cells, only the P2Y13 receptor is coupled to N-type Ca2+ channels via a PTX-sensitive G protein. Preliminary accounts of the data have appeared previously in abstract form (Wirkner et al., 2002).
Methods
Transfection procedures and culturing of HEK 293 cells
The plasmid pcDNA3 1/zeo(+) (Invitrogen, Carlsbach, CA, U.S.A.) was cleaved by EcoRI and ligated with cDNA encoding the rabbit N-type calcium channel α1B subunit to yield cDNA3.1/zeo(+)-α1B (Fujita et al., 1993). The plasmid pDouble one (IDEC, Osaka, Japan) was also cleaved by EcoRI and then ligated with the 1.6 kb EcoRI fragment from pCAS14 containing the rabbit muscle calcium channel β1 cDNA to yield pDouble one-β1. Afterwards, the plasmid pDouble one-β1 was cleaved by MunI and ligated with the 3.5 kb EcoRI fragment from pCAS7 containing the rabbit skeletal muscle calcium channel α2δ subunit cDNA to yield pDouble one-α2-β1.
These two expression plasmids, cDNA3.1/zeo(+)-α1B carrying the cDNAs encoding the rabbit N-type calcium channel α1B subunit and pDouble one-α2-β carrying the rabbit calcium channel α2δ and β1 subunits, were introduced into HEK 293 cells using the calcium phosphate method. HEK 293-N26 cells were maintained in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum, and were selected with 400 μg ml−1 active G418 (GibcoBRL, Eggenstein, Germany) and 250 μg ml−1 zeocin. Afterwards, the transfectants were screened for the expression of calcium channel subunits (α1B, α2δ and β1) by Northern blot analysis and the binding assay, using 125I-ω-conotoxin GVIA (NEN Life Science Products, Zaventem, Belgium).
Then, the cells were cultured in Dulbecco's minimal essential medium supplemented with 10% fetal bovine serum and 2 mM glutamine (Sigma, Deisenhofen, Germany) and 400 μg ml−1 G418. The cells were splitted 1 : 12 and replated into new flasks (Sarstedt, Nürnberg, Germany) two times a week. A 0.25% trypsin solution (GibcoBRL) was used to separate the cells for passage.
Recording of Ca2+ currents
N-type Ca2+ currents were recorded by the conventional whole-cell patch-clamp method. The external solution contained (mM): tetraethylammonium chloride (TEA) 120, KCl 3, MgCl2 1.5, CaCl2 5, HEPES 10 and glucose 11 (pH was adjusted to 7.4 with TEA-OH). In some experiments, CaCl2 was substituted by CoCl2 (2 mM). Patch electrodes (3–5 MΩ) were filled with a solution consisting of (mM): CsCl 110, MgCl2 3, HEPES 40, EGTA 3, Mg-ATP 1.5, Li-GTP 0.3 (pH adjusted to 7.4 with CsOH). When indicated, GTP was substituted with either GTP-γ-S or GDP-β-S (0.3 mM each). In some experiments, the concentration of EGTA was decreased from 3 to 0.3 mM and 2.7 mM CsCl was added to the pipette solution. This manipulation leads to a decrease of the Ca2+-buffering capacity.
Cells were voltage-clamped with an Axopatch 200B amplifier (Axon Instruments, Union City, CA, U.S.A.). The seal resistance of the whole-cell configuration was routinely ≈10 GΩ. Residual pipette capacitance was compensated in the cell-attached configuration using the negative circuit of the amplifier. Whole-cell capacitance was 23.8±1.1 pF (n=216). Series resistance (Rs) was calculated as τ (1/C), where τ was the time of constant for decay of the whole-cell capacity transient. The average values of τ and Rs were 286±7 μs and 12.2±0.2 MΩ, respectively. Rs was reduced by 40–80% using the inbuilt series resistance compensation circuit of the amplifier. The liquid junction potential (VLJ) between the bath and pipette solutions at 22°C was calculated according to Barry (1994); it was found to be −9.3 mV. Holding potential values given in this study were corrected for VLJ. The steady-state holding potential was −90 mV. Ca2+ channel currents were evoked routinely with a 100 ms depolarizing rectangular test pulse to −10 mV. Unless otherwise stated, step depolarizations to test potentials were delivered every 20 s; these stimulation rates were sufficiently low to minimize cumulative inactivation of the N-type Ca2+ channel. Current amplitudes were measured isochronically 10 ms from the onset of the test pulse near to the peak of the current. In some experiments, strong depolarizing prepulses to +120 mV from a holding potential of −90 mV were applied for 20 ms. The interval between the prepulse and the test pulse was 5 ms.
Currents were filtered at 5 kHz using the built-in low-pass Bessel filter of the amplifier, and sampled at 10 kHz via a Digidata 1200 interface (Axon Instruments). All recordings were corrected for linear capacitance and leakage currents by the −P/4 subtraction function of the pClamp 8.0 software, used also for data acquisition and analysis (Axon Instruments). Curve fits and figures were made with the help of Sigma Plot 6.0 (SPSS, Erkrath, Germany). The IC50 values were calculated based on fits using the following logistic function
where I is the steady-state inhibition produced by the agonist, Imax and Imin are the maximal and minimal inhibition, respectively, n is the Hill coefficient and IC50 is the concentration of agonist reducing Imax by 50%. Higher concentrations of adenosine 5′-O-(2-thiodiphosphate) (ADP-β-S) and α,β-methyleneadenosine 5′-triphosphate (α,β-meATP) than 100 μM were considered to be pharmacologically meaningless, and therefore not investigated.
Substance application
Stock solutions (1 or 10 mM) of P2 receptor agonists and antagonist were prepared in external solution, stored at −20°C and diluted immediately before use. The stock solution (1 mM) of ω-Conotoxin GVIA was dissolved in 100 mg ml−1 bovine serum albumin (Sigma) and stored at −20°C; further dilutions to the final concentration of 1 μM were made with an external medium.
Agonists were applied for 1–2 min by local superfusion using a fast, pressurized drug-application device system (DAD12, Adams and List, Westbury, NY, U.S.A.). HEK 293-N26 cells were continuously superfused with the standard external solution by one pressure-independent valve of this system. Each concentration of P2 receptor agonists was applied to individual HEK 293-N26 cells. Thereby, a possible desensitization developing during cumulative or repeated applications of increasing agonist concentrations was avoided. Only cells in which current amplitudes recovered to control values after washout were included in the evaluation.
The effects of various P2 receptor antagonists were investigated by applying the prototypic agonist ATP (300 μM) for 1 min before as well as 9 min after superfusion with suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), 2′deoxy-N6-methyladenosine-3′,5′-diphosphate (MRS 2179), 2-methylthioadenosine 5′-monophosphate (2-MeSAMP) and N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene-ATP (AR-C69931MX). The antagonist potencies were quantified by measuring the inhibitory effect of ATP after antagonist superfusion for 10 min. ω-Conotoxin GVIA (1 μM) was also pressure-applied onto the cells. In some experiments, activated PTX was intracellularly applied via the patch pipette. Immediately before use, PTX (2 μg ml−1) was dissolved in pipette solution containing dithiothreitol (5 mM) and incubated for 30 min at 37°C. Then, the pipette solution containing β-nicotinamide adenine dinucleotide (NAD, 5 mM) was added in a 1 : 1 volume. The final concentration of PTX was 1 μg ml−1. Nonactivated PTX was used as a negative control.
Reverse transcription and polymerase chain reaction
Total RNA was isolated from HEK 293-N26 cells (≈1.1 × 106 cells) 4 days after plating in culture dishes using a Total Quick RNA mini kit (BIOZOL, Eching, Germany). Twice, contamination with genomic DNA was removed by repeated digestion with RNase-free DNase I (Roche; 50 U) for 2 h at 37°C. According to standard protocols, first-strand cDNA was synthesized by reverse transcription of 1 μg of total RNA with Superscript™-II reverse transcriptase (Life Technologies). cDNA fragments of the various P2Y receptors were amplified by the use of a set of sense and antisense primers specific for the following receptor subtypes: PY1, PY2, PY4 and PY6 (Jin et al., 1998); PY11 (Conigrave et al., 2001); PY12 (Hollopeter et al., 2001) and PY13 (Communi et al., 2001). PCRs were run on a PTC-200 Thermocycler (MJ Research, Boston, MA, U.S.A.) in a final volume of 25 μl containing 1 μl of the first-strand cDNA, 1 u Ampli-Taq DNA Polymerase (Perkin-Elmer), and (anti)sense primers (200 nM, each) specific for the respective P2Y receptor subtypes after initial denaturation for 3 min at 95°C with 25 cycles (P2Y13) or 35 cycles (P2Y1–P2Y12). The annealing temperatures were 54°C, 55°C, 60°C, 58°C, 57°C, 57°C and 50°C for P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13, respectively. Negative controls lacking first-strand cDNA were run in parallel; positive controls were performed with cDNA from peripheral blood leukocytes or brain tissue. The amplification products were detected by ethidium bromide staining subsequent to agarose gel (1.5%) electrophoresis with 10 μl of PCR product.
Immunohistochemistry
After fixation, washing with Tris-buffered saline (TBS, 0.05 M; pH 7.6) and blocking with 5% fetal calf serum (FCS), the cells were incubated with the rabbit anti-P2Y receptor antibodies (anti-P2Y1: 1 : 1500, SmithKline Beecham Pharmaceuticals, Harlow, Essex, U.K.; anti-P2Y2: 1 : 1000, Laboratory of Kidney and Electrolyte Metabolism, National Heart, Lung and Blood Institute, Bethesda, MD, U.S.A.; anti-P2Y4: 1 : 1000, Alomone Labs, Jerusalem, Israel) with 0.1% Triton X-100, 5% FCS in TBS for 12 h at 4°C. The preparations were washed three times for 5 min each in TBS, and were then incubated with the secondary antibody Cy3-conjugated goat anti-rabbit IgG (1 : 800; Jackson ImmunoResearch, West Grove, PA, U.S.A.) in 5% FCS in TBS for 2 h. After intensive washing, the cultures were dehydrated in a series of graded ethanol, processed through n-butylacetate and covered with entellan (Merck, Darmstadt, Germany). The data obtained with the anti-P2Y1 and anti-P2Y2 receptor antibodies were confirmed by using similar antibodies from a commercial source (Alomone Labs); in this case, just as with the anti-P2Y4 receptor antibody, the supplier's certificate of analysis states that, in the appropriate Western blots, only a single band was observed. Control experiments were carried out without primary antibody or by preadsorption of the antibody with the immunizing peptides.
Microphotographs were taken by using a fluorescence microscope (Axioskop; Zeiss, Oberkochen, Germany; fluorescence filter set: BP 450–490, FT 510; LP 520) equipped with the Zeiss Axio Vision System 2.0.
Drugs
The following drugs and chemicals were used: ATP, adenosine 5′-diphosphate (ADP), uridine 5′-triphosphate (UTP), uridine 5′-diphosphate (UDP), α,β-meATP, 2-MeSAMP, 2-MeSATP, ADP-β-S, PTX, ω-conotoxin GVIA, guanosine 5′-O-(3-thiodiphosphate) (GDP-β-S), guanosine 5′-O-(3-thiotriphosphate) (GTP-γ-S), nicotine adenosine dinucleotide (NAD), PPADS, suramin and MRS 2179 (Sigma, Deisenhofen, Germany). Samples of ATP (99% ATP, 1% ADP) and UTP (82% UTP, 18% UDP) were checked for purity by HPLC before use. AR-C69931MX was a gift from AstraZeneca (M. Wayne, Wilmington, DE, U.S.A.).
Statistics
Data are presented as means±s.e.m. of n experiments. Statistical comparisons were made by unpaired Student's t-test (two groups) or by one-way analysis of variance (three or more groups), followed by the Bonferroni t-test. A probability level of 0.05 or less was considered to be statistically significant.
Results
Ca2+ channel currents and their voltage-dependent inhibition by ATP
When depolarizing voltage steps were applied in 10 mV increments to untransfected HEK 293 cells, from a holding potential of −90 mV and every 20 s, no inward current response was observed up to a maximal depolarization of +70 mV (n=25). Hence, under our conditions, native HEK 293 cells did not possess VACCs.
To investigate whether the properties of the transfected rabbit N-type calcium channels in HEK 293-N26 cells correspond to the pattern of native N-type calcium channels, voltage–current (I–V) relations between −90 and +50 mV were determined by applying a fast (280 ms) depolarizing ramp from a holding potential of −90 mV (Figure 1a). Maximal currents were usually observed after a depolarization to −10 mV. ATP (300 μM) did not alter the holding current, but caused a voltage-dependent inhibition of ICa(N) (n=8). In addition, a slight and reversible shift of the peak current to the right was observed in the presence of ATP.
Figure 1.
Characterization of the properties of the N-type calcium channel in HEK 293-N26 cells. Voltage-dependent inhibition by ATP and effects of Co2+. (a) Depolarizing voltage ramps of 280 ms duration from a holding potential of −90 to 50 mV were applied to evoke inward currents both in the absence and presence of ATP (300 μM), as well as after its washout. Application of ATP results in a slight shift of the voltage–current (I–V) relationship to the right. (b) Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. Substitution of Ca2+ by Co2+ (2 mM; n=6) in the superfusion medium abolished the current in this cell. The effect of a Ca2+-free medium supplemented with Co2+ was reversed by washout.
In all subsequent experiments, test pulses were delivered every 20 s from the holding potential of −90 to −10 mV for 100 ms. Pressure application of ω-conotoxin GVIA (1 μM) for 120 s nearly abolished ICa(N) (99.1±0.3% inhibition; n=5); this effect was not reversed by washout for 10 min. The application of an external medium onto cells which lacked the normal Ca2+ (5 mM) but contained Co2+ (2 mM) also led to complete blockade of the inward current within 20 s (Figure 1b). Recovery to control current amplitudes was observed after washout for 60 s (7.8±13.7% inhibition; n=6).
Effects of P2 receptor agonists and antagonists
Pressure application of ATP (30–1000 μM) to HEK 293 cells transfected with the rabbit N-type Ca2+ channel caused a concentration-dependent inhibition of Ca2+ currents (Figure 2A and B). A typical reversible slowing of the current kinetics was observed in the presence of higher concentrations of ATP (Figure 2Ab). A near steady-state inhibition was observed 60 s after starting the application of ATP; the inhibition was stable over a further 60 s and was reversed by washout within 20 s.
Figure 2.
Time- and concentration-dependence of the inhibition by ATP of N-type calcium channels in HEK 293-N26 cells. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. Current amplitudes were measured 10 ms after the onset of the test pulse. (A) After recording two stable control currents, ATP (30–1000 μM) was applied onto separate cells for 120 s each, followed by washout. (a) Representative tracings before (control) during and 40 s after (washout) the application of ATP (100 μM) for 1 min. (b) Representative tracings before, during and 40 s after the application of ATP (1000 μM) for 1 min. (B) Means±s.e.m. of 5–11 experiments similar to those shown in (A). The data were normalized with respect to the second control current recorded before the application of ATP. *P<0.05; statistically significant difference from the second control current. #P<0.05; statistically significant difference from the effect of ATP after 120 s application.
In addition to ATP, cells were exposed to a series of further P2 receptor agonists (Figure 3a and b). The concentration–response curves of the P2Y1,12,13 receptor agonists ADP (0.1–1000 μM) and ADP-β-S (0.1–100 μM) were remarkably similar and clearly differed from the concentration–response curve of ATP (1–1000 μM), which acts in addition at P2Y2,11 receptors. IC50 values were calculated for ADP and ATP, where the full concentration–response curves were established. Based on these values, ADP (6.7 μM) had a higher potency than ATP (116.6 μM) itself. Further agonists with selectivities for certain receptor types such as α,β-meATP (1–100 μM; P2X1,3), UTP and UDP (1–1000 μM each; P2Y2,4,6) inhibited ICa(N) maximally by about 10–20%.
Figure 3.
Concentration-dependent inhibition by P2 receptor agonists of N-type calcium channels in HEK 293-N26 cells. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. After recording of two stable control currents, increasing concentrations of various P2 receptor agonists were applied onto separate cells for 60 s each, followed by washout. Only cells that fully recovered are included. The agonist-induced depression of ICa(N) was calculated in comparison with the second control current amplitude. (a) Concentration–response relationships for ATP (n=5–11), ADP (n=5–8) and ADP-β-S (n=6–8). (b) Concentration–response relationships for α,β-meATP (n=5–7), UDP (n=7) and UTP (n=7–11). Means±s.e.m. of n experiments are shown in (a) and (b).
In the following experiments, a selected concentration of the prototypic agonist ATP (300 μM) was applied for 1 min before as well as 9 min after superfusion with various P2 receptor antagonists. The antagonist potencies were quantified by measuring the inhibitory ATP effect after antagonist superfusion for 10 min. In the absence of antagonists, the effect of ATP was reproducible 10 min after its first application (Figure 4Aa and B). The nonselective P2 receptor antagonist suramin (30 μM) and the P2Y12,13-selective antagonist AR-C6993MX (1 μM) markedly inhibited the depression by ATP (Figure 4Ab and B). In contrast, further receptor-selective antagonists such as PPADS (30 μM; P2X1,2,3,5/P2Y1), MRS 2179 (30 μM; P2Y1) and 2-MeSAMP (100 μM; P2Y12) failed to interfere with ATP (300 μM) (Figure 4B).
Figure 4.
Effects of P2 receptor antagonists on the ATP-induced inhibition of N-type Ca2+ channels in HEK-N26 cells. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. ATP (300 μM) was applied for 1 min before as well as 9 min after superfusion with various P2 receptor antagonists. The antagonistic potencies were quantified by measuring the inhibitory effect of ATP after antagonist superfusion for 10 min. (A) Representative tracings. (a) In the absence of antagonists, the ATP effect was reproducible 10 min after its first application. Complete recovery occurred upon washout. (b) Suramin (30 μM) abolished the ATP-induced inhibition of ICa(N). (B) Means±s.e.m. of the ATP-induced inhibition of ICa(N) both in the absence (control, n=8) and presence of antagonists (suramin 30 μM, n=6; PPADS 30 μM, n=7; MRS 2179 30 μM, n=5; 2-MeSAMP 100 μM, n=7; AR-C69931MX 1 μM, n=5). *P<0.05; statistically significant difference from the time-matching control current.
Since both agonist and antagonist studies unequivocally indicated the involvement of an ADP-preferring P2Y receptor, ADP rather than ATP was used as an agonist in the following experiments. Two subsequent applications of ADP (100 μM) for 1 min each and with 9 min intervals inhibited ICa by 56.2±6.3 and 40.8±4.1%, respectively (n=5). When MRS 2179 (30 μM) was present in the superfusion medium for 9 min before and during the second application of ADP (100 μM), there was no interaction of the antagonist with the ADP-induced inhibition of ICa (59.2±5.1 and 51.8±4.8% inhibition, respectively; n=6; P>0.05). Hence, N-type Ca2+ channels were definitely not affected via P2Y1 receptor-activation.
Characterization of the G protein
Since none of the P2 receptor agonists altered the holding current of HEK 293-N26 cells, the presence of an endogenous P2X receptor can be unequivocally excluded (see also Moore et al., 2001). In order to investigate the transduction mechanism of a G-protein-coupled P2Y receptor possibly involved in the blockade of ICa(N), instead of GTP, GDP-β-S (300 μM), an inhibitor of G-protein-dependent processes (Sternweis and Pang, 1990), was added to the standard pipette solution. Immediately after establishing whole-cell configuration, ATP (300 μM) exerted its usual inhibitory effect. However, 15 min later, when the cell was microdialyzed by GDP-β-S, ATP failed to cause any depression of ICa(N) (1.2±1.1% inhibition, n=12; Figure 5Ab), suggesting the participation of a G protein in the modulation of Ca2+ channels (compare with the time-matching control effect of ATP recorded with the standard GTP-containing micropipette; 40.6±5.4%, n=12; Figure 5Aa).
Figure 5.
Effect of intracellular GDP-β-S on the ATP-induced inhibition of N-type Ca2+ channels in HEK 293-N26 cells; inhibitory effect of intracellular GTP-γ-S. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. (A) Representative tracings. (a) Current responses before (control) during and 40 s after (washout) the application of ATP (300 μM) for 1 min; a standard pipette solution containing GTP (300 μM) was used. (b) Current responses recorded with a pipette solution containing GDP-β-S (300 μM) instead of GTP. The experimental protocol was identical with that used in (a). (B) The pipette solution contained GTP-γ-S (300 μM) instead of the standard GTP. A slowly developing inhibition of ICa(N) was observed in comparison with the second current response measured immediately after establishing whole-cell access. Means±s.e.m. of six experiments. *P<0.05; statistically significant difference from the second current response in this series.
In another series of experiments, GTP was substituted by GTP-γ-S (300 μM, Figure 5B), that irreversibly activates G proteins (Gilman, 1987). In fact, GTP-γ-S caused a slowly developing inhibition of ICa(N) which peaked at 50.7±8.5% (n=6), 4 min after establishing whole-cell configuration. This effect was comparable with the inhibition caused by ATP (300 μM; see above). Hence, Ca2+ channels appeared to be blocked both by direct (internal GTP-γ-S) and indirect (external ATP) G-protein activation (Dolphin, 1998). Moreover, a strongly depolarizing pre-pulse to +120 mV (20 ms duration) markedly counteracted the effects of ATP (3.2±4.8%; n=9; Figure 6A) and GTP-γ-S (10.3±0.2%; n=4; Figure 6B). Further, a reduction of the EGTA concentration in the pipette solution from 3 to 0.3 mM decreases the Ca2+ buffering capacity. However, in spite of this manipulation, the inhibitory effect of ATP (300 μM) on ICa was not altered (3 mM EGTA, 51.0±5.2%, n=6; 0.3 mM EGTA, 43.6±5.0%, n=7).
Figure 6.
Effect of a strongly depolarizing prepulse on the ATP- and GTP-γ-S-induced inhibition of N-type Ca2+ channels in HEK 293-N26 cell. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. In some experiments, the test pulse was preceded by a prepulse of 20 ms duration to +120 mV. The duration of the interpulse interval was 5 ms. The voltage protocols are illustrated on top of each plot. (A) Representative tracings illustrating the effect of ATP (300 μM) on ICa(N) evoked by a standard pulse alone (a) or by a standard pulse preceded by a prepulse (b). (B) Representative tracings illustrating the effect of intracellular GTP-γ-S (300 μM) on ICa(N) evoked by a standard pulse alone (a) or by a standard pulse preceded by a prepulse (b). The time period after establishing whole-cell configuration is indicated. Recordings both in (A) and (B) were from the same cell, respectively.
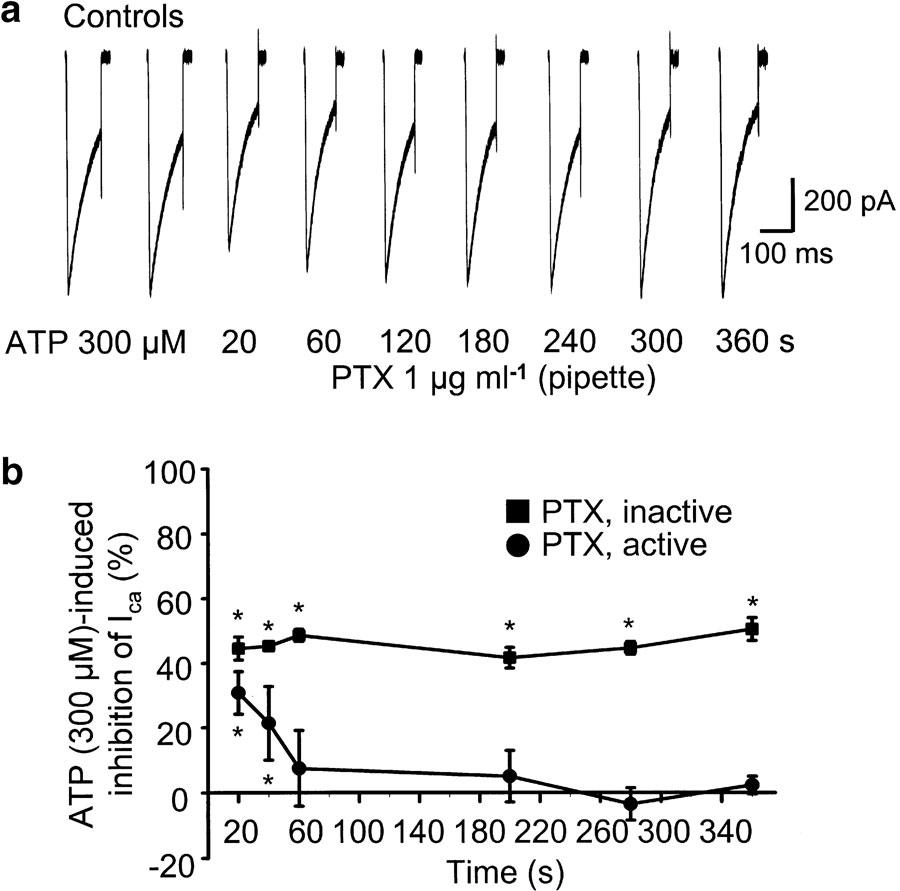
Finally, it was tested whether the inhibition of Ca2+ channels by P2Y receptor activation is associated with a G protein of the PTX-sensitive Gi/Go family. For this purpose, PTX was activated according to the protocol described in Methods and included into the pipette solution at a concentration of 1 μg ml−1. Immediately after establishing whole-cell access, ATP (300 μM) depressed Ca2+ current amplitudes as usual (Figure 7a and b). However, this effect gradually decreased and completely disappeared 60 s later. In contrast, the effect of ATP was stable over time, when PTX was not activated before inclusion into the pipette (Figure 7b), suggesting the involvement of a PTX-sensitive G protein in the modulation of Ca2+ channels by ATP.
Figure 7.
Effect of intracellular PTX on the ATP-induced inhibition of N-type Ca2+ channels in HEK 293-N26 cells. Cells were depolarized to −10 mV from a holding potential of −90 mV for 100 ms every 20 s to induce inward currents. Whole-cell recordings were performed with a pipette solution containing activated or nonactivated PTX (1 μg ml−1). Two control currents were recorded immediately after establishing whole-cell access. Then, ATP (300 μM) was applied for 6 min. (a) Representative tracings showing time-dependent decrease of the ATP-induced inhibition after application of activated PTX via the pipette solution. The presence of ATP in the superfusion medium is indicated by the number of seconds. (b) ATP-induced inhibition of ICa(N) with activated and nonactivated PTX in the pipette solution. Means±s.e.m. of 5–7 experiments. *P<0.05; statistically significant difference from the second current response in this series.
Presence of P2Y receptors
Using RT–PCR, the expression of mRNA encoding P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12 and P2Y13 was investigated in HEK 293-N26 cells cultured under identical conditions as those cells which were subjected to electrophysiological recordings. mRNAs coding for P2Y1, P2Y4, P2Y6, P2Y11 and P2Y13 were detected by the occurrence of 528, 431, 380, 410 and 575 bp amplification products, respectively (Figure 8A). No products were generated in RNA samples not subjected to cDNA synthesis, indicating that those samples were free from genomic DNA. No evidence was obtained for the expression of P2Y2 and P2Y12 receptors (Figure 8A). With the help of immunocytochemical staining, P2Y1 but not P2Y2 receptor subtypes were detected; in a few cells, especially within cell aggregates, P2Y4 receptor immunoreactivity was observed (Figure 8B). Since P2Y13 receptor antibodies were not available, the search for the presence of this particular receptor was not possible.
Figure 8.
P2Y receptor mRNA expression and immunohistochemistry in HEK 293-N26 cells. (A) Subsequent to total RNA extraction and RT–PCR amplification with primers specific for distinct P2Y receptor cDNA fragments (P2Y13, 25 cycles; P2Y1–P2Y12, 35 cycles), cDNA products were analyzed by agarose gel (1.5%) electrophoresis. A representative gel with ethidium bromide-stained cDNA fragments is shown: P2Y1 (528 bp), P2Y4 (431 bp), P2Y6 (380 bp), P2Y11 (410) and P2Y13 (575 bp). P2Y2 and P2Y12 transcripts were not detectable. (B) Representative fluorescence image of HEK 293-N26 cells after immunocytochemical labeling of P2Y1 (a) and P2Y4 receptors (c), with rabbit anti-P2Y1 and P2Y4 receptor antibodies (scale bar, 20 μm). P2Y2 receptor immunoreactivities (b) could not be detected.
Discussion
Modulation of VACCs by neurotransmitters is involved in the presynaptic regulation of neurotransmission (Hille, 1992,1994). In this respect, the inhibition of N-type Ca2+ channels following activation of G-protein-coupled receptors seems to be particularly important. Recent studies have shown that extracellular ATP may either facilitate transmitter release via P2X or depress transmitter release via P2Y receptor activation (von Kügelgen et al., 1993; Boehm, 1999). The exact transduction mechanism of P2Y receptors leading to the inhibition of neurosecretion is still unknown, although recent data suggest that, in bovine adrenal chromaffin cells, a modulation of VACCs underlies the inhibitory effect of ATP on exocytosis (Powell et al., 2000).
Native P2Y12 receptors depressed VACCs in neuronally differentiated rat PC12 cells (Kubista et al., 2003). Moreover, P2Y12 receptors inhibited the evoked entry of calcium through N-type calcium channels at the neuronal processes of these cells (Kulick & von Kügelgen, 2002). Finally, ATP and/or other nucleotides depressed N-type Ca2+ currents in superior cervical ganglion cells that were additionally transfected with P2Y1 (Filippov et al., 2000), P2Y2 (Filippov et al., 1998), P2Y4 (Filippov et al., 2003) or P2Y6 cRNA (Filippov et al., 1999). We used an alternative approach in that endogenous human P2Y receptors were activated to inhibit a recombinant rabbit N-type Ca2+ channel transfected into a HEK 293 cell line.
The HEK 293 cell line is a suitable and widely used system for the expression of VACCs. Untransfected HEK 293 cells do not contain high VACCs (McNaughton & Randall, 1997). Furthermore, Ca2+ currents, evoked in our transfected HEK 293-N26 cells, exhibited the electrophysiological characteristics described for N-type Ca2+ channels with a peak current of the I/V curve around −10 mV, tail current (McNaughton & Randall, 1997), inhibition by Co2+ ions (Wakamori et al., 1998) and sensitivity to ω-conotoxin GVIA that specifically binds to the N-type Ca2+ channel (Olivera et al., 1994).
Previous studies in neurons have identified different G-protein-dependent pathways for the inhibition of ICa(N) (Hille, 1994). One mechanism may involve diffusible second messengers and the subsequent activation of protein kinases (Boehm et al., 1996). A second mechanism is membrane-delimited and involves neurotransmitter receptors, G proteins and VACCs. In the present experiments, ATP time- and concentration-dependently inhibited Ca2+ currents and slightly shifted the voltage–current curve to the right. A shift in the voltage dependence of activation, a slowed activation kinetics and a reduced current amplitude of Ca2+ currents are characteristic properties following stimulation of G-protein-coupled receptors (Bean, 1989). Channels with these characteristics are described as being ‘reluctant' to open (Bean, 1989). However, a strong depolarizing pulse applied before the test pulse can reconvert ‘reluctant' into ‘willing' channels (Bean, 1989; Ikeda, 1991), a phenomenon called prepulse facilitation. In this study, application of a strongly depolarizing prepulse could prevent ATP-induced inhibition of the Ca2+ current, indicating a G-protein-mediated mechanism of Ca2+ channel modulation. Intracellular dialysis with GDP-β-S, an inhibitor of G-protein-dependent reactions (Sternweis & Pang, 1990), also prevented the inhibitory action of ATP. In order to mimic G protein stimulation by ATP, GTP-γ-S, an irreversible activator of G proteins (Gilman, 1987), was included into the pipette solution. Under these conditions, Ca2+ channel inhibition, comparable to the degree of inhibition induced by ATP, was observed 5 min after establishing whole-cell access. It is noteworthy that prepulse facilitation reversed the GTP-γ-S-induced inhibition as well. Since PTX inhibited the effect of ATP on ICa(N), the involvement of Gi,o can be assumed in this process.
Membrane-delimited pathways comprise a direct effect of the activated G protein subunit or subunits on the Ca2+ channel itself. A key role of the Gβγ rather than Gα subunits had been proposed (Herlitze et al., 1996; Ikeda, 1996). A Ca2+-dependent inactivation of the N-type Ca2+ channel (Budde et al., 2002), as a consequence of the P2Y receptor-induced increase of intracellular Ca2+, could be excluded by reducing the usual intrapipette concentration of EGTA. Under these conditions, the calcium-buffering capacity of EGTA decreases and a Gα-mediated triggering of the phospholipase C/inositol 1,4,5-trisphosphate (IP3)/Ca2+ pathway should lead to a larger inhibition of ICa(N). However, this was apparently not the case, indicating the involvement of Gβγ rather than Gα in this process.
Furthermore, it was of interest whether all types or only a single type of endogenous P2Y receptors expressed by HEK 293-N26 cells are involved in the modulation of ICa(N). Until now, eight P2Y receptors with molecularly distinct properties have been described (P2Y1–12, Burnstock & Williams, 2000; P2Y13, Communi et al., 2001; P2Y14, Abbracchio et al., 2003). Conflicting reports concerning the occurrence of P2Y receptor subtypes in HEK 293 cells exist. It has been suggested that HEK 293 cells exhibit IP3 responses to adenine nucleotides via activation of P2Y1 and P2Y2 receptor subtypes and furthermore mRNA for the P2Y1, but not for the P2Y4 subtype, was detected, using RT–PCR (Schachter et al., 1997). In a comprehensive study, copies of P2Y1, P2Y4 and P2Y11 mRNA, but not of P2Y2, and P2Y6 mRNA were determined (Moore et al., 2001). Finally, P2Y1 and P2Y4 receptor activation released Ca2+ from their intracellular storage sites in HEK 293 cells (Fischer et al., 2003).
The present data confirm the outcome of the study of Moore et al. (2001) by detecting P2Y1, P2Y4 and P2Y11 mRNAs in HEK 293-N26 cells using RT–PCR. In addition, P2Y6 and P2Y13 mRNA was found, whereas no evidence was obtained for the expression of P2Y2 and P2Y12 receptors. Accordingly, P2Y1 and P2Y4, but not P2Y2 receptor immunoreactivities, were identified by an immunocytochemical approach. The reported variability in the P2Y receptor endowment of HEK 293 cells may be due to the fact that different subcultures express different sets of P2Y receptors (i.e. for P2Y13, compare this study with Zhang et al., 2002).
In the present experiments, ADP and ADP-β-S were more potent than ATP; α,β-meATP, UDP and UTP were weak agonists only. ADP and ADP-β-S preferentially activate the human P2Y1, P2Y12 and P2Y13 receptor subtypes that are practically insensitive to UTP and UDP (von Kügelgen & Wetter, 2000; Communi et al., 2001). ATP and UTP are equipotent on P2Y2 receptors (von Kügelgen & Wetter, 2000), while the human P2Y4 and P2Y6 receptors are preferentially stimulated by UTP and UDP, respectively (von Kügelgen & Wetter, 2000). The low residual activity of UTP and UDP in the present study may be due to the interconversion of UDP to ADP by nucleoside diphosphokinase (Harden et al., 1997), and the subsequent activation of P2Y13 receptors by ADP. The failure of α,β-meATP to considerably inhibit ICa(N) was not surprising, because α,β-meATP is a P2X1,3 receptor-selective agonist (Khakh et al., 2001).
Whereas the agonist profile of the endogenous receptor present in HEK 293-N26 cells indicates a preference for ADP, its antagonist profile conforms with a P2Y13, but not with a P2Y1 or P2Y12 receptor. The P2Y1 receptor-selective antagonists MRS 2179 (Nandanan et al., 1999) and PPADS (von Kügelgen & Wetter, 2000; for high concentrations of PPADS, see Marteau et al., 2003) did not interfere with ATP. The P2Y12 receptor-preferential antagonist 2-MeSAMP (Hollopeter et al., 2001), which is a partial agonist at P2Y13 receptors with a low antagonistic potency (Marteau et al., 2003), also failed to alter the ATP effect. Moreover, AR-C69931MX, with selectivities for P2Y12 and P2Y13 receptors (Barnard & Simon, 2001; Boeynaems et al., 2003; Marteau et al., 2003), antagonized the ATP-induced inhibition of ICa(N). The incomplete blockade of the ATP response by AR-C6993MX may be due to the fact that this compound belongs to a class of antagonists which act in the nanomolar range at P2Y12, but only in the micromolar range at P2Y13 receptors (Boeynaems et al., 2003). The nonselective P2 receptor antagonist suramin was also effective. It is noteworthy that suramin has been reported not to discriminate between an endogenous adenosine receptor and the P2Y13 receptor transfected into 1321N1 cells coexpressing also Gα16 (Marteau et al., 2003). In view of the somewhat higher antagonistic potency of suramin in comparison with AR-C69931MX, and the existence of the P2Y11 receptor mRNA in HEK 293 cells, a minor contribution of this partly Gi-coupled receptor type (Ralevic & Burnstock, 1998) to the depression of ICa(N) cannot be excluded by the present experiments.
Our finding that PTX abolishes the inhibitory effect of ATP on N-type Ca2+ channels also assigns this effect to P2Y12 or P2Y13 in contrast to P2Y1 receptors. Whereas P2Y1 receptors couple to the PTX-insensitive Gq,11, P2Y12 and P2Y13 receptors couple to the PTX-sensitive Gi (Ralevic & Burnstock, 1998; Hollopeter et al., 2001; Zhang et al., 2002). Since P2Y13 receptor antibodies are not available, a direct proof for the presence of the P2Y13 receptor protein in HEK 293 cells is still missing. However, based on the combined pharmacological and RT–PCR evidence supporting the involvement of a PTX-sensitive P2Y receptor, it is suggested that endogenous P2Y13 receptors negatively interact with N-type Ca2+ channels, while P2Y1 and P2Y4 receptors release Ca2+ from intracellular pools subsequent to an increased production of IP3 (Fischer et al., 2003). Recently, analysis of the distribution of the P2Y13 receptor mRNA revealed high-level expression in tissues of several brain regions (Zhang et al., 2002). Hence, it appears to be appropriate to assume that a native P2Y13 or P2Y13-like receptor inhibits ICa(N) in neurons and thereby exerts a pre- and/or postsynaptic modulatory action.
Acknowledgments
This work was supported by the Bundesministerium für Bildung, Forschung und Technologie, Leitprojekt ‘Molekulare Medizin' (01GG981/0) and the Deutsche Forschungsgemeinschaft (IL 20/11-1). We are grateful to Dr Ch. Lange-Dohna (Rudolf-Boehm-Institute of Pharmacology and Toxicology, University of Leipzig, Germany) and K. Kameda (Department of Molecular and Cellular Biology, Nippon Boehringer Ingelheim Co. Ltd, Kawanishi Pharma Research Institute, Japan) for methodological help. We are grateful to M. Henschke and A. Sommer for excellent technical assistance.
Abbreviations
- α,β-meATP
α,β-methylene ATP
- ADP-β-S
adenosine 5′-O-(2-thiodiphosphate)
- AR-C69931MX
N6-(2-methylthioethyl)-2-(3,3,3-trifluoropropylthio)-β,γ-dichloromethylene-ATP
- bp
base pair
- HEK
human embryonic kidney
- IP3
inositol 1,4,5-trisphosphate
- 2-MeSAMP
2-methylthio AMP
- 2-MeSATP
2-methylthio ATP
- MRS 2179
2′deoxy-N6-methyladenosine-3′,5′-diphosphate
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- PTX
pertussis toxin
- RT–PCR
reverse transcription and polymerase chain reaction
- TEA
tetraethylammonium
- VACC
voltage-activated calcium channel
References
- ABBRACCHIO M.P., BOEYNAEMS J.-M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNARD E., SIMON J. An elusive receptor is finally caught: P2Y12, an important drug target in platelets. Trends Pharmacol. Sci. 2001;22:388–391. doi: 10.1016/s0165-6147(00)01759-4. [DOI] [PubMed] [Google Scholar]
- BARRY P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J. Neurosci. Meth. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- BEAN B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- BLEAKMAN D., BOWMAN D., BATH C.P., BRUST P.F., JOHNSON E.C., DEAL C.R., MILLER R.J., ELLIS S.B., HARPOLD M.M., HANS M. Characteristics of a human N-type calcium channel expressed in HEK 293 cells. Neuropharmacology. 1995;34:753–765. doi: 10.1016/0028-3908(95)00078-k. [DOI] [PubMed] [Google Scholar]
- BOEHM S. ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J. Neurosci. 1999;19:737–746. doi: 10.1523/JNEUROSCI.19-02-00737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEHM S., HUCK S., FREISSMUTH M. Involvement of a phorbol ester-insensitive protein kinase C in the α2 adrenergic inhibition of voltage-gated Ca2+ current in chick sympathetic neurons. J. Neurosci. 1996;16:4596–4603. doi: 10.1523/JNEUROSCI.16-15-04596.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEYNAEMS J.-M., WILKIN F., MARTEAU F., DUHANT X., SAVI P., GONZALEZ N.S., ROBAYE B., COMMUNI D. P2Y receptors: new subtypes, new functions. Drug Dev. Res. 2003;59:30–35. [Google Scholar]
- BUDDE T., MEUTH S., PAPE H.-C. Calcium-dependent inactivation of neuronal calcium channels. Nat. Rev. Neurosci. 2002;3:873–883. doi: 10.1038/nrn959. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic cotransmission. Brain Res. Bull. 1999;50:355–357. doi: 10.1016/s0361-9230(99)00103-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., WILLIAMS M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J. Pharmacol. Exp. Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- CHIZH B.A., ILLES P. P2X receptors and nociception. Pharmacol. Rev. 2000;53:553–568. [PubMed] [Google Scholar]
- COMMUNI D., GONZALEZ N.S., DETHEUX M., BREZILLON S., LANNOY V., PARMENTIER M., BOEYNAEMS J.M. Identification of a novel human ADP receptor coupled to Gi. J. Biol. Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- CONIGRAVE A.D., FERNANDO K.C., GU B., TASEVSKI V., ZHANG W., LUTTRELL B.M., WILEY J.S. P2Y11 receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur. J. Pharmacol. 2001;426:157–163. doi: 10.1016/s0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- DAVILA H.M. Molecular and functional diversity of voltage-gated calcium channels. Ann. N.Y. Acad. Sci. 1999;868:102–117. doi: 10.1111/j.1749-6632.1999.tb11281.x. [DOI] [PubMed] [Google Scholar]
- DOLPHIN A.C. Mechanisms of modulation of voltage-dependent calcium channels by G proteins. J. Physiol. 1998;506:3–11. doi: 10.1111/j.1469-7793.1998.003bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPPOV A.K., BROWN D.A., BARNARD E.A. The P2Y1 receptor closes the N-type Ca2+ channel in neurones, with both adenosine triphosphates and diphosphates as potent agonists. Br. J. Pharmacol. 2000;129:1063–1066. doi: 10.1038/sj.bjp.0703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPPOV A.K., SIMON J., BARNARD E.A., BROWN D.A. Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br. J. Pharmacol. 2003;138:400–406. doi: 10.1038/sj.bjp.0705043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPPOV A.K., WEBB T.E., BARNARD E.A., BROWN D.A. P2Y2 nucleotide receptors expressed heterologously in sympathetic neurons inhibit both N-type Ca2+ and M-type K+ currents. J. Neurosci. 1998;18:5170–5179. doi: 10.1523/JNEUROSCI.18-14-05170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILIPPOV A.K., WEBB T.E., BARNARD E.A., BROWN D.A. Dual coupling of heterologously-expressed rat P2Y6 nucleotide receptors to N-type Ca2+ and M-type K+ currents in rat sympathetic neurones. Br. J. Pharmacol. 1999;126:1009–1017. doi: 10.1038/sj.bjp.0702356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER W., WIRKNER K., WEBER M., EBERTS C., KÖLES L., REINHARDT R., FRANKE H., ALLGAIER C., GILLEN C., ILLES P. Characterization of P2X3, P2Y1 and P2Y4 receptors in cultured HEK 293-hP2X3 cells and their inhibition by ethanol and trichloroethanol. J. Neurochem. 2003;85:779–790. doi: 10.1046/j.1471-4159.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- FUJITA Y., MYNLIEFF M., DIRKSEN R.T., KIM M.S., NIDOME T., NAKAI J., FRIEDRICH T., IWABE N., MIYATA T., FURUICHI T. Primary structure and functional expression of the ω-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G. G proteins: transducers of receptor-generated signals. Ann. Rev. Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- HARDEN T.K., LAZAROWSKI E.R., BOUCHER R.C. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G protein-coupled P2 receptor agonist selectivity. Trends Pharmacol. Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- HERLITZE S., GARCIA D.E., MACKIE K., HILLE B., SCHEUER T., CATTERALL W.A. Modulation of Ca2+ channels by G protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- HILLE B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- HILLE B. Modulation of ion channel function by G protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- IKEDA S.R. Double-pulse calcium channel current facilitation in adult rat sympathetic neurons. J. Physiol. 1991;439:181–214. doi: 10.1113/jphysiol.1991.sp018663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IKEDA S.R. Voltage-dependent modulation of N-type calcium channels by G protein βγ-subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- ILLES P., KLOTZ K.-N., LOHSE M.J. Signaling by extracellular nucleotides and nucleosides. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:295–298. doi: 10.1007/s002100000308. [DOI] [PubMed] [Google Scholar]
- JIN J., DASARI V.R., SISTARE F.D., KUNAPULI S.P. Distribution of P2Y receptor subtypes on haematopoietic cells. Br. J. Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SEGUELA P., VOIGT M., HUMPHREY P.P. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KUBISTA H., LECHNER S.G., WOLF A.M., BOEHM S. Attenuation of the P2Y receptor-mediated control of neuronal Ca2+ channels in PC12 cells by antithrombotic drugs. Br. J. Pharmacol. 2003;138:343–350. doi: 10.1038/sj.bjp.0705037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULICK M.B., VON KÜGELGEN I. P2Y-receptors mediating an inhibition of the evoked entry of calcium through N-type calcium channels at neuronal processes. J. Pharmacol. Exp. Ther. 2002;303:520–526. doi: 10.1124/jpet.102.037960. [DOI] [PubMed] [Google Scholar]
- MARTEAU F., LE POUL E., COMMUNI D., COMMUNI D., LABOURET C., SAVI P., BOEYNAEMS J.-M., GONZALEZ S. Pharmacological characterization of the human P2Y13 receptor. Mol. Pharmacol. 2003;64:104–112. doi: 10.1124/mol.64.1.104. [DOI] [PubMed] [Google Scholar]
- MCNAUGHTON N.C., RANDALL A.D. Electrophysiological properties of the human N-type Ca2+ channel: I. Channel gating in Ca2+, Ba2+ and Sr2+ containing solutions. Neuropharmacology. 1997;36:895–915. doi: 10.1016/s0028-3908(97)00085-3. [DOI] [PubMed] [Google Scholar]
- MOORE D.J., CHAMBERS J.K., WAHLIN J.-P., TAN K.B., MOORE G.B., JANKINS O., EMSON P.C., MURDOCK P.R. Expression pattern of human P2Y receptor subtypes: a quantitative reverse transcription–polymerase chain reaction study. Biochem. Biophys. Acta. 2001;1521:107–119. doi: 10.1016/s0167-4781(01)00291-3. [DOI] [PubMed] [Google Scholar]
- NANDANAN E., CAMAIONI E., JANG S.Y., KIM Y.C., CRISTALLI G., HERDEWIJN P., SECRIST J.A., TIWARI K.N., MONRAHAM A., HARDEN T.K., BOYER J.L., JACOBSON K.A. Structure–activity relationships of bisphosphate nucleotide derivatives as P2Y1 receptor antagonists and partial agonists. J. Med. Chem. 1999;42:1625–1638. doi: 10.1021/jm980657j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NÖRENBERG W., ILLES P. Neuronal P2X receptors: localisation and functional properties. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:324–339. doi: 10.1007/s002100000311. [DOI] [PubMed] [Google Scholar]
- OLIVERA B.M., MILJANICH G.P., RAMACHANDRAN J., ADAMS M.E. Calcium channel diversity and neurotransmitter release: the ω-conotoxins and ω-agatoxins. Annu. Rev. Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- POELCHEN W., SIELER D., WIRKNER K., ILLES P. Co-transmission of ATP with noradrenaline in neurons of the rat nucleus locus coeruleus. Neuroscience. 2001;102:593–602. doi: 10.1016/s0306-4522(00)00529-7. [DOI] [PubMed] [Google Scholar]
- POWELL A.D., TESCHEMACHER A.G., SEWARD E.P. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J. Neurosci. 2000;20:606–616. doi: 10.1523/JNEUROSCI.20-02-00606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SCHACHTER L.B., SROMEK S.M., NICHOLAS R.A., HARDEN T.K. HEK 293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- SIMON J., FILIPPOV A.K., GÖRANSSON S., WONG Y.H., FRELIN C., MICHEL A.D., BROWN D.A., BARNARD E. Characterization and channel coupling of the P2Y12 nucleotide receptor of brain capillary endothelial cells. J. Biol. Chem. 2002;277:31390–31400. doi: 10.1074/jbc.M110714200. [DOI] [PubMed] [Google Scholar]
- STERNWEIS P.C., PANG I.H. The G protein-channel connection. Trends Neurosci. 1990;13:122–126. doi: 10.1016/0166-2236(90)90002-r. [DOI] [PubMed] [Google Scholar]
- VARADI G., STROBECK M., KOCH S., CAGLIOTI L., ZUCCHI C., PALYI G. Molecular elements of ion permeation and selectivity within calcium channels. Crit. Rev. Biochem. Mol. Biol. 1999;34:181–214. doi: 10.1080/10409239991209264. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., KURZ K., STARKE K. Axon terminal P2-purinoceptors in feedback control of sympathetic transmitter release. Neuroscience. 1993;56:263–267. doi: 10.1016/0306-4522(93)90330-i. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., STARKE K. Noradrenaline-ATP co-transmission in the sympathetic nervous system. Trends Pharmacol. Sci. 1991;12:319–324. doi: 10.1016/0165-6147(91)90587-i. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., WETTER A. Molecular pharmacology of P2Y receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WAKAMORI M., STROBECK M., NIIDOME T., TERAMOTO T., IMOTO K., MORI Y. Functional characterization of ion permeation pathway in the N-type Ca2+ channel. J. Neurophysiol. 1998;79:622–634. doi: 10.1152/jn.1998.79.2.622. [DOI] [PubMed] [Google Scholar]
- WHEELER D.B., RANDALL A.D., TSIEN R.W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M.E., BRUST P.F., FELDMAN D.H., PATTHI S., SIMERSON S., MAROUFI A., MYCUE A.F., VELICELEBRI G., ELLIS S.B., HARPOLD M.M. Structure and function of an ω-conotoxin sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- WIRKNER K., ALLGAIER C., SCHWEIGEL J., ILLES P. Interaction between P2Y receptors and N-type Ca2+-channels expressed in a HEK 293-N26 cell line. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:R116. [Google Scholar]
- ZAMPONI G.W., SNUTCH T.P. Modulation of voltage-dependent calcium channels by G proteins. Curr. Opin. Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- ZHANG F.L., LUO L., GUSTAFSON E., PALMER K., QIAO X., FAN X., YANG S., LAZ T.M., BAYNE M., MONSMA F. P2Y13: identification and characterization of a novel Gαi-coupled ADP receptor from human and mouse. J. Pharmacol. Exp. Ther. 2002;301:705–713. doi: 10.1124/jpet.301.2.705. [DOI] [PubMed] [Google Scholar]