Abstract
Tolerance to opioids frequently follows repeated drug administration and affects the clinical utility of these analgesics. Studies in simple cellular systems have demonstrated that prolonged activation of opioid receptors produces homologous receptor desensitization by G-protein receptor kinase mediated receptor phosphorylation and subsequent β-arrestin binding. To define the role of this regulatory mechanism in the control of the electrophysiological and behavioral responses to opioids, we used mice having a targeted disruption of the G-protein receptor kinase 3 (GRK3) gene.
Mice lacking GRK3 did not differ from wild-type littermates neither in their response latencies to noxious stimuli on the hot-plate test nor in their acute antinociceptive responses to fentanyl or morphine.
Tolerance to the electrophysiological response to the opioid fentanyl, measured in vitro in the hippocampus, was blocked by GRK3 deletion. In addition, tolerance to the antinociceptive effects of fentanyl was significantly reduced in GRK3 knockouts compared to wild-type littermate controls.
Tolerance to the antinociceptive effects of morphine was not affected by GRK3 deletion although morphine tolerance in hippocampal slices from GRK3 knockout mice was significantly inhibited. Tolerance developed more slowly in vitro to morphine than fentanyl supporting previous work in in vitro systems showing a correlation between agonist efficacy and GRK3-mediated desensitization.
The results of these studies suggest that GRK3-mediated mechanisms are important components of both electrophysiologic and behavioral opioid tolerance. Fentanyl, a high efficacy opioid, more effectively produced GRK3-dependent effects than morphine, a low efficacy agonist.
Keywords: GRK3, tolerance, withdrawal, phosphorylation, opioid receptor, receptor desensitization, fentanyl, morphine
Introduction
Although opioids have been used for thousands of years for the treatment of severe pain, prolonged or repeated exposure to these drugs can produce tolerance, physical dependence and addiction. The mechanisms underlying opioid analgesic tolerance are multifaceted and are likely to include receptor desensitization (Bohn et al., 2000), delayed receptor resensitization (Whistler et al., 1999; Finn & Whistler, 2001; He et al., 2002), and compensatory pharmacological (Gintzler & Chakrabarti, 2000), physiological (Mayer & Mao, 1999) or behavioral (Siegel et al., 2000) processes (for a review see Harrison et al., 1998; Liu and Anand, 2001; Kieffer & Evans, 2002). At the cellular level, responses to opioids are readily observed, and prolonged activation of opioid receptors leads to a gradual reduction in the amplitude of the opioid effect (see Nestler & Aghajanian, 1997). The mechanisms responsible for desensitization are not completely defined; however, opioid receptors are members of the G-protein-coupled receptor (GPCR) superfamily that includes rhodopsin and the β-adrenergic receptor. Desensitization of responses to agonists at the cellular level is mediated by G-protein-coupled receptor kinases (GRK) and subsequent arrestin binding to the phosphorylated receptors (e.g., Ferguson et al., 1996; Pitcher et al., 1998; Lowe et al., 2002). Similarly, reconstitution of mu- delta- or kappa-type opioid receptor (also called mu, delta and kappa opioid peptide receptors as per IUPHAR guidelines) signaling in mammalian cell lines (Yu et al., 1997) or Xenopus oocytes (Kovoor et al., 1997) has been found to produce opioid receptor mediated-responses in these expression systems that desensitize by GRK-dependent mechanisms. In particular, in vitro studies have demonstrated that GRK3 is the most effective member of this kinase family in producing agonist-dependent opioid receptor desensitization, although overexpression of GRK5 has also been found to be effective (Appleyard et al. 1997). In this study, we used mice lacking GRK3 (Peppel et al., 1997) to explore the relationship between homologous receptor desensitization in expression systems and opiate effects in situ using both electrophysiological and behavioral measures of opioid tolerance. In prior studies (Kovoor et al., 1998), we found that highly efficacious opioids such as fentanyl or DAMGO were more effective than lower efficacy opioids such as morphine in evoking GRK-dependent homologous desensitization of the mu opioid receptor. Thus, in these studies, we studied opioid tolerance using both a high efficacy and a low efficacy agonist.
Methods
Breeding and genotyping of mice
Homozygous GRK3 (−/−) mice were prepared by homologous recombination as previously described (Peppel et al., 1997). Animals were backcrossed with C57/B6 for at least six generations. Heterozygotes were then crossed to generate GRK3 (−/−) mice and littermate GRK3 (+/+) controls. By using wild-type littermates as controls, we assume that any residual genetic heterogeneity other than the GRK3 gene deletion will be randomly distributed among the tested animals. Mice were genotyped using tail sample DNA as a template in a pair of PCR reactions. One used a common primer (5′CAGGGCTAGGTGTGACTGTCATGT) that recognizes a sequence present in both wild-type and knockout mice along with a primer (5′CTTCCACAGCTGAGCATGAACGAC) based on a sequence within the GRK3 gene. The other used a primer (5′CTGACTAGGGGAGGAGTAGAAGGT) that recognizes a DNA sequence present in the neomycin (neo) resistance gene in the knockouts. GRK3 (−/−) mice were not visually distinguishable from their littermate GRK3 (+/+) controls, and thus all behavioral and electrophysiological studies were performed with the investigator blind to genotype.
Drugs
Fentanyl (Sigma, St Louis, MO, U.S.A.), morphine sulfate (Sigma), morphine sulfate pellets (NIDA, Bethesda, MD, U.S.A.), naloxone (Sigma).
Behavioral methods
4–5-week-old male knockout and wild-type littermate mice (20±4 g) were tested for baseline nociceptive responses using a 55°C hot-plate (hind-paw lick or jump). Mice of this age were chosen for consistency with the electrophysiological studies. We have previously reported that mice of this age from the background strains of these knockout mice do not differ from each other in their hot-plate latencies and demonstrate reproducible naloxone-reversible opiate-induced hot-plate antinociception (Varnado-Rhodes et al., 2000).
In the fentanyl studies, fentanyl was injected subcutaneously (0.01 ml g−1 volume) and hot-plate response latencies were measured every 15 min for the next hour. Animals were removed from the hot-plate following either hind-paw lick or 30 s at each time point. The 30 s was chosen as a maximum exposure to the hot-plate in order to minimize tissue damage and because pilot studies demonstrated that as many as 10 hot-plate tests as often as every 15 min did not produce long-term changes in hot-plate latencies in C57/B6 mice injected with fentanyl, morphine or saline.
After 3–7 days, under Halothane anesthesia, one Alzet mini-pump (100 μl pump; with delivery rate of 0.5 μl hr−1) filled with fentanyl (8 mg ml−1) was placed subcutaneously in the lumbar back of each animal using surgical scissors for incision, a curved hemostat for pocket formation and wound clips for wound closure.
Animals were tested for hot-plate pain responses (as above) before implantation and at 12, 24, 36, 42, 48, 52, 60, 72, 84 and/or 94 h after implantation. Animals appeared to behave normally within 30 min of surgery and the surgery itself did not effect hot-plate antinociception. GRK3 knockout and littermate wild-type mice (n=5) who underwent sham implantation using the same surgical technique but without opioid implantation did not show significant differences from their presurgical baseline responses (data not shown). All testing was performed during the dark phase of a 12 h on/12 h off light cycle as pain sensitivity is known to change during the sleep–wake cycle (Crockett et al., 1977). In separate replicates of the general experiment, implantation times were adjusted to allow testing intervals after implantation to coincide with the dark phase of the light cycle. Approximately equal numbers of GRK knockout and wild-type littermate control mice (n=5–6) were included in all experimental replicates. No animals during the study showed any evidence of foot damage caused by repeated hot-plate testing.
At 1 week after fentanyl pump implantation, mice were weighed and injected with naloxone 10 mg kg−1 s.c. and placed on a piece of previously weighed filter paper covered by an inverted 500 ml glass beaker. Mice were observed for 20 min noting five different measures associated with opioid withdrawal: total number of wet-dog shakes, total number of jumps and the number of minutes during which paw treading, paw tremor and teeth chattering behaviors occurred (Ledent et al., 1999). After the 20-min observation period, mice and filter paper were weighed again to quantify weight change and defecation (both were converted to % body weight). Saline injections in a subgroup of GRK3 knockout and wild-type littermate mice (n=3) induced none of these stereotypic opioid withdrawal behaviors. Similarly, sham-implanted (opioid naive) knockout and wild-type animals (n=5) showed no significant withdrawal behaviors following either naloxone or saline injections administered in a counterbalanced way.
In the morphine studies, morphine sulfate 10 mg kg−1, instead of fentanyl, was injected subcutaneously following baseline hot-plate testing and then hot-plate tests were conducted every 30 min for 150 min. The morphine tolerance and withdrawal studies were similar to those using fentanyl except that drug was administered using a 75-mg morphine pellet rather than a fentanyl containing minipump implanted in the back of each animal.
Hippocampal slice electrophysiology methods
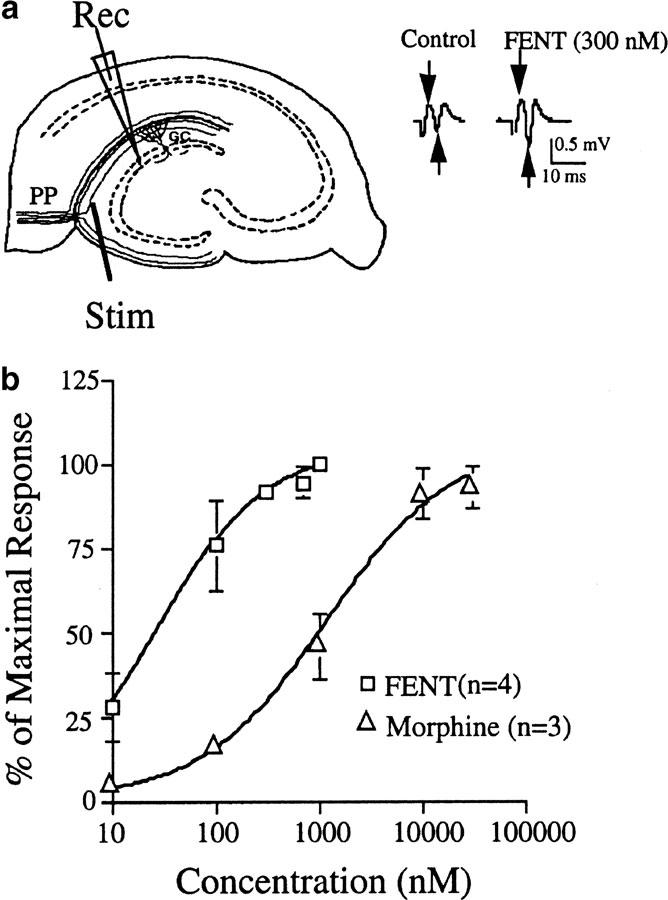
Male mice (4–5 weeks old) were decapitated, and the brains quickly removed, cooled, blocked and cut using a Vibratome (Technical Products International) into 400 μm transverse sections. Slices were transferred to a warmed (33°C), submerged tissue recording chamber perfused at 1 ml min−1 with modified artificial cerebrospinal fluid (with a composition in mM of: NaCl 120; KCl 3.5; CaCl2 2.5; MgCl2 2 1.3; NaH2PO4 1.25; NaHCO3 26 and glucose 10 saturated with 95% O2/5% CO2) (pH 7.4). Hippocampal slices were equilibrated in the recording chamber for at least 1 hour. A glass recording electrode (1-2 μm tip diameter) was filled with 3 M NaCl and placed in the granule cell layer of the hippocampal dentate gyrus. A 50 μm concentric bipolar stimulating electrode (SNE-100, Rhodes Medical Supply) was placed in the outer molecular layer at the apex of the dentate gyrus to stimulate the perforant path (see, for example, Figure 4a schematic). Population responses of granule cells were recorded with an Axopatch 200 (Axon Instruments). Axotape was used for acquiring the data, and Clampfit (Axon Instruments) was used for analysis. Stimulation consisted of paired-pulse stimulation (0.3 ms pulse duration and 40 ms interpulse interval). The stimulus intensity was adjusted to evoke a response one-third of the maximum (S1/3) and stimuli were administered at 1-min intervals throughout the experimental period.
Figure 4.
Hippocampal slice methodology. (a) Schematic diagram of a hippocampal slice with a stimulating electrode (Stim) placed in the perforant path fibers (PP) of the dentate gyrus. A recording electrode (Rec) is also pictured in the granular cell layer (GC) of the dentate gyrus. Representative examples of paired pulse stimulus-evoked field EPSPs in the dentate gyrus. (arrows illustrate peak-to-peak measurement of the second population spike amplitude) before (control) and after (FENT) opiate application. (b) Dose-dependent potentiation of dentate gyrus field potential amplitudes by morphine and fentanyl in C57/B6 mice.
Peak-to-peak amplitude of the second population response from each stimulus pair was measured as an indicator of cell excitability. Drugs were applied by perfusion in the modified artificial cerebrospinal fluid. In dose–response studies, up to three doses of drug were perfused for each slice in cumulative fashion with a total drug exposure of <30 min. Tolerance was operationally defined as the reduction in fentanyl-induced potentiation following 1 h of fentanyl exposure.
Statistical analysis
Analysis of variance was used to compare the data sets statistically. Separate group by trial two-way ANOVAs, with one repeated measure, were performed for baseline measurements, acute opioid (in vitro and in vivo) and opioid tolerance studies.
In the fentanyl tolerance studies, separate analyses were performed for each experimental replicate, although the data were merged for ease of presentation and a more detailed time course of the development of fentanyl analgesic tolerance. As different sets of animals were tested at different time points (in order to maintain dark-phase behavioral testing), we were able to evaluate the effect of repeated hot-plate tests on the development of analgesic tolerance in separate analyses. At no time point post-opioid implantation did hot-plate responses differ from one another as a function of the number of previous hot-plate tests to which the animals had been exposed. Similarly, in the morphine tolerance studies, separate wild-type mice tested for the first time 72 h after morphine pellet implantation demonstrated no difference in antinociceptive tolerance from implanted mice at that time point whom had already been repeatedly tested on the hot-plate (data not shown). Both sets of data support the use of the hot-plate test in repeated measure designs evaluating both short- and long-term drug effects (cf. Espejo & Mir, 1994).
The Newman–Keuls test was used for post hoc comparisons of between group differences at any one time point. One-way between-group ANOVAs were used to analyze the withdrawal data. An alpha value of P<0.05 was used to indicate significant differences.
Results
Behavioral effects of opioids in GRK3 knockout mice
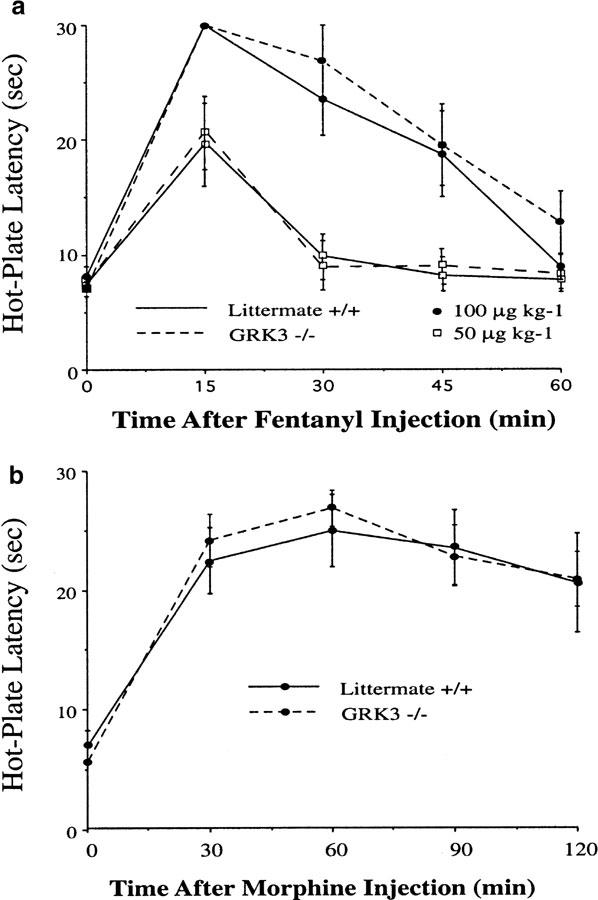
The acute analgesic effects of fentanyl and morphine in the GRK3 knockout mice and wild-type littermate controls were tested using the standard 55°C hot-plate assay by recording latency to hind-paw lick or jump. Our preliminary data (Varnado-Rhodes et al., 2000) demonstrated no significant differences in baseline hot-plate responses between the C57/B6 and 129 strains from which the GRK3 knockouts were derived (in agreement with Mogil et al., 1999). No significant differences in baseline hot-plate response latencies were observed between GRK3 knockout and wild-type littermate control mice on the hot-plate test prior to opioid administration (Figure 1a and b). GRK3 knockout mice and littermate control mice showed similar antinociception following subcutaneous injection of fentanyl (100 μg kg−1) (Figure 1a). As this dose of fentanyl produced a maximal response (the animals did not respond to the hot-plate within 30 s), we also tested the effects of a lower dose of fentanyl in another set of mice. Again, no significant difference in antinocieption was evident for GRK3 (+/+) and GRK3 (−/−) mice tested with a submaximal dose of fentanyl (50 μg kg−1) (Figure 1a). Moreover, as with the higher dose of fentanyl, the analgesia produced appeared to decrement at equal rates in the two groups. These results indicate that there were no significant pharmacokinetic differences or differences in opioid sensitivity between the groups following acute fentanyl administration. Similarly, the amplitude and time course of morphine (10 mg kg−1, s.c.) antinociception did not differ between the GRK3 (−/−) and (+/+) mice (Figure 1b). Thus, neither baseline nociception nor acute opioid sensitivity was affected by GRK3 deletion. The lack of effect of GRK3 knockout on the acute opioid response differs from the potentiation of morphine antinociception produced by β-arrestin 2 gene deletion (Bohn et al., 2000; 2002).
Figure 1.
GRK3 knockout mice (−/−) do not differ from wild-type littermate controls in baseline hot-plate nociceptive responses nor in fentanyl- or morphine-induced antinociception. (a) antinociception following fentanyl was unaffected by GRK3 knockout. Hind-paw lick or jump response latencies were measured on a 55°C hot-plate in GRK3 knockout (−/−) and GRK3 wild-type (+/+) littermate control mice (n=8) immediately before and every 15 min following a subcutaneous injection of either 100 or 50 μg kg−1 fentanyl. No significant effects of the GRK3 knockout were observed. Data are presented as means±s.e.m. (b) Morphine antinociception is unaffected in GRK3 knockout mice. Injection with 10 mg kg−1 morphine (subcutaneously; n=6) produced antinociceptive effects in both GRK3 knockout (−/−) and wild-type (GRK+/+) mice. No differences between groups at any time point were observed.
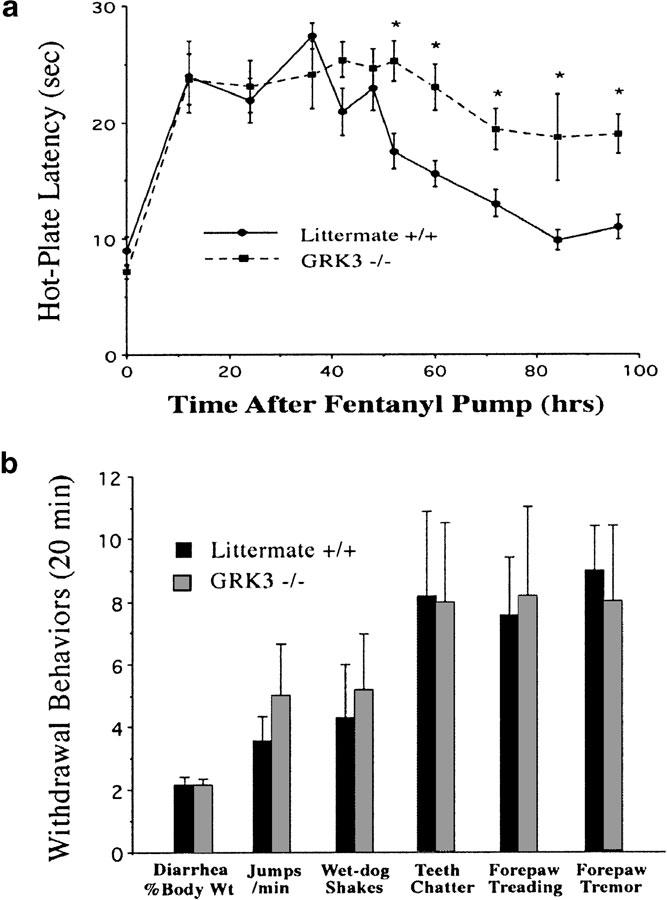
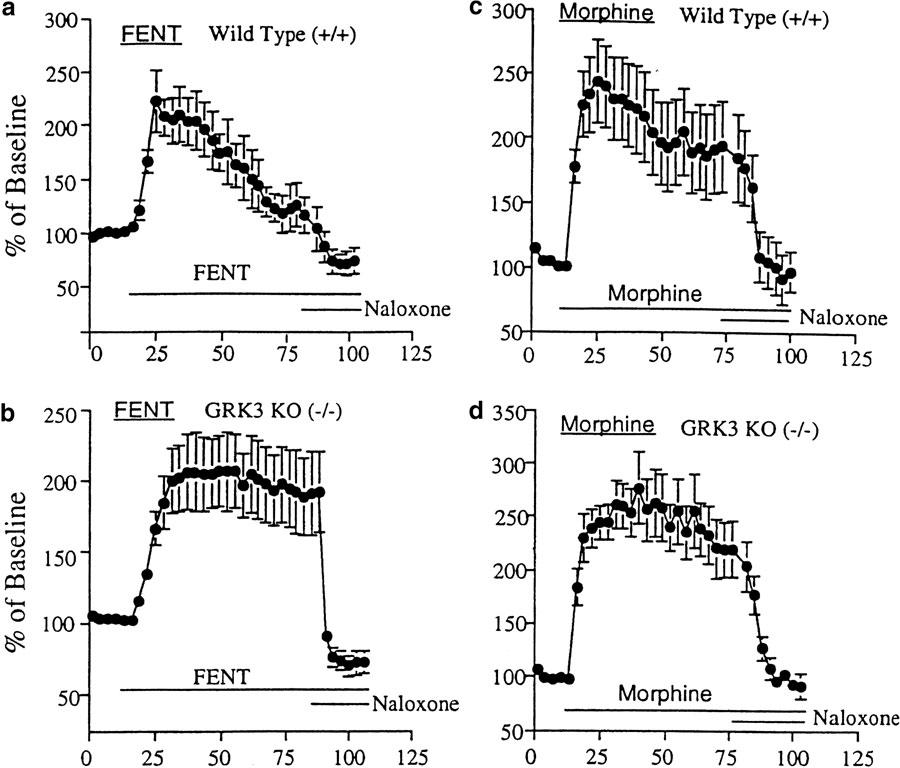
Following implantation of fentanyl-filled osmotic pumps, GRK3 (−/−) mice initially demonstrated antinociception equivalent to that seen in wild-type littermate controls (Figure 2a). These GRK3 (+/+) controls showed a slowly decrementing response (opioid tolerance) beginning about 50 h after the start of the fentanyl infusion and the response latencies for these mice returned to baseline after approximately 80 hrs of continuous fentanyl exposure. In contrast to wild-type controls, GRK3 (−/−) mice showed significantly less opioid tolerance. Even after 100 h of continuous exposure, there was little decrement in the analgesic effects of fentanyl.
Figure 2.
GRK3 knockout inhibits opioid analgesic tolerance development without effecting opioid withdrawal behaviors. (a) GRK3 (−/−) and GRK3 (+/+) littermate control mice (n=12 at each time point) were implanted with fentanyl-filled osmotic mini-pumps following baseline hot-plate testing (note the baseline hot-plate sensitivities were not different at time zero). Antinociception produced by sustained fentanyl declined during the third day of infusion in the GRK3 (+/+) mice. GRK3 (−/−) mice showed a significantly slower rate of analgesic tolerance induction (P<0.05). *signifies statistically significant group differences at that time point. (b) Withdrawal behaviors precipitated by naloxone injection (10 mg kg−1, subcutaneously) 7 days after fentanyl pump implantation. No significant difference in any withdrawal behavior was noted between knockout and wild-type mice. No significant withdrawal behaviors were observed in opioid-tolerant mice injected with saline or opioid naïve mice injected with naloxone (data not shown).
Although fentanyl tolerance was attenuated for the GRK3 (−/−) mice, withdrawal behaviors precipitated by injection of the opioid antagonist naloxone were not significantly different from those produced in the GRK3 (+/+) littermates (Figure 2b). No significant differences were observed between wild-type and GRK3 (−/−) littermates in any of the six naloxone precipitated opioid withdrawal behaviors evaluated (Figure 2b) following 1 week of fentanyl treatment. The results indicate that the compensatory adaptations produced by chronic opioid exposure unmasked during the drug withdrawal period were not dependent on GRK3 expression. Thus, GRK3 appears to be particularly important in the development of fentanyl analgesic tolerance without effecting the withdrawal behaviors produced by prolonged fentanyl administration and associated with physical dependence.
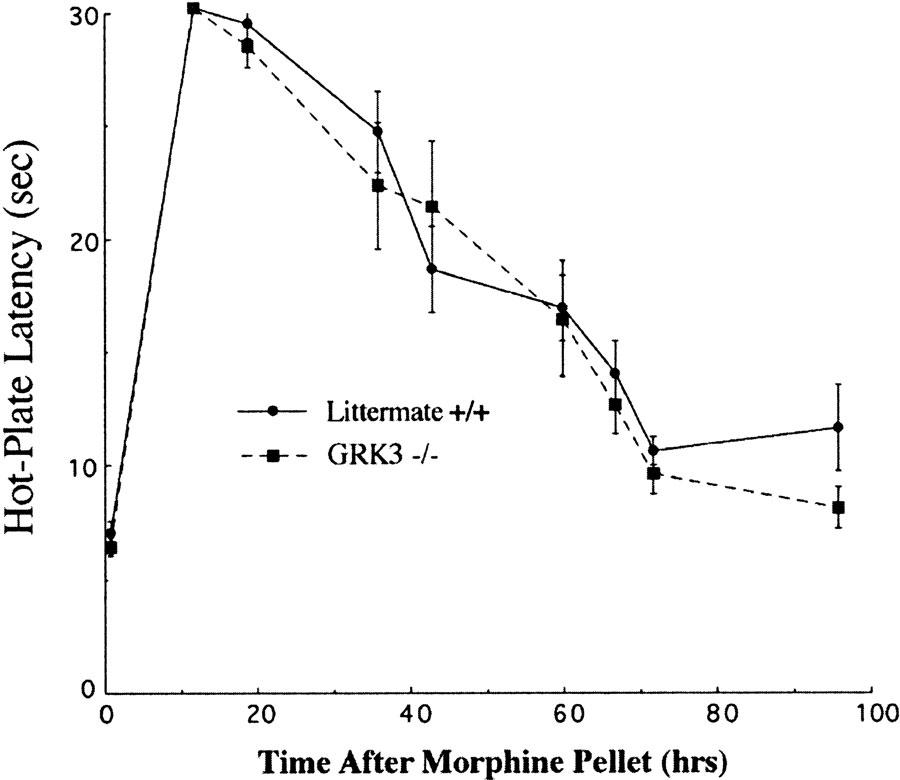
Following subcutaneous morphine pellet implantation, wild-type (GRK3 (+/+)) mice showed robust analgesic responses that slowly decremented despite continuous morphine exposure (Figure 3). The rate of tolerance to morphine analgesia was similar to that seen with fentanyl analgesia–with hot-plate responses returning to baseline after approximately 3 days. Unlike with fentanyl, however, analgesic tolerance to the opioid agonist morphine was not affected by GRK3 gene deletion (Figure 3); in that, the rate of reduction in response latency after morphine pellet implantation was not significantly different between wild-type and GRK3 knockout (−/−) mice. This result indicates that tolerance to morphine's analgesic effects are not dependent on GRK3 expression. Thus, in this standard assay of opioid analgesia, tolerance to the analgesic effects of morphine and fentanyl showed striking differences in their reliance on GRK3 mechanisms. As in the fentanyl studies, naloxone precipitated withdrawal behaviors following morphine administration did not differ for GRK(−/−) and GRK (+/+) mice.
Figure 3.
GRK3 knockout and wild-type littermate controls show no difference in morphine analgesic tolerance. Implantation of morphine pellets in knockout and wild-type mice produced similar antinociception in the two groups and analgesic tolerance developed at a similar rate.
The basis for the difference between our morphine and fentanyl tolerance results is not clear. Others have also observed differences between fentanyl and morphine analgesic tolerance. Bilsky et al. (1996), for example, reported that morphine tolerance has a greater sensitivity to NMDA receptor antagonism than does fentanyl tolerance. Duttaroy & Yoburn (1995) demonstrated that antinociception from continuously infused fentanyl developed tolerance at a slower rate than a continuous infusion of a equipotent dose of morphine–a result likely due to the fewer functioning receptors needed to produce analgesia by high efficacy agonists such as fentanyl (i.e., more spare receptors). The complex pharmacology (pharmacokinetic or spare receptor mechanisms) and physiology (learned or opponent processes including NMDA receptor mechanisms) involved in mediating opioid tolerance in vivo led us to extend our studies to a simpler, in vitro brain slice preparation.
Hippocampal slice physiology
The hippocampal slice preparation has, for many years, been studied because of its stability of neural responses for many hours. Electrical stimulation of the perforant path in the outer molecular layer of the dentate gyrus evokes a biphasic field potential recorded in the dentate granule cell layer (population spike, e.g., Figure 4a). Prior studies have established that mu opioid receptor activation in the rodent dentate gyrus produces a robust, increase in population spike amplitude (Figure 4a) caused by presynaptic inhibition of GABA release (disinhibition) (e.g., Simmons & Chavkin, 1996).
In our initial studies, we demonstrated this excitatory opioid effect in slices from C57/B6 mice using either fentanyl or morphine (Figure 4b); with fentanyl being the more potent opioid as expected. The effects of both opioids are dose-dependent (Figure 4b) and completely blocked by 100 nM naloxone (data not shown).
Our studies of the role of GRK3 in tolerance to the excitatory effects of opioids in the hippocampal slice were carried out using just-maximally effective doses of opioids (from Figure 4) and examining any changes in field potentials which took place during a 60-min infusion. When fentanyl (300 nM) was infused, for example (Figure 5a), the amplitude of the evoked response was initially increased, but sustained exposure resulted in a gradual reduction in the amplitude of the field potential towards predrug levels. Such tolerance to fentanyl's effects in slices from the GRK3 (+/+) mice (66±7.1% reduction after 60 min) was not different from that produced by the same treatment of slices from control, C57/B6 mice (60±8.3%, n=14, data not shown). Addition of 1 μM naloxone at the end of the 60-min fentanyl exposure returned the amplitude of the evoked potential to below baseline (71±5% of baseline at 10 min post-naloxone) (Figure 5a). Since repeated testing in the absence of fentanyl or after only brief (10 min) fentanyl exposure showed no change in baseline amplitudes during the same time interval (data not shown), the naloxone-induced evoked potential ‘overshoot' may represent an opioid withdrawal-like phenomenon. Compensatory changes in hippocampal synaptic responses have often been observed following a period of enhanced cellular excitability (e.g., Masukawa et al., 1997) and might account for such an overshoot.
Figure 5.
GRK3-mediated tolerance to opioid-induced population spike facilitation in hippocampal dentate gyrus. (a) Fentanyl (FENT) (300 nM) significantly increased the amplitude of the evoked response from perforant pathway stimulation in brain slices from wild-type GRK (+/+) mice. This potentiation was almost eliminated after 60 min of FENT exposure and completely reversed by naloxone (1 μM) at the end of the experiment. Indeed, naloxone produced a significant decrease in excitation (overshoot) compared to predrug baseline responses. (b) In slices from GRK (−/−) mice, the FENT-induced population spike enhancement showed minimal tolerance after 60 min although naloxone again completely reversed the FENT potentiation with a similar overshoot. (c) Like FENT, morphine (10 μM) produced a potentiated evoked response in slices from wild-type (+/+) mice. The 60 min of exposure to morphine produced less tolerance than that seen following 60 min of fentanyl, although naloxone again completely reversed the opioid-induced excitation. (d) In slices from GRK3 (−/−) mice, morphine again potentiated the evoked potentials in a naloxone reversible way. However, little tolerance was observed during the 60 min of morphine administration. Data are presented as means±s.e.m.
The maximal acute effect of 300 nM fentanyl in GRK3 knockout mice (208±27% increase, n=12) was not significantly different from that produced in slices from littermate GRK3 (+/+) controls (219±22%, n=8) (Figure 5a and b). This parallels the lack of effect of GRK3 deletion on acute opioid responses measured in the hot-plate assay. Similarly, as in the antinociception studies, prolonged treatment of hippocampal slices from GRK3 (−/−) mice with 300 nM fentanyl did not produce significant tolerance (Figure 5b). Indeed, following 60 min of fentanyl infusion, the population spike potentiation was reduced by only 5.6±5.7% from its initial fentanyl-induced excitatory response (n=12). As in the littermate wild-type control slices, infusion of 1 μM naloxone at the end of the 60 min fentanyl treatment returned the evoked response amplitude to below the original baseline (72±4% of baseline). Thus, the GRK3 gene deletion blocked fentanyl tolerance but not the naloxone-elicited withdrawal like responses in the mouse dentate gyrus much as it had in our studies in vivo.
A just-maximally effective dose of morphine produced similar increases in field potential amplitudes as fentanyl in the dentate gyrus of wild-type mice (219±22 and 243±28% of baseline, respectively) (Figure 5a and c) despite the known higher intrinsic efficacy of fentanyl at the mu opioid receptor compared to morphine (Kovoor et al., 1998). This is consistent with there being spare mu opioid receptors in the hippocampus. A 60 min of treatment of hippocampal slices from wild-type mice with morphine (10 μM) also produced a decrementing evoked response amplitude indicative of tolerance (Figure 5c). The rate of decline was significantly less than that produced by fentanyl exposure (Figure 5a and c, see also Figure 6), despite the similar initial effects of the two drugs. Interestingly, naloxone following sustained morphine did not produce a significant decrease from baseline responses as naloxone after fentanyl treatment had done (Figure 5c). Whether this decreased overshoot is related to or independent of the lesser tolerance produced by morphine in this model is unknown.
Figure 6.
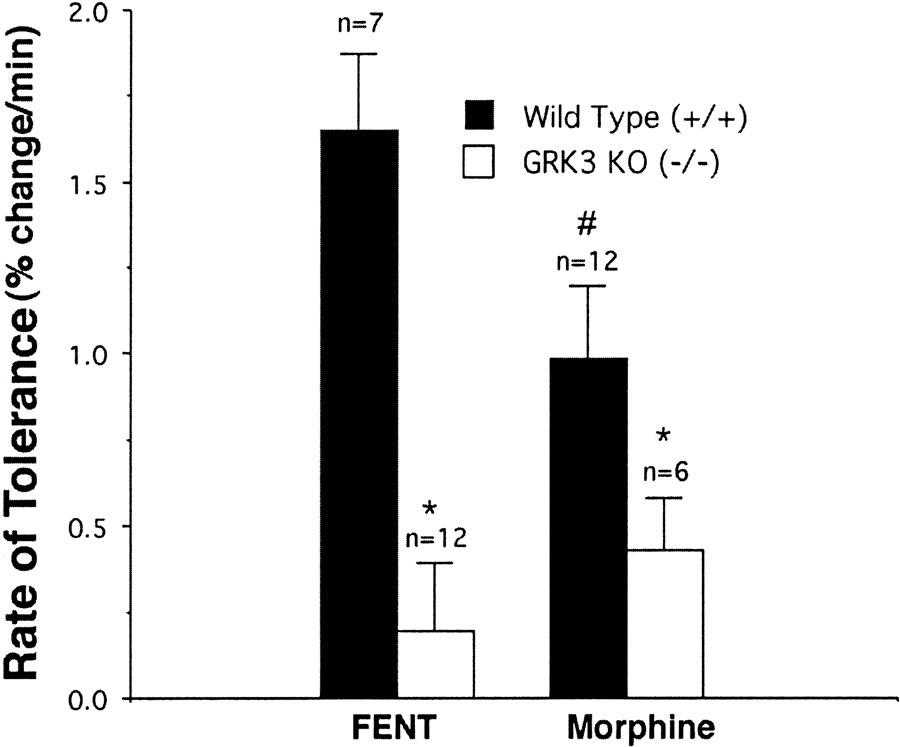
GRK3 gene deletion inhibits tolerance to both morphine- and fentanyl-induced excitation. desensitization rate was calculated as the decrement per minute in the opioid-induced potentiation during the last 50 min of opioid exposure. Data are presented as mean±s.e.m. Morphine produces less tolerance than fentanyl (FENT) in wild-type (GRK(+/+)) mice. # signifies a significant (P<0.05) difference between FENT- and morphine-exposed slices. Slices from GRK3 knockout (GRK3 (−/−)) mice showed a slower rate of tolerance than slices from their littermate wild-type controls (GRK (+/+)) following both morphine and pent. *signifies a significant (P<0.05) difference between slices from wild-type and knockout mice.
In slices from GRK3 (−/−) mice, acute morphine treatment produced the same potentiation of population spike amplitudes as seen in the GRK3 (+/+) slices (Figure 5d). The 60 min of morphine treatment of slices from the knockout animals, however, produced significantly less tolerance to the excitatory morphine effects than was seen in control slices (Figure 5d). Figure 6 illustrates the effects of GRK3 gene deletion on the rate of tolerance to the excitatory effects of fentanyl and morphine. Although morphine produced significantly slower induction of tolerance than fentanyl, GRK3 deletion significantly inhibited the rate of tolerance to both opioids. Thus, in the dentate gyrus GRK3 appears to contribute to tolerance to both fentanyl's and morphine's excitatory effects. This is in contrast to the GRK3 contribution to the analgesic tolerance of fentanyl but not morphine in the in vivo setting.
The slower rate of morphine tolerance induction (and lesser amount of naloxone-induced overshoot) when compared with fentanyl tolerance induction in the hippocampus is also different from our findings and those of Duttaroy & Yoburn (1995) in analgesia studies. Despite the presence of spare receptors in the hippocampus, the tolerance rate of the lower efficacy morphine was slower, not the same or faster, than the more efficacious opioid fentanyl. This is consistent with our findings in the Xenopus oocyte expression system showing that opioid receptor desensitization rate was correlated with agonist efficacy (Kovoor et al., 1998).
Discussion
The principal finding of this study is that GRK3 plays a significant role in the development of tolerance to the potent opioid fentanyl both in vitro in mammalian brain slices and in the in vivo analgesic response. In contrast, GRK3 gene deletion did not affect basal nociceptive responses, acute opioid effects or opioid withdrawal behaviors. The finding that GRK3 influences fentanyl tolerance is consistent with heterologous gene expression studies using Xenopus oocytes where agonist-induced, homologous desensitization of the opioid receptor is particularly dependent on expression of the GRK3 isozyme (Kovoor et al., 1998; Appleyard et al., 1999). In those studies, neither GRK2 nor GRK5 was as effective as GRK3 in producing opioid receptor desensitization (see however, Li & Wang, 2001). Opioid receptor desensitization in transfected cell lines is also known to follow phosphorylation by GRK and subsequent β-arrestin binding (Pak et al., 1997; Cen et al., 2001). In addition, the general role of GRK- and arrestin-mediated desensitization of G-protein-coupled receptors is well established (Ferguson et al., 1996; Pitcher et al., 1998). Thus, the novel finding that GRK3 knockout interferes with fentanyl-induced tolerance measured at both the electrophysiological and behavioral levels, supports the notion that GRK3-mediated desensitization is an important mechanism in opioid tolerance.
The finding that morphine-induced behavioral tolerance was not affected by the GRK3 gene deletion is consistent with our previous finding that morphine activates less GRK3 than fentanyl in the Xenopus oocyte in vitro (Kovoor et al., 1998) and that lower efficacy agonists produce less G-protein receptor phosphorylation (Benovic et al., 1988). Even in our hippocampal slice studies where morphine-induced tolerance was inhibited in the GRK3 knockout mice, the effect was much less robust than studies of fentanyl tolerance — largely because of a slower rate of tolerance with morphine. Differential rates of tolerance for different opioid agonists has also been linked to differences in receptor internalization in in vitro expression systems (Whistler et al., 1999; Finn & Whistler, 2001; He et al., 2002). Morphine produces little receptor internalization, although more efficacious agonists such as DAMGO, etorphine (Whistler et al., 1999) and fentanyl (Keith et al., 1998) produce potent receptor internalization. Methods that increase morphine-induced receptor internalization have been reported to inhibit morphine tolerance. Differences in agonist-induced internalization could conceivably mediate the differences that we observe between the influence of GRK3 on fentanyl and morphine tolerance if GRK3 were important in mediating fentanyl-induced receptor internalization. However, a specific role of GRK3 in the internalization process has not been defined (e.g., Patel et al., 2002) despite β-arrestin's well-known role in such receptor trafficking (Zhang et al., 1997). Moreover, in our studies, GRK3 deletion did significantly inhibit morphine tolerance development in the hippocampal slice, despite agonist efficacy-related differences in desensitization rate. It is likely that the differences we saw between the effects of GRK3 deletion on fentanyl and morphine analgesic tolerance were due to the complexity and redundancy of mechanisms mediating opioid tolerance in vivo.
Knockout mice are a useful tool for studying protein function in vivo and in vitro in the absence of specific pharmacological antagonists. Nevertheless, interpreting such knockout experiments requires the assumption that the only difference between knockout mice and their wild-type controls are the gene or genes targeted in producing the transgenic animal (the GRK3 enzyme in our studies). Such specificity is difficult to prove. In addition, many transgenic mice are generated using embryonic stem cells derived from one strain of mouse (a 129 strain in our knockout) and back-crossed into a different strain. Differences between mouse strains in behavioral studies of opioid sensitivity and tolerance induction are well documented (Mogil et al., 1996; Kolesnikov et al., 1998). For this reason, we back-crossed the GRK3 (−/−) mice to the C57/B6 background for more than six generations and studied only paired littermates. With this experimental design, the contributions of other possible genetic differences (e.g., the contribution of a gene closely linked to GRK3) were minimized, although admittedly not eliminated. Further, in our preliminary studies (Varnado-Rhodes et al., 2000), we showed that there were no differences in fentanyl tolerance in the two parental strains of the GRK3 knockout, again arguing against strain differences as causes for any opioid tolerance differences in subsequent studies. Finally, the identical baseline hot-plate responses and acute opioid responses seen in the GRK3(−/−), littermate control animals and parental strains (Varnado-Rhodes et al., 2000) simplifies the interpretation of the opioid tolerance differences seen between groups. In this regard, our findings of no differences in morphine analgesic tolerance between GRK3 knockout and wild-type mice, markedly differs from the report that knockout mice lacking β-arrestin 2 show dramatically reduced morphine analgesic tolerance (Bohn et al., 2000; 2002). It is possible that this is due to beta-arrestin acting downstream from the GRKs in the desensitization process and thus interacting with a variety of GRKs–not just the GRK3 studied here. However, direct comparison of the two data sets are complicated because, unlike in our GRK3 studies, β-arrestin knockout mice differ from their wild-type controls in their acute responses to morphine as well as in their tolerance to morphine's effects. Our numerous preliminary studies helped to focus our current studies on tolerance to opioids rather than on opioid effects per se.
Tolerance to opioid analgesics is known to be a complex process involving not only biochemical modifications in the opioid receptor signaling process but also compensatory behavioral mechanisms (Siegel et al., 2000) and physiologic adaptations (e.g., Mitchell et al., 2000) to the drug. Morphine tolerance, for example, has been known for many years to have an important learned component (for a review see Siegel et al., 2000). NMDA antagonists, known to inhibit learning in a variety of models (Morris et al., 1986), have also been reported to also inhibit opioid tolerance by several groups (e.g., Trujillo & Akil, 1991). In our studies, we specifically chose a tolerance paradigm using steady opioid infusions to reduce the pairing of environmental cues with drug administration and thereby minimize associational mechanisms of opioid tolerance (Kim et al., 1999). We can still not rule out the possibility that fentanyl tolerance is blocked by some yet undiscovered learning deficit in GRK3 knockout mice. However, NMDA antagonists have a greater inhibitory effect on morphine tolerance than on fentanyl tolerance (Bilsky et al., 1996). This does not support the hypothesis that learning mechanisms are more important in fentanyl tolerance than in morphine tolerance and that, therefore, the differences we see between morphine and fentanyl tolerance in our GRK3 knockout mice are due to a possible learning deficit in the GRK3 knockout mice. Indeed, the heightened importance of NMDA mediation in morphine tolerance may, in contrast, represent an example of redundant mechanisms of opioid tolerance in vivo by which, even with a GRK3 deletion, morphine tolerance is able to take place at a normal rate.
A number of compensatory physiological mechanisms have been suggested to mediate opioid tolerance in vivo. Neurochemicals, including glutamate at NMDA (Mayer & Mao 1999) and metabotropic receptors (Fundytus & Coderre 1999), dynorphin (Vanderah et al., 2001), cholecystokinin (Mitchell et al., 2000) and nitric oxide (Mao et al., 1995) have all been implicated in such compensatory mechanisms of opioid tolerance. When opioids are removed in such paradigms, hyperalgesia and other withdrawal symptoms are revealed (Harrison et al., 1998). Our findings of an important role for GRK3 in mediating fentanyl analgesic tolerance does not preclude the involvement of supplementary or redundant mechanisms activated by opioids. Indeed, our findings that naloxone-precipitated withdrawal effects were unaffected in GRK3 knockout mice despite an inhibition of analgesic tolerance suggests that compensatory mechanisms often associated with tolerance were still activated in the GRK3 animals. β-arrestin knockout mice have also been found to have normal naloxone-precipitated opioid withdrawal behaviors (Bohn et al., 2000) supporting our findings of independence between opioid withdrawal and receptor tolerance mechanisms (see also Nitsche et al., 2002).
Defining the role of GRK-mediated opioid receptor desensitization mechanisms causing opioid analgesic tolerance is complicated in vivo. Even in our hippocampal slice paradigm, NMDA antagonists can significantly inhibit residual desensitization in slices from GRK3 knockout mice (Jin et al., 2000) emphasizing the redundancy of tolerance mechanisms. In the studies reported here, we used a drug administration regimen and an opioid agonist thought, from our previous work in expression systems, to be most likely to facilitate receptor desensitization and thereby demonstrated a role for GRK3 in opioid tolerance both in vitro and in vivo.
However, we also found that, in vivo, GRK3 had no detectable influence on opioid tolerance to another agonist. We have previously demonstrated differences between such expression system models and in vivo drug effects. For example, our findings that agonist efficacy was directly correlated with the rate of opioid receptor desensitization in Xenopus oocytes (Kovoor et al., 1998) contrasts with studies in rats demonstrating that lower efficacy agonists (like morphine) show more rapid tolerance (Duttaroy & Yoburn, 1995). This difference is almost certainly due to the abundance of spare receptors in vivo and the resultant ability of high efficacy agonists to produce behavioral effects despite desensitization of the majority of opioid receptors. Agonist saturation of all opioid receptors (including spare receptors) in vivo would likely have lethal effects in the spontaneously breathing animal. On the other hand, saturation is easily attainable in vitro in expression systems and slice preparations and this may explain the much more rapid tolerance evident in our in vitro experiments compared to the tolerance we observed in vivo. Guignard et al. (2000) have reported an interesting clinical corollary to this finding. High-dose intraoperative infusions of the potent but very rapidly metabolized opioid, remifentanil, during general anesthesia seemed to produce analgesic tolerance in patients postoperatively. Rapid onset analgesic tolerance to high dose, high efficacy opioid agonist infusions has been described previously as ‘acute' tolerance (Kissin et al., 1996). However, the unique rapid metabolism of remifentanil now allows routine administration of opioid doses in the clinical setting that fully saturate even spare receptors without fear of long-term side effects such as respiratory depression. It will be interesting to observe the clinical significance of such rapid desensitization mechanisms in humans when both learning and spare receptor effects are factored out (by general anesthesia and saturating doses, respectively).
In conclusion, we find that in vivo opioid receptor tolerance is a complex phenomenon involving GRK3-mediated receptor desensitization mechanisms. Not surprisingly, a number of other mechanisms are also involved–at times, perhaps, obscuring the role of GRK3. In vitro studies of opioid receptors, in isolation from many behaviorally and physiologically relevant processes, are powerful tools for dissecting the mechanisms of opioid desensitization. The results of such studies, however, must eventually be interpreted in light of those same previously avoided more complicated in vivo processes. Future work on the mechanisms underlying opioid tolerance will benefit from studying the limitations as well as the strengths of particular mechanistic dogma.
Acknowledgments
The work was supported by USPHS Grants DA11672 (CC), DA00266 (GWT) and DA14588 (GWT) from the National Institute on Drug Abuse (NIDA). MGC and RJL are Investigators of the Howard Hughes Medical Institute. We thank the NIDA for the gift of morphine pellets. We thank Lisa Myers, Aaron Pattillo, Rose Brown and Brenda Murphy for assistance. We thank Krzysztoph Palczewski and Jeremy Celver for advice. Y.P. Cheong was a visiting scientist from the Department of Anesthesiology. Wonkwang University School of Medicine, Iksan, South Korea.
Abbreviations
- GRK
G-protein receptor kinase
References
- APPLEYARD S.M., CELVER J., PINEDA V., KOVOOR A., WAYMAN G.A., CHAVKIN C. Agonist-dependent desensitization of the kappa opioid receptor by G protein receptor kinase and beta-arrestin. J. Biol. Chem. 1999;274:23802–23807. doi: 10.1074/jbc.274.34.23802. [DOI] [PubMed] [Google Scholar]
- APPLEYARD S.M., PATTERSON T.A., JIN W., CHAVKIN C. Agonist-induced phosphorylation of the kappa-opioid receptor. J. Neurochem. 1997;69:2405–2412. doi: 10.1046/j.1471-4159.1997.69062405.x. [DOI] [PubMed] [Google Scholar]
- BENOVIC J.L., STANISZEWSKI C., MAYOR JR F., CARON M.G., LEFKOWITZ R.J. Beta-adrenergic receptor kinase activity of partial agonists for stimulation of adenylate cyclase correlates with ability to promote receptor phosphorylation. J. Biol. Chem. 1988;263:3893–3897. [PubMed] [Google Scholar]
- BILSKY E.J., INTURRISI C.E., SADEE W., HRUBY V.J., PORRECA F. Competitive and non-competitive NMDA antagonists block the development of antinociceptive tolerance to morphine, but not to selective mu or delta opioid agonists in mice. Pain. 1996;68:229–237. doi: 10.1016/s0304-3959(96)03185-5. [DOI] [PubMed] [Google Scholar]
- BOHN L.M., GAINETDINOV R.R., LIN F.T., LEFKOWITZ R.J., CARON M.G. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- BOHN L.M., LEFKOWITZ R.J., CARON M.G. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J. Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEN B., YU Q., GUO J., WU Y., LING K., CHENG Z., MA L., PEI G. Direct binding of beta-arrestins to two distinct intracellular domains of the delta opioid receptor. J. Neurochem. 2001;76:1887–1894. doi: 10.1046/j.1471-4159.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- CROCKETT R.S., BORNSCHEIN R.L., SMITH R.P. Diurnal variation in response to thermal stimulation: mouse-hotplate test. Physiol. Behav. 1977;18:193–196. doi: 10.1016/0031-9384(77)90120-2. [DOI] [PubMed] [Google Scholar]
- DUTTAROY A., YOBURN B.C. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- ESPEJO E.F., MIR D. Differential effects of weekly and daily exposure to the hot plate on the rat's behavior. Physiol. Behav. 1994;55:1157–1162. doi: 10.1016/0031-9384(94)90404-9. [DOI] [PubMed] [Google Scholar]
- FERGUSON S.S., BARAK L.S., ZHANG J., CARON M.G. G-protein-coupled receptor regulation: role of G-protein-coupled receptor kinases and arrestins. Can. J. Physiol. Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- FINN A.K., WHISTLER J.L. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- FUNDYTUS M.E., CODERRE T.J. Opioid tolerance and dependence: a new model highlighting the role of metabotropic glutamate receptors. Pain Forum. 1999;8:3–13. [Google Scholar]
- GINTZLER A.R., CHAKRABARTI S. Opioid tolerance and the emergence of new opioid receptor-coupled signaling. Mol. Neurobiol. 2000;21:21–33. doi: 10.1385/MN:21:1-2:021. [DOI] [PubMed] [Google Scholar]
- GUIGNARD B., BOSSARD A.E., COSTE C., SESSLER DI LEBRAULT C., ALFONSI P., FLETCHER D., CHAUVIN M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- HARRISON L.M., KASTIN A.J., ZADINA J.E. Opiate tolerance and dependence: receptors, G-proteins, and antiopiates. Peptides. 1998;19:1603–1630. doi: 10.1016/s0196-9781(98)00126-0. [DOI] [PubMed] [Google Scholar]
- HE L., FONG J., VON ZASTROW M., WHISTLER J.L. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- JIN W., ASSI A.-A., EASTMAN C TERMAN G.W., CHAVKIN C. Synaptic plasticity contributes to opioid tolerance in vitro. Soc. Neurosci. Abstr. 2000;26:636. [Google Scholar]
- KEITH D.E., ANTON B., MURRAY S.R., ZAKI P.A., CHU P.C., LISSIN D.V., MONTEILLET-AGIUS G., STEWART P.L., EVANS C.J., VON ZASTROW M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol. Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- KIEFFER B.L., EVANS C.J. Opioid tolerance–in search of the holy grail. Cell. 2002;108:587–590. doi: 10.1016/s0092-8674(02)00666-9. [DOI] [PubMed] [Google Scholar]
- KIM J.A., SIEGEL S., PATENALL V.R. Drug-onset cues as signals: intraadministration associations and tolerance. J. Exp. Psychol. Anim. Behav. Process. 1999;25:491–504. [PubMed] [Google Scholar]
- KISSIN I., LEE S.S., ARTHUR G.R., BRADLEY E.L., JR Time course characteristics of acute tolerance development to continuously infused alfentanil in rats. Anesth. Analg. 1996;83:600–605. doi: 10.1097/00000539-199609000-00029. [DOI] [PubMed] [Google Scholar]
- KOLESNIKOV Y., JAIN S., WILSON R., PASTERNAK G.W. Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J. Pharmacol. Exp. Ther. 1998;284:455–459. [PubMed] [Google Scholar]
- KOVOOR A., CELVER J.P., WU A., CHAVKIN C. Agonist induced homologous desensitization of mu-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol. Pharmacol. 1998;54:704–711. [PubMed] [Google Scholar]
- KOVOOR A., NAPPEY V., KIEFFER B.L., CHAVKIN C. Mu and delta opioid receptors are differentially desensitized by the coexpression of beta-adrenergic receptor kinase 2 and beta-arrestin 2 in xenopus oocytes. J. Biol. Chem. 1997;272:27605–27611. doi: 10.1074/jbc.272.44.27605. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VALVERDE O., COSSU G., PETITET F., AUBERT J.F., BESLOT F., BOHME G.A., IMPERATO A., PEDRAZZINI T., ROQUES B.P., VASSART G., FRATTA W., PARMENTIER M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- LI A.H., WANG H.L. G protein-coupled receptor kinase 2 mediates mu-opioid receptor desensitization in GABAergic neurons of the nucleus raphe magnus. J. Neurochem. 2001;77:435–444. doi: 10.1046/j.1471-4159.2001.00267.x. [DOI] [PubMed] [Google Scholar]
- LIU J.G., ANAND K.J. Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res. Brain Res. Rev. 2001;38:1–19. doi: 10.1016/s0165-0173(01)00057-1. [DOI] [PubMed] [Google Scholar]
- LOWE J.D., CELVER J.P., GUREVICH V.V., CHAVKIN C. mu-Opioid receptors desensitize less rapidly than delta-opioid receptors due to less efficient activation of arrestin. J. Biol. Chem. 2002;277:15729–15735. doi: 10.1074/jbc.M200612200. [DOI] [PubMed] [Google Scholar]
- MAO J., PRICE D.D., MAYER D.J. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- MASUKAWA L.M., O'CONNOR W.M., BURDETTE L.J., MCGONIGLE P., SPERLING M.R., O'CONNOR M.J., URUNO K. Mossy fiber reorganization and its possible physiological consequences in the dentate gyrus of epileptic humans. Adv. Neurol. 1997;72:53–68. [PubMed] [Google Scholar]
- MAYER D.J., MAO J. Mechanisms of opioid tolerance: a current view of cellular mechanisms. Pain Forum. 1999;8:14–18. [Google Scholar]
- MITCHELL J.M., BASBAUM A.I., FIELDS H.L. A locus and mechanism of action for associative morphine tolerance. Nat. Neurosci. 2000;3:47–53. doi: 10.1038/71120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOGIL J.S., KEST B., SADOWSKI B., BELKNAP J.K. Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: influence of nociceptive assay. J. Pharmacol. Exp. Ther. 1996;276:532–544. [PubMed] [Google Scholar]
- MOGIL J.S., WILSON S.G., BON K., LEE S.E., CHUNG K., RABER P., PIEPER J.O., HAIN H.S., BELKNAP J.K., HUBERT L., ELMER G.I., CHUNG J.M., DEVOR M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- MORRIS R.G., ANDERSON E., LYNCH G.S., BAUDRY M. selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J., AGHAJANIAN G.K. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- NITSCHE J.F., SCHULLER AG KING M.A., ZENGH M., PASTERNAK G.W., PINTAR J.E. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J. Neurosci. 2002;22:10906–10913. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAK Y., O'DOWD B.F., GEORGE S.R. Agonist-induced desensitization of the mu opioid receptor is determined by threonine 394 preceded by acidic amino acids in the COOH-terminal tail. J. Biol. Chem. 1997;272:24961–24965. doi: 10.1074/jbc.272.40.24961. [DOI] [PubMed] [Google Scholar]
- PATEL M.B., PATEL C.N., RAJASHEKARA V., YOBURN B.C. Opioid agonists differentially regulate mu-opioid receptors and trafficking proteins in vivo. Mol. Pharmacol. 2002;62:1464–1470. doi: 10.1124/mol.62.6.1464. [DOI] [PubMed] [Google Scholar]
- PEPPEL K., BOEKHOFF I., MCDONALD P., BREER H., CARON M.G., LEFKOWITZ RJ. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J. Biol. Chem. 1997;272:25425–25428. doi: 10.1074/jbc.272.41.25425. [DOI] [PubMed] [Google Scholar]
- PITCHER J.A., FREEDMAN N.J., LEFKOWITZ R.J. G protein-coupled receptor kinases. Annu. Rev. Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- SIEGEL S., BAPTISTA M.A., KIM J.A., McDONALD R.V., WEISE-KELLY L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp. Clin. Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- SIMMONS M., CHAVKIN C.Endogenous opioid regulation of hippocampal function International Review of Neurobiology 1996New York: Academic Press; 146–149.ed. Bradley, R., Harris, A. & Jenner, P. pp [DOI] [PubMed] [Google Scholar]
- TRUJILLO K.A., AKIL H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- VANDERAH T., OSSIPOV M., LAI J., MALAN T.J., PORECCA F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- VARNADO-RHODES Y., GUNTHER J., TERMAN G.W., CHAVKIN C. Mu opioid analgesia and analgesic tolerance in two mouse strains: C57BL/6 and 129/SvJ. Proc. West Pharmacol. Soc. 2000;43:15–17. [PubMed] [Google Scholar]
- WHISTLER J.L., CHUANG H.H., CHU P., JAN L.Y., VON ZASTROW M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction [see comments] Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- YU Y., ZHANG L., YIN X., SUN H., UHL G.R., WANG J.B. Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J. Biol. Chem. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- ZHANG J., FERGUSON S.S., BARAK L.S., ABER MJ GIROS B., LEFKOWITZ R.J., CARON M.G. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arresting in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]