Abstract
Stem cell factor (SCF) is a major growth factor for mast cells, promoting their differentiation and chemotaxis. Its expression is regulated by glucocorticoids in inflammatory conditions, showing an early increased protein expression, before the expected anti-inflammatory decrease (Da Silva et al., Br. J. Pharmacol. 2002:135,1634).
We here evaluated the early kinetic of SCF expression regulated by interleukin (IL)-1β, budesonide and the combination of both in human lung fibroblasts in culture.
Budesonide potentiated the IL-1β-enhanced expression of SCF mRNA (+103%) and protein (+98%) very shortly after treatment (at 30 min and 1 h, respectively). A gentle downregulation followed. This potentiating effect of budesonide was related to increased SCF mRNA stability and SCF gene transcription.
Deletion of a κB-like site that we identified in the first intron of the SCF gene, in a luciferase reporter system, abolished the potentiation by budesonide, as well as the effect of IL-1β alone, as compared to the wild-type construction activity.
All budesonide-induced effects were glucocorticoid-receptor dependent, since they were reproduced by dexamethasone and blocked by RU486.
IL-1β+budesonide did not affect the relative expression of the soluble and membrane-bound forms of SCF.
In conclusion, our results clearly show that glucocorticoids act very early to adversely increase the expression of SCF mRNA and protein in the inflammatory conditions created by IL-1β, and that this effect involves increased mRNA stability and increased gene expression through activation of the NF-κB-like responsive element.
Keywords: SCF, IL-1β, glucocorticoid, NF-κB, inflammation, fibroblast, mast cell, lung, asthma
Introduction
Stem cell factor (SCF) also termed Kit ligand, steel factor or mast cell growth factor (Huang et al., 1990; Martin et al., 1990; Zsebo et al., 1990), is the ligand of the c-kit proto-oncogene product. SCF is expressed in two forms, soluble (sSCF) and membrane-bound (mSCF), after alternative splicing of the sixth exon, which encodes a proteolytic cleavage site (Anderson et al., 1991; Flanagan et al., 1991). Since SCF acts as an important growth factor for human mast cells (Kirshenbaum et al., 1992; Rottem et al., 1994), it may be involved in many diseases associated with a local increase in the number (Iemura et al., 1994; Costa et al., 1996) and activation (Bischoff & Dahinden, 1992; Costa et al., 1996) of mast cells, as occurs in asthma.
The primary treatment for mast cell-associated diseases such as asthma remains the glucocorticoid family, which, as shown in the bronchial mucosa of asthmatic patients (Jeffery et al., 1992; Laitinen et al., 1992), reduces the number of mast cells. Since glucocorticoids' major therapeutic benefit is likely to occur through the regulation of the expression of proinflammatory genes (for review, see Adcock, 2001), we hypothesised an inhibitory effect of glucocorticoids on the mast cell growth factor SCF expression.
We recently reported that, in human lung fibroblasts in culture, glucocorticoids regulate the constitutive (Kassel et al., 1998) and interleukin (IL)-1β-induced (Da Silva et al., 2002) expression of SCF in an opposite manner. In the latter study, we noted that SCF protein expression surprisingly increased at 2.5 h after combined treatment with budesonide and IL-1β; this was surprising since SCF mRNA expression was decreased in the same circumstances. This observation suggested cellular events associated with an earlier increase in SCF mRNA expression. Intron 1 of the SCF gene contains a sequence marginally homologous to the responsive element of the transcription factor nuclear factor (NF)-κB, which is one of the major transcription factors involved in the inflammatory process. In particular, stimulation by IL-1β (Stylianou et al., 1992; Croston et al., 1995) leads to NF-κB activation and nuclear translocation (Israel, 1997). This κB-like site might therefore play a role in the IL-1β-related regulation of SCF gene expression.
We thus verified the effect of glucocorticoids on SCF gene expression in inflammatory conditions at earlier time (0–150 min), and studied the mechanism of this effect in human lung fibroblasts in culture. In particular, we evaluated the effect of the combination of IL-1β and budesonide on SCF mRNA stability and early gene transcription, and used a luciferase reporter assay system to assess the possible role of the κB-like site of the first intron in this regulation.
Methods
Culture of human lung fibroblasts
Human lung-derived fibroblasts were obtained by the explant technique, as previously described (Da Silva et al., 2002). Briefly, macroscopically normal human lung tissue was separated into fragments within 1 h of resection for bronchocarcinoma. Fibroblasts were cultured in Dulbecco's modified Eagle's medium (DMEM)/F-12 (1 : 1) (Gibco BRL, Cergy Pontoise, France), supplemented with 10% fetal calf serum (FCS), penicillin (50 U ml−1) and streptomycin (50 μg ml−1), and incubated in a humidified mixture of 95% air and 5% CO2 at 37°C. They were then replated in 25 cm2 tissue culture flasks (Costar, Acton, MA, U.S.A.) and were characterised as fibroblasts morphologically and by immunocytochemistry (with an anti-fibroblast monoclonal antibody (5B5) (DAKO S.A., Trappes, France). They subsequently were split 1 : 4 at confluence and passaged. Fibroblasts were used at passage 7.
Cell treatment
At confluence, fibroblasts were starved for 48 h. Human recombinant IL-1β (Roche Diagnostics, Meylan, France), prepared from a 2 μg ml−1 stock solution, was added at a final concentration of 400 pg ml−1. The glucocorticoid budesonide (kindly provided by Dr R. Brattsand, Astra, Lund, Sweden) was prepared from a 10 mM stock solution in absolute ethanol and used at 0.1 μM (Kassel et al., 1998). Dexamethasone (Sigma Chemicals, St Louis, MO, U.S.A.) was used at 1 μM in similar conditions. The glucocorticoid antagonist RU486 (Sigma Chemicals) was prepared from a 10 mM stock solution in absolute ethanol and incubated at 1 μM for 1 h before IL-1β and glucocorticoid treatment. Cells were treated for 0, 5, 15, 30, 60, 90 and 150 min. Similar experiments were performed with staggered treatment starts, to verify the effect of the duration of the culture. Supernatants were sampled and stored at –70°C until assayed for SCF protein. Fibroblasts were harvested for total RNA extraction.
To analyse transcript stability, confluent fibroblasts were treated with solvent alone (ethanol, as the control treatment; 1 μM), IL-1β (400 pg ml−1), budesonide (0.1 μM) or a combination of both for 30 min. Supernatants were replaced by the starving medium, which contained 5 μg ml−1 actinomycin D (Sigma Chemicals). Cells were harvested hourly from 0 to 4 h, and total RNA was extracted.
SCF ELISA
A sensitive ELISA procedure quantified immunoreactive SCF released into the supernatant of fibroblasts treated with IL-1β, glucocorticoid, RU486 or solvents (control). It used a capture anti-human SCF monoclonal antibody (clone 13302.6; R&D Systems Europe, Abingdon, U.K.) and a biotinylated detection anti-human SCF polyclonal antibody (R&D Systems Europe), revealed by extravidin – horseradish peroxidase and a 3,3′,5,5′-tetramethylbenzidine liquid substrate system (Sigma Chemicals). Standard curves were generated with recombinant human SCF (R&D Systems Europe) diluted in the starving medium and were linear from 3.9 to 500 pg ml−1.
Extraction of RNA and reverse transcription
Total RNA was extracted from control or treated fibroblasts with TriReagent® (Molecular Research Center Inc., Cincinnati, OH, U.S.A.). Isolated RNA was diluted in RNase-free water and quantified by absorbance measurement at 260 nm. A measure of 4 μg of total RNA was incubated with 0.5 μg of random primers for 5 min at 70°C and allowed to cool down at room temperature. RNA was subsequently reverse-transcribed in 1 × reverse transcription (RT) buffer (75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, and 50 mM Tris-HCl, pH 8.3), containing 1 U μl−1 RNasin ribonuclease inhibitor, 1 mM of each dNTP, and 10 U μl−1 RNase H(−)-Moloney leukaemia virus reverse transcriptase (all reagents from Promega, Madison, WI, U.S.A.). The reaction was conducted for 1 h at 37°C, and then the reverse transcriptase was heat-inactivated at 99°C for 5 min.
Quantification of total SCF mRNA
Quantification of total SCF mRNA (SCF149) was performed by polymerase chain reaction (PCR) after reverse transcription, and results were normalised to total cDNA.
PCR of total SCF cDNA (SCF149)
SCF149 cDNA was amplified from cDNA obtained after RT by on-line fluorescent PCR (LightCycler™-SYBR Green I, Roche Diagnostics), with primers located upstream of the alternatively spliced sixth exon to yield a single 149-bp PCR product. PCR reactions were performed in 1 × PCR reaction buffer (2 μl of the reaction mix, containing FastStart Taq DNA polymerase, dNTP mix, SYBR Green I, 3 mM MgCl2 (Roche Diagnostics), and 10 pmol of each primer:
- sense primer
5′-TGGATAAGCGAGATGGTAGT-3′
- antisense primer
5′-TTTTCTTTCACGCACTCCAC-3′,
in a 20 μl final volume for 35 cycles, with each cycle consisting of 15 s denaturation at 95°C, 10 s annealing at 53°C, and 10 s extension at 72°C. Amplified SCF149 cDNA and a standard SCF cDNA were analysed on-line by fluorescence (LightCycler™, Roche Diagnostics).
The standard SCF cDNA was obtained from lung fibroblast total cDNA. After amplification by PCR with the primers described above, the 149 bp PCR products were electrophoresed on a 2% agarose gel stained with ethidium bromide, purified on QIAEX II (QIAEX II gel extraction kit, QIAGEN, Courtaboeuf, France), and quantified by fluorescence (PicoGreen®, Molecular Probes Inc., Eugene, OR, U.S.A.) according to a standard curve obtained with a double stranded phage λ DNA (0.005-1 μg ml−1). This purified SCF cDNA was used as a standard curve from 1 to 300 fg ml−1.
Quantification of total cDNA
A volume of 1 μl of total cDNA was diluted (1 : 15) and added to 250 μl Tris-EDTA (TE) 1 × buffer (10 mM Tris-HCl, pH 7.8, 1 mM EDTA, pH 8). This reaction mix was incubated with 250 μl OliGreen® (1 : 200) (Molecular Probes Inc.) for 10 min in the dark. A standard single-stranded M13 phage DNA (Sigma) curve (0–500 ng ml−1) was obtained in the same conditions. The total cDNA was quantified in a fluorometer (Hitachi F-2000, Tokyo, Japan) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Total cDNA remained unchanged for all treatments.
Total SCF mRNA was expressed as the ratio of SCF149 cDNA total cDNA−1 calculated in each sample and expressed as fg SCF149 cDNA ng total cDNA−1.
Relative expression of sSCF and mSCF mRNA
The mRNA expression of both forms of SCF in fibroblasts treated with IL-1β, and/or glucocorticoids, was studied by PCR amplification after RT. We used primers spanning the alternatively spliced sixth exon:
- sense primer
5′-TGGATAAGCGAGATGGTAGT-3′
- antisense primer
5′-AGCCACAATTTACACTTCTT-3′,
to generate a 627-bp product from base 388 to base 1015 for sSCF cDNA and a 544-bp PCR product from base 388 to base 932 for mSCF cDNA, from the sequence reported by Martin et al. (1990). A measure of 1 μl (1 : 10) of cDNA after RT was amplified by PCR in 1 × PCR buffer containing 2.5 mM MgCl2, 0.1 mM dNTP, 10 pmol of each primer, and 1 U of Taq DNA polymerase (all reagents from Promega) in a 50-μl final volume for 40 cycles, each cycle consisting of 60 s denaturation at 94°C, 30 s annealing at 55°C, and 60 s extension at 72°C. After PCR, 15 μl of each reaction product was denatured by heating at 99°C for 10 min in the presence of 50% deionised formamide and resolved by electrophoresis on gels (50% urea/17% formamide/6% polyacrylamide), as was a 100-bp DNA ladder (Gibco BRL). Gels were stained with ethidium bromide, digitised under UV light with a high performance charge-coupled device camera (Cohu, San Diego, CA, U.S.A.), and analysed with the public domain NIH Image Program (available by anonymous FTP from http://rsb.info.nih.gov/nih-image/download.html). The ratio of sSCF to mSCF cDNA was calculated during the exponential phase of PCR amplification.
Nuclear Run-On assay
In vitro transcription
The Nuclei EZ Prep Nuclei isolation kit (Sigma Chemicals) enabled us to prepare nuclei from fibroblasts treated for 30 min with either solvent or IL-1β (400 pg ml−1) combined with budesonide (0.1 μM). Each reaction (final volume, 400 μl) took place in the presence of 5 × 107 isolated nuclei, 40 mM Tris-HCl, pH 8.3, 150 mM NH4Cl, 7.5 mM MgCl2, 0.625 mM ATP, 0.313 mM GTP, 0.313 mM CTP, 0.3 mCi [α-32P]UTP (800 Ci mmol−1) (Amersham Pharmacia Biotech Europe Gmbh, Freiburg, Germany), and 120 units ml−1 RNasin. Transcription reactions proceeded for 30 min at 27°C before the addition of 40 units of RNasin and 75 U of RQ-1 DNase (Promega). After DNase treatment, the radiolabelled RNA formed was purified after phenol–chloroform extraction, and precipitated three times in ethanol in the presence of 1.33 M ammonium acetate.
PCR of SCF cDNA
SCF cDNA was amplified from total cDNA by on-line fluorescent PCR (LightCycler™-SYBR Green I, Roche Diagnostics), as described above, with primers yielding a single 424-bp PCR product. Primers used were:
- sense primer
5′-CTCCAGAACAGCTAAACGGA-3′
- antisense primer
5′-ATGAGTTTTCTTTGACGCAC-3′,
in a 20 μl final volume for 35 cycles, with each cycle consisting of 15 s denaturation at 95°C, 10 s annealing at 52°C, and 10 s extension at 72°C. Amplified SCF cDNA was analysed on-line by fluorescence.
PCR of GAPDH cDNA
GAPDH cDNA was amplified from total cDNA by on-line fluorescent PCR (LightCycler™-SYBR Green I, Roche Diagnostics) with primers yielding a single 559-bp PCR product. PCR reactions were performed in 1 × PCR reaction buffer (2 μl of the reaction mix containing FastStart Taq DNA polymerase, dNTP mix, SYBR Green I, 3 mM MgCl2 (all from Roche Diagnostics)) and 10 pmol of each primer:
- sense primer
5′-GGCTGCTTTTAACTCTGGTA-3′
- antisense primer
5′-GATGTTCTGGAGAGCCCCGC-3′,
in a 20 μl final volume for 35 cycles, with each cycle consisting of 15 s denaturation at 95°C, 10 s annealing at 56°C, and 10 s extension at 72°C. Amplified GAPDH cDNA was analysed on-line by fluorescence (LightCycler™, Roche Diagnostics).
SCF and GAPDH cDNA probe construction
PCR products (424 and 559 bp, respectively), obtained as described above, were resolved on 2% agarose gel, extracted with Ultrafree®-DA (Millipore, St-Quentin, France), and subcloned in pGEM-T plasmid (Promega). Sequence analysis (IGBMC-Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) revealed 100% homology with the human SCF and GAPDH cDNAs and no significant homology with any other known gene (NCBI Blast program).
Hybridisation and quantification
Each sample of radiolabelled RNA was hybridised on individual membranes (Duralon UV, Stratagene, La Jolla, CA, U.S.A.), on which 10 μg of either pGEM-T plasmid (control) or plasmid-containing inserts of human SCF cDNA or GAPDH cDNA had been immobilised. After hybridisation for 72 h at 42°C, the membranes were washed at a final stringency of 0.1 × standard saline citrate and 0.1% sodium dodecyl sulphate at 55°C, including a 30 min digestion with 1 μg ml−1 RNase A and 10 U ml−1 RNase T1 (Boehringer-Mannheim, Mannheim, Germany) at 37°C to digest any single-stranded RNA not hybridised to DNA. After radioautography, films were digitised, and spot density was quantified with the NIH Image Program. The rate of SCF gene transcription was expressed as the ratio of SCF mRNA to GAPDH mRNA signals.
Transfection of human lung fibroblasts
Fibroblasts (650 000) were plated in six-well culture plates. After 24 h, they were placed into a quiescent state by reducing the FCS content to 0.3% (starving medium) and transiently transfected with 1 μg of pGL3e/SCF plasmid reporters (see section ‘plasmid constructs' below), together with 1 μg of Renilla luciferase pRL-TK vector as an internal standard (Promega). We used Fugene6™ (Roche Diagnostics) according to the manufacturer's instructions to perform this transfection. After 48 h, later cells were treated (see section ‘Cell treatment') and harvested. We measured luciferase with the dual luciferase assay system (Promega), according to the manufacturer's instructions.
Plasmid constructs
Genomic DNA purification
Cultured fibroblasts were washed with ice-cold PBS, scraped and centrifuged. The cell pellet was resuspended in an extraction buffer (100 mM Tris-HCl, pH 9, 400 mM LiCl, 25 mM EDTA, 1% SDS). After a phenol/chloroform/isoamylic alcohol mixture was added, cells were incubated on ice and centrifuged. The aqueous phase was incubated with isopropanol and centrifuged. The pellet then underwent this process again, in nearly identical conditions: it was dried and resuspended in a buffer (100 mM Tris-HCl, pH 8, 100 mM NaCl, 1 mM EDTA, 40 μg ml−1 Rnase A), incubated on ice, added to a phenol/chloroform/isoamylic alcohol mixture and centrifuged. DNA in the aqueous phase was precipitated with isopropanol and centrifuged. The DNA pellet was dried, washed and resuspended in TE1 × (10 mM Tris-HCl, pH 8, 1 mM EDTA).
Plasmid construction
Since we identified a sequence marginally homologous to the consensus sequence of the NF-κB-responsive element in intron 1 of the SCF gene: (+215) 5′-GGGAGCTCCC-3′ (+225) (TESS program: http://www.cbil.upenn.edu/tess), we subcloned the promoter region of the human SCF gene, including exon 1 and part of the intron 1 (position –2159 to +341), with nested PCR performed on genomic DNA. The amplified region was introduced into a pGL3-Enhancer vector (Promega) upstream of the firefly luciferase reporter gene, thus constructing a pGL3e/SCF-2159 plasmid. To optimise the luciferase gene activity induced by the SCF promoter, ATG sequences in phase with the luciferase ATG were point-mutated (QuikChange™ Site-Directed Mutagenesis Kit, Stratagene) to facilitate wild-type (WT) construction. The NF-κB-like-responsive element at position 2398 in the plasmid was deleted. The 2389–2549 portion in the WT plasmid containing the element was excised by restriction enzymes (AscI and XhoI), thereby generating a 160 bp fragment. We used high-fidelity PCR and primers that spanned the NF-κB-like sequence to create a PCR product of 150 bp without the κB-like site. After the WT construction was digested by AscI and XhoI and purified, this purified PCR product was reinserted into it by ligation: this produced the pGL3e/SCF-κB plasmid (κB), which had 100% sequence homology with the WT construction missing the κB-like element. To study the effect of the combination of IL-1β and budesonide in lung fibroblasts, the constructions were transfected into fibroblasts as described above.
Expression of results and statistical analysis
Results are expressed as means±s.e.m. and are represented as a percent of variation (%) of SCF cDNA and protein levels from fibroblasts treated with solvent alone for the same time period, since baseline levels progressively increase over time. Statistical analysis of the results used a Student–Newmann–Keuls test, or a paired Student's t–test when two variables were compared in the transfection experiments. Values of P<0.05 were considered significant.
Results
Effect of IL-1β on SCF expression
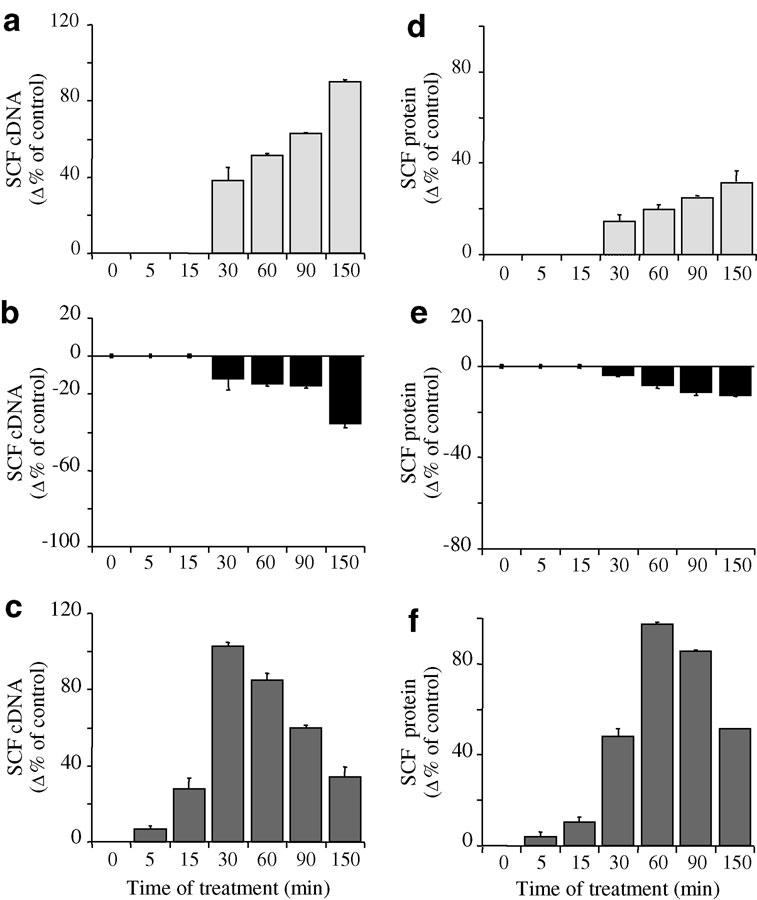
IL-1β (400 pg ml−1) significantly increased the expression of SCF mRNA by human lung fibroblasts in culture from 30 to 150 min (Figure 1a). This increase peaked at +91% at 150 min (128.2±5.4 and 245.9±9.4 fg ng total cDNA−1 for control and IL-1β-treated fibroblasts, respectively; P<0.001, n=4). The increase was significant from 30 to 150 min.
Figure 1.
Time-dependent effect of IL-1β (a, d) (400 pg ml−1; pale grey), budesonide (b, e) (0.1 μM; black) and IL-1β + budesonide (c, f) (dark grey) on SCF mRNA (a–c) and protein (d–f) expression by human lung fibroblasts in culture. SCF149 cDNA was quantified after total RNA RT by on-line fluorescent PCR. Results were normalised to total cDNA and expressed as fg SCF149 cDNA ng total cDNA−1. SCF protein levels were assessed in the supernatant by ELISA and the values are expressed in pg ml−1. Results are mean values (blocks)±s.e.m. (bars) of four experiments performed in duplicate on fibroblasts from three different donors. Values after treatment were expressed as a percent of variation (%) of SCF149 cDNA or protein levels from fibroblasts treated with solvent alone for the same time period.
Production of SCF protein increased slightly but significantly from 30 to 150 min after IL-1β treatment (Figure 1d). At 150 min, the increase reached +32% (36.8±1.6 and 48.7±3.3 pg ml−1 for control and IL-1β-treated fibroblasts, respectively; P<0.001, n=4). Results were similar when IL-1β treatment was staggered to stop the cell cultures at the same time, thereby ensuring that the effect we observed was not due to modification in the cell phenotype over time.
Effect of budesonide on SCF expression
From 30 to 150 min (Figure 1b) after budesonide (0.1 μM) treatment, SCF mRNA expression decreased significantly. This reduction reached a maximum of –36% at 150 min (85.7±35.7 fg ng total cDNA−1; P<0.001, n=4). In the same conditions and during the same period, SCF protein production also decreased, slightly but significantly (Figure 1e). A 13% decrease occurred at 150 min (32.1±1.7 pg ml−1 SCF, P<0.01, n=4).
Effect of budesonide on IL-1β-induced SCF production
Budesonide potentiated the IL-1β-induced increase of SCF mRNA expression very early after treatment, at 5–60 min (Figure 1c). The increase peaked (+103%) at 30 min (55.8±4.6 and 113.4±7.1 fg ng total cDNA−1 for control and IL-1β+budesonide-treated fibroblasts, respectively; P<0.001, n=4). From 60 to 150 min, this enhancement decreased in importance, with a 64% inhibition at 150 min (165.7±64.1 fg ng total cDNA−1; P<0.01, n=4). SCF expression was thus lower than with IL-1β alone (P<0.001, n=4).
SCF protein production followed the pattern of SCF mRNA expression (Figure 1f), with budesonide potentiating the IL-1β-enhanced SCF protein production early on, from 5 to 150 min (Figure 1f). Expression peaked (+98%) at 60 min (7.7±0.5 and 15.3±1.2 pg ml−1 for control and IL-1β+budesonide-treated fibroblasts, respectively; P<0.001, n=4). From 90 min to 150 min, enhancement decreased by 40% at 150 min (55.9±1.0 pg ml−1 SCF; P<0.001, n=4), although expression was still greater than with IL-1β alone.
GR-dependent glucocorticoid effect
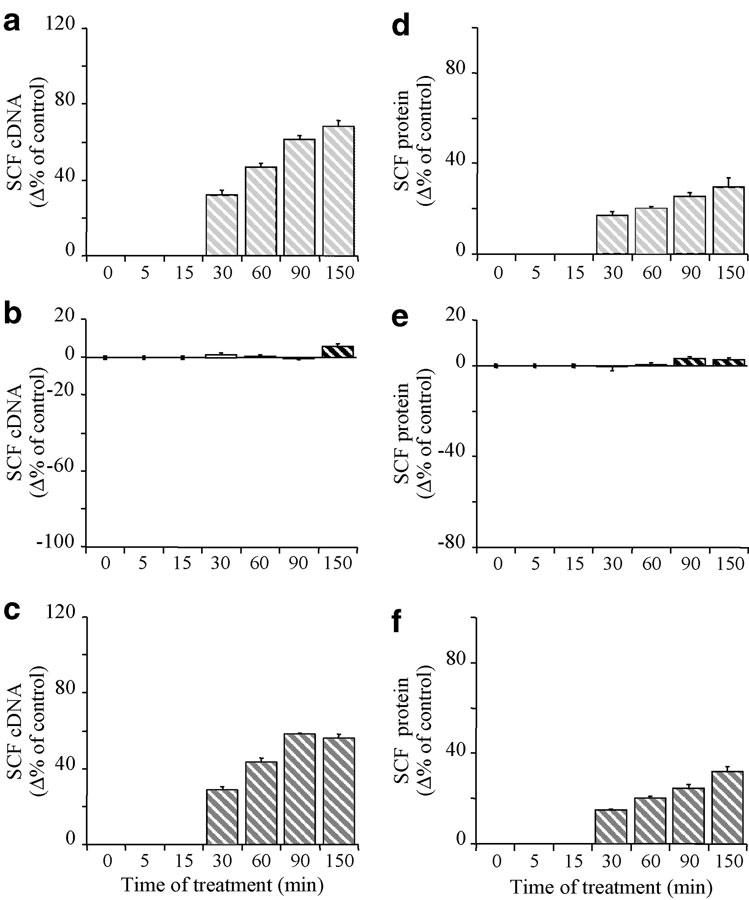
The glucocorticoid-receptor antagonist RU486 (1 μM) by itself did not affect the constitutive expression of SCF mRNA at any time studied (at 150 min for instance: 130.3±53.0 fg ng total cDNA−1, n=4) (Figure 2a and c). It antagonised the budesonide-induced decrease of SCF mRNA expression from 5 to 150 min (at 150 min; +6% 138.2±56.7 fg ng total cDNA−1, n=4) (Figure 2b). It also blocked the very early potentiating effect of budesonide on IL-1β-induced SCF mRNA expression (71% inhibition at 30 min; 72.7±5.9 fg ng total cDNA−1; P<0.001, n=4) (Figure 2c), leading to an effect nearly identical to that observed in response to RU486+IL-1β in the absence of budesonide (+39% and +33% at 30 min, respectively, NS; 78.4±9.3 and 73.6±6.9 fg ng total cDNA−1 respectively; P<0.001, n=4). The effect of RU486+IL-1β on SCF mRNA expression was, however, significantly less than that of IL-1β alone at 150 min (12% inhibition; 212.6±83.6 fg ng total cDNA−1; P<0.001, n=4) (Figure 1a and 2a), whereas it had no effect at earlier times (Figure 2a). Similarly, RU486 had no effect on the constitutive expression of SCF protein over time (e.g., at 150 min: 35.0±2.2 pg ml−1 SCF, n=4), but it totally abolished the effect of budesonide on this expression at any time (Figure 2e). In addition, RU486 abolished the very early potentiation by budesonide of the IL-1β-enhanced SCF protein expression (75% inhibition at 60 min, P<0.001; 28.3±5.3 and 32.9±6.2 pg ml−1 SCF for control and RU486+IL-1β+budesonide-treated fibroblasts, respectively, n=4) (Figure 2f). This effect again was nearly identical to that observed in response to IL-1β or IL-1β+RU486 without budesonide (+46% and 48% 11.4±1.0 and 11.42±1.1 pg ml−1 SCF; P<0.01, n=4) (Figure 2d). Finally, RU486 did not, at any time we studied, modify the effect of IL-1β alone on SCF protein expression (+30% at 150 min; 47.9±3.0 pg ml−1 SCF; P<0.001, n=4) (Figure 2d).Similar results were obtained with dexamethasone (Table 1).
Figure 2.
Time-dependent effect of IL-1β (a, d) (400 pg ml−1; hatched pale grey columns), budesonide (b, e) (0.1 μM; hatched black columns) and IL-1β + budesonide (c, f) (hatched dark grey columns) in the presence of RU486 (1 μM) on SCF mRNA (a–c) and protein (d–f) expression by human lung fibroblasts in culture. SCF149 cDNA was quantified after total RNA RT by on-line fluorescent PCR. Results were normalised to total cDNA and expressed as fg SCF149 cDNA ng total cDNA−1. SCF protein levels were assessed in the supernatant by ELISA and the are expressed in pg ml−1. Results are mean values (blocks)±s.e.m. (bars) of four experiments performed in duplicate on fibroblasts from three different donors. Values after treatment were expressed as a percent of variation (%) of SCF149 cDNA or protein levels from fibroblasts treated with solvent alone for the same time period.
Table 1.
Effect of dexamethasone on SCF expression
| Control | RU 486 | IL-1β | Dexa | IL-1β+Dexa | Ru 486+Dexa | RU 486+IL-1βDexa | |
|---|---|---|---|---|---|---|---|
| 30 min | 117.8±13.4 | 120.7±13.1 | 159.9±9.6 | 116.3±11.0 | 203.8±13.7 | 123.9±16.4 | 170.6±4.7 |
| SCF mRNA (fg ng−1) | NS | P<0.01 | NS | P<0.001 | NS | P<0.01 | |
| 60 min | 0 | 23.2±0.7 | 25.3±0.2 | 21.9±0.6 | 33.1±0.5 | 22.8±0.5 | 26.5±0.0 |
| SCF protein (pg ml−1) | NS | P<0.05 | NS | P<0.001 | NS | P<0.001 |
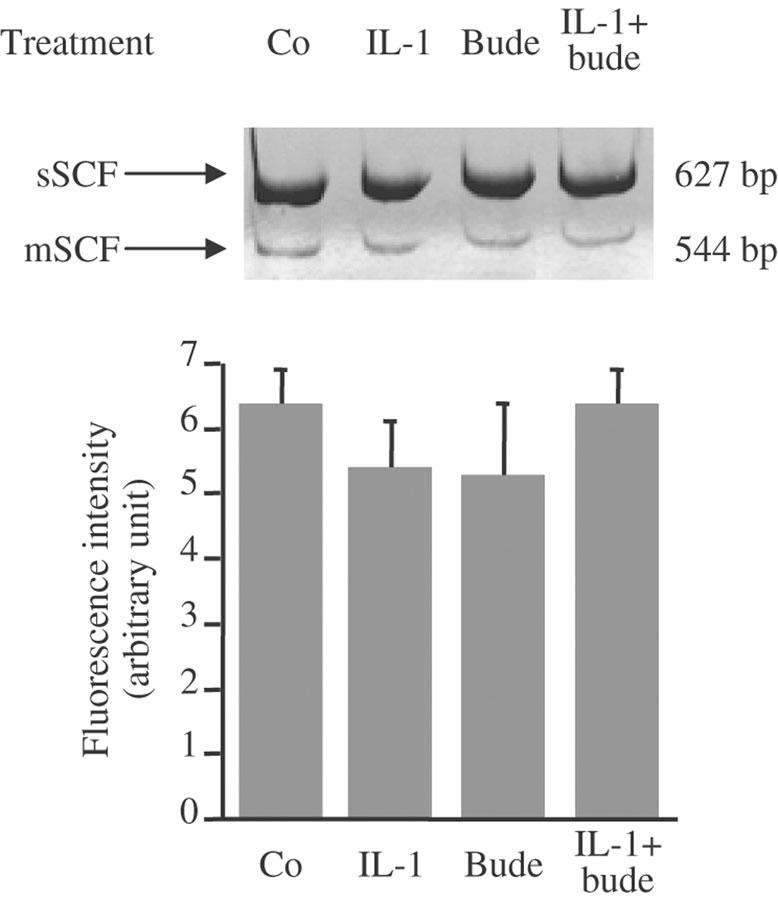
Relative expression of sSCF and mSCF mRNA
The effect of IL-1β and budesonide on the relative expression of the two forms of SCF mRNA was analysed at 30 min by on-line fluorescent PCR after RT, with primers spanning the alternatively spliced sixth exon. Their relative expression (Figure 3) after treatment with IL-1β, budesonide or both was unchanged.
Figure 3.
Effect of IL-1β (IL-1: 400 pg ml−1), budesonide (bude: 0.1 μM) or IL-1β+budesonide on the relative expression of sSCF and mSCF mRNA at 30 min in human lung fibroblasts in culture. Total RNA was extracted and reverse transcribed. sSCF and mSCF cDNA were amplified by PCR of RT products containing equal amounts of total SCF cDNA quantified by on-line fluorescent PCR. Ratios of sSCF to mSCF are expressed as means±standard errors of three different experiments performed in duplicate on fibroblasts from three different donors.
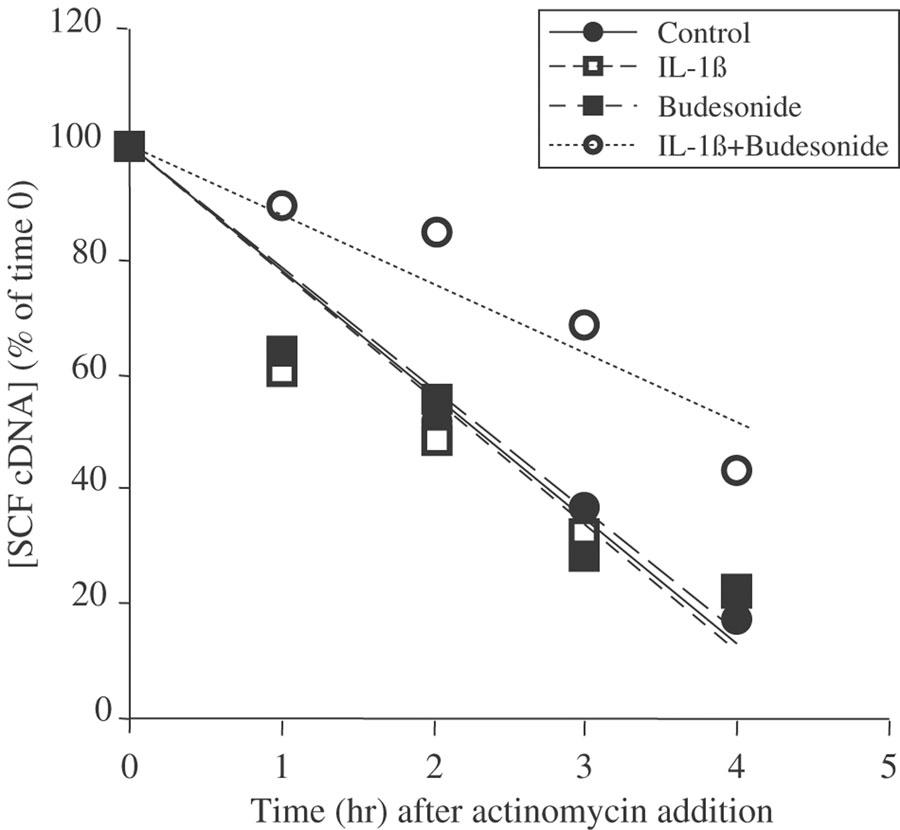
SCF mRNA stability
To assess the mechanism of the glucocorticoid-induced effects on IL-1β-treated fibroblasts, we evaluated the stability of SCF mRNA after addition of actinomycin D (5 μg ml−1). At 30 min, the estimated half-life of SCF mRNA was 2.3 h in control fibroblasts treated with solvent alone (Figure 4). Treatment with either IL-1β or budesonide did not change this (t1/2=2.3 h for both, NS compared with control cells). The combination treatment of IL-1β and budesonide, however, markedly increased the stability of SCF mRNA (t1/2=4.1 h, P<0.001, n=4).
Figure 4.
SCF mRNA stability. Fibroblasts were treated with IL-1β (400 pg ml−1) budesonide (0.1 μM), IL-1β+budesonide or ethanol (1 μM; control) for 30 min. Fibroblasts were then washed and treated with 5 μg ml−1 actinomycin D for the time indicated. SCF149 cDNA was quantified by on-line fluorescent PCR. Results are expressed as a percentage of the quantity of SCF149 cDNA measured for each treatment at time 0 of actinomycin D addition, which is represented as the 100% of each treatment. Data are means of three experiments performed in duplicate on fibroblasts from three different donors.
Effect of IL-1β and budesonide on the rate of SCF gene transcription
The nuclear run-on assay was performed on fibroblasts treated with the combination of IL-1β and budesonide or with solvent for 30 min. Gene transcription in fibroblasts treated with IL-1β and budesonide, expressed as the ratio of SCF gene to GAPDH gene transcription, was 40% greater than in fibroblasts treated with solvent alone (Figure 5).
Figure 5.
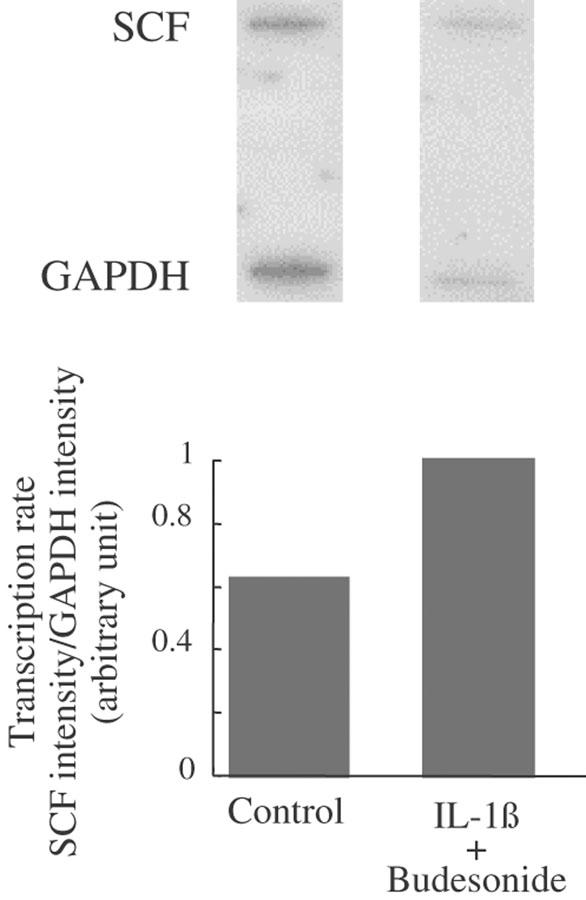
Effect of IL-1β+budesonide on SCF mRNA transcription rate. Fibroblasts were treated with ethanol (1 μM: control) or IL-1β (400 pg ml−1)+budesonide (0.1 μM) for 30 min. Nuclei were prepared, and SCF gene transcription rate was assessed after autoradiography of blots (top), and by the ratio of SCF mRNA signal to GAPDH mRNA signal (bottom), representative of two experiments perfomed on fibroblasts from two different donors.
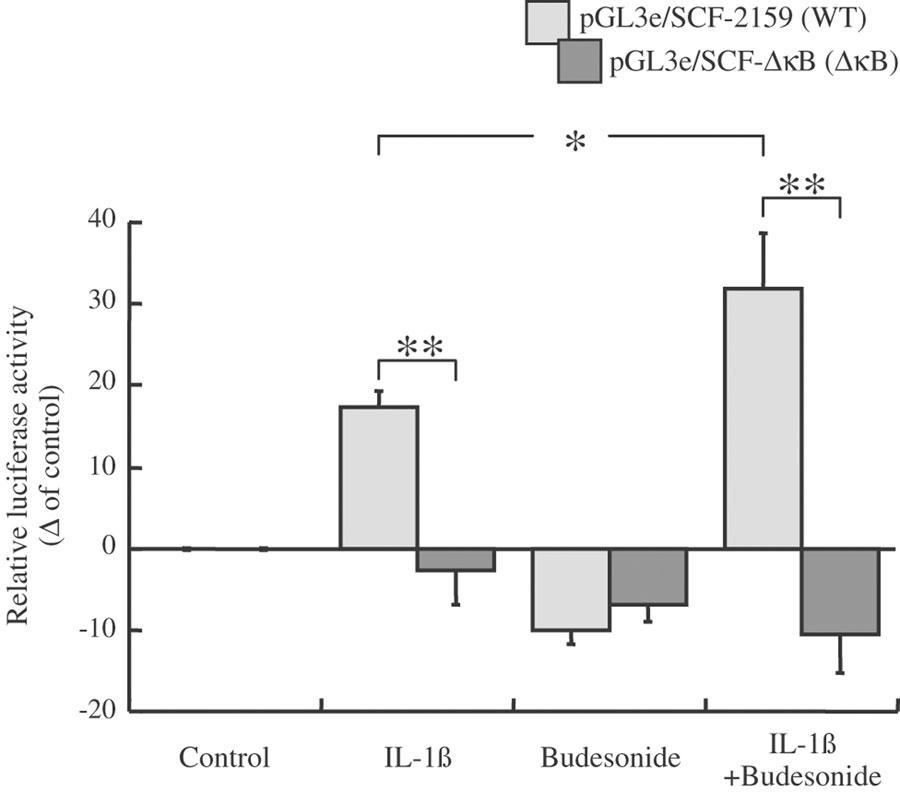
Effect of IL-1β and budesonide on fibroblasts transfected by the pGL3e/SCF-ΔκB (ΔκB) and pGL3e/SCF-2159 (WT) plasmids
IL-1β increased the luciferase reporter activity of the WT-SCF promoter gene transfected into lung fibroblasts at 30 min (+17%: from 4.2±0.2 and 5.0±0.1 RLU WT/RLU pRL-TK for control and IL-1β-treated fibroblasts, respectively; P<0.01, n=6) (Figure 6). In contrast, IL-1β had no effect when the κB-like sequence was deleted in the first intron (5.3±0.2 and 5.2±0.5 RLU ΔκB/RLU pRL-TK for control and IL-1β-treated fibroblasts, respectively; NS, n=6) (Figure 6). It is to be noticed that fibroblasts transfected with the ΔκB construction unexpectedly increased the luciferase reporter activity at baseline, compared with fibroblasts transfected with the WT construction (4.2±0.2 and 5.3±0.2 RLU pGL3e/RLU pRL-TK for cells transfected with WT and ΔκB, respectively).Budesonide slightly decreased activity of both the WT and ΔκB SCF genes at 30 min (−10% for WT: 3.8±0.1 RLU WT/RLU pRL-TK; P<0.001, n=6: −7% for ΔκB; 4.9±0.1 RLU ΔκB/RLU pRL-TK; P< 0.01, n=6), as expected. In contrast, budesonide potentiated the IL-1β induced WT-SCF gene activity by 20% at 30 min (5.6±0.3 RLU WT/RLU pRL-TK in IL-1β+budesonide-treated fibroblasts; P<0.01, n=6) (Figure 6). This potentiation was totally abolished for the ΔκB SCF gene activity, activity returning to that of budesonide alone (−11%: 4.7±0.2 RLU ΔκB/RLU pRL-TK; P<0.05, n=6).
Figure 6.
Effect of IL-1β and budesonide on SCF promoter gene activity. Human lung fibroblasts in culture were transiently cotransfected with pGL3e/SCF-2159 (WT: pale grey) or pGL3e/SCF-ΔκB (dark grey) firefly luciferase constructs in combination with a Renilla luciferase construct (pRL-TK) as an internal control. At 48 h after transfection, cells were stimulated with solvent (control: ethanol), IL-1β (400 pg ml−1), budesonide (0.1 μM) or a combination for 30 min. Then cells were harvested for luciferase activity measurements. The results are expressed as the level of WT and ΔκB constructions promoter-driven firefly luciferase expression after correcting for the transfection efficiency by pRL-TK luciferase measurements (relative luciferase activity), and represented as a percentage of control values. Results are mean (blocks)±s.e.m. (bars) of two independent experiments performed in triplicate in fibroblasts from three different donors *P<0.05, **P<0.01.
Discussion
Our study shows a marked and early (30 min) potentiation by budesonide of the IL-1β-induced increase in the expression of SCF mRNA and protein in human lung fibroblasts in culture. This effect occurs without affecting the relative expression of transcripts for the soluble and membrane-bound forms of SCF. It is related to the increased stability of SCF mRNA and to increased SCF gene transcription, as measured by a nuclear run-on assay. We also show with a luciferase reporter gene assay that the early transcription does not occur after the NF-κB-like responding site is deleted, suggesting that this responsive element plays a role in the early increase. The glucocorticoid effect all depends on the GR, since RU486, a glucocorticoid receptor antagonist, blocked all of these early glucocorticoid effects.
In this study, we first show that IL-1β alone increases SCF mRNA expression shortly after treatment, from 30 min onward, peaking at 150 min. This early increase (30 min) probably involves transcription of the SCF gene, since the SCF mRNA stability is not affected by IL-1β treatment. This finding is consistent with the reported IL-1β-induced increase in the expression of genes implicated in inflammatory processes. These include interleukin (IL)-6 in human lung primary fibroblasts (Ng et al., 1994) where the increase peaks at 150 min post-treatment, cyclooxygenase (COX)-2 mRNA expression by human pulmonary epithelial cells (Lin et al., 2000) or eotaxin mRNA expression in A549 airway epithelial cells (Jedrzkiewicz et al., 2000). These reports have proposed that the mechanism by which IL-1β increases this expression is related to the activation of NF-κB (Jedrzkiewicz et al., 2000; Lin et al., 2000). We have identified a NF-κB-like responsive element located in the first intron of the SCF gene (+215 to +225), and show that deletion of this NF-κB-like binding site leads to the abolition of the effect of IL-1β alone on SCF gene expression, thereby indicating that NF-κB might play a role in the IL-1β-induced SCF gene expression. This NF-κB-like responsive element, with original location in the first intron and activated by IL-1β stimulation, has not been described before and is one important finding of our paper. Activated NF-κB is detectable in the nucleus of cells within 10–20 min (Carlotti et al., 2000; Moynagh et al., 1993) and induces transcription of inhibitor (I)-κB and macrophage inflammatory protein (MIP)-2 genes at 10 and 20 min, respectively (Saccani et al., 2001). Our results are thus consistent with the expression of SCF mRNA in the first wave of NF-κB recruitment, as Saccani and coworkers proposed for MIP-2 (2001). We may thus submit SCF as an early regulated gene in inflammatory conditions in airway structural cells.
Our data also show that in the inflammatory conditions created by IL-1β in vitro, budesonide and dexamethasone markedly potentiate the IL-1β-induced increase in SCF expression shortly after treatment, from 0 to 30 min. Although nuclear accumulation of GRs is a rapid process occurring within 5-min and that target gene activation like the serine/threonine protein kinase gene (sgk) is observable within 30 min of addition of the hormone itself (Webster et al., 1993; Carey et al., 1996), no reports have described glucocorticoid potentiation of cytokines expression as early as observed here for SCF. One mechanism involved in the potentiation of SCF expression by glucocorticoids at 30 min is an increase in the stability of SCF mRNA. This effect is selectively related to the combination of IL-1β and budesonide, since neither alone, IL-1β or budesonide, changes this half-life from that of nonstimulated cells.
This combined effect of cytokines and glucocorticoids on mRNA stability has previously been described in rat glomerular mesangial cells, where dexamethasone increases inducible nitric oxide synthase (iNOS) mRNA stability in the presence of IL-1β (Kunz et al., 1996) although only slightly. It has been reported that IL-1β itself, like glucocorticoids (Paek & Axel, 1987), increase the process of polyadenylation (Stoeckle, 1992), which is related to increased mRNA stability. IL-1β alone has also been reported to stabilise mRNA through activation of p38 mitogen-activated protein (MAP) kinase (Ridley et al., 1998). Corticosterone has also been shown to stimulate extracellular-regulated kinase (ERK) in PC12 cells (Qiu et al., 2001). Accordingly, combining the effects induced by IL-1β and glucocorticoids on MAP kinases may work together to increase SCF mRNA stability although IL-1β or budesonide alone were inactive. On the other hand, this increased stability may involve other currently unidentified mechanisms. These may include the upregulation of poly-(A+) binding proteins (PABP) or of 5′-catabolite gene activator protein (CAP), which are reported to protect mRNA from degradation (Ross, 1995).
Our study also reports involvement of an additional mechanism in the potentiation by budesonide of IL-1β-enhanced SCF expression. The SCF gene transcription rate was increased at 30 min. Previous findings from luciferase reporter gene assays on rat hepatoma cells showed that dexamethasone and IL-6 act in synergy to increase metallothionein (mMT)-I gene expression (Kasutani et al., 1998). The authors hypothesised, based on previous findings (Nishio et al., 1993), that GR interacts directly with the Stat 3 transcription factor to increase mMT-I gene expression. In addition, increased expression of the mouse proliferin gene has been related to a direct interaction of GR with the jun–jun homodimer of activated protein (AP)-1 (Diamond et al., 1990). In our case, the luciferase reporter gene assay showed that the potentiating effect of glucocorticoids on the IL-1β-enhanced SCF expression was abolished when the NF-κB-like responsive element located in the first intron of the SCF gene was deleted. This indicates that potentiation of SCF transcription by budesonide is also related to activation of the NF-κB-like site by IL-1β very shortly after stimulation. This may indicate early and unexpected adverse effects of glucocorticoids on SCF expression in inflammatory conditions.
In contrast, after 60 min of budesonide incubation in the presence of IL-1β, SCF expression decreased markedly. This may reflect increased protein degradation, possibly due to budesonide promotion of protease expression or activity in inflammatory conditions, as previously reported for neutral endopeptidase (Piedimonte et al., 1991; Barnes, 1998). Such repression of IL-1β-enhanced expression by glucocorticoids has been documented for several other genes, including pro-inflammatory cytokines and chemokines (Ponta et al., 1992; Pfahl, 1993). On the other hand, a direct interaction between the activated GR and transcription factors like NF-κB or AP-1 has been suggested as an explanation for decreased gene transcription (for a review, see Cato & Wade, 1996; Adcock, 2001) that might be involved for SCF gene.
We noted that deletion of the κB-like element increased the baseline activity of the SCF promoter, which suggests that this NF-κB-like site may play a role in baseline regulation. We may then hypothesise that deletion of this site prevents a repressor factor from acting, as Baek et al. (2002) previously proposed for the nuclear receptor corepressor complex N-CoR. This regulatory activity would function together with other responsive elements in the SCF promoter, including cAMP responsive element (CRE), AP-1, and stress protein (Sp)1, all of which have previously been suggested to regulate baseline activity (Taylor et al., 1996).
In conclusion, our study clearly shows that in the inflammatory conditions mimicked in vitro by IL-1β, glucocorticoids act very early to adversely increase the expression of SCF mRNA and protein, and that this action implies increased mRNA stability, and increased gene transcription, involving activation of the NF-κB-like responsive element located in the first intron of the SCF gene. Such early glucocorticoid potentiation of the IL-1β-enhanced SCF expression is relatively uncommon since glucocorticoids are reported to decrease the expression of pro-inflammatory mediators; it provides new insights into the possible deleterious effects of glucocorticoids and the mechanism for them.
Abbreviations
- AP-1
activated protein-1
- CAP protein
5′-catabolite gene activator protein
- COX-2
cyclooxygenase type 2
- CRE
cyclic AMP responsive element
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- GR
glucocorticoid receptor
- I-κB
inhibitor κB
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- iNOS
inducible nitric oxyde synthase
- MAPKinase
mitogen-activated protein kinase
- MIP-2
macrophage inflammatory protein-2
- N-coR
nuclear receptor corepressor
- NF-κB
nuclear Factor-κB
- PABP
poly (A+) binding protein
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- RT
reverse transcription
- SCF
stem cell factor
- Sp1
stress protein 1
References
- ADCOCK I.M. Glucocorticoid-regulated transcription factors. Pulm. Pharmacol. Ther. 2001;14:211–219. doi: 10.1006/pupt.2001.0283. [DOI] [PubMed] [Google Scholar]
- ANDERSON D.M., WILLIAMS D.E., TUSHINSKI R., GIMPEL S., EISENMAN J., CANNIZZARO L.A., ARONSON M., CROCE C.M., HUEBNER K., COSMAN D., LYMAN S.D. Alternate splicing of mRNAs encoding human mast cell growth factor and localization of the gene to chromosome 12q22-q24. Cell Growth Differ. 1991;2:373–378. [PubMed] [Google Scholar]
- BAEK S.H., OHGI K.A., ROSE D.W., KOO E.H., GLASS C.K., ROSENFELD M.G. Exchange of N-CoR corepressor and Tip60 coactivator complexes links gene expression by NF-kappaB and beta-amyloid precursor protein. Cell. 2002;110:55–67. doi: 10.1016/s0092-8674(02)00809-7. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin. Sci. (Lond.) 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- BISCHOFF S.C., DAHINDEN C.A. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J. Exp. Med. 1992;175:237–244. doi: 10.1084/jem.175.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY K.L., RICHARDS S.A., LOUNSBURY K.M., MACARA I.G. Evidence using a green fluorescent protein-glucocorticoid receptor chimera that the Ran/TC4 GTPase mediates an essential function independent of nuclear protein import. J. Cell Biol. 1996;133:985–996. doi: 10.1083/jcb.133.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLOTTI F., DOWER S.K., QWARNSTROM E.E. Dynamic shuttling of nuclear factor kappa B between the nucleus and cytoplasm as a consequence of inhibitor dissociation. J. Biol. Chem. 2000;275:41028–41034. doi: 10.1074/jbc.M006179200. [DOI] [PubMed] [Google Scholar]
- CATO A.C., WADE E. Molecular mechanisms of anti-inflammatory action of glucocorticoids. Bioessays. 1996;18:371–378. doi: 10.1002/bies.950180507. [DOI] [PubMed] [Google Scholar]
- COSTA J.J., DEMETRI G.D., HARRIST T.J., DVORAK A.M., HAYES D.F., MERICA E.A., MENCHACA D.M., GRINGERI A.J., SCHWARTZ L.B., GALLI S.J. Recombinant human stem cell factor (kit ligand) promotes human mast cell and melanocyte hyperplasia and functional activation in vivo. J. Exp. Med. 1996;183:2681–2686. doi: 10.1084/jem.183.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSTON G.E., CAO Z., GOEDDEL D.V. NF-kappa B activation by interleukin-1 (IL-1) requires an IL-1 receptor- associated protein kinase activity. J. Biol. Chem. 1995;270:16514–16517. doi: 10.1074/jbc.270.28.16514. [DOI] [PubMed] [Google Scholar]
- DA SILVA C.A., KASSEL O., MATHIEU E., MASSARD G., GASSER B., FROSSARD N. Inhibition by glucocorticoids of the interleukin-1beta-enhanced expression of the mast cell growth factor SCF. Br. J. Pharmacol. 2002;135:1634–1640. doi: 10.1038/sj.bjp.0704617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND M.I., MINER J.N., YOSHINAGA S.K., YAMAMOTO K.R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- FLANAGAN J.G., CHAN D.C., LEDER P. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 1991;64:1025–1035. doi: 10.1016/0092-8674(91)90326-t. [DOI] [PubMed] [Google Scholar]
- HUANG E., NOCKA K., BEIER D.R., CHU T.Y., BUCK J., LAHM H.W., WELLNER D., LEDER P., BESMER P. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell. 1990;63:225–233. doi: 10.1016/0092-8674(90)90303-v. [DOI] [PubMed] [Google Scholar]
- IEMURA A., TSAI M., ANDO A., WERSHIL B.K., GALLI S.J. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am. J. Pathol. 1994;144:321–328. [PMC free article] [PubMed] [Google Scholar]
- ISRAEL A. Signal transduction. IkappaB kinase all zipped up [news; comment] Nature. 1997;388:519–521. doi: 10.1038/41433. [DOI] [PubMed] [Google Scholar]
- JEDRZKIEWICZ S., NAKAMURA H., SILVERMAN E.S., LUSTER A.D., MANSHARAMANI N., IN K.H., TAMURA G., LILLY C.M. IL-1beta induces eotaxin gene transcription in A549 airway epithelial cells through NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1058–L1065. doi: 10.1152/ajplung.2000.279.6.L1058. [DOI] [PubMed] [Google Scholar]
- JEFFERY P.K., GODFREY R.W., ADELROTH E., NELSON F., ROGERS A., JOHANSSON S.A. Effects of treatment on airway inflammation and thickening of basement membrane reticular collagen in asthma. A quantitative light and electron microscopic study. Am. Rev. Respir. Dis. 1992;145:890–899. doi: 10.1164/ajrccm/145.4_Pt_1.890. [DOI] [PubMed] [Google Scholar]
- KASSEL O., SCHMIDLIN F., DUVERNELLE C., DE BLAY F., FROSSARD N. Up- and down-regulation by glucocorticoids of the constitutive expression of the mast cell growth factor stem cell factor by human lung fibroblasts in culture. Mol. Pharmacol. 1998;54:1073–1079. doi: 10.1124/mol.54.6.1073. [DOI] [PubMed] [Google Scholar]
- KASUTANI K., ITOH N., KANEKIYO M., MUTO N., TANAKA K. Requirement for cooperative interaction of interleukin-6 responsive element type 2 and glucocorticoid responsive element in the synergistic activation of mouse metallothionein-I gene by interleukin-6 and glucocorticoid. Toxicol. Appl. Pharmacol. 1998;151:143–151. doi: 10.1006/taap.1998.8452. [DOI] [PubMed] [Google Scholar]
- KIRSHENBAUM A.S., GOFF J.P., KESSLER S.W., MICAN J.M., ZSEBO K.M., METCALFE D.D. Effect of IL-3 and stem cell factor on the appearance of human basophils and mast cells from CD34+ pluripotent progenitor cells. J. Immunol. 1992;148:772–777. [PubMed] [Google Scholar]
- KUNZ D., WALKER G., EBERHARDT W., PFEILSCHIFTER J. Molecular mechanisms of dexamethasone inhibition of nitric oxide synthase expression in interleukin 1 beta-stimulated mesangial cells: evidence for the involvement of transcriptional and posttranscriptional regulation. Proc. Natl. Acad. Sci. USA. 1996;93:255–259. doi: 10.1073/pnas.93.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAITINEN L.A., LAITINEN A., HAAHTELA T. A comparative study of the effects of an inhaled corticosteroid, budesonide, and a beta 2-agonist, terbutaline, on airway inflammation in newly diagnosed asthma: a randomized, double-blind, parallel-group controlled trial. J. Allergy Clin. Immunol. 1992;90:32–42. doi: 10.1016/s0091-6749(06)80008-4. [DOI] [PubMed] [Google Scholar]
- LIN C.H., SHEU S.Y., LEE H.M., HO Y.S., LEE W.S., KO W.C., SHEU J.R. Involvement of protein kinase C-gamma in IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells. Mol. Pharmacol. 2000;57:36–43. [PubMed] [Google Scholar]
- MARTIN F.H., SUGGS S.V., LANGLEY K.E., LU H.S., TING J., OKINO K.H., MORRIS C.F., MCNIECE I.K., JACOBSEN F.W., MENDIAZ E.A., BIRKETT N.C., SMITH K.A., JOHNSON M.J., PARKER V.P., FLORES J.C., PATEL A.C., FISHER E.F., ERJAVEC H.O., HERRERA C.J., WYPYCH J., SACHDEV R.J., POPE J.A., LESLIE A., WEN D., LIN C.H., CUPPLES R.L., ZSEBO K.M. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990;63:203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- MOYNAGH P.N., WILLIAMS D.C., O'NEILL L.A. Interleukin-1 activates transcription factor NF kappa B in glial cells. Biochem. J. 1993;294:343–347. doi: 10.1042/bj2940343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG S.B., TAN Y.H., GUY G.R. Differential induction of the interleukin-6 gene by tumor necrosis factor and interleukin-1. J. Biol. Chem. 1994;269:19021–19027. [PubMed] [Google Scholar]
- NISHIO Y., ISSHIKI H., KISHIMOTO T., AKIRA S. A nuclear factor for interleukin-6 expression (NF-IL6) and the glucocorticoid receptor synergistically activate transcription of the rat alpha 1-acid glycoprotein gene via direct protein-protein interaction. Mol. Cell Biol. 1993;13:1854–1862. doi: 10.1128/mcb.13.3.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAEK I., AXEL R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol. Cell Biol. 1987;7:1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFAHL M. Nuclear receptor/AP-1 interaction. Endocr. Rev. 1993;14:651–658. doi: 10.1210/edrv-14-5-651. [DOI] [PubMed] [Google Scholar]
- PIEDIMONTE G., MCDONALD D.M., NADEL J.A. Neutral endopeptidase and kininase II mediate glucocorticoid inhibition of neurogenic inflammation in the rat trachea. J. Clin. Invest. 1991;88:40–44. doi: 10.1172/JCI115302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTA H., CATO A.C., HERRLICH P. Interference of pathway specific transcription factors. Biochim. Biophys. Acta. 1992;1129:255–261. doi: 10.1016/0167-4781(92)90501-p. [DOI] [PubMed] [Google Scholar]
- QIU J., WANG P., JING Q., ZHANG W., LI X., ZHONG Y., SUN G., PEI G., CHEN Y. Rapid activation of ERK1/2 mitogen-activated protein kinase by corticosterone in PC12 cells. Biochem. Biophys. Res. Commun. 2001;287:1017–1024. doi: 10.1006/bbrc.2001.5691. [DOI] [PubMed] [Google Scholar]
- RIDLEY S.H., DEAN J.L., SARSFIELD S.J., BROOK M., CLARK A.R., SAKLATVALA J. A p38 MAP kinase inhibitor regulates stability of interleukin-1-induced cyclooxygenase-2 mRNA. FEBS Lett. 1998;439:75–80. doi: 10.1016/s0014-5793(98)01342-8. [DOI] [PubMed] [Google Scholar]
- ROSS J. mRNA stability in mammalian cells. Microbiol. Rev. 1995;59:423–450. doi: 10.1128/mr.59.3.423-450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTTEM M., OKADA T., GOFF J.P., METCALFE D.D. Mast cells cultured from the peripheral blood of normal donors and patients with mastocytosis originate from a CD34+/Fc epsilon RI- cell population. Blood. 1994;84:2489–2496. [PubMed] [Google Scholar]
- SACCANI S., PANTANO S., NATOLI G. Two waves of nuclear factor kappaB recruitment to target promoters. J. Exp. Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOECKLE M.Y. Removal of a 3′ non-coding sequence is an initial step in degradation of gro alpha mRNA and is regulated by interleukin-1. Nucleic Acids Res. 1992;20:1123–1127. doi: 10.1093/nar/20.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STYLIANOU E., O'NEILL L.A., RAWLINSON L., EDBROOKE M.R., WOO P., SAKLATVALA J. Interleukin 1 induces NF-kappa B through its type I but not its type II receptor in lymphocytes. J. Biol. Chem. 1992;267:15836–15841. [PubMed] [Google Scholar]
- TAYLOR W.E., NAJMABADI H., STRATHEARN M., JOU N.T., LIEBLING M., RAJAVASHISTH T., CHANANI N., PHUNG L., BHASIN S. Human stem cell factor promoter deoxyribonucleic acid sequence and regulation by cyclic 3′,5′-adenosine monophosphate in a Sertoli cell line. Endocrinology. 1996;137:5407–5414. doi: 10.1210/endo.137.12.8940364. [DOI] [PubMed] [Google Scholar]
- WEBSTER M.K., GOYA L., GE Y., MAIYAR A.C., FIRESTONE G.L. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol. Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZSEBO K.M., WILLIAMS D.A., GEISSLER E.N., BROUDY V.C., MARTIN F.H., ATKINS H.L., HSU R.Y., BIRKETT N.C., OKINO K.H., MURDOCK D.C., JACOBSEN F.W., LANGLEY K.E., SMITH K.A., TAKEISHI T., CATTANACH B.M., GALLI S.J., SUGGS S.V. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63:213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]