Abstract
The goal of this study was to elucidate the possible mechanisms by which protein kinase A (PKA)-mediated regulation of the sarcoplasmic reticulum (SR) via phospholambin protein phosphorylation is functionally impaired in streptozotocin-induced diabetic rats.
Phospholamban (PLB) protein and mRNA levels were 1.3-fold higher in diabetic than in control hearts, while protein expression of cardiac SR Ca2+-ATPase (SERCA2a) was unchanged.
Basal and isoprenaline-stimulated phosphorylation of PLB at Ser16 or Thr17 was unchanged in diabetic hearts. However, stronger immunoreactivity was observed at the basal level in diabetic hearts when antiphosphoserine antibody was used.
Basal 32P incorporation into PLB was significantly higher in diabetic than in control SR vesicles, but the extent of the PKA-mediated increase in PLB phosphorylation was the same in the two groups of vesicles.
Stimulation of Ca2+ uptake by PKA-catalyzed PLB phosphorylation was weaker in diabetic than in control SR vesicles. The PKA-induced increase in Ca2+ uptake was attenuated when control SR vesicles were preincubated with protein kinase C (PKC).
PKC activities were increased by more than two-fold in the membranous fractions from diabetic hearts in comparison with control values, regardless of whether Ca2+ was present. This was associated with increases in the protein content of PKCδ, PKCη, PKCι, and PKCλ in diabetic membranous fractions.
The changes observed in diabetic rats were reversed by insulin therapy.
These results suggest that PKA-dependent phosphorylation may incompletely counteract the function of PLB as an inhibitor of SERCA2a activity in diabetes in which PKC expression and activity are enhanced.
Keywords: Diabetes mellitus, protein kinase C, sarcoplasmic reticulum, phospholamban phosphorylation
Introduction
Patients with diabetes mellitus have an increased risk of developing congestive heart failure (CHF), even in the absence of coronary atherosclerosis or hypertension (Kannel et al., 1974). The precise mechanisms involved in the pathological process that leads from diabetes to CHF are still unknown. Defective cardiac responsiveness to catecholamines occurs in diabetic subjects (Berlin et al., 1986; Trovik et al., 1994), and may be associated with the onset of cardiac pump failure leading to CHF. Diverse mechanisms for impaired responsiveness to catecholamine in the diabetic heart have been reported in a variety of cardiac preparations from a number of animal models, including a reduction in β-adrenoceptor (ADR) density (Savarese & Berkowitz, 1979; Atkins et al., 1985; Nishio et al., 1988), uncoupling of β-ADRs from adenylate cyclase (Gøtzsche, 1983; Atkins et al., 1985; Wichelhaus et al., 1994), and/or elevated expression of inhibitory G proteins (Nishio et al., 1988; Roth et al., 1995). However, there appears to be no consistency among the different studies with regard to the presence or onset time of the changes in β-ADRs and their signal transduction systems in the diabetic heart (Tomlinson et al., 1992), and thus the exact nature of the link between the impairment of cardiac functional responses to catecholamines and the reduction in the number of β-ADRs or their uncoupling from subsequent signal transduction systems in diabetes remain poorly understood.
Although the density of myocardial β-ADRs is markedly reduced in diabetes (Gando et al., 1997; Matsuda et al., 1999), previous studies from our laboratory have demonstrated that the diminished functional responsiveness to β-ADR agonists in myocardial tissues from rats with streptozotocin-induced diabetes of 4–6 weeks duration is the result of a defect in the metabolic pathway between adenylate cyclase activation and the contractile machinery. Furthermore, we have shown that the increase in Ca2+ uptake by the sarcoplasmic reticulum (SR) in cardiomyocytes stimulated at both the β-ADR and postreceptor levels is blunted in the diabetic rat model, despite the fact that the Ca2+ current is increased normally, suggesting that impairment of the SR functions of uptake and release of Ca2+ may be a primary cause of diminished β-ADR responsiveness in the diabetic heart (Tamada et al., 1998).
In cardiomyocytes, the SR plays a critical role in excitation–contraction coupling through regulation of the intracellular free Ca2+ concentration (Tada et al., 1983). During contraction, the SR functions as a Ca2+ source, while during relaxation it serves as a Ca2+ sink. The transport of Ca2+ from the cytosol into the SR lumen is mediated by the enzyme cardiac SR Ca2+-ATPase (SERCA2a). The Ca2+-pumping activity of SERCA2a is strongly controlled by phospholamban (PLB), which in its dephosphorylated form depresses Ca2+ sequestration (Tada et al., 1983). In vitro studies have indicated that PLB can be phosphorylated on Ser10 by protein kinase C (PKC), on Ser16 by cyclic AMP-dependent protein kinase (protein kinase A, PKA), and on Thr17 by Ca2+-calmodulin-dependent protein kinase (CaMKII) (Simmerman et al., 1986; Koss & Kranias, 1996). Phosphorylation of PLB alleviates its inhibitory effects on SERCA2a, resulting in an overall increase in the affinity of SERCA2a for Ca2+, and thus an increase in the rate of Ca2+ uptake into the cardiac SR (Kranias, 1985). Recent studies have indicated that PKA-dependent phosphorylation of PLB and the resulting disinhibition of SERCA2a is likely to be one of the main molecular mechanisms for the inotropic and lusitropic effects of β-ADR agonists (Kuschel et al., 1999; Chu et al., 2000). The depressed uptake of Ca2+ into the SR in diabetic hearts is well documented (Penpargkul et al., 1981; Ganguly et al., 1983; Lopaschuk et al., 1983). In addition, our previous results and those of other investigators showing a deficiency in diabetic cardiomyocytes in reactions downstream of β-ADRs and adenylate cyclase suggest that there may be an alteration in the phosphorylation process in cardiac SR in diabetes (Yu et al., 1994; Tamada et al., 1998). Hence, changes in the activity of SERCA2a and its regulation by phosphorylation of PLB, and in the expression of these SR proteins, would be expected in the diabetic heart.
In the diabetic heart, the concentration of diacylglycerol, which is known to activate PKC, is increased (Okumura et al., 1988). Moreover, increases in the content of different PKC isozymes and the specific activity of PKC in the diabetic heart have been reported from a number of laboratories (Tanaka et al., 1991; Inoguchi et al., 1992; Xiang & McNeill, 1992; Liu et al., 1999). Since activation of PKC inhibits SR Ca2+ pump activity (Rogers et al., 1990) and decreases SERCA2a mRNA expression (Hartong et al., 1996; Qi et al., 1996), it is possible that any changes in the function and expression of SR proteins in the diabetic heart may be due to changes in PKC activity and/or PKC-mediated signal transduction mechanisms.
The present study aimed to investigate whether expression of PLB, site-specific phosphorylated PLB, and SERCA2a are altered in cardiac SR from rats with streptozotocin-induced diabetes of 4–6 weeks duration. Furthermore, we addressed the question of whether SR Ca2+ uptake under basal conditions and following PLB phosphorylation is actually altered in this diabetic model. Finally, we evaluated changes in PKC activity and PKC isoenzyme content in cytosolic and membranous fractions from diabetic hearts, in order to elucidate the role of PKC in the modulation of SR function that occurs in this pathological state.
Methods
Induction of diabetes
All procedures were in accordance with the regulations laid down by the Hokkaido University School of Medicine Animal Care and Use Committee.
Male Wister rats, 8-weeks old and weighing 180–200 g, were randomly assigned to two groups. One group of rats (the diabetic group) received a single tail-vein injection of streptozotocin (45 mg kg−1; Sigma Chemical, St Louis, MO, U.S.A.) under light anesthesia with diethyl ether. Streptozotocin was dissolved in a citrate buffer solution (0.1 M citric acid and 0.2 M sodium phosphate, pH 4.5). Another group (the control group) received an equivalent volume of citrate buffer alone. Control and diabetic rats were caged separately but housed under identical conditions. Both groups of animals were maintained on the same diet and given water ad libitum until they were used in experiments 4–6 weeks later. This incubation period was chosen because the cardiac alterations that take place during this time have been well characterized in previous studies from this laboratory (Gando et al., 1997; Tamada et al., 1998; Hattori et al., 2000). On the day of the experiments, blood samples were collected and serum glucose levels were measured before the animals were killed by exsanguination under anesthesia with gaseous diethyl ether. All rats injected with streptozotocin developed severe diabetes, as indicated by significantly increased serum glucose levels (P<0.001). Mean serum glucose levels were 160±6 and 544±17 mg dl−1 for control (n=32) and diabetic rats (n=30), respectively. Some diabetic rats were given subcutaneous injections of insulin (NPH Iszilin, 4 U day−1; Shimizu Pharmaceutical, Shizuoka, Japan). Insulin therapy was begun 1 day after the streptozotocin injection, and was continued up to the day before the animals were killed. The levels of serum glucose were significantly lower in rats given insulin therapy (163±11 mg dl−1, n=27, P<0.001).
Organ bath experiments
Experiments were performed as described previously (Gando et al., 1997; Ishitani et al., 2001). Briefly, left ventricular papillary muscles were isolated from the hearts of control and diabetic rats. The bathing solution contained (in mM): 119 NaCl, 4.8 KCl, 1.3 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 24.9 NaHCO3, 10.0 glucose, continuously gassed with 95% O2–5% CO2, and was maintained at a temperature of 35°C. The isometric force of contraction was measured after the muscle was preloaded to 0.5 g. We have confirmed that this resting tension produced >90% maximal force development in papillary muscles from both control and diabetic animals, based on resting tension/developed tension curves (Gando et al., 1997). The muscle was electrically stimulated at 1 Hz with rectangular pulses of 5-ms duration (Sanei-Sokki, 3F46, Tokyo, Japan), at a voltage 1.5 times greater than threshold. The preparations were allowed to equilibrate for at least 120 min before recording.
Preparations of SR-enriched membranes and SR vesicles
Membranes enriched in SR were prepared according to the procedure of Harigaya & Schwartz (1969), with some modifications. Briefly, rat hearts were removed and washed with ice-cold isotonic saline. After the removal of fatty and connective tissue, ventricular muscles were cut into small pieces with a pair of scissors, and homogenized in 5 volumes of 10 mM sodium bicarbonate with 5 mM sodium azaide in a polytron. The resulting homogenate was centrifuged at 8700 × g for 20 min at 4°C. The supernatant was reserved and centrifuged again, yielding a second supernatant fraction, which was centrifuged at 37,000 × g for 30 min at 4°C. The precipitate was suspended in 3 volumes of 20 mM Tris-HCl buffer (pH 6.8) containing 0.6 M KCl. The resulting suspension was then centrifuged at 37,000 × g for 30 min at 4°C. The harvested precipitate was finally suspended in a small volume of 20 mM Tris-HCl buffer (pH 6.8) containing 50 mM KCl. The protein concentration was determined by the method of Lowry et al. (1951) using bovine serum albumin (BSA) as standard.
SR vesicles were prepared using the method described by Osada et al. (1998). Rat hearts were removed and washed with ice-cold isotonic saline. After removal of fatty and connective tissues, ventricular muscles were cut into small pieces with a pair of scissors, and homogenized with a polytron in a solution containing (in mM): 10 NaHCO3, 5 NaN3, 15 Tris-HCl (pH 6.8). The resulting homogenate was centrifuged at 8700 × g for 20 min at 4°C. The supernatant fluid was centrifuged at 37,000 × g for 45 min at 4°C. The resulting pellet was resuspended in 0.6 M KCl and 10 mM Tris-HCl (pH 6.8), and centrifuged at 37,000 × g for 45 min at 4°C. The final pellet was resuspended in 250 mM sucrose and 10 mM histidine buffer.
Western blot analysis of PLB and SERCA2a
SR-enriched membrane samples (5 μg) were separated by SDS–polyacrylamide gels (PAGE) using 14% PAGE, and electroblotted onto a polyvinylidine difluoride (PVDF) membrane. To reduce nonspecific binding, the PVDF membrane was blocked for 60 min at room temperature in PBS-Tween buffer (137 mM NaCl, 2.7 mM KCl, 8.1 mM NH2PO4, 1.5 mM KH2PO4, 0.05% Tween 20) containing 1% BSA. The membrane was then incubated overnight at 4°C with mouse monoclonal anti-PLB antibody (Affinity BioReagents, Golden, CO, U.S.A.) at 1 : 500 dilution, or mouse monoclonal anti-SERCA2 antibody (Affinity BioReagents) at 1 : 1000 dilution in PBS-Tween buffer. After extensive washing with PBS-Tween buffer, the PVDF membrane was incubated with horseradish peroxidase-conjugated anti-mouse antibody (1 : 4000 dilution; Bio-Rad Laboratories, Hercules, CA, U.S.A.) for 60 min at room temperature. After being washed three times in PBS-Tween buffer for 10 min, the blots were visualized using an enhanced chemiluminescence detection system (Amersham, Little Chalfont, Buckinghamshire, U.K.), exposed to X-ray film and analyzed using free NIH Image software produced by Wayne Raeband (National Institutes of Health, Bethesda, MD, U.S.A.). The intensity of total protein bands per lane was evaluated by densitometry. Negligible loading/transfer variation was observed between samples.
Western blot analysis of site-specifically phosphorylated PLB
Rat hearts were perfused with Krebs–Henseleit solution using the Langendorff technique. The solution contained (in mM): 119 NaCl, 1.3 CaCl2, 4.8 KCl, 1.2 MgSO4, 0.234 KH2PO4, 27.2 NaHCO3, 10.0 glucose, continuously gassed with 95% O2–5% CO2, and was maintained at a temperature of 37°C. The flow rate was kept constant at 4.0 ml min−1. After 10 min of perfusion, isoprenaline (100 nM) was administrated by continuous infusion. After 5 min, the atria were removed and the hearts were immediately frozen with clamps which had been cooled with liquid nitrogen. Then, membranes enriched in SR were prepared from the frozen hearts using the same methods as described above.
For the immunological detection of site-specific-phosphorylated PLB, SR-enriched membranes (5 μg) were separated by SDS–PAGE using 14 PAGE, and electrotransferred to PVDF membranes. Processing for immunodetection was performed as described above. To detect the phosphorylation of PLB, rabbit polyclonal antiphospho-PLB (Ser16) antibody (1 : 500; Upstate Biotechnology, Lake Placid, NY, U.S.A.) or goat polyclonal antiphospho-PLB (Thr17) antibody (1 : 100; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) was used. The immunological signals were quantified by densitometry.
Some SR-enriched membranous fractions were precleared with 20 μl of protein G–sepharose (Sigma Chemical). After centrifugation, the precleared supernatant (100 μl) was incubated with monoclonal antiphosphoserine antibody (1 μg; Sigma Chemical) at 4°C for 60 min. Then, 20 μl of protein G–sepharose was added and incubated with gentle rocking at 4°C for 60 min. The protein was centrifuged at 4°C for 1 min, and the pellet was then washed three times. The associated proteins were characterized by Western blot analysis.
Western blot analysis of PKC isozymes
PKC expression patterns were determined in both the cytosol and membrane fractions. Membrane purification was performed as described by Inoguchi et al. (1992), with some modifications. Briefly, rat hearts were removed and washed with ice-cold Ca2+- and Mg2+-free Hank's solution containing (in mM): 137 NaCl, 5.4 KCl, 0.44 KH2PO4, 0.42 Na2HPO4, 4.17 NaHCO3, 5.55 glucose, 10 HEPES (pH 7.4). After the removal of fatty and connective tissue, ventricular muscles were cut into small pieces with a pair of scissors, and homogenized with a polytoron in buffer A containing: 2 mM EDTA, 0.5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 0.33 M sucrose, 20 mM Tris-HCl (pH 7.5), 25 μg ml−1 leupeptin, and 0.1 mg ml−1 aprotinin. The resulting homogenate was centrifuged at 1000 × g for 10 min at 4°C, and the supernatant was then subjected to ultracentrifugation at 100,000 × g for 30 min at 4°C. The resulting supernatant was designated the cytosolic fraction. The pellets were washed and resuspended in buffer B (buffer A without sucrose) and homogenized again. The homogenates were solubilized in buffer B with 1% Triton X-100. After incubation for 45 min, a soluble fraction was obtained by ultracentrifugation at 100,000 × g for 30 min at 4°C, and was designated the membranous fraction.
The membranous and cytosolic fractions (10 μg) were then separated on 7.5% polyacrylamide SDS gel, and electroblotted onto PVDF membranes. To detect PKC isozymes, a PKC Sampler Kit (BD Biosciences, Tokyo, Japan), a mouse monoclonal anti-PKCβI antibody (Sigma Chemical), and a mouse monoclonal anti-PKCβII antibody (Sigma Chemical) were used. The PVDF membrane was incubated overnight at 4°C with each anti-PKC isozyme antibody (1 : 250 dilution for η, θ, ι, and λ, 1 : 500 dilution for δ, 1 : 1000 dilution for α, γ, and ɛ, 1 : 8000 dilution for βI and βII), followed by incubation with horseradish peroxidase-conjugated anti-mouse antibody (1 : 2000–3000 dilution; Bio-Rad Laboratories) for 60 min at room temperature. The intensity of total protein bands per lane was evaluated by densitometry.
Poly(A)+ RNA purification and Northern blot analysis
Total RNA was extracted from the ventricular myocardium, using a guanidinium thiocyanate–phenol–chloroform method, according to the protocol of Chomczinski & Sacchi (1987). The frozen ventricles (about 200 mg) were placed in 1 ml ISOGEN (Nippon Gene, Toyama, Japan), and homogenized with a polytron. Chloroform (200 μl) was then added, and the mixture was shaken vigorously for 15 s and kept at room temperature for a few minutes. The mixture was then centrifuged at 12,000 × g for 15 min at 4°C, the aqueous phase was transferred to a fresh tube, 500 μl isopropanol was added, and the sample was centrifuged at 12,000 × g for 15 min at 4°C. The resulting pellet was washed twice with 75% ethanol by vortexing and subsequent centrifugation at 7500 × g for 15 min at 4°C. Total RNA was resuspended in diethyl pyrocarbonate (DEPC)-treated water. Then, Poly(A)+ RNA was purified from total RNA using an Oligotex™-dT30 〈Super〉 mRNA Purification kit (Takara, Ohtsu, Japan). The amount of extracted Poly(A)+ RNA was determined by UV absorption. The ratio of the optical density at 260 nm to that at 280 nm was ∼1.98–2.22 in all samples.
Rat PLB cDNA was isolated from gels after electrophoretic separation of the products which were amplified by the polymerase chain reaction using two oligodeoxynucleotide primers (TGACGATCACAGAAGCCAAG and GATGCAGATCAGCAGCAGAC). The cDNA probe was labeled with [32P]dCTP (6000 Ci ml−1; Amersham) using a random prime labeling system (redi prime™ II; Amersham). After being hybridized in a buffer (Rapid-hyb buffer; Amersham) containing 32P-labeled probe (107 c.p.m. ml−1) at 52°C for 2 h, the membrane was washed with 2 SCC–0.1% SDS at 42°C, and with 0.1 SCC–0.1% SDS twice at 65°C. The intensity of hybridization was visualized by autoradiography. The PLB mRNA was quantified by counting the radioactivity using a bioimaging analyzer system (Fujix BAS 2000; Fuji Photo Film, Tokyo, Japan), as previously described (Matsuda et al., 1999). In order to control for differences in RNA content, the membranes were sequentially probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Oncogene Research Products, Cambridge, MA, U.S.A.) after stripping. Thus, the amount of PLB mRNA was normalized to the mRNA of the constitutively expressed enzyme GAPDH on the same filter.
SR phosphorylation
Phosphorylation of cardiac SR was measured using the procedure of Movsesian et al. (1984), with some modifications. SR vesicles were suspended (1.0 mg ml−1) in a mixture containing (in mM): 5 MgCl2, 6 NaN3, 5 NaF, 0.2 CaCl2, and 40 MOPS (pH 6.8). Where indicated, 2 mM EGTA, 10–50 nM PKA catalytic subunit (Oncogene Research Products, Cambridge, MA, U.S.A.), or a combination of 18.7 μg ml−1 phosphatidylserine (Wako, Osaka, Japan) and 1–9 μg ml−1 PKC (Oncogene Research Products, Cambridge, MA, U.S.A.) were added to the mixture. Phosphorylation was initiated by the addition of 0.05 mM [γ-32P]ATP (Amersham) at 30°C. At 3 min, the reaction was stopped by the addition of an equal volume of SDS buffer, and aliquots were electrophoresed in 20% polyacrylamide SDS gels. The phosphorylated protein band per lane was quantified by counting the radioactivity using a Fujix BAS 2000 gamma counter.
SR Ca2+ uptake
Ca2+ uptake was measured using 45CaCl2 (Amersham), according to the procedure of Movsesian et al. (1984), with some modifications. The SR vesicles (0.1 mg ml−1 final concentration) were suspended in a reaction mixture containing (in mM): 5 MgCl2, 6 NaN3, 5 NaF, 0.6 45CaCl2, and 40 MOPS (pH 6.8). Phosphorylation was initiated by the addition of ATP to a final concentration of 5 mM. Where indicated, 10–50 nM PKA catalytic subunit or 1–9 μg ml−1 PKC (with 18.7 μg ml−1 phosphatidylserine) was present. After 1.5 min, Ca2+ uptake was initiated by the addition of an equal quantity of a solution containing (in mM): 10 oxalic acid (final concentration, 5), 5 MgCl2, 6 NaN3, 5 NaF, 240 KCl (final concentration, 120), 1.6 EGTA (final concentration, 0.8), and 40 MOPS (pH 6.8). The free 45Ca2+ concentration was calculated to be 207 μM during phosphorylation and 0.58 μM during 45Ca2+ uptake, according to dissociation constants published by Fabiato & Fabiato (1979). After 2 min, aliquots were removed and filtered through Millipore HAWP 025 00 discs (pore size 0.45 μm). The filter discs were washed with a solution containing (in mM): 120 KCl, 1 EGTA, 40 MOPS (pH 6.8), and then dried, and radioactivity was counted in a scintillation counter.
Measurement of PKC activity
In order to obtain a high-sensitivity measurement of PKC activity, PKC was partially purified from the membranous and cytosolic fractions which were prepared for immunoblotting of PKC isoenzymes. Both membranous and cytosolic fractions were passed through DEAE columns (Amersham), washed twice with buffer B, and then finally eluted with buffer B containing 200 mM NaCl. PKC activity was determined using a PKC enzyme assay system (Amersham) that measured the enzyme's ability to transfer 32P from [γ-32P]ATP (Amersham) into the specific substrate octapeptide (RKRTLRRL), which corresponds to the threonine phosphorylation sites (including Thr654) of epidermal growth factor receptor (residues 651–658), in the presence of Ca2+, phosphatidylserine, and phorbol 12-myristate 13-acetate. In some experiments, Ca2+ was omitted. After the reaction, assay discs were washed twice with 75 mM orthophosphoric acid for 5 min. The radioactivity of the discs was counted in a scintillation counter.
Data analysis
All data are quoted as means±s.e.m., along with the number of observations (n). Statistical significance was estimated by Student's t-test or ANOVA, followed by Scheffé's multiple comparison test to locate differences between groups. Differences were considered significant at a level of P<0.05.
Results
Isometric contractions in isolated papillary muscles
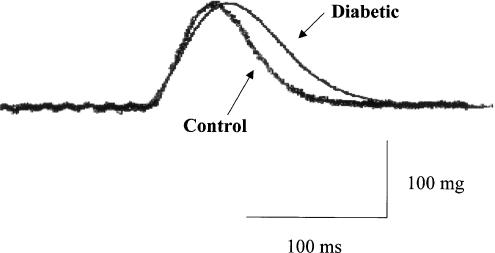
The basal force of contraction of the left ventricular papillary muscles isolated from diabetic rats (171±35 mg, n=7) was not significantly different from that of muscles from age-matched control animals (170±20 mg, n=7). However, a significant difference was observed in the time course of tension development and relaxation of individual isometric contractions. As illustrated in Figure 1, the time to peak tension and the half-relaxation time were significantly prolonged in diabetic muscles compared with controls (diabetic time to peak tension versus control, 75±3 versus 61±2 ms, P<0.001; diabetic half-relaxation time versus control, 58±2 versus 43±2 ms, P<0.001).
Figure 1.
Representative traces showing the isometric contraction curves obtained in papillary muscles from control and diabetic rat hearts.
Expressions of PLB and SERCA2a
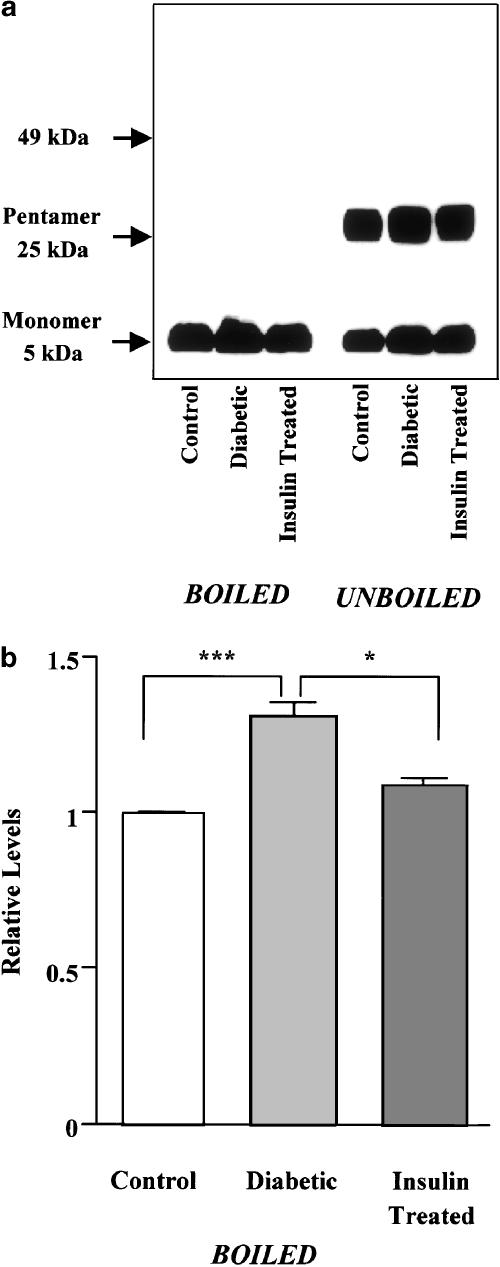
To determine changes in the protein levels of PLB and SERCA2a in diabetes, microsomal fractions enriched in SR membranes were subjected to Western blot analysis. About 45% of the PLB present in the microsomal preparations migrated as the monomeric form, and 55% migrated as the pentameric form (Figure 2a). The ratio of monomers to pentamers (45 : 55) in the microsomal preparations from diabetic rat hearts was similar to that in the preparations from controls (44 : 56), but the protein levels of monomeric and pentameric PLB were significantly higher in diabetic preparations than the corresponding control levels (monomeric PLB, 31±3% increase; pentameric PLB, 29±3% increase, n=4, P<0.001). Boiling of the samples before electrophoresis resulted in migration of all PLB as the monomeric form (Figure 2a). Quantification of the monomeric PLB protein levels revealed a 1.3-fold increase in diabetic hearts compared with controls (Figure 2b). The increased level of PLB protein observed in diabetes was reversed by insulin therapy (Figure 2).
Figure 2.
Western blot analysis of PLB expression levels in microsomal fractions enriched in SR membranes isolated from control, diabetic, and insulin-treated diabetic rat hearts. (a) Representative Western blots using the anti-mouse PLB monoclonal antibody show that PLB monomers and pentamers migrated at ∼5 and ∼25 kDa on SDS–PAGE, respectively (right panel). When the microsomal preparations were boiled for 5 min immediately before electrophoresis, all the PLB migrated as the monomeric form (left panel). (b) Quantification of total PLB protein levels, detected as the monomeric form after boiling. In each of the experiments, the SERCA2a level obtained from the control band is normalized as 1.0. Bars are means±s.e.m. of four separate experiments. *P<0.05, ***P<0.001.
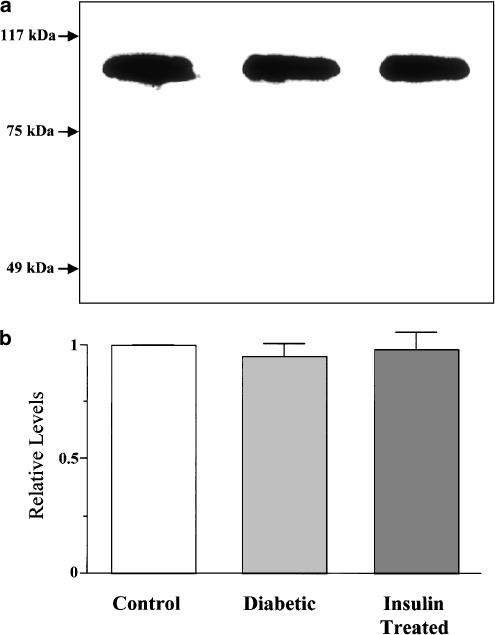
Typical immunoblots of SERCA2a protein in the SR-enriched microsomal preparations from control, diabetic, and insulin-treated diabetic rat hearts are illustrated in Figure 3a. The SERCA2a protein level was determined by measuring the density of a single band migrating at ∼110 kDa. As revealed by the cumulative data for quantitative immunoblotting (Figure 3b), the SERCA2a protein levels were similar in control and diabetic hearts.
Figure 3.
Western blot analysis of SERCA2a expression levels in microsomal fractions enriched in SR membranes isolated from control, diabetic, and insulin-treated diabetic rat hearts. (a) Representative Western blots showing immunochemical detection of SERCA2a, as a single protein band with an apparent molecular mass of ∼110 kDa. (b) Quantification of SERCA2a expression levels. Note that no significant difference was found among groups. In each of the experiments, the SERCA2a level obtained from the control band is normalized as 1.0. Bars are means±s.e.m. of six separate experiments.
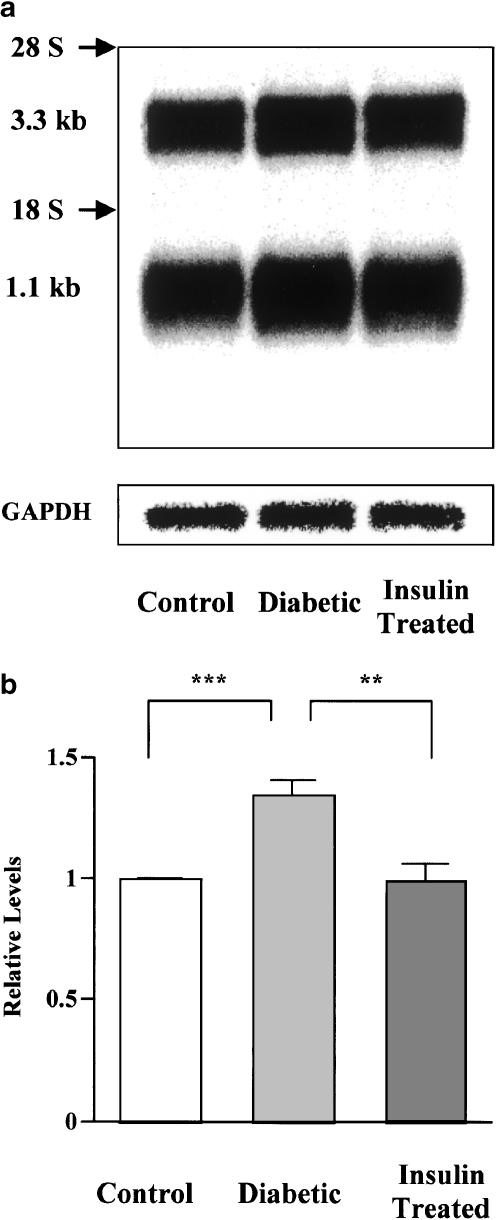
To determine whether the increase in expression of PLB in diabetes is regulated at the level of gene expression, we investigated PLB expression at the mRNA level. Northern blot analysis of Poly(A)+ RNA purified from total rat heart RNA revealed the presence of two endogenous PLB transcripts at 3.3 and 1.1 kb (Figure 4a). Both mRNAs appear to encode for the same protein. The difference between the mRNAs could be that they have distinct polyadenylation sites (Toyofuku & Zak, 1991). Multiple polyadenylation sites might be important as a protection against endonucleases. Thus, it is conceivable that diabetes affected the two PLB transcripts differently, and hence we quantified both PLB transcript bands either together, or each band separately. However, no differences were apparent (data not shown), and the sum of both transcripts is plotted in Figure 4b. The findings were qualitatively similar to those for protein levels. Namely, there was a 1.3-fold increase in PLB expression at the mRNA level in diabetic rat hearts compared with controls, which was substantially prevented by insulin therapy.
Figure 4.
Northern blot analysis of PLB mRNA levels in hearts isolated from control, diabetic, and insulin-treated diabetic rats. (a) Representative autoradiogram of Northern blots of PLB and GAPDH. Poly(A)+ RNA was purified from total RNA, blotted onto membranes, and then hybridized sequentially with specific 32P-labeled probes for PLB and GAPDH. Each lane contains PLB transcripts migrating at 3.3 and 1.1 kb. Both 18S and 28S ribosomal RNA are labeled. (b) Quantification of mRNA levels of PLB. Data on PLB mRNA expression levels (combined for the two transcripts) are normalized to GAPDH mRNA. Bars are means±s.e.m. of five separate experiments. **P<0.01, ***P<0.001.
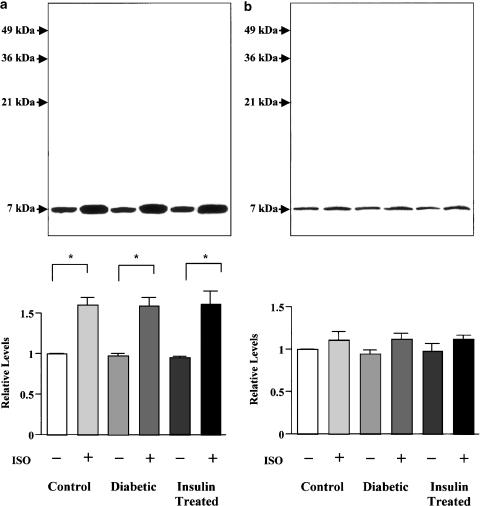
Isoprenaline-induced site-specific PLB phosphorylation
Figure 5a shows immunoblots of Ser16 phosphorylation of PLB in SR-enriched microsomal fractions from the perfused hearts of control, diabetic, and insulin-treated diabetic rats in the absence and presence of isoprenaline. The basal phosphorylation level at the Ser16 site of PLB was similar in the three groups. A 1.6-fold increase in Ser16 phosphorylation of PLB occurred after 5 min of exposure of the hearts to 100 nM isoprenaline in all three groups. No difference in the amount of Ser16-phosphorylated PLB was found among the groups.
Figure 5.
Immunoblots demonstrating basal and isoprenaline-stimulated levels of PLB protein phosphorylated at Ser16 (a) and Thr17 (b) in perfused hearts of control, diabetic, and insulin-treated diabetic rats. Isolated hearts were perfused with or without 100 nM isoprenaline (ISO) for 5 min, freeze-clamped, and processed to obtain SR-enriched microsomal fractions which were then subjected to Western blot analysis of site-specific phosphorylation of PLB. Original immunoblots (inset). PLB was present as the monomeric form migrating at ∼7 kDa, because the samples were boiled before electrophoresis. Bars represent cumulative data expressed as means±s.e.m. of 3–4 hearts in each group. In each of the experiments, the PLB obtained from control band is normalized as 1.0. *P<0.05.
As shown in Figure 5b, the basal phosphorylation level at the Thr17 site of PLB was also similar in SR-enriched microsomal fractions from the perfused hearts of control and diabetic rats. The increase in Thr17 phosphorylation of PLB after a 5 min exposure to isoprenaline was small and statistically insignificant in all the three groups.
When antiphosphoserine antibody was used for immunodetection of PLB phosphorylated at the serine residues, an evident increase in basal phosphorylation was found in diabetic SR-enriched membranes. Densitometric analysis revealed that the relative intensity of the PLB antiphosphoserine immunoreaction was increased 1.2-fold in diabetic compared with control preparations (n=4, P<0.05).
Phosphorylation of PLB by PKA and PKC
Using cardiac SR vesicles, we examined whether PKA- and PKC-mediated phosphorylation of PLB is altered in diabetes. On the electrophoretic gels, the bands that showed 32P incorporation corresponded to monomeric PLB with a molecular mass of approximately 7 kDa (upper traces in Figure 6). Basal phosphorylation of PLB was much higher in the diabetic than in the control group. Thus, the basal phosphorylation level was increased 1.9-fold in diabetes (P<0.01). Insulin treatment of diabetic rats nearly completely prevented the increase in the level of phosphorylated PLB (n=3). When SR vesicles were incubated in the presence of 10 nM purified catalytic subunit of PKA, a large amount of 32P was incorporated into PLB in both the control and diabetic groups (Figure 6a), though the PKA-mediated increase in PLB phosphorylation tended to be enhanced in diabetes. The effect of the PKA catalytic subunit at a concentration of 50 nM was also tested, and was found to be qualitatively similar to that produced by 10 nM (n=4). The addition of 1, 3, and 9 μg ml−1 PKC in the presence of phosphatidylserine caused a dose-dependent increase in phosphorylation of PLB in the two groups of SR vesicles (n=3). Figure 6b shows 32P incorporation into PLB in the control and diabetic groups when SR vesicles were incubated with 9 μg ml−1 PKC in the presence of phosphatidylserine. Although the level of incorporation in the presence of PKC was evidently higher in diabetic SR vesicles, there was no significant difference in the extent of the PKC-mediated increase in PLB phosphorylation above the basal value between control and diabetic vesicles.
Figure 6.
Comparison of PLB phosphorylation catalyzed by PKA (a) and PKC (b) in the SR vesicles prepared from control and diabetic rat hearts. Cardiac SR vesicles were phosphorylated in the reaction mixture containing EGTA (2 mM), PKA catalytic subunit (10 nM), or a combination of PKC (9 μg ml−1) and phosphatidylserine (18.7 μg ml−1). The reaction was carried out for 3 min, and aliquots were then electrophoresed in polyacrylamide SDS gels. SR vesicles were boiled prior to gel electrophoresis. Autoradiograms of 32P migrating with the 7-kDa bands, which were referred to as PLB, are shown in the upper panels. In each of the experiments, the PLB obtained from the control band is normalized as 1.0. Bars represent cumulative data of means±s.e.m. of three different preparations for PKA, and of four different preparations for PKC. *P<0.05.
Effects of phosphorylation by PKA and PKC on SR Ca2+ uptake
We next investigated whether diabetes modifies the effects of PKA- and PKC-mediated phosphorylation on oxalate-stimulated Ca2+ uptake by cardiac SR at a free Ca2+ concentration of 0.58 μM. As shown in Table 1, the basal Ca2+-uptake rate in diabetic SR vesicles was 1.4–1.9-fold higher than the control value when expressed per milligram of tissue protein. Insulin treatment of diabetic rats resulted in complete normalization of the basal Ca2+-uptake rate. Furthermore, the basal Ca2+-uptake rate in diabetic SR vesicles was markedly decreased from 48.4±4.4 to 18.5±1.7 nmol Ca2+ mg−1 min−1 (n=4, P<0.001) by 30 min preincubation with the PKC inhibitor GF109203X (5 μM), which also marginally affected the basal Ca2+ uptake in control SR vesicles. The rate of Ca2+ uptake was increased approximately 2.8-fold by exposure of control SR vesicles to 50 nM PKA catalytic subunit. A similar increase in Ca2+ uptake was seen when 10 nM PKA catalytic subunit was used (2.3-fold, n=4). However, the increase in Ca2+ uptake caused by PKA-catalyzed protein phosphorylation was significantly reduced in diabetic SR vesicles. The addition of 9 μg ml−1 PKC in the presence of phosphatidylserine slightly but significantly increased the Ca2+-uptake rate in control SR vesicles. By contrast, the Ca2+-uptake rate remained essentially the same in diabetic SR vesicles with and without PKC. The reductions in the effects of phosphorylation by PKA and PKC on Ca2+ uptake into the SR seen in diabetes were normalized to the control levels by insulin therapy. Preincubation with GF109203X also normalized the effect of PKA phosphorylation on Ca2+ uptake into diabetic SR vesicles (n=4).
Table 1.
Ca2+ uptake (nmol Ca2+ mg−1 min−1) by SR vesicles prepared from control, diabetic and insulin-treated diabetic rat hearts in the presence of PKA catalytic subunit or PKC
| Control | Diabetic | Insulin-treated diabetic | |
|---|---|---|---|
| PKA | |||
| Basal Ca2+-uptake rate | 26.51±3.81 | 50.03±5.71** | 27.70±1.52†† |
| Increase in Ca2+-uptake rate | 47.63±4.27 | 26.86±6.24* | 41.14±0.61† |
| PKC | |||
| Basal Ca2+-uptake rate | 25.07±0.45 | 34.93±0.78** | 25.64±1.94†† |
| Increase in Ca2+-uptake rate | 10.10±0.93 | 0.95±2.53** | 12.17±2.45† |
The concentration of free Ca2+ was 0.58 μM. Uptake was measured after incubation with 50 nM PKA catalytic subunit or 9 μg ml−1 PKC (in the presence of 18.7 μg ml−1 phosphatidylserine) under conditions favorable for phosphorylation, followed by the addition of 5 mM oxalic acid to the reaction mixture. Rates of Ca2+ uptake were determined by least-squares linear regression analysis of the data from triplicate experiments. Values are given as means±s.e.m. of 3–4 preparations from different animals in each group.
P<0.05
P<0.01 versus respective control values.
P<0.05
P<0.01 versus respective diabetic values.
When PKA catalytic subunit was added after incubation with PKC for 30 s in the presence of phosphatidylserine, the ability of PKA phosphorylation to increase Ca2+ uptake was markedly attenuated in control SR vesicles. Thus, the increase in the rate of SR Ca2+ uptake with PKA catalytic subunit was 42.66±3.51 nmol Ca2+ mg−1 min−1 (n=5) in the presence of phosphatidylserine and 16.31±2.34 nmol Ca2+ mg−1 min−1 (n=5, P<0.01) in the presence of PKC and phosphatidylserine.
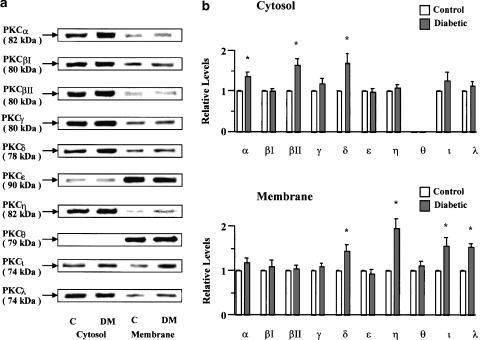
Subcellular distribution of PKC isozymes
The relative protein contents of 10 PKC isozymes in the cytosolic and membranous fractions of myocardium from control and diabetic rats were identified by Western blotting. The typical bands representing each of the PKC isozymes in these fractions of rat myocardium are illustrated in Figure 7a. All PKC isozymes, except PKCθ for which no significant immunoreactivity was found in the cytosolic fractions, were detected in both the cytosolic and membranous fractions of the rat myocardium. Densitometric analysis of the PKC isozyme bands revealed a significant increase in the relative protein contents of PKCα, PKCβII, and PKCδ in diabetic cytosolic fractions of 39, 64, and 71%, respectively, and of PKCδ, PKCη, PKCι, and PKCλ in diabetic membranous fractions of 46, 95, 57, and 53%, respectively, in comparison with the corresponding control values (Figure 7b).
Figure 7.
Western blot analysis of PKC isozymes in cytosolic and membranous fractions from control and diabetic rat hearts. (a) Representative immunoblots for PKC isozymes. The apparent molecular weight of each band was calculated from the molecular weight standards, and is indicated in parenthesis. C, control; DM, diabetic. (b) Densitometric analysis of Western blot. The protein level in each control band is normalized as 1.0. Values are means±s.e.m. of 4–6 preparations from different animals in each group. *Significantly different from control (P<0.05).
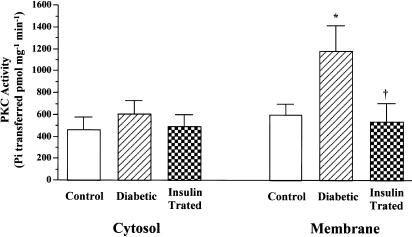
PKC activities
In the presence of Ca2+, phospholipid-dependent PKC activity was measured in partially purified cytosolic and membranous preparations. The results illustrated in Figure 8 showed a two-fold increase in PKC activity in the membranous fractions from diabetic myocardium, but no significant change in activity in the cytosolic fractions compared with the control values. Treatment of diabetic animals with insulin significantly reduced the PKC activity in membranous fractions to control levels. In the absence of Ca2+, phospholipid-dependent PKC activity in the membranous fractions from diabetic myocardium remained elevated: the PKC activity in the membranous fractions were 2.5-fold higher in diabetic rats than in controls (n=5 for each, P<0.05).
Figure 8.
PKC activities in partially purified cytosolic and membranous fractions from control, diabetic, and insulin-treated diabetic rat hearts. PKC activity was measured as the rate of transfer of 32P from [γ-32P]ATP into the specific substrate in the presence of Ca2+, phosphatidylserine, and phorbol 12-myristate 13-acetate. Values are means±s.e.m. of six separate experiments. *Significantly different from control (P<0.05). †Significantly different from diabetic (P<0.05).
Discussion
In the present study, a significant increase in PLB expression at the protein level was demonstrated in cardiac microsomal fractions enriched in SR membranes from rats 4–6 weeks after induction of diabetes with streptozotocin, in comparison with those from age-matched control rats. This is in good agreement with a recent report from another group (Zhong et al., 2001). PLB is proposed to exist as the pentameric form in native SR membranes, but is a more effective Ca2+-pump inhibitor in the monomeric form (Kadambi & Kranias, 1997). It seems unlikely that diabetes alters the equilibrium between the monomeric and pentameric states of PLB, since the ratios of the monomeric to pentameric forms of PLB were similar in our microsomal preparations from diabetic and control hearts. In the diabetic heart, the expression levels of PLB protein and mRNA increased in parallel. The straightforward explanation for these findings is that the alteration in PLB expression in diabetes occurs at the transcriptional level, and there is a specific transcription activator to regulate this gene that is more active in the diabetic heart. Promoter analysis of the murine PLB gene has suggested that PLB gene expression may be regulated by the interplay of cis-acting regulatory elements located within 5′-flanking and intronic regions (Haghighi et al., 1997). The overexpression of PLB at both the mRNA and protein levels observed in diabetes was completely reversed with insulin replacement, indicating that chronic changes in insulin receptor signaling may be involved in the regulation of PLB gene transcription. The present study provides evidence that the abundance of SERCA2a protein is unchanged in the diabetic heart. This confirms results previously presented by Zarain-Herzberg et al. (1994), although it has also been shown that the SERCA2a protein expression level appears to decrease with the duration of diabetes (Zhong et al., 2001). These findings suggest that an increase in the ratio of PLB to SERCA2a protein expression could underlie the slow rate of relaxation observed in papillary muscles, possibly due to a decrease in the apparent affinity of SR Ca2+ pump for Ca2+.
In this study, we report an increase in the basal level of phosphorylated PLB in the diabetic heart compared with control levels. Western blot analysis with antiphosphoserine antibody showed that the increase in baseline phosphorylation occurred at the serine residues of PLB. The increase appears to be unrelated to increased phosphorylation of Thr17 through a rise in the intrinsic activity of CaMKII, because the experiment using the antiphospho-PLB (Thr17) antibody showed that basal phosphorylation at the Thr17 site of PLB was unchanged in diabetes. Furthermore, selective binding of the antiphospho-PLB (Ser16) antibody to PLB demonstrated that there was no change in basal phosphorylation at the Ser16 site of PLB in the diabetic heart. This observation also implies that the increase in the basal level of phosphorylation of PLB in the diabetic heart cannot be explained solely by a pathological increase in the amount of this protein. Thus, it can logically be assumed that the increased basal level of phosphorylation of PLB in the diabetic heart results from Ser10 phosphorylation by endogenous PKC. We have previously found a significant increase in basal 32P incorporation into PLB in intact beating hearts from diabetic rats compared with control hearts (Gando et al., 1997). As phosphorylation of PLB by PKC activators has not been detected in vivo (Edes & Kranias, 1990), the regulation of PLB Ser10 phosphorylation in the intact heart may be strongly dependent on endogenous PKC isoform levels rather than exogenous activation of PKC.
We found an increased basal rate of Ca2+ uptake in SR vesicles from diabetic hearts. This is likely to result from endogenous PKC-dependent PLB phosphorylation, since the increase in basal Ca2+ uptake into diabetic SR was completely blocked by GF109203X, which inhibits all PKC isoenzymes, including novel and atypical isotypes (Martiny-Baron et al., 1993; Überall et al., 1999). However, the functional significance of this finding is questionable. Evidence has been presented, using fura-2, that basal intracellular Ca2+ concentration levels in quiescent diabetic cardiomyocytes are unchanged compared with controls (Yu et al., 1994). We have previously shown that the SR content of Ca2+, as assessed by measuring the peak intracellular Ca2+ transient induced by rapid application of caffeine, is significantly reduced in cardiomyocytes freshly isolated from the diabetic rats employed in this study, compared to the SR Ca2+ content of age-matched control cardiomyocytes (Tamada et al., 1998). Thus, it may be that the change in SR Ca2+ uptake at the basal level observed in this study does not reflect straightforward dynamic changes in the SR Ca2+ content, which is available for release in intact diabetic myocytes.
The principal new finding from the present study is that in diabetes there is an evident dissociation between PKA-catalyzed PLB phosphorylation and Ca2+ uptake by cardiac SR. Thus, PKA produced similar degrees of PLB phosphorylation in control and diabetic SR vesicles, while the increase in Ca2+ uptake caused by PKA-catalyzed phosphorylation was markedly reduced in diabetic SR vesicles. This suggests that PKA-dependent phosphorylation may incompletely reverse the function of PLB as an inhibitor of SERCA2a activity, leading to an impairment of the mechanism whereby PKA-dependent phosphorylation of PLB results in an increase in SR Ca2+ uptake. In support of this hypothesis, we have previously shown that the ability of isoprenaline, forskolin, and dibutyryl cyclic AMP to enhance the rise in the intracellular Ca2+ transient induced by rapid caffeine application is much reduced in diabetic cardiomyocytes compared to control cardiomyocytes (Tamada et al., 1998). Interestingly, we observed that the effect of PKA phosphorylation of increasing SR Ca2+ uptake was markedly attenuated by pre-exposure of control SR vesicles to PKC. PKA- and PKC-mediated phosphorylation appear to occur independently of each other (Iwasa & Hosey, 1988), but it is conceivable that phosphorylation at Ser10 may limit the effect of additional phosphorylation at Ser16 on the inhibition of SERCA2a activity by PLB. The present experiments do not allow us to speculate further about the exact interaction between PKA-dependent Ser16 phosphorylation and PKC-dependent Ser10 phosphorylation, and their effects on the coupling of PLB with SERCA2a. Nonetheless, our results suggest that the reduced ability of PKA-catalyzed PLB phosphorylation to increase SR Ca2+ uptake in the diabetic heart may involve PKC-mediated mechanisms.
In this study, we observed an increase in PKC activity in the partially purified membranous fraction obtained from the diabetic rat heart, but no significant change in the cytosolic fraction, regardless of whether Ca2+ was present in the reaction mixture. The increase in membranous PKC could be due to an increase in translocation and/or synthesis of PKC. Although an increase in cardiac PKC activity in the membranous fractions of diabetic rat hearts has also been observed by other investigators (Inoguchi et al., 1992; Xiang & McNeill, 1992), yet others have reported an increase in PKC activity in the cytosolic fractions only (Liu et al., 1999), or in both cytosolic and membranous fractions (Tanaka et al., 1991) obtained from diabetic animals. These differences in cardiac PKC activity in cytosolic and membranous fractions in diabetic rats between laboratories may be due to differences in the methods used to prepare the fractions, or extract the enzyme, and in the procedures employed for the assay of PKC activities. However, the changes observed in PKC activity in our diabetic heart preparations seem unlikely to be the result of an artifact, because we employed partially purified samples for the series of experiments. Moreover, treatment of diabetic animals with insulin was found to reverse the elevated level of cardiac PKC activity in the membranous fraction, but produced no change in the cytosolic fraction. The increased PKC activity in membranous fractions was associated with increases in the contents of PKCδ, PKCη, PKCι, and PKCλ isoforms. At present, at least 12 isoforms of PKC have been identified (Naruse & King, 2000). PKCδ and PKCη are classified as novel PKCs which are Ca2+-independent but activated by diacylglycerol and phosphatidylserine, and PKCι and PKCλ are classified as atypical PKCs which are Ca2+- and diacylglycerol-independent but are phosphatidylserine-sensitive (Naruse & King, 2000). It thus appears that the observed increase in cardiac PKC activity may be due to an increase in the protein content of these novel and atypical PKC isozymes in the membrane fractions of the diabetic heart. Except for PKCδ and PKCɛ, changes in novel and atypical PKC isoforms expressed in the diabetic myocardium have not been studied extensively. Diverse PKC isoforms, including PKCα, PKCβ, PKCɛ, and PKCδ, have been shown to increase in the diabetic rat heart (Inoguchi et al., 1992; Malhotra et al., 1997; Giles et al., 1998; Liu et al., 1999). However, it should be noted that discrepancies exist among laboratories as to whether the content of these PKC isozymes is altered in the cytosolic or membranous fractions of the diabetic heart. We, like Liu et al. (1999), found an increase in the contents of PKCα and PKCβII in the cytosolic fraction of the diabetic heart. The pathological significance of the increase in the content of these normally occurring PKC isoforms in cardiac cytosolic fractions, despite the lack of change in cytosolic PKC activity in the diabetic heart, needs to be investigated further.
In conclusion, we demonstrate a novel increase in PLB at both gene and protein levels, but no change in SERCA2a protein levels in the hearts of rats with streptozotocin-induced diabetes of 4–6 weeks duration. These observations suggest a molecular mechanism for the slow rate of relaxation of the diabetic heart. The most important discovery in this study is that the stimulatory effect of PKA-catalyzed phosphorylation of PLB at Ser16 on SR Ca2+ transport was depressed in the diabetic heart, possibly due to phosphorylation of the Ser10 site of PLB by PKC. Indeed, significantly increased PKC activity was found in the cardiac membranous fraction obtained from diabetic rats. This was associated with an increase in the protein contents of the PKCδ, PKCη, PKCι, and PKCλ isoforms in the membranous fraction. To our knowledge, this study is the first report showing a possible role for PKC in the subcellular mechanisms responsible for the cardiac dysfunction, especially the impaired inotropic responsiveness to β-ADR stimulation, in diabetes. However, whether phosphorylation of PLB by PKC can modify the PKA-dependent reversal of the inhibitory effect of PLB on SERCA2a through changes in the three-dimensional structure of PLB and SERCA2a remains to be elucidated.
Acknowledgments
We thank Drs Shigeaki Kobayashi, Toshiteru Ishitani, and Yukari Suzuki for preparing diabetic rats, Dr Xiao-Hong Zhang for insulin treatment of diabetic animals, and Ms Emi Okada and Emi Mizoguchi for their technical assistance. This study was supported by a Grant-in-Aid for Science Research from the Ministry of Education, Science, Sports and Culture of Japan.
Abbreviations
- ADR
adrenoceptor
- BSA
bovine serum albumin
- CaMKII
Ca2+-calmodulin-dependent protein kinase
- CHF
congestive heart failure
- DEPC
diethyl pyrocarbonate
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- PKA
protein kinase A
- PKC
protein kinase C
- PLB
phospholamban
- PVDF
polyvinylidine difluoride
- SERCA2a
cardiac sarcoplasmic reticulum Ca2+-ATPase
- SR
sarcoplasmic reticulum
References
- ATKINS F.L., DOWELL R.T., LOVE S. β-Adrenergic receptors, adenylate cyclase activity, and cardiac dysfunction in the diabetic rat. J. Cardiovasc. Pharmacol. 1985;7:66–70. doi: 10.1097/00005344-198501000-00011. [DOI] [PubMed] [Google Scholar]
- BERLIN I., GRIMALDI A., BOSQUEST F., PUECH A.J. Decreased adrenergic sensitivity in insulin-dependent diabetic subjects. J. Clin. Endocrinol. Metab. 1986;63:262–265. doi: 10.1210/jcem-63-1-262. [DOI] [PubMed] [Google Scholar]
- CHOMCZINSKI P., SACCHI N. Single step method of RNA isolation by guanidine thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHU G., LESTER J.W., YOUNG K.B., LUO W., ZHAI J., KRANIAS E.G. A single site (Ser16) phosphorylation in phospholamban is sufficient in mediating its maximal contractile responses to β-agonists. J. Biol. Chem. 2000;275:38938–38943. doi: 10.1074/jbc.M004079200. [DOI] [PubMed] [Google Scholar]
- EDES I., KRANIAS E.G. Phospholamban and troponin I are substrates for protein kinase C in vitro but not in intact beating guinea pig hearts. Circ. Res. 1990;67:394–400. doi: 10.1161/01.res.67.2.394. [DOI] [PubMed] [Google Scholar]
- FABIATO A., FABIATO F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- GANDO S., HATTORI Y., AKAISHI Y., NISHIHIRA J., KANNO M. Impaired contractile response to beta adrenoceptor stimulation in diabetic rat hearts: alterations in beta adrenoceptors-G protein-adenylate cyclase sysytem and phospholamban phosphorylation. J. Pharmacol. Exp. Ther. 1997;282:475–484. [PubMed] [Google Scholar]
- GANGULY P.K., PIERCE G.N., DHALLA K.S., DHALLA N.S. Defective sarcoplasmic reticular calcium transport in diabetic cardiomyopathy. Am. J. Physiol. 1983;244:E528–E535. doi: 10.1152/ajpendo.1983.244.6.E528. [DOI] [PubMed] [Google Scholar]
- GILES T.D., OUYANG J., KERUT E.K., GIVEN M.B., ALLEN G.E., MCLLWAIN E.F., GREENBERG S.S. Changes in protein kinase C in early cardiomyopathy and in gracilis muscle in the BB/Wor diabetic rat. Am. J. Physiol. 1998;274:H295–H307. doi: 10.1152/ajpheart.1998.274.1.H295. [DOI] [PubMed] [Google Scholar]
- GØTZSCHE O. The adrenergic β-receptor adenylate cyclase system in heart and lymphocytes from streptozotocin-diabetic rats: in vivo and in vitro evidence for a desensitized myocardial β-receptor. Diabetes. 1983;32:1110–1116. doi: 10.2337/diab.32.12.1110. [DOI] [PubMed] [Google Scholar]
- HAGHIGHI K., KADAMBI V.J., KOSS K.L., LUO W., HARRER J.M., PONNIAH S., ZHOU Z., KRANIAS E.G. In vitro and in vivo promoter analysis of the mouse phospholamban gene. Gene. 1997;12:199–207. doi: 10.1016/s0378-1119(97)00514-3. [DOI] [PubMed] [Google Scholar]
- HARIGAYA S., SCHWARTZ A. Rate of calcium binding and uptake in normal animal and failing human cardiac muscle. Circ. Res. 1969;25:781–794. doi: 10.1161/01.res.25.6.781. [DOI] [PubMed] [Google Scholar]
- HARTONG R.F., VILLARREAL F.J., GIORDANO F., HILALDANDAN R., MCDONOUGH P.M., DILLMANN W.H. Phorbol myristate acetate-induced hypertrophy of neonatal rat cardiac myocyte is associated with decreased sarcoplasmic reticulum Ca2+ ATPase (SERCA2) gene expression and calcium reuptake. J. Mol. Cell. Cardiol. 1996;28:2467–2477. doi: 10.1006/jmcc.1996.0239. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., MATSUDA N., KIMURA J., ISHITANI T., TAMADA A., GANDO S., KEMMOTSU O., KANNO M. Diminished function and expression of the cardiac Na+–Ca2+ exchanger in diabetic rats: implication in Ca2+ overload. J. Physiol. 2000;527:85–94. doi: 10.1111/j.1469-7793.2000.00085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOGUCHI T., BATTAN R., HANDLER E., SPORTSMAN J.R., HEATH W., KING G.L. Preferential elevation of protein kinase C isoform βII and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHITANI T., HATTORI Y., SAKURAYA F., ONOZUKA H., MAKINO T., MATSUDA N., GANDO S., KEMMOTSU O. Effects of Ca2+ sensitizers on contraction, [Ca2+]i transient and myofilament Ca2+ sensitivity in diabetic rat myocardium: Potential usefulness as inotropic agents. J. Pharmacol. Exp. Ther. 2001;298:613–622. [PubMed] [Google Scholar]
- IWASA Y., HOSEY M.M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J. Biol. Chem. 1988;259:534–540. [PubMed] [Google Scholar]
- KADAMBI V.J., KRANIAS E.G. Phospholamban: a protein coming of age. Biochem. Biophys. Res. Commun. 1997;239:1–5. doi: 10.1006/bbrc.1997.7340. [DOI] [PubMed] [Google Scholar]
- KANNEL W.B., HJORTLAND M., CASTELLI W.P. Role of diabetes in congestive heart failure: the Framingham Study. Am. J. Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- KOSS K.L., KRANIAS E.G. Phospholamban: a prominent regulator of myocardial contractility. Circ. Res. 1996;79:1059–1063. doi: 10.1161/01.res.79.6.1059. [DOI] [PubMed] [Google Scholar]
- KRANIAS E.G. Regulation of Ca2+ transport by cyclic 3′, 5′-AMP-dependent and calcium-calmodulin-dependent phosphorylation of cardiac sarcoplasmic reticulum. Biochim. Biophys. Acta. 1985;844:191–199. doi: 10.1016/0167-4889(85)90090-4. [DOI] [PubMed] [Google Scholar]
- KUSCHEL M., KARCZEWSKI P., HEMPEL P., SCHLEGEL W.-P., KRAUSE E.-G., BARTEL S. Ser16 prevails over Thr17 phospholamban phosphorylation in the β-adrenergic regulation of cardiac relaxation. Am. J. Physiol. 1999;276:H1625–H1633. doi: 10.1152/ajpheart.1999.276.5.H1625. [DOI] [PubMed] [Google Scholar]
- LIU X., WANG J., TAKEDA N., BINAGLIA L., PANAGIA V., DHALLA N.S. Changes in cardiac protein kinase C activities and isoenzymes in streptozotocin-induced diabetes. Am. J. Physiol. 1999;277:E798–E804. doi: 10.1152/ajpendo.1999.277.5.E798. [DOI] [PubMed] [Google Scholar]
- LOPASCHUK G.D., TAHILIANI A.G., VADLAMUDI R.V.S.V., KATZ S., MCNEILL J.H. Cardiac sarcoplasmic reticulum function in insulin or carnitine-treated diabetic rats. Am. J. Physiol. 1983;245:H969–H976. doi: 10.1152/ajpheart.1983.245.6.H969. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurment with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MALHOTRA A., REICH D., NAKOUZI A., SANGHI V., GREEN D.L., BUTTRICK P.M. Experimental diabetes is associated with functional activation of protein kinase C and phosphorylation of troponin I in the heart, which are prevented by angiotensin II receptor blockade. Circ. Res. 1997;81:1027–1033. doi: 10.1161/01.res.81.6.1027. [DOI] [PubMed] [Google Scholar]
- MARTINY-BARON G., KAZANIETZ M.G., MISCHAK H., BLUMBERG P.M., KOCHS G., HUG H., MARMÉ D., SCHÄCHTELE C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- MATSUDA N., HATTORI Y., GANDO S., AKAISHI Y., KEMMOTSU O., KANNO M. Diabetes-induced downregulation of β1-adrenoceptor mRNA expression in rat heart. Biochem. Pharmacol. 1999;58:881–885. doi: 10.1016/s0006-2952(99)00164-1. [DOI] [PubMed] [Google Scholar]
- MOVSESIAN M.A., NISHIKAWA M., ADELSTEIN R.S. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. J. Biol. Chem. 1984;259:8029–8032. [PubMed] [Google Scholar]
- NARUSE K., KING G.L. Protein kinase C and myocardial biology and function. Circ. Res. 2000;86:1104–1106. doi: 10.1161/01.res.86.11.1104. [DOI] [PubMed] [Google Scholar]
- NISHIO Y., KASHIWAGI A., KIDA Y., KODAMA M., ABE N., SEKI Y., SHIGETA Y. Deficiency of cardiac β-adrenergic receptor in streptozotocin-induced diabetic rats. Diabetes. 1988;37:1181–1187. doi: 10.2337/diab.37.9.1181. [DOI] [PubMed] [Google Scholar]
- OKUMURA K., AKIYAMA N., HASHIMOTO H., OGAWA K., SATAKE T. Alteration of 1,2-diacylglycerol content in myocardium from diabetic rats. Diabetes. 1988;37:1168–1172. doi: 10.2337/diab.37.9.1168. [DOI] [PubMed] [Google Scholar]
- OSADA M., NETTICADAN T., TAMURA K., DHALLA N.S. Modification of ischemia–reperfusion-induced changes in cardiac sarcoplasmic reticulum by preconditioning. Am. J. Physiol. 1998;274:H2025–H2034. doi: 10.1152/ajpheart.1998.274.6.H2025. [DOI] [PubMed] [Google Scholar]
- PENPARGKUL S., FEIN F., SONNENBLICK E.H., SCHEUER J. Depressed cardiac sarcoplasmic reticular function from diabetic rats. J. Mol. Cell. Cardiol. 1981;13:303–309. doi: 10.1016/0022-2828(81)90318-7. [DOI] [PubMed] [Google Scholar]
- QI M., BASSANI J.W., BERS D.M., SAMAREL A.M. Phorbol 12-myristate 13-acetate alters SR Ca2+-ATPase gene expression in cultured neonatal rat heart cells. Am. J. Physiol. 1996;271:H1031–H1039. doi: 10.1152/ajpheart.1996.271.3.H1031. [DOI] [PubMed] [Google Scholar]
- ROGERS T.B., GAA S.T., MASSEY C., DOSEMECI A. Protein kinase C inhibits Ca2+ accumulation in cardiac sarcoplasmic reticulum. J. Biol. Chem. 1990;265:4302–4308. [PubMed] [Google Scholar]
- ROTH D.A., WHITE C.D., HAMILTON C.D., HALL J.L., STANLEY W.C. Adrenergic desensitization in left ventricle from streptozotocin diabetic swine. J. Mol. Cell. Cardiol. 1995;27:2315–2325. doi: 10.1016/s0022-2828(95)91875-2. [DOI] [PubMed] [Google Scholar]
- SAVARESE J.J., BERKOWITZ B.A. β-Adrenergic receptor decrease in diabetic rat hearts. Life Sci. 1979;25:2075–2078. doi: 10.1016/0024-3205(79)90200-5. [DOI] [PubMed] [Google Scholar]
- SIMMERMAN H.K., COLLINS J.H., THEIBERT J.L., WEGENER A.D., JONES L.R. Sequence analysis of phospholamban: identification of phosphorylation sites and two major structural domains. J. Biol. Chem. 1986;261:13333–13341. [PubMed] [Google Scholar]
- TADA M., YAMADA M., OHMORI F., KUZUYA T., INUI M., ABE H. Transient state kinetic studies of Ca2+-dependent ATPase and calcium transport by cardiac sarcoplasmic reticulum: effect of cyclic AMP-dependent protein kinase-catalyzed phosphorylation of phospholamban. J. Biol. Chem. 1983;255:1985–1992. [PubMed] [Google Scholar]
- TAMADA A., HATTORI Y., HOUZEN H., YAMADA Y., SAKUMA I., KITABATAKE A., KANNO M. Effects of β-adrenoceptor simulation on contractility, [Ca2+]i, and Ca2+ currents in diabetic rat cardiomyocytes. Am. J. Physiol. 1998;266:H2334–H2342. doi: 10.1152/ajpheart.1998.274.6.H1849. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., KASHIWAGI A., OGAWA T., ABE T., ASAHINA T., IKEBUCHI M., TAKAGI Y., SHIGETA Y. Effect of verapamil on cardiac protein kinase C activity in diabetic rats. Eur. J. Pharmacol. 1991;200:353–356. doi: 10.1016/0014-2999(91)90595-h. [DOI] [PubMed] [Google Scholar]
- TOMLINSON K.C., GARDINER S.M., HEBDEN R.A., BENNETT T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol. Rev. 1992;44:103–150. [PubMed] [Google Scholar]
- TOYOFUKU T., ZAK R. Characterization of cDNA and genomic sequences encoding a chicken phospholamban. J. Biol. Chem. 1991;266:5375–5383. [PubMed] [Google Scholar]
- TROVIK T.S., JAEGER R., JORDE R., SAGER G. Reduced sensitivity to β-adrenoceptor stimulation and blockade in insulin dependent diabetic patients with hypoglycaemia unawareness. Br. J. Clin. Pharmacol. 1994;38:427–432. doi: 10.1111/j.1365-2125.1994.tb04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÜBERALL F., HELLBERT K., KAMFER S., MARY K., VILLUNGER A., SPITALER M., NWANJEWE J., BAIER-BITTERLICH G., BAIER G., GRUNICKE H.H. Evidence that atypical protein kinase C-λ and atypical protein kinase C-ζ participate in Ras-mediated reorganization of the F-actin cytoskeleton. J. Cell Biol. 1999;144:413–425. doi: 10.1083/jcb.144.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICHELHAUS A., RUSS M., PETERSEN S., ECKEL J. G protein expression and adenylate cyclase regulation in ventricular cardiomyocytes from STZ-diabetic rats. Am. J. Physiol. 1994;267:H548–H555. doi: 10.1152/ajpheart.1994.267.2.H548. [DOI] [PubMed] [Google Scholar]
- XIANG H., MCNEILL J.H. Protein kinase C activity is altered in diabetic rat hearts. Biochem. Biophys. Res. Commun. 1992;187:703–710. doi: 10.1016/0006-291x(92)91252-l. [DOI] [PubMed] [Google Scholar]
- YU Z., QUAMME G.A., MCNEILL J.H. Depressed [Ca2+]i responses to isoproterenol and cAMP in isolated cardiomyocytes from experimental diabetic rats. Am. J. Physiol. 1994;266:H2334–H2342. doi: 10.1152/ajpheart.1994.266.6.H2334. [DOI] [PubMed] [Google Scholar]
- ZARAIN-HERZBERG A., YANO K., ELIMBAN V., DHALLA N.S. Cardiac sarcoplasmic reticulum Ca2+-ATPase expression in streptozotocin-induced diabetic rat heart. Biochem. Biophys. Res. Commun. 1994;203:113–120. doi: 10.1006/bbrc.1994.2156. [DOI] [PubMed] [Google Scholar]
- ZHONG Y., AHMED S., GRUPP I.L., MATIB H.A. Altered SR protein expression associated with contractile dysfunction in diabetic rat hearts. Am. J. Physiol. 2001;281:H1137–H1147. doi: 10.1152/ajpheart.2001.281.3.H1137. [DOI] [PubMed] [Google Scholar]