Abstract
We have investigated the role of G protein-coupled receptor kinase 5 (GRK5) in the regulation of endosome sorting of human β2-adrenoceptors.
Expressing GRK5 at a high level significantly increased the extent of internalization of wild-type β2-adrenoceptors and of an internalization-defective mutant receptor, and increased receptor phosphorylation at serines 355 and 356 in the cytoplasmic tail.
Overexpressing GRK5 did not alter β2-adrenoceptor recycling as assessed by immunofluorescence microscopy and radioligand binding assays nor was there any change in receptor downregulation.
These data indicate that GRK5 does not regulate the sorting of β2-adrenoceptors in the endocytic pathway.
Keywords: β2-adrenoceptor, GRK5, phosphorylation, endosome sorting
Introduction
The signaling capacity of β2-adrenoceptors is attenuated by both acute and chronic desensitization processes involving several distinct but overlapping mechanisms. Agonist-induced desensitization of the β2-adrenoceptor initiates when the intracellular carboxyl-terminal (C-terminal) domain of agonist-occupied receptor is phosphorylated by G protein-coupled receptor kinases (GRK) (Seibold et al., 2000). GRK-phosphorylated receptors are then bound by nonvisual arrestins (β-arrestin1 and β-arrestin2), which causes the uncoupling of receptors from Gs and promotes rapid receptor endocytosis into early endosomes (Lefkowitz, 1993; 1998; Krupnick & Benovic, 1998). Intracellular receptors are dephosphorylated, possibly in early endosomes (Krueger et al., 1997; Seachrist et al., 2000), and most receptors recycle to the cell surface in a fully sensitized state (Yu et al., 1993). During chronic exposures to agonist, β2-adrenoceptors undergo continuous rounds of endocytosis and recycling to the plasma membrane. Over time, some receptors are sorted to lysosomes where they are downregulated (Gagnon et al., 1998; Moore et al., 1999b), although the mechanisms by which endosome sorting occurs remain unclear.
Recent evidence suggests that ezrin – radixin – moesin (ERM)-binding phosphoprotein-50 (EBP50), also known as the Na+/H+ exchanger regulatory factor (NHERF), interacts with the β2-adrenoceptor and regulates receptor recycling (Cao et al., 1999; Cong et al., 2001). EBP50 contains two protein-binding domains – a PDZ domain that apparently binds the β2-adrenoceptor via an interaction with the DSLL sequence at residues 410 – 413 of the receptor's C-terminal tail and an ERM-binding domain that can associate with the cortical actin cytoskeleton through interaction with ERM proteins (Reczek et al., 1997; Cao et al., 1999). Interruption of the β2-adrenoceptor – EBP50 interaction by the addition of a single alanine to the receptor's cytoplasmic tail, by replacing the serine in the DSLL domain (serine 411) with aspartic acid or alanine, or by overexpressing GRK5, which phosphorylates serine 411 in vitro, inhibits β2-adrenoceptor recycling and leads to the apparent missorting of receptors to lysosomes (Cao et al., 1999).
Other studies raise questions about the role of this GRK5-regulated interaction of EBP50 and receptors in mediating β2-adrenoceptor traffic. First, the addition of a 6-histidine or green fluorescent protein tag to the receptor C-terminal tail, maneuvers which ought to disrupt receptor interaction with EBP50, does not alter the rate or extent of receptor recycling to the plasma membrane when directly quantified (Seibold et al., 2000; Pan et al., 2003). Further, a β2-adrenoceptor mutated at leucine 412 (position −1 within the DSLL motif) maintains its ability to interact with EBP50 but is recycling deficient, suggesting that other proteins may be more important in the regulation of β2-adrenoceptor recycling (Cong et al., 2001). Finally, mutation of all six serine and threonine residues identified as in vitro substrates for GRK5 phosphorylation, including serine 411, to alanines does not affect receptor phosphorylation or the size of the steady-state pool of internalized receptors, which is a balance between receptor endocytosis and recycling (Seibold et al., 1998).
Given this conflicting data and our own data demonstrating serines 355, 356, and 364 as GRK phosphorylation sites (Seibold et al., 2000), we sought to define more clearly the role of GRK5 as a regulator of the endosome sorting of internalized β2-adrenoceptors by performing detailed measurements of receptor trafficking in cells transiently or inducibly overexpressing GRK5. Using these complementary approaches, we show that the overexpression of GRK5 has no effect on β2-adrenoceptor recycling, following both brief and prolonged exposures to agonist, or receptor downregulation. GRK5 enhances receptor internalization, but only when expressed at very high levels relative to receptor. Together, these data indicate that the intracellular trafficking of β2-adrenoceptors is not affected by the cellular levels of GRK5 and suggest that the endosome sorting of β2-adrenoceptors is GRK5 independent.
Methods
Cell culture and reagents
12β6 cells expressing human β2-adrenoceptors with an N-terminal HA tag (a gift of B. Kobilka, Stanford, CA, U.S.A.) and human embryonic kidney (HEK)293 cells (purchased from American Type Culture Collection, Manassas, VA, U.S.A.) were cultured in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS) and 200 μg ml−1 Geneticin (Life Technologies, Gaithersburg, MD, U.S.A.). HEK293 cells (EcR293 cells) expressing the ecdysone receptor subunits VgEcR and RXR were obtained from Invitrogen (Carlsbad, CA, U.S.A.), and cultured in DMEM with 10% FBS and 200 μg ml−1 of Zeocin (Invitrogen). COS-7 cells were obtained from the American Type Culture Collection (Manassas, VA, U.S.A.) and cultured in DMEM with high glucose and 10% FBS. Mouse monoclonal antibody against the HA tag (mHA.11) was obtained from Berkeley Antibody Co. (Berkeley, CA, U.S.A.) and the rabbit polyclonal antibodies against GRK5, the β2-adrenoceptor cytoplasmic tail, and β2-adrenoceptor phosphoserines 355, 356 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Species-appropriate goat secondary antibodies conjugated to Alexa564 and Alexa488 were purchased from Molecular Probes (Eugene, OR, U.S.A.). FuGene6 transfection reagent was obtained from Roche Molecular Biochemicals (Basel, Switzerland). The GRK2, GRK3, and GRK5 cDNAs cloned into pcDNA3 were kindly provided by Dr Jeffrey Benovic (Philadelphia, PA, U.S.A.). All other reagents were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.) unless otherwise noted.
Immunofluorescence microscopy of 12β6 cells transiently overexpressing GRK5
12β6 cells were plated onto poly-D-lysine-coated 22 mm glass coverslips at 50% confluence in six-well clusters. After 24 h and just prior to transfections, the media were removed and replaced with antibiotic-free media containing 3% FBS. Cells were transfected with 2 μg per well of empty vector as a control or GRK5 using FuGene 6 transfection reagent with a DNA : FuGene 6 ratio of 2 : 3. The transfection mixture remained on cells for 48 h, at which time the cells were treated as indicated in the figure legends.
Following treatment, the cells were washed with phosphate-buffered saline with 1.2% sucrose (PBSS), then fixed with 3.7% paraformaldehyde for 10 min. The fixed cells were incubated in 0.34% L-lysine, and 0.05% Na-m-periodate for 20 min, then washed with PBSS and permeabilized with 0.2% Triton X-100. After further wash, the cells were blocked with 10% normal goat serum for 15 min. Primary antibodies were diluted in PBSS with 0.2% normal goat serum and 0.05% Triton X-100, then added to the cells and left for 1 – 2 h. The cells were washed four times before labeling with secondary antibodies using the same procedure as for the primary antibodies. We used the following concentrations of antibodies: mHA.11, 5 μg ml−1; polyclonal anti-GRK5, 10 μg ml−1; Alexa488 anti-mouse IgG, 1 : 200 dilution; and Alexa564 anti-rabbit IgG, 1 : 500 dilution. The coverslips were mounted in Mowiol and viewed using a DeltaVision deconvolution microscopy system (Applied Precision Inc., Issaquah, WA, U.S.A.) equipped with a Zeiss Axiovert microscope. Imaging was performed using a Zeiss × 100 (1.4 numerical aperture) oil immersion lens and sections were collected at an optical depth of 150 nm in the z-plane. Images were optimized using DeltaVision deconvolution software and transferred to Adobe Photoshop 5.5 for the production of final figures.
Inducible overexpression of GRK5
EcR293 cells stably express the regulatory protein VgRXR, a chimeric steroid receptor that is activated by synthetic ecdysteroids such as ponasterone (No et al., 1996). Cells were initially transfected to overexpress human β2-adrenoceptors stably with an N-terminal FLAG epitope using FuGene transfection reagent as previously described, with selection for G418 resistance (Rosenfeld et al., 2001). The resulting cell line (EcR293:β2-adrenoceptor cells) expresses β2-adrenoceptors at a level of 1.3 pmol mg−1 of total protein. The GRK5 cDNA was cloned into the pIND-Hygro vector, which has a promoter element responsive to VgRXR and a hygromycin resistance gene. pIND-Hygro : GRK5 was stably transfected into EcR293:β2-adrenoceptor cells using FuGene6 with selection for hygromycin B (200 μg ml−1). For induction of GRK5 overexpression during experiments, cells were treated for 72 h with 5 μM ponasterone A, while control cells were treated with vehicle alone (0.125% ethanol).
Immunoblotting of HEK293 cells
Following the induction of GRK5 expression with ponasterone A as indicated in the figure legend, transfected cells were washed with PBS, then dissolved in Laemmli sample buffer (Laemmli, 1970). Samples were electrophoresed through a 4 – 15% Tris-HCl SDS – polyacrylamide gradient gel (Ready Gel, Bio-Rad, Hercules, CA, U.S.A.) and transferred to Immobilon-P membranes (Millipore, Bedford, MA, U.S.A.). The membranes were blocked in 5% dry milk in 0.2% Tween and probed with anti-GRK5 antibody at a dilution of 1 : 2000, and bound antibodies were detected by chemiluminescence (ECL-Pierce Chemical Co., Rockford, IL, U.S.A.). Immunoblots were quantified by densitometry using SigmaGel software (SPSS Science, Chicago, IL, U.S.A.) or using an Alpha Innotech FluorChem 800 imaging system (San Leandro, CA, U.S.A.).
Analysis of β2-adrenoceptor internalization and recycling
Stably transfected EcR293:β2-adrenoceptor cells growing on poly-D-lysine-coated 24-well clusters were cultured in the presence of ponasterone to induce GRK5 overexpression or vehicle for 72 h. Cells were exposed to 5 μM (−)-isoprenaline at 37°C for varying times up to 60 min to promote β2-adrenoceptor internalization. The cells were chilled, washed extensively with DMEM containing 20 mM HEPES, pH 7.4 (DMEM-H), and surface receptors measured by incubation in DMEM-H containing 6 nM [3H]CGP12177 at 0°C for 2 h, followed by washing with cold DMEM-H. The monolayers were then harvested in a solution containing 0.1% SDS and 0.1% NP-40, and the lysates counted by scintillation spectroscopy. For every experiment, binding at each time point was measured in triplicate. The fraction of receptors left on the surface was plotted versus time of exposure, and the curves fitted by nonlinear regression using GraphPad Prism software (version 3.0, San Diego, CA, U.S.A.) as previously described (Morrison et al., 1996). First-order rate constants for receptor endocytosis were determined using Equation (4) of Morrison et al. (1996) and the level of statistical significance was determined using a paired Student's t-test. Endocytic rate constants are presented as the means±s.e.m. of three independent experiments.
β2-adrenoceptor recycling was directly assessed in transfected cells by radioligand binding as previously described (Morrison et al., 1996). Following the induction of GRK5 overexpression with ponasterone or treatment with vehicle alone, cells were treated with isoprenaline for 15 min or 6 h, washed extensively and incubated in DMEM-H prewarmed to 37°C to allow recycling. At varying times up to 90 min, the cells were chilled and surface receptors measured by incubation in 6 nM [3H]CGP12177 as described above. The fraction of cell surface receptors was plotted versus time of recycling, and the curves were fitted by nonlinear regression and recycling rate constants where determined as previously described (Morrison et al., 1996). Recycling rate constants are presented as the means±s.e.m. of three separate experiments.
Determination of β2-adrenoceptor downregulation
Stably transfected EcR293 cells were cultured on 24-well clusters in the presence of ponasterone A or ethanol for 72 h as described above. During the final 24 h of incubation, cells were treated with 5 μM isoprenaline dissolved in 0.1 mM ascorbic acid and 1 mM thiourea (AT) carrier or with AT alone for the indicated times. Following agonist treatment, the cells were washed four times with ice-cold PBS, then incubated with 6 nM [3H]CGP12177 in DMEM-H containing 0.05% digitonin at 4°C for 90 min. The cells were washed twice with DMEM-H, then lysed in the wells with 0.1% Triton X-100, and the lysates counted by scintillation spectroscopy. Nonspecific binding of radioligand was determined by parallel incubations in the presence of propranolol (3 μM), and was less than 5% of total. Statistical significance was determined using a one-way ANOVA and defined by P<0.05.
Transient coexpression of β2-adrenoceptors and GRK5
HEK293 cells were plated into 100 mm dishes at a density of 35,000 cells per mm3 and grown in DMEM supplemented with 10% FBS. After 24 h and 30 min prior to transfection, the media was replaced and then cells were transfected with 0.6 μg of β2-adrenoceptor cDNA and 10 μg of GRK5 or plasmid cDNA using the calcium phosphate method. At 6 h following the transfection, cells were washed and grown in complete media for an additional 24 h. Cells were then reseeded into poly-D-lysine-coated 24-well dishes for recycling studies or six-well dishes for Western blot analysis and allowed to incubate for an additional 24 h prior to experiments. Immunofluorescence microscopy of transfected cells showed that all cells expressing β2-adrenoceptors also expressed GRK5.
To determine receptor recycling, cells were treated with 10 μM isoprenaline for 30 min at 37°C, washed thoroughly, and incubated in warm DMEM-H containing 6 nM [3H]CGP12177 for up to 60 min. Cells were harvested in 0.1% NP-40 and 0.1% SDS and the lysates counted by scintillation spectroscopy as described above.
For studies of the β2-adrenoceptor Y326A mutant, HEK293 cells were cotransfected with plasmids expressing the mutant receptors together with empty vector, or with plasmids expressing GRK2, GRK3, or GRK5 using FuGene 6 reagent as described above. After 48 h, cells were treated with isoprenaline (10 μM) for 30 min or left untreated and surface receptors determined using 6 nM [3H]CGP12177 as described.
Determination of β2-adrenoceptor phosphorylation at serines 355 and 356
HEK293 cells growing in six-well clusters were transfected with 0.6 μg of a plasmid expressing a β2-adrenoceptor with an N-terminus HA tag and a C-terminus 6-histidine tag and 2.4 μg of either empty vector, GRK2, or GRK5 using FuGene 6 transfection reagent as described above, with a DNA : FuGene six ratio of 3 : 4.5. The transfection mixture remained on cells for 48 h, at which time the cells were treated with 10 μM (−)-adrenaline in 0.1 mM ascorbate and 1 mM thiourea (AT) for 5 min or with AT carrier alone.
Cells were harvested, pelleted by centrifugation at 2000 rpm, and solubilized in buffer containing 20 mM HEPES, pH 7.4, 300 mM NaCl, 0.8% n-dodecyl-β-D-maltoside (DBM), 5 mM EDTA, 3 mM EGTA, 20 mM sodium pyrophosphate, 10 mM sodium fluoride, 25 mM imidazole, 10 μg ml−1 benzamidine, 10 μg ml−1 trypsin inhibitor, 100 nM okadaic acid, 10 μg ml−1 leupeptin, and 14 mM β-mercaptoethanol. The solubilized cells were centrifuged for 30 min at 45,000 rpm in a Beckman 50 Ti rotor, and the supernatant was applied to a Talon Co2+ – carboxymethylaspartate – agarose column (0.5 ml packed resin) that had been prewashed with Talon buffer (0.05% DBM, 20 mM HEPES, pH 7.4, 150 mM NaCl). The column was washed twice with Talon buffer, followed by washes with 10 and 20 mM imidazole in the same buffer. β2-adrenoceptors were eluted with 0.75 ml of Talon buffer containing 100 mM imidazole and concentrated to 50 μl in a centricon (Amicon; 30 kDa cutoff). The eluate was digested with 1500 U of N-glycosidase F (New England Biolabs, Beverly, MA, U.S.A.) for 2 h at 37°C and heated at 60°C for 15 min in SDS-sample buffer. Samples were resolved on SDS – polyacrylamide gel electrophoresis (12% gel) and transferred to nitrocellulose for immunoblotting. The membranes were probed with a rabbit β2-adrenoceptor phosphoserines 355 and 356-specific antibody at a dilution of 1 : 500 and detected by chemiluminescence. Following quantification by densitometry, the immunoblots were stripped and reprobed with a β2-adrenoceptor-specific rabbit antibody at a dilution of 1 : 1000 to quantify total receptors. The results reported in the text are the fold increase in receptor phosphorylation at serines 355, 356 in GRK-expressing cells compared with untreated cells expressing receptors alone and are normalized to total receptor load.
Results
Effect of GRK5 on β2-adrenoceptor trafficking in 12β6 cells
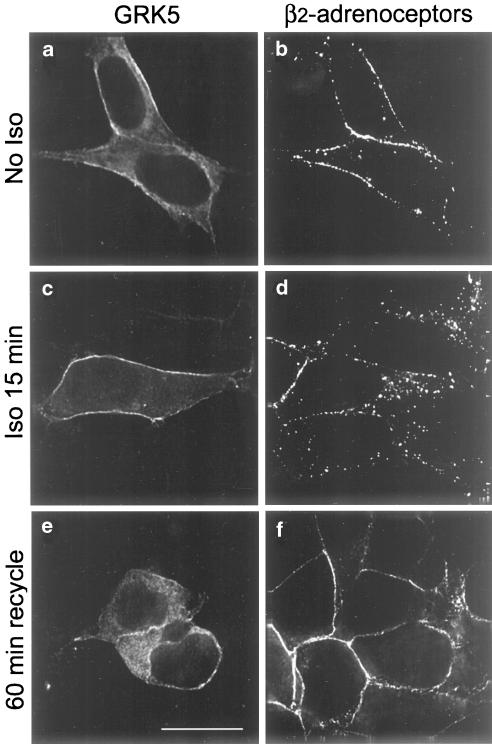
In HEK293 cells stably expressing HA-tagged human β2-adrenoceptors (12β6 cell line) and transiently overexpressing GRK5, GRK5 (Figure 1a, c and e) was localized to the plasma membrane and to the cytoplasm, while nontransfected cells did not express adequate endogenous levels of GRK5 to be detected with our antibody. In vector-transfected control cells (data not shown) and in cells overexpressing GRK5, β2-adrenoceptors localized to the plasma membrane in the basal state (Figure 1b) and internalized into punctate endosomes following a 15 min exposure to agonist (Figure 1d). After removal of agonist, β2-adrenoceptors were largely recycled to the plasma membrane within 60 min in both control (data not shown) and GRK5-overexpressing cells (Figure 1f).
Figure 1.
β2-adrenoceptor recycling in 12β6 cells transiently overexpressing GRK5. 12β6 cells transiently overexpressing GRK5 were left untreated (a, b) or treated with 5 μM isoprenaline for 15 min, then immediately fixed (c, d) or washed and incubated in the presence of propranolol (5 μM) for 60 min to allow β2-adrenoceptor recycling (e, f). Cells were immunolabeled with antibodies against the HA epitope to identify receptors and GRK5. The scale bar equals 10 μm.
Inducible overexpression of GRK5
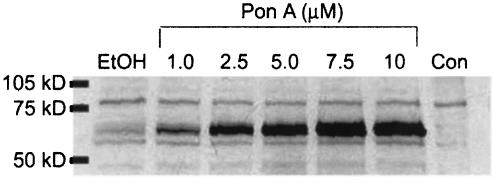
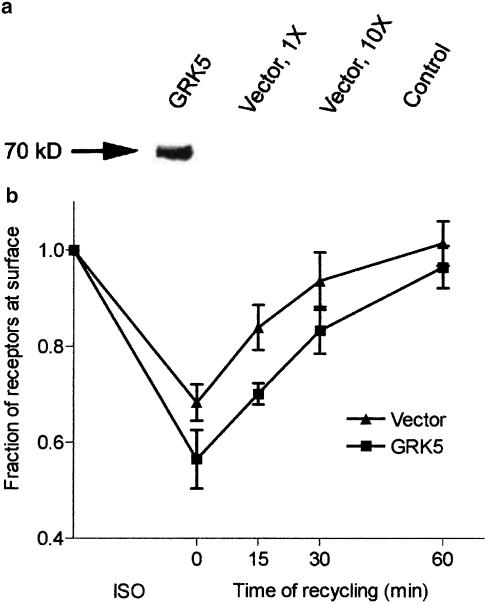
We created an EcR293 (EcR293:β2-adrenoceptor:GRK5) cell line stably expressing epitope-tagged β2-adrenoceptors in which GRK5 could be inducibly overexpressed upon addition of the insect hormone ponasterone A. Using immunoblot analysis (Figure 2), we found that GRK5 was significantly overexpressed following 72 h of induction by ponasterone A in a concentration-dependent manner. The degree of overexpression was difficult to determine precisely due to the very low level of endogenous expression in nontransfected control cells, but was estimated by densitometry to be at least eight- to 10-fold in cells treated with 5 μM ponasterone A. Although there was a small amount of GRK5 expression in the vehicle control group indicating some leak in the inducible system, there was an approximately five-fold additional increase in expression in cells induced with ponasterone.
Figure 2.
Inducible overexpression of GRK5. EcR293:β2-adrenoceptor:GRK5 cells were grown for 72 h in the presence of 0.125% ethanol (EtOH) or varying concentrations of ponasterone A (Pon A) up to 10 μM. The control lane (Con) is a lysate of EcR293:β2-adrenoceptor cells not transfected with GRK5. GRK5 migrates at approximately 69 kDa.
β2-adrenoceptor trafficking in cells inducibly overexpressing GRK5
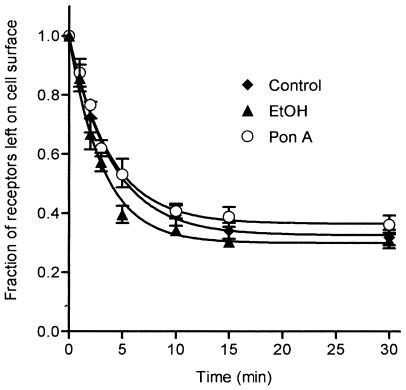
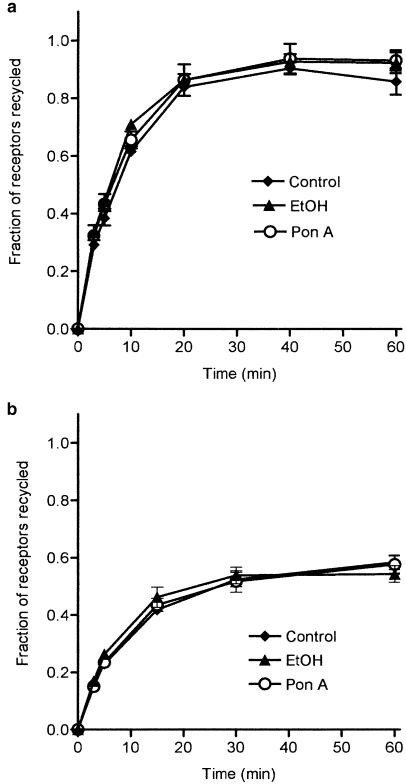
The inducible overexpression of GRK5 in EcR293:β2-adrenoceptor:GRK5 cells had no effect on the rate or extent of β2-adrenoceptor internalization (Figure 3), recycling following both brief (Figure 4a) and prolonged exposures to agonist (Figure 4b), or downregulation (Figure 5) as compared to noninduced cells and nontransfected EcR293:β2-adrenoceptor cells (see Table 1 for internalization and recycling rate constants). Consistent with our previously reported data (Moore et al., 1999a), the number of recyclable receptors in each group treated with isoprenaline for 6 h was reduced when compared to that observed following a brief exposure to agonist (see Figure 4). Internalization and recycling were also measured in a second GRK-transfected clone with similar results (data not shown).
Figure 3.
β2-adrenoceptor internalization in cells inducibly overexpressing GRK5. Following treatment with ponasterone (Pon A, 5 μM) or ethanol (EtOH) for 72 h, ECR293:β2-adrenoceptor:GRK5 cells were treated with 5 μM isoprenaline for varying times up to 30 min. Untransfected EcR293:β2-adrenoceptor cells (control) were similarly treated. Each point represents the means±s.e.m. of three independent experiments. The fraction of receptors left on the surface was plotted as a function of time and the curves fitted as previously described (Morrison et al., 1996).
Figure 4.
β2-adrenoceptor recycling in cells inducibly overexpressing GRK5. Receptor recycling in ponasterone- (Pon A, 5 μM) or vehicle- (EtOH) treated GRK5-transfected and -untranfected cell (control) was determined following agonist treatment (5 μM isoprenaline) for 15 min (a) or 6 h (b). Each point represents the means±s.e.m. of three independent experiments. The fraction of receptors left intracellular was plotted as a function of time and the curves fitted as previously described (Morrison et al., 1996).
Figure 5.
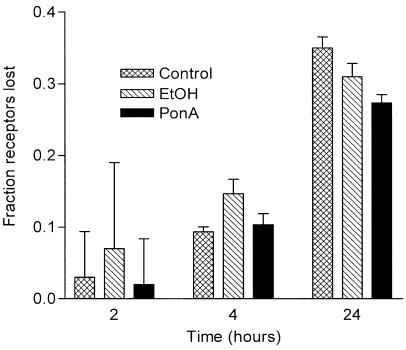
Effect of GRK5 overexpression on β2-adrenoceptor downregulation. Receptor downregulation was determined in ponasterone- (Pon A, 5 μM) or vehicle- (EtOH) treated GRK5-transfected and -untransfected cells (Control) following treatment with agonist (5 μM isoprenaline) or vehicle (AT) for the indicated times up to 24 h. Receptor levels in isoprenaline-treated cells were plotted against those from vehicle-treated cells to determine the fraction of β2-adrenoceptors lost. Individual values represent the means±s.e.m. from three independent experiments.
Table 1.
Endocytic and recycling rate constants (min−1) in EcR293:β2-adrenoceptor cell lines
| ke | kr (15 min) | kr (6 h) | |
|---|---|---|---|
| Untransfected | 0.168±0.013 | 0.121±0.009 | 0.086±0.007 |
| GRK5 (EtOH) | 0.229±0.013 | 0.139±0.009 | 0.118±0.013 |
| GRK5 (PON) | 0.197±0.009 | 0.124±0.011 | 0.085±0.010 |
Individual rate constants for β2-adrenoceptor endocytosis (ke) and recycling (kr) following 15 min and 6 h exposures to isoprenaline were determined as previously described (Morrison et al., 1996). Rate constants are expressed as means±s.e.m. from three independent experiments.
Effect of transiently coexpressing GRK5 and β2-adrenoceptors on receptor trafficking
Although the level of GRK5 overexpression was at least eight- to 10-fold in the inducible cell lines, we considered the possibility that even higher levels of GRK5 relative to that of β2-adrenoceptors are required to alter receptor traffic. To address this question, we transiently coexpressed GRK5 or empty vector and epitope-tagged β2-adrenoceptors in HEK293 cells using an almost 15-fold excess of GRK5 or vector cDNA relative to receptor. By Western blot analysis (Figure 6a), the level of GRK5 overexpression was much greater than 10-fold, as endogenous GRK5 could not be detected in the vector control lanes even with a × 10 protein load. Further, all cells that expressed epitope-tagged β2-adrenoceptors also overexpressed GRK5 when analyzed by immunofluorescence microscopy (data not shown).
Figure 6.
β2-adrenoceptor internalization and recycling in HEK293 cells transiently coexpressing receptors and GRK5. HEK293 cells were transfected with β2-adrenoceptor cDNA and GRK5 or plasmid cDNA. (a) Total cell extracts from GRK5-transfected and control cells were probed with anti-GRK5 antibody. (b) Receptor internalization and recycling were determined following treatment with isoprenaline (10 μM) for 30 min at 37°C. Each point represents the means±s.e.m. of three independent experiments.
Although overexpressing GRK5 at a very high level modestly, but significantly, enhanced receptor internalization (43.5%±0.061 in GRK-transfected cells versus 31.7%±0.038 in vector-transfected cells, P<0.05), recycling in cells transfected with GRK5 and with empty vector was almost identical (Figure 6b).
Effect of GRKs on internalization of mutant β2-adrenoceptors
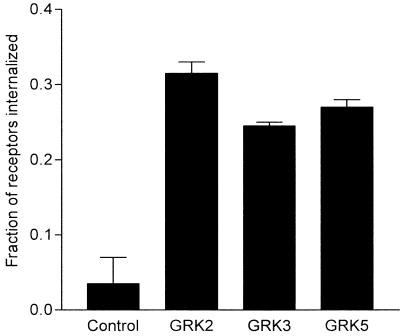
Since our results showed no or only modest effects of GRK5 overexpression on β2-adrenoceptor recycling and internalization, we sought to prove that the expression of the GRK5 plasmid used in these studies could alter some aspect of receptor trafficking. To this end, we determined if overexpressing GRK5 could enhance the internalization of a phosphorylation- and internalization-defective β2-adrenoceptor mutant (Y326A) in transiently cotransfected HEK293 cells. As has been previously shown (Menard et al., 1996), the overexpression of GRK2, GRK3, and GRK5 all significantly increased mutant receptor internalization (Figure 7).
Figure 7.
GRK overexpression and the internalization of mutant β2-adrenoceptors. The extent of receptor internalization was determined following 30 min of treatment with 10 μM isoprenaline in HEK293 cells transiently cotransfected with β2-adrenoceptor-Y326A and either vector, GRK2, GRK3, or GRK5. Results are means±s.d. of two independent experiments performed in triplicate.
GRK5 phosphorylation of β2-adrenoceptors
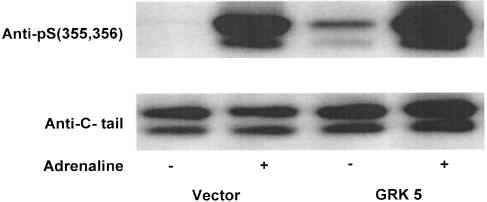
As a second control, we assessed the function of our GRK5 by measuring the effect of its overexpression on β2-adrenoceptor phosphorylation in HEK293 (Figure 8) and COS cells. While basal phosphorylation was minimal in HEK293 cells transiently expressing β2-adrenoceptors alone, a 5 min treatment with adrenaline (10 μM) induced a dramatic increase in phosphorylation of serines 355 and 356 in the receptor's cytoplasmic tail. In cells also transfected with GRK5, basal phosphorylation of serines 355 and 356 increased to approximately 33% of that seen with agonist stimulation. However, GRK5 overexpression did not further increase agonist-mediated receptor phosphorylation at these sites, suggesting that the endogenous levels of GRKs are adequate to saturate phosphorylation of serines 355 and 356 in this cell line. In COS cells, which have been reported to express lower levels of endogenous GRKs (Menard et al., 1997), overexpressing GRK5 had a more dramatic effect on basal phosphorylation of serines 355 and 356, which reached a comparable level to that observed with agonist stimulation (data not shown).
Figure 8.
GRK5 overexpression increases basal phosphorylation of β2-adrenoceptors in HEK293 cells. HEK293 cells transiently expressing β2-adrenoceptors alone (vector) or coexpressing GRK5 (GRK5) were exposed to vehicle (−) or 10 μM adrenaline (+) for 5 min and cellular extracts were probed with antiphosphoserines 355, 356 antibody. Results were normalized to total β2-adrenoceptor load as determined using an antibody directed against the terminal 15 amino acids of the receptor's cytoplasmic tail (anti-C-tail).
Discussion and conclusions
In this study, we used multiple techniques to more precisely define the role of GRK5 as a regulator of intracellular sorting of β2-adrenoceptors, given the central role of this process as a determinant of receptor activity. We transiently and inducibly overexpressed GRK5 in HEK293 cell lines stably expressing epitope-tagged β2-adrenoceptors and transiently coexpressed GRK5 and receptors using a high GRK5 : receptor cDNA molar ratio. Our data clearly show that the overexpression of GRK5 did not alter the recycling of intracellular receptors following both brief and prolonged exposures to agonist, nor did it alter receptor downregulation.
Previous work has identified serine 411 as part of a sorting signal comprised of the final four amino acids (DSLL) of the β2-adrenoceptor cytoplasmic tail and as a possible substrate for phosphorylation by GRK5 in vitro (Fredericks et al., 1996; Cao et al., 1999; Gage et al., 2001). It has been suggested that phosphorylation of serine 411 by GRK5 modulates β2-adrenoceptor recycling by disrupting receptor binding to EBP50. This protein then mediates the interaction of β2-adrenoceptors with the cortical actin cytoskeleton through the binding of an ERM protein, which is believed to be critical for the proper sorting of receptors to the recycling pathway (Cao et al., 1999; Hall et al., 1999). The conclusion that GRK5 regulates this process is based largely on the observation that β2-adrenoceptor recycling is impaired in cells transiently overexpressing GRK5, as assessed by immunofluorescence microscopy (Cao et al., 1999).
It is unclear why our results differ so strikingly from those previously reported, since both studies utilized HEK293 cells. Further, the GRK5 expression vector was obtained from the same source and clearly is functional as demonstrated both by its ability to stimulate receptor phoshorylation in HEK293 and COS cells and to increase the internalization of wild-type (Figure 6b) and mutant receptors (Figure 7) in HEK293 cells. It is possible that the differing results are due to variations in the HEK293 cell lines used in the two studies. Such a conclusion would require that the HEK293 cells used in this study expressed enough endogenous GRK5 to promote β2-adrenoceptor sorting to lysosomes fully, thus making overexpression irrelevant. However, in the current study, we used complementary methods to overexpress GRK5 in multiple independent HEK293 cell lines. Further, endogenous GRK5 was barely detectable in EcR293:β2-adrenoceptor cells (Figure 2) as assessed by Western blotting and undetectable in nontransfected HEK293 cells despite a 10-fold protein load (Figure 6a). Also, we assessed the endosome sorting of β2-adrenoceptors by quantitative radioligand binding following both the inducible and transient overexpression of GRK5, as well as by morphologic analysis. Despite these varied approaches, we were unable to observe any effect of GRK5 on receptor recycling.
However, our results indicate that overexpressing GRK5 can significantly enhance β2-adrenoceptor internalization, but only at very high expression levels relative to receptor. This finding is consistent with a recent report in which the extent of β2-adrenoceptor desensitization was approximately 20% greater in cells overexpressing GRK5 (Gray et al., 2001). However, in HEK293 cells, GRK2 normally is expressed at much higher levels as compared with GRK5, and it is likely that GRK2 is a more important determinant of β2-adrenoceptor desensitization under physiologic conditions (Menard et al., 1997).
We recently have shown that a serine cluster at positions 355, 356, and 364 is critical to rapid β2-adrenoceptor desensitization (Seibold et al., 2000). β2-adrenoceptors mutated at these sites are markedly impaired in their ability to undergo agonist-induced phosphorylation, desensitization, and endocytosis, indicating that these residues serve as in vivo substrates for GRK phosphorylation (Seibold et al., 2000). This study confirms that serines 355 and 356 are substrates for in vivo receptor phosphorylation by GRK5, as revealed by an increase in basal phosphorylation of these sites. We cannot exclude the possibility that other sites, such as serine 411, are also phosphorylated by GRK5 in vivo since in receptors in which serines 355, 356, and 364 are mutated to alanines, a small increase in receptor phosphorylation is observed following agonist treatment (Seibold et al., 2000). However, when β2-adrenoceptors in which all of the in vitro GRK5 sites (including serine 411) are mutated to alanine are expressed in vivo, mutant receptors undergo agonist-induced desensitization and phosphorylation similar to wild-type receptors (Seibold et al., 1998).
The role of the cytoplasmic DSLL domain as a signal for sorting endosomal β2-adrenoceptors is more established. Transplantation of this four amino-acid domain to the C-terminal tail of the nonrecycling δ opioid receptor creates a receptor chimera that is efficiently recycled (Gage et al., 2001). However, the mechanism by which this domain mediates receptor recycling is less clear. Both EBP50 and N-ethylmaleimide-sensitive factor (NSF) have been reported to interact with the β2-adrenoceptor via this DSLL region and regulate receptor recycling (Hall et al., 1998; Cao et al., 1999; Cong et al., 2001). Further, mutation of serine 411 to either aspartic acid, which may mimic the phosphorylated state, or to alanine disrupts receptor interactions with both EBP50 and NSF and causes a recycling defect (Cao et al., 1999; Cong et al., 2001). However, while mutation of leucine 412 to alanine also inhibits β2-adrenoceptor recycling, this mutation only disrupts receptor interaction with NSF, while the receptor – EBP50 interaction is not affected (Cong et al., 2001). Whichever mechanism regulates receptor recycling, our results clearly indicate that the endosome sorting of internalized β2-adrenoceptors is GRK5 independent.
Acknowledgments
This work was supported by National Institutes of Health Grant HL64934 (to R.H.M.) and HL50047 (B.J.K.). We wish to thank Dr Jeff Benovic (Philadelphia, PA, U.S.A.) for the gift of GRK2, GRK3, and GRK5 expression constructs.
Abbreviations
- AT
0.1 mM ascorbic acid and 1 mM thiourea
- DBM
n-dodecyl-β-D-maltoside
- EBP50
ezrin – radixin – moesin (ERM)-binding phosphoprotein-50
- GRK
G protein-coupled receptor kinase
- HEK
human embryonic kidney
- NHERF
Na+/H+ exchanger regulatory factor
- PBSS
phosphate-buffered saline with 1.2% sucrose
References
- CAO T.T., DEACON H.W., RECZEK D., BRETSCHER A., VON ZASTROW M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–289. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- CONG M., PERRY S.J., HU L.A., HANSON P.I., CLAING A., LEFKOWITZ R.J. Binding of the beta2 adrenergic receptor to N-ethylmaleimide-sensitive factor regulates receptor recycling. J. Biol. Chem. 2001;276:45145–45152. doi: 10.1074/jbc.M106087200. [DOI] [PubMed] [Google Scholar]
- FREDERICKS Z.L., PITCHER J.A., LEFKOWITZ R.J. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human β2-adrenergic receptor. J. Biol. Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- GAGE R.M., KIM K.A., CAO T.T., VON ZASTROW M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J. Biol. Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- GAGNON A.W., KALLAL L., BENOVIC J.L. Role of clathrin-mediated endocytosis in agonist-induced downregulation of the β2-adrenergic receptor. J. Biol. Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- GRAY J.A., SHEFFLER D.J., BHATNAGAR A., WOODS J.A., HUFEISEN S.J., BENOVIC J.L., ROTH B.L. Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine2A receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol. Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- HALL R.A., PREMONT R.T., CHOW C.-W., BLITZER J.T., PITCHER J.A., CLAING A., STOFFEL R.H., III, BARAK L.S., SHENOLIKAR S., WEINMAN E.J., GRINSTEIN S., LEFKOWITZ R.J. The β2-adrenergic receptor interacts with the Na+ – H+-exchanger regulatory factor to control N+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- HALL R.A., SPURNEY R.F., PREMONT R.T., RAHMAN N., BLITZER J.T., PITCHER J.A., LEFKOWITZ R.J. G protein-coupled receptor kinase 6A phosphorylates the Na+ – H+exchanger regulatory factor via a PDZ domain-mediated interaction. J. Biol. Chem. 1999;274:24328–24334. doi: 10.1074/jbc.274.34.24328. [DOI] [PubMed] [Google Scholar]
- KRUEGER K.M., DAAKA Y., PITCHER J.A., LEFKOWITZ R.J. The role of sequestration in G protein-coupled receptor resensitization. Regulation of β2-adrenergic dephosphorylation by vesicular acidification. J. Biol. Chem. 1997;272:5–8. doi: 10.1074/jbc.272.1.5. [DOI] [PubMed] [Google Scholar]
- KRUPNICK J.G., BENOVIC J.L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. G protein-coupled receptor kinases. Cell. 1993;74:409–412. doi: 10.1016/0092-8674(93)80042-d. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. G protein-coupled receptors III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- MENARD L., FERGUSON S.S., BARAK L.S., BERTRAND L., PREMONT R.T., COLAPIETRO A.M., LEFKOWITZ R.J., CARON M.G. Members of the G protein-coupled receptor kinase family that phosphorylate the beta2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35:4155–4160. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- MENARD L., FERGUSON S.S., ZHANG J., LIN F.T., LEFKOWITZ R.J., CARON M.G., BARAK L.S. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol. Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- MOORE R.H., HALL H.S., ROSENFELD J.L., DAI W., KNOLL B.J. Specific changes in β2-adrenoceptor trafficking kinetics and intracellular sorting during downregulation. Eur. J. Pharm. 1999a;369:113–123. doi: 10.1016/s0014-2999(99)00055-2. [DOI] [PubMed] [Google Scholar]
- MOORE R.H., TUFFAHA A., MILLMAN E.E., HALL H.S., DAI W., DICKEY B.F., KNOLL B.J. Agonist-induced sorting of human β2-adrenergic receptors to lysosomes during downregulation. J. Cell Sci. 1999b;112:329–338. doi: 10.1242/jcs.112.3.329. [DOI] [PubMed] [Google Scholar]
- MORRISON K.J., MOORE R.H., CARSRUD N.D.V., MILLMAN E.E., TRIAL J., CLARK R.B., BARBER R., TUVIM M., DICKEY B.F., KNOLL B.J. Repetitive endocytosis and recycling of the β2-adrenergic receptor during agonist-induced steady-state redistribution. Mol. Pharmacol. 1996;50:692–699. [PubMed] [Google Scholar]
- NO D., YAO T.P., EVANS R.M. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3346–3351. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN L., GUREVICH E.V., GUREVICH V.V. The nature of the arrestin × receptor complex determines the ultimate fate of the internalized receptor. J. Biol. Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- RECZEK D., BERRYMAN M., BRETSCHER A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin – radixin – moesin family. J. Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENFELD J.L., MOORE R.H., ZIMMER K.-P., ALPIZAR-FOSTER E., DAI W., ZARKA M.N., KNOLL B.J. Lysosome proteins are redistributed during expression of a GTP-hydrolysis defective rab5a. J. Cell Sci. 2001;114:4499–4508. doi: 10.1242/jcs.114.24.4499. [DOI] [PubMed] [Google Scholar]
- SEACHRIST J.L., ANBORGH P.H., FERGUSON S.S. Beta 2-adrenergic receptor internalization, endosomal sorting, and plasma membrane recycling are regulated by rab GTPases. J. Biol. Chem. 2000;275:27221–27228. doi: 10.1074/jbc.M003657200. [DOI] [PubMed] [Google Scholar]
- SEIBOLD A., JANUARY B.G., FRIEDMAN J., HIPKIN R.W., CLARK R.B. Desensitization of β2-adrenergic receptors with mutations of the proposed G protein coupled receptor kinase phosphorylation sites. J. Biol. Chem. 1998;273:7637–7642. doi: 10.1074/jbc.273.13.7637. [DOI] [PubMed] [Google Scholar]
- SEIBOLD A., WILLIAMS B., HUANG Z.F., FRIEDMAN J., MOORE R.H., KNOLL B.J., CLARK R.B. Localization of the sites mediating desensitization of the beta(2)-adrenergic receptor by the GRK pathway. Mol. Pharmacol. 2000;58:1162–1173. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- YU S.S., LEFKOWITZ R.J., HAUSDORFF W.P. Adrenergic receptor sequestration – a potential mechanism of receptor resensitization. J. Biol. Chem. 1993;268:337–341. [PubMed] [Google Scholar]