Abstract
Recently, environmental chemicals have appeared in daily human life, and these chemicals have been incidentally taken in by humans. The serum concentrations of some of these chemicals have been found to be associated with the onset and incidence rate of diabetes mellitus. It has been suggested that one of the environmental chemicals, bisphenol A (BPA), has hormone-like activity. It has also been demonstrated that some hormones affect insulin resistance and fat distribution in the body. To study the effects of these environmental chemicals on glucose metabolism, the effect of BPA on glucose transport in mouse 3T3-F442A adipocytes was investigated. The 3T3-F442A adipocytes were incubated with various concentrations of BPA in a medium. Deoxyglucose uptake assay was performed with and without insulin. Immunoblot analysis was performed with a glucose transporter (GLUT) 4-specific antibody and antiphosphotyrosine antibody. The BPA treatment enhanced basal and insulin-stimulated glucose uptake, and caused an increased amount of GLUT4 protein. Thus, the enhanced glucose uptake resulting from the BPA treatment was at least partially due to the increased amount of GLUT4. Tyrosine phosphorylation of insulin receptor substrate-1 with insulin stimulation was not significantly affected. In conclusion, it was demonstrated that BPA, one of the chemicals that we intake incidentally, affects the glucose transport in adipocytes, and also that the environmental chemicals may be identified as one of the environmental factors that affect diabetes and obesity.
Keywords: Bisphenol A, adipocyte, diabetes mellitus, environmental chemicals, glucose metabolism, glucose transport, glucose transporter 4, mouse 3T3-F442A cell, obesity
Introduction
Type II diabetes mellitus is a phenotypically and genetically heterogeneous disorder. This disease results from defects in insulin secretion and insulin action on its target tissues, such as muscles, adipose tissue, and the liver (Weir & Leahy, 1994). The development of type II diabetes is strongly influenced by genetic and environmental factors. Many environmental factors are described, such as the over-intake of food and drinks, a sedentary life, and obesity (Weir & Leahy, 1994).
Recently, there has been an increased exposure to environmental chemicals in daily human life and these chemicals have incidentally been taken in by humans. The serum concentrations of some of these chemicals have been found to be associated with the onset and incidence rate of diabetes (Longnecker & Michalek, 2000).
Some industrial chemicals are reported to affect animal physiology by mimicking the effects of endogenous hormones (Sonnenschein & Soto, 1998). Several of these compounds have been shown to have estrogenic activity in in vitro and in vivo bioassays (Krishnan et al., 1993; Soto et al., 1995; Ashby & Tinwell, 1998). One of those potential endocrine-disrupting chemicals, bisphenol A (BPA), is used to manufacture polycarbonate resin, and is detected in the coatings of food and beverage containers (Brotons et al., 1995), as well as in materials commonly used in dentistry (Olea et al., 1996). Exposure to BPA has been reported to accelerate puberty in female rats (Ashby & Tinwell, 1998) and produce constant estrus in adult ovariectomized rats, suggesting that BPA has estrogenic activity (Sonnenschein & Soto, 1998). It has also been demonstrated that estrogens affect insulin resistance and fat distribution in the body (Faustini-Fustini et al., 1999; Collison et al., 2000; Heine et al., 2000). However, it is not known whether BPA affects insulin action or glucose metabolism. In this study, the effects of BPA on glucose transport in mouse 3T3-F442A adipocytes were investigated.
Methods
Materials
BPA was purchased from Wako Pure Chemical Industries (Osaka, Japan). Its purity was no less than 99.0%. Estradiol and ICI 182,780 were purchased from Sigma Chemical (St Louis, MO, U.S.A.) and Tocris Cookson Limited (Avonmouth, U.K.), respectively. A monoclonal anti-glucose transporter (GLUT) 4 antibody was obtained from Genzyme-TECHNE (Minneapolis, MN, U.S.A.). An antiphosphotyrosine monoclonal antibody and anti-insulin receptor substrate (IRS)-1 antibody were obtained from Upstate Biotechnology (Lake Placid, NY, U.S.A.). [3H-]deoxy-D-glucose was purchased from NEN Life Science Products (Boston, MA, U.S.A.). ECL™ Western blotting detection reagent, an enhanced chemiluminescence reagent, was obtained from Amersham Pharmacia Biotech (Uppsala, Sweden). All other routinely used reagents were of analytical grade and were purchased from Sigma Chemical (St Louis, MO, U.S.A.) or Wako Pure Chemical Industries (Osaka, Japan).
Cell culture
The 3T3-F442A cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 μg ml−1) in a 5% humidified CO2 atmosphere at 37°C. The cells were differentiated in this medium with 5 μg ml−1 insulin (Saad et al., 1994) and used between 12 and 14 days after differentiation. The cells had acquired the characteristic adipocyte morphology before use. The cells were also incubated in a medium without insulin for 2 days before BPA exposure. Then, the cells were incubated with the indicated concentrations of BPA or estradiol with or without ICI 182,780 in a medium for 24 h in six multiwell plates. Before deoxyglucose uptake assay, the cells were washed with serum-free DMEM, and then incubated in serum-free DMEM with or without BPA or other compounds for 2 h.
Deoxyglucose uptake assay
Deoxyglucose uptake assay was performed as described previously (Frost & Lane, 1985), with some modifications. The cell monolayers cultured in six multiwell plates were rapidly washed three times with Krebs–Ringer phosphate buffer (KRP) at pH 7.4 (128 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 1.25 mM MgSO4, 5.0 mM Na2HPO4). The cells were incubated at 37°C in KRP for up to 10 min, at which time insulin (100 nM) was added. After 10 min, glucose uptake was initiated by the addition of 0.2 mM [3H-]-2-deoxy-D-glucose (92.5 kBq ml−1) for 10 min. The uptake was terminated by the addition of cold KRP with 100 μM phloretin, and then the cells were washed, using KRP with 100 μM phloretin. The washed cells were dissolved in 0.1% sodium dodecyl sulfate (SDS), and radioactivity was measured by a liquid scintillation counter. Nonspecific binding of [3H-]-2-deoxy-D-glucose was detected by means of incubation with 100 μM phloretin.
Immunoblotting of GLUT4
Protein extraction and immunoblot analysis were performed according to a method reported previously (Toyoda et al., 1999), with some modifications. The 3T3-F442A adipocytes grown in six multiwell plates were incubated in a medium with indicated concentrations of BPA with or without ICI 182,780 for 24 h. After this incubation, the cells were homogenized and dissolved in a buffer containing 42 mM HEPES, 150 mM NaCl, 5 mM EDTA, 0.5% Triton-X 100, 0.3 mg ml−1 phenylmethylsulfonyl fluoride (PMSF), 2 mM Na3VO4, 10 μg ml−1 aprotinin, 10 μg ml−1 leupeptin, 10 μg ml−1 pepstatin, pH 7.6, and rotated for 1 h at 4°C. The lysates were centrifuged to remove the insoluble materials. The supernatants (25 μg of protein) were then separated by 5–20% linear gradient gel SDS–polyacrylamide gel electrophoresis (PAGE), and transferred onto nitrocellulose membranes. The membranes were blocked in a buffer containing 10 mM Tris-HCl, 150 mM NaCl, 0.2% NP-40, 5% nonfat milk and pH 7.5 for 2 h at room temperature. The membranes were then probed with an anti-GLUT4 antibody for 16 h at 4°C. After the membranes were washed, the blots were incubated with a horseradish peroxidase-linked second antibody, followed by an enhanced chemiluminescence detection using an ECL™ reagent, according to the manufacturer's instructions. The intensity of the bands was quantified by LAS1000 (Fuji Film Co., Fuji, Japan).
Tyrosine phosphrylation of IRS-1
The 3T3-F442A adipocytes grown in six multiwell plates were incubated in a medium with indicated concentrations of BPA for 24 h. After this incubation, the cells were washed with serum-free DMEM, and incubated in serum-free DMEM with BPA for 2 h. Then, 100 nM insulin was added to each well. After 5 min, the cells were homogenized and dissolved for immunoprecipitation with an anti-IRS-1 antibody, followed by immunoblotting with the antiphosphotyrosine antibody. An amount of 250 μg of protein was used for immunoprecipitation. The membrane was reprobed by an anti-IRS-1 antibody. The intensity of the bands was measured by LAS1000 (Fuji Film). The ratio of intensities between an antiphosphotyrosine antibody image in insulin stimulation and an anti-IRS-1 antibody image was used for the analysis of tyrosine phosphorylation of IRS-1.
Statistical analysis
Data were expressed as mean (±s.e.m.). The pairwise differences were computed by Student's t-test. StatView-J version 5.0 (SAS Institute Inc., Cary, NC, U.S.A.) was used for the statistical analysis.
Results
Deoxyglucose uptake
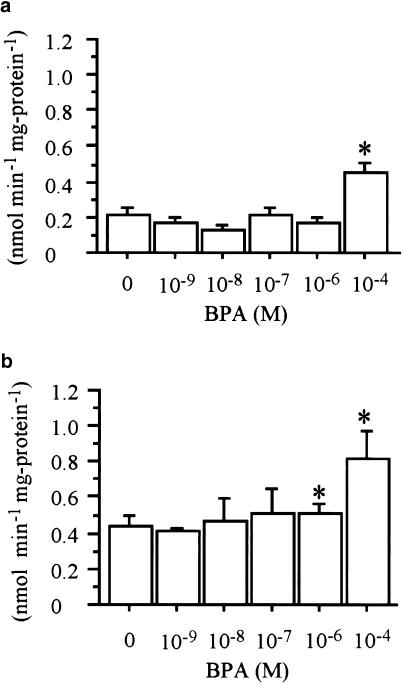
Differentiated mouse 3T3-F442A adipocytes were used to mimic the adipocyte function in a controlled environment, in order to determine whether BPA has any effects on glucose transport in adipocytes. We set out to examine the effect of BPA on glucose uptake as a model system. The cells were incubated with various concentrations of BPA or a vehicle (ethanol) for 24 h. Then, basal glucose uptake was assessed as described in the Methods section. As shown in Figure 1a, 10−4 M of BPA treatment significantly enhanced basal glucose uptake (0.22±0.031 vs 0.45±0.056 nmol min−1 mg protein−1, P<0.05, control vs 10−4 M). There was no change in morphology in the cells during this treatment. Next, the insulin-stimulated glucose uptake was examined (Figure 1b). It was found that the insulin-stimulated glucose uptake also increased due to the BPA treatment. Both 10−6 and 10−4 M of BPA significantly increased the insulin-stimulated glucose uptake (0.44±0.050 vs 0.51±0.059, 0.82±0.16 nmol min−1 mg protein−1, P<0.05, control vs 10−6, 10−4 M). These data show that BPA enhances basal and insulin-stimulated glucose uptake.
Figure 1.
Effect of BPA on 2-deoxy-D-glucose transport by 3T3-F442A adipocytes. (a, b) show [3H]-2-deoxy-glucose uptake in the absence (a) or presence (b) of 100 nM insulin. Treatment of 10−4 M BPA for 24 h caused a significant increase of 2-deoxy-D-glucose transport in the absence of 100 nM insulin. Treatment of 10−6 and 10−4 M BPA for 24 h caused a significant increase of 2-deoxy-D-glucose transport in the presence of 100 nM insulin. The values are means±s.e.m. from three or four independent experiments. *Denotes P<0.05 vs control (0 M BPA), using paired t-test.
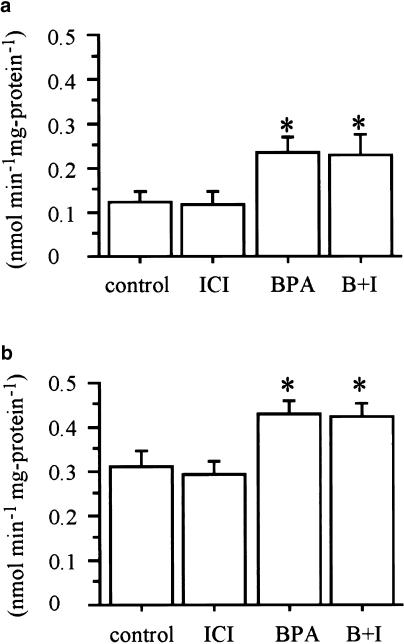
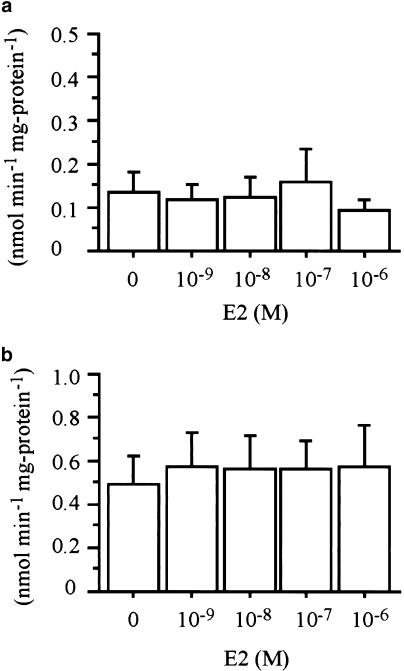
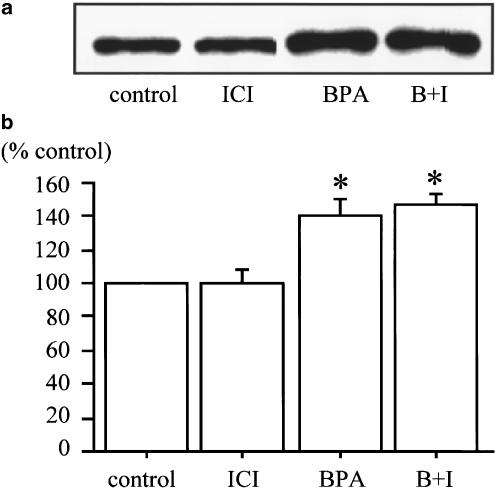
To determine whether the effects of BPA on glucose uptake are mediated by estrogen receptor, 3T3-F442A adipocytes were incubated with 10−4 M of BPA and 10−6 M of ICI 182,780 before assays. Although ICI 182,780 is one of the estrogen receptor inhibitors, ICI 182,780 could not inhibit the effects of BPA on deoxyglucose uptake (Figure 2). Estradiol also did not show significant effects on deoxyglucose uptake (Figure 3).
Figure 2.
Effect of estrogen receptor antagonist on increased 2-deoxy-D-glucose transport induced by BPA. Treatment of 10−4 M BPA (BPA), 10−6 M ICI 182,780 (ICI) and their combination (B+I) for 24 h was performed. ICI 182,780 could not inhibit the effect of BPA on 2-deoxy-D-glucose transport in the absence (a) or presence (b) of 100 nM insulin. Values are means±s.e.m. from three or four independent experiments. *Denotes P<0.05 vs control (0 M BPA), using paired t-test.
Figure 3.
Effect of estradiol (E2) on increased 2-deoxy-D-glucose transport. Treatment of indicated concentrations of E2 did not affect 2-deoxy-D-glucose transport in the absence (a) or presence (b) of 100 nM insulin. Values are means±s.e.m. from three or four independent experiments.
Effect on GLUT4 protein
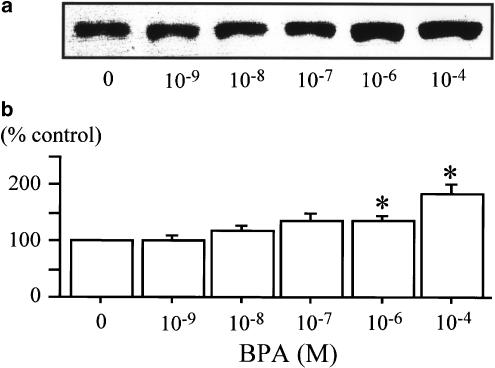
To determine whether the enhancement of GLUT activity was due to an increase in the transporter number, immunoblot analysis (Figure 4a) was performed using a GLUT4-specific antibody. The treatment with 10−6 and 10−4 M BPA caused an increased amount of GLUT4 protein significantly (Figure 4b). Thus, the enhanced glucose uptake resulting from the BPA treatment was at least partially due to the increased amount of GLUT4.
Figure 4.
GLUT4 protein levels after incubation with BPA in 3T3-F442A adipocytes. Cells were treated with BPA, as described in Methods. Treatment of 10−6 and 10−4 M BPA caused a significant increase of GLUT4 protein in 3T3-F442A adipocytes. (a) Immunoblot analysis with a typical experiment of five independent experiments. (b) Chemiluminescent intensity of GLUT4 measured by LAS 1000. Data are represented as a percentage of the control (vehicle), and are expressed as mean±s.e.m. from five independent experiments. *Denotes P<0.05 vs control, using paired t-test.
Incubation with ICI 182,780 did not have any significant effect on increase of GLUT4 protein by BPA (Figure 5).
Figure 5.
Effect of estrogen receptor antagonist on increased GLUT4 expression induced by BPA. Cells were treated with 10−4 M BPA (BPA), 10−6 M ICI 182,780 (ICI) and their combination (B+I), as described in Methods. ICI 182,780 could not inhibit the increased expression of GLUT4 expression induced by BPA. Chemiluminescent intensity measured by LAS 1000 was used for data calculation. (a) Immunoblot analysis with a typical experiment of three independent experiments. (b) Chemiluminescent intensity of GLUT4 measured by LAS 1000. Data are represented as a percentage of the control (vehicle), and are expressed as mean±s.e.m. from three independent experiments. *Denotes P<0.05 vs control, using paired t-test.
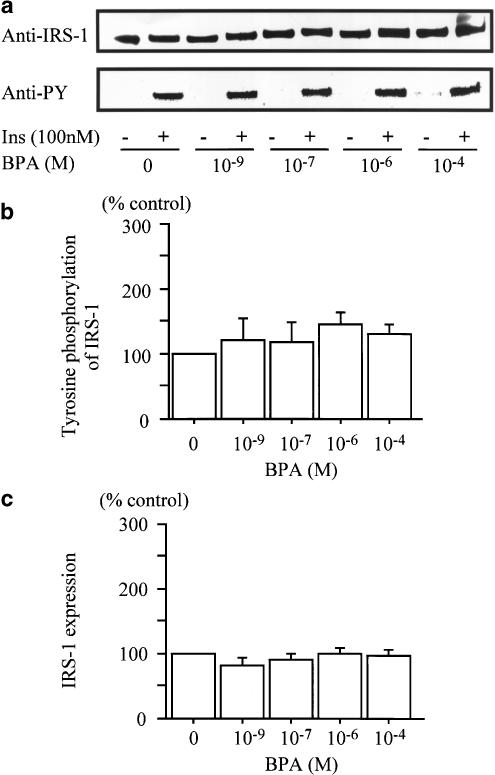
Tyrosine phosphorylation of IRS-1
To examine the effects of BPA on the insulin signal transduction, tyrosine phosphorylation of IRS-1 induced by insulin was investigated. As shown in Figure 6, each concentration of BPA did not significantly affect the insulin-induced tyrosine phosphorylation of IRS-1 (Figure 6b). The IRS-1 expression level also did not show significant changes with BPA treatment (Figure 6c).
Figure 6.
Effect of BPA on the tyrosine phosphorylation of IRS-1. Each concentration of BPA had no significant effect on insulin-stimulated IRS-1 phosphorylation and IRS-1 expression. (a) Immunoblot analysis with a typical experiment of four independent experiments. (b) Chemiluminescent intensity measured by LAS 1000. Data are calculated as ratios of anti-PY with insulin stimulation/anti-IRS-1 intensity, and are represented as a percentage of the control (vehicle). (c) Chemiluminescent intensity of IRS-1 measured by LAS 1000. Data are represented as a percentage of the control (vehicle), and are expressed as the mean±s.e.m. from three independent experiments.
Discussion
BPA was detected in the serum of a man who had been leading an ordinary life, at a concentration level of 0–0.16 ng ml−1 (Sajiki et al., 1999). Although the concentration level in that report was low compared with the one in our study, the authors did not mention about the exposure condition of the subjects to BPA and the concentration level of BPA in adipose tissue as well. It has been reported that orally administrated BPA arrived at the adipose tissue and was detected in a nonconjugated form (Snyder et al., 2000). However, the direct effects of BPA on glucose metabolism in adipose tissue have not yet been clarified.
In this study, the effects of BPA on 3T3-F442A adipocytes in cultures resulted in an increased basal- and insulin-stimulated glucose transport. An examination of the effects of BPA on GLUT4 protein expression was attempted and the results suggested that enhanced glucose uptake may be caused by an increased amount of GLUT4 protein. BPA has estrogenic activity (Sonnenschein & Soto, 1998), and the expression of the estrogen receptor alpha and beta was identified in 3T3-F442A adipocytes by reverse transcription PCR (RT–PCR) (data not shown). It is possible that the effect of BPA is brought about via estrogen receptors. However, high doses of estrogens reportedly reduced the mRNA of GLUT4 (Sugaya et al., 2000), and the stimulative effect of glucose uptake by BPA cannot be fully explained by the binding of BPA to the estrogen receptors. Moreover, in this study, biologically effective concentration of estradiol (Tremblay et al., 1998; Sugaya et al., 2000; Jensen et al., 2003) did not affect glucose transport. Although 10−6 M of ICI 182,780 had been reported to inhibit the estrogen receptor signal (Tremblay et al., 1998; Jensen et al., 2003), it had no significant inhibitory effects on the increase of glucose transport with and without insulin stimulation, caused by BPA in 3T3-F442A adipocytes. These results suggested that the effects of BPA on glucose transport were not mediated by estrogen receptor directly. ICI 182,780 showed no significant inhibition on the increase of GLUT4 protein expression caused by BPA in 3T3-F442A adipocytes, and it was suggested that the upregulation of GLUT4 protein by BPA was not mediated by estrogen receptor directly. The other possibility is that BPA binds to other nuclear receptors, such as human steroid and xenobiotic receptor (Takeshita et al., 2001). Some other factors may mediate these results caused by the BPA treatment. It was reported that the BPA treatment changes the expression of an orphan receptor NR4A1 in a Leydig tumor cell line (Song et al., 2002). It is possible that an increased amount of GLUT4 may be caused by the change of such nuclear receptor expressions and activities induced by BPA.
Insulin signaling involves a complex array of molecules that function in a tightly regulated fashion both spatially and temporally (Saltiel, 1996; Inoue et al., 1998). The activated insulin receptor phosphorylates several target proteins, notably IRS-1, which function as docking sites for the integration of subsequent cellular responses (Saltiel, 1996; Inoue et al., 1998). IRS-1 is a key molecule for the insulin signal pathway to GLUT4 translocation (Hill et al., 1999). An investigation on whether tyrosine phosphorylation of IRS-1 was changed by the BPA treatment was conducted. The results showed that the BPA treatment might not have a significant effect on tyrosine phosphorylation of IRS-1 induced by insulin. It is suggested that an increased insulin-stimulated glucose uptake by the BPA treatment could not be caused by the upregulation of insulin signaling through IRS-1. In the study of GLUT4 transgenic mice, an increased amount of GLUT4 causes increased basal- and insulin-stimulated glucose uptake in adipocytes (Deems et al., 1994). Therefore, an increased amount of GLUT4 caused by the BPA treatment could increase glucose uptake in adipocytes, without changing the tyrosine phosphorylation of IRS-1.
It was described that the GLUT4 expression increases in adipocyte differentiation (Macdougald & Lane, 1995). Recently, it was reported that BPA accelerates the conversion of 3T3-L1 fibroblasts to adipocytes (Masuno et al., 2002). In that paper (Masuno et al., 2002), 3T3-L1 preadipocytes were used and demonstrated that BPA in combination with insulin accelerates the differentiation. One of the derivatives of BPA, BPA diglycidyl ether (BADGE) affects the adipocyte differentiation through peroxisome proliferator-activated receptor-γ (PPAR-γ) (Wright et al., 2000). BPA may have an influence on the differentiation of 3T3-F442A cells.
BPA is one of the constituent substances used to coat food and beverage containers (Brotons et al., 1995), and the results of this experiment suggest that environmental chemicals which are taken into the human body continuously with food or drinks may influence the glucose metabolism of differentiated adipocytes. In conclusion, BPA, one of the chemicals which we intake constantly, affects glucose transport in matured adipocytes, and that these environmental chemicals may be identified as one of the environmental factors which affect diabetes and obesity.
Acknowledgments
This work was supported by a special coordination of funds for the Grant-in-Aid for Scientific Research on Priority Areas (A) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- BPA
bisphenol A
- DMEM
Dulbecco's modified Eagle's medium
- GLUT
glucose transporter
- IRS-1
insulin receptor substrate-1
- KRP
Krebs–Ringer phosphate buffer
- PMSF
phenylmethylsulfonyl fluoride
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
References
- ASHBY J., TINWELL H. Uterotrophic activity of bisphenol A in the immature rat. Environ. Health Perspect. 1998;106:719–720. doi: 10.1289/ehp.98106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROTONS J.A., OLEA-SERRANO M.F., VILLALOBOS M., PEDRAZA V., OLEA N. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 1995;103:608–612. doi: 10.1289/ehp.95103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLISON M., CAMPBELL I.W., SALT I.P., DOMINICZAK A.F., CONNELL J.M., LYALL H., GOULD G.W. Sex hormones induce insulin resistance in 3T3-L1 adipocytes by reducing cellular content of IRS proteins. Diabetologia. 2000;43:1374–1380. doi: 10.1007/s001250051541. [DOI] [PubMed] [Google Scholar]
- DEEMS R.O., EVANS J.L., DEACON R.W., HONER C.M., CHU D.T., BURKI K., FILLERS W.S., COHEN D.K., YOUNG D.A. Expression of human GLUT4 in mice results in increased insulin action. Diabetologia. 1994;37:1097–1104. doi: 10.1007/BF00418373. [DOI] [PubMed] [Google Scholar]
- FAUSTINI-FUSTINI M., ROCHIRA V., CARANI C. Oestrogen deficiency in men: where are we today. Eur. J. Endocrinol. 1999;140:111–129. doi: 10.1530/eje.0.1400111. [DOI] [PubMed] [Google Scholar]
- FROST S.C., LANE M.D. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3-L1 adipocytes. J. Biol. Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- HEINE P.A., TAYLOR J.A., IWAMOTO G.A., LUBAHN D.B., COOKE P.S. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILL M.M., CLARK S.F., TUCKER D.F., BIRNBAUM M.J., JAMES D.E., MACAULAY S.L. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell. Biol. 1999;19:7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE G., CHEATHAM B., EMKEY R., KAHN C.R. Dynamics of insulin signaling in 3T3-L1 adipocytes. Differential compartmentalization and trafficking of insulin receptor substrate (IRS)-1 and IRS-2. J. Biol. Chem. 1998;273:11548–11555. doi: 10.1074/jbc.273.19.11548. [DOI] [PubMed] [Google Scholar]
- JENSEN J.J., KITLEN J.W., BRIAND P., LABRIE F., LYKKESFELDT A.E. Effect of antiestrogens and aromatase inhibitor on basal growth of the human breast cancer cell line MCF-7 in serum-free medium. Steroid Biochem. Mol. Biol. 2003;84:469–478. doi: 10.1016/s0960-0760(03)00068-2. [DOI] [PubMed] [Google Scholar]
- KRISHNAN A.V., STATHIS P., PERMUTH S.F., TOKES L., FELDMAN D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- LONGNECKER M.P., MICHALEK J.E. Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology. 2000;11:44–48. doi: 10.1097/00001648-200001000-00010. [DOI] [PubMed] [Google Scholar]
- MACDOUGALD O.A., LANE M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- MASUNO H., KIDANI T., SEKIYA K., SAKAYAMA K., SHIOSAKA T., YAMAMOTO H., HONDA K. Bisphenol A in combination with insulin can accelerate the conversion of 3T3-L1 fibroblasts to adipocytes. J. Lipid Res. 2002;43:676–684. [PubMed] [Google Scholar]
- OLEA N., PULGAR R., PEREZ P., OLEA-SERRANO F., RIVAS A., NOVILLO-FERTRELL A., PEDRAZA V., SOTO A.M., SONNENSCHEIN C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996;104:298–305. doi: 10.1289/ehp.96104298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAAD M.J.A., FOLLI F., ARAKI E., HASHIMOTO N., CSERMELY P., KAHN C.R. Regulation of insulin receptor, insulin receptor substrate-1 and phosphatidylinosotol 3-kinase in 3T3-F442A adipocytes. Effects of differentiation, insulin, and dexamethasone. Mol. Endocrinol. 1994;8:545–557. doi: 10.1210/mend.8.5.7520127. [DOI] [PubMed] [Google Scholar]
- SAJIKI J., TAKAHASHI K., YONEKUBO J. Sensitive method for the determination of bisphenol-A in serum using two systems of high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1999;736:255–261. doi: 10.1016/s0378-4347(99)00471-5. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R. Diverse signaling pathways in the cellular actions of insulin. Am. J. Physiol. 1996;270:E375–E385. doi: 10.1152/ajpendo.1996.270.3.E375. [DOI] [PubMed] [Google Scholar]
- SNYDER R.W., MANESS S.C., GAIDO K.W., WELSCH F., SUMNER S.C.J., FENNELL T.R. Metabolism and disposition of bisphenol A in female rats. Toxicol. Appl. Pharmacol. 2000;168:225–234. doi: 10.1006/taap.2000.9051. [DOI] [PubMed] [Google Scholar]
- SONG K.H., LEE K., CHOI H.S. Endocrine disrupter bisphenol a induces orphan nuclear receptor Nur77 gene expression and steroidogenesis in mouse testicular Leydig cells. Endocrinology. 2002;143:2208–2215. doi: 10.1210/endo.143.6.8847. [DOI] [PubMed] [Google Scholar]
- SONNENSCHEIN C., SOTO A.M. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. Mol. Biol. 1998;65:143–150. doi: 10.1016/s0960-0760(98)00027-2. [DOI] [PubMed] [Google Scholar]
- SOTO A.M., SONNENSCHEIN C., CHUNG K.L., FERNANDEZ M.F., OLEA N., SERRANO F.O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ. Health Perspect. 1995;103 Suppl 7:113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGAYA A., SUGIYAMA T., YANASE S., SHEN X.X., MINOURA H., TOYODA N. Expression of glucose transporter 4 mRNA in adipose tissue and skeletal muscle of ovariectomized rats treated with sex steroid hormones. Life Sci. 2000;66:641–648. doi: 10.1016/s0024-3205(99)00636-0. [DOI] [PubMed] [Google Scholar]
- TAKESHITA A., KOIBUCHI N., OKA J., TAGUCHI M., SHISHIBA Y., OZAWA Y. Bisphenol-A, an environmental estrogen, activates the human orphan nuclear receptor, steroid and xenobiotic receptor-mediated transcription. Eur. J. Endocrinol. 2001;145:513–517. doi: 10.1530/eje.0.1450513. [DOI] [PubMed] [Google Scholar]
- TOYODA M., HASHIMOTO N., TOKITA K., GOLDSTEIN B.J., YOKOSUKA O., KANATSUKA A., SUZUKI Y., SAITO Y. Increased activity and expression of MAP kinase in HCC model rats induced by 3′-methyl-4-dimethylamino-azobenzene. J. Hepatol. 1999;31:725–733. doi: 10.1016/s0168-8278(99)80354-7. [DOI] [PubMed] [Google Scholar]
- TREMBLAY A., TREMBLAY G.B., LABRIE C., LABRIE F., GIGUERE V. EM-800, a novel antiestrogen, acts as a pure antagonist of the transcriptional functions of estrogen receptors α and β. Endocrinology. 1998;139:111–118. doi: 10.1210/endo.139.1.5702. [DOI] [PubMed] [Google Scholar]
- WEIR G.C., LEAHY J.L.Pathogenesis of non-insulin-dependent (type II) diabetes mellitus Joslin's Diabetes Mellitus 1994Media, PA, U.S.A.: Lippincott Williams & Wilkins; 240–264.ed. Kahn, C.R. & Weir, G.C., pp [Google Scholar]
- WRIGHT H.M., CLISH C.B., MIKAMI T., HAUSER S., YANAGI K., HIRAMATSU R., SERHAN C.N., SPIEGELMAN B.M. A synthetic antagonist for the peroxisome proliferator-activated receptor gamma inhibits adipocyte differentiation. J. Biol. Chem. 2000;275:1873–1877. doi: 10.1074/jbc.275.3.1873. [DOI] [PubMed] [Google Scholar]