Abstract
Platelet-activating factor (PAF) is known to stimulate a variety of neutrophil activities, including chemotaxis, phagocytosis, degranulation, reactive oxygen species production and intracellular pH increase. The purpose of this study was to investigate the effect of PAF on pH(i), specifically if these changes in pH are the result of phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK) pathway activation in bovine neutrophils.
PAF caused intracellular alkalinization in 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester-loaded bovine neutrophils. This phenomenon seems to be mediated by amiloride-sensitive Na+/H+ exchange, and is inhibited by WEB2086 (a selective PAF receptor antagonist), genistein (a tyrosine kinase inhibitor), wortmannin and LY294002 (PI3K inhibitors), and PD98059 and UO126 (MEK inhibitors).
PAF 100 nM induced an increase in tyrosine phosphorylation of proteins 62, 44 and 21 kDa with a maximum response at 2 min of incubation.
Unlike human neutrophils, bovine neutrophils are strongly stimulated by PAF via phosphorylation of ERK1/2 (extracellular-signal-regulated protein kinase) with an EC50 of 30 and 13 nM, respectively.
PAF MAPK activation was also inhibited by WEB2086, pertussis toxin (PTX), genistein, wortmannin, LY294002, PD98059 and UO126 in bovine neutrophils. The ERK1/2 activation is dependent on PI3K pathway, because protein kinase B was phosphorylated by PAF and inhibited by wortmannin and LY294002, but not by U0126.
Our results suggest that PAF induces intracellular alkalinization via PI3K–MAPK activation. This effect is upstream regulated by PAF receptor, PTX-sensitive G protein, tyrosine kinase, PI3K and MEK1/2 in bovine neutrophils.
Keywords: Na+/H+ exchanger, intracellular alkalinization, PAF, MAPK, PI3K
Introduction
Neutrophils play an essential role in host defense and represent one of the first lines of protection against potentially harmful microorganisms (Smith, 1994). Their repertoire of defense mechanism is complex, including phagocytosis as well as the production of reactive oxygen species, proteolytic enzymes and bactericidal peptides (Smith, 1994). Activation and regulation of neutrophil function is equally complex, involving an interplay of cytokines, adhesion molecules and chemoattractants (Bokoch, 1995).
A potent chemotactic factor and mediator of neutrophil functions is platelet-activating factor (PAF), a widely known biologically active phospholipid (1-0-alkyl-2(R) acetyl-glyceryl-3-phosphorylcholine). PAF is liberated by platelets, leukocytes and smooth muscle cells by the rupture of lipid precursors from the plasma membrane (Prescott et al., 2000). PAF participates in many pathological conditions such as inflammation, allergies, anaphylaxis and endotoxic shock (Ishii & Shimizu, 2000). PAF is also known to cause changes in pH in the microenvironments inside and outside the polymorphonuclear cells (PMN) (Naccache et al., 1986; Gronert et al., 1998). In particular, intracellular alkalinization has been linked to chemotaxis, degranulation and generation of free radicals (Simchowitz, 1985c; Simchowitz & Cragoe, 1986b; Wright et al., 1988; Gewirtz et al., 1998) and is NADPH oxidase-dependent (Grinstein et al., 1986). A number of chemotactic factors can activate an amiloride-sensitive Na+/H+ exchange (NHE), such as N-formylmethionine-leucyl-phenylalanine (fMLP), LTB4 and C5a (Simchowitz, 1985b; Osaki et al., 1989). Moreover, NHE blockers have been demonstrated to reduce PMN migration (Simchowitz & Cragoe, 1986a, 1986b). However, the cellular processes that link chemoattractant cell signaling pathways to cation exchange by neutrophils have received little attention.
The initial step in PAF modulating PMN inflammatory activity is binding to the seven transmembrane protein PAF receptor, which then activates the heterotrimeric Gq protein and thus the intracellular enzyme phospholipase C (PLC)-gamma. In doing so, PAF accelerates the production of diacylglycerol (DAG) and inositol triphosphate (IP3). The net effect of activating the PLC-gamma enzyme is intracellular protein phosphorylation (Chao & Olson, 1993; Ishii & Shimizu, 2000).
PAF has been linked to mitogen-activated protein kinase (MAPK) activation in human neutrophils (Nick et al., 1997; Ishii & Shimizu, 2000). Extracellular-signal-regulated protein kinase (ERK1/2) MAPK belongs to a family of 40–45 kDa protein serine/threonine kinases that are activated by many extracellular stimuli, including growth factors and hormones. MAPKs require phosphorylation on both threonine and tyrosine residues in Thr183-Glu-Tyr185 to become active (Payne et al., 1991; Granot et al., 1993). In neutrophils, the MAPK pathway controls several responses including priming, gene expression (Yaffe et al., 1999), phagocytosis and superoxide production (Downey et al., 1998). However, in contrast to other granulocytes, PAF has only a mild effect on neutrophil ERK1/2 phosphorylation (Nick et al., 1997).
It has been proposed that PAF induces MAPK activation by PI3K in neutrophils (Ferby et al., 1994). The PI3K is a heterodimeric enzyme (consisting of a 85 kDa regulatory and a 110 kDa catalytic subunit) that phosphorylates the D-3 position of inositol head of phosphoinositide lipids. The PI3K family comprises three classes (I, II and III) depending on substrate specificity and protein structure (Vanhaesebroeck et al., 1997; Fruman et al., 1998; Wymann & Pirola, 1998). Family I is divided into IA and IB subfamilies, and four known I PI3K isoforms (α, β, γ and δ), all expressed in leukocytes (Wymann & Pirola, 1998). Several experimental evidences suggest that PI3K is key in the chemotaxis, superoxide production and ERK phosphorylation induced by chemoattractants in PMNs (Okada et al., 1994; Hirsch et al., 2000; Sasaki et al., 2000).
On the other hand, little cell signaling work has been targeted toward bovine PMNs. However, one study has shed significant light on the bovine neutrophil biochemical response to PAF. At low concentrations (1 nM), PAF was demonstrated to lower the threshold for degranulation, expression of adhesion molecules, actin polymerization, rise in [Ca2+]i and altered membrane potential (Swain et al., 1998). At high concentrations (⩾100 nM), PAF caused more direct bactericidal responses, via the release of reactive oxygen species and granule enzymes (Swain et al., 1998).
Although both bovine neutrophils and human neutrophils play similar roles in host defense, a comparison of bovine and human neutrophils reveals distinct differences that might reflect variations in regulatory mechanisms. Bovine neutrophils show significant differences, such as the absence of fMLP receptors (Brown & Roth, 1991; Watson et al., 1995), low concentrations of lysozyme, a unique large granule not present in human neutrophils (Gennaro et al., 1983), and are poorly primed by PAF (Swain et al., 1998). Thus, one would expect differences in the response to PAF and signal transduction in bovine neutrophils. Therefore, it is important to study responses in bovine neutrophils in order to provide a broader understanding of the host defense processes.
In this study, we present evidence that PAF induces intracellular alkalinization through NHE controlled by the PI3K-ERK1/2 pathway.
Methods
Animals
Adult Holstein cows were obtained from the University herd. The cattle were maintained on ad lib grass diet with grain supplementation. All the experiments were conducted in accordance with institutional review board-approved protocols.
Isolation of neutrophils
Blood was collected by jugular venepuncture, and PMNs were isolated according to the method of Roth & Kaeberle (1981). Briefly, following collection into acid citrate dextrose (ACD) collection tubes, the blood was gently rocked for 5 min (Nutator, Becton Dickinson) and then centrifuged at 1000 × g at 20°C for 20 min. The plasma and buffy coat were aspirated and the remaining red blood cell and PMN pellet were resuspended in Hank's balanced salt solution (HBSS). The red blood cells were removed by flash hypotonic lysis with a cold phosphate-buffered water solution (0.0132 M, pH 7.2). Upon return to isotonicity with hypertonic phosphate buffer solution (0.0132 M, pH 7.2; 2.7% NaCl), the sample was centrifuged at 600 × g at 20°C for 10 min. The remaining PMN pellet was then washed with HBSS a total of three times. Cells were resuspended in approximately 5 ml of cold MD-RPMI 1640 at a density of approximately 2 × 106 cells ml−1. Viability was determined by trypan blue exclusion and was never less than 97%. Purity was at least 94%, as assessed by a dual-scatter flow cytometer (Becton Dickinson) and light microscopy following cytospin and differential staining.
Neutrophil extracellular acidification rate
PMN extracellular pH was measured in real time using the Cytosensor microphysiometer (Molecular Devices, Sunnyvale, CA, U.S.A.) (McConnell et al., 1992). PMNs were immobilized in 25% entrapment media, containing ∼1.5 × 105 PMN (7 μl). Cells were placed on non-tissue culture-treated transwell cell capsules (Transwell Inc.), assembled into the capsule chamber assembly unit, and loaded on to the Cytosensor. Cells were equilibrated for 90 min with MD-RPMI before the first exposure to PAF. Extracellular acidification rates were determined by 30 s potentiometric rate measurements (μV s−1) after an 80-s pump cycle with a 10 s delay (120 s total cycle time). PAF was perfused 30 s before the first rate measurement, and continued to do so for 30 min. The PMN extracellular acidification rate (μV s−1) was normalized to basal rates (100%) three cycles before PAF addition. Vehicle (EtOH) concentration did not exceed 0.1% v v−1 and did not cause statistically significant changes in the basal acidification rate. Unless otherwise indicated, each experiment represents the mean of four different animals.
Neutrophil intracellular pH
PMNs (2 × 107 cells ml−1) were suspended in a pH buffer (140 mM NaCl, 10 mM glucose, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 20 mM Hepes, pH 7.2) and incubated with 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester (BCECF-AM: 2.5 μM) for 30 min at 37°C. The cells were then washed twice and suspended at 4 × 106 cells ml−1. In all, 8 × 106 BCECF-loaded neutrophils were incubated with either vehicle (EtOH and DMSO, <0.01%) or varying concentrations of PD98059 (for 10 min), UO126 (for 10 min), genistein (for 30 min), wortmannin (for 10 min), LY294002 (for 10 min) or WEB2086 (for 30 min), followed by exposure to 100 nM PAF. Fluorescence was measured in a thermoregulated spectrofluorimeter (Kontron Instruments), with a magnetic stirrer at 439 and 505 nm excitation and 535 nm emission. Fluorescence was transformed to pH units using nigericin methods of calibration, described elsewhere (Grinstein et al., 1984).
Tyrosine phosphorylation immunoblot
A fixed number of PMNs (5 × 106) were incubated with 100 nm PAF for 0, 1, 2, 10 or 30 min at 37°C. The reaction was stopped by centrifugation and exposure to cold lysis buffer: 50 mM Tris-HCl, pH 7.4, 50 mM EDTA, 1 mM EGTA, protease inhibitors (leupeptin, aprotinin, pepstatin and trypsin inhibitor), 10 μg ml−1; 25 mM NaF; 2 mM NaVO4; 0.1 mM PMSF; 25 mM DTT; and 1.5% Triton X-100. The resultant proteins were centrifuged at 22,000 × g for 30 min at 4°C and quantified by Bradford's methods using BSA as standard, and analyzed by SDS/PAGE. A measure of 80 μg of protein was resolved in 12% SDS/PAGE and transferred to a nitrocellulose membrane for 14 h, at 35 mA. The membrane was then blocked with a buffer (1 × TBS, 0.1% Tween-20 and 5% nonfat dry milk) for 2 h at room temperature, washed, and incubated with an antiphosphotyrosine monoclonal antibody at a dilution of 1 : 5000. The membrane was incubated with HRP-conjugated secondary antibody for 2 h, and visualized using an enhanced chemiluminescence (ECL) system. Molecular weights of phosphoproteins were determined based on the mobility of prestained standards of known molecular weight. The Scion Image for Windows® 4.02 was used to analyze the blot.
ERK1/2 immunoblot
A fixed number of PMNs (5 × 106) were either incubated with increasing concentrations of PAF (1 × 10−11 to 1 × 10−6 M), or with 100 nM of PAF at different times 0, 1, 2, 10 and 30 min at 37°C. In some experiments, neutrophils were preincubated with different pharmacological tools (WEB2086 at 0.1, 1 and 10 μM for 10 min; pertussis toxin (PTX) at 200 and 500 ng ml−1 for 120 min; genistein at 0.1, 1 and 10 μM for 30 min; U0126 at 10 and 30 μM; PD98059 at 1, 10 and 50 μM; LY294002 at 10 and 30 μM; or wortmannin at 10, 50 and 100 nM for 10 min), and then treated with 100 nM PAF for 2 min. Phosphorylation of ERK1/2 was analyzed by blotting with a monoclonal anti-p-ERK1/2 at a dilution of 1 : 300 according to the manufacturer's instructions. Detection was enabled using an ECL system. As a control for p-ERK, the antibody was removed by incubation with stripping solution (100 mM 2-mercaptoethanol; 2% SDS; 62.5 mM Tris-HCl, pH 6.7) for 2 h at 50°C with agitation, followed by several washes with TBS-Tween 0.1%. The membrane was then incubated with anti-ERK antibody at a dilution of 1 : 5000, using a procedure similar to that described above. The Scion Image for Windows® 4.02 was employed to analyze the blot, and the pERK1/2 was normalized with the total ERK1/2.
PKB immunoblot
PMNs (5 × 106) were incubated with 100 nM PAF for 2 min at 37°C, or were preincubated with one of several physiologic antagonists (LY294002, wortmannin or U0126). Phosphorylation of PKB was analyzed by blotting with a polyclonal anti-p-PKB (Ser473) antibody and PKB antibody according to the manufacturer's instructions.
Cell viability
The cell toxicities of WEB2086, genistein, PTX, wortmannin, LY294002, PD98059 and U0126 on neutrophils, at the concentrations and times described in the above experiments, were assessed with trypan blue exclusion and the CytoTox 96® Non-radioactive Cytotoxicity Assay (Promega, Madison, U.S.A.).
Materials and reagents
PAF (C-16), PD98059 and genistein were obtained from Calbiochem (La Jolla, CA, U.S.A.). LY294002 and U0126 were purchased from Promega (Madison, U.S.A.). WEB2086 was a kind gift from Boehringer Ingelheim (Germany). All other reagents and chemicals were purchased from Merck (Darmstadt, Germany). MD-RPMI 1640 (l mM, pH 7.4) and low melting point agarose in MD-RPMI were purchased from Molecular Devices (Sunnyvale, CA, U.S.A.). Nitrocellulose membrane was purchased from Gibco BRL (Rockville, MD, U.S.A.). Phosphotyrosine monoclonal antibody, phospho-Akt antibody and Akt antibody were obtained from New England Biolabs (Beverly, MA, U.S.A.). Monoclonal antibody pERK1/2 (sc-7383), polyclonal antibody ERK1 (sc-94), anti-rabbit IgG-peroxidase (sc-2030) and anti-mouse IgG-HRP (sc-2005) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). ECL system was obtained from Amersham (U.S.A.). BCECF-AM was obtained from Molecular Probes (Oregon, U.S.A.). Amiloride (AM) was purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). Protease inhibitors were obtained from Roche Diagnostics GmbH. PAF was dissolved in ethanol at the stock concentration of 1 mM, and aliquots were kept at −70°C, used once for the experiment and discarded.
Statistical analysis
The results were expressed as a percentage of maximum response or pH increase as mean±s.e. EC50 values were calculated and dose–response curves were constructed using Graph Pad v2.0. A one-way analysis of variance (ANOVA) was performed and Dunnet's multiple comparison tests were applied using a significance level of 5%. The percentage was subjected to arcsin transformation before ANOVA.
Results
Effect of PAF on extracellular and intracellular pH
In order to assess the effects of PAF administration on changes in the neutrophil intracellular and extracellular microenvironments, two approaches were utilized. First, the extracellular acidification rate (ECAR) was measured by microphysiometry, and second the intracellular alkalinization was evaluated in BCECF-AM-loaded cells.
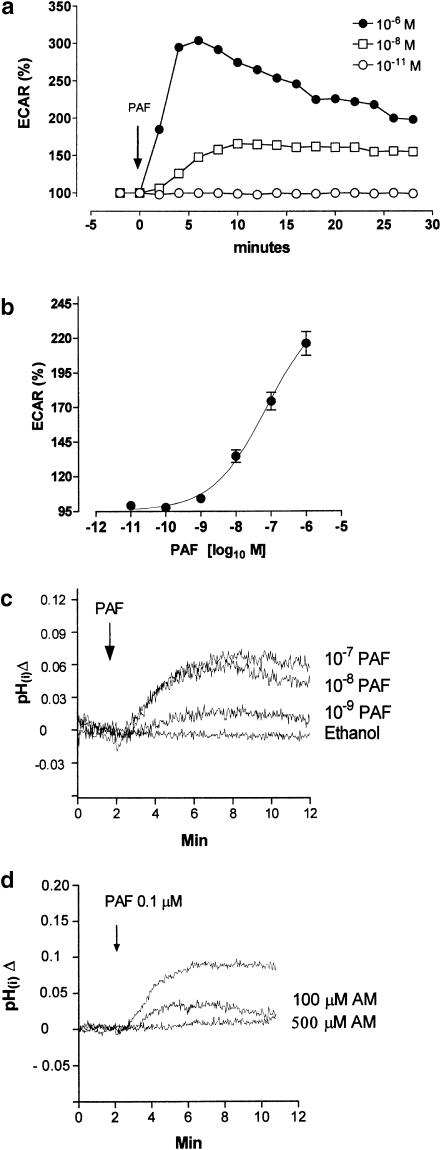
In bovine neutrophils, 1 μM of PAF caused a rapid, sustained (between 4 and 6 min postexposure) and transient response in ECAR (Figure 1a). The cumulative dose–response curves of PAF showed a maximum ECAR response of 116±8.5% above the basal rate (Figure 1b) and 1 nM (4.1±1.2%) was the lowest dose to elicit an effect (Figure 1b). A similar pH change was observed in BCECF-AM-loaded cells. Bovine neutrophils showed an intracellular pH of 7.20±0.01, and when 1 nM of PAF was added a mild intracellular alkalinization was recorded (Figure 1c). Concentrations between 1 and 100 nM can initiate a pH(i) increase that is maximum between 5 and 10 min (Figure 1c).
Figure 1.
Change of pH induced by PAF in bovine neutrophils. (a) Time course of PAF on ECAR stimulation. (b) Cumulative dose response of PAF on ECAR stimulation, 10 min of PAF stimulation. (c) Time course of PAF stimulation (10−7, 10−8 and 10−9 M) on pH(i) monitored fluorimetrically, in BCECF-AM-loaded neutrophils. (d) Effect of NHE1 inhibition by amiloride, in 10−7 M PAF stimulation on pH(i). Data represent the mean±s.e.m. of three independent experiments.
The intracellular alkalinization produced with PAF (0.1 μM) is dependent on NHE activity, because amiloride (0.1 and 0.5 mM) can block the response significantly (P<0.05) as shown in Figure 1d.
Intracellular alkalinization induced by PAF is dependant on PAF-R, tyrosine kinase, PI3K and MEK1/2 activation
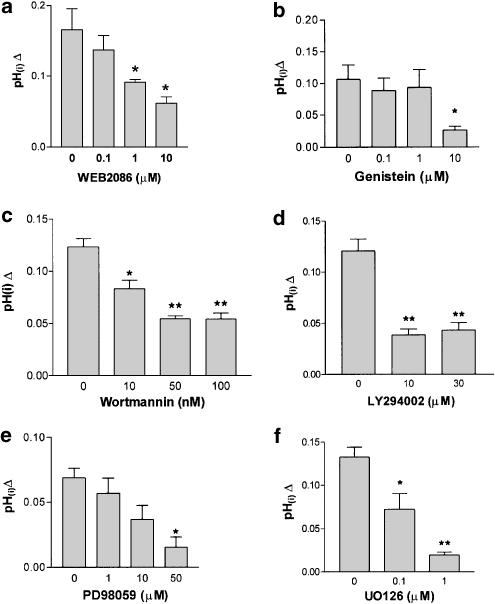
PAF induced an intracellular alkalinization in bovine neutrophils (Figure 1). This response can be attributed to the presence of a PAF-R. To assess this, BCECF-loaded neutrophils were stimulated with 100 nM of PAF in the presence of WEB2086. WEB2086 significantly reduced the intracellular alkalinization in a dose-dependent manner (Figure 2a), indicating the existence of a PAF-R. Recently, a bovine PAF-R has been sequenced which shares high homology with the human type (Yang et al., 2001). In neutrophils, tyrosine kinase inhibitors blocked cytosolic alkalinization after opsonized zymosan or FcgammaR (Fukushima et al., 1996), and because PAF induces a tyrosine kinase activity in neutrophils (Gomez-Cambronero et al., 1991), genistein was used to assess its possible role in pH changes. A measure of 10 μM genistein significantly reduced the intracellular alkalinization, but minor doses did not affect the pH changes induced by PAF (Figure 2b). The intracellular pH increases induced by PAF were analyzed using wortmannin, LY294002, PD98059 and U0126, in order to assess the role of PI3K and MEK1/2 in this response. Our results clearly indicate that the intracellular alkalinization induced by 100 nM of PAF was inhibited in a dose-dependent manner by wortmannin, LY294002, PD98059 and U0126 (Figure 2c–f). The concentrations of the inhibitors used in our experiments did not modify the cell viability measurement by trypan blue exclusion and CytoTox 96® Non-radioactive Cytotoxicity Assay (data not shown), and has been described elsewhere.
Figure 2.
Effect of a PAF receptor antagonist, PI3K inhibitor, tyrosine kinase inhibitor and MEK inhibitor on pH(i) induced by PAF. BCECF-loaded neutrophils were pretreated without or with WEB2086 (0, 0.1, 1 and 10 μM) for 30 min (a), genistein (0, 0.1, 1 and 10 μM) for 30 min (b), wortmannin (0, 1, 50 and 100 nM) for 10 min (c), LY294002 (0, 10 and 30 μM) for 10 min (d), PD98059 (0, 1, 10 and 50 μM) for 10 min (e) or UO126 (0, 0.1 and 1 μM) for 10 min (f) at 37°C, then stimulated with 100 nM PAF, and pH(i) was monitored fluorimetrically. Data represent the mean±s.e.m. of three experiments.
Effect of PAF on tyrosine phosphorylation and ERK1/2 phosphorylation
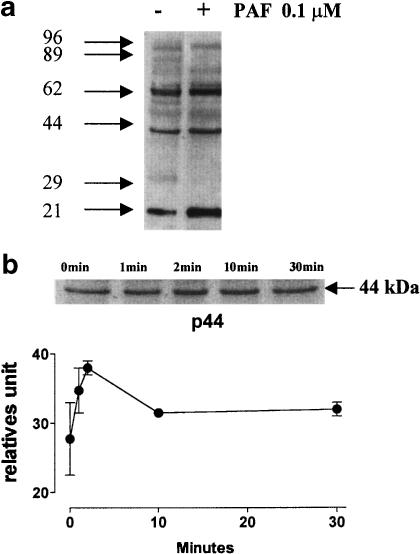
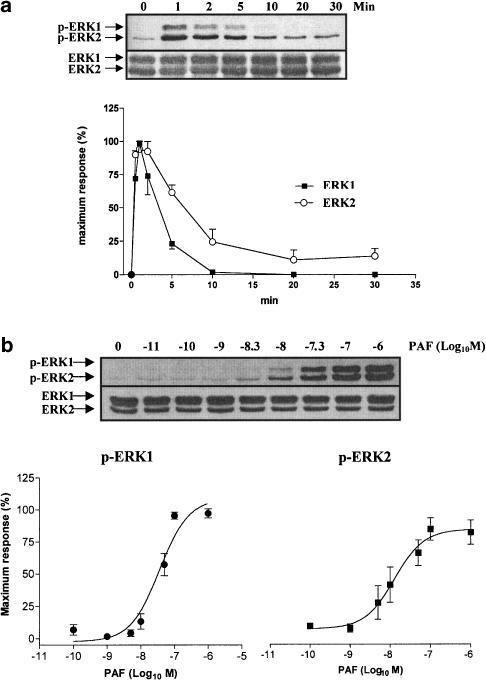
It has been suggested that in neutrophils, tyrosine kinase is involved in the NHE activation during phagocytosis (Fukushima et al., 1996) or cellular volume changes by hypertonic stress (Krump et al., 1997). To assess whether PAF caused a tyrosine phosphorylation, bovine neutrophils were stimulated with PAF (100 nM) at different times and analyzed by immunoblot using antiphosphotyrosine monoclonal antibody. PAF induced an increase in tyrosine phosphorylation of several proteins such as 62, 44 and 21 kDa (Figure 3a). The phosphorylation of pp44 kDa reached a maximum at 2 min and decreased slowly; however, it did not reach the basal value (Figure 3b). The proteins such as pp21 and pp62 were also phosphorylated at 2 min and this was sustained for 30 min incubation (i.e. pp21) (data not shown). Because of the possibility that the pp44 stimulated by PAF correspond to an MAPK, bovine neutrophils were incubated with PAF at different times or doses, and immunoblotting using a specific antibody against pERK1/2 was performed. PAF induced ERK1/2 phosphorylation with a similar time pattern of stimulation to pp44 (Figure 4a). A maximum response was observed at 1–2 min of incubation and decreased to the basal level between 10 and 30 min for pERK1 and pERK2 respectively. In bovine neutrophils a dose–response curve for ERK1/2 phosphorylation of PAF was constructed, and a sigmoid dose–response curve is depicted in Figure 4b, with an EC50 to pERK1 and pERK2 of 30 and 13 nM, respectively.
Figure 3.
Time course of protein phosphorylation induced by PAF. Cells were incubated with 100 nM PAF at 37°C for various time intervals. In all, 80 μg of proteins was fractionated by polyacrylamide gel electrophoresis in the presence of sodium dodecylsulfate and transferred to nitrocellulose membrane. Protein tyrosine phosphorylation was detected by immunoblotting with antiphosphotyrosine antibody, followed by incubation with an HRP-conjugated secondary antibody, and visualized with an ECL system. Molecular weights of phosphoproteins were determined based on the mobility of prestained standards of known molecular weight. (a) Protein tyrosine phosphorylation for 2 min. (b) Time response curve to the protein of 44 kDa. Data were represented as relative units mean of three independent experiments±s.e.m.
Figure 4.
Time course and dose response of PAF-dependent ERK1/2 phosphorylation. Neutrophils were incubated with vehicle or PAF at different times (0–30 min) (a) and concentrations (b). ERK1/2 activation was measured by SDS–PAGE and western blot with an antiphospho-ERK1/2 and anti-ERK1/2 antibody as described in Figure 3. Data shown are representative of three independent experiments, mean±s.e.
PAF-induced ERK1/2 phosphorylation is antagonized by WEB2086, PTX and genistein
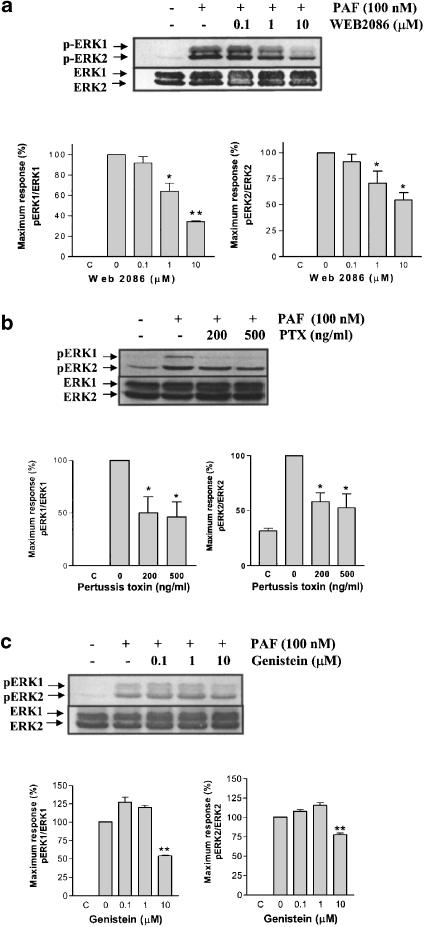
The role of a PAF receptor in the ERK1/2 phosphorylation was evaluated in bovine neutrophils. Cells incubated with a selective PAF antagonist, WEB2086, and stimulated with 100 nM of PAF showed an inhibition of ERK1/2 phosphorylation (Figure 5a). The IC50 of WEB2086 was estimated to be 1.14±0.4 μM, which indicates that the activation of pERK1/2 by PAF depends on the activation of a PAF receptor.
Figure 5.
Effect of WEB2086: gemistein PTX and on ERK1/2 phosphorylation induced by PAF. Neutrophils were pretreated without or with WEB2086 (0.1, 1 and 10 μM) for 30 min (a), PTX (200 and 500 ng ml−1) for 120 min (b) or genistein (0.1, 1 and 10 μM) for 30 min (c) at 37°C, and then stimulated with 100 nM PAF for 2 min. ERK1/2 phosphorylation was detected by immunoblotting as in Figure 4. Data shown are representative of three independent experiments, mean±s.e. *P<0.05, **P<0.01 compared to 0.
The PAF receptor is coupled to G protein, and as several cellular responses are PTX sensitive, this indicates a role of Gαi in the signal transduction. In order to assess the role of G proteins in the ERK1/2 activation, bovine neutrophils were preincubated with 200 and 500 ng ml−1 of PTX for 2 h. Afterwards, the cells were stimulated with 100 nM of PAF and the phosphorylation of ERK1/2 was detected by immunoblotting. The ERK1/2 phosphorylation induced by PAF was partially inhibited by 200 and 500 ng ml−1 PTX (Figure 5b), suggesting that PAF activates an MAPK pathway, in part through a PTX-sensitive G protein.
The ERK1/2 phosphorylation by PAF has been related to the activity of several tyrosine kinase proteins (Ishii & Shimizu, 2000), which cause MEK1/2 activation. Experiments using genistein, a tyrosine kinase inhibitor, demonstrated that the ERK1/2 phosphorylation induced by 100 nM of PAF was inhibited in a dose-dependent manner by genistein (Figure 5c).
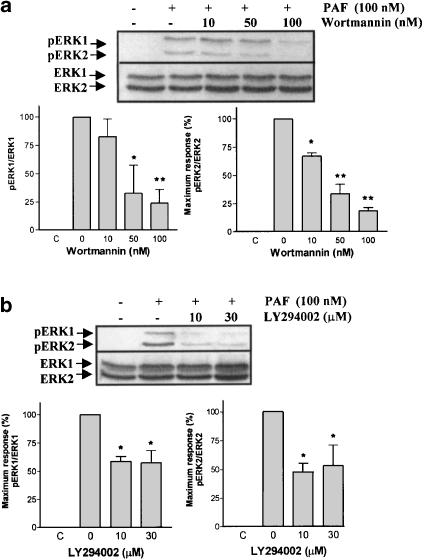
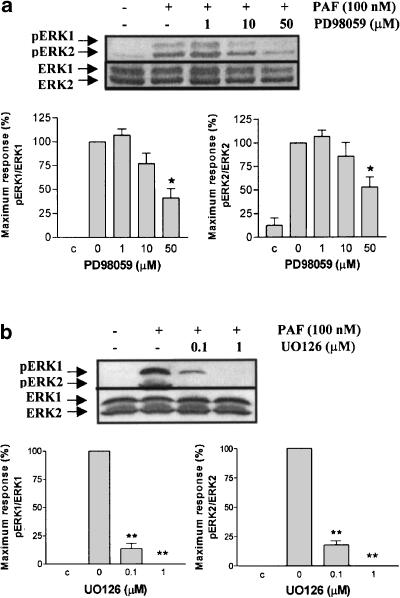
ERK1/2 phosphorylation induced by PAF is dependent on PI3K and MEK1/2 activation, but not PKC
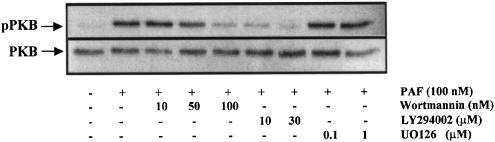
The PI3K pathway has been shown to participate in ERK1/2 activation by PAF in human eosinophils (Miike et al., 2000). A series of experiments using wortmannin and LY294002, PI3K inhibitors (Powis et al., 1994; Vlahos et al., 1994), and PD98059 and U0126, specific MEK1/2 inhibitors (Dudley et al., 1995; Favata et al., 1998), were conducted in order to evaluate these potential signal transduction pathways. The ERK1/2 phosphorylation induced by 100 nM of PAF was inhibited in a dose-dependent manner by wortmannin, LY294002 (Figure 6a and b), PD98059 and U0126 (Figure 7a and b), indicating that MAPK activation depends on PI3K and MEK1/2 pathway. In bovine neutrophils, PAF activates PI3K, because PKB is phosphorylated on serine 473. Wortmannin and LY294002, but not U0126, were able to reduce this response (Figure 8).
Figure 6.
Effect of wortmannin and LY294002 on ERK1/2 phosphorylation induced by PAF. Neutrophils were pretreated without or with wortmannin (10, 50 and 100 nM) for 10 min (a) or LY294002 (10 and 30 μM) for 10 min (b) at 37°C, and then stimulated with 100 nM PAF for 2 min. ERK1/2 phosphorylation was detected by immunoblotting as in Figure 4. Data shown are representative of three independent experiments, mean±s.e. *P<0.05, **P<0.01 compared to 0.
Figure 7.
Effect of PD98059 and UO126 on ERK1/2 phosphorylation induced by PAF. Neutrophils were pretreated without or with PD98059 (1, 10 and 50 μM) for 10 min (a) or UO126 (0.1 and 1 μM) for 10 min (b) at 37°C, then stimulated with 100 nM for 2 min. ERK1/2 phosphorylation was detected by immunoblotting as in Figure 4. Data shown are representative of three independent experiments, mean±s.e. *P<0.05, **P<0.01 compared to 0.
Figure 8.
Effect of PAF on PKB phosphorylation. Neutrophils were pretreated without or with wortmannin (10, 50 and 100 nM) for 10 min, LY294002 (10 and 30 μM) for 10 min or UO126 (0.1 and 1 μM) for 10 min at 37°C, and then stimulated with 100 nM PAF for 2 min. PKB phosphorylation was detected by immunoblotting with an anti-p-PKB (Ser473) antibody, after the blots were reprobed with anti-PKB. Experiment shown is representative of three independent experiments.
In order to demonstrate any role of PKC, bovine neutrophils were incubated with staurosporine or Gö6850 for 1 h and 30 min, respectively. Subsequently, 100 nM of PAF was added; however, neither of these PKC inhibitors was able to reduce the ERK1/2 phosphorylation (data not shown).
Discussion
Our results clearly show that 10 nM of PAF caused a significant increase in extracellular acidification rate, which correlated with an increase in intracellular alkalinization measured in BCECF-loaded cells (Figure 1). On the contrary, in human neutrophils, PAF induces only a mild ECAR and only 1 μM was able to increase ECAR above the basal rate (Gronert et al., 1998). Similarly, we have found that the intracellular alkalinization induced by PAF was blocked by amiloride, indicating the role of Na+/H+ ion exchange in the control of cellular pH response induced by PAF in bovine neutrophils. In human neutrophils, it has been demonstrated that the ECAR increases (Gronert et al., 1998) and the intracellular alkalinization stimulated by chemoattractant are controlled by an amiloride-sensitive Na+/H+ antiport (Simchowitz, 1985a; Naccache et al., 1986). Several experiments using different chemoattractant suggest that NHE is an important component in the regulation of cellular size changes and migration, due to the fact that amiloride and derivatives are able to reduce the chemotaxis and the pH(i) increase (Naccache et al., 1986; Simchowitz & Cragoe, 1986a, 1986b).
The signal transduction pathways involved in the intracellular alkalinization induced by PAF are unknown in neutrophils. ERK1/2 phosphorylation has been suggested to be involved in NHE activity (Fukushima et al., 1996). It is known that the MAPK pathway is coupled to G-protein receptor and the ERK1/2 phosphorylation is upstream regulated by tyrosine kinase, PI3K and MEK1/2 in eosinophils (Miike et al., 2000). We conducted several experiments to assess if the pH(i) increase is controlled by one of these pathways. Firstly, we demonstrated that WEB2086 inhibited in a dose-dependent manner the pH(i) increase induced by PAF suggesting the participation of a PAF receptor associated to this response. The pH(i) increase controlled by NHE can be activated in neutrophils by osmotic shrinkage, which induces intracellular alkalinization and increases tyrosine phosphorylation. Both effects are inhibited by genistein (Krump et al., 1997). We also demonstrated that genistein inhibited in a dose-dependent fashion the increase in pH(i), which suggests the role of some tyrosine kinase protein in the intracellular alkalinization induced by PAF in bovine neutrophils. Our results also demonstrated that the intracellular pH(i) increase is also under the control of PI3K activity, as this was inhibited by wortmannin and LY294002, and MEK1/2 activity, as this was inhibited by PD98059 and U0126. This experimental evidence suggests that PAF, through the PI3K–MAPK pathway activation, can modulate NHE activity in bovine neutrophils.
A series of experiments were conducted to elucidate the participation of MAP kinase pathways in bovine neutrophils. The results show that PAF induces tyrosine phosphorylation of several proteins. Specifically, a pp44 kDa was stimulated reaching a maximum at 2 min and declining to the basal level after 10 min. Differently, in human neutrophils, a 41 kDa tyrosine-phosphorylated protein induced by PAF (Gomez-Cambronero et al., 1991; 1992), granulocyte–macrophage colony-stimulating factor (GM-CSF) (McColl et al., 1991; Gomez-Cambronero et al., 1992), TNFα (Gomez-Cambronero et al., 1992; Waterman & Sha'afi, 1995), PMA (Huang et al., 1990), fMLP (Huang et al., 1990; Gomez-Cambronero et al., 1992; Grinstein & Furuya, 1992) and ionophore A23187 (Gomez-Cambronero et al., 1992) have been described. The fact that PAF induces the phosphorylation of ERK1/2 MAPK in bovine neutrophils is supported by the experiments shown in Figure 3. Studies of intracellular signaling also showed that PAF activates the 42- and 44-kDa mitogen-activated MAPK, ERK1 and ERK2, in human eosinophils (Miike et al., 2000) while PAF did not activate these MAPK in human neutrophils (Nick et al., 1997). Thus, there are quantitative and qualitative differences in cellular responses to PAF between human and bovine neutrophils.
The participation of a PAF receptor in the phosphorylation of ERK1/2 MAPK was suggested by the inhibition of WEB2086, a selective PAF receptor antagonist. The bovine PAF receptor has been sequenced and shares high homology with the human PAF receptor, suggesting that it is related to a G-protein-coupled receptor (Yang et al., 2001). The role of G proteins in the phosphorylation of PAF-induced ERK1/2 has been examined in bovine neutrophils by incubating cells with PTX, which ribosylates Gαi/Gαo in a αβγ heterotrimeric state-dependent fashion. In these experiments, PTX inhibited the ERK1/2 phosphorylation induced by PAF. However, this inhibition was partial, indicating that other G proteins could be involved in MAPK activation. In support of this, PAF induced both 42 and 44 kDa MAP kinase activity in CHO cells, transfected with a cloned guinea pig PAF receptor. This stimulus was differentially inhibited by PTX, strongly suggesting that the PAF receptor can activate MAP kinase by both PTX-sensitive and -insensitive G proteins (Honda et al., 1994). Furthermore, human neutrophils treated with PTX do not inhibit the p41 phosphorylation induced by PAF (Gomez-Cambronero et al., 1991). We have found that ERK1/2 phosphorylation induced by PAF is insensitive to staurosporine and Gö6850 (selective PKC inhibitors), discounting a role of PKC in MAPK activation (data not shown). A major β-isoform of PKC in comparison to a minor ξ-isoform of PKC has been described in bovine neutrophils (Stasia et al., 1990). Both staurosporine and Gö6850 are effective in reducing PKC activation induced with zymosan or PMA in bovine neutrophils (Yu & Czuprynski, 1996; Smits et al., 1997; Yamamori et al., 2000). In the absence of a PKC role in PAF-induced ERK1/2 phosphorylation, human eosinophils treated with staurosporine and calphostin (a PKC inhibitor) did not reduce significantly the ERK1/2 phosphorylation by PAF (Miike et al., 2000). However, our results do not disprove the participation of PKC isoforms in ERK1/2 phosphorylation induced by other stimuli.
In bovine neutrophils, the ERK1/2 phosphorylation induced by PAF is inhibited by genistein, indicating the participation of some tyrosine kinase protein. Our results are in accord with other studies in eosinophils, where the ERK1/2 pathway can be activated by PAF via the activation of a tyrosine kinase sensitive to genistein (Miike et al., 2000). In human platelets, PMA and arginine vasopressin (AVP) cause activation of MAPK. Moreover, both agonists stimulate the Na+/H+ exchange in a similar time frame and concentration dependence (Aharonovitz & Granot, 1996). The MAPK and NHE activities induced by PMA are inhibited by staurosporine, and by PD98059, but were not affected by genistein. In contrast, both AVP-induced MAPK and NHE activities are inhibited by genistein and MEK inhibitor, but not by staurosporine (Aharonovitz & Granot, 1996). Therefore, tyrosine kinase, but not PKC, upstream to MAPKs, is involved in the signaling pathway initiated by AVP (Aharonovitz & Granot, 1996). These findings are consistent with the hypothesis that tyrosine kinase activity is required for G-protein-coupled receptor Ras-dependent MAPK activation in rat fibroblasts and COS-7 cells (van Corven et al., 1993; Touhara et al., 1995).
We describe that PI3K participates in PAF-induced ERK1/2 phosphorylation in bovine neutrophils. This fact is supported as wortmannin and LY294002 were able to reduce the ERK1/2 phosphorylation induced by PAF. This result is consistent with other observations on the role of PI3K in the MAPK activation by other chemoattractans in granulocytes, such as fMLP, GM-CSF and C5a (Coffer et al., 1998; Miike et al., 2000; Sasaki et al., 2000). The Akt/PKB protein Ser/Thr kinase lies downstream of PI3K, as clearly demonstrated in neutrophils from gene-targeted mice lacking the p110 catalytic subunit of PI3Kγ. The PI3Kγ−/− mice granulocytes showed a poor response to PKB and ERK phosphorylation induced by fMLP and C5a (Sasaki et al., 2000).
Until now, the activation of Akt/PKB by PAF in neutrophil is unknown. We demonstrated for the first time a PKB phosphorylation in Ser473 by PAF in bovine neutrophils. Moreover, the PKB phosphorylation induced by PAF was reduced by wortmannin and LY294002, but not U0126 (Figure 8). Wortmannin is the major pharmacological tool to study PI3K activity. It is known that at nanomolar concentrations (1–100 nM) wortmannin is able to inhibit the class I and II of PI3K and at concentrations >100 nM it inhibits class III (Ward et al., 2003). However, at submicromolar concentrations, wortmannin also inhibits other kinases, such as PI4K (Nakanishi et al., 1995) and myosin light chain kinase (Nakanishi et al., 1992). For this reason, LY294002, a selective inhibitor of PI3K (at micromolar range), that has no detectable effect on other protein kinases or PI4K was used (Vlahos et al., 1994). LY294002 reduced only partially the ERK1/2 phosphorylation induced by PAF (Figure 6b) indicating that another pathway, different from PI3K, is involved.
Although PI3K-C2α is refractory to both inhibitors (Domin et al., 1997), neither wortmannin nor LY294002 exhibits any degree of selectivity on individual PI3K isoforms (Ward et al., 2003).
Finally, PD98059 and U0126 reduced the ERK1/2 phosphorylation induced with PAF in bovine neutrophils. U0126 was more potent in inhibiting the phosphorylation of ERK1/2 than PD98059 (Figure 7). Moreover, the inhibition of ERK1/2 phosphorylation with U0126 was identical on ERK1 and ERK2, and PD98059 had a more potent effect on ERK1 compared to ERK2 (Figure 7). This can be attributed to the fact that PD98059 is a specific MEK1/2 inhibitor with an IC50 values of 4 and 50 μM (MEK1 and MEK2, respectively) (Alessi et al., 1995). On the other hand, U0126 shows a similar and more potent MEK1 and MEK2 inhibition (MEK1 IC50=72 nM; MEK2 IC50=58 nM) (Favata et al., 1998). U0126 was also a more potent intracellular alkalinization inhibitor than PD98059 (Figure 2).
Other authors have demonstrated that the inhibition of the ERK1/2 MAPK signaling by PD98059 reduces by 50–60% the NHE1 activation in response to growth factors (Bianchini et al., 1997) and by 100% with arginine vasopressin in human platelets (Aharonovitz & Granot, 1996). Experiments using osmotic stimulation increase the NHE activity and ERK1/2 phosphorylation; however, under this condition, MEK inhibition did not reduce the intracellular alkalinization (Gillis et al., 2001). The alkalinization induced by PAF in bovine neutrophils was preceded by a mild and transitory period of intracellular acidification. This pattern is also observed in neutrophils activated with fMLP and TPA (Grinstein et al., 1986; Suszták et al., 1997). It has been demonstrated that sustained intracellular acidification increases the NHE1 activity by the ERK pathway activation (Haworth et al., 2003). Chemoattractants induce respiratory burst by NADPH oxidase eliciting an increase in the production of H+ (Grinstein et al., 1986; Suszták et al., 1997), and it is possible that the initial intracellular acidification could contribute directly to the NHE1 activity. However, using PD98059 or U0126, we did not observe an effect on the initial acidification rate induced by PAF in bovine neutrophils (data not shown), indicating that NHE activity is controlled by the ERK pathway.
In conclusion, in bovine neutrophils, PAF increases pHi via an NHE sensitive to amiloride, involving the activation of a PAF-R coupled to a PTX-sensitive Gαi/Gαo protein. This effect is upstream regulated by tyrosine kinase, PI3K and MEK1/2 activity. This regulation might be important in inflammation, due to the fact that NHE is ubiquitously expressed and is the target of multiple signaling pathways (Putney et al., 2002), as NHE participates in cytoskeletal organization and cellular migration.
Acknowledgments
We thank Dr R. Hermosilla for commenting on the manuscript, and Boehringer Ingelheim for WEB2086. This work was supported by grants from the Fondo Nacional de Ciencia y Tecnología, FONDECYT 1010204 and 7010204, CHILE.
Abbreviations
- BCECF-AM
2,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein acetoxymethyl ester
- DMSO
dimethylsulfoxide
- ECAR
extracellular acidification rate
- ERK1/2
extracellular-signal-regulated protein kinase
- fMLP
N-formylmethionine-leucyl-phenylalanine
- HBSS
Hank's balanced salt solution
- LTB4
leukotriene B4
- MAPK
mitogen-activated protein kinase
- MEK
MAPK kinase
- NHE
Na+/H+ exchange
- PAF
platelet-activating factor
- pHi
intracellular pH
- PI3K
phosphatidylinositol 3-kinase
- PKB
protein kinase B
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PMN
polymorphonuclear cells
- PTX
pertussis toxin
References
- AHARONOVITZ O., GRANOT Y. Stimulation of mitogen-activated protein kinase and Na+/H+ exchanger in human platelets. Differential effect of phorbol ester and vasopressin. J. Biol. Chem. 1996;271:16494–16499. doi: 10.1074/jbc.271.28.16494. [DOI] [PubMed] [Google Scholar]
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BIANCHINI L., L'ALLEMAIN G., POUYSSEGUR J. The p42/p44 mitogen-activated protein kinase cascade is determinant in mediating activation of the Na+/H+ exchanger (NHE1 isoform) in response to growth factors. J. Biol. Chem. 1997;272:271–279. doi: 10.1074/jbc.272.1.271. [DOI] [PubMed] [Google Scholar]
- BOKOCH G.M. Chemoattractant signaling and leukocyte activation. Blood. 1995;86:1649–1660. [PubMed] [Google Scholar]
- BROWN G.B., ROTH J.A. Comparison of the response of bovine and human neutrophils to various stimuli. Vet. Immunol. Immunopathol. 1991;28:201–218. doi: 10.1016/0165-2427(91)90115-s. [DOI] [PubMed] [Google Scholar]
- CHAO W., OLSON M.S. Platelet-activating factor: receptors and signal transduction. Biochem. J. 1993;292:617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COFFER P.J., GEIJSEN N., M'RABET L., SCHWEIZER R.C., MAIKOE T., RAAIJMAKERS J.A., LAMMERS J.W., KOENDERMAN L. Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochem. J. 1998;329:121–130. doi: 10.1042/bj3290121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMIN J., PAGES F., VOLINIA S., RITTENHOUSE S.E., ZVELEBIL M.J., STEIN R.C., WATERFIELD M.D. Cloning of a human phosphoinositide 3-kinase with a C2 domain that displays reduced sensitivity to the inhibitor wortmannin. Biochem. J. 1997;326:139–147. doi: 10.1042/bj3260139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNEY G.P., BUTLER J.R., TAPPER H., FIALKOW L., SALTIEL A.R., RUBIN B.B., GRINSTEIN S. Importance of MEK in neutrophil microbicidal responsiveness. J. Immunol. 1998;160:434–443. [PubMed] [Google Scholar]
- DUDLEY D.T., PANG L., DECKER S.J., BRIDGES A.J., SALTIEL A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVATA M.F., HORIUCHI K.Y., MANOS E.J., DAULERIO A.J., STRADLEY D.A., FEESER W.S., VAN DYK D.E., PITTS W.J., EARL R.A., HOBBS F., COPELAND R.A., MAGOLDA R.L., SCHERLE P.A., TRZASKOS J.M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- FERBY I.M., WAGA I., SAKANAKA C., KUME K., SHIMIZU T. Wortmannin inhibits mitogen-activated protein kinase activation induced by platelet-activating factor in guinea pig neutrophils. J. Biol. Chem. 1994;269:30485–30488. [PubMed] [Google Scholar]
- FRUMAN D.A., MEYERS R.E., CANTLEY L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- FUKUSHIMA T., WADDELL T.K., GRINSTEIN S., GOSS G.G., ORLOWSKI J., DOWNEY G.P. Na+/H+ exchange activity during phagocytosis in human neutrophils: role of Fcgamma receptors and tyrosine kinases. J. Cell Biol. 1996;132:1037–1052. doi: 10.1083/jcb.132.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENNARO R., DEWALD B., HORISBERGER U., GUBLER H.U., BAGGIOLINI M. A novel type of cytoplasmic granule in bovine neutrophils. J. Cell Biol. 1983;96:1651–1661. doi: 10.1083/jcb.96.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEWIRTZ A.T., SEETOO K.F., SIMONS E.R. Neutrophil degranulation and phospholipase D activation are enhanced if the Na+/H+ antiport is blocked. J. Leukocyte Biol. 1998;64:98–103. doi: 10.1002/jlb.64.1.98. [DOI] [PubMed] [Google Scholar]
- GILLIS D., SHRODE L.D., KRUMP E., HOWARD C.M., RUBIE E.A., TIBBLES L.A., WOODGETT J., GRINSTEIN S. Osmotic stimulation of the Na+/H+ exchanger NHE1: relationship to the activation of three MAPK pathways. J. Membr. Biol. 2001;181:205–214. doi: 10.1007/s00232-001-0023-3. [DOI] [PubMed] [Google Scholar]
- GOMEZ-CAMBRONERO J., HUANG C.K., GOMEZ-CAMBRONERO T.M., WATERMAN W.H., BECKER E.L., SHA'AFI R.I. Granulocyte–macrophage colony-stimulating factor-induced protein tyrosine phosphorylation of microtubule-associated protein kinase in human neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7551–7555. doi: 10.1073/pnas.89.16.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMEZ-CAMBRONERO J., WANG E., JOHNSON G., HUANG C.K., SHA'AFI R.I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J. Biol. Chem. 1991;266:6240–6245. [PubMed] [Google Scholar]
- GRANOT Y., ERIKSON E., FRIDMAN H., VAN PUTTEN V., WILLIAMS B., SCHRIER R.W., MALLER J.L. Direct evidence for tyrosine and threonine phosphorylation and activation of mitogen-activated protein kinase by vasopressin in cultured rat vascular smooth muscle cells. J. Biol. Chem. 1993;268:9564–9569. [PubMed] [Google Scholar]
- GRINSTEIN S., FURUYA W. Chemoattractant-induced tyrosine phosphorylation and activation of microtubule-associated protein kinase in human neutrophils. J. Biol. Chem. 1992;267:18122–18125. [PubMed] [Google Scholar]
- GRINSTEIN S., FURUYA W., BIGGAR W.D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J. Biol. Chem. 1986;261:512–514. [PubMed] [Google Scholar]
- GRINSTEIN S., GOETZ J.D., ROTHSTEIN A. 22Na+ fluxes in thymic lymphocytes. I. Na+/Na+ and Na+/H+ exchange through an amiloride-insensitive pathway. J. Gen. Physiol. 1984;84:565–584. doi: 10.1085/jgp.84.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRONERT K., COLGAN S.P., SERHAN C.N. Characterization of human neutrophil and endothelial cell ligand-operated extracellular acidification rate by microphysiometry: impact of reoxygenation. J. Pharmacol. Exp. Ther. 1998;285:252–261. [PubMed] [Google Scholar]
- HAWORTH R.S., MCCANN C., SNABAITIS A.K., ROBERTS N.A., AVKIRAN M. Stimulation of the plasma membrane Na+/H+ exchanger NHE1 by sustained intracellular acidosis: evidence for a novel mechanism mediated by the erk pathway. J. Biol. Chem. 2003;278:31676–31684. doi: 10.1074/jbc.M304400200. [DOI] [PubMed] [Google Scholar]
- HIRSCH E., KATANAEV V.L., GARLANDA C., AZZOLINO O., PIROLA L., SILENGO L., SOZZANI S., MANTOVANI A., ALTRUDA F., WYMANN M.P. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- HONDA Z., TAKANO T., GOTOH Y., NISHIDA E., ITO K., SHIMIZU T. Transfected platelet-activating factor receptor activates mitogen-activated protein (MAP) kinase and MAP kinase kinase in Chinese hamster ovary cells. J. Biol. Chem. 1994;269:2307–2315. [PubMed] [Google Scholar]
- HUANG C.K., BONAK V., LARAMEE G.R., CASNELLIE J.E. Protein tyrosine phosphorylation in rabbit peritoneal neutrophils. Biochem. J. 1990;269:431–436. doi: 10.1042/bj2690431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII S., SHIMIZU T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- KRUMP E., NIKITAS K., GRINSTEIN S. Induction of tyrosine phosphorylation and Na+/H+ exchanger activation during shrinkage of human neutrophils. J. Biol. Chem. 1997;272:17303–17311. doi: 10.1074/jbc.272.28.17303. [DOI] [PubMed] [Google Scholar]
- MCCOLL S.R., KRUMP E., NACCACHE P.H., POUBELLE P.E., BRAQUET P., BRAQUET M., BORGEAT P. Granulocyte–macrophage colony-stimulating factor increases the synthesis of leukotriene B4 by human neutrophils in response to platelet-activating factor. Enhancement of both arachidonic acid availability and 5-lipoxygenase activation. J. Immunol. 1991;146:1204–1211. [PubMed] [Google Scholar]
- MCCONNELL H.M., OWICKI J.C., PARCE J.W., MILLER D.L., BAXTER G.T., WADA H.G., PITCHFORD S. The cytosensor microphysiometer: biological applications of silicon technology. Science. 1992;257:1906–1912. doi: 10.1126/science.1329199. [DOI] [PubMed] [Google Scholar]
- MIIKE S., KURASAWA K., SAITO Y., IWAMOTO I. Platelet-activating factor activates mitogen-activated protein kinases through the activation of phosphatidylinositol 3-kinase and tyrosine kinase in human eosinophils. J. Leukocyte Biol. 2000;67:117–126. doi: 10.1002/jlb.67.1.117. [DOI] [PubMed] [Google Scholar]
- NACCACHE P.H., MOLSKI M.M., VOLPI M., SHEFCYK J., MOLSKI T.F., LOEW L., BECKER E.L., SHA'AFI R.I. Biochemical events associated with the stimulation of rabbit neutrophils by platelet-activating factor. J. Leukocyte Biol. 1986;40:533–548. doi: 10.1002/jlb.40.5.533. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S., CATT K.J., BALLA T. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5317–5321. doi: 10.1073/pnas.92.12.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANISHI S., KAKITA S., TAKAHASHI I., KAWAHARA K., TSUKUDA E., SANO T., YAMADA K., YOSHIDA M., KASE H., MATSUDA Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- NICK J.A., AVDI N.J., YOUNG S.K., KNALL C., GERWINS P., JOHNSON G.L., WORTHEN G.S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., SAKUMA L., FUKUI Y., HAZEKI O., UI M. Blockage of chemotactic peptide-induced stimulation of neutrophils by wortmannin as a result of selective inhibition of phosphatidylinositol 3-kinase. J. Biol. Chem. 1994;269:3563–3567. [PubMed] [Google Scholar]
- OSAKI M., SUMIMOTO H., TAKESHIGE K., CRAGOE E.J., JR, HORI Y., MINAKAMI S. Na+/H+ exchange modulates the production of leukotriene B4 by human neutrophils. Biochem. J. 1989;257:751–758. doi: 10.1042/bj2570751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE D.M., ROSSOMANDO A.J., MARTINO P., ERICKSON A.K., HER J.H., SHABANOWITZ J., HUNT D.F., WEBER M.J., STURGILL T.W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWIS G., BONJOUKLIAN R., BERGGREN M.M., GALLEGOS A., ABRAHAM R., ASHENDEL C., ZALKOW L., MATTER W.F., DODGE J., GRINDEY G. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- PRESCOTT S.M., ZIMMERMAN G.A., STAFFORINI D.M., MCINTYRE T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- PUTNEY L.K., DENKER S.P., BARBER D.L. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu. Rev. Pharmacol. Toxicol. 2002;42:527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- ROTH J.A., KAEBERLE M.L. Evaluation of bovine polymorphonuclear leukocyte function. Vet. Immunol. Immunopathol. 1981;2:157–174. doi: 10.1016/0165-2427(81)90047-7. [DOI] [PubMed] [Google Scholar]
- SASAKI T., IRIE-SASAKI J., JONES R.G., OLIVEIRA-DOS-SANTOS A.J., STANFORD W.L., BOLON B., WAKEHAM A., ITIE A., BOUCHARD D., KOZIERADZKI I., JOZA N., MAK T.W., OHASHI P.S., SUZUKI A., PENNINGER J.M. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- SIMCHOWITZ L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. I. Sodium fluxes. J. Biol. Chem. 1985a;260:13237–13247. [PubMed] [Google Scholar]
- SIMCHOWITZ L. Chemotactic factor-induced activation of Na+/H+ exchange in human neutrophils. II. Intracellular pH changes. J. Biol. Chem. 1985b;260:13248–13255. [PubMed] [Google Scholar]
- SIMCHOWITZ L. Intracellular pH modulates the generation of superoxide radicals by human neutrophils. J. Clin. Invest. 1985c;76:1079–1089. doi: 10.1172/JCI112061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMCHOWITZ L., CRAGOE E.J., JR Inhibition of chemotactic factor-activated Na+/H+ exchange in human neutrophils by analogues of amiloride: structure–activity relationships in the amiloride series. Mol. Pharmacol. 1986a;30:112–120. [PubMed] [Google Scholar]
- SIMCHOWITZ L., CRAGOE E.J., JR Regulation of human neutrophil chemotaxis by intracellular pH. J. Biol. Chem. 1986b;261:6492–6500. [PubMed] [Google Scholar]
- SMITH J.A. Neutrophils, host defense, and inflammation: a double-edged sword. J. Leukocyte Biol. 1994;56:672–686. doi: 10.1002/jlb.56.6.672. [DOI] [PubMed] [Google Scholar]
- SMITS E., BURVENICH C., HEYNEMAN R. Simultaneous flow cytometric measurement of phagocytotic and oxidative burst activity of polymorphonuclear leukocytes in whole bovine blood. Vet. Immunol. Immunopathol. 1997;56:259–269. doi: 10.1016/s0165-2427(96)05739-x. [DOI] [PubMed] [Google Scholar]
- STASIA M.J., STRULOVICI B., DANIEL-ISSAKANI S., PELOSIN J.M., DIANOUX A.C., CHAMBAZ E., VIGNAIS P.V. Immunocharacterization of beta- and zeta-subspecies of protein kinase C in bovine neutrophils. FEBS Lett. 1990;274:61–64. doi: 10.1016/0014-5793(90)81329-m. [DOI] [PubMed] [Google Scholar]
- SUSZTÁK K., MÓCSAI A., LIGETI E., CAPUZ A. Electrogenic H+ pathway contributes to stimulus-induced changes of internal pH and membrane potential in intact neutrophils: role of cytoplasmic phospholipase A2. Biochem. J. 1997;325:501–510. doi: 10.1042/bj3250501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWAIN S.D., BUNGER P.L., SIPES K.M., NELSON L.K., JUTILA K.L., BOYLAN S.M., QUINN M.T. Platelet-activating factor induces a concentration-dependent spectrum of functional responses in bovine neutrophils. J. Leukocyte Biol. 1998;64:817–827. doi: 10.1002/jlb.64.6.817. [DOI] [PubMed] [Google Scholar]
- TOUHARA K., HAWES B.E., VAN BIESEN T., LEFKOWITZ R.J. G protein beta gamma subunits stimulate phosphorylation of Shc adapter protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9284–9287. doi: 10.1073/pnas.92.20.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHAESEBROECK B., WELHAM MJ KOTANI K., STEIN R WARNE P.H., ZVELEBIL M.J., HIGASHI K., VOLINIA S., DOWNWARD J., WATERFIELD M.D. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN CORVEN E.J., HORDIJK P.L., MEDEMA R.H., BOS J.L., MOOLENAAR W.H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1257–1261. doi: 10.1073/pnas.90.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VLAHOS C.J., MATTER W.F., HUI K.Y., BROWN R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- WARD S., SOTSIOS Y., DOWDEN J., BRUCE I., FINAN P. Therapeutic potential of phosphoinositide 3-kinase inhibitors. Chem. Biol. 2003;10:207–213. doi: 10.1016/s1074-5521(03)00048-6. [DOI] [PubMed] [Google Scholar]
- WATERMAN W.H., SHA'AFI R.I. Effects of granulocyte–macrophage colony-stimulating factor and tumour necrosis factor-alpha on tyrosine phosphorylation and activation of mitogen-activated protein kinases in human neutrophils. Biochem. J. 1995;307:39–45. doi: 10.1042/bj3070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON G.L., SLOCOMBE R.F., ROBINSON N.E., SLEIGHT S.D. Definition of chemiluminescence and superoxide production responses of bovine neutrophils to selected soluble and particulate stimulants, and comparisons with the responses to Pasteurella haemolytica. Am. J. Vet. Res. 1995;56:1045–1054. [PubMed] [Google Scholar]
- WRIGHT J., MARIDONNEAU-PARINI I., CRAGOE E.J., JR, SCHWARTZ J.H., TAUBER A.I. The role of the Na+/H+ antiporter in human neutrophil NADPH-oxidase activation. J. Leukocyte Biol. 1988;43:183–186. doi: 10.1002/jlb.43.2.183. [DOI] [PubMed] [Google Scholar]
- WYMANN M.P., PIROLA L. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- YAFFE M.B., XU J., BURKE P.A., FORSE R.A., BROWN G.E. Priming of the neutrophil respiratory burst is species-dependent and involves MAP kinase activation. Surgery. 1999;126:248–254. [PubMed] [Google Scholar]
- YAMAMORI T., INANAMI O., NAGAHATA H., CUI Y., KUWABARA M. Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes. FEBS Lett. 2000;467:253–258. doi: 10.1016/s0014-5793(00)01167-4. [DOI] [PubMed] [Google Scholar]
- YANG W., DIEH J.R., ROUDEBUSH W.E. Comparison of the coding sequence of the platelet-activating factor receptor gene in three species. DNA Sequence. 2001;12:239–251. doi: 10.3109/10425170109024998. [DOI] [PubMed] [Google Scholar]
- YU P.W., CZUPRYNSKI C.J. Regulation of luminol-dependent chemiluminescence and degranulation by bovine neutrophils stimulated with opsonized zymosan. Vet. Immunol. Immunopathol. 1996;50:29–42. doi: 10.1016/0165-2427(95)05485-5. [DOI] [PubMed] [Google Scholar]