Abstract
We have previously shown that 11-keto boswellic acids (11-keto-BAs), the active principles of Boswellia serrata gum resins, activate p38 MAPK and p42/44MAPK and stimulate Ca2+ mobilisation in human polymorphonuclear leucocytes (PMNL).
In this study, we attempted to connect the activation of MAPK and mobilisation of Ca2+ to functional responses of PMNL, including the formation of reactive oxygen species (ROS), release of arachidonic acid (AA), and leukotriene (LT) biosynthesis.
We found that, in PMNL, 11-keto-BAs stimulate the formation of ROS and cause release of AA as well as its transformation to LTs via 5-lipoxygenase.
Based on inhibitor studies, 11-keto-BA-induced ROS formation is Ca2+-dependent and is mediated by NADPH oxidase involving PI 3-K and p42/44MAPK signalling pathways. Also, the release of AA depends on Ca2+ and p42/44MAPK, whereas the pathways stimulating 5-LO are not readily apparent.
Pertussis toxin, which inactivates Gi/0 protein subunits, prevents MAPK activation and Ca2+ mobilisation induced by 11-keto-BAs, implying the involvement of a Gi/0 protein in BA signalling.
Expanding studies on differentiated haematopoietic cell lines (HL60, Mono Mac 6, BL41-E-95-A) demonstrate that the ability of BAs to activate MAPK and to mobilise Ca2+ may depend on the cell type or the differentiation status.
In summary, we conclude that BAs act via Gi/0 protein(s) stimulating signalling pathways that control functional leucocyte responses, in a similar way as chemoattractants, that is, N-formyl-methionyl-leucyl-phenylalanine or platelet-activating factor.
Keywords: Boswellic acids, leucocytes, MAPK, Ca2+, reactive oxygen species, lipoxygenase, arachidonic acid
Introduction
Extracts of Boswellia serrata gum resins have been traditionally used as folk medicine to cure inflammatory and arthritic diseases (Safayhi & Sailer, 1997). It was found that B. serrata extracts suppress the formation of proinflammatory leukotrienes (LTs), and boswellic acids (BAs) were identified as the active principles targeting 5-lipoxygenase (5-LO), the key enzyme in LT biosynthesis (Safayhi et al., 1992,1995). In addition, human leucocyte elastase was found to be a target for BAs (Safayhi et al., 1997). Aside of these anti-inflammatory implications, BAs have been reported to influence the growth and differentiation of tumour cells. Thus, B. serrata extracts or isolated BAs induce the apoptosis of brain tumour cell lines (Glaser et al., 1999), meningioma cells (Park et al., 2002), rat gliomas (Winking et al., 2000), liver and colon cancer cell lines (Liu et al., 2002a), and also of leukaemic cells (Hoernlein et al., 1999). Caspase-8 (Liu et al., 2002a), topoisomerases (Hoernlein et al., 1999), and the p42/44MAPK pathway (Park et al., 2002) have been suggested as signalling molecules mediating the apoptotic effects of BAs.
With respect to inhibition of 5-LO, 3-acetyl-11-keto-BA (AKBA) was the most potent BA, whereas BAs lacking an 11-keto-group were weak 5-LO inhibitors (Safayhi et al., 1992). The IC50 values of AKBA for inhibition of 5-LO differ between different groups and appear to depend also on the cell type. Thus, the IC50 values were determined in the range of 1.5 μM for 5-LO in rat neutrophils (Safayhi et al., 1992) and 12–15 μM in differentiated HL60 and MM6 cells, respectively (Werz et al., 1997). In cell-free systems, the IC50 values of AKBA for 5-LO inhibition were around 50 μM, implying that, in intact cells, cellular components or mechanisms improve the efficacy of AKBA (Werz et al., 1997). Finally, low concentrations of ethanolic extracts of B. serrata potentiated 5-LO product formation in PMNL induced by ionophore (Safayhi et al., 2000), and 3-oxo-tirucallic acid, isolated from B. serrata extracts, stimulated 5-LO product synthesis in resting and agonist-challenged PMNL (Boden et al., 2001). Therefore, until today, inhibition of 5-LO as the main principle for the anti-inflammatory effects of B. serrata extracts, determined in several in vivo models (Gupta et al., 1992; Krieglstein et al., 2001) and pilot clinical studies (Gerhardt et al., 2001; Gupta et al., 2001), remains a matter of debate.
Chemotactic agonists, such as platelet-activating factor (PAF), N-formyl-methionyl-leucyl-phenylalanine (fMLP), or LTB4, bind to their specific G protein-coupled receptors (GPCR), leading to the activation of MAPK and the mobilisation of Ca2+, which are pivotal signalling molecules that regulate a number of functional processes of PMNL, including chemotaxis, degranulation, formation of reactive oxygen species (ROS), release of arachidonic acid (AA), and LT biosynthesis (Herlaar & Brown, 1999; Belcheva & Coscia, 2002; Johnson & Druey, 2002). Recently, we showed that 11-keto-BAs (AKBA, KBA, in the range of 10–30 μM) are potent activators of p42MAPK and p38 MAPK, and stimulate Ca2+ mobilisation in PMNL (Altmann et al., 2002). Comparison of these actions of BAs with those of chemotactic agonists led us to conclude that BAs may possibly function as ligands of a certain GPCR. In this study, we investigated if BAs are able to induce the functional cellular responses in PMNL and if a Gi/0-coupled heterotrimeric G protein mediates BA-induced MAPK/Ca2+ signalling. Finally, we demonstrate that BAs induce signalling responses depending on the cell type.
Methods
Materials
A-β-BA, β-BA, AKBA, and KBA were purchased from ChromaDex (Laguna Hills, CA, U.S.A.). Nycoprep was from PAA Laboratories (Linz, Austria); diphenyleneiodonium chloride (DPI), phorbol 12-myristate 13-acetate (PMA), Ca2+-ionophore A23187, and fMLP were from Sigma Chemical Co. (Deisenhofen, Germany); BAPTA/AM, LY-294002, SB203580, U0126, and Fura-2/AM were from Calbiochem (Bad Soden, Germany); RO-31-8425 was from Alexis (Grünberg, Germany); 2′,7′-dichlorofluorescein diacetate (DCF-DA) and lucigenin were from Molecular Probes European BV (Leiden, Netherlands); and wortmannin from Biotrend (Colonia, Germany). RPMI-1640 medium was from Gibco-BRL (Grand Island, NY, U.S.A.), and fetal calf serum was obtained from Boehringer Mannheim (Mannheim, Germany). Human TGF-β1 was purified from outdated platelets, as described (Werz et al., 1996). Calcitriol was kindly provided by Dr H. Wiesinger (Schering AG). [3H]AA was from Biotrend (Colonia, Germany); and high-performance liquid chromatography (HPLC) solvents were from Merck (Darmstadt, Germany).
Cells
Human PMNL were freshly isolated from leucocyte concentrates obtained at St Markus Hospital (Frankfurt, Germany). In brief, venous blood was taken from healthy adult donors and subjected to centrifugation for the preparation of leucocyte concentrates. PMNL were immediately isolated by dextran sedimentation, centrifugation on Nycoprep cushions, and hypotonic lysis of erythrocytes, as described (Werz et al., 2002a). PMNL were finally resuspended in PBS plus 1 mg ml−1 glucose (PG buffer), or alternatively in PBS plus 1 mg ml−1 glucose and 1 mM CaCl2 (PGC buffer), as indicated.
HL60 cells were maintained in RPMI 1640 medium supplemented with 10% fetal calf serum, 100 μg ml−1 streptomycin, and 100 U ml−1 penicillin. For differentiation towards granulocytic cells, cells were seeded at a density of 2 × 105 cells ml−1 and cultured in the presence of 1.5% (v v−1) dimethylsulphoxide, 500 pM calcitriol, and 2 ng ml−1 TGFβ for 4 days.
Mono Mac (MM) 6 cells were cultured and differentiated with TGFβ and calcitriol, as described (Werz et al., 1996).
Determination of release of [3H]-labelled AA from PMNL
Freshly isolated PMNL were resuspended at 2 × 106 in 1 ml RPMI 1640 medium containing 4.8 nM[3H]AA (corresponding to 0.25 μCi ml−1, specific activity 200 Ci mmol−1) and incubated for 120 min at 37°C in 5% CO2 atmosphere. Thereafter, the cells were collected by centrifugation, washed once with PBS and twice with PBS containing 2 mg ml−1 fatty acid-free albumin, to remove unincorporated [3H]AA. Labelled PMNL (5 × 106) were resuspended in 1 ml PGC buffer containing 2 mg ml−1 fatty acid-free albumin. The reaction was started by addition of the indicated stimuli. After 5 min at 37°C, the samples were placed on ice for 2 min and cells were centrifuged at 400 × g for 5 min at RT. Aliquots (100 μl) of the supernatants were measured in a beta-counter (Micro Beta Trilux, Perkin Elmer, Foster City, CA, U.S.A.) to detect the amounts of [3H]-labelled AA released into the medium.
Determination of cellular peroxide formation
Measurement of peroxides was conducted using the peroxide-sensitive fluorescence dye DCF-DA. Freshly isolated PMNL (1 × 107), HL 60 cells (1 × 107), or MM6 cells (5 × 106) were resuspended in 1 ml PGC buffer and preincubated with DCF-DA (1 μg ml−1 for PMNL and MM6; 10 μg ml−1 for HL 60 cells) for 2 min at 37°C in a thermally controlled (37°C) fluorimeter cuvette in a spectrofluorometer (Aminco-Bowman series 2, Thermo Spectronic, Rochester, NY, U.S.A.) with continuous stirring. The fluorescence emission at 530 nm was measured after excitation at 485 nm. The mean fluorescence data measured 5 min after stimulus addition are given as arbitrary units.
Detection of superoxide anion
Freshly isolated PMNL (2 × 105) were resuspended in 200 μl of 100 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 20 mM HEPES, pH 7.4, and transferred to a 96-well plate. Lucigenin (25 μM, final concentration) and 1 mM CaCl2 were added and cells were stimulated with the indicated stimuli. After stirring, samples were measured in duplicates at 37°C. The chemiluminescence (CL) was recorded using a Micro Luminat Plus LB 96 V (Berthold, Bad Wildbad, Germany) at intervals of 6 s (two cycles) in a total detection time of 3 min. The detected CL was summarised over two intervals and plotted versus blank values.
Measurement of intracellular Ca2+ levels
The determination of intracellular Ca2+ levels was performed as described previously (Werz, 2002a). In brief, freshly isolated PMNL (1 × 107), HL 60 cells (1 × 107 buffer), or MM6 cells (3 × 106) were resuspended in 1 ml PGC buffer and incubated with 2 μM Fura-2/AM for 30 min at 37°C. After washing, cells were finally resuspended in 1 ml PGC buffer and transferred into a thermally controlled (37°C) fluorimeter cuvette in a spectrofluorometer (Aminco-Bowman series 2, Thermo Spectronic, Rochester, NY, U.S.A.) with continuous stirring. The fluorescence emission at 510 nm was measured after excitation at 340 and 380 nm, respectively. Intracellular Ca2+ levels were calculated according to the method of Grynkiewicz et al. (1985), whereas Fmax (maximal fluorescence) was obtained by lysing the cells with 0.5% Triton-X 100 and Fmin by chelating Ca2+ with 10 mM EDTA.
Determination of MAPK activation by SDS–PAGE and Western blotting
Freshly isolated PMNL, HL60, or MM6 cells (5 × 106 each) were resuspended in PGC buffer; the final volume was 100 μl. After addition of the indicated stimuli, samples were incubated at 37°C and the reaction was stopped by addition of 100 μl of ice-cold 2 × sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) sample-loading buffer (SDS-b; 20 mM Tris/HCl, pH 8, 2 mM EDTA, 5% SDS (w v−1), 10% β-mercaptoethanol), vortexed and heated for 6 min at 95°C. Total cell lysates (20 μl) were mixed with 4 μl of glycerol/0.1% bromophenolblue (1 : 1, v v−1) and analysed by SDS–PAGE using a Mini Protean system (Bio-Rad, Hercules, CA, U.S.A.) on a 10% gel. After electroblot to nitrocellulose membrane (Amersham Pharmacia, Little Chalfont, U.K.), membranes were blocked with 5% nonfat dry milk in 50 mM Tris/HCl, pH 7.4, and 100 mM NaCl (Tris-buffered saline (TBS)) plus 0.1% Tween 20 for 1 h at RT. Membranes were washed and then incubated with primary antibody (AB) overnight at 4°C. Phospho-specific ABs recognising p44/42MAPK (Thr202/Tyr204) and p38 MAPK (Thr180/Tyr182) were obtained from New England Biolabs (Beverly, MA, U.S.A.), and used as 1 : 2000 dilution. The membranes were washed and incubated with 1 : 1000 dilution of alkaline phosphatase-conjugated immunoglobulin G (Sigma Chemical Co.) for 2 h at RT. After washing with TBS and TBS plus 0.1% NP-40, proteins were visualised with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma Chemical Co.) in detection buffer (100 mM Tris/HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2).
Determination of 5-LO product formation in PMNL
To determine 5-LO product formation in intact cells, 1 × 107 freshly isolated PMNL were finally resuspended in 1 ml PG buffer. When 5-LO product formation was assayed in the absence of Ca2+, PMNL were finally resuspended in 1 ml PG buffer and 1 mM EDTA and 30 μM BAPTA/AM were added. The reaction was started by simultaneous addition of exogenous AA with the indicated BAs. After 10 min at 37°C, the reaction was stopped with 1 ml of methanol, and 30 μl of 1 N HCl, and 200 ng prostaglandin B1 and 500 μl of PBS were added. Formed AA metabolites were extracted using C-18 solid-phase extraction columns and analysed by HPLC as described (Werz et al., 1997). 5-LO product formation is expressed as ng of 5-LO products per 106 cells, which includes LTB4 and its all-trans isomers, 5(S),12(S)-di-hydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid (5(S),12(S)-DiHETE), 5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HETE), and 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE). 5-HETE and 5-HPETE coelute as one major peak; integration of this peak represents both eicosanoids. Cysteinyl LTs (LTC4, D4, and E4) were not detected and oxidation products of LTB4 were not determined.
Statistics
The results are presented as mean±s.e. The program ‘GraphPad PRISM 3.0' was used for statistical comparisons. Statistical evaluation of the data was performed using Student's t-test for unpaired observations. A P-value of <0.05 was considered significant.
Results
11-Keto-BAs stimulate the release of superoxide anion and ROS in PMNL
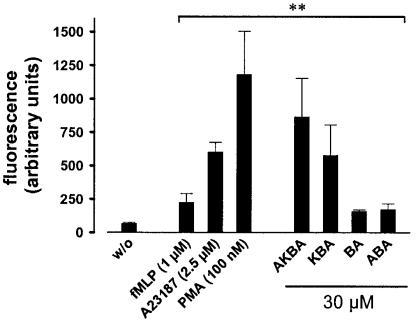
In order to explore the effects of BAs on PMNL, we sought to determine whether BAs are capable of eliciting functional processes connected to Ca2+ mobilisation and MAPK activation. Phagocytes undergo an oxidative burst in response to different agonists, resulting in the release of ROS via the NADPH oxidase. PMNL, preloaded with the ROS-sensitive dye DCF-DA, were stimulated with BAs (30 μM, each) and for comparison with 1 μM fMLP, 2.5 μM ionophore A23187, and 100 nM PMA. After 5 min, ROS formation was determined by analysing the fluorescence of the oxidised dye. In agreement with the literature (Roos et al., 1976; Simchowitz & Spilberg, 1979), fMLP, ionophore A23187, or PMA caused a rapid formation of ROS in PMNL (Figure 1). AKBA and KBA strongly upregulated the formation of ROS, whereas β-BA and A-β-BA had only moderate effects (Figure 1). Notably, the magnitude of AKBA- (or KBA-) induced ROS formation was much more pronounced as compared to fMLP, being in a close range of the efficacy of ionophore and PMA. Similar results were obtained when the formation of superoxide anion (O2−) was determined by measuring the chemiluminescence of the metabolised lucigenin. Thus, stimulation of PMNL with 30 μM AKBA resulted in a 12-fold increase in O2− formation compared to unstimulated cells, whereas fMLP (1 μM) and PMA (100 nM) caused 4- and 20-fold elevations, respectively (not shown).
Figure 1.
11-Keto-BAs induce the generation of ROS in PMNL. Freshly isolated PMNL (5 × 106 in 1 ml PGC buffer) were preincubated with DCF-DA (1 μg ml−1) for 2 min at 37°C, prior addition of the indicated stimuli. The generation of peroxides was measured as described. Data determined 5 min after addition of stimuli are expressed as the mean of the fluorescence given in arbitrary units ±s.e., n=4. Student's t-test; **P<0.01.
AKBA-induced ROS formation involves NADPH oxidase, PI 3-K, and p42/44MAPK, and requires Ca2+
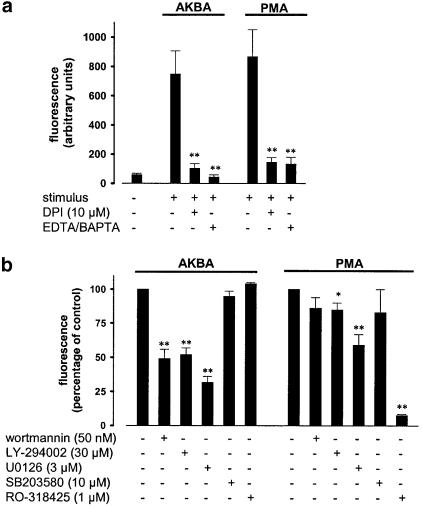
To further dissect the signalling pathways leading to ROS formation, pharmacological inhibitors of potential signalling molecules were examined using the DCF-DA fluorescence assay. PMA, used as a control, binds to PKC isoenzymes, stimulating NADPH oxidase by multiple phosphorylations of p47phox (Heyworth & Badwey, 1990). DPI, a direct inhibitor of NADPH oxidase (Hancock & Jones, 1987), almost completely abolished PMA- as well as AKBA-induced ROS formation (Figure 2a). Also, chelation of Ca2+ by 30 μM BAPTA/AM and 1 mM EDTA clearly reduced AKBA- or PMA-induced ROS formation (Figure 2a). As shown in Figure 2b, PMA-induced ROS formation was only slightly affected by wortmannin (50 nM) or LY-294002 (30 μM), inhibitors of phosphatidylinositol 3-kinase (PI 3-K), or by the p38 MAPK inhibitor SB203580 (10 μM). However, U0126 (3 μM) that blocks the activation of p42/44MAPK partially reduced ROS generation and the PKC inhibitor RO-318425 (1 μM) totally abolished the effects of PMA. In contrast, AKBA-induced ROS formation was not at all affected by PKC inhibition (RO-318425), but was clearly reduced by wortmannin or LY-294002, and by U0126, implying an involvement of PI 3-K and p42/44MAPK, respectively (Figure 2b). Also, for AKBA-induced ROS formation, a role for p38 MAPK is not apparent.
Figure 2.
AKBA-induced ROS formation involves NADPH oxidase, PI 3-K, and p42/44MAPK, and requires Ca2+. PMNL (5 × 106 in 1 ml PGC buffer) were preincubated with the indicated compounds for 20 min at RT, prior addition of DCF-DA (1 μg ml−1). After another 2 min, AKBA (30 μM) or PMA (100 nM) were added and the generation of peroxides was measured as described above. (a) Effects of DPI and Ca2+ depletion. Data determined 5 min after addition of stimuli are expressed as the mean of the fluorescence given in arbitrary units±s.e., n=3. Student's t-test; **P<0.01. (b) Effects of pharmacological protein kinase inhibitors. Data were determined 5 min after addition of stimuli and expressed as the percentage of the mean fluorescence±s.e., n=4, of control. Student's t-test; *P<0.05, **P<0.01. The control values (100%) in the absence of inhibitors for cells stimulated with AKBA were 425±57, and for PMA 675±103 arbitrary fluorescence units.
11-Keto-boswellic acids cause the release of [3H]AA from PMNL
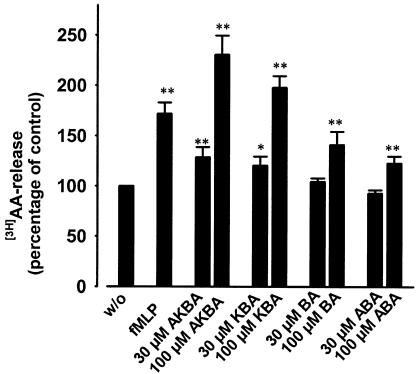
In PMNL, AA is released from phospholipids upon cell stimulation by the cytosolic PLA2 (cPLA2). Since cPLA2 is activated by phosphorylation at serine residues by members of the MAPK family and/or elevation of the intracellular Ca2+ levels (Gijon & Leslie, 1999), BAs were tested for their ability to elevate the liberation of AA from PMNL. AKBA and KBA considerably enhanced the release of [3H]-labelled AA, whereas β-BA and A-β-BA showed only weak effects (Figure 3). Also, under these experimental settings, AKBA and KBA caused even more pronounced responses as compared to 1 μM fMLP. The fMLP- as well as the AKBA-induced AA release was completely abolished when Ca2+ was removed by chelation using EDTA and BAPTA/AM (not shown). Experiments using SB203580 (10 μM) and U0126 (3 μM), in order to inhibit p38 MAPK and p42/44MAPK activities, respectively, were performed to estimate the importance of these MAPK for AA liberation. The effect of AKBA was partially suppressed by U0126 (53±4.6%), but was unaffected by SB203580 (not shown), implying that p42/44MAPK, but apparently not p38 MAPK, may contribute to the AKBA-induced AA release.
Figure 3.
11-Keto-BAs elicit the liberation of AA in PMNL. Freshly isolated PMNL (2 × 106 in 1 ml RPMI 1640 medium) were prelabelled with 0.25 μCi ml−1 [3H]AA for 120 min at 37°C and 5% CO2. After unincorporated [3H]AA was removed, cells (5 × 106 in 1 ml PGC buffer, containing 2 mg ml−1 fatty acid-free albumin) were treated with the indicated additives and incubated for 5 min at 37°C. Free (nonesterified) [3H]AA was determined as described in Methods. Results are given as mean±s.e. (n=4). Student's t-test; *P<0.05; **P<0.01.
Effects of 11-keto-boswellic acids on cellular 5-LO product formation in PMNL
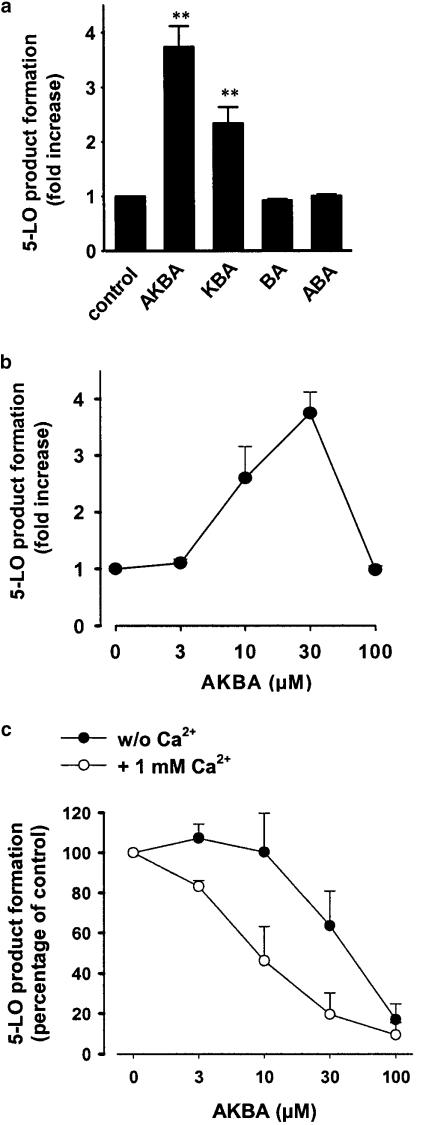
In intact cells, 5-LO can be activated upon elevation of the intracellular Ca2+ levels and/or phosphorylation by p38 MAPK-regulated MAPKAPKs and p42/44MAPK (Werz et al., 2000,2002a,2002b). Although 11-keto BAs are direct inhibitors of 5-LO (Safayhi et al., 1992,1995), we speculated that, due to their potential to mobilise Ca2+ and to activate MAPK, BAs could also stimulate 5-LO in intact cells. As shown in Figure 4a, when 11-keto-BAs were added to PMNL together with 20 μM AA, AKBA and KBA (30 μM each) caused 3.8- and 2.4-fold increases in 5-LO product formation versus control cells that had been stimulated with AA alone, whereas no upregulatory effects were observed for β-BA and A-β-BA. Notably, such upregulatory effects of the 11-keto-BAs were most prominent when Ca2+ was chelated by EDTA and BAPTA/AM, whereas, in the presence of Ca2+, AKBA increased 5-LO product formation only by 1.9-fold. Expanding the preincubation period with BAs prior addition of AA (>1 min) strongly reduced the effects of BAs (not shown). The dose–response curve shown in Figure 4b reveals that, at higher concentrations (100 μM), AKBA fails to stimulate 5-LO, possibly due to direct inhibitory enzyme interaction. In order to determine if p38 MAPK and/or p42/44MAPK are required for AKBA-induced 5-LO activation, PMNL were preincubated with 3 μM U0126 and/or 10 μM SB203580 prior stimulation. Upregulation of 5-LO product formation by AKBA (in the absence of Ca2+) was not significantly suppressed by these inhibitors (not shown), suggesting that MAPK are not determinants for AKBA-induced 5-LO activation.
Figure 4.
Effects of 11-keto-BAs on the formation of 5-LO metabolites. (a) Freshly isolated PMNL (5 × 106) were resuspended in 1 ml PG buffer containing 1 mM EDTA and preincubated with 30 μM BAPTA/AM for 15 min. Then, cells were stimulated with 20 μM AA alone or together with the indicated BAs (30 μM, each). After 10 min at 37°C, 5-LO products were determined by HPLC. Results are given as mean+s.e., n=4–6. Student's t-test; **P<0.01. (b) Dose–response curve of AKBA. PMNL (5 × 106) were resuspended in 1 ml PG buffer containing 1 mM EDTA and preincubated with 30 μM BAPTA/AM for 15 min. Then, cells were stimulated with 20 μM AA alone or together with the indicated amounts of AKBA. After 10 min at 37°C, 5-LO products were determined. Results are given as mean+s.e., n=4–5. (c) Inhibition of 5-LO. PMNL (5 × 106) were resuspended in either 1 ml PGC buffer or PG buffer containing 1 mM EDTA and 30 μM BAPTA/AM. After 15 min at 37°C, AKBA was added, and cells were incubated for another 30 min at 37°C. Then, CaCl2 was adjusted to 1 mM in all incubations and cells were immediately stimulated with 2.5 μM ionophore and 40 μM AA. After 10 min, 5-LO products were determined. Results are given as mean+s.e., n=3.
Prolonged exposure of 5-LO to elevated Ca2+ or oxidants such as ROS leads to a rapid inactivation of 5-LO (Ford-Hutchinson et al., 1994). Hence, it appeared possible, that the potent 5-LO inhibition by AKBA in intact cells after longer preincubation periods (30 min) prior cell stimulation is related to the prominent Ca2+ mobilisation and the accompanied ROS release. PMNL were preincubated for 30 min with AKBA in the presence of Ca2+, which results in Ca2+ mobilisation and ROS formation. Alternatively, Ca2+ was removed by 1 mM EDTA and 30 μM BAPTA/AM during this preincubation period, conditions where ROS release does not occur. Subsequently, after preincubation, all samples were adjusted to 1 mM Ca2+ and cells were immediately stimulated with ionophore and AA to induce 5-LO product formation in intact cells. As can be seen from Figure 4c, the efficacy of AKBA to inhibit 5-LO product formation was strongly impaired when cells were preincubated in the absence of Ca2+ (IC50 approx. 50 μM), as compared to conditions when Ca2+ was present (IC50 approx. 8 μM). Thus, potent 5-LO inhibitory effects of AKBA in intact cells by may require Ca2+-mediated processes, such as ROS formation.
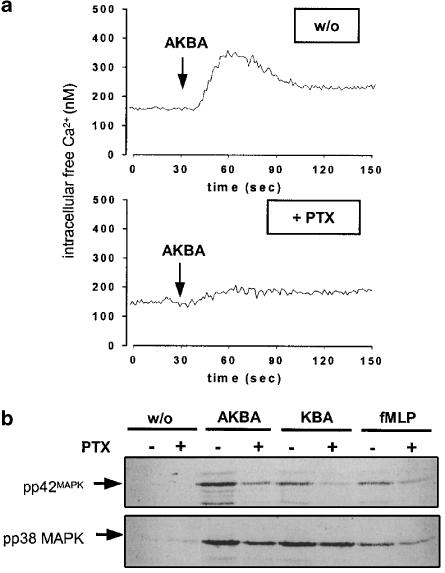
Pertussis toxin (PTX) attenuates boswellic acid-induced mobilisation of Ca2+ and MAPK activation in PMNL
The proximal signalling pathways involved, as well as the kinetics for Ca2+ mobilisation and MAPK activation, resembled those of chemotactic factors, such as fMLP, that act via G protein-coupled receptors (GPCR). In order to determine a possible role of Gi or G0 proteins in the AKBA-induced Ca2+ release and MAPK activation, the effects of PTX, an irreversible inhibitor of Gαi/0 subunits of heterotrimeric G proteins, were assessed in human isolated PMNL. As shown in Figure 5a, PTX (2 μg ml−1) suppressed the Ca2+ response induced by 30 μM AKBA in a similar fashion as compared to fMLP stimulation. Similarly, PTX clearly reduced the activation of p42MAPK in PMNL, stimulated with AKBA or KBA, whereas activation of p38 MAPK was only moderately reduced (Figure 5b). In control experiments (Table 1 and Figure 5b), PTX also partially decreased the fMLP-induced activation of MAPK and Ca2+ mobilisation, whereas MAPK activation (not shown) and Ca2+ mobilisation (Table 1) induced by ionomycin (that circumvents G protein signalling) were not suppressed by PTX.
Figure 5.
PTX suppresses Ca2+ mobilisation and activation of MAPK in PMNL induced by boswellic acids. (a) Ca2+ mobilisation. Freshly isolated PMNL (1±107 ml−1 PGC buffer) were loaded with 2 μM Fura-2 for 30 min at 37°C. Then, cells were preincubated with or without 2 μg ml−1 PTX for 60 min at 37°C. After addition of 30 μM AKBA, the fluorescence was measured and intracellular free Ca2+ was calculated as described. The monitored curves show one typical experiment out of four. (b) MAPK activation. Freshly isolated PMNL (5 × 106 in 100 μl PGC buffer) were preincubated with or without 2 μg ml−1 PTX for 60 min at 37°C and then stimulated with 30 μM AKBA, 30 μM KBA, 1 μM fMLP, or left untreated. After 1.5 min at 37°C, incubations were terminated by addition of the same volume of ice-cold SDS-b. Samples were electrophoresed and analysed for dually phosphorylated p38 MAPK or p44/42MAPK by Western blotting.
Table 1.
Effects of PTX on Ca2+ mobilisation
| Stimulus | Ca2+ mobilisation (percentage of control) |
|---|---|
| Ionomycin (2.5 μM) | 94.1±8.3 |
| fMLP (100 nM) | 39.6±9.7 |
| AKBA (30 μM) | 43.8±16.8 |
Freshly isolated PMNL (1 × 107 ml−1 PGC buffer) were loaded with 2 μM Fura-2 for 30 min at 37°C. Then, cells were preincubated with or without 2 μg ml−1 PTX for 60 min at 37°C. After addition of the indicated stimuli, the fluorescence was measured and intracellular free Ca2+ was calculated as described. Values (mean±s.e., n=4) are given as the percentage of Ca2+ mobilisation of PTX-treated cells versus control cells that received no PTX.
Moreover, PTX (2 μg ml−1) partially inhibited AKBA-induced ROS formation (21.1±7.6%), but did not at all affect ROS production in PMNL stimulated by PMA. On the other hand, PTX caused only marginal suppression (8.3±4.1%) of AKBA-induced 5-LO activity in PMNL.
Cell type-dependent induction of MAPK activation, Ca2+ mobilisation and ROS generation by BAs
The effects of BAs on the mobilisation of Ca2+, activation of MAPK and formation of ROS were further investigated in various haematopoietic cell lines. For monocytic MM6 cells differentiated with calcitriol and TGFβ, none of the four different BAs (up to 100 μM) induced Ca2+ mobilisation or activation of MAPK, and also no significant induction of ROS formation was observed (not shown). In control experiments, PAF, LTB4 or fMLP caused pronounced elevation of intracellular Ca2+, demonstrating that G protein-coupled Ca2+ mobilisation is operative in these cells. Similarly, BAs also failed to activate the B-lymphocytic cell line BL41-E-95-A in this respect (not shown).
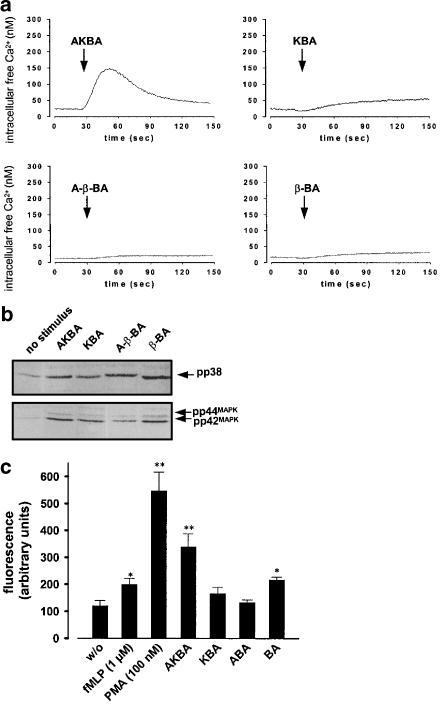
In contrast, the effects of BAs in differentiated granulocytic HL60 cells resembled those observed in PMNL from human blood. Thus, AKBA considerably induced mobilisation of Ca2+, stimulated activation of p38 MAPK and p42MAPK, and upregulated the formation of ROS (Figure 6). Interestingly, KBA was less effective, whereas β-BA and A-β-BA also stimulated HL60 cells for MAPK activation and release of ROS. Nevertheless, β-BA and A-β-BA failed to substantially mobilise Ca2+ in differentiated HL60 cells. It should be noted that undifferentiated HL60 cells did neither respond to AKBA nor to the other BAs (not shown), implying that a certain signalling component(s), induced during differentiation, is required to transduce the effects of BAs.
Figure 6.
Effects of BAs on Ca2+ release, MAPK activation, and ROS production in granulocytic HL60 cells. HL60 cells were differentiated towards granulocytic cells in the presence of 1.5% DMSO, 2 ng ml−1 TGFβ, and 500 pM calcitriol for 4 days. (a) Mobilisation of Ca2+. Cells (107 ml−1 PGC buffer) were loaded with 2 μM Fura-2 for 30 min at 37°C. After addition of BAs (50 μM, each) or 1 μM fMLP, the fluorescence was measured and intracellular free Ca2+ was calculated as described. The monitored curves show one typical experiment out of 4–5, respectively. (b) MAPK activation. Cells (5 × 106/100 μl PGC buffer) were stimulated with 30 μM of BAs, 1 μM fMLP, or left untreated. After 1.5 min at 37°C, incubations were terminated by addition of the same volume of ice-cold SDS-b. Samples were electrophoresed and analysed for dually phosphorylated p38 MAPK or p44/42MAPK by Western blotting. (c) ROS formation. Cells (5 × 107 ml−1 PGC buffer) were preincubated with DCF-DA (10 μg ml−1) for 2 min at 37°C prior addition of the indicated stimuli. The generation of peroxides was measured as described. Data determined 5 min after addition of stimuli are expressed as the mean of the fluorescence given in arbitrary units±s.e., n=4. Student's t-test; *P<0.05; **P<0.01.
Discussion
We have recently reported that 11-keto-BAs potently stimulate the elevation of intracellular Ca2+ levels and activate p38 MAPK as well as p42MAPK (Altmann et al., 2002), which are pivotal signalling events that regulate numerous effectors of PMNL. In the present study, we show that 11-keto-BAs in fact elicit functional responses of PMNL or granulocytic HL60 cells such as ROS generation, increased liberation of AA, and its subsequent metabolism by 5-LO. Investigation of the signalling molecules involved and comparison of the kinetics with those of chemotactic ligands for leukocytes, that is, fMLP, PAF or LTB4, led us to conclude that 11-keto-BAs may transduce their signals in a common way as chemotactic ligands that involve GPCR signalling (see Altmann et al. (2002) and references therein). Since elevated levels of AA and ROS are critical inducers of caspase-mediated apoptosis (Hampton et al., 1998; Taketo & Sonoshita, 2002), our findings may also provide a molecular basis for the 11-keto-BA-induced caspase activation (Liu et al., 2002a,2002b) and the apoptotic effects observed in various cancer cell lines (Shao et al., 1998; Glaser et al., 1999; Hoernlein et al., 1999; Jing et al., 1999; Liu et al., 2002a,2002b).
O2− is the precursor of ROS, which are essential for the host defence against microorganisms. The NADPH oxidase of leucocytes is the major source of O2− released upon agonist challenge (Chanock et al., 1994). Activation of NADPH oxidase requires the presence of Ca2+ and multiple phosphorylations of the subunit p47phox by certain PKC isoenzymes or other kinases (Heyworth & Badwey, 1990; Chanock et al., 1994; Dewas et al., 2000). We demonstrate that, according to their ability to stimulate Ca2+ mobilisation and MAPK activation (compare Altmann et al., 2002), 11-keto-BAs, but not BAs lacking the 11-keto group, induced a rapid and robust formation of O2− and of ROS in PMNL, as determined by lucigenin chemiluminescence and the oxidation of DCF-DA, respectively. Since the NADPH oxidase inhibitor DPI (Hancock & Jones, 1987) abolished ROS formation, ROS derived from the NADPH oxidase system may indeed be responsible. Pharmacological targeting of the proximal signalling pathways revealed that AKBA-induced ROS formation seemingly depends on PI 3-K and on the p42/44MAPK pathway, but does not require p38 MAPK or PKC. In this context, it is interesting that the fMLP-induced phosphorylation of the p47phox component in neutrophils was suppressed by inhibition of p42MAPK (Dewas et al., 2000) and PI 3-K (Ding et al., 1995), but not when p38 MAPK was blocked by SB203580 (Dewas et al., 2000). In contrast, our control experiments utilising PMA as a direct activator of PKC confirm the findings by others, showing that PKC, and to a minor extent also p42MAPK, are required for NADPH oxidase-dependent ROS formation (Cox et al., 1985; Heyworth & Badwey, 1990; Dewas et al., 2000), whereas a role of PI 3-K or p38 MAPK is not readily apparent. Notably, Ca2+ signalling pathways are determinants for ROS formation, since chelation of Ca2+ strongly suppressed the signals induced by AKBA and also by PMA. Together, 11-keto BAs may stimulate ROS formation by mobilisation of Ca2+ and by activation of p42/44MAPK, apparently involving PI 3-K.
The release of free AA by leukocytes is an important step in the onset of inflammatory reactions and the cPLA2, which is regulated by Ca2+ and phosphorylation by MAPK, appears to play a major role for AA liberation in PMNL (Gijon & Leslie, 1999). 11-keto BAs, but not β-BA and A-β-BA, induced the release of considerable amounts of AA, with a similar efficacy as fMLP. The doses of the respective BAs were somewhat higher than those needed for the formation of ROS, which might be due to the fact that cPLA2 favours a sustained Ca2+ influx (Qiu et al., 1998), which in turn requires higher concentrations of AKBA or KBA (unpublished observations). In agreement with findings reported for ligands acting via GPCR (Qiu et al., 1998), AKBA-induced AA liberation also depended on Ca2+ and p42/44MAPK.
Although BAs have been initially reported as direct-type inhibitors of the 5-LO enzyme, without reducing or iron-chelating properties (Safayhi et al., 1992,1995), we demonstrate that AKBA can paradoxically stimulate cellular 5-LO, when added to PMNL concomitantly with AA. Also, it was reported that low concentrations of B. serrata extracts enhanced ionophore-stimulated 5-LO product synthesis in PMNL (Safayhi et al., 2000), and 3-oxo-tirucallic acid, that acts as a direct 5-LO inhibitor in cell-free systems, induced and upregulated 5-LO activity in human neutrophils (Boden et al., 2001). In intact cells, mobilisation of Ca2+, and also phosphorylation by MAPKAPKs and by p42/44MAPK, activates 5-LO for product formation (Werz et al., 2000,2002a,2002b). Interestingly, AKBA-induced 5-LO activation was most prominent in the absence of Ca2+, and was not sensitive to MAPK inhibitors and hardly sensitive to PTX. Thus, additional unknown factors or pathways induced by AKBA seem operative, which still remain to be determined.
A discrepancy in the efficacy of AKBA is evident for suppression of 5-LO in intact cells (IC50≈2–5 μM) and cell-free systems (IC50≈15–50 μM) (Safayhi et al., 1995; Werz et al., 1997,1998). Thus, in intact cells, additional factors are operative for the suppression of 5-LO. Since prolonged exposure to elevated Ca2+ or oxidants rapidly inactivates 5-LO (Ford-Hutchinson et al., 1994), it appeared possible that the potent 5-LO inhibition by AKBA in intact cells is related to the prominent ROS release occurring during preincubation periods (15–30 min) prior cell stimulation. In fact, Ca2+ depletion, that prevents ROS formation, impaired the efficacy of AKBA in intact cells (Figure 4c), and the respective IC50 value fits well with those obtained in cell-free systems (Werz et al., 1998). Intriguingly, the efficacy of AKBA for suppression of 5-LO in MM6 cells (IC50≈15 μM), that are unable to mobilise Ca2+ and to produce ROS in response to AKBA, is significantly reduced as compared to PMNL (IC50≈2–5 μM).
Upon ligation of their specific GPCR, chemoattractants elicit various functional responses of different leucocytes involving Ca2+ mobilisation and activation of MAPK (Herlaar & Brown, 1999; Belcheva & Coscia, 2002). The putative BA receptor, operative in PMNL and mature HL60 cells, seems to be induced during differentiation towards granulocytic cells, since undifferentiated HL60 cells did not respond to BAs with Ca2+ mobilisation, MAPK activation or ROS formation. Along these lines, it was found that, in HL60 cells, the G protein-coupled fMLP receptor is also first induced after differentiation (Perez et al., 1992). Nevertheless, differentiated monocytic MM6 cells apparently do not possess any BA-inducible signalling pathways or BA receptor(s), although these cells respond to diverse ligands of GPCR (fMLP, LTB4 or PAF), implying intact G protein signalling pathways in differentiated MM6 cells. Intriguingly, differentiated MM6 cells also failed to mobilise Ca2+ in response to 5(S)-HETE or 5-oxo-ETE, which on the other side caused significant Ca2+ mobilisation in PMNL (unpublished observations). Accordingly, even the expression of the putative G protein coupled 5-oxo-ETE receptor was found to be cell type-dependent (O'Flaherty et al., 2000). Thus, we conclude that the expression of the target (–receptor) of AKBA may be restricted to certain cell types. Finally, it should be noted that, in contrast to PMNL, in differentiated HL60 cells also β-BA and A-β-BA caused cell stimulation (Figure 6a and b), suggesting that, at least in HL60 cells, putative receptor subtypes (not present in PMNL) may exist that accept BAs lacking the 11-keto group.
Our experiments using PTX suggest that a Gi/0-subunit of a heterotrimeric G protein mediates the effects of 11-keto-BAs in PMNL. Remarkably, the activation of p38 MAPK and of 5-LO was less affected by PTX, implying that, for example, PTX-insensitive Gq subunits, as in the case of the PAF receptor (Shimizu et al., 1996), are involved. Also, the PI 3-K inhibitor wortmannin differentially suppressed the activation of p38 MAPK and p42MAPK induced by AKBA (Altmann et al., 2002). It is yet unclear whether 11-keto-BAs act via a GPCR or alternatively interfere directly with a G protein. Notably, modulation of G proteins by low molecular weight compounds in the absence of a GPCR, both in a positive or a negative way, has been described (see Breitweg-Lehmann et al. (2002) and references therein).
The data presented here imply that BAs, at least in concentrations ⩾10 μM, are potent immunocompetent agents that might be regarded as proinflammatory stimuli. By contrast, cellular and pilot clinical studies indicate the anti-inflammatory effects of B. serrata extracts and BAs (Gupta et al., 1992,2001; Gerhardt et al., 2001; Krieglstein et al., 2001). It is conceivable that, at low concentrations, BAs may have antagonistic activity within certain signalling pathways induced by a second stimulus. In fact, at low concentrations, AKBA (2–8 μM) inhibited the activation of p42/44MAPK in meningioma cells stimulated with platelet-derived growth factor (Park et al., 2002), and, in our hands, BAs (0.3–1 μM) significantly suppressed the PAF-induced Ca2+ mobilisation in platelets (unpublished results). Further studies are required to identify the receptor(s) of BAs and the defined mechanisms leading to Ca2+ and MAPK signalling, and to reveal if BAs can act as partial agonists at receptors relevant for inflammatory processes. Such knowledge may help to unravel the molecular mechanisms of the anti-inflammatory actions of BAs, and may provide new concepts for the pharmacological intervention with inflammatory diseases.
Acknowledgments
We thank Astrid Neuß and Sven George for expert technical assistance. This study was supported by grants from the Fonds der Chemischen Industrie, the EU (LEUCHRON, QLRT-2000-01521), and the Deutsche Pharmazeutische Gesellschaft.
Abbreviations
- AA
arachidonic acid
- AB
antibody
- A-β-BA
3-O-acetyl-β-boswellic acid
- AKBA
3-O-acetyl-11-keto-β-boswellic acid
- β-BA
β-boswellic acid
- cPLA2
cytosolic phospholipase A2
- DCF-DA
2′,7′-dichlorofluorescein diacetate
- DPI
diphenyleneiodonium chloride
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GPCR
G protein-coupled receptor
- KBA
11-keto-β-boswellic acid
- 5-LO
5-lipoxygenase
- LT
leukotriene
- MAPK
mitogen-activated protein kinase
- PAF
platelet-activating factor
- PBS
phosphate-buffered saline
- PG buffer
PBS pH 7.4 containing 1 mg ml−1 glucose
- PGC buffer
PBS containing 1 mg ml−1 glucose and 1 mM CaCl2
- PI 3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- PMNL
polymorphonuclear leucocytes
- PTX
pertussis toxin
- ROS
reactive oxygen species
- SDS-b
2 × SDS–PAGE sample-loading buffer
- TGFβ
transforming growth factor β
- WB
Western blotting
References
- ALTMANN A., FISCHER L., SCHUBERT-ZSILAVECZ M., STEINHILBER D., WERZ O. Boswellic acids activate p42(MAPK) and p38 MAPK and stimulate Ca(2+) mobilization. Biochem. Biophys. Res. Commun. 2002;290:185–190. doi: 10.1006/bbrc.2001.6153. [DOI] [PubMed] [Google Scholar]
- BELCHEVA M.M., COSCIA C.J. Diversity of G protein-coupled receptor signaling pathways to ERK/MAP kinase. Neurosignals. 2002;11:34–44. doi: 10.1159/000057320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODEN S.E., SCHWEIZER S., BERTSCHE T., DUFER M., DREWS G., SAFAYHI H. Stimulation of leukotriene synthesis in intact polymorphonuclear cells by the 5-lipoxygenase inhibitor 3-oxo-tirucallic acid. Mol. Pharmacol. 2001;60:267–273. doi: 10.1124/mol.60.2.267. [DOI] [PubMed] [Google Scholar]
- BREITWEG-LEHMANN E., CZUPALLA C., STORM R., KUDLACEK O., SCHUNACK W., FREISSMUTH M., NURNBERG B. Activation and inhibition of G proteins by lipoamines. Mol. Pharmacol. 2002;61:628–636. doi: 10.1124/mol.61.3.628. [DOI] [PubMed] [Google Scholar]
- CHANOCK S.J., EL BENNA J., SMITH R.M., BABIOR B.M. The respiratory burst oxidase. J. Biol. Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- COX J.A., JENG A.Y., SHARKEY N.A., BLUMBERG P.M., TAUBER A.I. Activation of the human neutrophil nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase by protein kinase C. J. Clin. Invest. 1985;76:1932–1938. doi: 10.1172/JCI112190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEWAS C., FAY M., GOUGEROT-POCIDALO M.A., EL-BENNA J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J. Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- DING J., VLAHOS C.J., LIU R., BROWN R.F., BADWEY J.A. Antagonists of phosphatidylinositol 3-kinase block activation of several novel protein kinases in neutrophils. J. Biol. Chem. 1995;270:11684–11691. doi: 10.1074/jbc.270.19.11684. [DOI] [PubMed] [Google Scholar]
- FORD-HUTCHINSON A.W., GRESSER M., YOUNG R.N. 5-LIPOXYGENASE. Annu. Rev. Biochem. 1994;63:383–417. doi: 10.1146/annurev.bi.63.070194.002123. [DOI] [PubMed] [Google Scholar]
- GERHARDT H., SEIFERT F., BUVARI P., VOGELSANG H., REPGES R. Therapy of active Crohn disease with Boswellia serrata extract H 15. Z. Gastroenterol. 2001;39:11–17. doi: 10.1055/s-2001-10708. [DOI] [PubMed] [Google Scholar]
- GIJON M.A., LESLIE C.C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leukoc. Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- GLASER T., WINTER S., GROSCURTH P., SAFAYHI H., SAILER E.R., AMMON H.P., SCHABET M., WELLER M. Boswellic acids and malignant glioma: induction of apoptosis but no modulation of drug sensitivity. Br. J. Cancer. 1999;80:756–765. doi: 10.1038/sj.bjc.6690419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUPTA I., PARIHAR A., MALHOTRA P., GUPTA S., LUDTKE R., SAFAYHI H., AMMON H.P. Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med. 2001;67:391–395. doi: 10.1055/s-2001-15802. [DOI] [PubMed] [Google Scholar]
- GUPTA O.P., SHARMA N., CHAND D. A sensitive and relevant model for evaluating anti-inflammatory activity-papaya latex-induced rat paw inflammation. J. Pharmacol. Toxicol. Methods. 1992;28:15–19. doi: 10.1016/1056-8719(92)90060-e. [DOI] [PubMed] [Google Scholar]
- HAMPTON M.B., FADEEL B., ORRENIUS S. Redox regulation of the caspases during apoptosis. Ann. N.Y. Acad. Sci. 1998;854:325–328. doi: 10.1111/j.1749-6632.1998.tb09913.x. [DOI] [PubMed] [Google Scholar]
- HANCOCK J.T., JONES O.T. The inhibition by diphenyleneiodonium and its analogues of superoxide generation by macrophages. Biochem. J. 1987;242:103–107. doi: 10.1042/bj2420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERLAAR E., BROWN Z. p38 MAPK signalling cascades in inflammatory disease. Mol. Med. Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- HEYWORTH P.G., BADWEY J.A. Protein phosphorylation associated with the stimulation of neutrophils. Modulation of superoxide production by protein kinase C and calcium. J. Bioenerg. Biomembr. 1990;22:1–26. doi: 10.1007/BF00762842. [DOI] [PubMed] [Google Scholar]
- HOERNLEIN R.F., ORLIKOWSKY T., ZEHRER C., NIETHAMMER D., SAILER E.R., SIMMET T., DANNECKER G.E., AMMON H.P. Acetyl-11-keto-beta-boswellic acid induces apoptosis in HL-60 and CCRF-CEM cells and inhibits topoisomerase I. J. Pharmacol. Exp. Ther. 1999;288:613–619. [PubMed] [Google Scholar]
- JING Y., NAKAJO S., XIA L., NAKAYA K., FANG Q., WAXMAN S., HAN R. Boswellic acid acetate induces differentiation and apoptosis in leukemia cell lines. Leuk. Res. 1999;23:43–50. doi: 10.1016/s0145-2126(98)00096-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON E.N., DRUEY K.M. Heterotrimeric G protein signaling: role in asthma and allergic inflammation. J. Allergy Clin. Immunol. 2002;109:592–602. doi: 10.1067/mai.2002.122636. [DOI] [PubMed] [Google Scholar]
- KRIEGLSTEIN C.F., ANTHONI C., RIJCKEN E.J., LAUKOTTER M., SPIEGEL H.U., BODEN S.E., SCHWEIZER S., SAFAYHI H., SENNINGER N., SCHURMANN G. Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int. J. Colorectal Dis. 2001;16:88–95. doi: 10.1007/s003840100292. [DOI] [PubMed] [Google Scholar]
- LIU J.J., NILSSON A., OREDSSON S., BADMAEV V., DUAN R.D. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int. J. Mol. Med. 2002b;10:501–505. [PubMed] [Google Scholar]
- LIU J.J., NILSSON A., OREDSSON S., BADMAEV V., ZHAO W.Z., DUAN R.D. Boswellic acids trigger apoptosis via a pathway dependent on caspase-8 activation but independent on Fas/Fas ligand interaction in colon cancer HT-29 cells. Carcinogenesis. 2002a;23:2087–2093. doi: 10.1093/carcin/23.12.2087. [DOI] [PubMed] [Google Scholar]
- O'FLAHERTY J.T., TAYLOR J.S., KUROKI M. The coupling of 5-oxo-eicosanoid receptors to heterotrimeric G proteins. J. Immunol. 2000;164:3345–3352. doi: 10.4049/jimmunol.164.6.3345. [DOI] [PubMed] [Google Scholar]
- PARK Y.S., LEE J.H., BONDAR J., HARWALKAR J.A., SAFAYHI H., GOLUBIC M. Cytotoxic action of acetyl-11-keto-beta-boswellic acid (AKBA) on meningioma cells. Planta Med. 2002;68:397–401. doi: 10.1055/s-2002-32090. [DOI] [PubMed] [Google Scholar]
- PEREZ H.D., KELLY E., HOLMES R. Regulation of formyl peptide receptor expression and its mRNA levels during differentiation of HL-60 cells. J. Biol. Chem. 1992;267:358–363. [PubMed] [Google Scholar]
- QIU Z.H., GIJON M.A., DECARVALHO M.S., SPENCER D.M., LESLIE C.C. The role of calcium and phosphorylation of cytosolic phospholipase A(2) in regulating arachidonic acid release in macrophages. J. Biol. Chem. 1998;273:8203–8211. doi: 10.1074/jbc.273.14.8203. [DOI] [PubMed] [Google Scholar]
- ROOS D., GOLDSTEIN I.M., KAPLAN H.B., WEISSMANN G. Dissociation of phagocytosis, metabolic stimulation and lysosomal enzyme release in human leukocytes. Agents Actions. 1976;6:256–259. doi: 10.1007/BF01972218. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., SAILER E.R. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997;63:487–493. doi: 10.1055/s-2006-957748. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., BODEN S.E., SCHWEIZER S., AMMON H.P. Concentration-dependent potentiating and inhibitory effects of Boswellia extracts on 5-lipoxygenase product formation in stimulated PMNL. Planta Med. 2000;66:110–113. doi: 10.1055/s-2000-11136. [DOI] [PubMed] [Google Scholar]
- SAFAYHI H., MACK T., SABIERAJ J., ANAZODO M.I., SUBRAMANIAN L.R., AMMON H.P. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J. Pharmacol. Exp. Ther. 1992;261:1143–1146. [PubMed] [Google Scholar]
- SAFAYHI H., RALL B., SAILER E.R., AMMON H.P. Inhibition by boswellic acids of human leukocyte elastase. J. Pharmacol. Exp. Ther. 1997;281:460–463. [PubMed] [Google Scholar]
- SAFAYHI H., SAILER E.R., AMMON H.P. Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-beta-boswellic acid. Mol. Pharmacol. 1995;47:1212–1216. [PubMed] [Google Scholar]
- SHAO Y., HO C.T., CHIN C.K., BADMAEV V., MA W., HUANG M.T. Inhibitory activity of boswellic acids from Boswellia serrata against human leukemia HL-60 cells in culture. Planta Med. 1998;64:328–331. doi: 10.1055/s-2006-957444. [DOI] [PubMed] [Google Scholar]
- SHIMIZU T., MORI M., BITO H., SAKANAKA C., TABUCHI S., AIHARA M., KUME K. Platelet-activating factor and somatostatin activate mitogen-activated protein kinase (MAP kinase) and arachidonate release. J. Lipid Mediat. Cell. Signal. 1996;14:103–108. doi: 10.1016/0929-7855(96)00515-9. [DOI] [PubMed] [Google Scholar]
- SIMCHOWITZ L., SPILBERG I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J. Lab. Clin. Med. 1979;93:583–593. [PubMed] [Google Scholar]
- TAKETO M.M., SONOSHITA M. Phospholipase A2 and apoptosis. Biochim. Biophys. Acta. 2002;1585:72–76. doi: 10.1016/s1388-1981(02)00326-8. [DOI] [PubMed] [Google Scholar]
- WERZ O., BRUNGS M., STEINHILBER D. Purification of transforming growth factor beta1 from human platelets. Pharmazie. 1996;51:893–896. [PubMed] [Google Scholar]
- WERZ O., BUERKERT E., FISCHER L., SZELLAS D., DISHART D., RÅDMARK O., SAMUELSSON B., AND STEINHILBER D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. 2002b;16:1441–1443. doi: 10.1096/fj.01-0909fje. [DOI] [PubMed] [Google Scholar]
- WERZ O., BUERKERT E., SAMUELSSON B., RÅDMARK O., AND STEINHILBER D. Activation of 5-lipoxygenase by cell stress is calcium-independent in human polymorphonuclear leukocytes. Blood. 2002a;99:1044–1052. doi: 10.1182/blood.v99.3.1044. [DOI] [PubMed] [Google Scholar]
- WERZ O., KLEMM J., SAMUELSSON B., RÅDMARK O. 5-Lipoxygenase is phosphorylated by p38 kinase dependent MAPKAP kinases. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERZ O., SCHNEIDER N., BRUNGS M., SAILER E.R., SAFAYHI H., AMMON H.P.T., STEINHILBER D. A test system for leukotriene synthesis inhibitors based on the in-vitro differentiation of the human leukemic cell lines HL-60 and Mono Mac 6. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:441–445. doi: 10.1007/pl00005074. [DOI] [PubMed] [Google Scholar]
- WERZ O., SZELLAS D., HENSELER M., STEINHILBER D. Nonredox 5-lipoxygenase inhibitors require glutathione peroxidase for efficient inhibition of 5-lipoxygenase activity. Mol. Pharmacol. 1998;54:445–451. doi: 10.1124/mol.54.2.445. [DOI] [PubMed] [Google Scholar]
- WINKING M., SARIKAYA S., RAHMANIAN A., JODICKE A., BOKER D.K. Boswellic acids inhibit glioma growth: a new treatment option. J. Neurooncol. 2000;46:97–103. doi: 10.1023/a:1006387010528. [DOI] [PubMed] [Google Scholar]