Abstract
Matrix metalloproteinase-2 (MMP-2) plays a role in agonist- and tumour cell-induced platelet aggregation (TCIPA).
MMP-2 is synthesized as a proenzyme and is activated at the cell surface by membrane type-1 matrix metalloproteinase (MT1-MMP, MMP-14).
The significance of tumour cell-associated MT1-MMP for TCIPA was investigated using human breast carcinoma MCF7 cells stably coexpressing the integrin αvβ3 with MT1-MMP, cells expressing αvβ3 alone and mock-transfected cells.
Western blot and zymography confirmed that αvβ3/MT1-MMP cells expressed MT1-MMP and efficiently processed proMMP-2 to MMP-2.
Aggregometry, phase-contrast and transmission electron microscopy and flow cytometry were used to characterize TCIPA induced by MCF7 cell lines.
The aggregating potency of cells was: αvβ3/MT1-MMP >αvβ3=mock cells, as shown by aggregometry and phase-contrast microscopy.
Electron microscopy revealed close, membrane–membrane interactions between activated platelets and αvβ3/MT1-MMP cells during TCIPA.
Inhibition of MMP-2 with the neutralizing anti-MMP-2 antibody (5 μg ml−1) and o-phenanthroline (100 μM) reduced aggregation induced by αvβ3/MT1-MMP cells.
TCIPA induced by αvβ3/MT1-MMP cells was also reduced by inhibiting the generation and actions of ADP with apyrase (250 μg ml−1) and 2-methylthio-AMP (2-MeSAMP) (30 μM), but not N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179) (30 μM).
Flow cytometry demonstrated that TCIPA enhanced expression of glycoprotein (GP) Ib and IIb/IIIa receptors not only on platelets but also on breast cancer cells.
Thus, (a) human breast carcinoma cell surface-associated MT1-MMP, via activating proMMP-2, stimulates TCIPA; (b) ADP amplifies the effects of MMPs via stimulation of P2Y12 receptors and (c) both tumour- and platelet-derived GPIb and GPIIb/IIIa are involved in the aggregatory effects of MT1-MMP.
Keywords: Platelets, TCIPA, haematogenous metastasis, matrix metalloproteinases, ADP, receptor glycoproteins
Introduction
Tumour cell induced-platelet aggregation (TCIPA) plays an important role in the haematogenous spread of cancer and this ability of tumour cells is an indicator of their metastatic potential (Trousseau, 1865; Billroth, 1878; Gasic, 1984; Nash et al., 2002).
There are several mechanisms involved in TCIPA, and these can vary among different tumour cells. For example, tumour cells can activate platelets by tumour cell-induced thrombin generation, releasing ADP (Camez et al., 1986), thromboxane A2 (TXA2) (Honn et al., 1987) and MMP-2 (Jurasz et al., 2001a).
Matrix metalloproteinases (MMPs) comprise a family of structurally related, zinc-dependent endopeptidases, which are involved in degradation of extracellular matrix (Sternlicht & Werb, 2001). In addition to their general matrix-degrading abilities, individual MMPs specifically cleave peptide hormones, cytokines, cell adhesion receptors and other functionally important proteins including big-endothelin-1(Fernandez-Patron et al., 1999), chemokine monocyte chemoattractant protein-3 (Overall et al., 2002), integrin αvβ3 (Deryugina et al., 2000; Deryugina et al., 2002; Ratnikov et al., 2002), transglutaminase (Belkin et al., 2001) and the cytokine TGF-β1 (Mu et al., 2002).
Recent evidence has shown that in addition to embryonic development, morphogenesis, physiological and pathological remodelling, MMPs also act as signalling molecules regulating processes such as vascular reactivity, leukocyte activation and platelet function (Sternlicht & Werb, 2001; Jurasz et al., 2002). Indeed, we and others have previously described novel pathways of platelet aggregation that are mediated via the release of matrix metalloproteinase-1 (MMP-1) and MMP-2 from platelets (Sawicki et al., 1997; Sawicki et al., 1998; Kazes et al., 2000; Martinez et al., 2001; Radomski et al., 2001; Chung et al., 2002; Galt et al., 2002; Jurasz et al., 2002). Interestingly, MMP-2 release from platelets and cancer cells stimulates TCIPA (Jurasz et al., 2001a).
MMPs are synthesized as zymogens and require proteolytic activation for their catalytic activity. The activation of MMP-2 is unique among MMPs since it involves the formation of the trimolecular complex proMMP-2•TIMP-2•MT1-MMP (Sato et al., 1994; Strongin et al., 1995; Deryugina et al., 2001). In addition to its role as an activator of MMP-2, membrane-tethered membrane type-1 matrix metalloproteinase (MT1-MMP) (MMP-14, GenBank™ U41078) is a key enzyme in tumour cell migration and invasion (Hotary et al., 2000; Koshikawa et al., 2000; Belkin et al., 2001; Itoh et al., 2001). MT1-MMP is also present on the surface of collagen-aggregated platelets (Kazes et al., 2000).
The mechanisms of MMP-mediated stimulation of platelets and tumour cells are not clear, but interactions between these proteinases and glycoprotein (GP) receptors such as GPIIb/IIIa (αIIbβ3), GPIb and vitronectin receptor αvβ3 may underlie the effects of MMPs (Deryugina et al., 2000; Martinez et al., 2001; Galt et al., 2002).
GPIIb/IIIa is the major integrin receptor on the surface of platelets and it plays a key role in platelet aggregation (Shattil et al., 1998). GPIIb/IIIa is a multifunctional receptor involved in both tumour cell–matrix and tumour cell–platelet interactions (Grossi et al., 1988; Trikha et al., 2002b). In addition, GPIbα, the von Willebrand factor-binding subunit of the GPIb/V/IX receptor of platelets, is an important adhesion molecule that initiates diverse signal transduction pathways. These pathways lead to activation of the integrin receptor GPIIb/IIIa (Ruggeri, 1993; Zaffran et al., 2000). Interestingly, both GPIIb/IIIa and GPIb are also expressed on the surface of breast carcinoma MCF7 cells (Oleksowicz et al., 1995; Oleksowicz et al., 1997a).
For these reasons, the major objective of our present study was to investigate the functional significance of tumour cell-associated MT1-MMP in TCIPA. Simultaneously, we studied the relationship between MT1-MMP and the expression of GPIIb/IIIa and GPIb in both platelets and tumour cells. For this purpose, we employed human breast carcinoma MCF7 cells, which were transfected with MT1-MMP, as an experimental model in our investigation.
Methods
Reagents
All reagents were purchased from Sigma (Oakville, ON, Canada) unless otherwise indicated. Collagen was obtained from Chronolog (Havertown, PA, U.S.A.). Acetylsalicylic acid, apyrase, 2-methylthio-AMP (2-MeSAMP), prostacyclin and o-phenanthroline were purchased from Sigma Chemical Co (St Louis, MO, U.S.A.). N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179) was purchased from Tocris Cookson Inc. (Ellisville, MO, U.S.A.). Rabbit polyclonal antibody against the catalytic domain of MT1-MMP was purchased from Chemicon (Temecula, CA, U.S.A.). Fluorescein-isothiocyanate (FITC)-conjugated monoclonal antibody against high-affinity GPIIb/IIIa (PAC-1-FITC) was purchased from Becton Dickinson Biosciences (Mississauga, ON, Canada). R-phycoerythrin (PE)-conjugated monoclonal antibody against human platelet GPIb (CD42b-PE) was purchased from DAKO Diagnostics (Mississauga, ON, Canada).
Tumour cell culture
We used a series of doubly transfected human breast carcinoma MCF7 cells (Deryugina et al., 2001). Briefly, human MT1-MMP and β3 integrin subunit cDNAs were cloned into the mammalian expression vectors pcDNA3-zeo and pcDNA3-neo, respectively (Invitrogen, San Diego, CA, U.S.A.). Parental MCF7 cells (ATCC, Rockville, MD, U.S.A.) were first transfected with either the original neo-plasmid (mock vector) or the neo-plasmid carrying the β3 integrin subunit. The selected MCF7-neo and MCF7-β3 cells were then each transfected with either the original zeo-plasmid (mock vector) or the zeo-plasmid carrying MT1-MMP. Accordingly, MCF7-neo/zeo (mock-transfected cells), MCF7-β3/zeo, MCF7-neo/MT and MCF7-β3/MT cells were generated after selection.
Parental MCF7 cells, and consequently, MCF7-neo/zeo and MCF7-neo/MT, express the αv, but not the β3 integrin subunit. In turn, MCF7 cells transfected with the neo-plasmid carrying the β3 integrin subunit were able to express functional integrin αvβ3 (Deryugina et al., 2000; Deryugina et al., 2001).
Transfected cells were cultured as monolayers in 250 ml culture flasks at 37°C in a humidified atmosphere with 5% CO2 and grown in Dulbecco's MEM supplemented with 10% fetal calf serum and 0.25 mg ml−1 zeocin (Invitrogen Canada, Burlington, Canada) and 0.25 mg ml−1 G418 (Sigma). The cells were supplied with fresh medium and subcultured three times each week. When confluent, cells were detached from the flasks using EDTA (7 mM) (Jurasz et al., 2001a) in DMEM with 10% fetal bovine serum. Cells were washed in Tyrode's buffer to remove EDTA and resuspended at a concentration of 3 × 107 cells ml−1 in fresh Tyrode's buffer.
Western blotting
Western blotting was used in order to investigate membrane-tethered MT1-MMP in doubly transfected MCF7-β3/MT breast carcinoma cells. MCF7-neo/zeo cells (MT1-MMP-deficient) were used as negative control. To study the presence of soluble MT1-MMP protein, the MCF7-conditioned media were also examined by Western blotting. Rabbit polyclonal antibody (AB8345, Chemicon, Temecula, CA, U.S.A.) raised against the catalytic region of MT1-MMP was used in these experiments. This antibody recognizes the full-length proenzyme and the active enzyme. Immunoblot analysis was performed as previously described (Sawicki et al., 1997). Briefly, the samples were homogenized in homogenization buffer (containing 320 mM sucrose, 10 mM HEPES, 0.1 mM EDTA, 1 mM DL-dithiothreitol, 10 mM CHAPS, 10 μg ml−1 leupeptin, 10 μg ml−1 soybean trypsin inhibitor and 2 μg ml−1 aprotinin), sonicated, and centrifuged at 10,000 × g for 20 min at 4°C. The resultant supernatants and the samples of MCF7-conditioned media were subjected to 12% SDS–PAGE. Following electrophoresis and transfer of samples onto PVDF membranes (Bio-Rad, Hercules, U.S.A.), the blots were blocked overnight in blocking buffer and then incubated with the primary antibody (2 μg ml−1). The immunoreactive bands were revealed by means of an enhanced chemiluminescence kit, SuperSignal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, U.S.A.), and the images acquired by the ChemiDoc XRS System (Bio-Rad, Hercules, CA, U.S.A.).
Gelatine zymography
Gelatine zymography (Sawicki et al., 1997; Sawicki et al., 1998; Jurasz et al., 2001a; Martinez et al., 2001; Radomski et al., 2001) was used to detect MMP-2 in the conditioned media of MCF7 cells. Subconfluent neo/zeo, neo/MT, β3/zeo and β3/MT cells were incubated in serum-free medium for 24 h. Conditioned medium was harvested and centrifuged at 300 × g for 5 min at room temperature. The resulting supernatant was stored at −20°C. Gelatine zymography was performed using 8% SDS–PAGE with copolymerized gelatine (2 mg ml−1). The samples (20 μg protein per lane) were subjected to electrophoresis. Following electrophoresis, gels were washed in 2.5% Triton X-100 for 1 h (three times, 20 min each) and incubated for 48 h in enzyme assay buffer (25 mM Tris, pH 7.5, 5 mM CaCl2, 0.9% NaCl, 0.05% Na3N). The gelatinolytic activities were detected as transparent bands against the background of Coomassie blue-stained gelatine. MMP-2 was identified by its molecular weight when compared to standards. The media conditioned by human fibrosarcoma parental HT-1080 and HT-1080 cells stably over-expressing MT1-MMP (HT-1080-MT cells), which were diluted 100-fold, were used as MMP-2 standards (Deryugina et al., 2001).
Preparation of human washed platelets
Blood was collected from healthy volunteers who had not taken any drugs known to affect platelet function for at least 14 days prior to the study. Washed platelet suspensions (2.5 × 1011 platelets l−1) were prepared from blood (Radomski & Moncada, 1983).
Platelet aggregation
The interactions between platelets and tumour cells were measured by light aggregometry (Radomski et al., 1991; Jurasz et al., 2001a, 2001b). Briefly, platelet samples were placed in a whole blood ionized calcium lumi-aggregometer (Chronolog Corp., Havertown, PA, U.S.A.), and incubated for 2 min at 37°C, with stirring at 900 r.p.m., prior to the addition of aggregating agents. For most experiments, collagen at a concentration that resulted in maximal aggregation (5 μg ml−1) was used as a control agonist. TCIPA was initiated by the addition of tumour cells (0.08 × 106–0.5 × 106 cell ml−1), and the reaction was monitored and analysed by Aggro-Link data processing system (Chronolog) for up to 30 min. Platelet aggregation was expressed as the percentage of aggregation at a time when the highest concentration of tumour cells increased the light transmittance by 50% (Jurasz et al., 2001a). The EC50 values were determined from the analysis of the concentration–response curves with GraphPad Prism software (GraphPad Software, San Diego, CA, U.S.A.) and represent the concentration of tumour cells that induced 50% of the maximum response. The lag-phase, that is the time elapsing from the addition of tumour cells to the half-maximal aggregation, was also used to characterize the aggregatory ability of tumour cells. To study the involvement of TXA2-, ADP- and MMP-2-dependent pathways in MCF7-induced platelet aggregation, platelets were preincubated for 2 min with inhibitors of these pathways such as aspirin, apyrase and o-phenanthroline (Sawicki et al., 1997; Jurasz et al., 2001a), and aggregation was induced with 0.3 × 106 cells ml−1. We used the antagonist of P2Y1 and P2Y12 receptors MRS2179 (Baurand et al., 2001) and 2-MeSAMP (Jantzen et al., 1999; Takasaki et al., 2001), respectively, to further characterize the ADP-dependent pathway of MCF7 cell-induced platelet aggregation. In some experiments, resting platelets were preincubated for 30 min with the neutralizing anti-MMP-2 antibody or control IgG (each 5 μg ml−1).
Microscopy of TCIPA
The structure of platelet–tumour cell aggregates was studied using both light and electron microscopy. Briefly, MCF7 cells (0.2 × 106 cells ml−1) were added to the platelet suspension and aggregation was terminated either at the peak of the shape change or at 50% maximal aggregation, as determined using the aggregometer. The samples were fixed by adding an equal volume of 2% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4, and then incubated for 1 h at room temperature.
Aliquots of each sample were then taken for phase-contrast microscopy examination using an Olympus CKX41 microscope (Olympus America Inc., Melville, NY, U.S.A.). Photomicrographs were captured using a digital camera and MicroFire (Olympus America Inc.) software.
The remaining samples were then prepared for transmission electron microscopy examination (Muller et al., 1994). After fixation, samples were centrifuged and washed three times in PB, pH 7.4. Pellets were postfixed with 1% buffered osmium tetroxide for 1 h and stained with 1% Millipore-filtered uranyl acetate. The samples were then dehydrated in a series of graded ethanol solutions, infiltrated, and embedded in epoxy resin. Ultrathin sections were cut in an LKB Ultracut microtome (Leica, Deerfield, IL, U.S.A.), stained with uranyl acetate and lead citrate in an LKB Ultrostainer, and examined in a JEM 1010 transmission electron microscope (JEOL, U.S.A., Inc., Peabody, MA, U.S.A.) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Advantage digital CCD camera system (Advanced Microscopy Techniques Corp, Danvers, MA, U.S.A.).
All the images were imported into Adobe Photoshop version 7.0 (San Jose, CA, U.S.A.), cropped and corrected for brightness and contrast, but not otherwise manipulated.
Flow cytometry analysis
The abundance of GPIIb/IIIa and GPIb on the surface of platelets and tumour cells during TCIPA was measured by flow cytometry (Chung et al., 2002). Platelets were activated with either collagen (5 μg ml−1, positive control) or tumour cells (0.3 × 106 cells ml−1), and then diluted 10-fold with physiological saline when the light transmittance reached 20%. Resting platelets and MCF7 cells alone were both used as controls. Samples were then incubated, in the dark for 15 min at room temperature, in the presence of saturating concentrations (10 μg ml−1) of FITC-antiGPIIb/IIIa (PAC-1-FITC) or PE-antiGPIb (CD42b-PE). The activated GPIIb/IIIa platelet receptors were measured using PAC-1 monoclonal antibody. PAC-1 specifically recognizes an epitope on the high-affinity GPIIb/IIIa complex of activated platelets at or near the platelet fibrinogen receptor (Abrams et al., 1990).
Following incubation, samples were diluted in FACS Flow fluid and analysed within 5 min using a FACSCalibur flow cytometer (Becton Dickinson, CA, U.S.A.) equipped with a 488 nm wavelength argon laser, and 525 and 575 nm band pass filters for the detection of FIT and PE fluorescence. The instrument was set up to measure the size (forward scatter), granularity (side scatter) and cell fluorescence. A two-dimensional analysis gate based on forward and side scatter was drawn in order to include single platelets or tumour cells and exclude platelet–tumour cell aggregates and microparticles. Antibody binding was measured by analysing individual platelets for fluorescence. The mean fluorescence intensity was determined after correction for cell autofluorescence. For each sample, the fluorescence was analysed using a logarithmic scale. Fluorescence histograms were obtained for 10,000 individual events. Data were analysed using CellQuest software and expressed as a percentage of control fluorescence in arbitrary units.
Statistics
The data were analysed using one-way analysis of variance (GraphPad Prism software). The results are expressed as mean±s.e.m. of at least three independent experiments. Tukey–Kramer multiple comparisons test, and paired and unpaired Student's t-tests were performed, where appropriate. Statistical significance was considered when P<0.05.
Results
MT1-MMP and MMP-2 in MCF7 cells
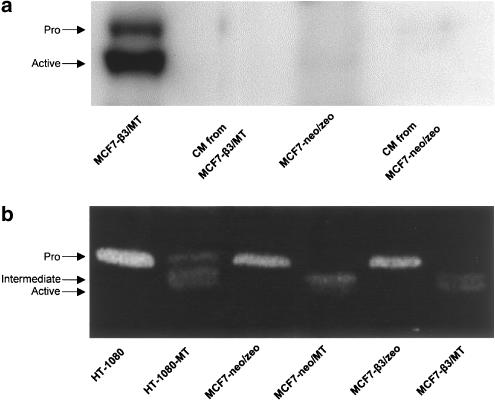
Western blotting studies revealed the presence of both the 63 kDa proMT1-MMP and the 60 kDa active MT1-MMP enzymes in MCF7-β3/MT cells (Figure 1a). MT1-MMP immunoreactive bands were undetectable in the conditioned medium from MCF7-β3/MT cells or in the lysates or conditioned medium of MCF7-neo/zeo cells, transfected with the original pcDNA3 -neo and -zeo plasmids.
Figure 1.
Western blot and zymography of MCF7 cells. (a) Western blotting revealing the 63 kDa proMT1-MMP and the 60 kDa active MT1-MMP enzymes in MCF7-β3/MT cells. No immunoreactive bands are present in the conditioned medium from MCF7-β3/MT cells or in the lysates or conditioned medium of MCF7-neo/zeo cells. (b) Zymogram showing the activity of MMP-2 in the conditioned media of MCF7 cells. Gelatinolytic activity at approximately 68 kDa was visualized, indicating the presence of the proenzyme, in the media from neo/zeo and β3/zeo cells. The activation intermediate (64 kDa) was the main form found in the medium from neo/MT cells. Zymography showed both the activation intermediate and the active form of MMP-2 (62 kDa) in the medium of β3/MT cells. The media obtained from wild-type HT-1080 and HT-1080-MT1-MMP were used as standards. Representative Western blot and zymogram from three separate experiments.
As demonstrated by gelatine zymography of conditioned media, mock neo/zeo and β3/zeo cells constitutively produced, but failed to efficiently activate, proMMP-2. In contrast, neo/MT cells generated the 64 kDa activation intermediate of MMP-2 as the main activation product. The conversion of the 64 kDa intermediate to the mature 62 kDa MMP-2 enzyme was relatively minor in neo/MT cells. Only β3/MT cells, expressing both αvβ3 and MT1-MMP, were able to efficiently activate and release both the intermediate and mature active forms of MMP-2 (Figure 1b).
TCIPA
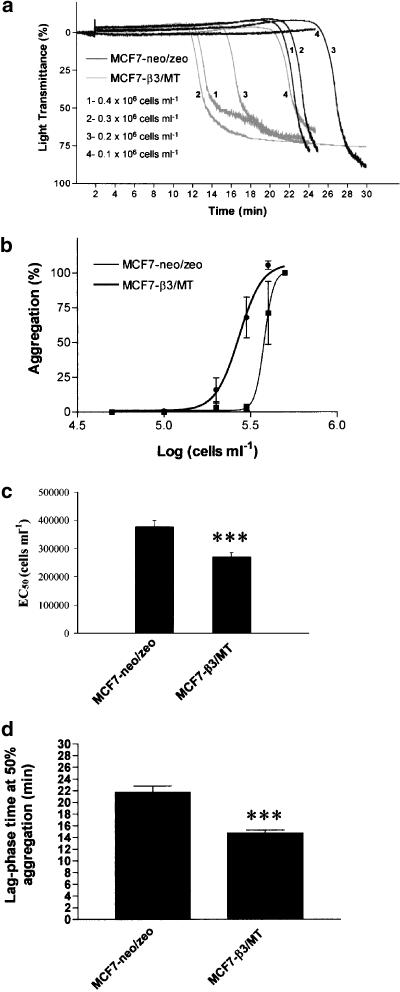
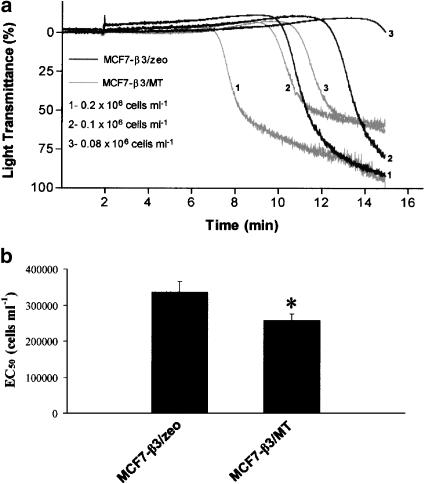
MCF7 breast carcinoma cells were tested for their ability to induce platelet aggregation. No platelet aggregation was detected in the presence of conditioned medium from tumour cells (n=4). In contrast, MCF7 cells induced activation of platelets, leading to shape change and concentration-dependent aggregation. This aggregation was preceded by a lag-phase of several minutes that increased as the concentration of tumour cells decreased (Figure 2a). The cells transfected with MT1-MMP and β3 (β3/MT) aggregated platelets following shorter lag-phase and were more potent to induce aggregation (P<0.001; n=8) when compared to control cells transfected only with empty vectors (neo/zeo) (Figure 2b–d). In order to discriminate between the relative contributions of integrin αvβ3 and MT1-MMP to TCIPA, the aggregating potencies of β3/MT and β3/zeo cells were compared. The cells transfected with MT1-MMP and β3 subunit were more potent in inducing TCIPA than the cells expressing αvβ3 (P<0.05, n=4) (Figure 3a and b). Moreover, no significant differences in aggregation were observed between β3/neo and neo/zeo cells (n=3).
Figure 2.
Comparison of platelet aggregatory potency of breast carcinoma MCF7 β3/MT and mock (neo/zeo) cells. (a) Representative traces showing TCIPA at different concentrations of tumour cells (0.1 × 106–0.4 × 106 cells ml−1). (b) Concentration–response curves for neo/zeo- and β3/MT-induced platelet aggregation and (c) the corresponding EC50 values. (d) Lag-phase time measured at 50% aggregation induced by 0.2 × 106 cells ml−1 of neo/zeo and β3/MT cells. Bars are means±s.e.m. from eight separate experiments. ***, P<0.001.
Figure 3.
Comparison of platelet aggregatory potency of breast carcinoma MCF7 β3/MT and β3/zeo cells. (a) Representative traces showing TCIPA (0.08 × 106–0.2 × 106 cells ml−1) and (b) the corresponding. EC50 values. Bars are means±s.e.m. from four separate experiments. *, P<0.05.
Light and electron microscopy of TCIPA
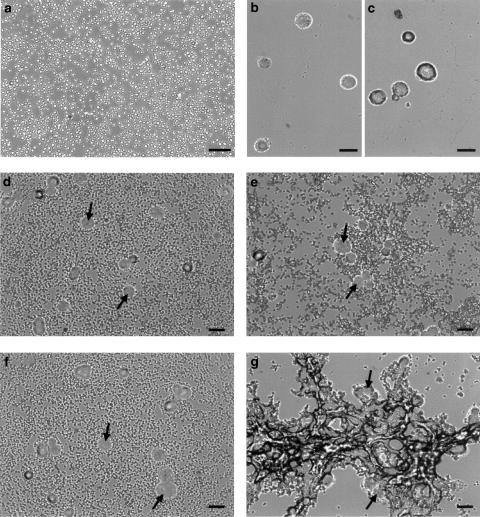
Figure 4 shows the morphology of resting platelets (panel a), mock control MCF7-neo/zeo (panel b) and MCF7-β3/MT (panel c) cells viewed using phase-contrast microscopy. When examined during the corresponding stages of activation (shape change, d and e) and 50% maximal aggregation (panels f and g), platelet aggregation stimulated with β3/MT cells was more pronounced than that induced by controls.
Figure 4.
Light microscopy of platelet–tumour cell aggregates. Phase-contrast photomicrographs of (a) resting platelets, (b) mock control MCF7-neo/zeo and (c) MCF7-β3/MT cells. Platelet–MCF7-neo/zeo and MCF-β3/MT cell aggregates were examined during shape change (d and e) and 50% maximal aggregation (f and g). Arrows indicate MCF7 cells. Scale bars=20 μm.
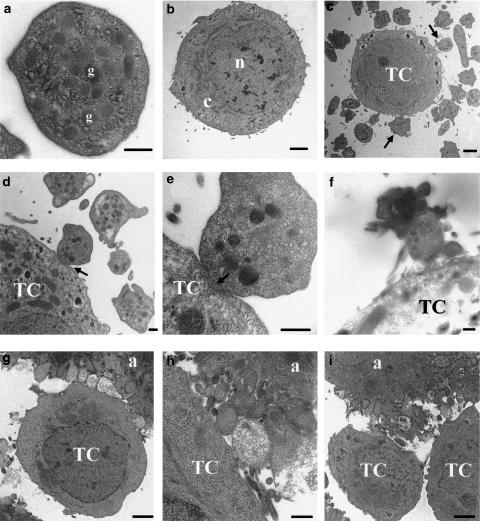
Transmission electron microscopy was then used to examine the ultrastructural features of MCF7-β3/MT-induced platelet aggregation. Figure 5 shows the morphology of a resting platelet illustrating the internal organization with numerous granules, dense bodies, mitochondria and glycogen particles randomly distributed throughout the cytoplasm (panel a) and typical morphological features of cancer cells such as MCF7-β3/MT cells (panel b). Following addition of β3/MT cells to platelet suspensions, low-power magnification micrographs showed that platelets underwent shape change and formed pseudopodia in close association with β3/MT cells (panels c and d), corresponding to morphological signs of activation. At this stage, the majority of platelets remained solitary except those associated with tumour cells. A very close interaction between platelet and tumour cell membranes was evident at high-power magnification (panel e).
Figure 5.
Transmission electron microscopy of MCF7-β3/MT-induced aggregation. (a) High-power micrograph from a typical section of a resting platelet, with well-preserved granular structure (g). (b) Low-power micrograph showing an MCF7-β3/MT cell, with the nucleus (n) and cytoplasm (c). (c) Tumour cell surrounded by platelets (arrows) of various shapes (discoid forms without pseudopodia and spherical forms with pseudopodia). (d and e) Low- and high-power magnification of membrane–membrane heterotypic interactions between platelets and MCF7 cells (arrows). (f) Interactions between small platelet aggregates and tumour cell. (g and h) Large tight platelet aggregates associated with tumour cell membrane. (i) Heterotypic, tumour–platelet and homotypic, tumour–tumour, interactions. TC=tumour cell. a=platelet aggregates. Scale bars=500 nm (a, d, e, f, h), 2 μm (b, c, g).
Following shape change, a partial centralization of α-granules and some small-sized aggregates, composed of loosely packed platelets, could be observed (panel f). Subsequently, remaining individual platelets were recruited into larger aggregates in which platelets at the periphery of the aggregate had undergone secretion and appeared to be more degranulated than those within the core of the aggregate (panel g and h). In final stages of TCIPA, tumour–platelet and tumour–tumour interactions could also be observed (panel i).
Inhibition of MCF7-induced platelet aggregation
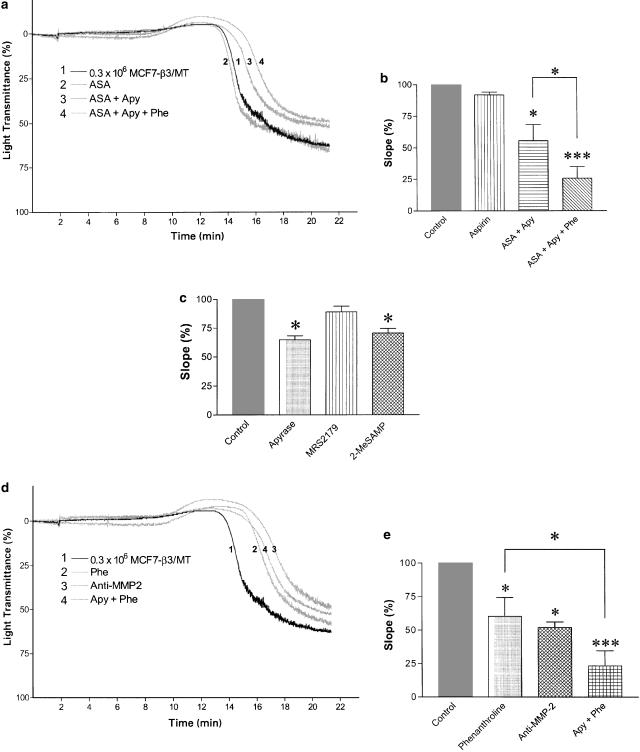
We used selective inhibitors of three major platelet-aggregating pathways to characterize the mechanism of MCF7-induced platelet aggregation. Acetylsalicylic acid (100 μM), apyrase (250 μg ml−1) and o-phenanthroline (100 μM) were used to inhibit TXA2-, ADP- and MMP-2-mediated pathways of aggregation, respectively (Sawicki et al., 1997; Jurasz et al., 2001a; Radomski et al., 2001). The lag-phase and the slope of the curve, that is changes in aggregation per minute, were significantly affected by o-phenanthroline, but not by acetylsalicylic acid (Figure 6a and b). Apyrase, an ADP scavenger, and 2-MeSAMP (30 μM), a P2Y12 receptor antagonist (Jantzen et al., 1999; Takasaki et al., 2001), inhibited TCIPA to a similar extent (n=5, P>0.05) (Figure 6c). No statistically significant changes in the slope of the curve for platelet aggregation induced by MCF7 cells were observed with the P2Y1 receptor antagonist MRS2179 (30 μM) (Figure 6c). However, combinations of apyrase and aspirin (Figure 6a and b) or apyrase and o-phenanthroline resulted in enhanced inhibition of TCIPA (P<0.05; n=6) (Figure 6d and e).
Figure 6.
Inhibition of TCIPA induced by β3/MT cells with selective inhibitors of three major pathways of platelet aggregation. (a) Representative traces showing inhibition of TCIPA (induced by 0.3 × 106 tumour cells ml−1) by acetylsalicylic acid (100 μM), apyrase (250 μg ml−1) and o-phenanthroline (100 μM). (b) The corresponding bar graphs, expressed as a percentage of slope, with the statistical analysis. (c) Bar graphs showing the effects of apyrase (250 μg ml−1), MRS2179 (30 μM) and 2-MeSAMP (30 μM) on MCF7 cell-induced platelet aggregation, expressed as a percentage of slope, with statistical analysis. (d) Representative traces showing inhibition of MMP-2-mediated pathway of TCIPA (induced by 0.3 × 106 tumour cells ml−1) with o-phenanthroline (Phe, 100 μM) and the neutralizing anti-MMP-2 antibody (5 μg ml−1), and the inhibitory effects of combination of phenanthroline and apyrase. (e) Bar graphs showing the results expressed as a percentage of slope. Bars are means±s.e.m. from three to six separate experiments. *, P<0.05; ***, P<0.001.
Since MCF7-β3/MT cell-induced TCIPA was o-phenantroline-sensitive (Figure 6a and b), we studied the specific contribution of the MMP-2-mediated pathway to this reaction. Incubation of platelets with a neutralizing anti-MMP-2 antibody, but not with control IgG (5 μg ml−1, each), significantly inhibited (P<0.05; n=3) aggregation induced by β3/MT cells (Figure 6d and e).
Measurement of adhesion receptors during TCIPA
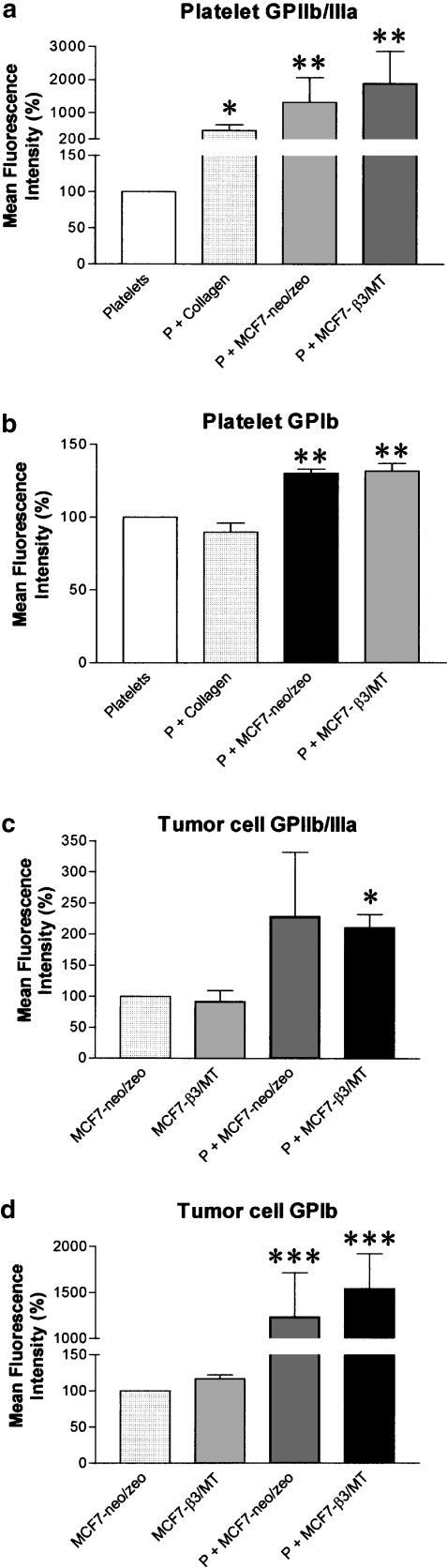
Platelet activation with MCF7 cells (0.3 × 106 cells ml−1) resulted in a significant (P<0.01; n=4) increase in the number of copies of the GPIIb/IIIa and GPIb on platelets, as shown by the increase in mean fluorescence intensity (Figure 7a and b). Flow cytometric analysis also demonstrated the presence of GPIIb/IIIa and GPIb on the surface of MCF7 cells (Figure 7c and d). Furthermore, the interactions of platelets with tumour cells induced a significant (P<0.05; n=4) increase in the number of copies of activated GPIIb/IIIa on β3/MT cells (Figure 7c). Moreover, TCIPA led to a significant (P<0.001; n=4) increase of GPIb on the surface of neo/zeo and β3/MT cells (Figure 7d).
Figure 7.
Flow cytometry analysis of activated GPIIb/IIIa and GPIb on platelets and MCF7 cells during TCIPA (at 20% maximal aggregation). TCIPA induced by mock and β3/MT cells (0.3 × 106 cells ml−1) significantly enhanced expression of GPIIb/IIIa and GPIb integrin receptors on platelets (a and b) and on MCF7 cells (c and d). Bars are mean±s.e.m. from four separate experiments. *, P<0.05; **, P<0.01 and ***, P<0.001.
Discussion and conclusions
We have studied the role of MT1-MMP in aggregation of human platelets induced by MCF7 human breast carcinoma cells. Platelet activation by tumour cells is a complex process involving different pathways of platelet aggregation. The relative contribution of each pathway responsible to TCIPA varies between cancer cells of different origin. Some of the proposed mechanisms involve generation of proaggregatory factors including TXA2 and ADP (Bastida & Ordinas, 1988). The data concerning the effects of ADP on TCIPA induced by human breast cancer cells are contradictory such that both major and minor roles for ADP in this aggregation have been suggested (Camez et al., 1986; Abecassis et al., 1987; Oleksowicz et al., 1995). During the course of our investigation, we have found that platelet aggregation induced by MCF7 human breast carcinoma cells was aspirin-insensitive indicating that selective inhibition of TXA2 does not reduce TCIPA. In contrast, apyrase (ADP scavenger) and phenanthroline (MMP inhibitor) attenuated TCIPA. Therefore, we focused on MMPs and ADP to unravel the mechanisms involved in the proaggregatory effects of MCF7 human breast cancer cells.
Recent results demonstrate that MMP-2 mediates, in part, platelet aggregation induced by HT-1080 human fibrosarcoma cells, indicating that this MMP plays an important role in TCIPA (Jurasz et al., 2001a). We have previously shown the requirement for activated MMP-2 to induce the MMP-2-dependent pathway both in agonist- and tumour cell-induced platelet aggregation (Sawicki et al., 1997; Jurasz et al., 2001a). The mechanism by which MMP-2 is activated at the surface of cancer cells is fully dependent on the activity of MT1-MMP (Sato et al., 1994; Strongin et al., 1995). Recently, Kazes et al. (2000) showed that resting platelets express the latent form of MT1-MMP on their surface and this is activated during collagen-induced platelet aggregation. The authors hypothesized that MT1-MMP may contribute to collagen-induced platelet aggregation. In our experiments, we used MCF7 human breast carcinoma cells transfected with β3 integrin and MT1-MMP, transfected with β3 alone, as well as mock-transfected cells, in order to study the functional significance of MT1-MMP in TCIPA. Parental MCF7 cells are MT1-MMP deficient (Deryugina et al., 2001). The coexpression of αvβ3 and MT1-MMP in β3/MT cells ensures stimulation of the maturation process of proMMP-2 leading to fully active MMP-2 (Deryugina et al., 2001). Indeed, our Western blotting and zymography data demonstrated the presence of membrane-tethered MT1-MMP in MT1-MMP-transfected cells and an increased activation of proMMP-2 to MMP-2 by these cells. Using aggregometry and phase-contrast microscopy, it was found that MCF7-β3/MT cells were clearly more potent in inducing platelet aggregation than mock controls. The increased aggregatory activity of β3/MT cells was due to the presence of MT1-MMP, but not αvβ3, because the cells expressing only this integrin were much less efficient in inducing TCIPA than those coexpressing αvβ3 and MT1-MMP. It is concluded that MT1-MMP stimulates TCIPA and the activation of proMMP-2 to MMP-2 by MT1-MMP may be responsible for these proaggregatory effects of MT1-MMP. This conclusion is supported by pharmacological experiments showing inhibition of β3/MT-induced aggregation with a neutralizing anti-MMP-2 antibody and o-phenanthroline.
Interestingly, apyrase amplified the inhibitory effects of phenanthroline on TCIPA indicating the involvement of the ADP pathway. Pharmacological studies using selective antagonists showed that ADP-induced platelet aggregation is largely mediated by two purinergic P2 receptors. The P2Y1 receptors initiate aggregation through mobilization of calcium stores, whereas the P2Y12 receptors coupled to adenylyl cyclase inhibition are essential for a full aggregation response to ADP and the stabilization of aggregates (Gachet, 2001). We used MRS2179, a selective P2Y1 receptor antagonist (Baurand et al., 2001), and 2-MeSAMP, a selective P2Y12 receptor antagonist (Jantzen et al., 1999), to further characterize the ADP-dependent pathway of MCF7 cell-induced platelet aggregation. We found that both 2-MeSAMP and apyrase inhibited aggregation to a similar extent, while MRS2179 was ineffective as inhibitor of TCIPA. These results suggest that P2Y12 receptors largely mediate the effects of ADP on MCF7 cell-induced platelet aggregation.
We have also characterized mechanism(s) of proaggregatory effects of MT1-MMP expressing cells at the ultratructural and receptor levels. The studies using transmission electron microscopy showed that platelet activation by these cells was associated with close membrane–membrane interactions, suggesting that platelet/tumour cell receptors could be involved in MT1-MMP-related TCIPA.
Cell surface receptors such as GPIIb/IIIa and GPIb play an important role in tumour cell–host cell interactions, tumour cell interactions with components of the subendothelial matrix, and subsequent tumour metastasis (Felding-Habermann et al., 2001). We have recently found that TCIPA results in profound changes in the surface abundance of these receptor glycoproteins in platelets (Jurasz et al., 2001b). These findings prompted us to study GPIIb/IIIa and GPIb on platelets and MCF7 cells during TCIPA.
GPIIb/IIIa is constitutively expressed as an inactive receptor in resting platelets. Upon platelet aggregation, GPIIb/IIIa undergoes activation to a high-affinity state, and in its active conformation the integrin binds plasma protein ligands including fibrinogen, von Willebrand factor, fibronectin and vitronectin (Abrams & Shattil, 1991; Hynes, 1992). The interactions between GPIIb/IIIa and fibrinogen are crucial for the formation of stable platelet aggregates (Bennett, 2001). Our results demonstrate the activation of platelets by MCF7 cells, as shown by increased number of copies of the high-affinity (activated) state of GPIIb/IIIa on the surface of platelets. Interestingly, we have recently shown that the release of MMP-2 is associated with the activation of GPIIb/IIIa receptor (Martinez et al., 2001). Therefore, the MT1-MMP-MMP-2 system could be involved in activation of platelet GPIIb/IIIa during TCIPA.
We found similar levels of activated GPIIb/IIIa on the surface of unstimulated neo/zeo and β3/MT cells. Thus, the transfection with the β3 integrin subunit apparently did not enhance the abundance of activated GPIIb/IIIa in breast carcinoma cells. However, TCIPA significantly increased the numbers of activated GPIIb/IIIa in β3/MT cells. The presence of this integrin in tumour cells is believed to be involved in TCIPA (Oleksowicz et al., 1995), tumour cell adhesion to endothelial cells and subendothelial matrix (Timar et al., 1996), invasion of tumour cells through a reconstituted basement membrane (Trikha et al., 1996) and increased cancer cell survival and growth in vivo (Trikha et al., 2002a). Moreover, Chang et al. (1992) demonstrated a positive correlation between GPIIb/IIIa and metastatic potential of B16a cells . Furthermore, we have previously found that inhibition of GPIIb/IIIa receptor function with a selective antagonist reduced TCIPA in vitro (Jurasz et al., 2001a). Finally, the combined blockade of GPIIb/IIIa and αvβ3 with blocking antibodies exerts significant antiangiogenic and antitumour effects in vivo (Trikha et al., 2002a). Therefore, platelets have the capacity to increase activation of GPIIb/IIIa in tumour cells, and this underscores the importance of platelet–tumour cells interactions for the regulation of GPIIb/IIIa during cancer growth and metastasis.
The GPIb/IX/V receptor complex is also constitutively expressed on the platelet surface (Michelson et al., 1994). The function of these receptors in vascular haemostasis is to anchor platelets, using von Willebrand factor as a bridge, to the damaged portion of the vascular wall (Ruggeri, 1999). In our experiments, we used a specific monoclonal antibody against the α subunit of GPIb. We have previously shown that the dynamic changes (from upregulation to downregulation) of platelet surface numbers of GPIb mark the transition of agonist- and tumour cell-stimulated platelets from adhesion to aggregation (Jurasz et al., 2001b; Radomski et al., 2001). In agreement with these observations, we have measured decreased platelet surface numbers of GPIb in collagen-aggregated platelets during the course of our present experiments. We have also shown that GPIb is upregulated on the platelet surface during aggregation with both cotransfected and mock MCF7 cells. The reasons for this persistent upregulation of GPIb during aggregation of platelets by MCF7 cells are not clearly understood. It has been shown that the plasma membrane receptor GPIb is present on the platelet α-granule membranes and, to a lesser extent, on the dense granules (Berger et al., 1996; Youssefian et al., 1997). Therefore, the upregulation of GPIb induced by MCF7 cells could be explained by the vesicle trafficking between the α-granules and plasma membrane. Interestingly, human recombinant MMP-2 increases GPIb in platelets adhering to von Willebrand factor (Radomski et al., 2001) and such a mechanism could also operate during TCIPA induced by MCF7 cells.
GPIb was initially believed to be expressed only in cells from the megakaryocytic lineage; there is, however, increasing evidence for its ectopic expression in different tumour cell lines. Indeed, GPIbα is present on the surface of MCF7 breast carcinoma cells, and this receptor may be involved in the initial adhesive interactions between platelets and tumour cells (Oleksowicz et al., 1995; Oleksowicz et al., 1997b). In our studies, neo/zeo and β3/MT cells showed GPIb on their surface, and there was a strong (10-fold) increase in the number of copies of GPIb during TCIPA. However, a four-fold increase in GPIb was only achieved after 84 h of incubation with phorbol-12-myristate 13-acetate that stimulates protein kinase C (Oleksowicz et al., 1997a). The mechanism(s) of GPIb upregulation in our experiments is unlikely to involve de novo protein synthesis due to the short time course (30 min) of our experiments. It is more likely that GPIb is translocated to the plasma membrane from an intracellular pool. Indeed, HeLa cells have been shown to exocytotically upregulate β1 integrins from an intracellular pool to the cell surface through a PKC-mediated mechanism (Chun et al., 1997). Moreover, GPIIb/IIIa activation during TCIPA could help to stimulate this PKC-dependent translocation of GPIb, via activation of PKC downstream of GPIIb/IIIa (Giuliano et al., 2003).
Clinically, the expression of MMP-2 and MT1-MMP in breast cancer cells has been proposed as an index of malignant behaviour and as a prognostic marker of aggressive and metastasizing tumour growth. (Talvensaari-Mattila et al., 1998; 1999; Mimori et al., 2001).
Thus, our results show that the expression of functionally active MT1-MMP on breast carcinoma cells stimulates TCIPA. The proaggregatory effects of MT1-MMP are enhanced by ADP and are associated with upregulation of platelet and tumour adhesion receptors, GPIb and GP IIb/IIIa.
Acknowledgments
We are grateful to Dr. P. Jurasz for his helpful advice regarding cell culture and aggregometry experiments, and to Dr. A. Radomski for her help with the Western blotting studies. The study was supported by the Canadian Institutes of Health Research (CIHR) grant to MWR and the Secretaria de Estado de Educacion y Universidades fellowship, cofunded by the European Social Fund to DAE. DAE is a post-doctoral fellow of Spanish Ministry of Education, MWR is a CIHR scientist. AYS is supported by NIH Grants CA83017 and CA77470, California Breast Cancer Res. Program Grant 5JB0094 and Susan G. Komen Breast Cancer Foundation Grant 9849. The electron microscopy studies were carried out at the High Resolution Electron Microscopy Facility, UTMDACC (Institutional Core Grant #CA16672).
Abbreviations
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type-1 matrix metalloproteinase
- TCIPA
tumour cell-induced platelet aggregation
- TIMP
tissue inhibitor of metalloproteinases
- GP
glycoprotein
- 2-MeSAMP
2-methylthio-AMP
- MRS2179
N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate
References
- ABECASSIS J., BERETZ A., MILLON-COLLARD R., FRICKER J.P., EBER M., CAZENAVE J.P. In vitro interactions between human breast cancer cells MCF-7 and human blood platelets. Thromb Res. 1987;47:693–698. doi: 10.1016/0049-3848(87)90108-3. [DOI] [PubMed] [Google Scholar]
- ABRAMS C.S., ELLISON N., BUDZYNSKI A.Z., SHATTIL S.J. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990;75:128–138. [PubMed] [Google Scholar]
- ABRAMS C., SHATTIL S.J. Immunological detection of activated platelets in clinical disorders. Thromb. Haemost. 1991;65:467–473. [PubMed] [Google Scholar]
- BASTIDA E., ORDINAS A. Platelet contribution to the formation of metastatic foci: the role of cancer cell-induced platelet activation. Haemostasis. 1988;18:29–36. doi: 10.1159/000215780. [DOI] [PubMed] [Google Scholar]
- BAURAND A., RABOISSON P., FREUND M., LEON C., CAZENAVE J.-P., BOURGUIGNON J.-J., GACHET C. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur. J. Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- BELKIN A.M., AKIMOV S.S., ZARITSKAYA L.S., RATNIKOV B.I., DERYUGINA E.I., STRONGIN A.Y. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J. Biol. Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- BENNETT J.S. Platelet-fibrinogen interactions. Ann. N.Y. Acad. Sci. 2001;936:340–354. doi: 10.1111/j.1749-6632.2001.tb03521.x. [DOI] [PubMed] [Google Scholar]
- BERGER G., MASSE J.M., CRAMER E.M. Alpha-granule membrane mirrors the platelet plasma membrane and contains the glycoproteins Ib, IX, and V. Blood. 1996;87:1385–1395. [PubMed] [Google Scholar]
- BILLROTH T. Lectures on Surgical Pathology and Therapeutics: a Handbook for Students and Practitioners 1878London: New Sydenham Society; 355p [Google Scholar]
- CAMEZ A., DUPUY E., BELLUCCI S., CALVO F., BRYCKAERT M.C., TOBELEM G. Human platelet–tumor cell interactions vary with the tumor cell lines. Invas. Metast. 1986;6:321–334. [PubMed] [Google Scholar]
- CHANG Y.S., CHEN Y.Q., TIMAR J., NELSON K.K., GROSSI I.M., FITZGERALD L.A., DIGLIO C.A., HONN K.V. Increased expression of alpha IIb beta 3 integrin in subpopulations of murine melanoma cells with high lung-colonizing ability. Int. J. Cancer. 1992;51:445–451. doi: 10.1002/ijc.2910510318. [DOI] [PubMed] [Google Scholar]
- CHUN J., AUER K.A., JACOBSON B.S. Arachidonate initiated protein kinase C activation regulates HeLa cell spreading on a gelatin substrate by inducing F-actin formation and exocytotic upregulation of beta 1 integrin. J. Cell Physiol. 1997;173:361–370. doi: 10.1002/(SICI)1097-4652(199712)173:3<361::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- CHUNG A.W.Y., JURASZ P., HOLLENBERG M.D., RADOMSKI M.W. Mechanisms of action of proteinase-activated receptor agonists on human platelets. Br. J. Pharmacol. 2002;135:1123–1132. doi: 10.1038/sj.bjp.0704559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DERYUGINA E.I., BOURDON M.A., JUNGWIRTH K., SMITH J.W., STRONGIN A.Y. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int. J. Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- DERYUGINA E.I., RATNIKOV B., MONOSOV E., POSTNOVA T.I., DISCIPIO R., SMITH J.W., STRONGIN A.Y. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp. Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- DERYUGINA E.I., RATNIKOV B.I., POSTNOVA T.I., ROZANOV D.V., STRONGIN A.Y. Processing of integrin alpha(v) subunit by membrane type 1 matrix metalloproteinase stimulates migration of breast carcinoma cells on vitronectin and enhances tyrosine phosphorylation of focal adhesion kinase. J. Biol. Chem. 2002;277:9749–9756. doi: 10.1074/jbc.M110269200. [DOI] [PubMed] [Google Scholar]
- FELDING-HABERMANN B., O'TOOLE T.E., SMITH J.W., FRANSVEA E., RUGGERI Z.M., GINSBERG M.H., HUGHES P.E., PAMPORI N., SHATTIL S.J., SAVEN A., MUELLER B.M. Integrin activation controls metastasis in human breast cancer. Proc. Natl. Acad. Sci. USA. 2001;98:1853–1858. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERNANDEZ-PATRON C., MARTINEZ-CUESTA M.A., SALAS E., SAWICKI G., WOZNIAK M., RADOMSKI M.W., DAVIDGE S.T. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb. Haemost. 1999;82:1730–1735. [PubMed] [Google Scholar]
- GACHET C. Identification, characterization, and inhibition of the platelet ADP receptors. Int. J. Hematol. 2001;74:375–381. doi: 10.1007/BF02982079. [DOI] [PubMed] [Google Scholar]
- GALT S.W., LINDEMANN S., ALLEN L., MEDD D.J., FALK J.M., MCINTYRE T.M., PRESCOTT S.M., KRAISS L.W., ZIMMERMAN G.A., WEYRICH A.S. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ. Res. 2002;90:1093–1099. doi: 10.1161/01.res.0000019241.12929.eb. [DOI] [PubMed] [Google Scholar]
- GASIC G.J. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metast. Rev. 1984;3:99–114. doi: 10.1007/BF00047657. [DOI] [PubMed] [Google Scholar]
- GIULIANO S., NESBITT W., ROONEY M., JACKSON S. Bi-directional integrin alpha IIbbeta 3 signaling regulating platelet adhesion under flow: contribution of protein kinase C. Biochem. J. 2003;372:163–172. doi: 10.1042/BJ20020868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSI I.M., HATFIELD J.S., FITZGERALD L.A., NEWCOMBE M., TAYLOR J.D., HONN K.V. Role of tumor cell glycoproteins immunologically related to glycoproteins Ib and IIb/IIIa in tumor cell–platelet and tumor cell–matrix interactions. FASEB J. 1988;2:2385–2395. doi: 10.1096/fasebj.2.8.2452113. [DOI] [PubMed] [Google Scholar]
- HONN K.V., STEINERT B.W., MOIN K., ONODA J.M., TAYLOR J.D., SLOANE B.F. The role of platelet cyclooxygenase and lipoxygenase pathways in tumor cell induced platelet aggregation. Biochem. Biophys. Res. Commun. 1987;145:384–389. doi: 10.1016/0006-291x(87)91333-7. [DOI] [PubMed] [Google Scholar]
- HOTARY K., ALLEN E., PUNTURIERI A., YANA I., WEISS S.J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYNES R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- ITOH Y., TAKAMURA A., ITO N., MARU Y., SATO H., SUENAGA N., AOKI T., SEIKI M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20:4782–4793. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANTZEN H.M., GOUSSET L., BHASKAR V., VINCENT D., TAI A., REYNOLDS E.E., CONLEY P.B. Evidence for two distinct G-protein-coupled ADP receptors mediating platelet activation. Thromb. Haemost. 1999;81:111–117. [PubMed] [Google Scholar]
- JURASZ P., CHUNG A.W.Y., RADOMSKI A., RADOMSKI M.W. Nonremodeling properties of matrix metalloproteinases: the platelet connection. Circ. Res. 2002;90:1041–1043. doi: 10.1161/01.res.0000021398.28936.1d. [DOI] [PubMed] [Google Scholar]
- JURASZ P., SAWICKI G., DUSZYK M., SAWICKA J., MIRANDA C., MAYERS I., RADOMSKI M.W. Matrix metalloproteinase 2 in tumor cell-induced platelet aggregation: regulation by nitric oxide. Cancer Res. 2001a;61:376–382. [PubMed] [Google Scholar]
- JURASZ P., STEWART M.W., RADOMSKI A., KHADOUR F., DUSZYK M., RADOMSKI M.W. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. Br. J. Pharmacol. 2001b;134:1104–1112. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZES I., ELALAMY I., SRAER J.D., HATMI M., NGUYEN G. Platelet release of trimolecular complex components MT1-MMP/TIMP2/MMP2: involvement in MMP2 activation and platelet aggregation. Blood. 2000;96:3064–3069. [PubMed] [Google Scholar]
- KOSHIKAWA N., GIANNELLI G., CIRULLI V., MIYAZAKI K., QUARANTA V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTINEZ A., SALAS E., RADOMSKI A., RADOMSKI M.W. Matrix metalloproteinase-2 in platelet adhesion to fibrinogen: interactions with nitric oxide. Med. Sci. Monit. 2001;7:646–651. [PubMed] [Google Scholar]
- MICHELSON A.D., BENOIT S.E., KROLL M.H., LI J.M., ROHRER M.J., KESTIN A.S., BARNARD M.R. The activation-induced decrease in the platelet surface expression of the glycoprotein Ib-IX complex is reversible. Blood. 1994;83:3562–3573. [PubMed] [Google Scholar]
- MIMORI K., UEO H., SHIRASAKA C., MORI M. Clinical significance of MT1-MMP mRNA expression in breast cancer. Oncol. Rep. 2001;8:401–403. doi: 10.3892/or.8.2.401. [DOI] [PubMed] [Google Scholar]
- MU D., CAMBIER S., FJELLBIRKELAND L., BARON J.L., MUNGER J.S., KAWAKATSU H., SHEPPARD D., BROADDUS V.C., NISHIMURA S.L. The integrin {alpha}v{beta}8 mediates epithelial homeostasis t. J. Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLER H.K., BUCANA C.D., KRIPKE M.L., COX P.A., SAIJO S., STRICKLAND F.M. Ultraviolet irradiation of murine skin alters cluster formation between lymph node dendritic cells and specific T lymphocytes. Cell. Immunol. 1994;157:263–276. doi: 10.1006/cimm.1994.1221. [DOI] [PubMed] [Google Scholar]
- NASH G., TURNER L., SCULLY M., KAKKAR A. Platelets and cancer. Lancet Oncol. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- OLEKSOWICZ L., DUTCHER J.P., DELEON-FERNANDEZ M., ETKIND P. A GPIb alpha-related protein is expressed by fresh human breast carcinoma tissue and is regulated by a PKC-sensitive mechanism. Exp. Cell Res. 1997a;237:110–117. doi: 10.1006/excr.1997.3784. [DOI] [PubMed] [Google Scholar]
- OLEKSOWICZ L., DUTCHER J.P., DELEON-FERNANDEZ M., PAIETTA E., ETKIND P. Human breast carcinoma cells synthesize a protein immunorelated to platelet glycoprotein-Ib alpha with different functional properties. J. Lab. Clin. Med. 1997b;129:337–346. doi: 10.1016/s0022-2143(97)90182-7. [DOI] [PubMed] [Google Scholar]
- OLEKSOWICZ L., MROWIEC Z., SCHWARTZ E., KHORSHIDI M., DUTCHER J.P., PUSZKIN E. Characterization of tumor-induced platelet aggregation: the role of immunorelated GPIb and GPIIb/IIIa expression by MCF-7 breast cancer cells. Thromb. Res. 1995;79:261–274. doi: 10.1016/0049-3848(95)00113-6. [DOI] [PubMed] [Google Scholar]
- OVERALL C.M., MCQUIBBAN G.A., CLARK-LEWIS I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: a new tool for degradomics. Biol. Chem. 2002;383:1059–1066. doi: 10.1515/BC.2002.114. [DOI] [PubMed] [Google Scholar]
- RADOMSKI A., STEWART M.W., JURASZ P., RADOMSKI M.W. Pharmacological characteristics of solid-phase von Willebrand factor in human platelets. Br. J. Pharmacol. 2001;134:1013–1020. doi: 10.1038/sj.bjp.0704345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADOMSKI M., MONCADA S. An improved method for washing of human platelets with prostacyclin. Thromb. Res. 1983;30:383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- RADOMSKI M.W., JENKINS D.C., HOLMES L., MONCADA S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073–6078. [PubMed] [Google Scholar]
- RATNIKOV B.I., ROZANOV D.V., POSTNOVA T.I., BACIU P.G., ZHANG H., DISCIPIO R.G., CHESTUKHINA G.G., SMITH J.W., DERYUGINA E.I., STRONGIN A.Y. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- RUGGERI Z.M. Mechanisms of shear-induced platelet adhesion and aggregation. Thromb. Haemost. 1993;70:119–123. [PubMed] [Google Scholar]
- RUGGERI Z.M. Structure and function of von Willebrand factor. Thromb. Haemost. 1999;82:576–584. [PubMed] [Google Scholar]
- SATO H., TAKINO T., OKADA Y., CAO J., SHINAGAWA A., YAMAMOTO E., SEIKI M. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- SAWICKI G., SALAS E., MURAT J., MISZTA-LANE H., RADOMSKI M.W. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386:616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- SAWICKI G., SANDERS E.J., SALAS E., WOZNIAK M., RODRIGO J., RADOMSKI M.W. Localization and translocation of MMP-2 during aggregation of human platelets. Thromb. Haemost. 1998;80:836–839. [PubMed] [Google Scholar]
- SHATTIL S.J., KASHIWAGI H., PAMPORI n. Integrin signaling: the platelet paradigm. Blood. 1998;91:2645–2657. [PubMed] [Google Scholar]
- STERNLICHT M.D., WERB Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRONGIN A.Y., COLLIER I., BANNIKOV G., MARMER B.L., GRANT G.A., Goldberg G.I. Mechanism of cell surface activation of 72-kDa type IV collagenase. J. Biol. Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- TAKASAKI J., KAMOHARA M., SAITO T., MATSUMOTO M., MATSUMOTO S.-i., OHISHI T., SOGA T., MATSUSHIME H., FURUICHI K. Molecular cloning of the platelet P2TAC ADP receptor: pharmacological comparison with another ADP receptor, the P2Y1 receptor. Mol. Pharmacol. 2001;60:432–439. [PubMed] [Google Scholar]
- TALVENSAARI-MATTILA A., PAAKKO P., HOYHTYA M., BLANCO-SEQUEIROS G., TURPEENNIEMI-HUJANEN T. Matrix metalloproteinase-2 immunoreactive protein: a marker of aggressiveness in breast carcinoma. Cancer. 1998;83:1153–1162. doi: 10.1002/(sici)1097-0142(19980915)83:6<1153::aid-cncr14>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- TALVENSAARI-MATTILA A., PAAKKO P., TURPEENNIEMI-HUJANEN T. MMP-2 positivity and age less than 40 years increases the risk for recurrence in premenopausal patients with node-positive breast carcinoma. Breast Cancer Res. Treat. 1999;58:287–293. doi: 10.1023/a:1006326513176. [DOI] [PubMed] [Google Scholar]
- TIMAR J., TRIKHA M., SZEKERES K., BAZAZ R., TOVARI J., SILLETTI S., RAZ A., HONN K.V. Autocrine motility factor signals integrin-mediated metastatic melanoma cell adhesion and invasion. Cancer Res. 1996;56:1902–1908. [PubMed] [Google Scholar]
- TRIKHA M., TIMAR J., LUNDY S.K., SZEKERES K., TANG K., GRIGNON D., PORTER A.T., HONN KV. Human prostate carcinoma cells express functional alphaIIb(beta)3 integrin. Cancer Res. 1996;56:5071–5078. [PubMed] [Google Scholar]
- TRIKHA M., TIMAR J., ZACHAREK A., NEMETH J.A., CAI Y., DOME B., SOMLAI B., RASO E., LADANYI A., HONN K.V. Role for beta3 integrins in human melanoma growth and survival. Int. J. Cancer. 2002a;101:156–167. doi: 10.1002/ijc.10521. [DOI] [PubMed] [Google Scholar]
- TRIKHA M., ZHOU Z., TIMAR J., RASO E., KENNEL M., EMMELL E., NAKADA M.T. Multiple roles for platelet GPIIb/IIIa and {alpha}v{beta}3. Cancer Res. 2002b;62:2824–2833. [PubMed] [Google Scholar]
- TROUSSEAU A. Clinique Medicale de L'Hotel-Dieu Paris. London, New Sydenham Society; 1865. pp. 94–96. [Google Scholar]
- YOUSSEFIAN T., MASSE J.M., RENDU F., GUICHARD J., CRAMER E.M. Platelet and megakaryocyte dense granules contain glycoproteins Ib and IIb-IIIa. Blood. 1997;89:4047–4057. [PubMed] [Google Scholar]
- ZAFFRAN Y., MEYER S.C., NEGRESCU E., REDDY K.B., FOX J.E. Signaling across the platelet adhesion receptor glycoprotein Ib-IX induces alpha IIbbeta 3 activation both in platelets and a transfected Chinese hamster ovary cell system. J. Biol. Chem. 2000;275:16779–16787. doi: 10.1074/jbc.275.22.16779. [DOI] [PubMed] [Google Scholar]