Abstract
The fatty acid amide class of compounds, which include the endocannabinoid anandamide and the sleep-inducing compound oleamide, have been shown in vitro to have a multiplicity of actions upon different neurochemical systems. In the present issue of this journal, Leggett et al present data indicating that oleamide functionally activates CB1 cannabinoid receptors in vitro. The significance of their finding is discussed in this commentary.
Keywords: Oleamide, cannabinoid, vanilloid
Among the fatty acid amide chemical class of compounds are a number of important endogenous biologically active agents including palmitoylethanolamide (an anti-inflammatory agent), anandamide (arachidonoylethanolamide, an antinociceptive and possibly neuroprotective agent, among other actions) and oleamide (a sleep-inducing agent) (see Bezuglov et al, 1998; Rice, 2001). Despite the obvious chemical similarity of the compounds (see Figure 1), they show widely divergent pharmacological profiles. Thus, for example, anandamide activates cannabinoid receptors but is devoid of activity at the nuclear receptor PPAR-α, whereas the reverse is true for its homologue oleoylethanolamide (Fu et al, 2003). A second important feature of the compounds is their multiplicity of actions. The most well studied of these, anandamide, has in addition to its cannabimimetic activity effects upon other molecular targets, of which the vanilloid receptors have received the most attention, not the least in this journal (for a review, see Ross, 2003). When it is considered that the ethanolamine subgroup of fatty acid amides are synthesised on demand and released together, and that they can affect the actions of one another (see e.g. Smart et al, 2002 and references cited therein), it is clear that dissection of the predominant mechanisms of in vivo action of individual compounds becomes rather difficult. Even for a well-investigated compound like anandamide, there is debate at present as to whether this compound is both an endocannabinoid and an ‘endovanilloid', or alternatively whether its vanilloid effects are pharmacologically possible but physiologically irrelevant (see Di Marzo et al, 2001).
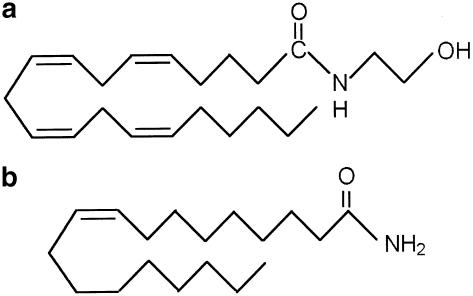
Figure 1.
Chemical structures of (a) anandamide and (b) oleamide. The simplest nomenclature of these compounds is to annotate the length of the acyl chain and the number of bonds together with a note as to whether the compound is an amide or an ethanolamide. Thus, anandamide is C20:4 ethanolamide and oleamide is C18:1 amide. Other related endogenous compounds with biological activity include palmitoylethanolamide (C16:0 ethanolamide), stearoylethanolamide (C18:0 ethanolamide), oleoylethanolamide (C18:1 ethanolamide) and erucamide (C22:1 amide) (see Bezuglov et al, 1998).
Oleamide is no exception to the rule of ‘single compound, multiple actions', producing effects in vitro upon a variety of targets including gap junction communication, serotonin 5-HT1A, 5-HT2A/2C, 5-HT7 and GABAA receptors (see Leggett et al, 2003). The ability of oleamide to interact with cannabinoid receptors has, however, been a matter of controversy. In this issue, Leggett et al present data indicating that oleamide functionally activates CB1 cannabinoid receptors in vitro. Thus, the authors demonstrate among other findings that oleamide is able to: (a) inhibit agonist and antagonist ligand binding to CB1 receptors; (b) increase via CB1 receptors the binding of [35S]GTPγS to membranes, an effect associated with G-protein coupled receptors; and (c) inhibit forskolin-stimulated cyclic AMP production in a manner blocked by a CB1 cannabinoid receptor antagonist and by pertussis toxin. In vitro potencies of lipophilic compounds like anandamide and oleamide are notoriously variable between laboratories, a point well made by Leggett et al. However, relative potencies between compounds with similar physicochemical properties determined in the same laboratory have value, and their finding that oleamide inhibits agonist binding to CB1 receptors with a potency only three-fold lower than seen for anandamide may be of potential importance in neurons, at least on the basis of the relative levels of the two compounds in neuroblastoma cells (Bisogno et al, 1997). This assumes, of course, that the concentrations of the compounds at the biophase under the assay conditions used reflect the situation in vivo, a rather large assumption given that factors such as metabolic processes may occur during the incubation times used. Both anandamide and oleamide are avidly metabolised by fatty acid amide hydrolase present in membrane fractions, and the relative potencies of the two compounds in binding assays may be entirely different under conditions where FAAH is inhibited, or absent, than in its presence (Lichtman et al, 2002).
While the data presented here by Leggett et al convincingly demonstrate that in their hands oleamide is capable of interacting with and activating CB1 receptors in vitro, their conclusion that this compound ‘is a directly acting endogenous cannabinoid with selectivity for the CB1 receptor' can be interpreted in many ways. Certainly, the compound is endogenous, and certainly it shows in vitro selectivity for the CB1 receptor over the CB2 receptor. A wider interpretation that the compound acts as an endocannabinoid in vivo is perhaps premature. The in vivo data so far reported in the literature give conflicting information regarding the contribution of CB1 receptors at least for some of the actions of exogenously applied oleamide (see Leggett et al, 2003) and there is, to my knowledge, no evidence to indicate that the compound is involved in the endocannabinoid tone that is believed to modulate neurotransmitter release, neuroprotection and other important physiological events (see Wilson & Nicoll, 2002; Marsicano et al, 2003). Nevertheless, the study of Leggett et al raises important issues that can but stimulate further research into an exciting area of pharmacology.
Abbreviations
- CB
cannabinoid
References
- BEZUGLOW V.V., BOBROV M.Y.U., ARCHAKOV A.V. Bioactive amides of fatty acids. Biochemistry (Moscon) 1998;63:22–30. [PubMed] [Google Scholar]
- BISOGNO T., SEPE N., DE PETROCELLIS L., MECHOULAM R., DI MARZO V. The sleep inducing factor oleamide is produced by mouse neuroblastoma cells. Biochem. Biophys. Res. Commun. 1997;239:473–479. doi: 10.1006/bbrc.1997.7431. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L. Anandamide: some like it hot. Trends Pharmacol. Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- FU J., GAETANI S., OVEISI F., LO VERME J., SERRANO A., RODRÍGUEZ DE FONSECA F., ROSENGATH A., LUECKE H., DI GIACOMO B., TARZIA G., PIOMELLI D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- LEGGETT J.D., ASPLEY S., BECKETT S.R.G., D'ANTONA A.M., KENDALL D.A., KENDALL D.A.Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors Br. J. Pharmacol. 2003 10.1038/sj.bjp.0705607advance online publication, December 22, 2003, doi [DOI] [PMC free article] [PubMed]
- LICHTMAN A.H., HAWKINS E.G., GRIFFIN G., CRAVATT B.F. Pharmacological activity of fatty acid amides is regulated, but not mediated, by fatty acid amide hydrolase in vivo. J. Pharmacol. Exp. Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- MARSICANO G., GOODENOUGH S., MONORY K., HERMANN H., EDER M., CANNICH A., AZAD S.C., CASCIO M.G., GUTIÉRREZ S.O., VAN DER STELT M., LÓPEZ-RODRÍGUEZ M.L., CASANOVA M.L., SCHÜTZ G., ZIEGLGÄNSBERGER W., DI MARZO V., BEHL C., LUTZ B. CB1 cannabinoid receptors and on-demand defense against excitoxocity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- RICE A.S.C. Cannabinoids and pain. Curr. Opin. Invest. Drugs. 2001;2:399–414. [PubMed] [Google Scholar]
- ROSS R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JONSSON K.-O., VANDEVOORDE S., LAMBERT D.M., FOWLER C.J. ‘Entourage' effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]