Abstract
This study characterises the binding of a novel nonpeptide antagonist radioligand, [3H]SB-674042 (1-(5-(2-fluoro-phenyl)-2-methyl-thiazol-4-yl)-1-((S)-2-(5-phenyl-(1,3,4)oxadiazol-2-ylmethyl)-pyrrolidin-1-yl)-methanone), to the human orexin-1 (OX1) receptor stably expressed in Chinese hamster ovary (CHO) cells in both a whole cell assay and in a cell membrane-based scintillation proximity assay (SPA) format.
Specific binding of [3H]SB-674042 was saturable in both whole cell and membrane formats. Analyses suggested a single high-affinity site, with Kd values of 3.76±0.45 and 5.03±0.31 nM, and corresponding Bmax values of 30.8±1.8 and 34.4±2.0 pmol mg protein−1, in whole cell and membrane formats, respectively. Kinetic studies yielded similar Kd values.
Competition studies in whole cells revealed that the native orexin peptides display a low affinity for the OX1 receptor, with orexin-A displaying a ∼five-fold higher affinity than orexin-B (Ki values of 318±158 and 1516±597 nM, respectively).
SB-334867, SB-408124 (1-(6,8-difluoro-2-methyl-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea) and SB-410220 (1-(5,8-difluoro-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea) all displayed high affinity for the OX1 receptor in both whole cell (Ki values 99±18, 57±8.3 and 19±4.5 nM, respectively) and membrane (Ki values 38±3.6, 27±4.1 and 4.5±0.2 nM, respectively) formats.
Calcium mobilisation studies showed that SB-334867, SB-408124 and SB-410220 are all functional antagonists of the OX1 receptor, with potencies in line with their affinities, as measured in the radioligand binding assays, and with approximately 50-fold selectivity over the orexin-2 receptor.
These studies indicate that [3H]SB-674042 is a specific, high-affinity radioligand for the OX1 receptor. The availability of this radioligand will be a valuable tool with which to investigate the physiological functions of OX1 receptors.
Keywords: Orexin, hypocretin, [3H]SB-674042, SB-334867, SB-408124, SB-410220, radioligand binding
Introduction
Orexin-A and orexin-B (hypocretin-1 and -2) are 33 and 28 amino-acid peptides, respectively, which have been isolated from the rat hypothalamus (Sakurai et al., 1998). Both are derived from a 130 amino-acid precursor, prepro-orexin, and are located predominantly in the hypothalamus and locus coeruleus (Sakurai et al., 1998; Evans et al., 1999). The orexins have been implicated in a number of physiological functions, including energy metabolism and control of feeding (Sakurai et al., 1998), modulation of neuroendocrine function (Van den Pol et al., 1998; Smart, 1999) and regulation of arousal (Hagan et al., 1999) and the sleep–wake cycle (Piper et al., 2000).
Both orexin peptides are ligands for two receptors, orexin-1 (OX1) and orexin-2 (OX2). Both orexin-A and orexin-B bind to and activate OX2 with similar affinities and potencies, whereas orexin-B has 10-fold lower affinity and ∼100-fold lower potency at OX1 compared with orexin-A (Sakurai et al., 1998). OX1 and OX2 receptors are members of the seven-transmembrane G-protein-coupled cell surface receptor superfamily, and binding of the ligands is associated with an increase in intracellular calcium ([Ca2+]i).
Examination of the functional roles of the orexin receptors has been hampered by the lack of a suitable antagonist radioligand. Several groups have used [125I]orexin-A or other agonist peptide analogues to label the receptors (Sakurai et al., 1998; Wieland et al., 2002), but these have undesirable physico-chemical properties such as adhesion to plasticware and glass-fibre filters, which have usually prevented their use in membrane-based assays; to date, most radioligand-binding studies with these peptides have been performed using whole cells (Sakurai et al., 1998; Wieland et al., 2002).
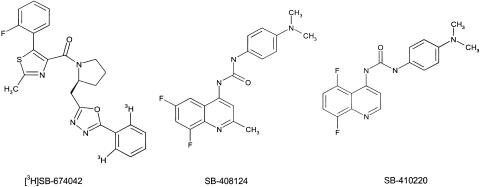
SB-334867 has been previously described as a selective, nonpeptide antagonist of the OX1 receptor (Smart et al., 2001). Here we present the first example of a selective, OX1 receptor nonpeptide antagonist radioligand, [3H]SB-674042 (1-(5-(2-fluoro-phenyl)-2-methyl-thiazol-4-yl)-1-[(S)-2-(5-phenyl-[1,3,4]oxadiazol-2-ylmethyl)-pyrrolidin-1-yl)-methanone; Figure 1) and characterise its binding to the OX1 receptor both in membrane and whole-cell-based assay formats. We also describe two further nonpeptide antagonists of the OX1 receptor, SB-408124 (1-(6,8-difluoro-2-methyl-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea) and SB-410220 (1-(5,8-difluoro-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea; Porter et al., 2001).
Figure 1.
Structures of SB-674042, SB-408124 and SB-410220.
Methods
Cloning of orexin receptors and stable cell lines
The Chinese hamster ovary (CHO)-K1-OX1 cell line was produced by PCR amplification of full-length cDNA corresponding to the human OX1 receptor, followed by subcloning into the SmaI site of pBS. An EcoRI/NotI fragment of pBS-OX1R was then subcloned into pcDNA3 and transfected into CHO-K1 cells using Lipofectamine (Life Technologies, Paisley, U.K.). Clones were selected using 400 μg ml−1 G418 (Life Technologies) and single-cell clones were produced by limiting dilution cloning. Dideoxy sequencing of the PCR product confirmed an identical sequence to accession number AH009943.
CHO-DG44-OX1 and OX2 cell lines were produced by PCR from in-house foetal and adult brain cDNA libraries, respectively, using primers located across the start and stop codons. The receptors were subcloned into the pCDN vector (with neomycin resistance) and transfected into CHO cells using Lipofectamine (Life Technologies). All PCR products were confirmed by full-length dideoxy sequencing.
Cell culture for radioligand binding
CHO cells stably expressing the OX1 receptor (CHO-K1_OX1) were cultured in DMEM/F12, supplemented with 5% FBS and 2 mM L-glutamine under 5% CO2 at 37°C. For whole-cell-binding assays, cells were seeded onto 96-well Packard CulturPlates at 3 × 104 cells well−1 24 h prior to assay. For membrane-based SPA assays, cells were scraped, pelleted at 500 × g for 5 min and stored at −80°C until use for membrane preparation.
Membrane preparations
Membranes were prepared as follows; all procedures were performed at 4°C. The cell pellet was resuspended in 10 v buffer containing 25 mM HEPES, 2 mM EDTA, 1 × Complete™ serine and cysteine protease inhibitor tablet 50 ml−1, pH 7.5. The cell suspension was disrupted by use of a glass-teflon homogeniser and centrifuged at 50,000 × g for 30 min. The supernatant was discarded and the pellets resuspended in buffer followed by homogenisation and centrifugation, as previously described. The resulting pellets were resuspended in buffer at a concentration of 4 mg protein ml−1 and stored at −80°C until further use.
[3H]SB-674042 whole cell binding assays
After overnight culture in 96-well Packard Cultur plates, the medium was discarded and cells were incubated in buffer containing 150 mM NaCl, 20 mM HEPES and 0.5% bovine serum albumin (pH 7.4) for 60 min at 25°C. Saturation studies were carried out by incubating cells with a range of concentrations of [3H]SB-674042 (0.2–24 nM); the total assay volume was 250 μl. Protein content was assayed by lysing cells with 0.1 M NaOH and using the Bradford method (Bradford, 1976) with bovine serum albumin (BSA) as a standard.
Association kinetic studies were performed by measuring the specific binding of [3H]SB-674042 (3 nM) at 1–60 min after addition of [3H]SB-674042. For dissociation studies, cells were first incubated with [3H]SB-674042 (3 nM) for 60 min. Specific binding was then measured at 2–120 min after the addition of 3 μM SB-408124. Competition studies were performed by incubating cells with [3H]SB-674042 (3 nM) and a range of concentrations of the test compound. All assays were terminated by washing the cells three times with 250 μl ice-cold phosphate-buffered saline. A volume of 100 μl of Microscint 40 was added to each well and the plate was left at room temperature for 2 h. Cell-associated radioactivity was then measured using a Packard Topcount, with a count time of 2 min well−1.
[3H]SB-674042 membrane-based SPA binding assays
CHO-K1_OX1 cell membranes (75 μg ml−1) were precoupled by shaking with wheatgerm-agglutinin polyvinyltoluene (WGA-PVT) scintillation proximity assay (SPA) beads (5 mg ml−1) in buffer containing 25 mM HEPES, 2.5 mM MgCl2, 0.5 mM EDTA and 0.025% bacitracin (pH 7.4) at 4°C for 1 h. The bead-membrane suspension was centrifuged at 300 × g and resuspended in the same volume of room temperature assay buffer. A volume of 100 μl of bead-membrane suspension was incubated with [3H]SB-674042 (5 nM) in a total assay volume of 200 μl in a 96-well Packard Optiplate to give a final protein concentration of 7.5 μg well−1. Nonspecific binding was measured as that remaining in the presence of 3 μM SB-408124. Assay plates were shaken for 10 min and then incubated at room temperature for 4 h before being counted on a Packard TopCount scintillation counter (count time 2 min well−1).
Saturation studies were carried out by incubating bead-membranes (equivalent to 7.5 μg protein well−1 and 2.5 mg beads ml−1) with a range of concentrations of [3H]SB-674042 (0.1–20 nM). Protein content was assayed using the Bradford method (Bradford, 1976) using bovine serum albumin as a standard. Association kinetic studies were performed by measuring specific binding of [3H]SB-674042 (5 nM) at 1–30 min after addition of bead-membranes (equivalent to 7.5 μg protein well−1 and 2.5 mg beads ml−1). For dissociation studies, bead-membranes were first incubated with [3H]SB-674042 (5 nM) for 30 min. Specific binding was then measured at 2–120 min after the addition of 3 μM SB-408124. Competition studies were performed by incubating bead-membranes (equivalent to 7.5 μg protein well−1 and 2.5 mg beads ml−1) with [3H]SB-674042 (5 nM) and a range of concentrations of the test compound.
Calcium mobilisation studies
CHO-DG44_OX1 or CHO-DG44_OX2 cells were routinely grown as monolayers in MEM-Alpha medium supplemented with 10% foetal calf serum and 400 μg ml−1 G418, and maintained under 95%/5% O2/CO2 at 37°C. Cells were passaged every 3–4 days. CHO-DG44_OX1 or CHO-DG44_OX2 cells were seeded into black-walled clear-base 96-well plates (Costar, U.K.) at a density of 20,000 cells well−1 in MEM-Alpha medium supplemented as above, and cultured overnight. The cells were then incubated with MEM-Alpha medium containing the cytoplasmic calcium indicator Fluo-3AM (4 μM; Teflabs, Austin, TX, U.S.A.) and 2.5 mM probenecid at 37°C for 60 min. The cells were washed four times with, and finally resuspended in, Tyrode's medium containing 2.5 mM probenecid and 0.1% gelatine, before being incubated for 30 min at 37°C with either buffer alone (control) or buffer containing antagonist (0.1 nM–10 μM). The plates were then placed into a FLIPR (Molecular Devices, U.K.) to monitor the fluorescence (λex=488 nm, λEM=540 nm) before and after the addition of orexin-A (10 nM).
Materials
CHO-K1 and CHO-DG44 cells stably transfected with OX1 or OX2 receptor were produced by GlaxoSmithKline Pharmaceuticals (Harlow, U.K.). Human orexin-A, human orexin-B, SB-334867 (1-(2-methyl-benzooxazol-6-yl)-3-[1,5]naphthyridin-4-yl-urea hydrochloride), SB-408124 (1-(6,8-difluoro-2-methyl-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea) and SB-410220 (1-(5,8-difluoro-quinolin-4-yl)-3-(4-dimethylamino-phenyl)-urea) were synthesised in house. Other drugs and chemicals were purchased from Sigma-Aldrich (Poole, U.K.), Boeringher Mannheim (Mannheim, Germany), Fisher Scientific (Loughborough, U.K.) or BDH (Poole, U.K.). 1-(5-(2-fluoro-phenyl)-2-methyl-thiazol-4-yl)-1-((S)-2-(5-phenyl-(1,3,4)oxadiazol-2-ylmethyl)-pyrrolidin-1-yl)-methanone ([3H]SB-674042; specific activity 27 Ci mmol−1) was synthesised by Amersham Pharmacia (Cardiff, U.K.).
Data analysis
Specific saturation-binding data were analysed using the program Radlig (Biosoft) to provide estimates of Kd and Bmax values. Kinetic data were analysed by the program GraFit (Erithacus Software) to provide estimates of Kon and Koff values. Competition curves were analysed by nonlinear least-squares fitting to a four-parameter logistic equation in Microsoft Excel in order to determine IC50 values (Bowen & Jerman, 1995). Ki values were then derived from the IC50 values using the Kd value obtained from saturation studies (Cheng & Prussoff, 1973).
For calcium mobilisation studies, the peak stimulation (minus basal) was plotted versus the concentration of test compound and iteratively curve fitted using a four-parameter logistic equation (Grafit, Erithacus Software) to assess the antagonist potency and maximal response. Results are given as means (±s.e.m.) of at least three independent experiments.
Results
Saturation studies
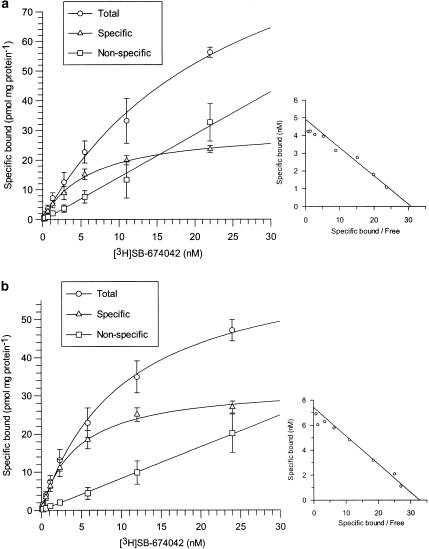
Specific binding of [3H]SB-674042 to both CHO-K1_OX1 whole cells and membranes was saturable and represented more than 70 and 80% of total binding, respectively, whereas nonspecific binding increased linearly with radioligand concentration. No specific binding was seen in membranes of CHO-K1 wild-type cells (data not shown). Analysis of whole cell-binding data revealed that [3H]SB-674042 bound to one site on the CHO-K1_OX1 cells, with a Kd value of 5.03±0.31 nM and a Bmax value of 34.4±2.0 pmol mg protein−1 (Figure 2). Analysis of membrane-based SPA data gave similar results, with a Kd value of 3.76±0.45 nM and a Bmax value of 30.8±1.8 pmol mg protein−1 (Figure 2).
Figure 2.
Total, specific and nonspecific binding of [3H]SB-674042 to (a) CHO-K1 whole cells stably expressing the OX1 receptor and (b) membranes from CHO-K1 cells expressing the OX1 receptor in SPA format, with increasing radioligand concentration. Nonspecific binding was defined as that remaining in the presence of 3 μM SB-408124. Vertical lines show s.e.m. The inset graphs show Scatchard transformations of the specific binding data. Data are the mean of 3–4 experiments.
Kinetic studies
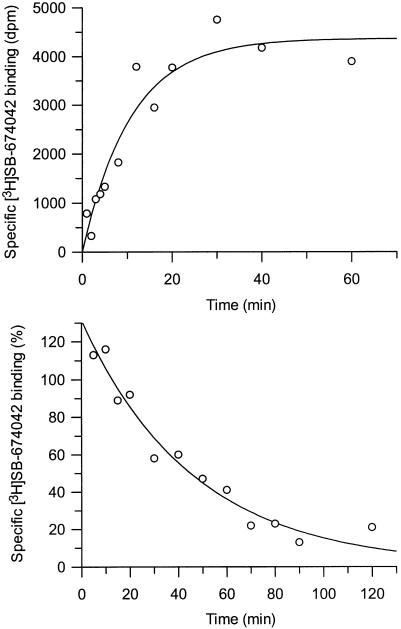
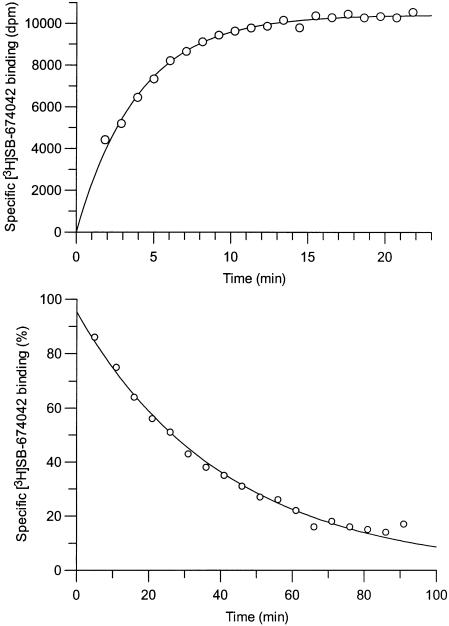
Association studies indicated that [3H]SB-674042 binding was monophasic and equilibrium was reached within 60 min for whole cells (Figure 3) and 30 min for membranes (Figure 4). Dissociation upon addition of excess SB-408124 appeared monophasic, with >80% of specific binding being dissociated after 120 min in whole cells and 90 min in membrane assays (Figures 3 and 4). The specific binding of [3H]SB-674042 was shown to be stable for up to 5 h (data not shown). Single-site analysis of membrane-binding results gave a Kobs of 0.177±0.005 min−1 and a Koff of 0.0277±0.0004 min−1, leading to a calculated Kon value of 23.2±2.1 min−1 μM−1. Similar analysis of whole-cell binding gave a Kobs of 0.0433±0.0180 min−1 and a Koff of 0.0197±0.0026 min−1, leading to a calculated Kon value of 16.9±5.5 min−1 μM−1. The Kd values derived from these data were 1.07 and 1.16 nM for membrane and whole-cell binding, respectively, which are close to the values determined by saturation analyses.
Figure 3.
Time course for association and dissociation of [3H]SB-674042 binding to CHO-K1_OX1 whole cells. Data shown are from a single experiment, which was replicated three times with similar results.
Figure 4.
Time course for association and dissociation of [3H]SB-674042 binding to CHO-K1_OX1 membranes in SPA format. Data shown are from a single experiment, which was replicated three times with similar results.
Competition studies
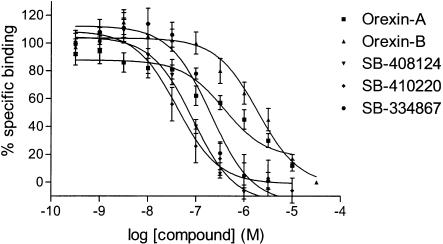
Specific binding represented ∼80 and ∼60% of total binding in membrane SPA and whole-cell formats, respectively. Human orexin-A and orexin-B displayed weak affinity for the OX1 receptor in the whole-cell competition assay, with Ki values of 318±158 and 1516±596 nM, respectively (Table 1; Figure 5). Furthermore, orexin-A displayed a shallow inhibition curve. In contrast, no inhibition of [3H]SB-674042 binding could be detected with the orexin peptides up to 10 μM in the membrane-based SPA format, even in the presence of 0.5% BSA (data not shown).
Table 1.
Affinities of orexin-A, orexin-B, SB-334867, SB-410220, SB-408124 and SB-674042, as measured by [3H]SB-674042 binding or calcium mobilisation
| Orexin-A | Orexin-B | SB-334867 | SB-408124 | SB-410220 | SB-674042 | |
|---|---|---|---|---|---|---|
| Kb, OX1 (nM) | nd | nd | 27.8±2.6 | 21.7±2.3 | 8.7±0.8 | 1.1±0.1 |
| Kb, OX2 (nM) | nd | nd | 1704±266 | 1405±284 | 503±90 | 129±15 |
| Ki, SPA OX1 (nM) | dnb | dnb | 38.7±3.6 | 26.9±4.1 | 4.5±0.2 | nd |
| Ki, whole-cell OX1 (nM) | 318±158 | 1516±596 | 57±8.3 | 99±18 | 18.5±4.5 | nd |
nd – value not determined; dnb – did not bind up to 10 μM.
Figure 5.
Competition for [3H]SB-674042 binding to CHO-K1_OX1 whole cells by orexin-A, orexin-B, SB-334867, SB-408124 and SB-410220. Data are the mean of at least three independent experiments; vertical lines show s.e.m.
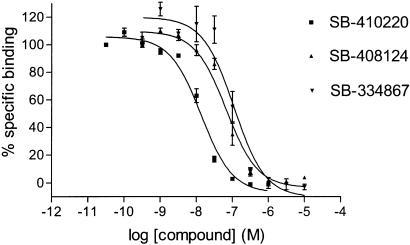
SB-334867, SB-408124 and SB-410220 bound to the OX1 receptor with high affinity in the membrane SPA format (Table 1; Figure 6). Slightly lower affinities were observed in the whole-cell assay format (Table 1; Figure 5).
Figure 6.
Competition for [3H]SB-674042 binding to membranes from CHO-K1_OX1 cells in SPA format by SB-334867, SB-408124 and SB-410220. Data are the mean of at least three independent experiments; vertical lines show s.e.m.
Calcium mobilisation studies
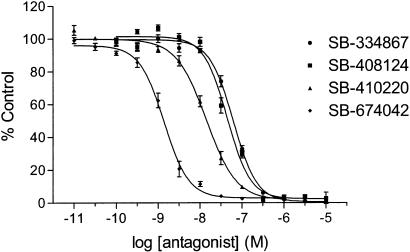
Calcium mobilisation studies using FLIPR technology demonstrated that SB-334867, SB-408124, SB-410220 and SB-674042 were all competitive, functional antagonists at the OX1 receptor (Table 1, Figure 7). SB-334867, SB-408124 and SB-410220 displayed approximately 50-fold selectivity over the human OX2 receptor, whereas SB-674042 was >100-fold selective (Table 1). Furthermore, SB-674042 displayed no significant affinity for a range of serotonergic, dopaminergic, adrenergic and purinergic receptors at concentrations up to 10 μM (data not shown).
Figure 7.
Concentration-dependent inhibition of OX1 receptor-mediated calcium responses by SB-334867, SB-408124, SB-410220 and SB-674042. Data are the mean of at least three independent experiments; vertical lines show s.e.m.
Discussion
These results indicate that [3H]SB-674042 is a high-affinity radioligand for the OX1 receptor. Whole-cell saturation studies suggest that [3H]SB-674042 binds to a single, high-affinity binding site on the OX1 receptor (Kd value 5.03±0.31 nM). Similar results were observed using a membrane-based SPA format (Kd value 3.76±0.45 nM). Kinetic studies also suggest both monophasic association and dissociation; single-site analysis of the data produced Kd values of 1.16 and 1.07 nM for whole-cell assays and membrane-based SPA, respectively. These values are close to those revealed by the saturation studies.
Competition studies in whole cell assay format showed that both human orexin-A and orexin-B demonstrate weak affinity for the OX1 receptor, while orexin-A displays a shallow inhibition curve. The Ki values for orexin-A and orexin-B (318±158 and 1516±596 nM, respectively) are in line with their differential efficacy in stimulating calcium mobilisation (Smart et al., 1999), and are similar to the Ki values derived for inhibition of [125I]-orexin-A binding to membranes from CHO cells stably expressing the human OX1 receptor and membranes from the rat anterior hypothalamus (Kane et al., 2000).
No displacement of [3H]SB-674042 could be detected in the membrane SPA format with the orexin peptides at concentrations of up to 10 μM, even in the presence of 0.5% BSA. It is possible that the unfavourable physico-chemical properties (e.g. adhesion to plasticware) of the orexin peptides were not compatible with the SPA format of the assay. It also possible that [3H]SB-674042, an antagonist, binds to all available OX1 receptor-binding sites, whereas the agonist peptides only bind to a small fraction of coupled receptors. To a certain extent, this may also explain the relatively low affinity of the orexin peptides in the whole-cell binding assay compared to their functional potency at the OX1 receptor in calcium mobilisation studies (Smart et al., 1999).
The OX1 receptor selective antagonist SB-334867 (Smart et al., 2001) inhibits [3H]SB-674042 binding in both whole-cell assay and membrane SPA formats, with Ki values of 38.7±3.6 and 99±18 nM, respectively. These values are in good agreement with the potency of SB-334867 to inhibit rhodamine green-tagged orexin-A binding to CHO cells stably expressing the OX1 receptor (Smart et al., 2001). We also describe two novel, nonpeptide OX1 receptor antagonists, SB-408124 and SB-410220, which display Ki values of 26.9±4.1 and 4.5±0.2 nM, respectively, in the membrane SPA format, and 57±8.3 and 18.5±4.5 nM, respectively in the whole-cell assay. Consistent with the data for SB-334867, these compounds show an approximate half-log unit lower affinity when tested against whole cells rather than membranes in SPA format.
All the three nonpeptide compounds were shown to be potent, functional antagonists in a calcium mobilisation assay. SB-334867, SB-408124 and SB-410220 displayed Kb values of 27.8±2.6, 21.7±2.3 and 8.7±0.8 nM, respectively, values that correlate well with their radioligand-binding affinities. SB-334867 displays 10-fold higher affinity for the inhibition of orexin-induced calcium mobilisation than for the inhibition of orexin-A- and orexin-B-induced cell firing in the noradrenergic neurons of the locus coeruleus of the rat, which is reported to be under the control of the OX1 receptor (Soffin et al., 2002). However, this level of discrepancy is not unusual for values measured in a highly expressing recombinant system versus a native tissue slice preparation. SB-674042 was also shown to be a competitive, functional antagonist of the OX1 receptor in the calcium mobilisation assay, displaying a Kb value of 1.1±0.1 nM, which is in close agreement with the Kd values derived from the radioligand-binding assays.
In summary, our results demonstrate that [3H]SB-674042 is a high-affinity radioligand suitable for labelling human OX1 receptors stably expressed in CHO cells. Our studies have characterised the binding of [3H]SB-674042 to OX1 receptors in both a whole cell and membrane-based SPA format.
We have also identified two novel, nonpeptide antagonists, SB-408124 and SB-410220, and characterised their interaction with the OX1 receptor with both the radioligand-binding assays and a calcium mobilisation assay. Both compounds are potent, high-affinity antagonists of the OX1 receptor. These tool compounds, along with [3H]SB-674042, should assist in further probing the localisation and function of OX1 receptors.
Abbreviations
- CHO
Chinese hamster ovary cells
- DMEM
Dulbecco's modified Eagle's medium
- FLIPR
fluorometric imaging plate reader
- MEM
modified Eagle's medium
- OX1
orexin-1 receptor
- OX2
orexin-2 receptor
- SPA
scintillation proximity assay
- WGA-PVT
wheatgerm-agglutinin polyvinyltoluene
References
- BOWEN W.P., JERMAN J. Nonlinear regression using spreadsheets. Trends Pharmacol. Sci. 1995;16:413–417. doi: 10.1016/s0165-6147(00)89091-4. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- EVANS M.E., HARRIES M., PATEL S., BENHAM C. Orexin-A depolarises neurones in the rat locus coeruleus brain slice in vitro. J. Physiol. 1999;515:121. [Google Scholar]
- HAGAN J.J., LESLIE R.A., PATEL S., EVANS M.L., WATTAM T.A., HOLMES S., BENHAM C.D., TAYLOR S.G., ROUTLEDGE C., HEMMATI P., MUNTON R.P., ASHMEADE T.E., SHAH A.S., HATCHER J.P., HATCHER P.D., JONES D.N., SMITH M.I., PIPER D.C., HUNTER A.J., PORTER R.A., UPTON N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANE J.K., TANAKA H., PARKER S.L., YANAGISAWA M., LI M.D. Sensitivity of orexin-A binding to phospholipase C inhibitors, neuropeptide Y and secretin. Biochem. Biophys. Res. Commun. 2000;272:959–965. doi: 10.1006/bbrc.2000.2880. [DOI] [PubMed] [Google Scholar]
- PIPER D.C., UPTON N., SMITH M.I., HUNTER A.J. The novel brain neuropeptide, orexin-A, modulates the sleep–wake cycle of rats. Eur. J. Neurosci. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- PORTER R.A., CHAN W.N., COULTON S., JOHNS A., HADLEY M.S., WIDDOWSON K., JERMAN J.C., BROUGH S.J., COLDWELL M., SMART D., JEWITT F., JEFFREY P., AUSTIN N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg. Med. Chem. Lett. 2001;11:1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., AMEMIYA A., ISHII M., MATSUZAKI I., CHEMELLI R.M., TANAKA H., WILLIAMS S.C., RICHARDSON J.A., KOZLOWSKI G.P., WILSON S., ARCH J.R., BUCKINGHAM R.E., HAYNES A.C., CARR S.A., ANNAN R.S., MCNULTY D.E., LIU W.S., TERRETT J.A., ELSHOURBAGY N.A., BERGSMA D.J., YANAGISAWA M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- SMART D. Orexins: a new family of neuropeptides. Br. J. Anaesth. 1999;83:695–697. doi: 10.1093/bja/83.5.695. [DOI] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., BROUGH S.J., RUSHTON S.L., MURDOCK P.R., JEWITT F., ELSHOURBAGY N.A., ELLIS C.E., MIDDLEMISS D.N., BROWN F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., SABIDO-DAVID C., BROUGH S.J., JEWITT F., JOHNS A., PORTER R.A., JERMAN J.C. SB-334867-A: the first selective orexin-1 receptor antagonist. Br. J. Pharmacol. 2001;132:1179–1182. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOFFIN E.M., EVANS M.L., GILL C.H., HARRIES M.H., BENHAM C.D., DAVIES C.H. SB-334867 antagonises orexin mediated excitation in the locus coeruleus. Neuropharmacology. 2002;42:127–133. doi: 10.1016/s0028-3908(01)00156-3. [DOI] [PubMed] [Google Scholar]
- VAN DEN POL A.N., GAO X.B., OBRIETAN K., KILDUFF T.S., BELOUSOV A.B. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIELAND H.A., SOLL R.M., DOODS H.N., STENKAMP D., HURNAUS R., LAMMLE B., BECK-SICKINGER A.G. The SK-N-MC cell line expresses an orexin binding site different from recombinant orexin 1-type receptor. Eur. J. Biochem. 2002;269:1128–1135. doi: 10.1046/j.0014-2956.2001.02739.x. [DOI] [PubMed] [Google Scholar]