Abstract
The functional changes in mesenteric arterioles of streptozotocin-induced diabetes were investigated by intravital microscopy. The mesentery was exteriorized from anesthetized rats, spread in a chamber, and superfused with Tyrode solution. All drugs tested were applied to the superfusing Tyrode solution.
Compared with age-matched controls, the diabetic rats showed enhanced vascular sensitivity to phenylephrine, an α1-adrenoceptor agonist. The preincubation of the mesentery with NG-nitro-L-arginine (L-NNA), a nitric oxide synthase (NOS) inhibitor, shifted the phenylephrine-concentration–response curves to the left in both the diabetic and control rats. Even in the presence of L-NNA, the sensitivity to phenylephrine was higher in the diabetic rats than in the control.
Acetylcholine relaxed the mesenteric arterioles in both groups, but to a significantly greater extent in the control than in the diabetic rats. However, the L-NNA-induced constriction of arterioles did not differ significantly between the groups. In contrast, the amplitude of the constrictions of mesenteric arterioles induced by S-ethylisothiourea, an inducible NOS (iNOS) inhibitor, was significantly greater in the diabetic rats than in the control.
Immunostaining of the mesentery with a specific antibody for iNOS revealed iNOS in the microvessels of only the diabetic rats.
These results suggest that constrictor responses to α1-adrenoceptor stimulation are sensitized in the mesenteric arterioles of STZ-diabetic rats, and that iNOS expressed in the arteriolar smooth muscle plays a role in suppressing the basal tone and the reactivity of the arterioles in STZ-diabetic rats.
Keywords: Streptozotocin, mesenteric arterioles, intravital microscopy, immunohistochemistry, nitric oxide, iNOS, NG-nitro-L-arginine, S-ethylisothiourea, acetylcholine, α1-adrenoceptor
Introduction
A number of in vitro studies have shown altered arterial contractility in streptozotocin (STZ)-induced diabetic rats. These experiments have been done extensively in isolated large arteries such as the aorta and superior mesenteric artery. However, there are considerable discrepancies among the results reported thus far: Some studies have demonstrated increased vasoconstrictor responses to noradrenaline in STZ-diabetic rats, while others showed decreased or unchanged reactivity to the amine. In contrast, all of the in vivo studies with STZ-diabetic rats have revealed only decreased pressor responses to noradrenaline (see reviews by Öztürk et al., 1996; Cooper et al., 2001).
Endothelial dysfunction has also been reported in STZ-diabetic rats. A number of in vitro studies have investigated endothelium-dependent relaxation of vascular smooth muscle in experimental diabetes. However, these studies have provided conflicting observations of decreased, unchanged, or increased responses to acetylcholine, which causes an endothelium-dependent vasorelaxation, in the arterial ring preparations from STZ-diabetic rats (Öztürk et al., 1996; Cooper et al., 2001). In vivo studies with STZ-diabetic rats have also revealed conflicting results, that is, decreased (Bucala et al., 1991) or unchanged (Kiff et al., 1991) depressor responses to acetylcholine in STZ-diabetic rats. The reasons underlying these discrepancies have not been elucidated.
Unlike the case with arteries, little information is available about the influence of diabetes on resistance arterioles. Several studies have shown the effects of diabetes in isolated perfused mesentery, in which changes in the resistance across the mesenteric arterial tree were measured by monitoring the perfusion pressure (Diederich et al., 1994; Taylor et al., 1994b; Makino & Kamata, 1998). Although the arterial arch, including the arteriole, is thought to be a main determinant of the mesenteric vascular resistance (Fenger-Gron et al., 1997), it is possible that arteries other than the arterial arch together contribute to an alteration of the perfusion pressure in response to some stimulation. The present study was thus undertaken to elucidate whether or not STZ-induced diabetes alters in vivo responses of resistance arterioles. To this end, we used intravital microscopy to observe STZ-diabetic rats' mesenteric arterioles with an inner diameter of around 20 μm, and investigated the modulatory role of nitric oxide (NO), particularly that derived via inducible NO synthase (iNOS), in both the basal tone and vasoconstrictor response to the α-adrenergic stimulation.
Methods
Drugs
The following drugs were used: acetylcholine hydrochloride, fluorescein isothiocyanate (FITC)-isomer 1, NG-nitro-L-arginine (L-NNA), streptozotocin (STZ), and L-phenylephrine hydrochloride (Sigma, St Louis, MO, U.S.A.); and S-ethylisothiourea hydrobromide (EIT; BIOMOL Research Laboratories, Plymouth Meeting, PA, U.S.A.). The Tyrode solution had the following composition (mM): NaCl 158.3, KCl 4.0, NaHCO3 10.0, NaH2PO4 0.42, CaCl2 2.0, MgCl2 0.42, and glucose 5.6.
STZ was dissolved in 0.1 M sodium citrate. Acetylcholine, EIT, L-NNA, and phenylephrine were dissolved in the Tyrode solution.
Induction of experimental diabetes
Animals were treated as approved by the Institutional Animal Care and Use Committee, and according to the Guidelines for Animal Experiments established by the Japanese Pharmacological Society.
Male Wistar rats, 3 weeks old, obtained from SLC (Hamamatsu, Japan) were randomized to receive an intraperitoneal injection of STZ (65 mg kg−1) or vehicle (0.1 M sodium citrate, pH 4.0) under light ether anesthesia. The rats were housed in a temperature- and light-controlled environment, and were allowed access to food and water ad libitum. The experiments were performed 10 weeks after the injections. STZ-treated animals were considered diabetic and retained for the experiments if their blood glucose was greater than 300 mg dl−1. Blood samples, for the measurement of glucose concentration, were obtained on the day of the experiment. Plasma glucose concentration was determined by the O-toluidine method (Glucose Test Wako, Wako Pure Chemical Industries, Osaka, Japan).
Preparations
The rats were anesthetized with α-chloralose (40 mg kg−1, i.v.) and urethane (400 mg kg−1, i.v.). A polyethylene tube was placed in the right carotid artery for the measurement of blood pressure and heart rate, which were recorded on a polygraph system (model RM-6000, Nihon Kohden, Tokyo, Japan) via a pressure transducer (model TDN-R, Gould, Oxnard, CA, U.S.A.). After receiving a midline abdominal incision, the animal was placed on its right side on a microscope stage. The small intestine and mesentery were exteriorized, and the mesentery was carefully spread over a glass plate in a chamber. The chamber was connected to a reservoir that allowed continuous superfusion of the mesentery with warm (37±1°C) Tyrode solution aerated with 97% O2–3% CO2 at a rate of 10 ml min−1 by a peristaltic pump. After insertion of the mesentery in the chamber, the preparation was allowed to equilibrate for 30–40 min.
Intravital microscopy
Quantitative microscopic observations were performed on the arterioles in the mesentery under an intravital fluorescence microscope system (BH-2; Olympus, Tokyo, Japan) with an × 20 water-immersion objective (UMPlanFl; Olympus) and a CCD camera (DXC-108; Sony, Tokyo, Japan) or an SIT camera (C2400; Hamamatsu Photonics, Hamamatsu, Japan) for the measurement of vessel diameter or blood flow velocity, respectively, as previously described (Nakayama et al., 1991,1999). The arteriole (10–30 μm in inner diameter) corresponded to the third or fourth branch enumerated from the stem mesenteric artery, and was located 6–8 mm from the internal border of the intestinal arch. Video images were monitored and recorded with a digital video recorder for playback analyses. The recorded video images were analyzed on a Sony PCV-R70 personal computer. To measure the inner diameter of the arterioles, still video frames were captured and the inner diameter at each of five points per arteriole was measured with graphics software (Canvas 7, Deneba Software, Miami, FL, U.S.A.) and averaged. For the measurement of vascular contractility, arterioles with an inner diameter of around 20 μm were selected (19.4±1.5 and 22.8±1.6 μm in the control and diabetic rats, respectively; each n=18).
To visualize the blood flow, a portion of erythrocytes was labeled fluorescently and injected intravenously, as previously described (Suzuki et al., 1996). The arterial blood (0.2 ml) of a donor rat was collected from a catheter placed in a right carotid artery into a 3 ml test tube containing heparin (100 U) for anticoagulation. The erythrocytes were separated from the plasma by centrifugation, and were washed twice with a physiological saline solution. These erythrocytes were then incubated at room temperature with a phosphate-buffered saline (PBS) solution that was adjusted to pH 7.8 and contained 1 mg ml−1 FITC-isomer 1. After 60 min of incubation, the labeled cells were then washed twice with a saline solution containing 1% bovine serum albumin to remove uncombined fluorescent dyes. The final volume concentration of the labeled cells was adjusted to 50% by adding an isotonic saline solution. These suspensions were injected intravenously through a catheter placed in the jugular vein. The erythrocyte flow velocity was calculated by a frame-by-frame analysis on the personal computer.
All drugs were added to the superfusing Tyrode solution. We examined the response of one arteriole per rat.
Immunohistochemistry
Each mesentery, including the arterioles, was excised and fixed in 1% paraformaldehyde for 3 h, permeabilized with 1% Triton X-100 for 3 h, and blocked with 5% skim milk for 1 h at room temperature. The mesentery was then incubated overnight at 4°C with a mixture of a rabbit anti-iNOS antibody (diluted 1 : 25; sc-650, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and a mouse anti-α-smooth muscle actin (diluted 1 : 400; Sigma) or antimacrophage (diluted 1 : 50; Biomeda, Foster City, CA, U.S.A.). After washing with PBS containing 0.5% Triton X-100, a mixture of Alexa Fluor 488 anti-rabbit IgG (diluted 1 : 200; Molecular Probes, Eugene, OR, U.S.A.) and Alexa Fluor 546 anti-mouse IgG (diluted 1 : 200; Molecular Probes) was allowed to stand for 2 h at room temperature. After being washed three times, the mesentery was dipped in 75% glycerol and then observed under a laser-scanning confocal microscope (LSM510; Carl Zeiss, Oberkochen, Germany). The Alexa Fluor 488 and 546 were excited with 488 nm argon and 543 nm helium–neon lasers, respectively, and the resultant fluorescence images were acquired through 505–530 and 560–615 nm band pass filters, respectively. For immunohistochemical controls, the mesentery was incubated without the primary antibody or with preimmune serum.
Statistics
The amplitude of vasoconstriction was expressed as a percentage of the inner diameter before the application of the drugs; that is, the initial inner diameter was normalized to 100%. The amplitude of vasorelaxation was expressed as a percentage of the amplitude of preconstriction induced by phenylephrine; that is, the initial inner diameter, minus the inner diameter after the application of phenylephrine, was normalized to 100%.
Results were expressed as means±s.e.m. Comparisons were made by Student's t-test for unpaired data. A probability of P<0.05 was accepted as the level of statistical significance.
Results
Characteristics of the animals
The blood glucose levels in the diabetic rats (441.3±19.2 mg dl−1; n=7) were about four times higher than those in the control rats (116.3±3.4 mg dl−1; n=10) 10 weeks after the injection of STZ or vehicle (Table 1). The STZ injection retarded the rats' increase in body weight (Table 1). Fundamental cardiovascular parameters were measured 10 weeks after the injection of STZ or vehicle. The diabetic rats had significantly lower blood pressure than the control rats, and exhibited a significant reduction of blood flow velocity in the mesenteric arterioles, with an inner diameter of around 20 μm (Table 1).
Table 1.
Characteristics of rats 10 weeks after injection with streptozotocin (STZ), compared with age-matched control rats
| Body weight (g) | Serum glucose (mg dl−1) | Mean blood pressure (mmHg) | Blood flow velocity (mm s−1) | |
|---|---|---|---|---|
| Control | 309±3 | 121.2±7.5 | 132.7±5.9 | 2.64±0.41 |
| STZ | 130±4** | 443.7±13.3** | 99.9±5.9** | 1.43±0.22** |
Blood flow velocity was measured in mesenteric arterioles with an inner diameter of around 20 μm. Each value represents the mean±s.e.m. for nine rats.
P<0.01 compared to the corresponding control value.
Constrictor response to phenylephrine
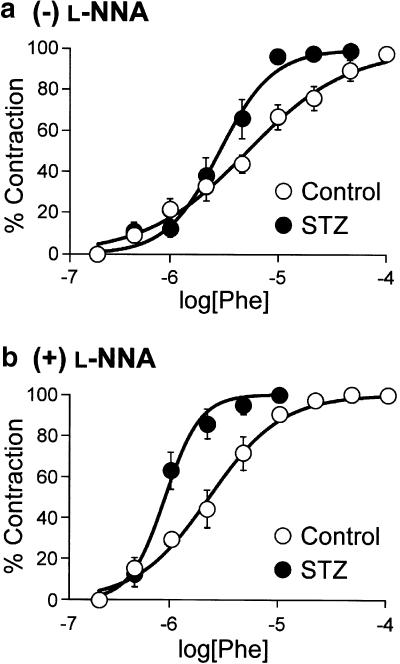
The effect of phenylephrine, an α1-adrenergic agonist, on the mesenteric arterioles was compared between the STZ-induced diabetic rats and the age-matched control rats. As shown in Figure 1, phenylephrine caused concentration-dependent constrictions of mesenteric arterioles in both groups. Although the pEC50 value for phenylephrine was significantly greater in the diabetic arterioles than in the control, there was no significant difference in the maximal response between the two groups (Table 2).
Figure 1.
Concentration–response curves for phenylephrine (Phe)-induced constriction of mesenteric arterioles in the absence (a) and presence (b) of L-NNA in the control and STZ-diabetic rats. Each point represents the mean±s.e.m. of six rats.
Table 2.
Effect of phenylephrine on mesenteric arteriolar diameter in the absence and presence of NG-nitro-L-arginine (L-NNA) in streptozotocin-diabetic and control rats
| pEC50 | Rmax (%) | n | |
|---|---|---|---|
| L-NNA (−) | |||
| Control | 5.17±0.07 | 53.9±9.0 | 6 |
| Streptozotocin | 5.54±0.06** | 52.7±3.8 | 6 |
| L-NNA (+) | |||
| Control | 5.59±0.09## | 58.6±4.0 | 6 |
| Streptozotocin | 6.07±0.03**## | 57.9±3.8 | 6 |
Each value represents the mean±s.e.m. for the number (n) of experiments. pEC50: −log EC50. Rmax: The maximal response to phenylephrine is shown as a percentage of the inner diameter before the application of the drug. L-NNA (0.1 mM) was applied 15 min before the application of phenylephrine.
P<0.01 compared to the corresponding control value.
P<0.01 compared to the corresponding value of L-NNA (−).
After the concentration–response relationship for phenylephrine was obtained, about 30 min were allowed to pass to allow recovery from the constrictor response. The mesentery was then incubated with L-NNA (0.1 mM) for 15 min, and again phenylephrine was cumulatively applied. L-NNA (0.1 mM) caused a constriction of the mesenteric arterioles in both the diabetic and control rats. The amplitude of constriction was comparable between the two groups (14.6±2.8 and 17.7±3.2% in the control and diabetic rats, respectively; n=6). Preincubation of the mesentery with L-NNA for 15 min shifted the phenylephrine-concentration–response curves to the left in both the diabetic and control rats (Figure 1), associated with a significant increase in the pEC50 values (Table 2). Even in the presence of L-NNA, the sensitivity to phenylephrine was higher in the diabetic rats than in the control (Figure1b and Table 2). L-NNA did not change the maximal response (Table 2). Furthermore, we confirmed that the vasodilatation of the mesenteric arterioles in response to acetylcholine was nearly abolished after the treatment with L-NNA (0.1 mM) in both groups (data not shown).
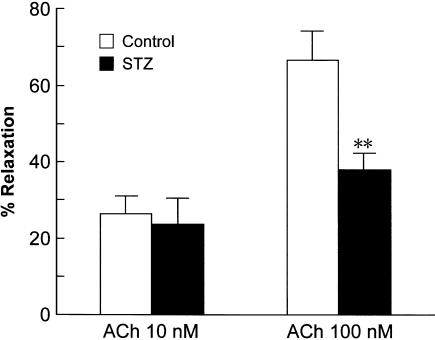
Dilator response to acetylcholine
Acetylcholine caused relaxations in both the diabetic and control mesenteric arterioles that were submaximally constricted with phenylephrine (5 or 10 μM). Since the acetylcholine-induced relaxation was not sustained, cumulative application was not used. Successive concentrations (10 and 100 nM) of acetylcholine were applied to the mesentery for 10 min each, with an interval of 30 min. As shown in Figure 2, there was no difference in the relaxation response to 10 nM acetylcholine between the diabetic and control mesenteric arterioles. However, the relaxation induced by 100 nM acetylcholine was significantly greater in the control than in the diabetic rats (Figure 2). L-NNA (0.1 mM) nearly abolished the vasodilatation of the mesenteric arterioles in response to acetylcholine, but indomethacin (10 μM), an inhibitor of cyclooxygenase, had no effect (data not shown). This suggested that NO, but not prostanoids, mediates the endothelium-dependent vasodilator response to acetylcholine in rat mesenteric arterioles.
Figure 2.
Vasodilator responses of mesenteric arterioles to ACh in control and STZ-diabetic rats. Each bar represents the mean±s.e.m of six rats. **P<0.01 compared to the control.
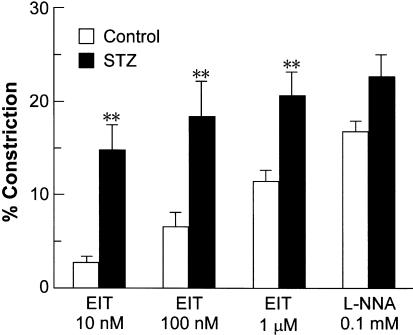
Constrictor responses to NOS inhibitors
EIT, which is a NOS inhibitor that is more selective for iNOS than for endothelial NOS (eNOS) or neuronal NOS (Nakane et al., 1995), was used to investigate the contribution of iNOS to the regulation of the vascular diameter of mesenteric arterioles. EIT at 10 nM caused a remarkable constriction of the mesenteric arterioles in the diabetic rats, whereas it induced a slight constriction in the control rats (Figure 3). At 100 nM and 1 μM, EIT also caused significantly greater constrictions in the diabetic than in the control rats (Figure 3). After the response to 1 μM EIT was measured, the nonselective NOS inhibitor L-NNA (0.1 mM) was finally applied to the mesentery. Unlike the case with EIT, L-NNA-induced constriction of the diabetic mesenteric arterioles did not significantly differ from the control (Figure 3).
Figure 3.
Vasoconstrictor responses of mesenteric arterioles to S-ethylisothiourea (EIT), an inducible NO synthase inhibitor, and L-NNA, a nonselective NO synthase inhibitor, in control and STZ-diabetic rats. Each bar represents the mean±s.e.m of seven rats. **P<0.01 compared to the corresponding control.
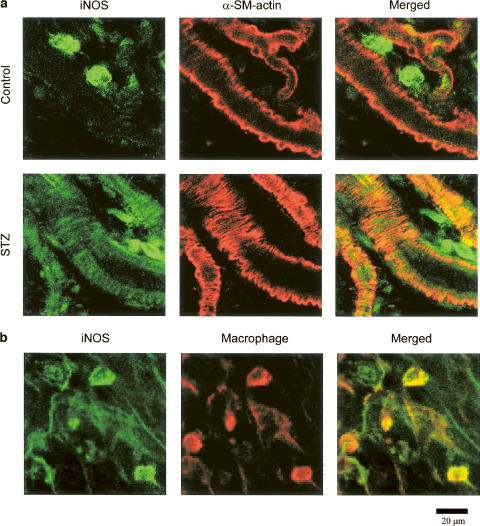
iNOS distribution in the mesentery
Immunohistochemical analysis was performed to investigate whether iNOS could be detected in the mesenteric arterioles of the control and STZ-diabetic rats. Double-immunostaining with specific antibodies for iNOS and α-smooth muscle actin showed that the diabetic mesenteries contained iNOS in both the smooth muscle layer of the arterioles and in the macrophages in the diabetic mesentery, whereas the control mesenteries showed iNOS only in the macrophages (Figure 4a). The positive signals for iNOS in the macrophages were confirmed by the double-immunostaining with antibodies for iNOS and macrophage (Figure 4b).
Figure 4.
Immunohistochemical localization of iNOS in the mesenteries of control and STZ-diabetic rats. (a) The mesenteries isolated from the control (upper panels) and the STZ-diabetic rats (lower panels) were double-immunolabeled for iNOS (green fluorescence; left panels) and α-smooth muscle (SM) actin (red fluorescence; center panels). (b) The mesenteries isolated from the control rats were double-immunolabeled for iNOS (green fluorescence; left panel) and macrophage (red fluorescence; center panel). The yellowish area in each right panel is produced by the coincidence of both fluorescences.
Discussion
Using intravital microscopy, we investigated the effects of experimental diabetes on the function of mesenteric arterioles, whose inner diameter was around 20 μm. The present study produced several important findings. First, the constriction responses of the arterioles to the α1-adrenergic agonist phenylephrine were sensitized in the STZ-diabetic rats. The enhanced response to phenylephrine does not appear to be attributable to a reduced production of NO in the diabetic rats. Secondly, the arterioles' relaxation responses to acetylcholine were impaired in the STZ-diabetic rats. Finally, regulation of the basal diameter of the arterioles by endogenous NO was altered during diabetes mellitus. iNOS, rather than eNOS, appears to profoundly contribute to the regulation of arteriolar diameters in diabetic rats.
Effects of phenylephrine
In the present study, the mesenteric arterioles of STZ-diabetic rats exhibited increased sensitivity to the α1-adrenergic agonist phenylephrine, compared with that of age-matched control rats. These data are consistent with previous studies in isolated perfused mesenteric arterial beds and in isolated mesenteric resistant arteries, which have shown enhanced contractile responses to noradrenaline in STZ-diabetic rats (Taylor et al., 1992,1994b; Van Buren et al., 1998). In line with this, a number of studies have shown that diabetes mellitus enhances the contractile responses of isolated large arteries, such as the aorta and superior mesenteric artery, to noradrenaline and phenylephrine. In contrast, conflicting results, that is, decreased or unchanged reactivity of the α-adrenoceptor agonists, have also been reported in isolated arteries, including mesenteric arteries (Öztürk et al., 1996; Cooper et al., 2001), and even in the perfused mesentery (Longhurst & Head, 1985; Takiguchi et al., 1989; Makino & Kamata, 1998). The precise reason for the discrepancy is not entirely clear. One possibility is that the duration and the severity of diabetes influence the vasocontractility to α-adrenoceptor agonists: In isolated mesenteric arteries, the sensitivity to noradrenaline has been shown to be significantly enhanced after a long-term (15–40 weeks) but not a short-term (1–4 weeks) diabetic state (Jackson & Carrier, 1981; Macleod & Mcneill, 1985; Van Buren et al., 1998).
Other researchers have suggested that the enhanced contractile response to α-adrenoceptor agonists observed in diabetic arteries is attributable to reduced NO release (Taylor et al., 1992; Kamata & Makino, 1997). However, it is unlikely that insufficient release of endothelium-derived NO could explain the enhanced contractile response to phenylephrine in the mesenteric arterioles of our STZ-diabetic rats. The present study clearly demonstrated a significantly enhanced contractile response to phenylephrine in the diabetic arterioles compared to the control, even in the presence of L-NNA. These results are consistent with previous investigations of endothelium-denuded mesenteric arteries from diabetic rats, in which the responsiveness to α-adrenoceptor stimulation was enhanced (Harris & Macleod, 1988). Other mechanisms for the enhanced reactivity to α-adrenoceptor agonists in diabetic arteries have also been proposed; for example, enhanced Ca2+ influx (Abebe et al., 1994; Tam et al., 1997), increased activity of G proteins and phospholipase C-β (Weber & Macleod, 1997), and/or increased Ca2+ sensitivity to the contractile protein (Chow et al., 2001).
The present study showed that STZ-diabetic rats had a significantly lower blood pressure, compared with control rats, which is in good agreement with the previous studies (Chang & Lund, 1986; Hicks et al., 1998). The hypotension in STZ-diabetic rats may be related to the damage of sympathetic nerve fibers and impaired sympathetic function by hyperglycemia (Monckton & Pehowich, 1980; Maeda et al., 1995). Furthermore, the sympathetic activity may have been altered by the anesthetics used in the present study. It has recently been shown that α-chloralose reduces the sympathetic activity in the rat (Maignan et al., 2000) and leads to unstable systemic hemodynamics when associated with urethane (Jong et al., 2002). Therefore, we would have to consider the alternative possibility that the increased sensitivity to phenylephrine in the diabetic arterioles is attributable to the changed baseline sympathetic activity. We have previously reported the in vivo preparation of rats acutely denervated by the treatment with TTX (Chino et al., 2000). Further analysis under the conditions where the sympathetic activity is totally eliminated by use of such procedures would be needed to verify the possibility.
Effects of acetylcholine
Acetylcholine relaxed the rat mesenteric arterioles in both groups. This appeared to be mediated primarily by NO, because of its sensitivity to the NOS inhibitor L-NNA. Indomethacin, a cyclooxygenase inhibitor, had no effect on the acetylcholine-induced relaxation, suggesting that prostanoids are not involved in the effect of acetylcholine in rat mesenteric arterioles. The relaxant response to acetylcholine was attenuated in the STZ-diabetic rats. The impaired relaxation to acetylcholine in the diabetic arterioles agrees with the majority of studies in isolated conduit arteries, but several studies have shown unchanged or augmented relaxant responses to muscarinic agonists in diabetic arteries (Öztürk et al., 1996; Cooper et al., 2001). The reason for these discrepant findings is not obvious, but variations in the duration and severity of the diabetes may be contributory. It has been shown that the attenuated relaxant responses to acetylcholine in diabetic rat aorta are dependent on the duration of the diabetes (Orie et al., 1993; Pieper, 1999).
Although exposure to elevated glucose levels impairs acetylcholine-induced relaxation, it has been observed that free radical scavengers, such as superoxide dismutase, prevent this impairment (Tesfamariam & Cohen, 1992; Taylor & Poston, 1994a), and oxygen-derived free radicals have been shown to abolish endothelium-dependent relaxation in both normal (Gryglewski et al., 1986; Rubanyi & Vanhoutte, 1986) and diabetic (Pieper & Gross, 1988; Tesfamariam & Cohen, 1992; Chang et al., 1993) blood vessels. Moreover, superoxide dismutase has been shown to normalize endothelium-dependent relaxation in response to acetylcholine in the perfused mesenteric arterial beds of STZ-diabetic rats (Diederich et al., 1994). These observations suggest that hyperglycemia increases the degradation of NO secondary to its enhancement of oxygen-derived free radical production. It has been postulated that hyperglycemia produces free radicals in diabetic arteries by forming advanced glycation end products (AGEs) (Baynes, 1991), altering the polyol pathway activity (Tesfamariam, 1994), and activating NAD(P)H oxidase (Inoguchi et al., 2000). In addition, the elevated expression of iNOS in the mesentery of STZ-diabetic rats shown in the present study may also contribute to the production of free radicals. It has recently been shown that iNOS generates superoxide in L-arginine-depleted macrophages (Xia & Zweier, 1997).
Contribution of iNOS in diabetic rats
Nonselective inhibition of NOS with L-NNA resulted in a leftward shift of the phenylephrine-concentration–response curves in both the control and diabetic arterioles. These results suggest that NO suppressed the contractility to the α1-adrenergic agonist in both the control and diabetic rats. NO produced by eNOS in endothelial cells is thought to be of primary importance in the regulation of vessel diameter. However, the reduced activity of acetylcholine in causing vasorelaxation suggested a dysfunction of endothelium-derived NO in the diabetic mesenteric arterioles. Therefore, we can infer the possible involvement of other NOS isoforms in the vascular regulation of the diabetic rats. Recently, the induction of iNOS has been shown in cardiomyocytes (Smith et al., 1997) and the superior mesenteric arteries (Bardell & MacLeod, 2001) of STZ-diabetic rats.
The results of the present study provide several lines of evidence that iNOS is functionally expressed in the mesenteric arterioles of STZ-diabetic rats. First, EIT, which is a NOS inhibitor that is more selective for iNOS than for eNOS or nNOS (Nakane et al., 1995), caused greater constriction of the diabetic mesenteric arterioles compared with the control arterioles. Second, immunohistochemical analyses clearly demonstrated a positive signal for iNOS expression in the smooth muscle layer of mesenteric microvessels in the diabetic rats. This is well consistent with the recent study by Bardell & MacLeod (2001), which showed strong positive signals for iNOS immunoreactivity in the medial and adventitial layers of the superior mesenteric artery of STZ-diabetic rats. These results suggest that iNOS is expressed in the mesentery of STZ-diabetic rats and contributes to the basal tone and the reactivity of the arterioles. Increased NO production via iNOS may act in a protective manner in a pathophysiological aspect, by preventing the enhancement of vasoconstriction of diabetic arterioles. This is supported by the present results showing that the sensitivity of diabetic arterioles to phenylephrine was significantly enhanced by the NOS inhibitor L-NNA.
In the present study, we used EIT as a selective inhibitor of iNOS. However, EIT has been reported to be only 10–40-fold more selective for iNOS than for eNOS (Nakane et al., 1995). Therefore, we investigated the concentration–response relation for the constrictor response to iNOS in the arterioles. At 10 nM, EIT caused only a slight constriction in the control rats, whereas it caused a remarkable constriction in the diabetic rats; the constriction of the control arterioles was less than 20% of that of the diabetic ones. In contrast, EIT at 1 μM induced remarkable constrictions in both the diabetic and control rats; the constriction of the control arterioles was more than 50% of that in the diabetic ones. It is likely therefore that 10 nM EIT caused a constriction by mainly inhibiting iNOS, whereas 1 μM EIT inhibited not only iNOS but also eNOS in rat mesenteric arterioles.
In conclusion, the present investigation provides evidence for the enhanced constrictor responses to α1-adrenoceptor stimulation in mesenteric arterioles of STZ-diabetic rats. In addition, the data suggest that iNOS expressed in arteriolar smooth muscle plays a role in suppressing the basal tone and the reactivity of the arterioles in STZ-diabetic rats.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research from Japan Society for the Promotion of Science. We would like to thank Dr N. Ohshima at the University of Tsukuba, Ibaraki, Japan, for advice on the measurement of blood flow velocity.
Abbreviations
- AGEs
advanced glycation end products
- EIT
S-ethylisothiourea
- eNOS
endothelial nitric oxide synthase
- FITC
fluorescein isothiocyanate
- iNOS
inducible nitric oxide synthase
- L-NNA
NG-nitro-L-arginine
- NO
nitric oxide
- PBS
phosphate-buffered saline
- STZ
streptozotocin
References
- ABEBE W., HARRIS K.H., MACLEOD K.M. Role of extracellular Ca2+ in the selective enhancement of contractile responses of arteries from diabetic rats to noradrenaline. Can. J. Physiol. Pharmacol. 1994;72:1544–1551. doi: 10.1139/y94-222. [DOI] [PubMed] [Google Scholar]
- BARDELL A.L., MACLEOD K.M. Evidence for inducible nitric-oxide synthase expression and activity in vascular smooth muscle of streptozotocin-diabetic rats. J. Pharmacol. Exp. Ther. 2001;296:252–259. [PubMed] [Google Scholar]
- BAYNES J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- BUCALA R., TRACEY K.J., CERAMI A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J. Clin. Invest. 1991;87:432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG K.C., CHUNG S.Y., CHONG W.S., SUH J.S., KIM S.H., NOH H.K., SEONG B.W., KO H.J., CHUN K.W. Possible superoxide radical-induced alteration of vascular reactivity in aortas from streptozotocin-treated rats. J. Pharmacol. Exp. Ther. 1993;266:992–1000. [PubMed] [Google Scholar]
- CHANG K.S., LUND D.D. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J. Mol. Cell. Cardiol. 1986;18:617–624. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- CHINO D., AKIMARU S., KATAHA K., ISHII K., NAKAYAMA K. Specific augmentation of plantar skin blood flow by lipo-PGE1 assessed in tetrodotoxin- and NG-nitro-L-arginine-treated rats. J. Cardiovasc. Pharmacol. 2000;35:630–637. doi: 10.1097/00005344-200004000-00017. [DOI] [PubMed] [Google Scholar]
- CHOW W.L., ZHANG L., MACLEOD K.M. Noradrenaline-induced changes in intracellular Ca2+ and tension in mesenteric arteries from diabetic rats. Br. J. Pharmacol. 2001;134:179–187. doi: 10.1038/sj.bjp.0704221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER M.E., BONNET F., OLDFIELD M., JANDELEIT-DAHM K. Mechanisms of diabetic vasculopathy: an overview. Am. J. Hypertens. 2001;14:475–486. doi: 10.1016/s0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]
- DIEDERICH D., SKOPEC J., DIEDERICH A., DAI F.X. Endothelial dysfunction in mesenteric resistance arteries of diabetic rats: role of free radicals. Am. J. Physiol. 1994;266:H1153–H1161. doi: 10.1152/ajpheart.1994.266.3.H1153. [DOI] [PubMed] [Google Scholar]
- FENGER-GRON J., MULVANY M.J., CHRISTENSEN K.L. Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J. Physiol. 1997;498:215–224. doi: 10.1113/jphysiol.1997.sp021852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRYGLEWSKI R.J., PALMER R.M., MONCADA S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- HARRIS K.H., MACLEOD K.M. Influence of the endothelium on contractile responses of arteries from diabetic rats. Eur. J. Pharmacol. 1988;153:55–64. doi: 10.1016/0014-2999(88)90587-0. [DOI] [PubMed] [Google Scholar]
- HICKS K.K., SEIFEN E., STIMERS J.R., KENNEDY R.H. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J. Auton. Nerv. Syst. 1998;69:21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- INOGUCHI T., LI P., UMEDA F., YU H.Y., KAKIMOTO M., IMAMURA M., AOKI T., ETOH T., HASHIMOTO T., NARUSE M., SANO H., UTSUMI H., NAWATA H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- JACKSON C.V., CARRIER G.O. Supersensitivity of isolated mesenteric arteries to noradrenaline in the long-term experimental diabetic rat. J. Auton. Pharmacol. 1981;1:399–405. doi: 10.1111/j.1474-8673.1981.tb00079.x. [DOI] [PubMed] [Google Scholar]
- JONG W.M., ZUURBIER C.J., DE WINTER R.J., VAN DEN HEUVEL D.A., REITSMA P.H., TEN CATE H., INCE C. Fentanyl-fluanisone-midazolam combination results in more stable hemodynamics than does urethane α-chloralose and 2,2,2-tribromoethanol in mice. Contemp. Top. Lab. Anim. Sci. 2002;41:28–32. [PubMed] [Google Scholar]
- KAMATA K., MAKINO A. A comparative study on the rat aorta and mesenteric arterial bed of the possible role of nitric oxide in the desensitization of the vasoconstrictor response to an α1-adrenoceptor agonist. Br. J. Pharmacol. 1997;120:1221–1228. doi: 10.1038/sj.bjp.0701031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIFF R.J., GARDINER S.M., COMPTON A.M., BENNETT T. Selective impairment of hindquarters vasodilator responses to bradykinin in conscious Wistar rats with streptozotocin-induced diabetes mellitus. Br. J. Pharmacol. 1991;103:1357–1362. doi: 10.1111/j.1476-5381.1991.tb09793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LONGHURST P.A., HEAD R.J. Responses of the isolated perfused mesenteric vasculature from diabetic rats: the significance of appropriate control tissues. J. Pharmacol. Exp. Ther. 1985;235:45–49. [PubMed] [Google Scholar]
- MACLEOD K.M., MCNEILL J.H. The influence of chronic experimental diabetes on contractile responses of rat isolated blood vessels. Can. J. Physiol. Pharmacol. 1985;63:52–57. doi: 10.1139/y85-009. [DOI] [PubMed] [Google Scholar]
- MAEDA C.Y., FERNANDES T.G., TIMM H.B., IRIGOYEN M.C. Autonomic dysfunction in short-term experimental diabetes. Hypertension. 1995;26:1100–1104. doi: 10.1161/01.hyp.26.6.1100. [DOI] [PubMed] [Google Scholar]
- MAIGNAN E., DONG W.X., LEGRAND M., SAFAR M., CUCHE J.L. Sympathetic activity in the rat: effects of anaesthesia on noradrenaline kinetics. J. Auton. Nerv. Syst. 2000;80:46–51. doi: 10.1016/s0165-1838(00)00075-8. [DOI] [PubMed] [Google Scholar]
- MAKINO A., KAMATA K. Possible modulation by endothelin-1, nitric oxide, prostaglandin I2 and thromboxane A2 of vasoconstriction induced by an alpha-agonist in mesenteric arterial bed from diabetic rats. Diabetologia. 1998;41:1410–1418. doi: 10.1007/s001250051086. [DOI] [PubMed] [Google Scholar]
- MONCKTON G., PEHOWICH E. Autonomic neuropathy in the streptozotocin diabetic rat. Can. J. Neurol. Sci. 1980;7:135–142. doi: 10.1017/s0317167100023519. [DOI] [PubMed] [Google Scholar]
- NAKANE M., KLINGHOFER V., KUK J.E., DONNELLY J.L., BUDZIK G.P., POLLOCK J.S., BASHA F., CARTER G.W. Novel potent and selective inhibitors of inducible nitric oxide synthase. Mol. Pharmacol. 1995;47:831–834. [PubMed] [Google Scholar]
- NAKAYAMA K., HORIKAWA N., OGAWA T., KOHNO F., ISHII K., KUBO K., IMABEPPU S. Arteriolar and venular vasodilating properties of benidipine hydrochloride, a 1,4-dihydropyridine Ca2+ antagonist with long-lasting action, assessed in rat mesenteric microcirculation. J. Cardiovasc. Pharmacol. 1999;33:540–548. doi: 10.1097/00005344-199904000-00005. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA K., WATANABE N., YAMAZAWA T., TAKESHITA N., TANAKA Y., YANAIHARA N. Effects of porcine galanin on the mesenteric microcirculation and arteriolar smooth muscle in the rat. Eur. J. Pharmacol. 1991;193:75–80. doi: 10.1016/0014-2999(91)90202-2. [DOI] [PubMed] [Google Scholar]
- ORIE N.N., ALOAMAKA C.P., IYAWE V.I. Duration-dependent attenuation of acetylcholine- but not histamine-induced relaxation of the aorta in diabetes mellitus. Gen. Pharmacol. 1993;24:329–332. doi: 10.1016/0306-3623(93)90311-k. [DOI] [PubMed] [Google Scholar]
- ÖZTÜRK Y., ALTAN V.M., YILDIZOĞLU-ARI N. Effects of experimental diabetes and insulin on smooth muscle functions. Pharmacol. Rev. 1996;48:69–112. [PubMed] [Google Scholar]
- PIEPER G.M., GROSS G.J. Oxygen free radicals abolish endothelium-dependent relaxation in diabetic rat aorta. Am. J. Physiol. 1988;255:H825–H833. doi: 10.1152/ajpheart.1988.255.4.H825. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia. 1999;42:204–213. doi: 10.1007/s001250051140. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- SMITH J.M., PAULSON D.J., ROMANO F.D. Inhibition of nitric oxide synthase by L-NAME improves ventricular performance in streptozotocin-diabetic rats. J. Mol. Cell. Cardiol. 1997;29:2393–2402. doi: 10.1006/jmcc.1997.0474. [DOI] [PubMed] [Google Scholar]
- SUZUKI T., YANAGI K., OOKAWA K., HATAKEYAMA K., OHSHIMA N. Flow visualization of microcirculation in solid tumor tissues: intravital microscopic observation of blood circulation by use of a confocal laser scanning microscope. Front. Med. Biol. Eng. 1996;7:253–263. [PubMed] [Google Scholar]
- TAKIGUCHI Y., SATOH N., HASHIMOTO H., NAKASHIMA M. Reversal effect of thyroxine on altered vascular reactivity in diabetic rats. J. Cardiovasc. Pharmacol. 1989;13:520–524. [PubMed] [Google Scholar]
- TAM E.S., FERGUSON D.G., BIELEFELD D.R., LORENZ J.N., COHEN R.M., PUN R.Y. Norepinephrine-mediated calcium signaling is altered in vascular smooth muscle of diabetic rat. Cell Calcium. 1997;21:143–150. doi: 10.1016/s0143-4160(97)90038-5. [DOI] [PubMed] [Google Scholar]
- TAYLOR P.D., POSTON L. The effect of hyperglycaemia on function of rat isolated mesenteric resistance artery. Br. J. Pharmacol. 1994a;113:801–808. doi: 10.1111/j.1476-5381.1994.tb17064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P.D., MCCARTHY A.L., THOMAS C.R., POSTON L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of streptozotocin-induced diabetic rats. Br. J. Pharmacol. 1992;107:393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR P.D., WICKENDEN A.D., MIRRLEES D.J., POSTON L. Endothelial function in the isolated perfused mesentery and aortae of rats with streptozotocin-induced diabetes: effect of treatment with the aldose reductase inhibitor, ponalrestat. Br. J. Pharmacol. 1994b;111:42–48. doi: 10.1111/j.1476-5381.1994.tb14021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESFAMARIAM B., COHEN R.A. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am. J. Physiol. 1992;263:H321–H326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAM B. Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 1994;16:383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- VAN BUREN T., VLEEMING W., KRUTZEN M.M., VAN DE KUIL T., GISPEN W.H., DE WILDT D.J. Vascular responses of isolated mesenteric resistance and basilar arteries from short- and long-term diabetic rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:663–670. doi: 10.1007/pl00005309. [DOI] [PubMed] [Google Scholar]
- WEBER L.P., MACLEOD K.M. Influence of streptozotocin diabetes on the alpha-1 adrenoceptor and associated G proteins in rat arteries. J. Pharmacol. Exp. Ther. 1997;283:1469–1478. [PubMed] [Google Scholar]
- XIA Y., ZWEIER J.L. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]