Abstract
ATP-sensitive potassium channel in the mitochondrial inner membrane (mitoKATP channel) rather than in the sarcolemma (sarcKATP channel) appears to play an important role in cardioprotection. We examined the effect of minoxidil, a potent antihypertensive agent and hair growth stimulator, on sarcKATP and mitoKATP channels in guinea-pig ventricular myocytes.
Minoxidil activated a glybenclamide-sensitive sarcKATP channel current in the whole-cell recording mode with an EC50 of 182.6 μM. Minoxidil reversibly increased the flavoprotein oxidation, an index of mitoKATP channel activity, in a concentration-dependent manner. The EC50 for mitoKATP channel activation was estimated to be 7.3 μM; this value was notably ≈25-fold lower than that for sarcKATP channel activation.
Minoxidil (10 μM) significantly attenuated the ouabain-induced increase of mitochondrial Ca2+ concentration, which was measured by loading cells with rhod-2 fluorescence. Furthermore, pretreatment with minoxidil (10 μM) before 20-min no-flow ischaemia significantly improved the recovery of developed tension measured after 60 min of reperfusion in coronary perfused guinea-pig ventricular muscles. These cardioprotective effects of minoxidil were completely abolished by the mitoKATP channel blocker 5-hydroxydecanoate (500 μM).
Our results indicate that minoxidil exerts a direct cardioprotective effect on heart muscle cells, an effect mediated by the selective activation of mitoKATP channels.
Keywords: KATP channel, minoxidil, mitochondria, cardioprotection
Introduction
Cardiac myocytes contain ATP-sensitive potassium (KATP) channels in both sarcolemmal plasma membrane (sarcKATP channels) and in mitochondrial inner membrane (mitoKATP channels) (Noma, 1983; Garlid et al., 1996; Liu et al., 1998). SarcKATP channels have been molecularly defined as an octameric complex of four pore-forming Kir6.2 and four SUR2A sulphonylurea receptors (Inagaki et al., 1996; Clement et al., 1997). On the other hand, the molecular cloning of mitoKATP channel has not yet been achieved, although recent studies using Kir6.1- and Kir6.2-deficient mice suggest that neither of these subunits is an essential component of the cardiac mitoKATP channel in mice (Miki et al., 2002; Suzuki et al., 2002).
MitoKATP channels possess a distinct pharmacological profile, while sharing some pharmacological properties with sarcKATP channels. Notably, diazoxide opens mitoKATP channels ≈2000-fold more potently than sarcKATP channels in cardiac myocytes (Garlid et al., 1996). Consistent with this, Liu et al. (1998) have demonstrated that diazoxide oxidizes the mitochondrial matrix redox potential via opening of mitoKATP channels in rabbit hearts, whereas sarcKATP channels are resistant to diazoxide. Zang et al. (2001) have also demonstrated that diazoxide increases the open probability of reconstituted myocardial mitoKATP channels in lipid bilayers. Using diazoxide as a pharmacological tool, recent studies have suggested that mitoKATP channels rather than sarcKATP channels are involved in cardioprotection (Garlid et al., 1997; Liu et al., 1998; Sato et al., 2000b). However, diazoxide has been reported to inhibit succinate dehydrogenase (Schäfer et al., 1969; Hanley et al., 2002), suggesting that the interpretation of the effect of diazoxide may not be straightforward. Accordingly, to further elucidate the functional role of mitoKATP channel, it is desirable to look at another mitoKATP channel-specific agent.
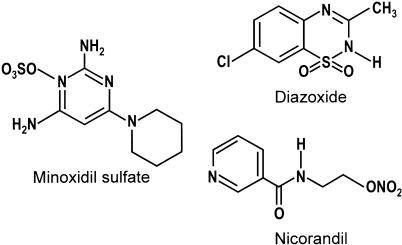
Minoxidil (chemical structure shown in Figure 1) is a potent KATP channel opener, and has been shown to act as a vasodilating agent (Campese, 1981; Leblanc et al., 1989), and the drug is used externally for treatment of androgenetic alopecia at present (DeVillez, 1990). Hayashi et al. (1993) reported that a relatively high concentration of minoxidil opened the sarcKATP channel in guinea-pig ventricular myocytes. Contrarily, the effect of minoxidil on cardiac mitoKATP channel remains unclear. Although minoxidil has been shown to improve the contractile function after ischaemia–reperfusion in dog hearts (Yamamoto et al., 2002), cardioprotective action of minoxidil is not well understood. In the present study, we therefore examined the effects of minoxidil on mitoKATP channels by measuring flavoprotein fluorescence in guinea-pig ventricular myocytes. The results show that minoxidil confers cardioprotection via preferential activation of mitoKATP channels.
Figure 1.
Chemical structures of minoxidil sulphate, diazoxide, and nicorandil.
Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1985).
Cell preparation
Adult guinea-pig ventricular myocytes were isolated by collagenase digestion, as previously described (Tohse et al., 1992). Once isolated, the cells were suspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal calf serum at room temperature until use. The cells used in the present experiments had a regular shape with clear cross-striation.
Membrane current measurement
The patch-clamp technique was used in whole-cell patch or nystatin-perforated patch configuration, as previously described (Sakamoto et al., 1998). Single ventricular cells were superfused with HEPES-buffered Tyrode's solution containing (in mM): NaCl 143, KCl 5.4, CaCl2 1.8, NaH2PO4 0.33, MgCl2 0.5, glucose 5.5, and HEPES 5 (pH 7.4) at 37°C. For whole-cell patch recording, the internal pipette solution (solution A) contained (in mM) K-aspartate 110, KCl 20, CaCl2 1.4, MgCl2 1, EGTA 10, HEPES 5, phosphocreatinine 1, and, unless otherwise noted, K2-ATP 1 (pH 7.4). In a separate series of whole-cell clamp experiments, 100 μM ADP was added to the solution A. For nystatin-perforated patch recording, the pipette solution (solution B) contained (in mM) K-aspartate 110, KCl 20, CaCl2 1, MgCl2 1, EGTA 0.1, HEPES 5 (pH 7.4), and nystatin. A stock solution of nystatin was added to the pipette solution to a final concentration of 300 μg ml−1 just before the experiments. In both whole-cell and nystatin-perforated patch recordings, the membrane potential was held at −40 mV and depolarized first to +50 mV and then hyperpolarized to −100 mV with a slope of −60 mV s−1. This ramp-pulse protocol was repeated every 5 s. The quasi-steady state membrane current was plotted against the membrane potential during hyperpolarizing voltage ramps. The current signals were filtered at 3 kHz with a digital Gaussian filter and digitized at 2 kHz for data analysis with pClamp software (Axon Instruments, Foster City, CA, U.S.A.). These experiments were performed at 36°C.
Flavoprotein fluorescence measurement
To index the mitoKATP channel activity, flavoprotein fluorescence was measured by a modification of method described by Sato et al. (1998). Briefly, the cells were superfused with a bath solution containing (mM): NaCl 143, KCl 5.4, CaCl2 1.8, NaH2PO4 0.33, MgCl2 0.5, and HEPES 5 (pH 7.4) at room temperature (≈22°C). Flavoprotein fluorescence was excited at 480 nm (for 200 ms) and emitted at 520 nm. At the end of each experiment, cells were exposed to the mitochondrial uncoupler 2,4-dinitrophenol (DNP, 100 μM) to obtain maximal flavoprotein oxidation. The emitted fluorescence was monitored with a cooled charge-coupled device (CCD) digital camera (Hamamatsu Photonics, Hamamatsu, Japan). The imaging of flavoprotein was analysed for average pixel intensities of regions of interest drawn to include the whole cell, and expressed as a percentage of the DNP-induced maximal oxidation, using an Aquacosmos image-processing system (Hamamatsu Photonics).
[Ca2+]m measurement
The Ca2+ fluorophore rhod-2 was used to measure the changes of mitochondrial Ca2+ concentration ([Ca2+]m). For rhod-2 loading, cells were plated on uncoated 35 mm Falcon culture dishes with a medium based on a 1 : 1 mixture of DMEM and HEPES-buffered Tyrode's solution, supplemented with 10% foetal calf serum. Then, cells were loaded with 10 μM rhod-2 acetoxymethyl ester for 120 min at 4°C. After cold loading, cells were incubated for 30 min at 37°C. This two-step cold loading/warm incubation protocol achieves exclusive loading of rhod-2 into the mitochondria (Trollinger et al., 2000). Cells loaded with rhod-2 were perfused with a HEPES-buffered Tyrode's solution containing 2.7 mM CaCl2 at 37°C. Rhod-2 fluorescence was excited at 540 nm (for 100 ms), with emission monitored through a 605-nm (55-nm bandpass) barrier filter. The imaging of rhod-2 was analysed for the average pixel intensities of regions of interest drawn to include the whole cell, following correction for background, using an Aquacosmos image-processing system (Hamamatsu Photonics).
Coronary-perfused right ventricular myocardium
The isolated coronary-perfused guinea-pig right ventricular free wall was prepared as described previously (Shigematsu et al., 1995). In brief, the preparation was mounted in the recording chamber and pinned to the floor of the chamber. The coronary artery was perfused with oxygenated Tyrode's solution containing (in mM) NaCl 136.7, NaHCO3 11.9, KCl 5.4, NaH2PO4 0.42, MgCl2 0.5, CaCl2 1.8, and glucose 11 (pH 7.35–7.40 when gassed with 97% O2 and 3% CO2). The flow rate was maintained at 1.0±0.2 ml min−1 g−1 wet weight using a roller pump (MP-3; Tokyo Rikakikai, Tokyo, Japan). The surface of the preparation was superfused with glucose-free hypoxic Tyrode's solution (10 ml min−1) to minimize direct O2 diffusion from the surface of the preparations into the muscles. The composition of the hypoxic Tyrode's solution was the same as above, except that it contained no glucose and was gassed with 97% N2 and 3% CO2. The temperatures of these solutions were maintained at 37±0.5°C. The basal portion of the preparation was stimulated at 3 Hz throughout the experiment and contractile tension was recorded using a force transducer (TB-612T; Nihon Kohden, Tokyo, Japan) connected to the apical end of the preparation. Resting tension was adjusted to obtain the optimal developed tension. The contractile tension was monitored on a multibeam oscilloscope (VC-9A; Nihon Kohden) and recorded on a multichannel thermal array corder (WT-645G; Nihon Kohden).
After equilibration for 90 min, the preparations were assigned to the study groups. Control (n=5): the preparations were subjected to 20 min of no-flow ischaemia followed by 60 min of reperfusion. Minoxidil (n=4): the preparations were treated for 5 min with minoxidil (10 μM) followed by a 10-min washout before no-flow ischaemia. Minoxidil+5-HD (n=4): pretreatment with the mitoKATP channel blocker 5-hydroxydecanoate (5-HD, 500 μM) (Sato et al., 1998) starting 5 min prior to and continued during minoxidil treatment. Minoxidil+HMR (n=3): pretreatment with the sarcKATP channel blocker HMR 1098 (HMR, 30 μM) (Sato et al., 2000b) starting 5 min prior to and continued during minoxidil treatment.
Chemicals
Minoxidil sulphate was a kind gift from Taisho Pharmaceutical (Omiya, Japan). HMR 1098 (HMR) was a kind gift from Aventis Pharma (Tokyo, Japan). Pinacidil, sodium 5-hydroxydecanoic acid (5-HD), glybenclamide, and ouabain were purchased from Sigma (St Louis, MO, U.S.A.). Rhod-2 acetoxymethyl ester was purchased from Molecular Probes (Eugene, OR, U.S.A.). Nystatin and 2,4-dinitrophenol (DNP) were purchased from Wako Pure Chemical (Osaka, Japan). Minoxidil sulphate and glybenclamide were dissolved as a 100 mM stock solution in dimethyl sulphoxide, and the final concentration of solvent was ⩽0.1%. Pinacidil was dissolved as a 50 mM stock solution in 0.1 N HCl+saline. A stock solution of nystatin dissolved in methanol at a concentration of 10 mg−1 ml−1 was prepared fresh before each experiment. Ouabain, 5-HD, HMR, and DNP were dissolved in the perfusate.
Data analysis
Data are presented as mean±s.e.m., and the number of cells or experiments is shown as n. Concentration–response data were fit with a four-parameter logistic equation:
where Y is the current (Figure 2b) or the flavoprotein oxidation (Figure 5), A1 is the minimum current (Figure 2b) or the minimum flavoprotein oxidation (Figure 5), A2 is the maximum current (Figure 2b) or the maximum flavoprotein oxidation (Figure 5), [minoxidil] is the concentration of minoxidil, and n is the Hill coefficient. Curve fits were performed with Origin 7J software (OriginLab, Northampton, MA, U.S.A.). Intergroup comparisons are made by Student's t-test for two groups and by ANOVA followed by Fisher's post hoc test for multiple groups. A value of P<0.05 was regarded as significant.
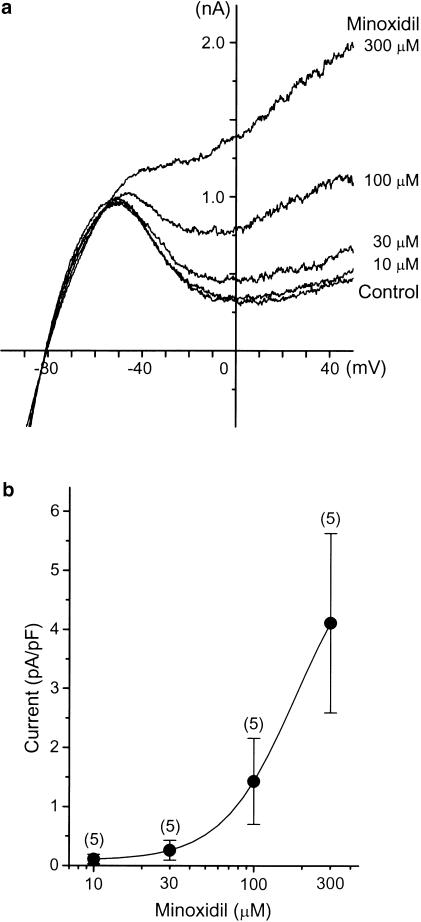
Figure 2.
Effects of minoxidil on the sarcKATP channels. (a) Representative current–voltage relationships recorded under whole-cell patch clamp. Each concentration of minoxidil was applied for 5 min. (b) Dose–response curve for minoxidil-induced sarcKATP channel currents. The current traces obtained in each drug concentration were subtracted from the control current tracing. The amplitude of minoxidil-sensitive current at 0 mV was normalized to the cell capacitance of each cell. All data resented as mean±s.e.m., with numbers of cells given in parentheses.
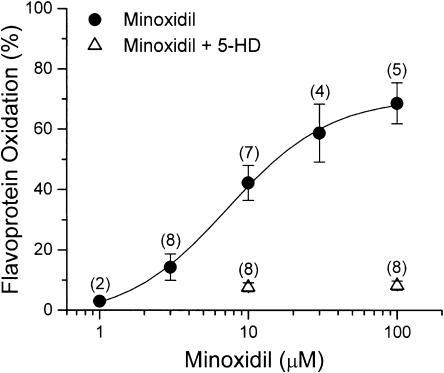
Figure 5.
Summarized dose–response data for minoxidil-induced flavoprotein oxidation. Values are expressed as percents relative to those obtained with DNP. All data are presented as mean±s.e.m., with numbers of cells given in parentheses.
Results
Effect of minoxidil on membrane currents
To test the effect of minoxidil on sarcKATP channel, membrane current was recorded with patch-clamp techniques. Figure 2a shows the representative current traces recorded in the whole-cell configuration. When 1 mM ATP was included in the pipette solution, cumulative application of minoxidil (10–300 μM) resulted in a concentration-dependent increase in the quasi-steady-state outward current. The minoxidil-induced outward current was completely inhibited by subsequent application of 10 μM glybenclamide (data not shown), indicating that minoxidil is an activator of sarcKATP channel. Figure 2b illustrates the dose–response curve for minoxidil-induced currents measured at the membrane potential of 0 mV. The estimated EC50 value for minoxidil in activating glybenclamide-sensitive outward current was 182.6 μM.
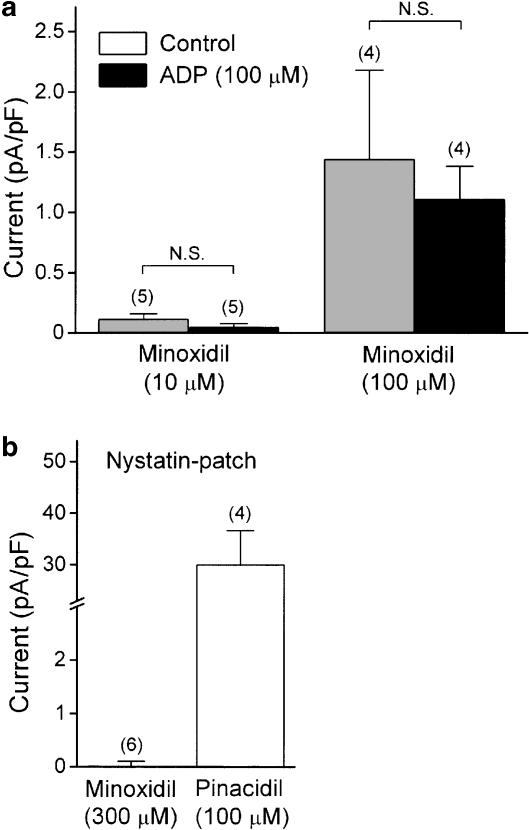
To determine if intracellular ADP modulates the effect of minoxidil, the quasi-steady-state membrane current was recorded by adding ADP to the internal pipette solution. ADP (100 μM) per se did not affect the outward current and the current amplitude measured at 0 mV was 2.7±1.3 (n=11) and 2.9±1.2 pA pF−1 (n=5) in the absence and presence, respectively, of ADP in the pipette. As shown in Figure 3a, there was no significant change in the amplitude of minoxidil-induced outward current, when the patch pipette contained ADP. In a nystatin-perforated patch configuration, as shown in Figure 3b, significant current activation could not be detected even at a high concentration of minoxidil (300 μM), whereas pinacidil at a concentration of 100 μM produced a robust increase in outward current.
Figure 3.
(a) Summarized effect of intracellular ADP on minoxidil-induced sarcKATP channel current. ADP (100 μM) was added to the pipette in the whole-cell recording mode. (b) Comparative effect of minoxidil and pinacidil on sarcKATP channel current recorded in the nystatin-perforated patch configuration. In each panel, the amplitude of sarcKATP channel at 0 mV was normalized to the cell capacitance of each cell. Each bar represents the mean±s.e.m., with numbers of cells given in parentheses.
Effect of minoxidil on flavoprotein oxidation
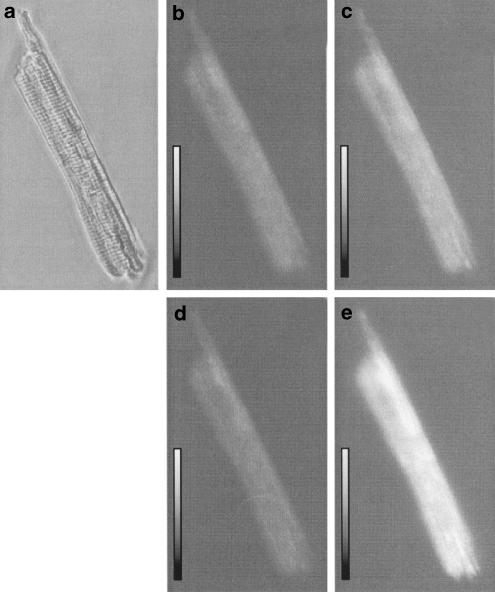
Figure 4 shows the representative images of flavoprotein fluorescence in a cell exposed to minoxidil. Flavoprotein fluorescence was low under control condition (Figure 4b) in agreement with earlier reports (Liu et al., 1998; Romashko et al., 1998). Exposure to minoxidil (10 μM) oxidized flavoprotein and increased the fluorescence (Figure 4c), which was reversible on washout (Figure 4d). Subsequent exposure to DNP (100 μM) led to increase in flavoprotein fluorescence (Figure 4e). As summarized in Figure 5, minoxidil increased flavoprotein fluorescence in a concentration-dependent manner. The estimated EC50 value for minoxidil to induce flavoprotein oxidation was 7.3 μM. Coadministration of 5-HD (500 μM), a selective mitoKATP channel blocker (Sato et al., 1998), virtually abolished the minoxidil-induced flavoprotein oxidation. These results indicate that minoxidil is an opener of mitoKATP channels.
Figure 4.
Imaging of flavoprotein fluorescence in guinea-pig ventricular myocyte. (a) Transmitted image. (b–e) A pseudocolour palette was applied to visualize the relative increase in flavoprotein oxidation, to yield images of cell at control (b), after 7-min exposure to 10 μM minoxidil (c), washing out of minoxidil (d), and 2 min after exposure to 100 μM DNP (e).
Effect of minoxidil on mitochondrial Ca2+ overload
A previous study demonstrated that the opening of mitoKATP channels by diazoxide attenuated the mitochondrial Ca2+ overload (Ishida et al., 2001). We therefore examined the effect of minoxidil on mitochondrial Ca2+ overload. As summarized in Figure 6, treatment of myocytes with ouabain (1 mM) evoked mitochondrial Ca2+ overload and the intensity of rhod-2 fluorescence significantly increased from 27.6±1.3 to 75.7±8.5 a.u. after 30-min exposure to ouabain (P<0.001). Coadministration of minoxidil (10 μM) significantly prevented the ouabain-induced increase in rhod-2 fluorescence to 40.3±3.0 a.u. (P<0.001 vs ouabain alone). The effect of minoxidil was antagonized by both 5-HD (500 μM) and glybenclamide (10 μM). These results indicate that opening of mitoKATP channels by minoxidil attenuates the ouabain-induced Ca2+ overload in mitochondria.
Figure 6.
Summarized effect of minoxidil on ouabain-induced mitochondrial Ca2+ overload. In each group, drugs (minoxidil, 10 μM; 5-HD, 500 μM; glybenclamide, 10 μM) were applied together with ouabain (1 mM), and the resultant fluorescence was collected at 15 and 30 min after exposure to ouabain. Each point indicates the mean±s.e.m. *P<0.001 vs baseline; #P<0.001 vs ouabain; ¶P<0.001 vs ouabain+minoxidil.
Effect of minoxidil on contractile function during ischaemia/reperfusion
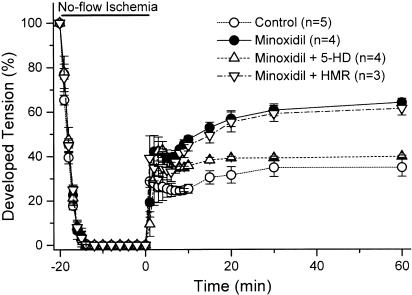
To test whether minoxidil confers cardioprotection in guinea-pig hearts, coronary perfused right ventricular preparations were subjected to 20-min no-flow ischaemia, followed by 60-min reperfusion. Table 1 summarizes the changes in developed tension before ischaemia. Neither 5-HD (500 μM) nor HMR (30 μM) alone had any significant effect on developed tension. Although not statistically significant, the developed tension was slightly depressed by minoxidil (10 μM). Figure 7 shows the time courses of developed tension during ischaemia/reperfusion. Pretreatment with 10 μM minoxidil prior to ischaemia significantly improved the recovery of contractility after 60 min of reperfusion, compared with controls (64.0±1.8 vs 34.8±4.1%, P<0.01). This cardioprotective effect of minoxidil was blocked by 5-HD (39.7±0.5%, P<0.01 vs minoxidil alone), but not by HMR (61.3±3.1%, P=NS vs minoxidil alone), suggesting that the cardioprotective effect of minoxidil results from mitoKATP channel activation.
Table 1.
Changes of developed tension before ischemia
| Drug | ||||
|---|---|---|---|---|
| Group | Stabilization | 5-HD or HMR | Minoxidil | Preischemia |
| Control (n=5) | 11.6±0.8 mN | — | — | 11.3±0.9 mN |
| Minoxidil (n=4) | 11.8±0.8 mN | — | 11.0±0.9 mN | 11.8±0.8 mN |
| Minoxidil+5-HD (n=4) | 12.0±0.8 mN | 12.9±1.0 mN | 12.0±0.8 mN | 12.0±0.8 mN |
| Minoxidil+HMR (n=4) | 11.6±1.0 mN | 11.8±1.0 mN | 11.1±0.9 mN | 11.4±1.0 mN |
Values are mean±s.e.m. Stabilization: at the end of 90 min of stabilization; 5-HD or HMR: 5 min after treatment with 5-hydroxydecanoate or HMR 1098; minoxidil; 5 min after treatment with minoxidil in the absence and presence of 5-hydroxydecanoate or HMR 1098; preischemia: immediately before the onset of ischaemia.
Figure 7.
Time courses of changes in developed tension during 20-min no-flow ischaemia and 60-min reperfusion. Each point indicates the mean±s.e.m. for 3–5 preparations, and is expressed as a percentage of the preischaemic value.
Discussion
Minoxidil is a potent KATP channel opener and has diverse actions ranging from vasodilation to promotion of hair growth (Campese, 1981; Leblanc et al., 1989; DeVillez, 1990). Although significant side effects, such as ventricular arrhythmia and severe hypotension, may limit the clinical utility of the KATP channel openers, these compounds are effective in protecting cells from ischaemic injury, and thus merit further investigation. The major finding of the present study is that minoxidil could open mitoKATP channels ≈25-fold more potently than sarcKATP channels in guinea-pig ventricular myocytes, and thereby attenuated mitochondrial Ca2+ overload and improved contractile recovery after ischaemia/reperfusion.
In agreement with the previous report (Hayashi et al., 1993), minoxidil activated the sarcKATP channels in a concentration-dependent fashion, with an EC50 value of 182.6 μM when assessed by whole-cell recording in the presence of 1 mM ATP. We further found that, in contrast to pinacidil, minoxidil evoked only a small outward current even at high concentration (Figure 3b), when sarcKATP current recordings were performed with nystatin in the pipette solution (nystatin-perforated patch). Therefore, minoxidil is less potent than pinacidil in activating sarcKATP channel current. The perforated-patch technique allows the exchange of monovalent cations and anions, whereas it maintains intracellular metabolites intact (Horn & Marty, 1988). In this respect, the activity of minoxidil to open sarcKATP channels is dependent on the intracellular ATP concentrations, and the drug can be expected to have no effect on the sarcKATP channel under normal condition. Recently, diazoxide and nicorandil, a putative mitoKATP channel opener (Liu et al., 1998; Sato et al., 2000a), have been shown to activate sarcKATP channels during simulated ischaemia or when intracellular ADP is raised (D'hahan et al., 1999; Matsuoka et al., 2000). Such an ADP-dependent activation of sarcKATP channel has been proposed to underlie the cardioprotective effect against ischaemia-induced contractile dysfunction of mouse heart (Suzuki et al., 2003). In the present study, we found that minoxidil did not enhance the sarcKATP channel activity even when the patch pipette contained 100 μM ADP (Figure 3a). These results indicate that minoxidil may not open sarcKATP channels even when intracellular ADP was considerably increased, a condition to be encountered, for example, during ischaemia.
MitoKATP channel activity was indexed by measuring flavoprotein fluorescence (Liu et al., 1998). It is so far the only method that can be used to assess mitoKATP channel activity in intact cells. However, two previous studies (Lawrence et al., 2001; Hanley et al., 2002) have failed to demonstrate the oxidation of flavoprotein by diazoxide. These discrepancies are likely to reflect the different experimental conditions. They used freshly isolated myocytes and measured flavoprotein fluorescence in the presence of glucose. In our experiments, the cells were kept in a culture medium until use to stabilize the mitochondrial redox state. Moreover, since mitoKATP channel-induced flavoprotein oxidation is detectable only if uncompensated by increased production of electron donor such as NADH (Chance et al., 1972), we used the glucose-free Tyrode's solution for measurement of flavoprotein fluorescence. Under our experimental conditions, the mitoKATP channel opener diazoxide oxidized flavoprotein in guinea-pig ventricular myocytes (Sato et al., 2003). The present study demonstrated that minoxidil reversibly oxidized the flavoprotein in a concentration-dependent manner (Figures 4, 5). Moreover, the mitoKATP channel blocker 5-HD completely abolished the minoxidil-induced flavoprotein oxidation. The estimated EC50 value for minoxidil-induced flavoprotein oxidation was 7.3 μM. This value was notably ≈25-fold lower than that for sarcKATP channel activation assessed by whole-cell recording (182.6 μM), suggesting that minoxidil primarily activates mitoKATP channels in cardiac cell. Thus, a pharmacological profile of minoxidil is qualitatively similar to that of diazoxide and nicorandil.
Ca2+ uptake into mitochondria is driven primarily by the large negative electrical potential of the matrix (Gunter & Pfeiffer, 1990). Therefore, depolarization of the mitochondrial membrane via opening of mitoKATP channels reduces the driving force for Ca2+ influx and, hence, results in the prevention of mitochondrial Ca2+ overload. In agreement with this hypothesis, we have reported that, in rat cardiomyocytes, opening of mitoKATP channels by diazoxide attenuates the ouabain-induced mitochondrial Ca2+ overload, and such an effect is associated with the depolarization of the mitochondrial membrane (Ishida et al., 2001). In the present study, using the same experimental design, we found that minoxidil could prevent the ouabain-induced Ca2+ overload in mitochondria (Figure 6). The concentration of minoxidil used in these experiments (10 μM) was close to the EC50 for flavoprotein oxidation. The mitoKATP channel blocker 5-HD completely abolished the effects of minoxidil. Moreover, the degree of protection conferred by minoxidil was comparable to that seen with diazoxide (data not shown). Here, we used diazoxide and 5-HD as the mitoKATP channel-selective agents. However, it has also been claimed that diazoxide inhibits succinate dehydrogenase and 5-HD is converted to 5-HD-CoA (Schäfer et al., 1969; Hanley et al., 2002; Lim et al., 2002). Thus, the interpretation of our results may not be straightforward. So far, there is no report suggesting that minoxidil, like diazoxide, inhibits succinate dehydrogenase. The specific succinate dehydrogenase inhibitor malonate could not prevent the ouabain-induced mitochondrial Ca2+ overload (our unpublished data). Furthermore, the nonselective KATP channel blocker glybenclamide abolished the effect of minoxidil, in a manner similar to 5-HD (Figure 6). We therefore propose that cardioprotective effects of minoxidil are mediated by the opening of mitoKATP channels, although mitoKATP channel-independent action cannot be completely excluded.
We found that brief exposure to minoxidil prior to ischaemia improved the recovery of developed tension after reperfusion. In the present study, the coronary flow rate was maintained at 1.0±0.2 ml min−1 g−1 wet weight using a roller pump. It is therefore reasonable to assume that, under our experimental condition, cardioprotective effects of minoxidil are not due to vasodilation resulting from vascular sarcKATP channel activation. As described above, minoxidil can hardly open sarcKATP channels at the concentration used. In addition, the putative mitoKATP channel blocker 5-HD but not the putative sarcKATP channel blocker HMR completely abolished the effect of minoxidil (Figure 7). These results further support the notion that minoxidil confers cardioprotection via preferential activation of mitoKATP channels.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research, the Mitsui Life Social Welfare Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research. We thank M. Tamagawa, Y. Reien, and I. Sakashita for excellent technical and secretarial assistance.
Abbreviations
- [Ca2+]m
mitochondrial Ca2+ concentration
- DNP
2,4-dinitrophenol
- 5-HD
5-hydroxydecanoate
- KATP
ATP-sensitive potassium
- mitoKATP
mitochondrial KATP
- sarcKATP
sarcolemmal KATP
References
- CAMPESE V.M. Minoxidil: a review of its pharmacological properties and therapeutic use. Drugs. 1981;22:257–278. doi: 10.2165/00003495-198122040-00001. [DOI] [PubMed] [Google Scholar]
- CHANCE B., SALKOVITZ I.A., KOVACH A.G. Kinetics of mitochondrial flavoprotein and pyridine nucleotide in perfused heart. Am. J. Physiol. 1972;223:207–218. doi: 10.1152/ajplegacy.1972.223.1.207. [DOI] [PubMed] [Google Scholar]
- CLEMENT J.P., JR, KUNJILWAR K., GONZALEZ G., SCHWANSTECHER M., PANTEN U., AGUILAR-BRRYAN L., BRYAN J. Association and stoichiometry of KATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- DEVILLEZ R.L. The therapeutic use of topical minoxidil. Dermatol. Clin. 1990;8:367–375. [PubMed] [Google Scholar]
- D'HAHAN N., MOREAU C., PROST A.L., JACQUET H., ALEKSEEV A.E., TERZIC A., VIVAUDOU M. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc. Natl. Acad. Sci. U.S.A. 1999;96:12162–12167. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLID K.D., PAUCEK P., YAROV-YAROVOY V., MURRAY H.N., DARBENZIO R.B., D'ALONZO A.J., SMITH M.A., GROVER G.J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels: possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- GARLID K.D., PAUCEK P., YAROV-YAROVOY V., SUN X., SCHINDLER P.A. The mitochondrial KATP channel as a receptor for potassium channel openers. J. Biol. Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- GUNTER T.E., PFEIFFER D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- HANLEY P.J., MICKEL M., LÖFFLER M., BRANDT U., DAUT J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., HORIE M., OKADA Y. Ionic mechanism of minoxidil-induced shortening of action potential durations in guinea pig ventricular myocytes. J. Pharmacol. Exp. Ther. 1993;265:1527–1533. [PubMed] [Google Scholar]
- HORN R., MARTY A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;94:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INAGAKI N., GONOI T., CLEMEMT J.P., Jr, WANG C.Z., AGUILAR-BRYAN L., BRYAN J., SEINO S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- ISHIDA H., HIROTA Y., GENKA C., NAKAZAWA H., NAKAYA H., SATO T. Opening of mitochondrial KATP channels attenuates the ouabain-induced calcium overload in mitochondria. Circ. Res. 2001;89:856–858. doi: 10.1161/hh2201.100341. [DOI] [PubMed] [Google Scholar]
- LAWRENCE C.L., BILLUPS B., RODRIGO G.C., STANDEN N.B. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. Br. J. Pharmacol. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEBLANC N., WILDE D.W., KEEF K.D., HUME J.R. Electrophysiological mechanisms of minoxidil-induced vasodilation of rabbit portal vein. Circ. Res. 1989;65:1102–1111. doi: 10.1161/01.res.65.4.1102. [DOI] [PubMed] [Google Scholar]
- LIM K.H., JAVADOV S.A., DAS M., CLARKE S.J., SULEIMAN M.S, HALESTRAP A.P. The effect of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J. Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Y., SATO T., O'ROURKE B., MARBAN E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- MATSUOKA T., MATSUSHITA K., KATAYAMA Y., FUJITA A., INAGEDA K., TANEMOTO M., INANOBE A., YAMASHITA S., MATSUZAWA Y., KURACHI Y. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K+ channels. Circ. Res. 2000;87:873–880. doi: 10.1161/01.res.87.10.873. [DOI] [PubMed] [Google Scholar]
- MIKI T., SUZUKI M., SHIBASAKI T., UEMURA H., SATO T., YAMAGUCHI K., KOSEKI H., IWANAGA T., NAKAYA H., SEINO S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat. Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- NOMA A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- ROMASHKO D., MARBAN E., O'ROURKE B. Subcellular metabolic transients and mitochondrial redox waves in heart cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1618–1623. doi: 10.1073/pnas.95.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAMOTO N., UEMURA H., HARA Y., SAITO T., MASUDA Y., NAKAYA H. Bradykinin B2-receptor-mediated modulation of membrane currents in guinea-pig cardiomyocytes. Br. J. Pharmacol. 1998;125:283–292. doi: 10.1038/sj.bjp.0702060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO T., O'ROURKE B., MARBÁN E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ. Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- SATO T., SASAKI N., O'ROURKE B., MARBÁN E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J. Am. Coll. Cardiol. 2000a;35:514–518. doi: 10.1016/s0735-1097(99)00552-5. [DOI] [PubMed] [Google Scholar]
- SATO T., SASAKI N., SEHARASEYON J., O'ROURKE B., MARBÁN E. Selective pharmacological agents implicate mitochondrial but not sarcolemmal KATP channels in ischemic cardioprotection. Circulation. 2000b;83:110–114. doi: 10.1161/01.cir.101.20.2418. [DOI] [PubMed] [Google Scholar]
- SATO T., TAKIZAWA T., SAITO T., KOBAYASHI S., HARA Y., NAKAYA H.Amiodarone inhibits sarcolemmal but not mitochondrial KATP channels in guinea-pig ventricular cells J. Pharmacol. Exp. Ther. 2003. October 8, 2003; DOI:10.1124/jpet.103.055863 [DOI] [PubMed]
- SCHÄFER G., WEGENER C., PORTENHAUSER R., BOJANOVSKI D. Diazoxide, an inhibitor of succinate oxidation. Biochem. Pharmacol. 1969;18:2678–2681. [PubMed] [Google Scholar]
- SHIGEMATSU S., SATO T., ABE T., SAIKAWA T., SAKATA T., ARITA M. Pharmacological evidence for the persistent activation of ATP-sensitive K+ channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation. 1995;92:2266–2275. doi: 10.1161/01.cir.92.8.2266. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., SAITO T., SATO T., TAMAGAWA M., MIKI T., SEINO S, NAKAYA H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., SASAKI N., MIKI T., SAKAMOTO N., OHMOTO-SEKINE Y., TAMAGAWA M., SEINO S., MARBÁN E., NAKAYA H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J. Clin. Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOHSE N., NAKAYA H., KANNO M. α1 Adrenoceptor stimulation enhances the delayed rectifier K+ current of guinea pig ventricular cells through the activation of protein kinase C. Circ. Res. 1992;71:1441–1446. doi: 10.1161/01.res.71.6.1441. [DOI] [PubMed] [Google Scholar]
- TROLLINGER D.R., CASCIO W.E., LEMASTERS J.J. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca2+-indicating fluorophores. Biophys. J. 2000;79:39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO A., SATOH K., ICHINOSAWA K., KANETA S., KANO S., ICHIHARA K. Effects of minoxidil on ischemia-induced mechanical and metabolic dysfunction in dog myocardium. Jpn. J. Pharmacol. 2002;90:173–180. doi: 10.1254/jjp.90.173. [DOI] [PubMed] [Google Scholar]
- ZANG D.X., CHEN Y-F., CAMPBELL W.B., ZOU A-P., GROSS G.J., LI P.-L. Characteristics and superoxide-induced activation of reconstituted myocardial mitochondrial ATP-sensitive potassium channels. Circ. Res. 2001;89:1177–1183. doi: 10.1161/hh2401.101752. [DOI] [PubMed] [Google Scholar]