Abstract
Adenosine A1, A2A, and A3 receptors (ARs) and extracellular signal-regulated kinase 1/2 (ERK1/2) play a major role in myocardium protection from ischaemic injury. In this study, we have characterized the adenosine receptor subtypes involved in ERK1/2 activation in newborn rat cardiomyocytes.
Adenosine (nonselective agonist), CPA (A1), CGS 21680 (A2A) or Cl-IB-MECA (A3), all increased ERK1/2 phosphorylation in a time- and dose-dependent manner. The combined maximal response of the selective agonists was similar to adenosine alone. Theophylline (nonselective antagonist) inhibited completely adenosine-mediated ERK1/2 activation, whereas a partial inhibition was obtained with DPCPX (A1), ZM 241385 (A2A), and MRS 1220 (A3).
PD 98059 (MEK1; ERK kinase inhibitor) abolished all agonist-mediated ERK1/2 phosphorylation. Pertussis toxin (PTX, Gi/o blocker) inhibited completely CPA- and partially adenosine- and Cl-IB-MECA-induced ERK1/2 activation. Genistein (tyrosine kinase inhibitor) and Ro 318220 (protein kinase C, PKC inhibitor) partially reduced adenosine, CPA and Cl-IB-MECA responses, without any effect on CGS 21680-induced ERK1/2 phosphorylation. H89 (protein kinase A, PKA inhibitor) abolished completely CGS 21680 and partially adenosine and Cl-IB-MECA responses, without any effect on CPA response.
Cl-IB-MECA-mediated increases in cAMP accumulation suggest that A3AR-induced ERK1/2 phosphorylation involves adenylyl cyclase activation via phospholipase C (PLC) and PKC stimulation.
In summary, we have shown that ERK1/2 activation by adenosine in cardiomyocytes results from an additive stimulation of A1, A2A, and A3ARs, which involves Gi/o proteins, PKC, and tyrosine kinase for A1 and A3ARs, and Gs and PKA for A2AARs. Moreover, the A3AR response also involves a cAMP/PKA pathway via PKC activation.
Keywords: Adenosine A1 receptors, adenosine A3 receptors, adenosine A2A receptors, extracellular signal-regulated kinase 1/2, adenylyl cyclase, cardiomyocytes
Introduction
Four subtypes of adenosine receptors (ARs) have been characterized and classified into A1, A2A, A2B, and A3 (Fredholm et al., 2001). These receptors belong to the G-protein-coupled receptor (GPCR) superfamily. A1 and A3ARs activate Gi/o protein and inhibit adenylyl cyclase, whereas A2A and A2BARs signal through Gs and activate adenylyl cyclase. Previous studies have shown that A1, A2A, and A3ARs are expressed in rat ventricular cardiomyocytes (Martens et al., 1987; Linden, 1994; Dixon et al., 1996; Xu et al., 1996; Dobson & Fenton, 1997). In the heart, A1ARs mediate an antiadrenergic effect as well as a protective effect from ischaemic injury (Mubagwa & Flameng, 2001). Likewise, it has been shown that A3ARs play a cardioprotective role in rat cardiomyocytes (Safran et al., 2001). A2AARs mediate a positive inotropic effect (Xu et al., 1996; Dobson & Fenton, 1997) and counteract the antiadrenergic effects of A1ARs (Norton et al., 1999) in ventricular cardiomyocytes. The role of A2AARs in cardioprotection involves an anti-inflammatory effect rather than a direct effect on cardiomyocytes (Cargnoni et al., 1999). Furthermore, it has been reported that A2AAR activation increases the cell death during ischaemia in chick cardiomyocytes (Strickler et al., 1996).
Extracellular signal-regulated kinases 1/2 (ERK1/2) are isoforms of the mitogen-activated protein kinase (MAPK) family, which can be activated by tyrosine kinase receptors and Gi/o-, Gq-, and Gs-coupled receptors via a range of different signalling pathways (Lowes et al., 2002). ERK1/2 are involved in cardiac hypertrophy and can play a protective role in ischaemic myocardium (Michel et al., 2001).
Adenosine has been found to activate ERK1/2 in perfused rat heart (Haq et al., 1998). It is noteworthy that this physiological agonist is released during myocardial hypoxia and ischaemia, and possesses cardioprotective properties (Sommerschild & Kirkeboen, 2000). Although human A1, A2A, A2B, and A3ARs transfected in Chinese hamster ovary cells activate ERK1/2 (Schulte & Fredholm, 2000), nothing is known about the regulation of the ERK1/2 signalling pathway by the different subtypes of ARs expressed in the heart, both of which are involved in cardioprotection.
Thus, the aim of the present study was to characterize which AR subtypes activate ERK1/2 and to determine the mechanisms implicated in the ERK1/2 signalling pathway by these receptors in newborn rat cardiomyocytes. We present evidence that ERK1/2 activation by adenosine in newborn rat cardiomyocytes results from an additive stimulation of A1, A2A, and A3ARs. ERK1/2 phosphorylation is mainly mediated by A1 and A3ARs, and involves Gi/o proteins, protein kinase C (PKC), and tyrosine kinase. In addition, the A3AR response also involves a crosstalk between PLC/PKC and cAMP/protein kinase A (PKA) signalling pathways. The A2AAR activates ERK1/2 through Gs/PKA pathway.
Methods
Cell culture
Neonatal ventricular myocytes were prepared from 1–4 day-old Wistar rats using the Neonatal Cardiomyocyte Isolation System (Worthington Biochemical Corporation, Lornes laboratories, Reading, U.K.). The cells were preplated three times for 30 min in a humidified incubator (95% air/5% CO2 at 37°C) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 2 mM L-glutamine, 10% (v v−1) foetal calf serum, and penicillin/streptomycin (100 U ml−1), in order to minimize fibroblast contamination. Cardiomyocyte-rich cultures (>90%) were plated onto fibronectin-coated plates at a final density of 1.25 × 105 cells cm−2 in supplemented DMEM. For ERK1/2 phosphorylation detected by Western blot analysis, cardiomyocytes were plated onto 12-well plates. For cAMP accumulation assay, the cells were plated onto 24-well plates. After 3 days, confluent and spontaneously beating cells were serum-starved overnight before the experiments.
Western blot analysis
After serum starvation of cardiomyocytes (overnight), assays were carried out in serum-free DMEM in a humidified incubator (95% air/5% CO2 at 37°C). Agonists, antagonists, and/or inhibitors according to the experiments were added as described in the figure legends. Following stimulation, cardiomyocytes were washed with ice-cold phosphate-buffered saline (PBS) and lysed in ice-cold lysis buffer (150 mM NaCl, 50 mM Tris–HCl, 5 mM EDTA, 1% (v v−1) IGEPAL CA-630, 0.5% (w v−1) sodium deoxycholate, 0.1% (w v−1) sodium dodecyl sulphate (SDS), 1 mM Na3VO4, 1 mM NaF, 1 mM benzamidine, 0.1 mM phenylmethylsulphonylfluoride (PMSF), 10 μg ml−1 aprotinin, and 5 μg ml−1 leupeptin). Cell lysates were clarified by centrifugation (5 min; 12, 000 rpm) in an Eppendorf microcentrifuge. A measure of 100 μl of the cell lysate was removed and stored at −20°C until required. Protein concentration was determined using Bio-Rad DC Protein assay (Bio-Rad laboratories, Hertfordshire, U.K.) with bovine serum albumin as the standard. Samples in lysis buffer were heated at 95°C in SDS–polyacrylamide gel electrophoresis (PAGE) sample buffer (v v−1). Proteins (20–30 μg) were separated by SDS/PAGE(12% acrylamide gel) using a Bio-Rad Mini-Protean II system (1 h at 200 V). Proteins were transferred to nitrocellulose membranes using a Bio-Rad Trans-Blot system (1 h at 100 V in 25 mM Tris, 192 mM glycine, and 20% MeOH). Following transfer, the membranes were washed with tris-buffered saline (TBS) and blocked for 1 h at room temperature in blocking buffer (TBS, 5% (w v−1) skimmed milk powder, 0.1% Tween-20). Blots were then incubated overnight at 4°C with primary antibody against ERK1/2 at 1 : 1000 dilution in blocking buffer. The primary antibody was removed and the blot extensively washed three times for 5 min in TBS/Tween 20. Blots were then incubated for 1 h at room temperature with the goat anti-mouse secondary antibody coupled to horseradish peroxidase (DAKO Ltd, Cambridge, U.K.) at 1 : 1000 dilution in blocking buffer. Following removal of the secondary antibody, blots were extensively washed as above and developed using the Enhanced Chemiluminescence Detection System (Amersham Pharmacia Biotech, Little Chalfont, U.K.) and quantified using the programme QuantiScan (BioSoft, Cambridge, U.K.). The uniform transfer of proteins to the nitrocellulose membrane was routinely monitored by transiently staining the membranes with Ponceau S stain (Sigma Chemical Co.) prior to application of the primary antibody. In addition, replicate samples from each experiment were analysed on separate blots using an antibody (1 : 1000) that recognize unphosphorylated (total) ERK1/2. In these experiments, the uniformity of protein loading was confirmed by measuring total ERK1/2 (data omitted from the appropriate figures for clarity).
cAMP accumulation assay
After serum-starvation of cardiomyocytes (overnight), assays were carried out in serum-free DMEM in a humidified incubator (95% air/5% CO2 at 37°C). Agonists and/or inhibitors according to the experiments were added as described in the figure legends. The cells were incubated for 3 h in a humidified incubator (95% air/5% CO2 at 37°C) with 500 μl of serum-free DMEM containing [3H]adenine (37 kBq/well). [3H]adenine-labelled cells were washed twice with Hanks/HEPES buffer and then incubated in 500 μl/well serum-free DMEM containing the cyclic AMP phosphodiesterase inhibitor rolipram (10 μM) for 15 min at 37°C in a humidified incubator. Agonists were added (in 10 μl of medium) 5 min prior to the incubation with 1.5 μM forskolin (10 min). Pathway inhibitors were added 30 min before the agonist. Incubations were terminated by the addition of 500 μl 5% trichloroacetic acid, after removing the medium. [3H]cyclic AMP was isolated by sequential Dowex-alumina chromatography, as previously described (Dickenson & Hill, 1998). After elution, the levels of [3H]cyclic AMP were determined by liquid scintillation counting.
Statistical analysis
Results are expressed as means±s.e.m. Dose–response and inhibition response curves were analysed by computer-assisted iteration using the GraphPad Prism (GraphPad software, San Diego, U.S.A.). Statistical significance was determined by analysis of variance (ANOVA) followed by Dunnet's test, and P<0.05 was considered as the limit of statistical significance.
Materials
Bovine serum albumin, DMEM, foetal calf serum, IGEPAL CA-630 ((octylphenoxy)polyethoxyethanol), leupeptin, adenosine (9-β-D-ribofuranosyladenine), forskolin, N6-cyclopentyladenosine (CPA), 1,3-dipropylcyclopentylxanthine (DPCPX), theophylline, phorbol 12-myristate 13-acetate (PMA), epidermal growth factor (EGF), pertussis toxin (PTX), phospho-specific ERK1/ERK2 (Thr202/Tyr204), and non-phospho-specific ERK1/2 antibodies were obtained from Sigma Chemical Co. (Poole, Dorset, U.K.). Trichloroacetic acid, genistein, 3-{1-[3-(2-isothioureido) propyl]indol-3-yl}-4-(1-methylindol-3-yl)-3-pyrrolin-2,5-dione (Ro 31-8220), 2′-amino-3′-methoxyflavone (PD 98059), N-[2-(p-bromocinamylamine)ethyl]-5-isoquinolisesulphonamide (H-89), rolipram and {1-[6-((17β-3 methoxyestra-1,3,5(10)-trien-17-yl)amoni)hexyl]-1H-pyrrole-2,5-dione} (U-73122) were purchased from Calbiochem (Nottingham, U.K.). 4-[2-[[-6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid (CGS 21680), 1-[2-chloro-6[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide (2-Cl-IB-MECA), 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5ylamino]ethyl)phenol (ZM 241385), and N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-benzeneacetamide (MRS 1220) were from Tocris (Bristol, U.K.). [8-3H]adenine was obtained from Amersham (Bucks, U.K.).
Results
Adenosine A1, A2A, and A3 receptor-induced ERK1/2 activation in rat cardiomyocytes
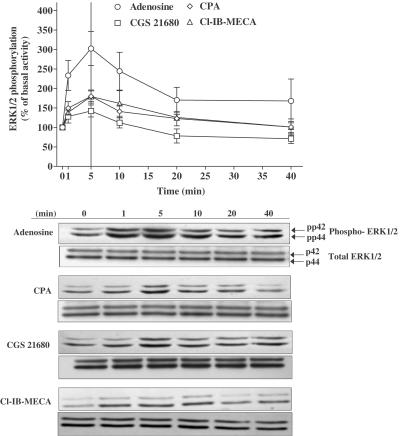
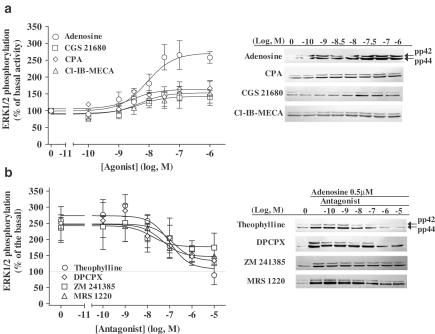
In order to investigate which subtype of ARs activates ERK1/2 in newborn rat cardiomyocytes, we used the following agonists: adenosine (nonselective agonist), CPA (A1 selective agonist), CGS 21680 (A2A selective agonist) or Cl-IB-MECA (A3 selective agonist). All agonists induced ERK1/2 phosphorylation in a time- (Figure 1) and dose-dependent (Figure 2a) manner. Although each AR agonist stimulated increases in ERK1 and ERK2 phosphorylation, activation of the ERK1 isoform (p42 MAPK) was dominant. Furthermore, due to the variation in the basal level of ERK2 phosphorylation, all data are presented as the increase in combined ERK1/2 phosphorylation. For each agonist, ERK1/2 activation reached a maximal value at 5 min and decreased back to the basal level after 10 min. The rank order of the maximal stimulation (% over basal activity) was adenosine (203±44, n=5)≫CPA (78±14, n=5, P<0.05 versus adenosine)=Cl-IB-MECA (79±17, n=4, P<0.05 versus adenosine)>CGS 21680 (42±15, n=4, P<0.01 versus adenosine). When the responses of the A1, A2A, and A3 agonists were added, the value (199%) was consistent with the response obtained with adenosine alone (203%). In the same way, from the dose–response curves (Figure 2a), the combined maximal response (Emax) of all the three selective agonists (185%; CPA: 65±4, n=5; CGS 21680: 54±19, n=4; Cl-IB-MECA: 66±31, n=4; P<0.01 versus adenosine) was similar to the adenosine Emax value (175±20%, n=5). These data suggest that adenosine induces ERK1/2 phosphorylation by stimulating the three adenosine subtypes A1, A2A, and A3.
Figure 1.
Time course of AR agonist-induced ERK1/2 phosphorylation in newborn rat cardiomyocytes. Serum-starved cardiomyocytes were stimulated with 1 μM adenosine (○), CPA (◊), CGS 21680 (□) or Cl-IB-MECA (▵) for the indicated periods of time. Data are expressed as the percentage of the basal level of ERK1/2 phosphorylation (100%). Samples were also analysed on a separate blot, using an antibody that recognizes nonphosphorylated (total) ERK1/2 to confirm equal protein loading. Each point represents the mean±s.e.m. of 4–6 independent experiments. The immunoblots are representative of the experiments summarized in the graph.
Figure 2.
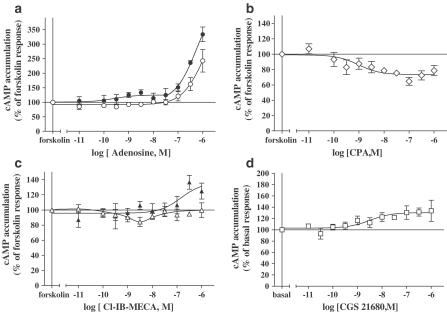
Dose–response curve of ERK1/2 phosphorylation (a) after stimulation for 5 min with adenosine (○), CPA (◊), CGS 21680 (□) or Cl-IB-MECA (▵), and inhibition of 0.5 μM adenosine-induced ERK1/2 phosphorylation (b) by theophylline (○), DPCPX (◊), ZM 241385 (□) or MRS 1220 (▵) in newborn rat cardiomyocytes. From panel (a), agonist potencies were evaluated by their EC50 values (concentration of agonist inducing 50% of maximal ERK1/2 phosphorylation) expressed as −log10 EC50 (adenosine: 8.23±0.42, n=5; CPA: 8.91±0.15, n=5; CGS 21680 7.98±0.30, n=4; Cl-IB-MECA: 8.61±0.34, n=4). In panel (b), serum-starved cardiomyocytes were preincubated for 30 min with the different antagonists before stimulating was 148±5%. Data are expressed as the percentage of the basal level of ERK1/2 phosphorylation (100%). Each point represents the mean±s.e.m. of 4–6 independent experiments. The immunoblots are representative of the experiments summarized in the graph.
To further characterize the AR subtypes involved in ERK1/2 activation in newborn rat cardiomyocytes, we studied the inhibition of the 0.5 μM adenosine response by theophylline (nonselective antagonist), DPCPX (A1 selective antagonist), ZM 241385 (A2A selective antagonist) or MRS 1220 (A3 selective antagonist) (Figure 2b). Theophylline completely inhibited adenosine-induced ERK1/2 activation (98±3%, n=4), whereas the inhibition by the selective antagonists was partial (DPCPX: 67±8%, n=4, P<0.05 versus theophylline; ZM 241385: 27±10%, n=3, P<0.01 versus theophylline; MRS 1220: 54±12%, n=3, P<0.01 versus theophylline). The potencies of antagonists were evaluated by their IC50 (concentration of antagonist inhibiting 50% of ERK1/2 activation by adenosine) expressed as −log10 IC50. They showed that DPCPX (7.31±0.79%) and MRS 1220 (7.43±0.21%) were six and nine times more potent, respectively, than theophylline (6.45±0.0.35%) in inhibiting adenosine-induced ERK1/2 phosphorylation, and both were more efficient than ZM 241385 (6.95±0.61%). These results suggest that adenosine activates ERK1/2 phosphorylation mainly via A1 and A3ARs.
Effect of signalling pathway inhibitors on adenosine A1, A2A, and A3 receptor-induced ERK1/2 activation in rat cardiomyocytes
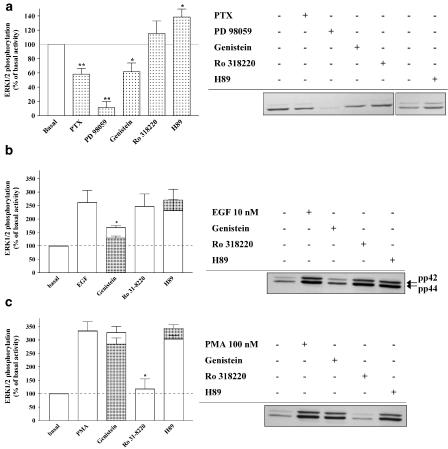
Gi/o-, Gq-, and Gs-PCRs have been shown to activate ERK1/2 via a range of signalling pathways involving tyrosine kinase, PKC, and PKA (Kolch et al., 1993; Hawes et al., 1995; Lowes et al., 2002). In order to determine the mechanisms implicated in ERK1/2 activation by the three subtypes of ARs, cardiomyocytes were pretreated with PTX (100 ng ml−1, 16 h) to block the Gi/o protein, and inhibitors of MEK1 (PD 98059; 50 μM, 30 min), tyrosine kinase (genistein; 100 μM, 30 min), PKC (Ro 318220; 10 μM, 30 min), and PKA (H89; 10 μM, 30 min). Initially, we performed the relevant and essential controls required for the interpretation of data relating to the characterization of ERK1/2 signalling pathways induced by ARs (Figure 3). Indeed, the effects of these inhibitors on the basal level of ERK1/2 phosphorylation were investigated (Figure 3a). Furthermore, we also determined the effects of genistein, Ro 318220, and H89 on ERK1/2 activation induced by EGF (tyrosine kinase-dependent, Figure 3b) and the PKC activator PMA (Figure 3c). As shown in Figure 3b, genistein selectively inhibited the robust increase in ERK1/2 phosphorylation induced by EGF. Similarly, Ro 318220 selectively inhibited PMA-induced increases in ERK1/2 phosphorylation (Figure 3c). Importantly, the PKA inhibitor H89 had no significant effect on EGF or PMA-induced ERK1/2 phosphorylation, indicating that H89 is not inhibiting tyrosine kinase and PKC-dependent pathways of ERK1/2 activation in cardiomyocytes. These observations clearly indicate that genistein and Ro 318220 are selective for tyrosine kinase and PKC-dependent pathways of ERK1/2 activation in rat cardiomyocytes.
Figure 3.
Effect of PTX and protein kinase inhibitors on the basal level of ERK1/2 phosphorylation and ERK1/2 activation triggered by EGF and PMA. Serum-starved cardiomyocytes were pretreated for 16 h with 100ng ml−1 PTX (Gi/o protein blocker) and 30 min with 50 μM PD 98059, 100 μM genistein 10 μM Ro 318220 or 10 μM H89. The effects of PTX, PD 98059, genistein, Ro 318220, and H89 on the basal level of ERK1/2 phosphorylation are shown in (a). Panels (b) and (c) show the effect of genistein, Ro 318220, and H89 on 10 nM EGF (b) and 100 nM PMA (c) -induced ERK1/2 phosphorylation (both 5 min stimulation). Data are expressed as the percentage of the basal level ERK1/2 phosphorylation (100%). Each bar represents the mean±s.e.m. of four independent experiments. Filled bars indicate the data obtained from the immunoblot, and open bars represent the data after removing the effect of the inhibitors on the basal level of ERK1/2 phosphorylation for each individual experiment and that illustrated in (a). The immunoblots are representative of the experiments summarized in the bar graph. *P<0.05 and **P<0.01 versus basal level of ERK1/2 phosphorylation (panel (a)) and versus EGF or PMA-induced ERK1/2 activation (panels (b) and (c)).
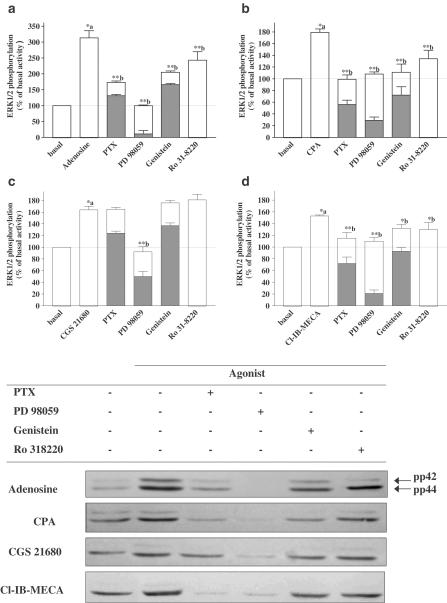
The effects of PTX, PD 98059, genistein, Ro 318220, and H89 on agonist-stimulated ERK1/2 phosphorylation are illustrated in Figures 4 and 5. ERK1/2 activation by CPA (A1 selective agonist) was completely abolished by PTX. PTX partially inhibited ERK1/2 phosphorylation (72%) induced by Cl-IB-MECA (A3 selective agonist), indicating that ERK1/2 activation by A3AR stimulation involves PTX-sensitive (Gi/o) and insensitive (Gq and/or Gs) G protein. PTX was ineffective on CGS 21680 (A2A selective agonist)-induced ERK1/2 phosphorylation. ERK1/2 phosphorylation induced by adenosine was partially inhibited by PTX (66%), indicating that adenosine induces ERK1/2 phosphorylation by stimulating these three subtypes.
Figure 4.
Effect of PTX (Gi/o protein blocker), PD 98059 (MEK1 inhibitor), genistein (tyrosine kinase inhibitor), and Ro 318220 (PKC inhibitor) on adenosine (a), CPA (b), CGS 21680 (c) or Cl-IB-MECA (d)-induced ERK1/2 phosphorylation. Serum-starved cardiomyocytes were pretreated for 16 h with 100 ng ml−1 PTX and 30 min with 50 μM PD 98059, 100 μM genistein or 10 μM Ro 318220, before stimulating for 5 min with 1 μM adenosine, CPA, CGS 21680 or Cl-IB-MECA. Data are expressed as the percentage of the basal level of ERK1/2 phosphorylation. Each bar represents the mean±s.e.m. of 5–6 independent experiments. Filled bars indicate the data obtained from the immunoblot, and open bars represent the data after removing the effect of the inhibitors on the basal level of ERK1/2 phosphorylation for each individual experiment, and illustrated in Figure 3a. The immunoblots are representative of the experiments summarized in the bar graph. *P<0.05 and **P<0.01, (a) versus basal activity and (b) versus agonist stimulation.
Figure 5.
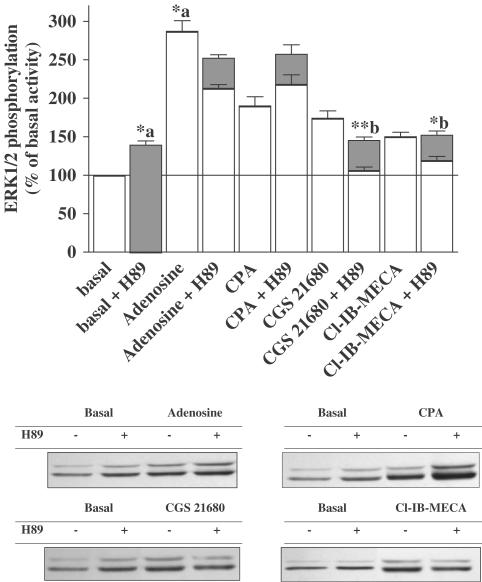
Effect of H89 (PKA inhibitor) on adenosine, CPA, CGS 21680, and Cl-IB-MECA-induced ERK1/2 phosphorylation. Serum-starved cardiomyocytes were pretreated for 30 min with 10 μM H89 before stimulating for 5 min with 1 μM adenosine, CPA, CGS 21680 or Cl-IB-MECA. Data are expressed as the percentage of the basal level of ERK1/2 phosphorylation. H89 alone elicited a small but significant increase in the basal level of ERK1/2 phosphorylation. Each bar represents the mean±s.e.m. of 4–5 independent experiments. Filled bars indicate the data obtained from the immunoblot and open bars represent the data after removing the effect of the inhibitors on the basal level of ERK1/2 phosphorylation for each individual experiment. The immunoblots are representative of the experiments summarized in the bar graph. *P<0.05 and **P<0.01, (a) versus basal activity and (b) versus agonist stimulation.
As expected, PD 98059 (MEK1 inhibitor) completely abolished the ERK1/2 phosphorylation mediated by all the agonists, showing that MEK1 activation is the common and converging pathway to the three subtypes of ARs.
Following genistein pretreatment, adenosine, CPA, and Cl-IB-MECA-induced ERK1/2 activation were reduced by 51, 86, and 40%, respectively. These data suggest that ERK1/2 activation via the A1ARs is mainly tyrosine kinase-dependent, whereas the A3ARs appear to activate ERK1/2 via a tyrosine kinase-dependent and independent pathway. As expected, adenosine showed an intermediate response, taking into account the lack of genistein effect on the CGS 21680 response.
Ro 318220 inhibited the response induced by adenosine, CPA, and Cl-IB-MECA, respectively, by 33, 57, and 43%, without affecting CGS 21680-induced ERK1/2 phosphorylation. These data indicate that A1 and A3ARs stimulate ERK1/2 phosphorylation via a pathway that is partially dependent upon PKC.
CGS 21680-induced ERK1/2 phosphorylation was completely inhibited by H89, whereas CPA-stimulated ERK1/2 activation was unaltered. A partial inhibition (about 50%) was achieved with adenosine and Cl-IB-MECA, suggesting that ERK1/2 activation induced by A3ARs in cardiomyocytes also involves coupling to the Gs/PKA pathway.
Effect of adenosine A1, A2A, and A3 agonists on cAMP accumulation in rat cardiomyocytes
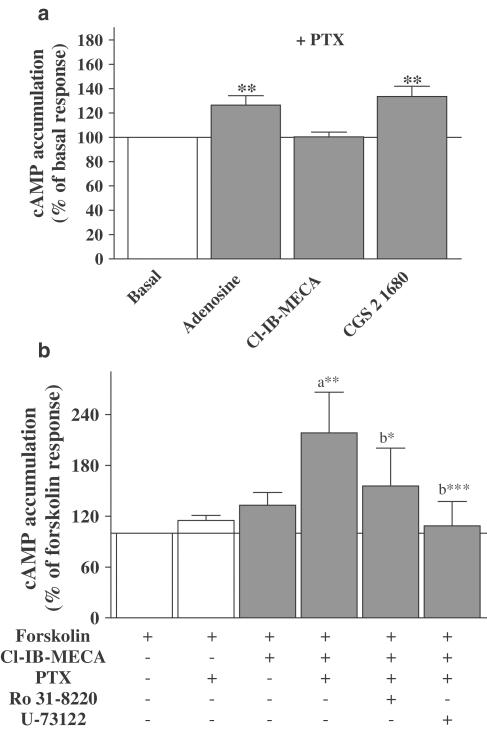
In view of the role of cAMP/PKA in coupling of the A3ARs to ERK1/2, we investigated the effect of A3AR activation on cAMP accumulation. A1 and A3ARs classically activate Gi/o protein and inhibit adenylyl cyclase (Fredholm, 2001). The effects of adenosine (nonselective), CPA (A1 selective), and Cl-IB-MECA (A3 selective) on forskolin-stimulated cAMP accumulation are shown in Figures 6a–c, respectively. In addition, we measured cAMP accumulation in response to CGS 21680 (A2A selective; Figure 6d). Adenosine did not inhibit forskolin-induced cAMP accumulation, but increased cAMP formation at concentrations above 100 nM (Figure 6a). The dose–response effects of adenosine presumably reflect A1, A2A, and A3AR costimulation, as observed with ERK1/2 activation. As expected, CPA elicited a dose-dependent inhibition of forskolin-stimulated cAMP accumulation by 26±1%, with a potency of 8.8±0.34 (1.5 nM; Figure 6b). The A2AAR agonist CGS 21680 stimulated an increase in cAMP accumulation of 28% above the basal accumulation, with a pEC50 value of 8.50±0.46 (3.18 nM; Figure 6d). Noradrenaline, which was used as a positive control for Gs/PKA pathway activation, stimulated a robust increase in cAMP accumulation (301±29% over basal), with a potency of 7.15±0.23 (71 nM). Interestingly, the dose–response curve for Cl-IB-MECA-induced inhibition of forskolin-stimulated cAMP accumulation was biphasic (Figure 7c). Inhibition of forskolin-stimulated cAMP accumulation was observed at lower concentrations of Cl-IB-MECA (maximal inhibition occurring at 3 nM; about 18%), with an approximate pIC50 of 9.7±0.8 (0.7 nM). At concentrations above 3 nM, A3AR stimulation can also induce a reversal of the inhibitory effect on cAMP accumulation. PTX treatment abolished Cl-IB-MECA-mediated inhibition of forskolin-induced cAMP accumulation (observed at low concentrations), and produced an increase in cAMP formation (about 14%) at 1 μM agonist (Figure 6c), indicating that A3ARs are coupling to the Gi protein and Gs/PKA pathway. PTX treatment also abolished CPA-induced inhibition of forskolin-induced cAMP accumulation, confirming the A1 AR coupling to Gi/o proteins (data not shown). Finally, the inhibition of Gi by PTX produced an increase in adenosine-stimulated cAMP production by 25% in a dose-dependent manner between 0.01 and 30 nM, indicating A2AAR stimulation (Figure 6a). In order to investigate whether the A3AR directly couples to Gs-protein, PTX-treated cardiomyocytes were stimulated with Cl-IB-MECA in the absence of forskolin. As shown in Figure 7a, 1 μM Cl-IB-MECA (concentration used to determine ERK1/2 signalling pathway activation) did not increase cAMP accumulation, whereas adenosine and CGS 21680 similarly increased cAMP accumulation by 27±3 and 34±5%, respectively.
Figure 6.
Effect of AR agonists on cAMP accumulation in isolated rat cardiomyocytes. In panels (a)–(c), cells were initially prestimulated for 5 min with the indicated concentrations of adenosine (a), CPA (b), and Cl-IB-MECA (c), prior to stimulation with 1.5 μM forskolin for 10 min in the absence (opened symbols) and presence (filled symbols) of PTX. Cells were pretreated with 100 ng ml−1 PTX for 16 h to block Gi/Go-protein-dependent pathways. Data are expressed as the percentage of the forskolin response (in the absence of agonist=100%). In panel (d), cells were stimulated with the indicated concentrations of CGS 21680 for 15 min in the absence of forskolin. Data are expressed as the percentage of the basal cAMP accumulation (100%). The results represent mean±s.e.m. of 4–6 independent experiments performed in duplicate. Standard error bars not shown are within the symbol.
Figure 7.
Effect of adenosine, Cl-IB-MECA, and CGS 21680 on basal cAMP accumulation (a), and the effects of Ro 318220 (PKC inhibitor) and U-73122 (PLC inhibitor) on Cl-IB-MECA-mediated augmentation of forskolin-stimulated cAMP accumulation (b). In panel (a), PTX-treated cells (100 ng ml−1 for 16 h) were stimulated with 1 μM adenosine, Cl-IB-MECA, and CGS 21680 for 15 min in the absence of forskolin. Data are expressed as the percentage of the basal cAMP accumulation (100%). The results represent mean±s.e.m. of 4–5 independent experiments performed in duplicate. **P<0.01 vs basal cAMP accumulation. In panel (b), cells were pretreated with PTX (100 ng ml−1 for 16 h) to block Gi/Go-protein-dependent pathways. In addition, the indicated cells were incubated for 30 min with 10 μM Ro 318220 and 10 μM U-73122, inhibitors of PKC and PLC, respectively. Cells were then stimulated for 5 min with 1 μM Cl-IB-MECA prior to stimulation with 1.5 μM forskolin for 10 min in the continued presence of Cl-IB-MECA. Data are expressed as the percentage of the forskolin response in the absence of Cl-IB-MECA (100%). The results represent the mean±s.e.m. of four independent experiments performed in duplicate. *P<0.05, **P<0.01, ***P<0.001, (a) versus Cl-IB-MECA/forskolin response and (b) versus Cl-IB-MECA/forskolin/PTX response.
Since the Gq/PLC/PKC pathway can stimulate cAMP production (Cordeaux & Hill, 2002), and given that A3ARs activate PLC in cardiomyocytes (Parsons et al., 2000), we determined the role of PKC and PLC in A3AR-mediated augmentation of forskolin-induced cAMP accumulation in the presence of PTX. Pretreatment with Ro 31822 and U-73122, inhibitors of PKC and PLC, respectively, significantly decreased 1 μM Cl-IB-MECA-mediated cAMP production in the presence of forskolin and PTX (Figure 7b). Overall, these data strongly indicate that A3ARs are not directly coupled to Gs protein in cardiomyocytes, but rather increase cAMP accumulation via PLC/PKC.
Discussion
Adenosine released during myocardial ischaemia plays an important cardioprotective role, via the activation of cell surface ARs belonging to the GPCR superfamily (Sommerschild & Kirkeboen, 2000). Previous studies have shown that A1, A2A, and A3ARs are functionally expressed in cardiomyocytes (Martens et al., 1987; Linden, 1994; Dixon et al., 1996; Xu et al., 1996; Dobson & Fenton, 1997). Extracellular signal-regulated kinase 1/2 (ERK1/2) pathway, which can be activated by tyrosine kinase receptors and Gi/o-, Gq-, and Gs-PCRs (Lowes et al., 2002), is involved in cardiac hypertrophy and myocardium protection (Michel et al., 2001). Given the important role of adenosine and ERK1/2 in the heart, the aims of the present study were to characterize the AR subtypes involved in the ERK1/2 activation and to determine the signalling mechanisms implicated in ERK1/2 activation by these receptors in newborn rat cardiomyocytes.
Our study has shown for the first time that stimulation of A1, A2A, and A3ARs activated ERK1/2 in newborn rat cardiomyocytes. Indeed, adenosine (nonselective agonist), CPA (A1 selective agonist), CGS 21680 (A2A selective agonist), and Cl-IB-MECA (A3 selective agonist), all induced ERK1/2 phosphorylation in a time- and dose-dependent manner (Figures 1 and 2a). These observations are in agreement with other studies on human A1, A2A, and A3ARs in transfected Chinese hamster ovary cells (Dickenson et al., 1998; Schulte & Fredholm, 2000; Graham et al., 2001) and endogenous A1ARs in DDT1MF-2 cells (Robinson & Dickenson, 2001) and A2AARs human umbilical venous endothelial cells (Sexl et al., 1997).
It is notable that the combined response of all the three selective agonists was similar to the value obtained with adenosine alone, indicating that this physiological agonist stimulates all the receptor subtypes when it is released in pathological situations or in response to physiological adaptation. This finding is supported by previous studies showing an additive protection against ischaemia induced by a simultaneous activation of A1 and A3ARs in cardiomyocytes (Jacobson et al., 2000; Safran et al., 2001). Furthermore, A1AR-mediated antiadrenergic effects are modulated by costimulation of the A2AAR in rat cardiomyocytes and whole heart (Norton et al., 1999). Adenosine-mediated ERK1/2 activation via the three AR receptor subtypes was confirmed by using adenosine-receptor antagonists (Figure 2b). Theophylline (nonselective antagonist) completely inhibited the adenosine-induced ERK1/2 activation, whereas DPCPX (A1 selective antagonist), ZM 241385 (A2A selective agonist), and MRS 1220 (A3 selective agonist) elicited a partial inhibition of this response by 67, 54, and 27%, respectively. The efficiency to inhibit adenosine-mediated ERK1/2 activation was higher using A1 and A3 than A2A antagonists. These data suggest that adenosine-induced ERK1/2 phosphorylation in newborn rat cardiomyocytes is highly dependent on A1 and A3AR stimulation.
Experiments investigating the signalling pathways associated with ERK1/2 activation revealed that A1- and A3AR-mediated ERK1/2 phosphorylation involves a genistein-sensitive tyrosine kinase and MEK1 activation (Figures 4b and d). In addition, A1AR-mediated ERK1/2 activation was completely abolished by PTX (hence Gi/o protein-mediated), whereas the response to Cl-IB-MECA was partially sensitive to PTX (72%). These data are in agreement with previous studies showing that Gi/o-PCR-mediated ERK1/2 activation, including the A1AR, involves G protein-derived βγ subunits and genistein-sensitive Src-related tyrosine kinase(s) (Faure et al., 1994; Della Rocca et al., 1997). Also, A1- and A3AR-induced increases in ERK1/2 activation were partially sensitive to the PKC inhibitor Ro 318220 (57 and 43% inhibition, respectively), indicating the involvement of a PKC-dependent pathway. There is increasing evidence for a role of PKC in Gi/o-PCR-mediated ERK1/2 activation (Takeda et al., 1999). The involvement of PKC in A1AR-induced ERK1/2 activation may reflect the coupling of the A1AR to PLC/PKC activation in cardiomyocytes (Parsons et al., 2000; Lee et al., 2001). Furthermore, the A1AR is known to activate PLC via βγ subunits released from PTX-sensitive Gi/o proteins (Dickenson & Hill, 1998). Therefore, the PKC-sensitive component of A1AR-induced ERK1/2 activation in cardiomyocytes may involve βγ subunit-mediated PLC/PKC activation. A similar pathway has been reported for α2A adrenergic receptor-mediated ERK1/2 activation (Della Rocca et al., 1997).
The PKC-dependent pathway associated with A3AR-induced ERK1/2 activation (PTX-insensitive) may reflect the reported coupling of the A3AR to Gq and PLC (Palmer et al., 1995; Parsons et al., 2000), since PKC is associated with ERK1/2 activation via Gq-PCRs (Hawes et al., 1995; Lowes et al., 2002). We conclude that A1 and A3ARs in cardiomyocytes mediate ERK1/2 activation predominantly via a Gi/o/tyrosine kinase/MEK1 pathway. In addition, A1AR-activated ERK1/2 phosphorylation also involves a Gi/o/PLC/PKC-dependent pathway. Furthermore, it is conceivable that the PTX-insensitive component of the A3AR-mediated ERK1/2 phosphorylation can involve Gq/PLC-mediated PKC activation.
It is notable that A3AR-mediated ERK1/2 activation was also sensitive to the PKA inhibitor H89, suggesting the involvement of a Gs/PKA/cAMP-dependent pathway. Interestingly, A3ARs have been shown to induce an increase in intracellular calcium and potentiate Ca2+ currents via PKA activation in A6 renal cells (Reshkin et al., 2000) and hippocampal CA3 pyramidal neuronal cells (Fleming & Mogul, 1997). In addition, the A3AR stimulates cAMP production in human eosinophils (Ezeamuzie & Philips, 2003). Based on these previous studies and the sensitivity of A3AR-mediated ERK1/2 activation to H89, we investigated whether the A3AR couples to Gs/cAMP in cardiomyocytes. Interestingly, the dose–response curve for Cl-IB-MECA-induced inhibition of forskolin-stimulated cAMP accumulation was biphasic (Figure 6c). Inhibition of forskolin-stimulated cAMP accumulation was observed at lower concentrations of Cl-IB-MECA (<3 nM), whereas, at concentrations above 3 nM, the inhibitory effect was reversed and potentiated in the presence of PTX (Figure 6c), indicating that A3AR stimulation can also induce an increase in cAMP accumulation. This increase does not involve direct coupling of the A3AR to Gs or the nonselectivity of Cl-IB-MECA (via A2A activation), since this agonist did not induce cAMP accumulation in cells treated with PTX in the absence of forskolin (Figure 7a). Since PKC can directly activate certain isoforms of adenylyl cyclase (Cordeaux & Hill, 2002), and given the evidence for A3AR coupling to PLC in cardiomyocytes, we determined the role of PLC/PKC in Cl-IB-MECA-mediated augmentation of forskolin-induced cAMP accumulation. Ro 31-8220 (PKC inhibitor) and U-73122 (PLC inhibitor) inhibited the potentiation of forskolin-induced cAMP production by Cl-IB-MECA (Figure 7b). Therefore, these data suggest that the cAMP/PKA-dependent pathway associated with A3AR-mediated ERK1/2 activation in cardiomyocytes may involve PKC via Gq/PLC and not direct coupling to Gs protein. A3AR coupling to cAMP/PKA may explain the induction of apoptosis in cardiomyocytes after stimulation with high concentration (⩾10 μM) of Cl-IB-MECA (Shneyvays et al., 2000). The cardioprotective effects of Cl-IB-MECA are only observed using concentrations below 1 μM (Safran et al., 2001).
A2AAR-mediated increases in ERK1/2 phosphorylation in cardiomyocytes appear to involve coupling to Gs protein and, subsequently, PKA activation, since the PKA inhibitor H89 completely blocked the responses to CGS 21680 (Figure 5). These data are strengthened by the activation of cAMP accumulation by CGS 21680, as shown in Figure 6d. In addition, the MEK1 inhibitor PD 98059 blocked A2AR-mediated ERK1/2 activation. Previous studies have shown that the A2AAR activates ERK1/2 via a cascade composed of Gs, adenylyl cyclase, PKA, and MEK1, although additional pathways independent of Gs coupling and PKA activation have also been observed (Sexl et al., 1997; Seidel et al., 1999).
In conclusion, this study has shown for the first time that the activation of ERK1/2 by adenosine in newborn rat cardiomyocytes arises from an additive stimulation of A1, A2A, and A3ARs endogenously expressed in these cells. Adenosine-induced ERK1/2 phosphorylation is mainly mediated by A1 and A3ARs, and involves Gi/o proteins, PKC, and tyrosine kinase. In addition, the A3AR response also involves a cAMP/PKA-dependent pathway triggered by A3AR-induced PKC activation. The A2AAR activates ERK1/2 via Gs and PKA. The role of the different ERK1/2 pathways activated by the three AR subtypes and their possible interaction in cardioprotection induced by adenosine remain to be established. However, we have recently obtained preliminary data which indicate that ERK1/2 are involved in A1AR- and A3AR-mediated cardioprotection in isolated rat cardiomyocytes (Germack et al., 2003).
Acknowledgments
This work was funded by the Wellcome Trust (grant reference 058137/z/99/z). We wish to thank Annerose Schneider for technical assistance in performing the cAMP accumulation assay.
Abbreviations
- AR
adenosine receptor
- CGS 21680
(4-[2-[[-6-amino-9-(N-ethyl-β-D-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid)
- 2-Cl-IB-MECA
(1-[2-chloro-6[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-D-ribofuranuronamide)
- CPA
N6-cyclopentyladenosine
- DMEM
Dulbecco's modified Eagle's medium
- DPCPX
1,3-dipropylcyclopentylxanthine
- EGF
epidermal growth factor
- ERK1/2
extracellular signal-regulated kinases
- GPCR
G-protein-coupled receptor
- H-89
N-[2-(p-bromocinamylamine)ethyl]-5-isoquinolisesulphonamide
- MRS 1220
(N-[9-chloro-2-(2-furanyl)[1,2,4]-triazolo[1,5-c]quinazolin-5-benzeneacetamide)
- PD 98059
2′-amino-3′-methoxyflavone
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- PMA
phorbol 12-myristate 13-acetate
- PTX
pertussis toxin
- Ro 31-8220
(3-{1-[3-(2-isothioureido) propyl]indol-3-yl}-4-(1-methylindol-3-yl)-3-pyrrolin-2,5-dione)
- U-73122
({1-[6-((17β-3 methoxyestra-1,3,5(10)-trien-17-yl)amoni)hexyl]-1H-pyrrole-2,5-dione})
- ZM 241385
(4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5ylamino]ethyl)phenol)
References
- CARGNONI A., CECONI C., BORASO A., BERNOCCHI P., MONOPOLI A., CURELLO S., FERRARI R. Role of A2A receptor in the modulation of myocardial reperfusion damage. J. Cardiovasc. Pharmacol. 1999;33:883–893. doi: 10.1097/00005344-199906000-00008. [DOI] [PubMed] [Google Scholar]
- CORDEAUX Y., HILL S.J. Mechanisms of cross-talk between G-protein-coupled receptors. Neurosignals. 2002;11:45–57. doi: 10.1159/000057321. [DOI] [PubMed] [Google Scholar]
- DELLA ROCCA G.J., VAN BIESEN T., DAAKA Y., LUTTRELL D.K., LUTTRELL L.M., LEFKOWITZ R.J. Ras-dependent mitogen-activated protein kinase activation by G protein-coupled receptors. Convergence of Gi- and Gq-mediated pathways on calcium/calmodulin, Pyk 2, and Src kinase. J. Biol. Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- DICKENSON J.M., BLANK J.L., HILL S.J. Human adenosine A1 receptor and P2Y2-purinoceptor-mediated activation of the mitogen-activated protein kinase cascade in transfected CHO cells. Br. J. Pharmacol. 1998;124:1491–1499. doi: 10.1038/sj.bjp.0701977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENSON J.M., HILL S.J. Involvement of G-protein βγ subunits in coupling the adenosine A1 receptor to phospholipase C in transfected CHO cells. Eur. J. Pharmacol. 1998;355:85–93. doi: 10.1016/s0014-2999(98)00468-3. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., GUBITZ A.K., SIRINATHSINGHJI D.J.S., RICHARDSON P.J., FREEMAN T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBSON J.G., FENTON R.A. Adenosine A2 receptor function in rat ventricular myocytes. Cardiovasc. Res. 1997;34:337–347. doi: 10.1016/s0008-6363(97)00023-0. [DOI] [PubMed] [Google Scholar]
- EZEAMUZIE C.I., PHILIPS E. Positive coupling of atypical adenosine A3 receptors on human eosinophils to adenylyl cyclase. Biochem. Biophys. Res. Commun. 2003;300:712–718. doi: 10.1016/s0006-291x(02)02910-8. [DOI] [PubMed] [Google Scholar]
- FAURE M., VOYONO-YASENETSKAYA T.A., BOURNE H.R. cAMP and βγ subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- FLEMING K.M., MOGUL D.J. Adenosine A3 receptors potentiate hippocampal calcium current by a PKA-dependent/PKC-independent pathway. Neuropharmacology. 1997;36:353–362. doi: 10.1016/s0028-3908(97)83762-8. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERIAAN A.P., JACOBSON K.A., KLOTZ K.N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- GERMACK R., MARSHALL C., GRIFFIN M., DICKENSON J.M. Role of PKB and ERK1/2 in A1 and A3 adenosine receptor-mediated cardioprotection in newborn rat cardiomyocytes. Eur. J. Biochem. 2003;270 Suppl 1:180. [Google Scholar]
- GRAHAM S., COMBES P., CRUMIERE M., KLOTZ K.N., DICKENSON J.M. Regulation of p42/p44 mitogen-activated protein kinase by the human adenosine A3 receptor in transfected CHO cells. Eur. J. Pharmacol. 2001;420:19–26. doi: 10.1016/s0014-2999(01)00976-1. [DOI] [PubMed] [Google Scholar]
- HAQ S.E.A., CLERK A., SUGDEN P.H. Activation of mitogen-activated protein kinases (p38-MAPKs, SAPKs/JNKs and ERKs) by adenosine in the perfused rat heart. FEBS Lett. 1998;434:305–308. doi: 10.1016/s0014-5793(98)01000-x. [DOI] [PubMed] [Google Scholar]
- HAWES B.E., VAN BIESEN T., KOCH W.J., LUTTRELL L.M., LEFKOWITZ R.J. Distinct pathways of Gi- and Gq-mediated mitogen-activated protein kinase activation. J. Biol. Chem. 1995;270:17148–17153. doi: 10.1074/jbc.270.29.17148. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A., XIC R., YOUNG L., CHANG L., LIANG B.T. A novel pharmacological approach to treating cardiac ischemia. Binary conjugates of A1 and A3 adenosine receptor agonists. J. Biol., Chem. 2000;275:30272–30279. doi: 10.1074/jbc.M001520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLCH W., HEIDECKER G., KOCHS G., HUMMEL R., VAHIDI H., MISCHAK H., FINKENZELLER G., MARME D., RAPP U.R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- LEE J.E., BOKOCH G., LIANG B.T. A novel cardioprotective role of RhoA: new signalling mechanism for adenosine. FASAB J. 2001;15:1886–1894. doi: 10.1096/fj.01-0212com. [DOI] [PubMed] [Google Scholar]
- LINDEN J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol. Sci. 1994;15:298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- LOWES V., IP N., WONG Y. Integration of signals from receptor tyrosine kinases and G protein-coupled receptors. Neurosignals. 2002;11:5–19. doi: 10.1159/000057317. [DOI] [PubMed] [Google Scholar]
- MARTENS D., LOHSE M.J., RAUCH B., SCHWABE U. Pharmacological characterization of A1 adenosine receptors in isolated rat ventricular myocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:342–348. doi: 10.1007/BF00172688. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., LI Y., HEUSHCH G. Mitogen-activated protein kinases in the heart. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:245–266. doi: 10.1007/s002100000363. [DOI] [PubMed] [Google Scholar]
- MUBAGWA K., FLAMENG W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc. Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- NORTON G.R., WOODIWISS A.J., McGINN R.J., LORBAR M., CHUNG E.S., HONEYMAN T.W., FENTON R.A., DOBSON J.G., MEYER T.E. Adenosine A1 receptor-mediated antiadrenergic effects are modulated by A2A receptor activation in rat heart. Am. J. Physiol. 1999;276:H341–H349. doi: 10.1152/ajpheart.1999.276.2.H341. [DOI] [PubMed] [Google Scholar]
- PALMER T.M., GETTYS T.W., STILES G.L. Differential interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J. Biol. Chem. 1995;270:16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- PARSONS M., YOUNG L., LEE J.E., JACOBSON K.A., LIANG B.T. Distinct cardioprotective effects of adenosine mediated by differential coupling of receptor subtypes to phospholipases C and D. FASEB J. 2000;14:1423–1431. doi: 10.1096/fj.14.10.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESHKIN S.J., GUERRA L., BAGORDA A., DEBELLIS L., CARDONE R., LI A.H., JACOBSON K.A., CASAVOLA V. Activation of A3 adenosine receptor induces calcium entry and chloride secretion in A6 cells. J. Membr. Biol. 2000;178:103–113. doi: 10.1007/s002320010018. [DOI] [PubMed] [Google Scholar]
- ROBINSON A.J., DICKENSON J.M. Regulation of p42/p44 MAPK and p38 MAPK by the adenosine A1 receptor in DDT1MF-2 cells. Eur. J. Pharmacol. 2001;413:151–161. doi: 10.1016/s0014-2999(01)00761-0. [DOI] [PubMed] [Google Scholar]
- SAFRAN N., SHNEYVAYS V., BALAS N., JACOBSON K.A., NAWRATH H., SHAINBERG A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol. Cell. Biochem. 2001;217:143–152. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTE G., FREDHOLM B.B. Human adenosine A1, A2A, A2B and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol. Pharmacol. 2000;58:477–482. [PubMed] [Google Scholar]
- SEIDEL M., KLINGER M., FREISSMUTH M., HÖLLER C. Activation of mitogen-activated protein kinase by A2A-adenosine receptor via a rap1-dependent and via a p21rea-dependent pathway. J. Biol. Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- SEXL V., MANCUSI G., HOLLER C., GLORIA-MAECKER E., SCHUTZ W., FREISSMUTH M. Stimulation of mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J. Biol. Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- SHNEYVAYS V., JACOBSON K.A., LI A.H., NAWRATH H., ZINMAN T., ISAAC A., SHAINBERG A. Induction of apoptosis in rat cardiocytes by A3 adenosine receptor activation and its suppression by isoproterenol. Exp. Cell Res. 2000;257:111–126. doi: 10.1006/excr.2000.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMERSCHILD H.T., KIRKEBOEN K.A. Adenosine and cardioprotection during ischemia and reperfusion – an overview. Acta Anaesthesiol. Scand. 2000;44:1038–1055. doi: 10.1034/j.1399-6576.2000.440903.x. [DOI] [PubMed] [Google Scholar]
- STRICKLER J., JACOBSON K.A., LIANG B.T. Direct preconditioning of cultured chick ventricular myocytes novel functions of cardiac adenosine A2a and A3 receptors. J. Clin. Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEDA H., MATOZAKI T., TAKADA T., NOGUCHI T., YAMAO T., TSUDA M., OCHI F., FUKUNAGA K., INAGAKI K., KASUGA M. PI3-kinase γ and protein kinase C-ζ mediate RAS-independent activation of MAP kinase by a Gi protein-coupled receptor. EMBO J. 1999;18:386–395. doi: 10.1093/emboj/18.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU H., STEIN B., LIANG B. Characterization of a stimulatory adenosine A2A receptor in adult rat ventricular myocyte. Am. J. Physiol. 1996;270:H1655–H1661. doi: 10.1152/ajpheart.1996.270.5.H1655. [DOI] [PubMed] [Google Scholar]