Abstract
This study shows for the first time the presence of interstitial cells of Cajal (ICC) and their possible role in the initiation of spontaneous excitation in the corporal tissue of the guinea-pig penis. ICC, which were identified by their c-kit immunoreactivity, were abundantly distributed in the corporal smooth muscle meshwork. Spontaneous increases in the intracellular calcium concentration ([Ca2+]i; calcium transients) were visualized in preparations loaded with the fluorescent dye fura-2. Ca transients originated from the boundary of muscle bundles and then spread throughout the meshwork (Ca waves). Ca waves were strongly suppressed by either CPA (10 μM), ryanodine (50 μM) or 2-APB (10 μM), and their synchronicity was disrupted by 18β-GA (30 μM). These results suggest that ICC in the corporal tissue may have a role as pacemakers to drive the bulk of smooth muscles, and that intracellular Ca2+ stores and gap junctions are critical for the generation of spontaneous excitation.
Keywords: Interstitial cells of Cajal (ICC), corporal tissue, calcium wave, gap junction
Introduction
Penile erection and detumescence result from the increased activity of parasympathetic and sympathetic nerves, respectively (Andersson & Wagner, 1995). There is general agreement that nitric oxide (NO) released from both autonomic nerves and endothelium is the principal mediator for the relaxation of corporal smooth muscles and erection. Detumescence predominantly results from the activation of α-adrenorecptors by neurally released noradrenaline, which contracts the corporal smooth muscles. Continuous input from sympathetic nerves and spontaneous ‘myogenic activity' may both contribute to the sustained smooth muscle tone in the flaccid penis (Andersson & Wagner, 1995).
Corporal smooth muscle strips taken from several species, including man, exhibit spontaneous contractions. These contractions rely largely on Ca2+ influx through L-type Ca2+ channels, and are inhibited either by L-type Ca2+ channel blockers or by K+ channel openers, which hyperpolarize the membrane (Holmquist et al., 1990; Christ et al., 1990; Hashitani, 2000; Hashitani et al., 2002). Underlying the contractions are spontaneous action potentials and associated Ca2+ transients (Hashitani et al., 2002). However, the origin and basis of spontaneous excitation in corporal smooth muscles is still to be elucidated.
Interstitial cells of Cajal (ICC) are now considered to play a key role in pacemaking and signal transmission in gastrointestinal tissues (Sanders, 1996; Hirst & Ward, 2003). ICC-like cells have also been identified in the urinary tract of the guinea-pig and mouse, in which spontaneous activity is generated, either by their immunoreactivity for c-kit, a cell surface marker specific for ICC, or through morphological criteria (Klemm et al., 1999; Sergeant et al., 2000; McCloskey & Gurney, 2002; Pezzone et al., 2003). Since corporal smooth muscles are also spontaneously active, it would be reasonable to speculate that ICC are involved in the generation of spontaneous excitation in corporal tissue.
ICC generate electrical slow waves which coordinate activity in the bulk of smooth muscle cells. Regardless of the varied sensitivity of slow waves for L-type Ca2+ channel blockers (Hashitani et al., 1996; Suzuki & Hirst, 1999; Yoneda et al., 2002), they are readily blocked by chemicals which disrupt the function of either intracellular Ca2+ stores or mitochondria (Hashitani et al., 1996; Fukuta et al., 2002; Yoneda et al., 2002). Consistently, the underlying currents recorded from isolated ICC are also highly sensitive to these chemicals, indicating that Ca2+ handling by intracellular Ca stores and mitochondria is crucial in generating slow waves (Ward et al., 2000; Sergeant et al., 2001). Although spontaneous excitation in corporal smooth muscles is known to be blocked by Ca2+ channel blockers, the contribution of intracellular Ca handling to their generation remains unknown.
Generally, gap junctions play a key role in signal transmission, and thus signals generated from either ICC or smooth muscles require intact gap junctions in coordinating multicellular units. Cultured corporal smooth muscles are shown to be electrically coupled to each other through gap junction channels (Christ et al., 1992). Consistently, in intact corporal smooth muscle preparations, electrical coupling between two separate cells measured using independent electrodes was blocked by 18β-glycyrrhetinic acid (18β-GA), a gap junction blocker (Hashitani, 2000). However, we have not yet elucidated how the blockade of gap junctions alters the propagation of spontaneous excitation in the corporal tissue.
In the present study, first, ICC were identified in corporal tissues of the guinea-pig, using an antibody for c-kit, a selective marker for ICC. Second, Ca waves in intact corporal tissues were visualized, and the effects of a gap junction blocker and inhibitors for the function of intracellular Ca2+ stores were examined. The possible roles of ICC in generating spontaneous excitation in the corporal tissue will be discussed.
Methods
General
The procedures described have been approved by the animal experimentation ethics committee at Nagoya City University Medical School. Male guinea-pigs weighing 400–600 g were killed by a blow to the head, followed by cervical dislocation. After removal of the bulbospongiosum muscle, which covered the bottom of the penile shaft, the tunica albuginea was slit open with a lateral incision and the corpus spongiosum was dissected free. Corpus spongiosum preparations were pinned out in a dissecting chamber and several smooth muscle layers were carefully removed, leaving a single layer of the meshwork of corporal smooth muscle. These procedures did not cause disruption of either corporeal smooth muscles or nerves (Hashitani, 2000; Hashitani et al., 2002).
Immunohistochemistry
To visualize cells expressing c-kit immunoreactivity, preparations were incubated for 1 h in physiological saline containing rat monoclonal antibodies raised against the c-kit protein (ACK-2, diluted 1 : 100, Cymbus Biotechnology Ltd, Hants, U.K.). The tissue was washed and then incubated for another 1 h in anti-rat IgG antibody labelled with a fluorescent marker (IgG-Alexa Fluor 488, diluted 1 : 100, Molecular Probes, OR, U.S.A.). After washing with physiological saline, the preparations were observed using confocal laser microscopy (LSM5 PASCAL, Zeiss, Germany).
Intracellular calcium measurements
For measurements of changes in the concentration of intracellular calcium ([Ca2+]i),preparations were pinned out on a block of Sylgard (silicone elastomer, Dow Corning Corporation, Midland, MI, U.S.A.) which had a window of some 3 mm × 3 mm in the centre. The Sylgard block was turned over and then placed at the bottom of the recording chamber, so that the preparation faced a glass coverslip. After 30 min incubation with warmed (35°C) physiological saline, spontaneous movements of the tissues were detected visually, and then the preparations were loaded with fluorescent dye, fura-2, by incubation in nominally Ca2+-free solution containing 10 μM fura-2 AM for 1 h at room temperature. After loading, the preparations were superfused with dye-free, warmed (35°C) physiological saline, at a constant flow (about 2 ml min−1) for 30 min. Preparations, loaded with fura-2, were viewed under either an oil-immersion objective (UPlanApo 40, Olympus) or a normal objective (UplanApo 10 and 20, Olympus), and were illuminated with two periods of ultraviolet light, wave lengths 340 and 380 nm, alternating at a frequency of higher than 40 Hz. The ratio of the emission fluorescence (R340/380) in a desired size of rectangular window was measured through a barrier filter of 510 nm (sampling interval 25–100 ms), using a micro-photoluminescence measurement system (ARGUS/HiSCA, Hamamatsu Photonics, Hamamatsu, Japan).
Solutions
The composition of physiological saline was (in mM): NaCl, 120; KCl, 5.9; MgCl2, 1.2; CaCl2, 2.5; NaHCO3, 15.5; KH2PO4, 1.2 and glucose, 11.5. The solution was bubbled with 95% O2 and 5% CO2, and pH was 7.2–7.3.
The drugs used were 2-aminoethoxydiphenylborate (2-APB), fura-2 AM and ryanodine (from Calbiochem-Novabiochem Ltd, San Diego, CA, U.S.A.), cyclopiazonic acid (CPA), 18β-GA, nifedipine (from Sigma, St Louis, MO, U.S.A.). Nifedipine was dissolved in 100% ethanol; 2-APB, CPA, 18β-GA and ryanodine were dissolved in dimethyl sulphoxide (DMSO). The final concentration of these solvents in the physiological saline did not exceed 1 : 1000.
Results
Identification of c-kit-positive cells in the corporal tissue
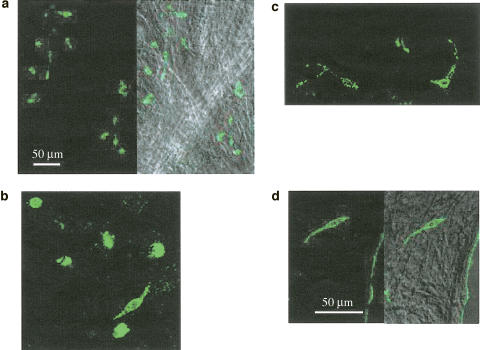
Staining using antibody for c-kit identified two different cell types in the corporal tissue (Figure 1a). Based on their morphological characteristics, these were determined to be ICC and mast cells. ICC had a spindle-shaped cell body, some 50 μm in length and less than 10 μm width (Figure 1b), and sometimes had a few processes (Figure 1c). Mast cells, which are known to be another c-kit-positive cell type (Hagger et al., 1998), had a round-shaped cell body, some 10 μm in diameter (Figure 1b). ICC were preferentially located near the boundaries of the smooth muscle meshwork rather than in the middle (Figure 1d).
Figure 1.
Identification of ICC in corporal tissue of the guinea-pig penis. ICC identified using ACK2 antibody against c-kit labelled with Alexa 488 are seen in the corporal tissue of the guinea-pig (a). The left panel shows fluorescent images of ICC and the right panel shows the image superimposed on the plane image of smooth muscle bundles. Two distinct populations of cells expressed c-kit immunoreactivity (b). Spindle-shaped cells were identified as ICC, while round-shaped cells were considered to be mast cells. ICC had a spindle-shaped cell body, some 50 μm in length and less than 10 μm in width, and sometimes had a few processes (c). ICC are preferentially located near the boundary of the smooth muscle meshwork (d). The scale bar in (a) refers to a. The scale bar in (d) refers to b–d.
Propagation of Ca waves in corporal smooth muscles
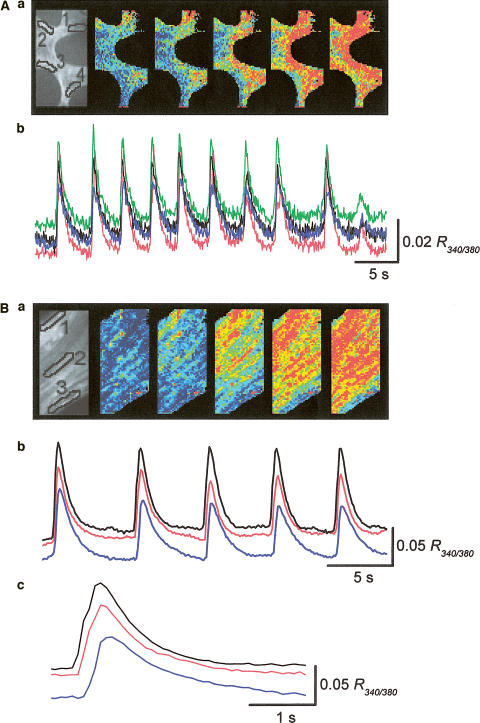
When changes in [Ca2+]i were visualized in the corporal smooth muscle meshwork, increases in [Ca2+]i generally arose from either single or multiple sites near the boundary of muscle bundles and then spread throughout the meshwork (Ca waves). Figure 2Aa shows the propagation of Ca waves originating from two different sites. When changes in [Ca2+]i were recorded from separate areas with a distance between areas of some 100 μm, synchronous Ca signals were recorded in all areas (Figure 2Ab). Figure 2Ba shows a Ca wave that originated from one boundary of the muscle bundle and spread to the other boundary (Figure 2Ba). When changes in [Ca2+]i were recorded from separate areas with a distance between areas of some 40 μm, Ca signals concurrently occurred in all areas (Figure 2Bb). However, on a fast time scale, there was a delay of several hundred ms between the peaks of the Ca signal recorded from one boundary and that from the other boundary (Figure 2Bc). The estimated conduction velocities of Ca waves ranged between 0.44 and 1.2 mm s−1 (mean 0.73±0.23 mm s−1, n=8), and were not very different from the values reported in gastrointestinal and urinary tissues (Stevens et al., 1999; Hashitani et al., 2001).
Figure 2.
Calcium waves in the corporal smooth muscle meshwork. (A) Changes in [Ca2+]i were simultaneously recorded from separate areas located with a separation between each area of some 100 μm. The series of frames show Ca waves originating from two separate sites and spreading throughout the tissue within the imaged area (Aa). When changes of [Ca2+]i were simultaneously recorded from four different areas, transient increase in [Ca2+]i occurred synchronously in all areas (Ab). (B) Changes in [Ca2+]i were simultaneously recorded from three separate areas located on the muscle bundle, with a separation between each area of some 40 μm. The series of frames demonstrate a Ca wave originating from the boundary of the muscle bundle and spreading to the other boundary (Ba). When changes of [Ca2+]i were simultaneously recorded from each area, synchronous increase in [Ca2+]i occurred in all areas (Bb). Ca2+ transients recorded from the areas had almost identical time courses, but a delay of some 200 ms was observed between the rising phases of Ca transients in both boundaries (Bc). Black, red and blue traces were recorded from areas 1, 2 and 3, respectively.
Role of gap junctions and intracellular Ca stores in Ca waves
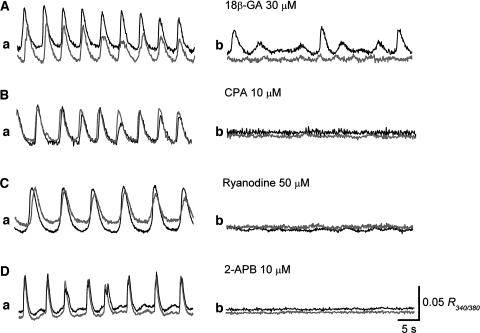
To investigate the role of gap junctions in the propagation of Ca waves, the effects of 18β-GA, a gap junction blocker (Hashitani, 2000), were examined. In control conditions, synchronous Ca transients were recorded from two separate areas located some 100 μm apart (Figure 3Aa). 18β-GA (30 μM) reduced the amplitude of Ca transients recorded from the area in which the leading Ca transients were generated, while almost completely suppressing the follower Ca signals (Figure 3Ab), indicating that cell-to-cell communication was effectively disrupted by 18β-GA.
Figure 3.
Role of gap junctions and intracellular Ca stores in the generation of Ca waves. (A) In control solution, synchronous Ca waves were recorded from two areas separated by a distance of some 100 μm (Aa). 18β-GA (30 μM) reduced the amplitude of Ca transients in the leading area, while almost completely suppressing Ca transients in the follower area (Ab). (B) In control solution, Ca waves occurred synchronously in two separate areas (Ba). After some 10 min application of CPA (10 μM), Ca waves in both areas were almost completely suppressed (Bb). (C) In a different preparation, 20 min of application of ryanodoine (50 μM) strongly inhibited Ca waves, which had synchronously arisen from two separate areas (Cb). (D) 2-APB (10 μM) abolished Ca waves recorded from two areas in some 10 min (Db). The scale bar on the right of Db refers to all traces.
As shown previously, Ca transients recorded from corporal smooth muscles were readily abolished by nifedipine (1 μM, n=4), indicating that they resulted from Ca influx through L-type Ca channels (Hashitani et al., 2002). To investigate the cellular basis of Ca transients in corporal tissues further, the role of intracellular Ca stores in the generation Ca waves was examined.
In control conditions, synchronous Ca transients were recorded from separate areas at a distance of some 100 μm apart (Figure 3Ba). CPA (10 μM), an inhibitor of the sarcoplasmic reticulum Ca2+ ATPase, reduced the amplitude of Ca transients and almost completely suppressed them in about 10 min (Figure 3Bb). Ryanodine (50 μM), a blocker of Ca-induced Ca release from the store, also reduced the amplitude of Ca transients and strongly suppressed them in some 20 min (Figure 3Cb). 2-APB (10 μM), a known blocker for InsP3-induced Ca release (Maruyama et al., 1997), reduced the frequency of Ca transients and prevented their generation in about 10 min (Figure 3Db).
Discussion
In the present study, ICC have for the first time been identified in corporal tissue of the guinea-pig penis using an antibody for c-kit, a specific marker of ICC. Spontaneous Ca signals originated from the boundary of muscle bundles and spread throughout the muscle meshwork to generate synchronous Ca waves. The Ca waves were blocked by CPA, ryanodine and 2-APB, suggesting that interplay between the receptors for InsP3 and ryanodine on intracellular Ca store membrane is crucial in generating Ca waves. The synchronicity of Ca waves was disrupted by 18β-GA, indicating a key role of gap junctions in signal transmission in the corporal tissue.
ICC can be identified from their ultrastructural characteristics with electron microscopy, but since these cells in both the gastrointestinal and urinary tracts also express immunoreactivity for c-kit, a surface tyrosin kinase receptor (Dickens et al., 1999; McCloskey & Gurney, 2002; Yoneda et al., 2002), staining using anti-c-kit antibody is the most convenient way to identify ICC. In the corporal tissue, two populations of c-kit positive cells were identified. The other cell type known to express c-kit immunoreactivity is the mast cell (Hagger et al., 1998). Based on morphological criteria, spindle-shaped cells with some branches were considered to be ICC, while round-shaped cells were considered to be mast cells. In the canine corpus cavernosum, mast cells have been identified by toluidine blue staining (Iwamoto et al., 2001). ICC are now believed to play a key role in pacemaking and signal transmission in the gastrointestinal and urinary tracts (Sanders, 1996; Hirst & Ward, 2003), and this may also be the case in the corporal tissue. Mast cells are also known to release a number of biologically active substances such as prostaglandins and histamine (Ennis et al., 1984). Furthermore, they are often closely associated with nerves and are possibly themselves innervated (Newson et al., 1983; Stead et al., 1989). It has indeed been reported that spontaneous contractions in human corporal tissues are markedly reduced by indomethacin, suggesting an involvement of cyclo-oxygenase products in the generation of the contractions (Christ et al., 1990). Therefore, not only ICC but also mast cells may play an important role in pacemaking or the transmission of spontaneous excitation in the corporal tissues.
Spontaneous contractions in corporal smooth muscles result from action potentials and associated Ca transients, indicating the critical role of Ca influx through L-type Ca channels (Hashitani, 2000; Hashitani et al., 2002). In the present study, Ca waves in corporal smooth muscles of the guinea-pig penis were inhibited by chemicals, which disrupt the function of intracellular Ca stores. Unlike action potentials, slow waves originating from ICC in gastrointestinal tissues are generally insensitive to L-type Ca channel blockers (Dickens et al., 1999). In the mouse colon, however, slow waves were blocked by both Ca antagonists and blockers for the function of Ca stores, indicating an interplay between L-type Ca channels and Ca release from the stores (Yoneda et al., 2002). In the urethra, the plateau phase, but not the initial component of slow waves recorded in multicellular preparations, was blocked by nifedipine (Hashitani et al., 1996). Therefore, although ICC are widely distributed in visceral smooth muscle tissues, the basic mechanisms involved in generating spontaneous excitation appear to vary between tissues. In corporal tissue, as in the case of the mouse colon, both the activation of L-type Ca channels and the Ca release from intracellular stores may be required in generating spontaneous excitation.
Interestingly, both 2-APB and ryanodine strongly suppressed the Ca waves. It is generally agreed that Ca2+ handling by intracellular stores and mitochondria play a critical role in the generation of slow waves (Ward et al., 2000; Sergeant et al., 2001; Fukuta et al., 2002). Accumulated evidence suggests a central role of InsP3 receptors (Suzuki et al., 2000; Van Helden et al., 2000), while the role of ryandoine receptors appears to be controversial. In gastrointestinal tissues, ryanodine had almost no effects on spontaneous activity (Liu et al., 1995; Van Helden et al., 2000; Fukuta et al., 2002). Conversely, spontaneous currents recorded from isolated ICC in the urethra were readily blocked by ryanodine (Sergeant et al., 2001). In corporal tissues, there seems to be a complex interplay between L-type Ca channels, InsP3 and ryanodine receptors in the generations of spontaneous excitation.
18β-GA, a gap junction blocker, almost completely suppressed Ca signals in the follower area, while only reducing the amplitude of Ca signals in the leading area, suggesting that 18β-GA inhibited signal transmission through gap junctions rather than the generation of Ca signals. Both cultured and intact corporal smooth muscles have been shown to be coupled to each other via gap junctions (Christ et al., 1992; Hashitani, 2000). Although we do not have any information about the coupling between ICC and corporal smooth muscles, we have confirmed that the transmission of both electrical and calcium signals requires intact gap junctions.
In conclusion, ICC identified using their immunoreactivity for c-kit may be crucial in pacemaking and signal transmission in corporal tissue, and thus contribute to the corporal muscle tone to maintain detumescence in the penis. ICC are also thought to be involved in neuromuscular transmission, including inhibitory transmission mediated by NO (Ward et al., 1998; Hirst et al., 2002); therefore, they may also play an important role in the NO-mediated relaxation of corporal smooth muscles, which appears to be fundamental for the penile erection. Further investigation of the physiological role of corporal ICC might enable advances in the pharmacological treatment of erectile dysfunction.
Acknowledgments
We wish to thank Professor A.F. Brading for her critical reading of the manuscript. This work was supported by a grant from the Japan Society for the Promotion of Science (No. 15591704) to H.H.
Abbreviations
- 2-APB
2-aminoethoxydiphenylborate
- 18β-GA
18β-glycyrrhetinic acid
- [Ca2+]i
intracellular concentration of free calcium ions
- CICR
calcium-induced calcium release
- CPA
cyclopiazonic acid
- DMSO
dimethyl sulphoxide
- ICC
interstitial cells of Cajal
- InsP3
inositol 1,4,5-trisphosphate
References
- ANDERSSON K.-E., WAGNER G. Physiology of penile erection. Physiol. Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., MORENO A.P., MELMAN A., SPRAY D.C. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am. J. Physiol. 1992;263:C373–C383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., MAAYANI S., VALCIC M., MELMAN A. Pharmacological studies of human erectile tissue: characteristics of spontaneous contractions and alterations in alpha-adrenoceptor responsiveness with age and disease in isolated tissues. Br. J. Pharmacol. 1990;101:375–381. doi: 10.1111/j.1476-5381.1990.tb12717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKENS E.J., HIRST G.D.S., TOMITA T. Identification of rhythmically active cells in guinea-pig stomach. J. Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS M., BARROW S.E., BLAIR I.A. Prostaglandin and histamine release from stimulated rat peritoneal mast cells. Agents Actions. 1984;14:397–400. doi: 10.1007/BF01973836. [DOI] [PubMed] [Google Scholar]
- FUKUTA H., KITO Y., SUZUKI H. Spontaneous electrical activity and associated changes in calcium concentration in guinea-pig gastric smooth muscle. J. Physiol. 2002;540:249–260. doi: 10.1113/jphysiol.2001.013306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGGER R., GHARAIE S., FINLAYSON C., KUMAR D. Regional and transmural density of interstitial cells of Cajal in human colon and rectum. Am. J. Physiol. 1998;275:G1309–G1316. doi: 10.1152/ajpgi.1998.275.6.G1309. [DOI] [PubMed] [Google Scholar]
- HASHITANI H. Neuroeffector transmission to different layers of smooth muscle in the rat penile bulb. J. Physiol. 2000;524:549–563. doi: 10.1111/j.1469-7793.2000.t01-2-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., FUKUTA H., DICKENS E.J., SUZUKI H. Cellular mechanisms of nitric oxide-induced relaxation of corporal smooth muscle in the guinea-pig. J. Physiol. 2002;538:573–581. doi: 10.1113/jphysiol.2001.013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., FUKUTA H., TAKANO H., KLEMM M., SUZUKI H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea-pig urinary bladder. J. Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHITANI H., VAN HELDEN D.F., SUZUKI H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br. J. Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G.D.S., DICKENS E.J., EDWARDS F.R. Pacemaker shift in the gastric antrum of guinea-pigs produced by excitatory vagal stimulation involves intramuscular interstitial cells. J. Physiol. 2002;541:917–928. doi: 10.1113/jphysiol.2002.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST G.D.S., WARD S.M. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J. Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQUIST F., ANDERSSON K.-E., FOVAEUS M., HEDLUND H. K+-channel openers for relaxation of isolated penile erectile tissue from rabbit. J. Urol. 1990;144:146–151. doi: 10.1016/s0022-5347(17)39398-9. [DOI] [PubMed] [Google Scholar]
- IWAMOTO Y., SONG K., TAKAI S., YAMADA M., JIN D., SAKAGUCHI M., UEDA H., KATSUOKA Y., MIYAZAKI M. Multiple pathways of angiotensin I conversion and their functional role in the canine penile corpus cavernosum. J. Pharmacol. Exp. Therap. 2001;298:43–48. [PubMed] [Google Scholar]
- KLEMM M.F., EXINTARIS B., LANG R.J. Identification of the cells underlying pacemaker activity in the guinea-pig upper urinary tract. J. Physiol. 1999;519:867–884. doi: 10.1111/j.1469-7793.1999.0867n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L.W., THUNEBERG L., HUIZINGA J.D. Cyclopiazonic acid, inhibiting the endoplasmic reticulum calcium pump, reduces the canine colonic pacemaker frequency. J. Pharmacol. Exp. Therap. 1995;275:1058–1068. [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- MCCLOSKEY K.D., GURNEY A.M. Kit positive cells in the guinea pig bladder. J. Urol. 2002;168:832–836. [PubMed] [Google Scholar]
- NEWSON B., DAHLSTROM A., ENERBACK L., AHLMAN H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983;10:565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- PEZZONE M.A., WATKINS S.C., ALBER S.M., KING W.E., DE GROAT W.C., CHANCELLOR M.B., FRASER M.O. Identification of c-kit-positive cells in the mouse ureter: the interstitial cells of Cajal of the urinary tract. Am. J. Physiol. 2003;284:F925–G929. doi: 10.1152/ajprenal.00138.2002. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- SERGEANT G.P., HOLLYWOOD M.A., MCCLOSKEY K.D., MCHALE N.G., THORNBURY K.D. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am. J. Physiol. 2001;280:C1349–C1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- SERGEANT G.P., HOLLYWOOD M.A., MCCLOSKEY K.D., THORNBURY K.D., MCHALE N.G. Specialised pacemaking cells in the rabbit urethra. J. Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEAD R.H., DIXON M.F., BRAMWELL N.H., RIDDELL R.H., BIENENSTOCK J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- STEVENS R.J., WEINERT J.S., PUBLICOVER N.G. Visualization of origins and propagation of excitation in canine gastric smooth muscle. Am. J. Physiol. 1999;277:C448–C460. doi: 10.1152/ajpcell.1999.277.3.C448. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., HIRST G.D.S. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. J. Physiol. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI H., TAKANO H., YAMAMOTO Y., KOMURO T., SAITO M., KATO K., MIKOSHIBA K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J. Physiol. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HELDEN D.F., IMTIAZ M.S., NURGALIYEVA K., VON DER WEID P., DOSEN P.J. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J. Physiol. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., MORRIS G., REESE L., WANG X.Y., SANDERS K.M. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- WARD S.M., ORDOG T., KOH S.D., BAKER S.A., JUN J.Y., AMBERG G., MONAGHAN K., SANDERS K.M. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YONEDA S., TAKANO H., TAKAKI M., SUZUKI H. Properties of spontaneously active cells distributed in the submucosal layer of mouse proximal colon. J. Physiol. 2002;542:887–897. doi: 10.1113/jphysiol.2002.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]