Abstract
Since the roles of thromboxane A2 (TXA2), prostacyclin (PGI2) and 8-isoprostane F2α in mediating vascular O2•− formation and its relation to adult respiratory distress syndrome (ARDS) is unknown, the effects of these eicosanoids on the expression of gp91phox (catalytic subunit of NADPH oxidase) and O2•− release from cultured pig pulmonary artery (PA) segments, PA vascular smooth muscle cells (PAVSMCs) and PA endothelial cells (PAECs) were investigated.
PA segments, PAVSMCs and PAECs were incubated with the TXA2 analogue, U46619, (±LPS, tumour necrosing factor-alpha (TNF-α) or IL-1α), 8-isoprostane F2α and±iloprost (a stable PGI2 analogue) for 16 h. The formation of superoxide dismutase-inhibitable O2•− was then measured spectrophotometrically and gp91phox expression assessed using Western blotting. In parallel experiments, whole PA segments were treated with LPS, TNF-α and IL-α after which time TXA2, PGI2, PGF2α and 8-isoprostane F2α formation was measured using enzyme-linked immunoassays.
U46619, PGF2α and 8-isoprostane F2α promoted the formation of O2•− in PA segments, PAVSMCs and PAECs, an effect inhibited by diphenyleneiodonium and apocynin (both NADPH oxidase inhibitors) and upregulated the expression of gp91phox in PAECs and PAVSMCs. These effects were augmented by LPS, TNF-α and IL-1α but inhibited by iloprost. Under identical incubation conditions, IL-1α, LPS and TNF-α all induced an increase in the formation of TXA2, PGF2α and 8-isoprostane F2α but reduced the concomitant formation of PGI2.
These data demonstrate that LPS and cytokines influence the relative balance of TXA2, PGI2, PGF2α and 8-isoprostane F2α in pig PA, which in turn alter NADPH oxidase expression and O2•− formation. These novel findings have implications in devising effective strategies for treating ARDS.
Keywords: Superoxide, thromboxane, prostacyclin, isoprostane, pig pulmonary artery
Introduction
Oxidative stress (OS) plays a central role in the aetiology of adult respiratory distress syndrome (ARDS) (Chabot et al., 1998), a condition characterised by a time-dependent worsening of intrapulmonary inflammation and hypertension (Weinacker & Vaszar, 2001). Principal among the reactive oxygen species (ROS) generated by OS is superoxide (O2•−), which reacts with nitric oxide (NO) to produce peroxynitrite (ONOO−), promoting not only vasoconstriction but also the adhesion of leucocytes and platelets (Stuart-Smith & Jeremy, 2001).
In sepsis-induced ARDS, endotoxins trigger the adhesion of platelets and leucocytes to the pulmonary vascular endothelium, which then release a battery of cytokines including tumour necrosing factor-alpha (TNF-α), interleukins (ILs) and thromboxane A2 (TXA2) (Jeremy et al., 1994b). TXA2 is a potent vasoconstrictor and promoter of platelet aggregation and its involvement in ARDS is well established (Jeremy et al., 1994b; Ermert et al., 2000a,2000b). Other vasoconstrictors, such as angiotensin II and endothelin-1 have also been shown to upregulate NADPH oxidase that generates O2•− (Sorescu et al., 2001). OS also promotes the formation of isoprostanes, which exert similar effects as TXA2 (Jourdan et al., 1997; Minuz et al., 1998; Audoly et al., 2000; Ekmekcioglu et al., 2002; Morrow & Roberts, 2002; Roberts & Morrow, 2002), and have been implicated in the pathophysiology of ARDS (Jourdan et al., 1997; Minuz et al., 1998; Audoly et al., 2000; Ekmekcioglu et al., 2002; Morrow & Roberts, 2002; Roberts & Morrow, 2002). It is reasonable to suggest that such a TXA2/isoprostane-mediated effect may play an additional role in the aetiology of ARDS.
In contrast to TXA2, vascular tissues also generate the protective prostanoid, prostacyclin (PGI2) a vasodilator and inhibitor platelet and leucocyte adhesion (Jeremy et al., 1997; Ullrich et al., 2001). The importance of PGI2 in ARDS is exemplified by the therapeutic benefits of inhalational PGI2 administration to patients with ARDS (Lowson, 2002). Cytokines also augment the formation of TXA2 but reduce the formation of PGI2 in vascular tissue (Jeremy et al., 1994b). Whether PGI2 modulates O2•− formation or NADPH oxidase expression is also unknown.
In order to study this area further, the effect of endotoxin and cytokines on the formation of PGI2, TXA2, PGF2α and 8-isoprostane F2α by porcine pulmonary arteries was investigated. The effect of TXA2 analogue, U46619, 8-isoprostane F2α and PGF2α on O2•− formation and the expression of gp91phox, an active catalytic subunit of NADPH oxidase (Sorescu et al., 2001), was also investigated. The effect of the PGI2 analogue, iloprost, on O2•− formation and the expression of gp91phox in response to cytokines, endotoxin, U46619, 8-isoprostane F2α and PGF2α was then studied.
Methods
Dissection and incubation of pulmonary arteries
Lungs were obtained from White Landrace male pigs of body weight ranging from 20 to 35 kg. All animals were given humane care in compliance with the rules and regulations of Bristol University and the UK Home Office. Pigs were anaesthetised with an intravenous injection of ketamine hydrochloride (10 mg kg−1; Ketaset Injection, Fort Dodge Animal Health, Southampton, U.K.) and inhaled halothane (1–2% in oxygen), exsanguinated and lungs removed. Pulmonary arteries (PA; 3–4 mm diameter) were dissected out and placed in Dulbecco's minimum essential medium with Glutamax-1 (DMEM; GibcoBRL, Paisley, Scotland) and cut into 2 mm2.
Pulmonary artery vascular smooth muscle cells (PAVSMCs) and pulmonary artery endothelial cells (PAECs) were prepared as previously described (Chaudhari et al., 1990; Southgate et al., 1992). PAECs were grown in an endothelial cell growth medium (PromoCell, Heidelberg, Germany) at 37°C in a 95% air–5% CO2 incubator. PAVSMCs were maintained in DMEM (containing 10% foetal-calf serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin) at 37°C in a 95% air–5% CO2 incubator. Subconfluent cultures of pulmonary VSMCs were growth-arrested by washing in sterile phosphate-buffered saline (PBS, GibcoBRL) and incubating in a quiescing medium (serum-free DMEM supplemented with 0.5% lactalbumin hydrolysate, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin) for 72 h.
Effect of LPS and cytokines on eicosanoid formation by intact PA segments
PA segments were incubated in serum-free DMEM (supplemented with 100 U ml−1 of penicillin and 100 μg ml−1 of streptomycin) and containing LPS (1 μg ml−1; Escherichia coli;026:B6; Sigma Chemical Co., Poole Dorset, U.K.), human recombinant IL-1α (10 ng ml−1; R&D Systems, Abingdon, U.K.) or human recombinant TNF-α (10 ng ml−1; R&D Systems) for 16 h at 37°C in a 95% air–5% CO2 incubator. It was essential to use serum-free conditions since serum contains large amounts of platelet and leucocyte release substances, including cytokines and eicosanoids, which in turn would have rendered responses to cytokines and eicosanoids indiscernible in the present study.
After washing, segments were placed in Hank's balanced salt solution (HBSS; GibcoBRL) and eicosanoid formation were stimulated with calcium ionophore A23187 (10 μM final; Sigma Chemical Co.) since basal eicosanoid release was below the lower limit of detection of the assay. A23187 elicits an increase in intracellular calcium through the formation of artificial calcium channels. Ca2+ then activates phosholipase A2, which releases arachidonic acid from endogenous phospholipid stores (Jeremy et al., 1994a). Arachidonic acid is then converted to different eicosanoids by cyclooxygenase and different synthesising enzymes, including PGI2 and TXA2 synthase (Jeremy et al., 1994a).
After incubation for 30 min at 37°C, supernatants were removed and aliquots taken for the measurement of TXA2 (as TXB2) and PGI2 (as 6-keto-PGF1α), 8-isoprostane F2α and PGF2α (Alexis Corporation, U.K.) using enzyme-linked immunoassay kits. The roles of O2•− and NO were explored using apocynin (1 μM; Sigma Chemical Co. an inhibitor of NADPH oxidase (Stolk et al., 1994)) diphenyleneiodonium chloride (DPI; 10 μM; Sigma Chemical Co.; another NADPH oxidase inhibitor (Griendling et al., 1994)), allopurinol (100 μM; Sigma Chemical Co.; a xanthine oxidase inhibitor (Greene & Paller, 1992), rotenone (10 μM; Sigma Chemical Co.; an electron transfer chain inhibitor (Meier et al., 1989)), L-NAME (100 μM; Sigma Chemical Co.; nitric oxide synthase inhibitor (Rees et al., 1990)) and aspirin (100 μM; Sigma Chemical Co.; a cyclooxygenase inhibitor (Tate et al., 1984)).
Effect of U46619 and 8-isoprostane F2α (±LPS, TNF-α or IL-1α) on O2•− formation
PAVSMCs, PAECs or PA segments (±endothelium) were incubated with the TXA2 analogue, U46619 or 8-isoprostane F2α (±LPS, TNF-α or IL-1α ) for 16 h at 37°C in a 95% air–5% CO2 incubator. In order to determine the source of the O2•−, segments or cells were incubated with DPI, rotenone, L-NAME, aspirin or allopurinol for 1 h prior to the measurement of O2•− using ferricytochrome c reduction (Muzaffar et al., 2003). Following incubation, segments or cells were equilibrated in DMEM without phenol red for 10 min at 37°C in a 95% air–5% CO2 incubator (Heraeus, Hera Cell, Kandro Laboratory Products, Germany). In all, 20 μM horseradish cytochrome c (Sigma Chemical Co.) with or without 500 U ml−1 copper–zinc superoxide dismutase (SOD; Sigma Chemical Co.) was added and incubated at 37°C in a 95% air–5% CO2 incubator for an hour. The reaction medium was removed and reduction of cytochrome c determined at 550 nm in an anthos Lucy 1 spectrometer (Lab-tech International, Ringmer, East Sussex, U.K.) and converted to nanomoles of O2•−, using ΔE550 nm = 21.1 mM−1 cm−1 as the extinction coefficient. The reduction of cytochrome c that was inhibitable with SOD reflected actual O2•− release. Segments were blotted, dried and weighed, data being expressed as nanomoleses of O2•− mg tissue−1 h−1. Cells were rinsed in PBS, lysed with 0.1% v v–1 Triton X-100 and total protein content measured using BCA-protein assay kit (Pierce, Rockford, IL, U.S.A.). The results were expressed as micromoles of O2•− mg protein−1 h−1.
Effect of iloprost on LPS-, cytokine- and U46619-induced O2•− formation and gp91phox expression
PAVSMCs or PAECs were seeded, quiesced and incubated with the TXA2 analogue, U46619 (10 nM), PGF2α (100 nM), 8-isoprostane F2α (100 nM), LPS (1 μg ml−1), IL-1α (10 ng ml−1) and TNF-α (10 ng ml−1), alone and in combination for 16 h and with or without iloprost (100 ng ml−1; Schering, Berlin, Germany). Cells were then washed three times in PBS and O2•− formation, and then measured using the cytochrome c assay as described above. In order to determine whether iloprost possesses a direct O2•− quenching capacity, 100 ng ml−1 iloprost was incubated with xanthine (100 μM)/xanthine oxidase (0.15 U ml−1) mixture (Sigma Chemical Co.), which generates a steady flux of superoxide radicals (Greene & Paller, 1992), and assayed for O2•− formation using identical conditions as above.
For Western analysis, cells were lysed with Tris buffer (50 mM pH 7.4) containing 1% v v–1 Triton X-100, EDTA (10 mM), PMSF (1 mM) pepstatin (0.05 mM) and leupeptin (0.2 mM). Extracts were boiled at a 1 : 1 ratio with Tris (50 mM; pH 6.8 containing 4% w v–1 sodium dodecyl sulphate; 10% v v–1 glycerol; 4% v v–1 2-mercaptoethanol; 2 mg ml−1 bromophenol blue). Samples of equal protein (100 μg) were loaded onto 12% Tris–glycine sodium dodecyl sulphate gels and separated by electrophoresis. After transfer to nitrocellulose, the blots were primed with a specific human antineutrophil gp91phox antibody (2.5 μg ml−1 final concentration) raised in mouse (a kind gift from Professor D. Roos, CLB, Amerstdam, The Netherlands). The blots were then incubated with goat antimouse immunoglobulin (Dako, Cambridgeshire, U.K.) conjugated to horseradish peroxidase (1 : 1000 dilution) and developed by enhanced chemiluminescence (Amersham International). Rainbow markers (14–220 kDa; Amersham) were used for molecular weight determination.
Data analysis
Data are expressed as mean±s.e.m. and n indicates the number of animals used. Student's unpaired t-test or one-way factorial ANOVA was used to determine the difference in the data. A P-value of less than 0.05 was considered statistically significant. Multiple group comparisons were made using one-way ANOVA.
Results
Effect of LPS and cytokines on eicosanoid formation
In PA segments, LPS, IL-1α and TNF-α elicited a statistically significant increase in the formation of TXA2 PGF2α and 8-isoprostane F2α but a statistically significant decrease in PGI2 formation (Figure 1). The amount of eicosnoids produced was substantial and all well above the lower limits of detection of the ELISAs. The PGI2 : TXA2 ratio was markedly altered from 10 : 1 in controls to the following in treated tissues: LPS, 2.4 : 1; IL-1α, 2.1 : 1; TNF-α, 1.3 : 1. The ratio of PGF2α to 8-isoprostane F2α, both in control and treated segments was 10 : 1 (Figure 1).
Figure 1.
Effect of LPS (1 μg ml−1), IL-1α (10 ng ml−1) and TNF-α (10 ng ml−1) on the formation of: (a) prostacyclin (PGI2) [as 6 keto-PGF1α], (b) TXA2 [as TXB2], (c) PGF2α and (d) 8-isoprostane F2α [8-IPF2α] stimulated with calcium ionophore A23187 by intact pulmonary artery (PA) segments, following a 16-h incubation. Data = mean (pg eicosanoid –1mg tissue –1minute–1)±s.e.m.; n=6. *P<0.05; comparing treated with (untreated) controls.
Effect of eicosanoids on O2•− formation
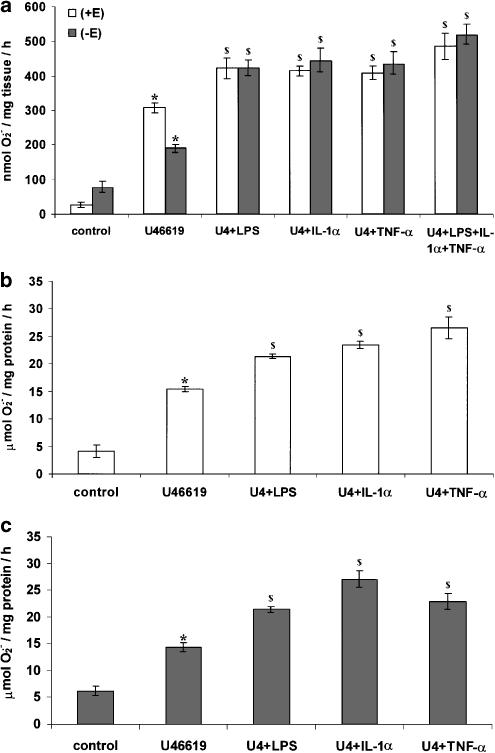
U46619 promoted a statistically significant increase in the formation of O2•− from PA segments compared to controls (Figure 2). Removal of the endothelium caused a statistically significant reduction in the formation of O2•− compared to segments in which the endothelium was intact (Figure 2a). In both PAECs and PAVSMCs, U46619 promoted a statistically significant increase in the formation of O2•− in a concentration-dependent manner (Figure 2b and 2c). Following a 16-h incubation, the combination of U46619 with LPS, IL-1α or TNF-α, potentiated the formation of O2•− in PA segments, PAVSMCs and PAECs (Figure 3). Following a 16-h incubation, U46619-stimulated O2•− formation was inhibited by DPI, apocynin and L-NAME but not by allopurinol or rotenone in PA segments, PAVSMCs and PAECs (Figure 4).
Figure 2.
Effect of U46619 on O2•− formation by (a) whole pig PA segments (with [+E] or without [–E] endothelium), (b) cultured PAVSMCs and (c) cultured PAECs following a 16-h incubation. Data = mean±s.e.m.; n=6. *P<0.05; comparing treated with untreated controls. $P< 0.05; comparing with endothelium and without endothelium (in PA segments (a) only).
Figure 3.
Effect of 10 nM U46619 (U4) in combination with LPS (1 μg ml−1), IL-1α (10 ng ml−1) or TNF-α (10 ng ml−1) on SOD-inhibitable O2•− formation by: (a) pig pulmonary arterial segments with (+E) and without (–E) endothelium, (b) cultured PAECs and (c) PAVSMCs following a 16-h incubation. Data = mean±s.e.m.; n=6. *P<0.05; comparing treated with controls. $P<0.05; comparing combinations with U46619 alone.
Figure 4.
Effect of DPI (10 μM), apocynin (1 μM), allopurinol (100 μM), rotenone (10 μM) or L-NAME (100 μM) on U46619 (10 nM)-stimulated O2•− formation by: (a) PA segments (with and without endothelium), (b) PAVSMCs and (c) PAECs. Data = mean±s.e.m.; n=6. $P<0.05; comparing basal to U46619-induced levels in the absence of inhibitors. *P< 0.05; significantly inhibited compared to U46619-treated segments or cells.
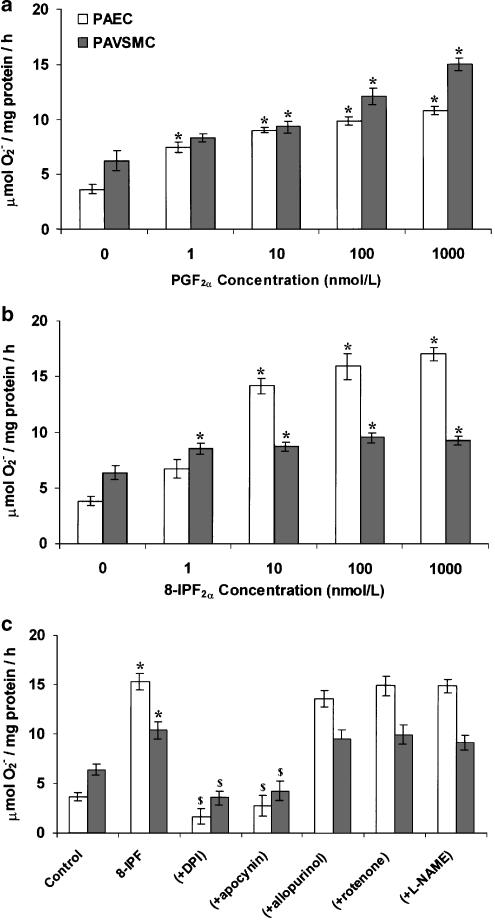
Similar effects as U46619 on the formation of O2•− were observed with 8-isoprostane F2α and PGF2α in PAECs and PAVSMCs (Figure 5). O2•− formation in response to 8-isoprostane F2α was inhibited by DPI and apocynin but not by rotenone or, L-NAME or allopruinol (Figure 5c), indicating that NADPH oxidase mediates this effect.
Figure 5.
Effect of (a) PGF2α (b) 8-isoprostane F2α [8-IPF2α] and (c) 8-IPF2α (±10 μM DPI, 1 μM apocynin, 100 μM allopurinol, 10 μM rotenone or 100 μM L-NAME) on SOD-inhibitable O2•− formation by cultured PAVSMCs or cultured PAECs following a 16-h culture. Data = mean±s.e.m.; n=6. *P<0.05; comparing treated with controls. $P< 0.05; significantly inhibited compared to 8-IPF2α-treated cells only.
The formation of 8-isoprostane F2α was inhibited by the aspirin, DPI and L-NAME but not by allopurinol (Figure 6). In contrast, PGF2α formation was only inhibited by aspirin (Figure 6).
Figure 6.
Effect of aspirin (100 μM), DPI (10 μM), L-NAME (100 μM) and allopurinol (100 μM) on PGF2α and 8-isoprostane F2α [8-IPF2α] formation induced with (a) 1 μg ml−1 LPS, (b) 10 ng ml−1 IL-1α or (c) 10 ng ml−1 TNF-α. Data = mean±s.e.m.; n=6. $P<0.05; comparing basal to LPS- or cytokine-induced levels in the absence of inhibitors. *P<0.05; significantly inhibited compared to LPS-/cytokine-treated only.
Iloprost (100 ng ml−1) inhibited the formation of O2•− induced not only by U46619 but also by LPS, IL-1α, TNF-α following a 16-h incubation with PAECs and PAVSMCs (Figure 7). Iloprost, at the highest concentration studied (100 ng ml−1), did not directly quench O2•− generated by xanthine/xanthine oxidase. The O2•− generated by xanthine/xanthine oxidase system in the absence of iloprost was 900±30.6 nmol h–1 and in the presence of iloprost was 889±75.9 nmol h–1, a difference that was not statistically significant. The basal value in the absence of xanthine oxidase was 261±40.5 nmol h–1.
Figure 7.
Effect of iloprost (100 ng ml−1) on O2•− formation by cultured PAVSMCs and PAECs derived from pig PA after a 16-h incubation with (a) 1 μg ml−1 LPS, (b) 10 ng ml−1 IL-1α, (c) 10 ng ml−1 TNF-α or (d) 10 nM U46619. Data = mean±s.e.m., n=6. *P< 0.05; comparing controls to LPS, cytokine or U46619 induced levels in the absence of iloprost. $P<0.05; comparing LPS, cytokines or U46619 treated in the presence of iloprost to those in the absence of iloprost.
Effect of eicosanoids (±LPS, TNF-α or IL-1α) on gp91phox expression
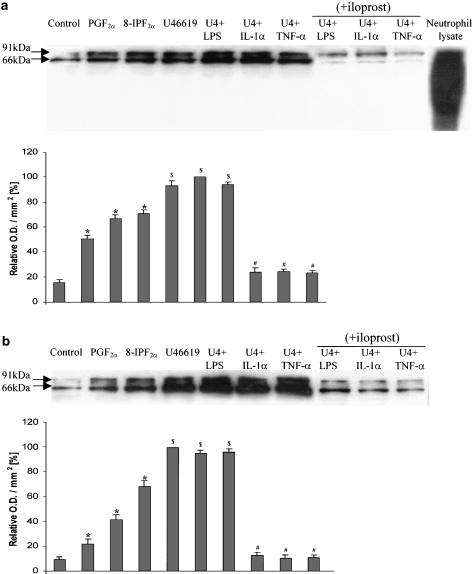
Following a 16-h incubation, the protein expression of gp91phox, the active catalytic subunit of NADPH oxidase was upregulated by U46619, PGF2α and 8-isoprostane F2α in PAECs and PAVSMCs (Figure 8). The combination of U46619 with LPS, IL-1α or TNF-α, further augmented gp91phox protein expression in PAECs and PAVSMCs (Figure 8). Coincubation with iloprost over the 16-h incubation caused a statistically significant inhibition of gp91phox expression (Figure 8).
Figure 8.
Western analysis of NAD(P)H oxidase in (a) PAECs (b) PAVSMCs using a monoclonal antibody directed against the gp91phox-subunit of human neutrophil NAD(P)H oxidase (MoAb 48). Cells were either not treated or treated for 16 h with one of the following: PGF2α (100 nM); 8-isoprostane F2α (8-IPF2α; 100 nM); U46619 (as U4;10 nM) + LPS (1 μg ml−1), IL-1α (10 ng ml−1) or TNF-α (10 ng ml−1) either in the absence or presence of iloprost (100 ng ml−1). The bands detected are the 91 kDa for the heavily glycosylated form of gp91phox and the 66 kDa for the less-glycosylated form of gp91phox. The upper panels show the representative blots and the lower panels the results of the densitometric analyses of six blots (expressed as relative optical density (OD) mm–2). Pig neutrophil lysates were used as positive controls. *P<0.05; comparing treated with control. $P< 0.05; significantly inhibited compared to combinations of LPS or cytokines with U46619.
Discussion
The primary trigger for the inflammatory cascades associated with ARDS is the release of endotoxins from bacteria destroyed by neutrophils, which then promote the adhesion of platelets and leucocytes to vessel walls (Jeremy et al., 1994b; Chabot et al., 1998; Ermert et al., 2000b; Stuart-Smith & Jeremy, 2001; Weinacker & Vaszar, 2001). Adherent and activated platelets and leucocytes then release a number of potent inflammogens, including cytokines and TXA2 (Jeremy et al., 1994b,1997), which may influence OS in ARDS. Thus, it was first found that the stable TXA2 analogue, U46619, promoted the formation of O2•− in intact pulmonary arteries and PAVSMCs and PAECs, an effect augmented by LPS, IL-1α and TNF-α. Since elevated plasma levels of TXA2, IL-1α and TNF-α, coexist in ARDS patients (Reines et al., 1982; Jeremy et al., 1994b), these interactions may be important in the progression and outcome of the syndrome.
Apocynin and DPI, both inhibitors of NADPH oxidase, blocked O2•− formation in response to U46619, both alone and in combination with LPS, IL-1α and TNF-α. In contrast, the inhibition of xanthine oxidase and mitochondrial respiration had little effect. U46619 also upregulated the expression of gp91phox, an active catalytic subunit of NAPDH oxidase, in PAVSMCs and PAECs. It is concluded, therefore, that U46619 increases O2•− formation through an upregulation of NADPH oxidase expression/activity in pulmonary arterial cells.
Since LPS and cytokines have long been known to alter vascular eicosanoid formation (Jeremy et al., 1994a,1994b), the effect of LPS and cytokines on the formation of TXA2 and PGI2 in intact pig pulmonary arteries was explored. Not only did LPS and cytokines augment TXA2 production but concomitantly diminished PGI2 formation following a 16-h incubation. The possible importance of PGI2 status in ARDS is exemplified by the beneficial effects of aerosolised PGI2 to treat ARDS (Lowson, 2002). Since PGI2 has diametrically opposite effects to TXA2 (Ullrich et al., 2001) it was reasonable to speculate that PGI2 may inhibit NADPH oxidase expression. Indeed, in the present study the stable PGI2 analogue, iloprost, inhibited the induction of gp91phox expression and O2•− formation elicited by U46619, LPS and cytokines. A reduction of PGI2 formation may result in diminished ‘protection' against the O2•−-promoting effects of TXA2, LPS and cytokines.
In the present studies, LPS and cytokines also augmented the formation of 8-isoprostane F2α and PGF2α in intact pulmonary arteries. Isoprostanes, formed via oxidative pathways are potent agonists of the thromboxane/endoperoxide receptor (Morrow & Roberts, 2002; Roberts & Morrow, 2002). It was further demonstrated here that both 8-isoprostane F2α and PGF2α, upregulated the expression of NADPH oxidase expression/activity and increased the formation of O2•−. The formation of 8-isoprostane F2α was inhibited not only by aspirin but also by DPI and L-NAME, whereas PGF2α (the parent compound of 8-isoprostane F2α) was inhibited only by aspirin. This indicates that not only O2•− derived from NADPH oxidase but also that NO and possibly reactive nitrogen species (RNS), influence the formation of isoprostanes. It has been suggested that isoprostanes are sequestered into phospholipids and are then released by NO or that NO modifies arachidonic acid moiety in phospholipids such that when it is released and metabolised by cyclooxygenase, 8-isoprostane F2α is formed (Morrow & Roberts, 2002; Roberts & Morrow, 2002). However, since L-NAME also indirectly inhibits RNS formation, it is possible that RNS also modulates the formation of 8-isoprostane F2α.
To summarise, the TXA2 analogue U46619 upregulates NAPDH oxidase activity and O2•− formation by pulmonary arterial tissues and cells. In ARDS, therefore, the initial release of TXA2 by adherent platelets would promote intrapulmonary OS. Concomitantly, LPS and cytokines (released by adherent leucocytes) increase TXA2-synthesising capacity but decrease that of PGI2 in the pulmonary arterial tissue. Since TXA2 increases O2•− formation through an upregulation of NADPH oxidase protein expression, which is prevented by PGI2, this imbalance may render the vessel susceptible to further augmentation of O2•−-mediated pathology. A self-perpetuating cascade may ensue, whereby O2•− and or RNS promote the release of arachidonic acid, fuelling the formation of TXA2, which in turn would further augment the formation of O2•− via upregulation of NADPH oxidase. Finally, O2•− (and/or NO or RNS) also increases the formation of 8-isoprostane F2α, which also engenders NADPH oxidase protein expression, and O2•− formation. Together, these events constitute a novel self-perpetuating pathway that would amplify and worsen the progress of ARDS.
From a clinical perspective, ARDS is an intractable condition to treat and many drugs, including NSAIDS and thromboxane formation inhibitors, have proved ineffective in treating the condition (The ARDS Network, 2000; Tasaka et al., 2002). An innate problem with treating ARDS is its rapidity of onset (Jeremy et al., 1994b; Stuart-Smith & Jeremy, 2001; Weinacker & Vaszar, 2001), such that by the time ARDS has been diagnosed and interventions implemented, the upregulation of NAPDH oxidase may already have occurred. Thus, one may be ‘shutting the therapeutic door after the pathological horse has bolted' when one administers drugs such as ketoconazole. Furthermore, since inflammation associated with ARDS is promulgated by many disparate factors, then the inhibition of any one factor (e.g. TXA2 formation) may not prevent the progress of the disease. To exemplify this point, several disparate factors have been shown in this study to promote the upregulation of NAPDH oxidase. However, since this is a common denominator effect of all these factors, it is reasonable to suggest that the pharmacological inhibition of NAPDH oxidase activity and/or inhibition of further upregulation of the enzyme may constitute a possible effective approach to treating ARDS. In support of this and in the context of the present study, inhalational PGI2 and NO (Klinger, 2002; Lowson, 2002) have proved effective in treating ARDS and are both inhibited by O2•−-mediated mechanisms.
Acknowledgments
This research was funded by the British Heart Foundation (Grant No. FS/2001041).
Abbreviations
- ARDS
adult respiratory distress syndrome
- O2•−
superoxide
- OS
oxidative stress
- PAECs
pulmonary artery endothelial cells
- PAVSMCs
pulmonary artery vascular smooth muscle cells
References
- AUDOLY L., ROCCA B., FABRE J., KOLLER B., THOMAS D., LOEB A., COFFMAN T., FITZGERALD G. Cardiovascular responses to the isoprostane iPF2-III and iPE2-III are mediated via the thromboxane A2receptor. Circulation. 2000;101:2833–2840. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- CHABOT F., MITCHELL J.A., GUTTERIDGE J.M.C., EVANS A.M. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998;11:745–757. [PubMed] [Google Scholar]
- CHAUDHARI A., PEDRAM A., KIRSCHENBAUM M.A. Prostanoid biosynthesis in cultured rabbit renal microvascular smooth muscle cells. Effect of arachidonic acid, calcium, and A23187. Lab. Invest. 1990;63:30–37. [PubMed] [Google Scholar]
- EKMEKCIOGLU C., SCHWEIGER B., STRAUSS-BLASCHE G., MUNDIGLER G., SIOSTRZONEK P., MARKTL W. Urinary excretion of 8-iso-PGF(2 alpha) in three patients during sepsis, recovery and state of health. Prostaglandins Leukotr. Essent. Fatty Acids. 2002;66:441–442. doi: 10.1054/plef.2002.0371. [DOI] [PubMed] [Google Scholar]
- ERMERT L., EMRERT M., DUNCKER H.R., GRIMMINGER F., SEEGER W. In situ localisation and regulation of thromboxane A2 synthase in normal and LPS-primed lungs. Am. J. Physiol. 2000a;278:L744–L753. doi: 10.1152/ajplung.2000.278.4.L744. [DOI] [PubMed] [Google Scholar]
- ERMERT M., MERKLE M., MOOTZ R., GRIMMINGER F., SEEGER W., ERMERT L. Endotoxin priming of the cyclooxygenase-2-thromboxane axis in isolated rat lungs. Am. J. Physiol. 2000b;278:L1195–L1203. doi: 10.1152/ajplung.2000.278.6.L1195. [DOI] [PubMed] [Google Scholar]
- GREENE E.L., PALLER M.S. Xanthine oxidase produces superoxide in posthypoxic injury of renal epithelial cells. Am. J. Physiol. 1992;263:F251–F255. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., MINIERI C.A., OLLERENSHAW J.D., ALEXANDER R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cellls. Circ. Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., MEHTA D., BRYAN A.J., LEWIS D., ANGELINI G.D. Platelets and saphenous vein graft failure following coronary artery bypass surgery. Platelets. 1997;8:295–309. doi: 10.1080/09537109777168. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., MIKHAILIDIS D.P., KARATAPANIS S., HARRY D., BURROUGHS A.K., MCINTYRE N., STANSBY G., JACOBS M., MCCORMICK A. Altered prostacyclin synthesis by aortae from hepatic portal vein-constricted rats: evidence for effects on protein kinase C and calcium. J. Hepatol. 1994a;21:1017–1022. doi: 10.1016/s0168-8278(05)80611-7. [DOI] [PubMed] [Google Scholar]
- JEREMY J.Y., NYSTROM M.L., BARRADAS M.A., MIKHAILIDIS D.P. Eicosanoids and septicaemia. Prostaglandins Leukotr. Essent. Fatty Acids. 1994b;50:287–297. doi: 10.1016/0952-3278(94)90235-6. [DOI] [PubMed] [Google Scholar]
- JOURDAN K.B., MITCHELL J.A., EVANS T.W. Release of isoprostanes by human pulmonary artery in organ culture: a cyclooxygenase and nitric oxide dependent pathway. Biochem. Biophys. Res. Commun. 1997;233:668–672. doi: 10.1006/bbrc.1997.6523. [DOI] [PubMed] [Google Scholar]
- KLINGER J.R. Inhaled nitric oxide in ARDS. Crit. Care Clin. 2002;18:45–68. doi: 10.1016/s0749-0704(03)00064-2. [DOI] [PubMed] [Google Scholar]
- LOWSON S.M. Inhaled alternatives to nitric oxide. Anesthesiology. 2002;96:1504–1513. doi: 10.1097/00000542-200206000-00034. [DOI] [PubMed] [Google Scholar]
- MEIER B., RADEKE H.H., SELLE S., YOUNES M., SIES H., RESCH K., HABERMEHL G.G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem. J. 1989;263:539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINUZ P., ANDRIOLI G., DEGAN M., GAINO S., ORTOLANI R., TOMMASOLI R., ZULIANI V., LECHI A., LECHI C. The F2-isoprostane 8-epiprostaglandin F2alpha increases platelet adhesion and reduces the antiadhesive and antiaggregatory effects of NO. Arterioscl. Thromb. Vasc. Biol. 1998;18:1248–1256. doi: 10.1161/01.atv.18.8.1248. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., ROBERTS L.J. The isoprostanes: their role as an index of oxidant stress status in human pulmonary disease. Am. J. Respir. Crit. Care Med. 2002;166:S25–30. doi: 10.1164/rccm.2206011. [DOI] [PubMed] [Google Scholar]
- MUZAFFAR S., JEREMY J.Y., ANGELINI G.D., STUART-SMITH K., SHUKLA N. Role of the endothelium and nitric oxide synthases in modulating superoxide formation induced by endotoxin and cytokines in porcine pulmonary arteries. Thorax. 2003;58:598–604. doi: 10.1136/thorax.58.7.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINES H.D., HALUSHKA P.V., COOK J.A., WISE W.C., RAMBO W. Plasma thromboxane concentrations are raised in patients dying with septic shock. Lancet. 1982;2:174–175. doi: 10.1016/s0140-6736(82)91027-3. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.J., II, MORROW J.D. Products of the isoprostane pathway: unique bioactive compounds and markers of lipid peroxidation. Cell Mol. Life Sci. 2002;59:808–820. doi: 10.1007/s00018-002-8469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORESCU D., SZOCS K., GRIENDLING K.K. NAD(P)H oxidases and their relevance to atherosclerosis. TCM. 2001;11:124–131. doi: 10.1016/s1050-1738(01)00097-4. [DOI] [PubMed] [Google Scholar]
- SOUTHGATE K.M., DAVIES M., BOOTH R.F., NEWBY A.C. Involvement of extracellular-matrix-degrading metalloproteinases in rabbit aortic smooth-muscle cell proliferation. Biochem J. 1992;288:93–99. doi: 10.1042/bj2880093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLK J., HILTERMANN T.J., DIJKMAN J.H.V.A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- STUART-SMITH K., JEREMY J.Y. Microvessel damage in acute respiratory distress syndrome: the answer may not be NO. Br. J. Anaesth. 2001;87:272–279. doi: 10.1093/bja/87.2.272. [DOI] [PubMed] [Google Scholar]
- TASAKA S., HASEGAWA N., ISHIZAKA A. Pharmacology of acute lung injury. Pulm. Pharmacol. Ther. 2002;15:83–95. doi: 10.1006/pupt.2001.0325. [DOI] [PubMed] [Google Scholar]
- TATE R.M., MORRIS H.G., SCHROEDER W.R., REPINE J.E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J. Clin. Invest. 1984;74:608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THE ARDS NETWORK AUTHORS FOR THE ARDS NETWORK Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2000;283:1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- ULLRICH V., ZOU M.H., BACHSCHMID M. New physiological and pathophysiological aspects on the thromboxane A(2)-prostacyclin regulatory system. Biochim. Biophys. Acta. 2001;1532:1–14. doi: 10.1016/s1388-1981(01)00126-3. [DOI] [PubMed] [Google Scholar]
- WEINACKER A.B., VASZAR L.T. Acute respiratory distress syndrome: physiology and new management strategies. Annu. Rev. Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]