Abstract
Melatonin deprival in young rats induces alterations in cerebral arteriolar wall similar to those observed during aging: atrophy and a decrease in distensibility. In this study, we examined the effects of melatonin treatment on cerebral arteriolar structure and distensibility and on the lower limit of cerebral blood flow autoregulation (LLCBF) in old rats.
We measured cerebral blood flow (arbitrary unit, laser Doppler, open skull preparation) prior to and during stepwise hypotension (SH) in adult (12/13 months) and old (24/25 months) IcoWI and WAG/Rij male rats. Old rats were untreated or treated for 3 months with melatonin (0.39 (IcoWi) and 0.44 (Wag/Rij) mg kg−1 day−1, drinking water). Stress–strain relationships were determined using cross-sectional area (CSA, μm2, histometry) and values of arteriolar internal diameter (μm) obtained during a second SH following arteriolar deactivation (EDTA, 67 mmol l−1).
Aging induced (a) atrophy of the arteriolar wall in IcoWI (616±20 vs 500±27 μm2, P<0.05) but not in WAG/Rij rats (328±25 vs 341±20 μm2), (b) a decrease in arteriolar wall distensibility and (c) an increase in the LLCBF in both strains (67±10 mmHg in 12-month-old vs 95±6 mmHg in 24-month-old IcoWi, P<0.05 and 53±2 mmHg in 13-month-old vs 67±6 mmHg in 25-month-old WAG/Rij).
Melatonin treatment induced in IcoWI and WAG/Rij rats (a) hypertrophy of the arteriolar wall (643±34 and 435±25 μm2, respectively), (b) an increase in arteriolar wall distensibility and (c) a decrease in the LLCBF (64±6 and 45±4 mmHg, respectively).
Melatonin treatment of old rats induced hypertrophy of the arteriolar wall, prevented the age-linked decrease in cerebral arteriolar distensibility and decreased the LLCBF.
Keywords: Lower limit of cerebral blood flow autoregulation, atrophy, hypertrophy, distensibility, cerebral arterioles, aging
Introduction
Aging alters the structure and function of cerebral arterioles. In old rats, the cerebral arteriolar wall becomes thinner and stiffer (Hajdu et al., 1990). This decrease in distensibility of the cerebral arteriolar wall may reduce the arteriolar vasodilatory capacity, thus contributing to the increase in the lower limit of cerebral blood flow (CBF) autoregulation observed in old rats (Lartaud et al., 1993). This, together with an increase in blood pressure variability (Zito et al., 1991), may contribute to vascular dementia in aging (Shuaib, 1992; Skoog, 1998; Atkinson, 2001).
Following our previous finding that melatonin vasoconstricts cerebral arterioles via activation of either MT1 and MT2 G-protein-linked membrane receptors (Regrigny et al., 1999), we suggested that the age-related decrease in the plasma concentration of the pineal hormone, melatonin, starting at an age of 60 days until at least 17 months (Pang et al., 1990), could contribute to the development of vascular wall atrophy in old rats. This hypothesis is based on previous reports showing that some G-protein-linked membrane receptors are also coupled to intracellular pathways (Koch et al., 1994; Somlyo & Somlyo, 2000) leading to increase in cell size, number or both (Gottshall et al., 1997; Finkel, 1999). Assuming that melatonin MT1 and MT2 G-protein-linked membrane receptors share these characteristics, melatonin deprival could decrease the smooth muscle component of the wall, thus inducing vascular wall atrophy. Furthermore, we hypothesized that the decrease in the vascular smooth muscle component, defined as a distensible element of the wall by Baumbach et al. (1988), would be associated with a reduction in distensibility and an increase in the lower limit of CBF autoregulation. We have to remain cautious, however, as, to our knowledge, there is no direct evidence that melatonin MT1 and MT2 G-protein-linked membrane receptors are also coupled to intracellular pathways leading to an increase in cell size, number or both. Nevertheless, these hypotheses were partly confirmed by our finding that melatonin deprival in young rats induces atrophy and decreases the distensibility of the cerebral arteriolar wall (Regrigny et al., 2001b). However, the decrease in distensibility was not associated with a shift in the lower limit of CBF autoregulation (Regrigny et al., 2001b). This could be explained by the fact that the autoregulatory capacity in young rats is capable of adapting to a change in wall distensibility. This may not be the case in old animals in which autoregulatory capacity is reduced.
The aim of this study was to examine the effects of melatonin treatment in old rats, a model with atrophy and stiffening of cerebral arteriolar wall (Hajdu et al., 1990) and an increase in the lower limit of CBF autoregulation (Lartaud et al., 1993). We performed the experiments on two strains of Wistar rats, the IcoWI strain used for the pinealectomy experiments (Regrigny et al., 2001b) and the WAG/Rij strain, often used to study the effects of age in rats.
Methods
Animals and operative procedures
The experiments were conducted on 12- and 24-month-old male IcoWI rats (Ico: WI, IOPS AF/Han; Iffa-Credo, l'Arbresle, France) and 13- and 25-month-old male WAG/Rij rats (CEA, Saclay, France). The ages of the animals used in this study were chosen according to a previous experiment where we observed an increase in the lower limit of CBF autoregulation between 14 and 23 months in rats (Lartaud et al., 1993). Three months before experiments, the rats were divided into six groups as follows: 12-month-old control IcoWI rats (n=9), 24-month-old control IcoWI rats (n=9), 24-month-old melatonin-treated IcoWI rats (10 mg l−1 in drinking water, n=11), 13-month-old control WAG/Rij rats (n=11), 25-month-old control WAG/Rij rats (n=13) and 25-month-old melatonin-treated WAG/Rij rats (10 mg l−1 in drinking water, n=8). We treated the animals for 3 months assuming that such a period would allow for the structural changes to take place. The dose of melatonin used in the present experiment was adjusted as a function of water consumption in order to obtain average daily intakes of melatonin (0.39±0.01 and 0.44±0.01 mg kg−1 day−1 for IcoWI and WAG/Rij rats, respectively) similar to the one obtained in our previous report (Regrigny et al., 2001b). It has already been reported that treatment with melatonin (4 mg l−1) in the drinking water produces a nocturnal plasma melatonin level 15 times higher than that in untreated rats (Rasmussen et al., 1999). Consecutively, we would expect our higher dose of 10 mg l−1 of melatonin in the drinking water to produce plasma melatonin level in treated rats at least 15 times higher than that in untreated rats. Thus, as rats drink mainly during the night, the physiological day/night rhythm in melatonin concentration was maintained, but blood levels were probably elevated beyond the physiological range and should be considered as pharmacological levels. Melatonin was prepared three times a week by dissolving the drug (10 mg) in ethanol (1 ml; 100% v v−1). This solution was then diluted with demineralized water to a final concentration of 10 mg l−1 (concentration of ethanol: 0.1% v v−1). A preliminary study showed that melatonin is stable in such a solution for up to 3 days (high-performance liquid chromatography separation with fluorescence detection, unpublished results). The two control groups received solvent. Fluid and food consumption as well as body weight were determined twice a week. Fluid and food consumption were similar in all groups (results not shown).
Animals were housed at 24°C, exposed to 12 h of light (lights on at 0600 and off at 1800) and allowed free access to food and fluid. Experiments were performed in accordance with the guidelines of the French Ministry of Agriculture, Paris, France (permits 54-4 and 03575).
After 3 months of treatment, we evaluated CBF autoregulation and the structure and function of cerebral arterioles. Animals were anesthetized with sodium pentobarbitone (60 mg kg−1, i.p.), and a polyethylene cannula (Merck Biotrol, Chennevieres, France) was introduced into the right femoral artery; the cannula was connected to a low-volume, strain-gauge transducer (Baxter, Bentley Laboratories, Europe) for measurement of blood pressure and heart rate. A second cannula was introduced into the left femoral artery to obtain blood samples for the measurement of arterial blood gases at baseline and for withdrawal of blood to produce hypotension. A silicone catheter (Sigma Medical, Nanterre, France) introduced into the right femoral vein was connected to a pump (Bioblock Scientific, Paris, France) for continuous infusion of sodium pentobarbitone (0.25 ml h−1; 20 mg kg−1 h−1) to maintain anesthesia throughout the experiment. Animals were intubated and mechanically ventilated with room air (50 strokes per minute, tidal volume 3.0 ml) to maintain blood gases (pH, pCO2, pO2; blood gas analyzer 238, Ciba Corning, Cergy Pontoise, France) in the physiological range. The depth of anesthesia was periodically evaluated by testing the corneal reflex. Rectal temperature was maintained at 37–38°C with a heating pad.
Measurement of arteriolar diameter
We measured the internal diameter of first-order arterioles of the cerebrum (Chillon et al., 1997) through an open skull preparation (Chillon et al., 1996). The head was placed in an adjustable head holder and a 1 cm skin incision made to expose the skull. A dam of dental acrylic was constructed around the exposed skull and ports were placed for inflow and outflow of artificial cerebrospinal fluid (CSF). Craniotomy was performed over the left parietal cortex, and the dura was incised to expose cerebral vessels. The exposed brain was continuously suffused with artificial CSF, warmed to 37–38°C and equilibrated with a gas mixture of 5% CO2–95% N2. The composition of the CSF was (mmol l−1) KCl, 3.0; MgCl2, 0.6; CaCl2, 1.5; NaCl, 131.9; NaHCO3, 24.6; urea, 6.7; and glucose, 3.7 in order to mimic as closely as possible the composition of naturally produced CSF (Baumbach et al., 1988).
Arteriolar diameter was monitored through a microscope (Stemi 200-C, Carl Zeiss Jena GMBH, Jena, Germany) connected to a closed-circuit video system with a final magnification of × 400. Images were digitized using a video frame grabber and diameter measured using image analysis software (Saisam®, Microvision Instruments, Evry, France). The precision of this system is 0.5 μm.
Measurement of cerebral blood flow
Relative changes in CBF were measured by laser Doppler flowmetry using a BLF 21 system (Transonic Systems Inc., Ithaca, NY, U.S.A.) equipped with a 1.2 mm diameter needle probe (Fujii et al., 1991). The probe was placed in the CSF of the cranial window, 1–2 mm above the brain surface. CBF was expressed as arbitrary units (a.u.) or as percentage (of baseline) changes in CBF (during stepwise hypotension).
Measurement of cerebral arteriolar pressure
Cerebral arteriolar pressure was measured in WAG/Rij but not in IcoWI rats due to equipment availability. Cerebral arteriolar pressure was measured with a micropipette connected to a servo-null pressure-measuring device (model 5A, Vista Electronics Company, Ramona, CA, U.S.A.). Pipettes were sharpened to a beveled tip, 3–5 μm in diameter, filled with 1.5 mmol l−1 sodium chloride, and inserted into the lumen of a cerebral arteriole with a micromanipulator (Chillon et al., 1997). The presence of the pipette tip in the vessel wall had no discernible effect on the diameter of cerebral arterioles.
Experimental protocol
At 30 min after completion of surgery, cerebral arteriolar diameter was measured under baseline conditions. Stepwise hypotension (10 mmHg per step) down to a systemic mean arterial pressure of 20–30 mmHg was induced by controlled withdrawal of blood. At each step, systemic pressure, arteriolar diameter, CBF and blood gases were measured 1 min after the fall in blood pressure. The time interval between each step was about 3 min. After the final step, blood was reinjected to restore blood pressure.
Vascular smooth muscle was then deactivated by suffusion of cerebral vessels with artificial CSF containing EDTA (67 mmol l−1). This concentration of EDTA produces complete deactivation of smooth muscle in cerebral arterioles (Baumbach et al., 1988). The maximal vasodilator response was measured and systemic arterial pressure–diameter relationships and cerebral arteriolar pressure–diameter relationships were obtained in deactivated cerebral arterioles for IcoWI and WAG/Rij rats, respectively. Systemic arterial pressure–diameter relationships were obtained between mean arterial pressures of 110 and 20 mmHg and cerebral arteriolar pressure–diameter relationships were obtained between mean arteriolar pressures of 40 and 5 mmHg, using hemorrhage to reduce pressure in steps of 10 mmHg (systemic) or 5 mmHg (arteriolar). At each pressure step, arteriolar diameter reached a steady state within 15 s and internal diameter was measured 30 s later.
After the final pressure step, blood was reinfused to restore pressure to control levels. Suffusion of cerebral vessels with artificial CSF containing EDTA was stopped, and the maximally dilated arterioles were fixed by suffusion with glutaraldehyde (2.25% v v−1 in 0.10 mol l−1 cacodylate buffer). Arterioles were considered to be adequately fixed when blood flow through the arteriole had ceased.
The animal was killed with a sodium pentobarbitone overdose (250 mg kg−1), and the arteriolar segment used for pressure–diameter measurements was removed with a microsurgery knife and processed for paraffin embedding and light microscopy. The cross-sectional area (CSA) of the wall was measured in 7 μm sections using the video image analyzing system described above. Luminal and total (lumen plus vessel wall) CSA of the arteriole were measured by tracing the inner and outer edges of the vessel wall. Wall CSA was calculated by subtraction of luminal from total CSA.
Calculations
We determined the lower limit of CBF autoregulation individually for each animals. For this, CBF was represented as a function of systemic mean pressure. We then used a method that fits two linear regressions: one for the autoregulation plateau and one for the decrease in CBF with pressure when systemic mean pressure is lower than the lower limit of CBF. The lower limit of CBF was determined as the intersection between these two slopes. The security margin (%), which indicates the degree to which mean arterial pressure may fall before CBF starts to decrease, was defined as ((baseline mean arterial blood pressure−lower limit of CBF/autoregulation)/baseline mean arterial blood pressure) × 100 (Lartaud et al., 1993).
Circumferential stress (σ) at each pressure step was calculated from mean pressure (APm), systemic mean arterial pressure (IcoWI) and cerebral mean arteriolar pressure (WAG/Rij), internal diameter of the cerebral arterioles (ID) and wall thickness (WT):
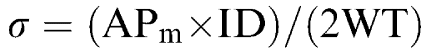 |
Mean pressure was converted from millimeters of mercury to newtons per square meter (1 mmHg=1.334 × 102 N m−2). Because the volume of the vessel wall does not change during changes in intravascular pressure (Dobrin & Rovick, 1969), we assumed that the CSA of the vessel wall remains constant despite the changes in arteriolar diameter induced by hypotension. This enabled us to calculate wall thickness from CSA and internal cerebral arteriolar diameter: 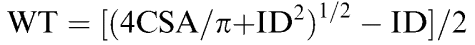
Histological determination of CSA was used in all calculations of wall thickness and circumferential stress.
Circumferential strain (ε) was calculated as: 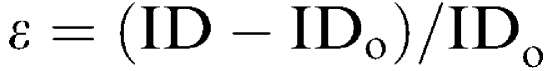 where IDo is the original internal diameter defined as the diameter at 5–10 mmHg.
where IDo is the original internal diameter defined as the diameter at 5–10 mmHg.
Substances used
Melatonin, glutaraldehyde and cacodylate were purchased from Sigma Chemical Company (St Louis, MO, U.S.A.). Nitrogen and carbon dioxide were purchased from Air Liquide (Nancy, France). Sodium pentobarbitone was purchased from Sanofi Santé Animale (Libourne, France). KCl, MgCl2, CaCl2, NaCl, NaHCO3, urea and glucose were purchased from Merck KGaA (Darmstadt, Germany).
Statistical analysis
Results are expressed as means±s.e.m. The experimental protocol was designed for the use of an ANOVA with the variable ‘group' (12–13-, 24–25- and 24–25-month-old melatonin-treated rats). The stress values were pooled by strain, and an ANOVA using the variables ‘group' and ‘strain' was performed. Significant differences between means were determined using the Bonferroni test. The probability level chosen was P≤0.05.
Results
Baseline values
Body weight, blood pressure, heart rate and blood gases were similar in all groups of IcoWI (Table 1) and WAG/Rij rats (Table 2). In WAG/Rij rats, mean, systolic, diastolic and pulse cerebral arteriolar pressures were not influenced by age or melatonin (Table 2). CBF and ID before deactivation with EDTA were also similar in the various groups of IcoWI (Table 1) and WAG Rij rats (Table 2). In IcoWI rats, wall CSA significantly decreased with age and significantly increased after treatment with melatonin to a value similar to the one observed in 12-month-old control rats (Table 1). In contrast, in WAG/Rij rats, wall CSA was not modified by age but increased significantly after treatment with melatonin (Table 2).
Table 1.
Baseline values in 12-month-old control (n=9), 24-month-old control (n=9) and 24-month-old melatonin-treated IcoWI rats (0.39 mg kg−1 in drinking water, n=11)
| Parameters | 12-month-old control | 24-month-old control | 24-month-old + melatonin | |
|---|---|---|---|---|
| Body weight | 626±29 | 645±33 | 631±22 | |
| Systemic arterial pressure (mmHg) | ||||
| Systolic | 134±4 | 128±3 | 126±5 | |
| Diastolic | 97±5 | 87±2 | 88±4 | |
| Mean | 108±6 | 102±3 | 102±4 | |
| Pulse | 39±3 | 41±2 | 38±2 | |
| Heart rate (beats min−1) | 370±12 | 376±10 | 358±12 | |
| Arterial blood gases | ||||
| pCO2 (mmHg) | 33±1 | 32±1 | 33±1 | |
| pO2 (mmHg) | 93±4 | 92±2 | 90±1 | |
| pH | 7.48±0.01 | 7.51±0.01 | 7.50±0.01 | |
| CBF (arbitrary units) | 16.3±0.9 | 15.2±1.1 | 13.9±1.1 | |
| Lower limit of CBF autoregulation (mmHg) | 67±10 | 95±6† | 64±6‡ | |
| Security margin (%) | 37±11 | 7±4† | 36±7‡ | |
| Cerebral arterioles before deactivation | ||||
| Internal diameter (μm) | 46.6±2.5 | 39.3±2.4 | 39.2±2.2 | |
| Cerebral arterioles after deactivation | ||||
| CSA of wall (μm2) | 616±20 | 500±27† | 643±34‡ |
Values are means±s.e.m. CBF, cerebral blood flow; CSA, cross-sectional area.
P≤0.05 vs 12-month-old control.
‡P≤0.05 vs 24-month-old control.
Table 2.
Baseline values in 13-month-old control (n=8), 25-month-old control (n=11) and 25-month-old melatonin-treated WAG/Rij rats (0.44 mg kg−1 in drinking water, n=7)
| Parameters | 13-month-old control | 25-month-old control | 25-month-old+melatonin |
|---|---|---|---|
| Body weight | 380±8 | 372±11 | 358±9 |
| Systemic arterial pressure (mmHg) | |||
| Systolic | 126±2 | 127±4 | 120±6 |
| Diastolic | 90±2 | 91±3 | 85±5 |
| Mean | 103±2 | 105±4 | 99±5 |
| Pulse | 36±1 | 36±1 | 35±2 |
| Heart rate (beats min−1) | 347±11 | 327±11 | 322±10 |
| Arterial blood gases | |||
| pCO2 (mmHg) | 30±1 | 31±1 | 31±1 |
| pO2 (mmHg) | 74±2 | 81±3 | 75±4 |
| pH | 7.52±0.01 | 7.50±0.02 | 7.51±0.01 |
| Cerebral arteriolar pressure (mmHg) | |||
| Systolic | 44±2 | 49±4 | 49±4 |
| Diastolic | 39±2 | 42±4 | 42±4 |
| Mean | 41±2 | 46±4 | 46±4 |
| Pulse | 6±1 | 7±1 | 7±1 |
| CBF (arbitrary units) | 13.7±0.9 | 12.4±0.9 | 10.9±0.3 |
| Lower limit of CBF autoregulation (mmHg) | 53±2 | 67±6 | 45±4‡ |
| Security margin (%) | 48±2 | 36±6 | 54±4 |
| Cerebral arterioles before deactivation | |||
| Internal diameter (μm) | 41.6±3.2 | 45.0±2.4 | 45.9±2.9 |
| Cerebral arterioles after deactivation | |||
| CSA of wall (μm2) | 328±25 | 341±20 | 435±25†‡ |
Values are means±s.e.m. CBF, cerebral blood flow; CSA, cross-sectional area.
P≤0.05 vs 13-month-old control.
P≤0.05 vs 25-month-old control.
Mechanics of cerebral arterioles
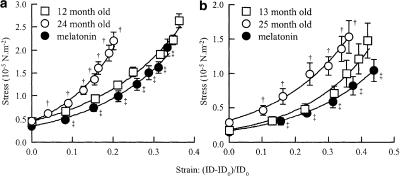
In both strains, after deactivation of cerebral arterioles with EDTA, the stress–strain curves of the old (24- and 25-month-old) rats were significantly shifted to the left with respect to the curves of the mature (12- and 13-month-old) rats (Figure 1), that is, cerebral arterioles were stiffer in 24- and 25-month-old rats than in 12- and 13-month-old rats. Melatonin treatment shifted the stress–strain curve back to values similar to those of 12- and 13-month-old untreated rats (Figure 1). Thus, melatonin treatment reduced the age-related increase in stiffness of the cerebral arterioles.
Figure 1.
Stress–strain relationships in 12-month-old (n=9), 24-month-old (n=9), and 24-month-old melatonin-treated (0.39 mg kg−1 in drinking water, n=11) IcoWI rats (a) and in 13-month-old (n=8), 25-month-old (n=11), and 25-month-old melatonin-treated (0.44 mg kg−1 in drinking water, n=6) WAG/Rij rats (b). Values are means±s.e.m. ID, internal diameter of cerebral arterioles for systemic mean pressure steps of 10 mmHg (20–29 to 100–109 mmHg) (a) and for mean cerebral arteriolar pressure steps of 5 mmHg (10–14 to 35–39 mmHg) (b) IDo, internal diameter at the lowest pressure step. †P≤0.05 vs 12–13-month-old control. ‡P≤0.05 vs 24–25-month-old control.
Lower limit of CBF autoregulation, security margin and hypotension-induced vasodilatation
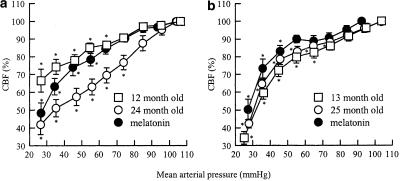
In both IcoWI and WAG/Rij rats, the lower limit of CBF autoregulation increased with age (Figure 2, Tables 1 and 2). However, this increase was statistically significant only for IcoWI rats. On the contrary, in both strains, melatonin treatment significantly decreased the lower limit of CBF autoregulation in old rats (Figure 2, Tables 1 and 2). The security margin was significantly decreased in 24-month-old compared to 12-month-old IcoWI rats (Table 1). Treatment of old IcoWI rats with melatonin increased the security margin to a value similar to the one obtained in mature rats (Table 1). In WAG/Rij rats, the security margin values followed the same pattern without reaching statistical significance (Table 2).
Figure 2.
CBF autoregulation during stepwise hypotensive hemorrhage in 12-month-old (n=9), 24-month-old (n=9), and 24-month-old melatonin-treated (0.39 mg kg−1 in drinking water, n=11) IcoWI rats (a) and in 13-month-old (n=8), 25-month-old (n=11), and 25-month-old melatonin-treated (0.44 mg kg−1 in drinking water, n=6) WAG/Rij rats (b). CBF values (percent baseline±s.e.m.) are grouped by mean arterial pressure ranges of 10 mmHg (20–29 to 120–129 mmHg) (Barry et al., 1982). *P≤0.05 vs baseline in the same group.
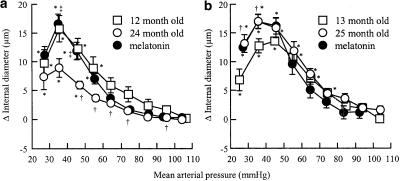
Hypotension-induced vasodilatation was significantly modified by age or melatonin in IcoWI rats (Figure 3a). Maximal vasodilatation was obtained at 30–39 mmHg for the three groups and was significantly reduced in 24-month-old (8.9±1.6 μm) compared to 12-month-old control IcoWI rats (15.7±2.3 μm). Treatment with melatonin restored maximal vasodilatation in 24-month-old IcoWI rats to a value similar to the one observed in 12-month-old control IcoWI rats (16.6±1.7). In 12-month-old control, 24-month-old control and 24-month-old melatonin-treated IcoWI rats, cerebral arterioles dilated significantly at pressures <60, <50 and <60 mmHg, respectively (Figure 3a).
Figure 3.
Variation in internal diameter of cerebral arterioles at systemic arterial mean pressure steps between 20 and 130 mmHg, during hypotensive hemorrhage before EDTA in 12-month-old (n=9), 24-month-old (n=9), and 24-month-old melatonin-treated (0.39 mg kg−1 in drinking water, n=11) IcoWI rats (a) and in 13-month-old (n=8), 25-month-old (n=11), and 25-month-old melatonin-treated (0.44 mg kg−1 in drinking water, n=6) WAG/Rij rats (b). Values are means±s.e.m. *P≤0.05 vs baseline in the same group. †P≤0.05 vs 12–13-month-old control. ‡P≤0.05 vs 24–25-month-old control.
In contrast, hypotension-induced vasodilatation was not significantly modified by age or melatonin in WAG/Rij rats (Figure 3b). Maximal dilatation of cerebral arterioles was obtained at 40–49 mmHg in 13-month-old rats (13.6±0.7 μm) and at 30–39 mmHg in 25-month-old rats treated (17.0±1.2 μm) or not (16.0±1.5 μm) with melatonin. In 13-month-old control, 25-month-old control and 25-month-old melatonin-treated WAG/Rij rats, cerebral arterioles dilated significantly at pressures <80, <70 and <50 mmHg, respectively (Figure 3b).
Discussion
This study shows that in IcoWI rats, aging is associated with atrophy and a decrease in distensibility of the cerebral arteriolar wall, a decrease in hypotension-induced vasodilatation and a shift in the lower limit of CBF autoregulation toward higher levels of blood pressure. Melatonin treatment of old IcoWI rats induced hypertrophy of the cerebral arteriolar wall and prevented the age-related decrease in arteriolar wall distensibility. These changes in arteriolar structure and mechanics following melatonin treatment were accompanied by an increase in hypotension-induced vasodilatation and an improvement of the cerebral autoregulatory function with a shift in the lower limit of CBF autoregulation.
In WAG/Rij rats, aging was also associated with a decrease in distensibility of the cerebral arteriolar wall and an increase in the lower limit of CBF autoregulation. However, in WAG/Rij old rats, we did not observe atrophy of the cerebral arteriolar wall or a decrease in hypotension-induced vasodilatation. As in IcoWI rats, melatonin treatment of old WAG/Rij rats induced hypertrophy of the cerebral arteriolar wall and prevented the age-related decrease in arteriolar wall distensibility. However, despite these changes in arteriolar structure and mechanics, in WAG/Rij rats, melatonin had no significant effect on hypotension-induced vasodilatation, but improved cerebral autoregulatory function by shifting the lower limit of CBF autoregulation to lower values of blood pressure.
Effects of age and melatonin on cerebral arteriolar wall structure and mechanics
It has been reported that in old rats, the cerebral arteriolar wall becomes thinner and stiffer (Hajdu et al., 1990). The results obtained in the present experiment partly confirm this observation as we observed atrophy of the cerebral arteriolar wall with age in IcoWI but not in WAG/Rij rats. This discrepancy may be explained by the different rat strains used in these studies (Fischer 344 rats for Hadju, IcoWI and WAG/Rij rats in the present experiment). In WAG/Rij rats, there may be no atrophy of the cerebral arteriolar wall with age, or atrophy may appear earlier than in Fischer 344 or IcoWI rats. In contrast, in all strains, aging is accompanied by a decrease in the cerebral arteriolar wall distensibility. It has already been shown that vascular wall distensibility in cerebral arterioles may be related to the ratio of compliant (smooth muscle cells, elastin, endothelial cells) to stiff (collagen, basement membrane) components of the cerebral arteriolar wall (Baumbach et al., 1988). Thus, in old IcoWI rats, cerebral arteriolar wall atrophy, by reducing the number or size of smooth muscle cells, a compliant component of the vessel wall, may contribute to the decrease of the cerebral arteriolar wall distensibility. In contrast, in old WAG/Rij rats, this decrease in passive distensibility is not related to atrophy of the arteriolar wall and cannot be explained by a loss of such material. The decrease in distensibility may be related to a modification of the relative composition of the arteriolar wall. The proportion of compliant components may have been reduced without any change in the total wall mass. Another possibility is that the intrinsic properties of the different components of the wall, or the way in which they interact with each other, was modified with age.
The results obtained in the present experiment confirm our hypothesis that melatonin has a trophic effect on the cerebral arteriolar wall in old rats. Melatonin increased CSA of cerebral arteriolar wall in old IcoWI and WAG/Rij rats. One of the mechanisms that may explain this trophic effect of melatonin is a direct action on cell growth and/or proliferation. The activation of the MT1 and/or MT2 melatonin receptors leads to constriction of cerebral arteries and arterioles (Geary et al., 1997; Regrigny et al., 1999) via a Gi- or Go-protein-linked mechanism, at least in the middle cerebral artery (Geary et al., 1997). Similar G proteins can activate the Ras or Rho transduction pathways via a cross-talk mechanism (Koch et al., 1994; Somlyo & Somlyo, 2000) which can lead to hypertrophy or proliferation of the smooth muscle cell (Gottshall et al., 1997; Finkel, 1999). Thus, melatonin could induce hypertrophy of the arteriolar wall directly through activation of MT1 and/or MT2 receptors. This increase in vascular wall mass following an increase in the proportion of smooth muscle cells, a compliant component of the vessel wall (Baumbach et al., 1988), may contribute to the increase in distensibility observed in old rats treated with melatonin. We have to remain cautious, however, as, to our knowledge, there is no report that MT1 and/or MT2 melatonin receptors are coupled to intracellular pathways that can lead to hypertrophy or proliferation of smooth muscle cells. The fact that some G-protein-coupled membrane receptors are coupled to such pathways does not imply that all G-protein-coupled membrane receptors share the same characteristics. Furthermore, there is also no direct evidence for the expression of MT1 and/or MT2 receptors' mRNA or protein in the cerebral arterioles examined in the present experiment. There is indirect pharmacological evidence of the expression of such receptors in the cerebral arterioles: melatonin-induced contraction of the cerebral arteriole is antagonized by luzindole, an MT1 and MT2 receptor antagonist (Regrigny et al., 1999).
Decreases in distensibility and hypotension-induced vasodilatation
In the present experiment, results obtained concerning the relation between distensibility and hypotension-induced vasodilatation of cerebral arterioles are contradictory. In IcoWI rats, the decrease in distensibility is accompanied by a reduction in hypotension-induced vasodilatation. Furthermore, melatonin treatment increased distensibility and hypotension-induced vasodilatation. In contrast, in WAG/Rij rats, despite a decrease in distensibility, hypotension-induced vasodilatation was not impaired in 25- compared to 13-month-old WAG/Rij. Furthermore, melatonin treatment increased distensibility but not hypotension-induced vasodilatation in old WAG/Rij rats. One of the possible explanations of this discrepancy may be that the decrease in distensibility is more important in old IcoWI than WAG/Rij rats, possibly due to the loss of smooth muscle cells following the vascular wall atrophy. Thus, in IcoWI rats but not in WAG/Rij rats, this reduction in distensibility could have an impact on hypotension-induced dilatation. We have to remain prudent, however, as the decrease in distensibility and hypotension-induced vasodilatation in old IcoWI rats are not necessarily related. To our knowledge, there is no direct evidence that a decrease in vascular wall distensibility is associated with a decrease in vasodilatation to different stimuli such as hypotension or vasodilator agonists. For example, it has been reported that vasodilatation induced by nitroglycerin, an endothelium independent vasodilator, is not altered in cerebral arterioles of old rats (Mayhan et al., 1990) despite a decrease in distensibility (Hajdu et al., 1990). Furthermore, we cannot rule out the possibility that melatonin affects other factors that might modify the vessels' response to hemorrhage, such as adrenergic innervation, for example.
Effects of age and melatonin on CBF autoregulation
In the present experiments, the lower limit of CBF autoregulation increased with age, and melatonin treatment shifted the lower limit of CBF autoregulation to lower values of blood pressure in both IcoWI and WAG/Rij rats. This result confirms previous reports showing an increase of the lower limit of CBF autoregulation with age in awake rats using hydrogen clearance to measure CBF (Lartaud et al., 1993; 1994). However, these variations of the lower limit of CBF autoregulation are linked to the variation in hypotension-induced vasodilatation in IcoWI but not in WAG/Rij rats. We have already observed that acute intravenous administration of melatonin improves the lower limit of CBF autoregulation but not cerebral arteriolar dilatation (Regrigny et al., 2001a). Factors other than hypotension-induced vasodilatation of cerebral arterioles may explain this shift of the lower limit of CBF autoregulation in WAG/Rij rats. Cerebral arterioles dilated at lower blood pressure steps in the 25-month-old melatonin-treated rats compared with 13- and 25-month-old control rats. According to Johnson (1986), a segmental response occurs during autoregulation of CBF, larger vessels dilating earlier than smaller arteries during hypotension. As melatonin constricts the middle cerebral artery (Geary et al., 1997), this hormone may shift the lower limit of CBF autoregulation in WAG/Rij rats by improving the vasodilatory capacity of the vessels upstream of the arterioles.
Conclusion and implications
Treatment of old rats with pharmacological doses of melatonin induces hypertrophy of cerebral arteriolar wall and prevents the age-related decrease in cerebral arteriolar distensibility. Such a treatment also improves CBF autoregulation and the security margin in old rats.
The age-associated increase in the lower limit of CBF autoregulation (Lartaud et al., 1993) and the increase in blood pressure variability with age (Zito et al., 1991) may lead to vascular dementia (Shuaib, 1992; Skoog, 1998; Atkinson, 2001). Thus, this study suggests that pharmacological treatment with melatonin or synthetic analogs may slow the development of vascular dementia in the elderly. This hypothesis remains to be tested.
Acknowledgments
This study was funded by grants from the French Ministry of Education, Research and Technology (EA3448, Paris, France), the Regional Development Committee (Metz, France), the Greater Nancy Urban Council (Nancy, France), Henri Poincaré University (Nancy, France) and Institut de Recherches Internationales Servier (Courbevoie, France).
Abbreviations
- APm
mean pressure
- CBF
cerebral blood flow
- CSA
cross-sectional area
- CSF
cerebrospinal fluid
- ID
internal diameter
- LLCBF
lower limit of cerebral blood flow autoregulation
- SH
stepwise hypotension
- WT
wall thickness
References
- ATKINSON J. Cerebrovascular structure and dementia: new drug targets. Trends Pharmacol Sci. 2001;22:630–635. doi: 10.1016/s0165-6147(00)01866-6. [DOI] [PubMed] [Google Scholar]
- BARRY D.I., STRANDGAARD S., GRAHAM D.I., BRAENDSTRUP O., SVENDSEN U.G., VORSTRUP S., HEMMINGSEN R., BOLWIG T.G. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab. 1982;2:347–353. doi: 10.1038/jcbfm.1982.35. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., DOBRIN P.B., HART M.N., HEISTAD D.D. Mechanics of cerebral arterioles in hypertensive rats. Circ Res. 1988;62:56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., GHONEIM S., BAUMBACH G.L. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in rats. Hypertension. 1997;30:1097–1104. doi: 10.1161/01.hyp.30.5.1097. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., HEISTAD D.D., BAUMBACH G.L. Effects of endothelin receptor inhibition on cerebral arterioles in hypertensive rats. Hypertension. 1996;27:794–798. doi: 10.1161/01.hyp.27.3.794. [DOI] [PubMed] [Google Scholar]
- DOBRIN P.B., ROVICK A.A. Influence of vascular smooth muscle on contractile mechanics and elasticity of arteries. Am J Physiol. 1969;217:1644–1651. doi: 10.1152/ajplegacy.1969.217.6.1644. [DOI] [PubMed] [Google Scholar]
- FINKEL T. Myocyte hypertrophy: the long and winding RhoA'd. J Clin Invest. 1999;103:1619–1620. doi: 10.1172/JCI7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJII K., HEISTAD D.D., FARACI F.M. Role of the basilar artery in regulation of blood flow to the brain stem in rats. Stroke. 1991;22:763–767. doi: 10.1161/01.str.22.6.763. [DOI] [PubMed] [Google Scholar]
- GEARY G.G., KRAUSE D.N., DUCKLES S.P. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am J Physiol. 1997;273:H1530–1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- GOTTSHALL K.R., HUNTER J.J., TANAKA N., DALTON N., BECKER K.D., ROSS J., CHIEN K.R. Ras-dependent pathways induce obstructive hypertrophy in echo-selected transgenic mice. Proc Natl Acad Sci U.S.A. 1997;94:4710–4715. doi: 10.1073/pnas.94.9.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAJDU M.A., HEISTAD D.D., SIEMS J.E., BAUMBACH G.L. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res. 1990;66:1747–1754. doi: 10.1161/01.res.66.6.1747. [DOI] [PubMed] [Google Scholar]
- JOHNSON P.C. Autoregulation of blood flow. Circ Res. 1986;59:483–495. doi: 10.1161/01.res.59.5.483. [DOI] [PubMed] [Google Scholar]
- KOCH W.J., HAWES B.E., ALLEN L.F., LEFKOWITZ R.J. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U.S.A. 1994;91:12706–12710. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARTAUD I., BRAY DES BOSCS L., CHILLON J.M., ATKINSON J., CAPDEVILLE-ATKINSON C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am J Physiol. 1993;264:H851–858. doi: 10.1152/ajpheart.1993.264.3.H851. [DOI] [PubMed] [Google Scholar]
- LARTAUD I., MAKKI T., BRAY DES BOSCS L., NIEDERHOFFER N., ATKINSON J., CORMAN B., CAPDEVILLE-ATKINSON C. Effect of chronic ANG I-converting enzyme inhibition on aging processes. IV. Cerebral blood flow regulation. Am J Physiol. 1994;267:R687–694. doi: 10.1152/ajpregu.1994.267.3.R687. [DOI] [PubMed] [Google Scholar]
- MAYHAN W.G., FARACI F.M., BAUMBACH G.L., HEISTAD D.D. Effects of aging on responses of cerebral arterioles. Am J Physiol. 1990;258:H1138–1143. doi: 10.1152/ajpheart.1990.258.4.H1138. [DOI] [PubMed] [Google Scholar]
- PANG S.F., TSANG C.W., HONG G.X., YIP P.C., TANG P.L., BROWN G.M. Fluctuation of blood melatonin concentrations with age: result of changes in pineal melatonin secretion, body growth, and aging. J Pineal Res. 1990;8:179–192. doi: 10.1111/j.1600-079x.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN D.D., BOLDT B.M., WILKINSON C.W., YELLON S.M., MATSUMOTO A.M. Daily melatonin administration at middle age suppresses male rat visceral fat, plasma leptin, and plasma insulin to youthful levels. Endocrinology. 1999;140:1009–1012. doi: 10.1210/endo.140.2.6674. [DOI] [PubMed] [Google Scholar]
- REGRIGNY O., DELAGRANGE P., SCALBERT E., ATKINSON J., CHILLON J.M. Melatonin increases pial artery tone and decreases the lower limit of cerebral blood flow autoregulation. Fund Clin Pharmacol. 2001a;15:233–238. doi: 10.1046/j.1472-8206.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- REGRIGNY O., DUPUIS F., ATKINSON J., LIMINANA P., SCALBERT E., DELAGRANGE P., CHILLON J.M. Cerebral arteriolar structure and function in pinealectomized rats. Am J Physiol Heart Circ Physiol. 2001b;281:H1476–1480. doi: 10.1152/ajpheart.2001.281.4.H1476. [DOI] [PubMed] [Google Scholar]
- REGRIGNY O., DELAGRANGE P., SCALBERT E., LARTAUD-IDJOUADIENE I., ATKINSON J., CHILLON J.M. Effects of melatonin on rat pial arteriolar diameter in vivo. Br J Pharmacol. 1999;127:1666–1670. doi: 10.1038/sj.bjp.0702714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUAIB A. Alteration of blood pressure regulation and cerebrovascular disorders in the elderly. Cerebrovasc Brain Metab Rev. 1992;4:329–345. [PubMed] [Google Scholar]
- SKOOG I. Status of risk factors for vascular dementia. Neuroepidemiology. 1998;17:2–9. doi: 10.1159/000026147. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522Pt2:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZITO M., PARATI G., OMBONI S., CERVONE C., ULIAN L., D'AVIERO M., ABATE G., MANCIA G. Effect of ageing on blood pressure variability. J Hypertens Suppl. 1991;9:S328–329. [PubMed] [Google Scholar]