Abstract
There is circumstantial evidence suggesting that 5-hydroxytryptamine (5-HT) could be biotransformed by enzymatic systems other than monoamino oxidase A, and that the isoforms of cytochrome P450 may be a source of nitric oxide. This study aimed to assess whether cytochrome P450 contributes to 5-HT biotransformation, and to provide evidence that 5-HT metabolism generates nitric oxide.
Addition of 5-HT to cultured hepatocytes yielded 5-hydroxyindol acetic acid, a formation modulated by cytochrome P450 enzyme inducers and inhibitors. Recombinant human CYP2B6, 2C9 and 2C19 biotransformed 5-HT in 5-hydroxyindol acetic acid, but not CYP1A2, 2D6 or 3A4.
Cultured hepatocytes with 5-HT generated nitric oxide, the amount of which was altered by cytochrome P450 enzyme inducers and inhibitors. In the presence of CYP2B6, 2C9 and 2C19, 5-HT relaxed precontracted isolated aortic rings, with or without endothelium, an effect prevented by the addition of methylene blue and an inhibitor of catalase, but not by myoglobin. In the absence of catalase, hydroxylamine was always assayed as a byproduct of 5-HT metabolism.
In conclusion, CYP2B6, 2C9 and 2C19 biotransform 5-HT, yielding hydroxylamine, which is converted to nitric oxide in the presence of catalase.
Keywords: Cytochrome P450, 5-hydroxytryptamine, nitric oxide, CYP2B6, CYP2C9, CYP2C19, hydroxylamine, hepatocytes
Introduction
There is clear evidence that the isoforms of the cytochrome P450 (P450) superfamily are implicated in the formation of vasoactive substances, primarily through the biotransformation of arachidonic acid (Capdevila et al., 1981). Enzymes of the CYP4A, 4B and 4F subfamilies catalyse the ω-hydroxylation of fatty acids, and enzymes of the CYP1A, 2B, 2C, 2D, 2E, 2J, 3A and 4A subfamilies catalyse the formation of epoxyeicosatrienoic acids (EETs) (Roman, 2002). An endothelium-derived hyperpolarizing factor (EDHF), the 11,12-EET is a metabolite of arachidonic acid synthesized by CYP2C8/34 (Fisslthaler et al., 1999). Enzymes of P450 have also been implicated in the formation of nitric oxide (NO•), particularly the CYP3A subfamily (Boucher et al., 1992).
5-Hydroxytryptamine (5-HT) is an effector on various types of tissue receptors. Contraction of blood vessels to 5-HT is mediated by smooth muscle 5-HT1B/1D and/or 5-HT2A receptors (Saxena et al., 1998). On the other hand, 5-HT is a vasodilator. The vasodilatation elicited by 5-HT is mediated by endothelial 5-HT receptors (Glusa & Pertz, 2000) and smooth muscle 5-HT receptors that appear coupled to endothelial cells to release NO• (Mylecharane, 1990; Bruning et al., 1993). However, the role of NO• remains questionable because 5-HT-induced relaxation is not inhibited by rubbing the endothelium or by N,G-nitro-L-arginine, a nitric oxide synthase inhibitor (Tsuru et al., 1998).
5-HT is believed to be primarily metabolized by monoamine oxidase A (MAO A), which deaminates 5-HT, yielding 5-hydroxyindole acetaldehyde that is converted to 5-hydroxyindole acetic acid (5-HIAA) by an aldehyde dehydrogenase. However, there is indirect data suggesting that 5-HT may also be biotransformed by other enzymatic systems. Metyrapone and ketoconazole (KTZ) show an antidepressant profile (Murphy, 1997; Healy et al., 1999), possibly associated with enhanced postsynaptic response to 5-HT (Kennett et al., 1985). Moreover, in rats, metyrapone treatment increases 5-HT and its metabolites in the brain (Leret et al., 1998). Since metyrapone (Hildebrandt, 1972) and KTZ (Lomaestro & Piatek, 1998) are known P450 enzyme inhibitors, we speculated that, besides MAO A, 5-HT is biotransformed by enzymes of P450. Supporting such hypothesis, it has been shown in vitro that 5-HT inhibits the oxidase activity of CYP1A2, CYP2C9 and CYP3A4 (Gervasini et al., 2001). On the other hand, to explain why rubbing the endothelium and N,G-nitro-L-arginine do not prevent 5-HT relaxation (Tsuru et al., 1998), we hypothesized that deamination of 5-HT by P450 generates NO•. By combining experiments conducted in hepatocytes, human recombinant P450 isoforms and in aortic rings, we demonstrate that 5-HT is biotransformed by CYP2B6, CYP2C9 and CYP2C19 to hydroxylamine, which in the presence of catalase generates nitric oxide.
Methods
Biotransformation of 5-HT by P450 isoforms
Animals and hepatocyte collection
Male New Zealand rabbits (1.8–2.2 kg, Charles Rivers, St-Constant, Québec, Canada) were maintained on Purina Laboratory Chow and water ad libitum for at least 7 days before any experimental work was undertaken. All the experiments were conducted according to the Canadian Council on Animal Care guidelines for the use of laboratory animals. Hepatocytes were isolated according to the two-step liver perfusion method of Seglen (1976), with minor modifications (El-Kadi et al., 1997). Viability was assessed by trypan blue exclusion to ensure that it was greater than 90%. Cell concentration was adjusted to 1 × 106 ml−1.
Experimental protocols
Hepatocytes were incubated for 72 h with cytochrome P450 enzyme inducers, rifampicin (RIF, 5–25 μM) and phenobarbital (PB, 50–200 μM), and enzyme inhibitors, such as KTZ (10–50 μM), omeprazole (OME, 10–50 μM) and β-phenylethyl isothiocyanate (PEITC, 10–50 μM), with the nitric oxide synthase inhibitor L-NAME (0.1–1 mM), and with the MAO A inhibitor chlorgyline (10–50 μM). At 4 h before ending the incubation period, for example, at 68 h, L-arginine (L-Arg, 0.1–1 mM) and 5-HT (10–125 μM) were added. Finally, following the 72 h incubation period, 5-HT biotransformation, protein expression of CYP1A1/1A2, CYP2B6, CYP2C9/2C19, CYP3A6 and nitric oxide synthase 2 (NOS2), as well as NO• concentration were documented.
Human recombinant P450 isoforms CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 were incubated with 5-HT (10–125 μM) and the P450 inhibitors in the presence of an NADPH regenerating system, as described elsewhere (Projean et al., 2003), for 55 min, and 5-HT, 5-HIAA, NO• and hydroxylamine were assayed.
Western blot analysis
The amount of proteins in hepatocytes was measured in cell lysates (Lowry et al., 1951). For Western blot analysis, 40 μg of cell lysate was separated by SDS–polyacrylamide gel electrophoresis, as described elsewhere (Fradette et al., 2002). CYP1A1 and 1A2 were detected with a polyclonal anti-rabbit CYP1A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.) and visualized with an alkaline phosphatase-conjugated secondary antibody, using nitro blue tetrazolium as substrate. Specific antibodies against CYP3A6 (Oxford Biochemical Research, Oxford, MI, U.S.A.), CYP2B6, CYP2C9/2C19 (Research Diagnostics, Inc., Flanders, NJ, U.S.A.) and NOS2 (BD Transduction Laboratories, Mississauga, Canada) were used and identified with a secondary antibody conjugated with horseradish peroxidase enzyme, and visualized by autoradiography. All antibodies had crossreactivity for rabbit proteins. Western blot results were normalized with a protein sample to allow for comparisons between measurements. Intensity of the bands was quantified with the software Un-Scan-It-Gel (Silk Scientific Inc., Orem, UT, U.S.A.), and are presented in arbitrary units.
5-HT and 5-HIAA assay
5-HT and its metabolite 5-HIAA were assayed in cells and recombinant P450 isoform supernatants by high-performance liquid chromatography with an electrochemical detector, as described by Yamaguchi (1993).
NO• and hydroxylamine assays
NO• was determined by converting nitrate into nitrite in the culture media, and total nitrite was assayed spectrophotometrically using the Griess reaction (Grisham et al., 1996).
Hydroxylamine could only be assayed in the absence of catalase, for example, in experiments conducted with recombinant CYP2B6, CYP2C9 and CYP2C19, via a transglutaminase activity assay (Folk & Chung, 1985). The assay is based on the fact that transglutaminase catalyses the formation of hydroxamate from CBZ-glutaminylglycine and hydroxylamine at pH 6.0. The formed hydroxamate was measured spectrophotometrically at 525 nm.
Vasorelaxant activity of 5-HT byproduct
Functional studies in isolated aortic rings
Functional studies with aortic rings were conducted as described elsewhere (Thollon et al., 2002). Rings of thoracic aorta of Sprague–Dawley rats of approximately 2.5 mm in length were mounted under 2 × g resting tension in the chamber bath. After equilibration, to test their ability to contract, rings were initially contracted with 100 nM phenylephrine (PE), followed by the addition of 1 μM acetylcholine, to test the presence of the endothelium. After re-equilibration, 50 μM of ketanserin (5-HT receptor antagonist) was added, and 10 or 100 nM PE was used to generate a sustained tension in vessels without and with endothelium, respectively. Thereafter, 10–125 μM of 5-HT was added into the chamber bath. All experiments were conducted in the presence or absence of recombinant CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 (25 pM). Each experiment included a control relaxation with 1 μM sodium nitroprusside(SNP), an NO• donor.
To further characterize the nature of the relaxing agent, aortic rings were incubated with OME (50 μM), methylene blue (30 μM), myoglobin (50 μM) and 3-amino-1,2,4-triazole (3-AT, 50 mM). Tissues were precontracted with 5 nM of PE. Since pretreatment with 3-AT depresses PE-induced tension by 32.2±2.4% (n=6), PE concentration was 30 nM.
Drugs and chemicals
All reagents, enzymes and substrates were purchased from Sigma Chemicals (Sigma, St Louis, MO, U.S.A.). Recombinant human P450 CYP1A2, CYP3A4, CYP2D6, CYP2C9, CYP2C19 and CYP2B6 were purchased from Gentest (BD Biosciences Company, Woburn, MA, U.S.A.).
Calculation of the maximal effect and statistical analysis
The predicted maximal concentration of NO• (Cmax) generated by 5-HT was estimated from the dose–response curves by using the Emax model with a subroutine written in FORTRAN for the computer program WinNonlin (Scientific Consulting Inc., Apex, NC, U.S.A.). All results are presented as mean±s.e. Comparison of results from the various experimental groups and their corresponding controls was carried out by a one-way analysis of variance (ANOVA), followed by the Newman–Keuls post hoc test. Differences were considered significant when P<0.05.
Results
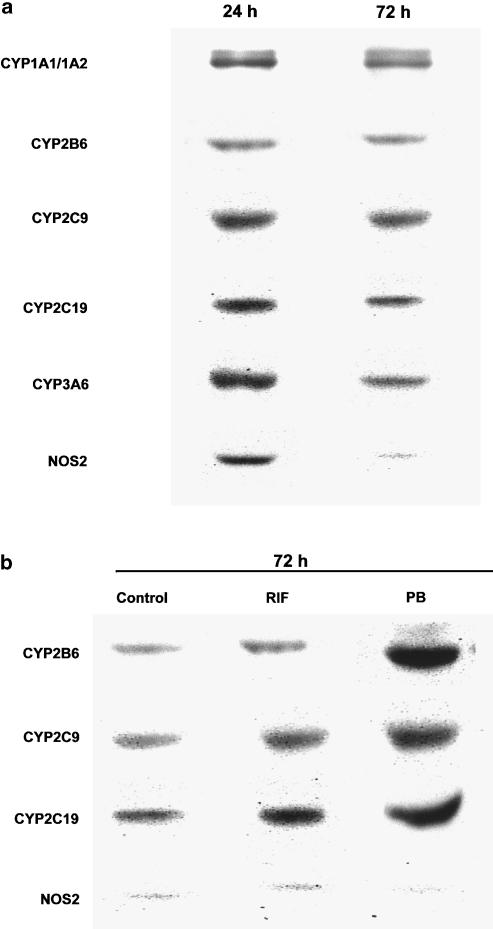
Following 24 h incubation, hepatocytes from control rabbits expressed CYP1A1, CYP1A2, CYP2B6, CYP2C9, CYP2C19 and CYP3A6, as well as NOS2. After 72 h of incubation, NOS2 expression was almost undetectable. The time of incubation did not affect the expression of P450 isoforms (Figure 1a). Therefore, all the experiments reported herein were conducted with hepatocytes incubated for 72 h, for example, in the absence of NOS2. Incubation of hepatocytes for 72 h with the enzyme inducer RIF increased the expression of CYP2C9 from 0.79±0.12 to 0.98±0.15 (P<0.05), that of CYP2C19 from 0.95±0.15 to 1.88±0.15 (P<0.05), and that of CYP2B6 from 0.55±0.09 to 0.84±0.08 (P<0.05); PB, another enzyme inducer, increased the expression of CYP2C9 from 0.79±0.12 to 1.59±0.11 (P<0.05), that of CYP2C19 from 0.95±0.15 to 2.25±0.21 (P<0.05), and that of CYP2B6 from 0.55±0.09 to 2.35±0.11 (P<0.05). Neither RIF nor PB affected NOS2 expression (Figure 1b).
Figure 1.
(a) Immunoblot analysis of CYP1A1/1A2, CYP2B6, CYP2C9, CYP2C19, CYP3A6 and NOS2 in rabbit's hepatocytes incubated for 24 and 72 h. (b) Immunoblot analysis of CYP2B6, CYP2C9, CYP2C19 and NOS2 in rabbit's hepatocytes following 72 h incubation with RIF (10 μM) and PB (125 μM).
Biotransformation of 5-HT
Rabbit's hepatocytes
In the supernatant of cultured hepatocytes, baseline concentrations of 5-HT and 5-HIAA were 0.045±0.005 and 0.120±0.007 μM, respectively (n=8). The enzyme inducers RIF and PB dose dependently increased the production of 5-HIAA by 118 and 72% (P<0.05), respectively, while 5-HT was decreased to an undetectable level by both inducers (n=8). On the other hand, KTZ, an unspecific P450 inhibitor, OME, an inhibitor of CYP2C9 and 2C19, and PEITC, an inhibitor of CYP2B6, reduced baseline concentrations of 5-HIAA by 42, 75 and 58% (P<0.05, n=8), respectively, whereas the concentrations of 5-HT increased by 167, 390 and 227% (P<0.05, n=8), respectively. Chlorgyline, a specific inhibitor of MAO A, dose-dependently decreased 5-HIAA concentrations up to 15% baseline values.
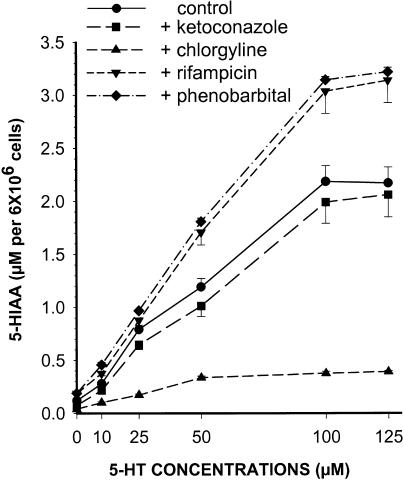
In hepatocytes incubated with 5-HT (0–125 μM), there was a dose-dependent increase in 5-HIAA production (Figure 2). The enzyme inducers RIF and PB dose dependently increased the production of 5-HIAA by around 50% (P<0.05, n=8), and slightly reduced 5-HT. On the other hand, KTZ, OME and PEITC reduced the concentrations of 5-HIAA by 10–30% and increased 5-HT concentrations by 10–50%. Finally, in hepatocytes incubated with increasing concentrations of 5-HT, chlorgyline reduced the formation of 5-HIAA by 79% (Figure 2).
Figure 2.
5-HIAA concentrations following 4 h of incubation of increasing concentrations of 5-HT with control hepatocytes and hepatocytes exposed for 72 h to KTZ (50 μM), chlorgyline (25 μM), RIF (10 μM) and PB (125 μM). Each point is mean±s.e. of n=8.
Human recombinant P450 isoforms
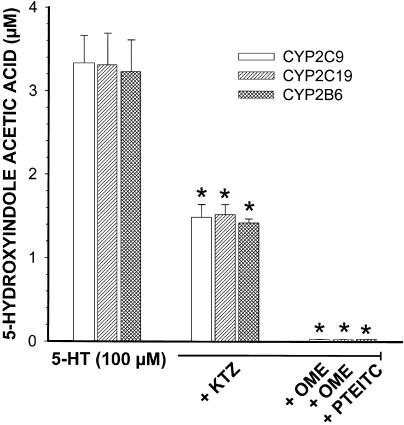
Incubation of 5-HT (0–125 μM) with human recombinant CYP2C9, CYP2C19 and CYP2B6 dose-dependently increased the concentrations of 5-HIAA to plateau at around 3.5 μM, with 5-HT concentrations of 100 μM or higher. Human recombinant CYP1A2, CYP3A4 and CYP2D6 did not biotransform 5-HT to 5-HIAA. KTZ reduced the formation of 5-HIAA in CYP2C9, CYP2C19 and CYP2B6 by 54, 52 and 55% (P<0.05, n=8), whereas OME and PEITC impeded 5-HIAA production almost completely (Figure 3).
Figure 3.
Formation of 5-HIAA by human recombinant CYP2B6, CYP2C9 and CYP2C19 after incubation with 5-HT (100 μM) alone or in the presence of KTZ (50 μM), OME (50 μM) and PEITC (50 μM). Each point is mean±s.e. of n=8.
NO• production
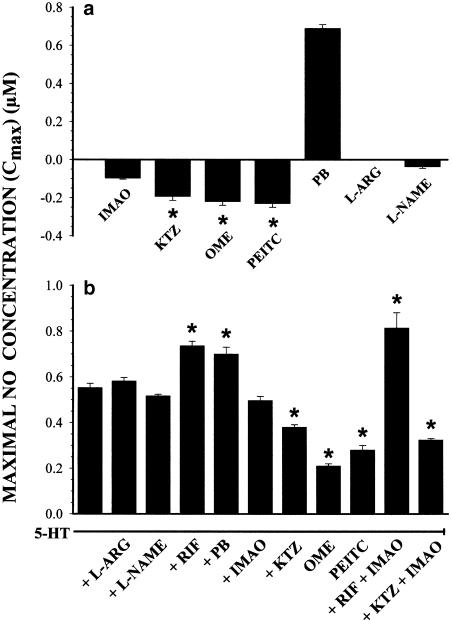
The mean baseline NO• concentration in 72 h hepatocytes was 0.28±0.02 μM. L-Arg and L-NAME did not affect baseline NO•. However, NO• concentrations were dose-dependently decreased by KTZ, OME and PEITC up to 32, 18 and 23% baseline values, respectively (P<0.05, n=8). In addition, chlorgyline reduced NO• concentrations by 21%. On the other hand, PB enhanced baseline NO• concentrations dose-dependently to a maximum increase of 316% (P<0.05, n=4). The predicted maximal effect or efficacy on baseline NO• concentrations (Cmax) of these enzyme inhibitors and inducers is depicted in Figure 4a.
Figure 4.
(a) Predicted maximal concentrations of baseline NO• (Cmax) in hepatocytes incubated for 72 h with chlorgyline (IMAO, 10–50 μM), KTZ (10–50 μM), OME (10–50 μM), β-phenylethylisothiocyanate (PEITC, 10–50 μM), PB (50–200 μM), L-Arg (0.1–1 mM) and L-NAME (0.1–1 mM) added to the incubation media at 68 h. (b) Predicted NO• Cmax in hepatocytes incubated for 72 h with L-ARG (0.5 mM), L-NAME (0.5 mM), RIF (10 μM), PB (125 μM), IMAO (25 μM), KTZ (25 μM), OME (50 μM) and PEITC (50 μM) in the presence of 5-HT (10–125 μM) added at 68 h. Vertical bars are mean±s.e. of n=4.
In hepatocytes, 5-HT increased the concentration of NO• dose-dependently, and predicted Cmax reached 0.6 μM. L-Arg and L-NAME did not modify the NO• concentrations or predicted Cmax in hepatocytes incubated with 5-HT. On the other hand, chlorgyline, KTZ, OME and PEITC reduced the production of NO• induced by 5-HT, as well as predicted Cmax of NO•. Finally, the enzyme inducers RIF and PB increased the concentrations of NO• generated by 5-HT, as well as predicted Cmax of NO• (Figure 4b).
Dynamic studies
Vasorelaxant effect of 5-HT metabolite
Preliminary experiments confirmed that, in rat aorta rings with endothelium, 50 μM of 5-HT produced a spontaneous contraction of 197±17% (n=6). However, when 5-HT (50 μM) was incubated in the presence of 50 μM ketanserin, the precontracted aorta ring showed a discrete relaxation of 11±0.89% (P=0.07; n=6) (data not shown). Interestingly, in the presence of ketanserin, the mild relaxation elicited by 5-HT was totally abolished in endothelium-denuded aorta rings (relaxation of 0%) (n=6) (data not shown). Chlorgyline (25 μM) did not modify the ability of 5-HT to induce a relaxation of the precontracted rat aorta ring (data not shown).
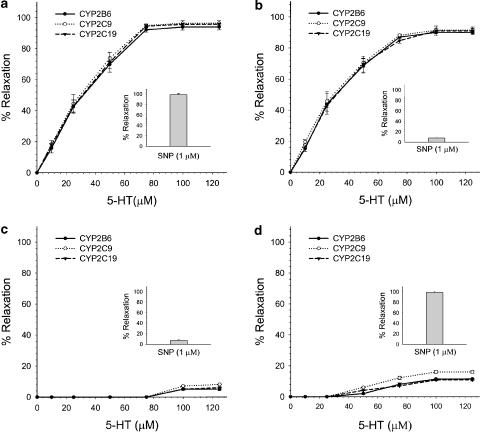
In rat aorta rings with endothelium precontracted by PE (1.6±0.1 g), 50 μM of 5-HT produced a powerful relaxation in the presence of human recombinant CYP2C9, CYP2C19 and CYP2B6, reaching a maximal relaxation of around 95±8.6% (n=6). The relaxation generated by 5-HT in the presence of CYP2C9, CYP2C19 and CYP2B6 was abolished by 50 μM of OME (n=3). The presence of CYP1A2, CYP3A4 or CYP2D6 did not induce any relaxation. In endothelium-denuded segments, a lower concentration of PE (10 nM) was required to induce a tension similar to that produced in endothelium-containing segments (1.55±0.12 × g), and a greater concentration of 5-HT (75 μM) was necessary to produce maximal relaxations of around 95±11% (n=6) (Figure 5a). The addition of CYP1A2, CYP3A4 or CYP2D6 did not produce relaxation. SNP induced a 100% relaxation of precontracted aorta rings with and without endothelium (n=6).
Figure 5.
(a) Concentration–response curves of the relaxation induced by 5-HT in the presence of CYP2B6, CYP2C9 and CYP2C19 in endothelium-denuded aortic rings precontracted with PE (100 nM). The inside histogram represents the relaxation elicited by SNP. (b) Effect of myoglobin (50 μM) on concentration–response curves of 5-HT- and SNP-induced relaxation in the presence of CYP2B6, CYP2C9 and CYP2C19 in endothelium-denuded rings precontracted with PE (5 mM). (c) Effect of methylene blue (30 μM) on concentration–response curves of 5-HT- and SNP-induced relaxation in the presence of CYP2B6, CYP2C9 and CYP2C19 in endothelium-denuded rings precontracted with PE (5 nM). (d) Effect of the catalase inhibitor 3-AT (50 mM), on concentration–response curves of curves of 5-HT- and SNP-induced relaxation in the presence of CYP2B6, CYP2C9 and CYP2C19 in endothelium-denuded rings precontracted with PE (5 nM). Each point is the mean±s.e. of n=6.
Characterization of the mechanism underlying the vasorelaxation induced by 5-HT
Myoglobin (NO• scavenger) added to the medium containing CYP2C9, CYP2C19 and CYP2B6 abolished SNP-induced relaxation of PE precontracted aorta rings. In contrast, myoglobin did not affect the vasorelaxation elicited by 5-HT (n=6) (Figure 5b). On the other hand, the relaxation induced by both SNP and 5-HT was abolished by methylene blue, an inhibitor of soluble guanylate cyclase (Figure 5c). These results suggested that the vasorelaxant factor produced by 5-HT is not NO•, but rather a byproduct that has to be converted to NO• in the smooth muscle.
Assuming that the product resulting from 5-HT deamination by CYP2C9, CYP2C19 and CYP2B6 was hydroxylamine (NH2OH), which is not scavenged by myoglobin (Taira et al., 1997), we hypothesized that hydroxylamine is transformed to NO• by catalase in the presence of hydrogen peroxide (H2O2). Pretreatment of the aortic rings with the catalase inhibitor 3-AT (Mian & Martin, 1995) almost completely abolished 5-HT-induced relaxations of PE precontracted endothelium-denuded rings, and that independently of the P450 isoforms present, for example, CYP2C9, CYP2C19 and CYP2B6 (n=6). As expected, 3-AT did not affect SNP-induced relaxation (Figure 5d).
When 5-HT was incubated with recombinant human P450 isoforms, NO• was not detected. Assuming that the product generated by P450 isoforms was hydroxylamine, recombinant human CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 were incubated with catalase (1000 U ml−1) and H2O2 (1 mM), in the presence of a NADPH regenerating system and several concentrations of 5-HT. Under these experimental conditions, 5-HT dose-dependently generated NO•. With CYP2C9, predicted Cmax of NO• was 2.04±0.07 μM (n=7), significantly greater (P<0.05) than the predicted Cmax for CYP2C19 and CYP2B6, for example, 1.48±0.04 and 1.30±0.10 μM, respectively (n=7). In the presence of catalase and H2O2, CYP1A2, CYP2D6 and CYP3A4 did not generate NO• (data not shown). In the presence of KTZ, the production of NO• and predicted Cmax was reduced to 0.496±0.030, 0.230±0.014 and 0.132±0.016 μM for CYP2C9, CYP2C19 and CYP2B6, respectively (n=7, P<0.05). On the other hand, OME and PEITC totally prevented the production of NO• by CYP2C9, CYP2C19 and CYP2B6.
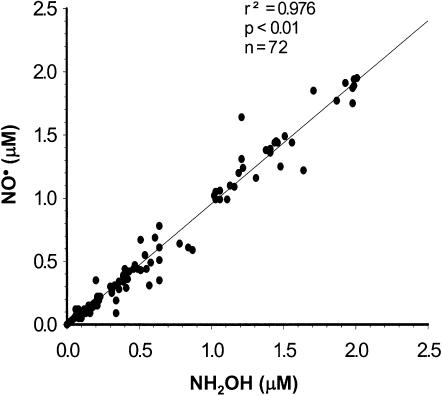
To confirm that the byproduct generated by P450 isoforms was hydroxylamine, recombinant human CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6 and CYP3A4 were incubated without catalase and H2O2, in the presence of the NADPH regenerating system, and several concentrations of 5-HT. Under these experimental conditions, hydroxylamine was generated dose-dependently only by CYP2B6, CYP2C9 and CYP2C19, and KTZ reduced its production, while OME and PEITC totally abrogated hydroxylamine production. There was a close relationship between the NO• produced by CYP2B6, CYP2C9 and CYP2C19 in the presence of 5-HT and catalase, and the concentration of hydroxylamine assayed in the absence of catalase (Figure 6).
Figure 6.
Concentration of NO• generated by the biotransformation of 5-HT by CYP2B6, CYP2C9 and CYP2C19 in the presence of catalase, as a function of hydroxylamine concentration produced by CYP2B6, CYP2C9 and CYP2C19 in the absence of catalase.
Discussion
The above investigations provide evidence that P450 isoforms CYP2C9, CYP2C19 and CYP2B6 biotransform 5-HT generating 5-HIAA and NH2OH, which is converted to NO• by catalase. It has always been assumed that 5-HT is exclusively biotransformed by MAO A; however, the present results demonstrate that several isoforms of the P450 contribute to 5-HT degradation. Incubation of hepatocytes with an IMAO A, chlorgyline, shows that P450 contributes with around 20% of the 5-HIAA produced. However, in the presence of P450 inducers, RIF and PB, the contribution of P450 to 5-HIAA formation is similar to that of MAO A. Can 5-HT biotransformation by P450 occur in the central nervous system? Circumstantial evidence supports this eventuality. Firstly, CYP2C9, CYP2C19 and CYP2B6 are expressed in different regions of the brain (Miksys & Tyndale, 2002), and, secondly, P450 inhibitors metyrapone and KTZ increase 5-HT concentrations in the brain (Kennett et al., 1985; Murphy, 1997; Leret et al., 1998; Healy et al., 1999). Moreover, phenytoin, a drug biotransformed by CYP2C9 and CYP2C19 (Giancarlo et al., 2001), increases 5-HT in the brain without enhancing its synthesis (Chadwick et al., 1978; Pratt et al., 1985), suggesting that phenytoin competitively inhibits 5-HT biotransformation.
The biotransformation of 5-HT by P450 yields hydroxylamine, which in the presence of catalase and H2O2 generates NO•. In hepatocytes incubated for 72 h, NOS2 was barely detectable, and the amount of NO• was independent of L-Arg and L-NAME, supporting that the source of NO• was primarily the P450. Moreover, specific inhibitors of CYP2B6, 2C9 and 2C19 reduced baseline NO• by around 80%. The source of the 20% remaining NO• is probably by the MAO A pathway. The experiments conducted with isolated aortic rings provide evidence that the relaxation elicited by 5-HT depends upon the formation of NO•. The relaxation is not due to hydroxylamine, because inhibition of catalase prevents the 5-HT-induced relaxation as well as the formation of NO•. The fact that methylene blue, an inhibitor of soluble guanylate cyclase (Gruetter et al., 1979), prevents the aortic relaxation triggered by SNP, a NO• donor (Cellek et al., 1996), as well as that elicited by 5-HT in the presence of P450 isoforms, further supports that NO• is responsible for the vasorelaxation by activating soluble guanylate cyclase (Murad, 1994).
In hepatocytes incubated for 72 h, the maximal efficacy to generate NO• from 5-HT, as illustrated by the predicted Cmax, was 0.55±0.02 μM. As a reference, in hepatocytes incubated for 24 h where NOS2 was expressed, the predicted Cmax of NO• was 0.95±0.04 μM in the presence of increasing doses of L-arg. The difference in NO• production between the two sources P450 and NOS2 was reduced when hepatocytes were exposed to P450 enzyme inducers. More than 15 years ago, the group of Moncada reported that EDRF was NO• which was generated from L-arg by NOS (Palmer et al., 1987). The present study is evidence that NO• can also be generated from 5-HT by CYP2C9, CYP2C19 and CYP2B6 in the presence of catalase. The actual results confirm the hypothesis raised by Mansuy and Boucher, who proposed that cytochrome P450 isoforms might be good candidates to catalyse oxidations similar to those performed by NOS, because of the great analogy between these two classes of heme-thiolate proteins (Morgan et al., 2001).
At the present time, there seems to be a fairly clear consensus that at least four distinct receptor types are involved in the endothelium-dependent relaxant effect of 5-HT. Relaxation to 5-HT is mediated via activation of smooth muscle 5-HT7 receptors (Hoyer et al., 2002) and 5-HT4 receptors (Prins et al., 1999). In addition, it has been proposed that endothelial 5-HT2B receptors mediate vascular relaxation by the release of NO• and increase in cyclic GMP in smooth muscle cells (Glusa & Pertz, 2000). Moreover, 5-HT counteracts smooth muscle contraction by stimulating sodium influx and hyperpolarizing the membrane, an effect mediated by 5-HT2A (Rhoden et al., 2000). On the other hand, several reports have shown that 5-HT triggers the relaxation of smooth muscle cells by mechanisms independent of 5-HT receptors, such as the relaxation induced in isolated guinea-pig gallbladder strips and in the rat anococcygeus muscle (Emre et al., 2000; Emre-Aydingoz et al., 2001). The present results add another mechanism to explain the receptor-independent smooth muscle relaxation elicited by 5-HT. It is noteworthy that, in the presence of ketanserin, 5-HT produced a slight relaxation (P=0.07) of precontracted aorta rings, an effect completely abolished in denuded aorta rings, suggesting that endothelial cytochrome P450 biotransformed 5-HT. Supporting this hypothesis, CYP2C and 2B are expressed in the endothelial cells of arteries (Fisslthaler et al., 2000; Earley et al., 2003).
The fact that myoglobin, an NO• scavenger (Taira et al., 1997), hinders SNP- but not 5-HT-induced relaxation, and that incubation of 5-HT with recombinant CYP2C9, 2C19 and 2B6 does not generate NO•, even if 5-HIAA is produced dose-dependently, is further proof that 5-HT biotransformation does not generate NO• directly. We speculate that the deamination of 5-HT generates ammonia, which is spontaneously transformed into water and hydroxylamine in the presence of H2O2. Hydroxylamine is a well-known vasodilator in endothelium-denuded arterial segments, via its decomposition to NO• (DeMaster et al., 1989). In agreement with the actual results, the relaxation of smooth muscle induced by hydroxylamine is endothelium-independent, requires catalase to be transformed to NO• (Craven et al., 1979), and the relaxation is blocked by methylene blue (Gruetter et al., 1979). Further supporting that 5-HT-induced vasorelaxation is mediated by a catalase-dependent by-product is the fact that addition of 5-HT, catalase and H2O2 to the incubation media with recombinant human CYP2C9, 2C19 and 2B6 generated 5-HIAA and NO•.
P450 is heavily implicated in the metabolism of arachidonic acid generating EETs, dihydroxyeicosatetraenoic acids (diHETEs) and hydroxyeicosatetranoic acid (HETE) derivatives with an important role as paracrine factors and second messengers in the regulation of vascular function. EETs are potent vasodilators, and CYP2C9 contributes to 50% of their production (Roman, 2002). The present report adds to the known role of CYP2C9, 2C19 and 2B6 in the formation of vasoactive substrates. Moreover, since NO• modulates the release of neurotransmitters, such as acetylcholine, catecholamines, excitatory and inhibitory amino acids, 5-HT, histamine and adenosine (Prast & Philippu, 2001), the present results raise several questions, primarily which is the role of the cytochrome P450 expression and activity in diseases such as depression and stress (McLeod et al., 2001), aggressive behaviour (Chiavegatto et al., 2001), hyperphagia (Yamada et al., 2000), hypertension (de Wardener, 2001), learning and memory (Yamada et al., 1995) and cephalalgia (D'Andrea, 1999).
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (MOP – 43925 and MT-10605). We are grateful to Ms Lucie Héroux, Sanäe Yamaguchi and Linda Beaupré for their excellent technical assistance.
Abbreviations
- 3-AT
3-amino-1,2,4-triazole
- EDHF
endothelium-derived hyperpolarizing factor
- EETs
epoxyeicosatrienoic acids
- H2O2
hydrogen peroxide
- HETE
hydroxyeicosatetranoic acid
- diHETEs
dihydroxyeicosatetraenoic acids
- 5-HIAA
5-hydroxyindole acetic acid
- 5-HT
5-hydroxytryptamine
- KTZ
ketoconazole
- L-Arg
L-arginine
- L-NAME
N,G-nitro-L-arginine methyl ester
- MAO A
monoamine oxidase A
- NO•
nitric oxide
- NOS2
nitric oxide synthase 2
- OME
omeprazole
- PB
phenobarbital
- PE
phenylephrine
- PEITC
β-phenylethyl isothiocyanate
- RIF
rifampicin
- SNP
sodium nitroprusside
References
- BOUCHER J.L., GENET A., VADON S., DELAFORGE M., HENRY Y., MANSUY D. Cytochrome P450 catalyzes the oxidation of N omega-hydroxy-L-arginine by NADPH and O2 to nitric oxide and citrulline. Biochem. Biophys. Res. Commun. 1992;187:880–886. doi: 10.1016/0006-291x(92)91279-y. [DOI] [PubMed] [Google Scholar]
- BRUNING T.A., CHANG P.C., BLAUW G.J., VERMEIJ P., VAN ZWIETEN P.A. Serotonin-induced vasodilatation in the human forearm is mediated by the ‘nitric oxide-pathway': no evidence for involvement of the 5-HT3-receptor. J. Cardiovasc. Pharmacol. 1993;22:44–51. doi: 10.1097/00005344-199307000-00008. [DOI] [PubMed] [Google Scholar]
- CAPDEVILA J., CHOCOS N., WERRINGLOER J., PROUGH R.A., ESTABROOK R.W. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. U.S.A. 1981;78:5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELLEK S., KASAKOV L., MONCADA S. Inhibition of nitrergic relaxations by a selective inhibitor of the soluble guanylate cyclase. Br. J. Pharmacol. 1996;118:137–140. doi: 10.1111/j.1476-5381.1996.tb15376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHADWICK D., GORROD J.W., JENNER P., MARSDEN C.D., REYNOLDS E.H. Functional changes in cerebral 5-hydroxytryptamine metabolism in the mouse induced by anticonvulsant drugs. Br. J. Pharmacol. 1978;62:115–124. doi: 10.1111/j.1476-5381.1978.tb07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIAVEGATTO S., DAWSON V.L., MAMOUNAS L.A., KOLIATSOS V.E., DAWSON T.M., NELSON R.J. Brain serotonin dysfunction accounts for aggression in male mice lacking neuronal nitric oxide synthase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1277–1281. doi: 10.1073/pnas.031487198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVEN P.A., DERUBERTIS R.F., PRATT W.D. Electron spin resonance study of the role of NO• catalase in the activation of guanylate cyclase by NaN3 and NH2OH. Modulation of enzyme responses by heme proteins and their nitrosyl derivatives. J. Biol. Chem. 1979;254:8213–8222. [PubMed] [Google Scholar]
- D'ANDREA G. Nitric oxide pathway, Ca2+ and serotonin. Cephalalgia. 1999;19:767. doi: 10.1046/j.1468-2982.1999.19097656.x. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H.E. The hypothalamus and hypertension. Physiol. Rev. 2001;81:1599–1658. doi: 10.1152/physrev.2001.81.4.1599. [DOI] [PubMed] [Google Scholar]
- DEMASTER E.G., RAIJ L., ARCHER S.L., WEIR E.K. Hydroxylamine is a vasorelaxant and a possible intermediate in the oxidative conversion of L-arginine to nitric oxide. Biochem. Biophys. Res. Commun. 1989;163:527–533. doi: 10.1016/0006-291x(89)92169-4. [DOI] [PubMed] [Google Scholar]
- EARLEY S., PASTUSZYN A., WALKER B.R. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H127–H136. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- EL-KADI A.O.S., MAURICE H., ONG H., DU SOUICH P. Down regulation of the hepatic cytochrome P450 by an acute inflammatory reaction: implication of human and animal serum, and intrahepatic mediators. Br. J. Pharmacol. 1997;121:1164–1170. doi: 10.1038/sj.bjp.0701232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMRE S., ERDEM S.R., TUNCER M. Does serotonin relax the rat anococcygeus muscle via 5-HT7 receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:96–100. doi: 10.1007/s002100000245. [DOI] [PubMed] [Google Scholar]
- EMRE-AYDINGOZ S., ERDEM S.R., TUNCER M. Relaxation induced by serotonin and sumatriptan in isolated guinea pig gallbladder strips. Res. Exp. Med. 2001;200:175–182. [PubMed] [Google Scholar]
- FISSLTHALER B., HINSCH N., CHATAIGNEAU T., POPP R., KISS L., BUSSE R., FLEMING I. Nifedipine increases cytochrome P4502C expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension. 2000;36:270–275. doi: 10.1161/01.hyp.36.2.270. [DOI] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FOLK J.E., CHUNG S.I. Transglutaminases. Methods Enzymol. 1985;113:358–375. doi: 10.1016/s0076-6879(85)13049-1. [DOI] [PubMed] [Google Scholar]
- FRADETTE C., BLEAU A.M., PICHETTE V., CHAURET N., DU SOUICH P. Hypoxia-induced down-regulation of CYP1A1/1A2 and up-regulation of CYP3A6 involves serum mediators. Br. J. Pharmacol. 2002;137:881–891. doi: 10.1038/sj.bjp.0704933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERVASINI G., MARTINEZ C., AGUNDEZ J.A., GARCIA-GAMITO F.J., BENITEZ J. Inhibition of cytochrome P450 2C9 activity in vitro by 5-hydroxytryptamine and adrenaline. Pharmacogenetics. 2001;11:29–37. doi: 10.1097/00008571-200102000-00004. [DOI] [PubMed] [Google Scholar]
- GIANCARLO G.M., VENKATAKRISHNAN K., GRANDA B.W., VON MOLTKE L.L., GREENBLATT D.J. Relative contributions of CYP2C9 and 2C19 to phenytoin 4-hydroxylation in vitro: inhibition by sulfaphenazole, omeprazole, and ticlopidine. Eur. J. Clin. Pharmacol. 2001;57:31–36. doi: 10.1007/s002280100268. [DOI] [PubMed] [Google Scholar]
- GLUSA E., PERTZ H.H. Further evidence that 5-HT-induced relaxation of pig pulmonary artery is mediated by endothelial 5-HT(2B) receptors. Br. J. Pharmacol. 2000;130:692–698. doi: 10.1038/sj.bjp.0703341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISHAM M.B., JOHNSON G.G., LANCASTER J.R., JR Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268:237–246. doi: 10.1016/s0076-6879(96)68026-4. [DOI] [PubMed] [Google Scholar]
- GRUETTER C.A., BARRY B.K., MCNAMARA D.B., GRUETTER D.Y., KADOWITZ P.J., IGNARRO L.J. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J. Cyclic Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- HEALY D.G., HARKIN A., CRYAN J.F., KELLY J.P., LEONARD B.E. Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacology (Berl.) 1999;145:303–308. doi: 10.1007/s002130051062. [DOI] [PubMed] [Google Scholar]
- HILDEBRANDT A.G. The binding of metyrapone to cytochrome P-450 and its inhibitory action on microsomal hepatic mixed function oxidation reactions. Biochem. Soc. Symp. 1972;34:79–102. [PubMed] [Google Scholar]
- HOYER D., HANNON J.P., MARTIN G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- KENNETT G.A., DICKINSON S.L., CURZON G. Central serotonergic responses and behavioural adaptation to repeated immobilisation: the effect of the corticosterone synthesis inhibitor metyrapone. Eur. J. Pharmacol. 1985;119:143–152. doi: 10.1016/0014-2999(85)90290-0. [DOI] [PubMed] [Google Scholar]
- LERET M.L., ANTONIO M.T., ARAHUETES R.M. Effect of metyrapone administration in pregnant rats on monoamine concentration in fetal brain. Life Sci. 1998;62:1943–1948. doi: 10.1016/s0024-3205(98)00163-5. [DOI] [PubMed] [Google Scholar]
- LOMAESTRO B.M., PIATEK M.A. Update on drug interactions with azole antifungal agents. Ann. Pharmacother. 1998;32:915–928. doi: 10.1345/aph.17271. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MCLEOD T.M., LOPEZ-FIGUEROA A.L., LOPEZ-FIGUEROA M.O. Nitric oxide, stress, and depression. Psychopharmacol. Bull. 2001;35:24–41. [PubMed] [Google Scholar]
- MIAN K.B., MARTIN W. The inhibitory effect of 3-amino-1,2,4-triazole on relaxation induced by hydroxylamine and sodium azide but not hydrogen peroxide or glyceryl trinitrate in rat aorta. Br. J. Pharmacol. 1995;116:3302–3308. doi: 10.1111/j.1476-5381.1995.tb15139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKSYS S.L., TYNDALE R.F. Drug-metabolizing cytochrome P450s in the brain. J. Psychiatry Neurosci. 2002;27:406–415. [PMC free article] [PubMed] [Google Scholar]
- MORGAN E.T., ULLRICH V., DAIBER A., SCHMIDT P., TAKAYA N., SHOUN H., MCGIFF J.C., OYEKAN A., HANKLE C.J., CAMBELL W.B., PARK C.S., KANG J.S., YI H.G., CHA Y.N., MANSUY D., BOUCHER J.L. Cytochromes P450 flavin monoxygenases-targets and sources of nitric oxide. Drug Met. Disp. 2001;29:1366–1376. [PubMed] [Google Scholar]
- MURAD F. Regulation of cytosolic guanylyl cyclase by nitric oxide: the NO-cyclic GMP signal transduction system. Adv. Pharmacol. 1994;26:19–33. doi: 10.1016/s1054-3589(08)60049-6. [DOI] [PubMed] [Google Scholar]
- MURPHY B.E. Antiglucocorticoid therapies in major depression: a review. Psychoneuroendocrinology. 1997;22 Suppl 1:S125–S132. [PubMed] [Google Scholar]
- MYLECHARANE E.J. Mechanisms involved in serotonin-induced vasodilatation. Blood Vessels. 1990;27:116–126. doi: 10.1159/000158802. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PRAST H., PHILIPPU A. Nitric oxide as modulator of neuronal function. Prog. Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. [DOI] [PubMed] [Google Scholar]
- PRATT J.A., JENNER P., MARSDEN C.D. Comparison of the effects of benzodiazepines and other anticonvulsant drugs on synthesis and utilization of 5-HT in mouse brain. Neuropharmacology. 1985;24:59–68. doi: 10.1016/0028-3908(85)90096-6. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., VAN HASELEN J.F., LEFEBVRE R.A., BRIEJER M.R., AKKERMANS L.M., SCHUURKES J.A. Pharmacological characterization of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle. Br. J. Pharmacol. 1999;127:1431–1437. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROJEAN D., BAUNE B., FARINOTTI R., FLINOIS J.P., BEAUNE P., TABURET A.M., DUCHARME J. In vitro metabolism of chloroquine identification of CYP2C8, CYP3A4 and CYP2D6 as the main isoforms catalyzing N-desethylchloroquine formation. Drug Metab. Dispos. 2003;31:748–754. doi: 10.1124/dmd.31.6.748. [DOI] [PubMed] [Google Scholar]
- RHODEN K.J., DODSON A.M., KY B. Stimulation of the Na(+)–K(+) pump in cultured guinea pig airway smooth muscle cells by serotonin. J. Pharmacol. Exp. Ther. 2000;293:107–112. [PubMed] [Google Scholar]
- ROMAN R.J. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol. Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- SAXENA P.R., DE VRIES P., VILLALÓN C.M. 5-HT1-like receptors: a time to bid goodbye. Trends Pharmacol. Sci. 1998;19:311–316. doi: 10.1016/s0165-6147(98)01228-0. [DOI] [PubMed] [Google Scholar]
- SEGLEN P.O. Preparation of isolated rat liver cells. Methods Cell. Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- TAIRA J., MISIK V., RIESZ P. Nitric oxide formation from hydroxylamine by myoglobin and hydrogen peroxide. Biochem. Biophys. Acta. 1997;1336:502–508. doi: 10.1016/s0304-4165(97)00064-0. [DOI] [PubMed] [Google Scholar]
- THOLLON C., FOURNET-BOURGUIGNON M.P., SABOUREAU D., LESAGE L., REURE H., VANHOUTTE P.M, VILAINE J.P. Consequences of reduced production of NO on vascular reactivity of porcine coronary arteries after angioplasty: importance of EDHF. Br. J. Pharmacol. 2002;136:1153–1161. doi: 10.1038/sj.bjp.0704828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURU H., NAKAI S., UCHIYAMA T., TERANISHI Y. Endothelium-independent relaxant effect of 5-hydroxytryptamine (5-HT) on the isolated rabbit facial vein. J. Smooth Muscle Res. 1998;34:101–110. doi: 10.1540/jsmr.34.101. [DOI] [PubMed] [Google Scholar]
- YAMADA J., SUGIMOTO Y., KUNITOMO M. A nitric oxide synthase inhibitor reduces hyperphagia induced in rats by the 5-HT(1A) receptor agonist, 8-OH-DPAT, independently of hypothalamic serotonin metabolism. Eur. J. Pharmacol. 2000;402:247–250. doi: 10.1016/s0014-2999(00)00507-0. [DOI] [PubMed] [Google Scholar]
- YAMADA K., NODA Y., NAKAYAMA S., KOMORI Y., SUGIHARA H., HASEGAWA T., NABESHIMA T. Role of nitric oxide in learning and memory and in monoamine metabolism in the rat brain. Br. J. Pharmacol. 1995;115:852–858. doi: 10.1111/j.1476-5381.1995.tb15011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI N. In vivo evidence for adrenal catecholamine release mediated by nonnicotinic mechanism: local medullary effect of VIP. Am. J. Physiol. Regulat. Integrat. Comp. Physiol. 1993;265:R766–R771. doi: 10.1152/ajpregu.1993.265.4.R766. [DOI] [PubMed] [Google Scholar]