Abstract
Bovine adrenal medulla 22 (BAM22) peptide is one of the cleavage products of proenkephalin A. It binds with high affinity to both opioid receptors and a newly discovered receptor in vitro. This latter receptor was first named sensory neuron-specific receptor and is here named BAM peptide-activated receptor with non-opioid activity (BPAR). BPAR is uniquely distributed in small-diameter DRG neurons, most of which are associated with the IB4 class of nociceptor afferent.
The present study examined the effects of intrathecal administration of BAM22 on formalin-induced nocifensive behaviors and tail-withdrawal latency in the rat.
Intrathecal (i.t.) administration of BAM22 decreased nocifensive behavior scores, measured as the sum of flinching and lifting/licking, in the first and second phases of the formalin test. This decrease was partially attenuated by systemic injection of naloxone.
In the presence of naloxone, i.t. BAM22 produced a dose-dependent suppression of the nocifensive behaviors observed during the formalin test. The ratio of the efficacy of BAM22 (5 nmol) in the presence of naloxone over that in the absence of naloxone was 0.65 for flinching and 0.74 for lifting/licking in the second phase.
BAM22 at a dose of 5 nmol increased the tail-withdrawal latency by 193 and 119% of baseline in the absence and presence of naloxone, respectively.
Systemic administration of naloxone alone enhanced the nocifensive behaviors in the second, but not in the first phase of the formalin test. Naloxone treatment did not alter the tail-withdrawal latency.
These data confirm earlier in vitro data showing that BAM22 has both opioid and non-opioid biological actions. The non-opioid action of BAM22 involves inhibition of acute and persistent nociceptive behaviors at the spinal level, presumably mediated via BPAR.
The name suggested for this novel receptor, its potential physiological function and its ligand are discussed.
Keywords: BAM22, intrathecal, formalin test, tail-withdrawal test, acute pain, persistent pain, spinal cord
Introduction
Bovine adrenal medulla 22 (BAM22) is a peptide with 22 amino acids that is one of the cleavage products of proenkephalin A, the precursor of Leu- and Met-enkephalin, in the adrenal medulla (Dores et al., 1990). BAM22 is also found in the central nervous system (Khachaturian et al., 1983; Pittius et al., 1984), including the cerebral cortex, caudate putamen (Bloch et al., 1983), hippocampus (McGinty, 1985), hypothalamus (Hollt et al., 1982), periaqueductal gray (Merchenthaler et al., 1986), midbrain and spinal cord (Reiner, 1987; Pittius et al., 1984). Nerve fiber- and terminal-like processes containing BAM22 have been found to be concentrated in the superficial laminae of the dorsal horn in the lumbar spinal cord (Maderdrut et al., 1986). However, the function of the peptide remains unclear.
BAM22 has the classical opioid YGGFM (Met-enkephalin) motif. It binds to μ- (Garzon et al., 1983; Dray et al., 1985), δ- (Lembo et al., 2002) and κ-opioid receptors (Quirion & Weiss, 1983; Davis et al., 1990; Boersma et al., 1994) with a high affinity; in vitro and in vivo, and this binding is naloxone-displaceable (Boersma et al., 1994). The BAM22 peptide exhibits opioid activity. For example, it inhibits electrically stimulated contraction of the ileum and vas deferens (Sanchez-Blazquez & Garzon, 1985) and reflex bladder contraction (Dray et al., 1985). Moreover, BAM22-induced effects are antagonized by naloxone (Davis et al., 1990). BAM22 may also exert a protective action, as its level in plasma is increased during stress such as injury, shock and stroke (Swain et al., 1994). The opioid activity of BAM22 implies that it may play a role in nociceptive processing. However, very little has been done to determine the involvement of BAM22 in pain mechanisms, and the limited results reported have been controversial. It was found that i.c.v. administration of BAM22 in mice produced a substantial analgesic effect, with a high potency similar to morphine (Hollt et al., 1982). This result was brought into question by a study showing that i.c.v. administration of BAM22 produced antinociception, but not in a dose-dependent manner, and intrathecal (i.t.) administration had no effect in mice (Fang et al., 1986).
It was recently found that BAM22 exerts a dual action in vitro. Besides activating opioid receptors, BAM22 also binds to a novel receptor, named the sensory neuron-specific receptor (SNSR). This receptor is uniquely expressed in a subpopulation of small-diameter neurons in the dorsal root and trigeminal ganglia in rat and human spinal cord (Lembo et al., 2002). This receptor may be more appropriately named the BAM peptide-activated receptor with non-opioid activity (BPAR, see Discussion). As the small-diameter neurons in the dorsal root and trigeminal ganglia are believed to mediate nociceptive transmission (Snider & McMahon, 1998), it is possible that BPAR is involved in nociceptive processing or modulation. The present study was designed to examine the effects of i.t. administration of BAM22 on nociceptive responses observed during the formalin and tail-withdrawal (TW) tests. Particular attention was focused on identifying a potential function for BPAR.
Methods
Experimental animals
Adult male Sprague–Dawley rats weighing 230–300 g were housed three per cage in a room maintained at 22±0.5°C with an alternating 12-h light–dark cycle. Food and water were available ad libitum. Animals were used only once and were always carefully handled throughout the experiments to minimize behavioral stress. The study was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee, and was approved by the Ethics Committee on Animal Experimentation of the University. Prior to behavioral testing, the rats were acclimatized to the laboratory and habituated to individual observation boxes or devices every day for at least 5 days. The behavioral studies were conducted using blind testing protocols, and were repeated by different observers.
Acute i.t. administration
BAM22 or vehicle was administered i.t. by percutaneous lumbar puncture (Hylden & Wilcox, 1980; Mestre et al., 1994; De la Calle & Paino, 2002). Rats were briefly anesthetized with halothane and placed on a board in such a way that the spine was curved at the level of the L4–L5 vertebrae. Lumbar puncture was performed using a 50-μl microinjection syringe (Hamilton) and a 27-gauge needle was inserted between the L5 and L6 vertebrae. A characteristic tail flick indicated penetration of the subarachnoid space and i.t. delivery of the drug or vehicle. Following injection of 20 μl of drug solution, the animal was returned to the testing chamber for recovery. All animals recovered from anesthesia within 1 min of completion of the lumbar puncture.
Formalin test
The formalin test was conducted in a clear plastic chamber (30 × 30 × 30 cm3) with a mirror placed at a 45° angle beneath the floor to allow an unobstructed view of the paws. Nociceptive behaviors were induced by injecting 50 μl of a 2.5% formalin solution subcutaneously into the plantar surface of one hindpaw with a 27-gauge needle. Immediately after the formalin injection, the animal was returned to the testing box. The nocifensive scores were recorded for 60 min after formalin injection using the following criteria: flinching–the paw was rapidly and briefly withdrawn or shaken; lifting–the injected paw was elevated and not in contact with floor; licking–the paw was licked or bitten. The formalin-evoked response was divided into two phases. The first phase was characterized by an initial burst of nociceptive behaviors after the chemical injection, which lasted approximately 10 min. This was followed by a silent period of 5 min. The second phase started 15 min after the formalin injection and the responses peaked at 35–40 min and disappeared at 55–60 min. Early (15–40 min) and late (40–60 min) subdivisions of the second phase were also monitored because pilot experiments suggested that the effect of BAM22 in the presence of naloxone lasted 40–50 min. Formalin-induced pain behaviors were quantified by recording the time in seconds that was spent lifting plus licking (lifting/licking), and counting the number of flinches. Two rats were scored at the same time using a computer program developed in-house. The cumulative response time spent lifting and licking the injected paw and the number of flinches were recorded for each 5 min block. At the end of the observation period, the animals were immediately killed with an overdose of barbiturate.
Tail-withdrawal test
Rats were placed in a device that held the body without restraining the head or legs. The distal 5 cm of the tail was blackened and dipped into warm water, and the time that elapsed before the rat flicked its tail was recorded as the TW latency. The water temperature was adjusted to 51°C as this temperature produced an average baseline TW latency of approximately 6 s in naive rats. The TW latency for any test time point was derived from the average of three measurements taken at 1.5 min intervals. A cutoff time was set at 20 s to prevent tissue damage. Baseline latency was measured 10 min before drug or vehicle administration, and was determined by averaging five measurements.
Drugs
BAM22 was obtained from Bachem (AG, Switzerland) and naloxone hydrochloride from Sigma (St Louis, MO, U.S.A.). The drugs were dissolved in sterile saline. Naloxone was administered intraperitoneally at a dose of 1 mg kg−1 (Nozaki-Taguchi & Yaksh, 1999; Orii et al., 2002; Shannon & Lutz, 2002) in a total volume of 1 ml kg−1. Formalin (2.5%) was prepared from a saturated solution of formaldehyde (38%, Shengong Chemicals, Shanghai, China) with sterile saline.
Statistical analysis
Formalin test
The time-course data are presented as mean values for flinches and lifting/licking±s.e.m. per 5 min. For the dose–response analysis, data from the first (0–10 min) and second phases (15–60 min) were summed separately. The second phase was further divided into early (15–40 min) and later (40–60 min) periods. One-way ANOVA was used to evaluate dose dependence. For multiple comparisons, Tukey's test was used to compare the nociceptive behaviors between control and the drug-treated groups. The efficiency of the drug was defined as (1−after-treatment level/pre-treatment level) × 100%.
TW test
The unpaired t-test (two-tailed) was used to detect the difference in TW latencies between the drug-treated and vehicle groups. Data for TW latencies are presented as percentages of baseline latency (% baseline). Differences between values for which P<0.05 were considered statistically significant.
Results
Formalin test
In animals administered vehicle (saline) i.t. followed by an injection of 2.5% formalin 10 min later, there was a highly reliable biphasic display of flinching and lifting/licking of the injected paw (Figure 1; n=8). These behaviors were comparable to those previously reported (Hong & Abbott, 1996), and provided a sensitive measure of both increases and decreases in pain levels.
Figure 1.
Effects of i.t. administration of BAM22 on the nocifensive response to an intraplantar injection of formalin. (a) and (b) show the time course of the effects of BAM22 (1.5 and 5 nmol) on flinching (a) and lifting/licking (b) evoked by 2.5% formalin. Saline or drug was administered i.t. 10 min before the formalin injection. The number of flinches and the time spent lifting/licking per 5 min were plotted. Each data point and error bar represent the group mean±s.e.m. (c) and (d) are the histograms representing formalin-induced nocifensive behaviors, summed as the number of flinches (c) and time spent lifting licking (d). The data are presented as group means±s.e.m. averaged over the first (0–10 min) and second (15–60 min) phases. The second phase was further divided into early (15–40 min) and later (40–60 min) periods. *P<0.05, **P<0.01 and ***P<0.001, compared with the response of saline-treated rats (one-way ANOVA followed by Tukey's multiple comparison post hoc test).
BAM22 administered i.t. at a dose of 1.5 nmol 10 min before 2.5% formalin had no effect on the formalin-induced flinching and lifting/licking (P>0.05, n=7; Figure 1). However, 5 nmol of BAM22 significantly inhibited these nocifensive behaviors (flinching and lifting/licking; Figure 1; n=6). At this dose, the drug decreased flinching by 49.8% (P<0.01 compared to the vehicle group), but did not change lifting/licking in the first phase. In the second phase, flinching was reduced by 48.9% (P<0.01) and lifting/licking by 41.7% (P<0.05). The decreases in lifting/licking were 42.5 and 40.3% in, respectively, the early (15–40 min) and later periods (40–60 min; both P<0.05) of the second phase.
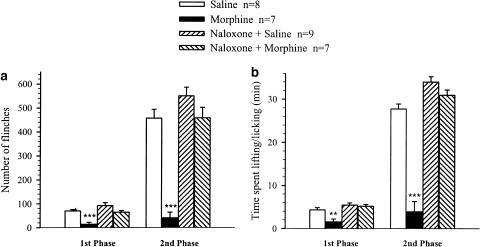
The non-selective opioid receptor antagonist naloxone was given to block opioid receptors and thus determine if BAM22 has dual activity in vivo. As an initial step, to determine whether 1 mg kg−1 of naloxone was sufficient to block opioid receptors, the effect of naloxone was tested on morphine-induced antinociception. Morphine at a dose of 20 μg was administered intrathecally 10 min before 2.5% formalin. Figure 2 illustrates that in the presence of morphine formalin produced very few flinching and lifting/licking behaviors (n=7). When 1 mg kg−1 of naloxone was administered i.p. 2 min before morphine, subsequent injection of formalin evoked clear nociceptive behaviors (n=7, Figure 2), which resembled those in the group pretreated with naloxone (1 mg kg−1, i.p.) plus saline (20 μl, i.t.; P>0.05, n=9; Figure 2). In the latter group, naloxone augmented the formalin-induced nocifensive behaviors in the second, but not the first phase, compared to the group without naloxone. The numbers of flinches were 457±27 and 562±34 (P<0.05), and the times spent lifting/licking were 27.8±1.1 and 34.1±1.2 min (P<0.01) in the groups with and without naloxone, respectively.
Figure 2.
Effects of i.t. administration of morphine on formalin-induced nocifensive behaviors in the absence and presence of naloxone. Naloxone (1 mg kg−1) was injected i.p. 2 min before i.t. morphine (20 μg) or saline (20 μl), followed by formalin injection 10 min later. The number of flinches (a) and the time spent in lifting/licking (b) were summed over the first (0–10 min) and second (15–60 min) phases. Pretreatment with naloxone completely abolished the analgesic effects of morphine. **P<0.01 and ***P<0.001, compared with the respective responses (unpaired t-test).
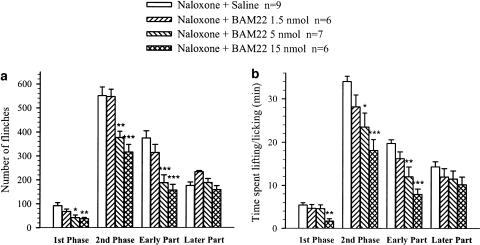
The effects of i.t. BAM22 following pretreatment with naloxone were examined. Figure 3 shows that i.t. administration of BAM22 produced a dose-related suppression of flinching and lifting/licking behaviors in the presence of naloxone. At a dose of 1.5 nmol, BAM22 had no effect on the formalin-induced behaviors (P>0.05; n=6). However, administration of 5 nmol of BAM22 reduced the number of flinches by 49.8 and 55.5% in the first (P<0.01, n=7) and second (P<0.001) phases, respectively. The drug decreased the time spent lifting/licking only in the second phase by 31% (P<0.05). Interestingly, the decreases in flinching and lifting/licking mainly occurred in the early period of the second phase (50 and 39%, P<0.01 and 0.05, respectively) and not in the later period. At a dose of 15 nmol, BAM22 suppressed all the nocifensive behaviors in both the first and second phases; flinching was reduced by 60 and 44%, respectively, while lifting/licking was reduced by 69 and 47%, respectively (P<0.01–0.001, n=6). Again, the changes were uniformly expressed over the early, but not later, period of the second phase (see Figure 3).
Figure 3.
Dose–response plot for the effects of i.t. administration of BAM22 on formalin-induced nocifensive behaviors in the presence of naloxone. Rats were treated with naloxone (1 mg kg−1, i.p.) 2 min before i.t. BAM22 (1.5, 5 and 15 nmol) or saline, followed by formalin injection 10 min later. Histograms represent the sums of formalin-induced flinching (a) and lifting/licking (b) behaviors. The data are presented as group means±s.e.m. averaged over the first (0–10 min) and second (15–60 min) phases, and the early (15–40 min) and later (40–60 min) periods of the second phase. *P<0.05, **P<0.01 and ***P<0.001, compared with the response of saline-treated rats (one-way ANOVA followed by Tukey's multiple comparison post hoc test).
The efficacy of i.t. BAM22 was determined by analyzing the effect of the drug on nocifensive behaviors with and without pretreatment with naloxone. Data are presented in Table 1. The efficacy of BAM22 on flinching in the first phase was similar in the absence and presence of naloxone: BAM22 reduced flinching by 49.5 and 54.5%, respectively, of the respective control values. However, in the second phase, BAM22 reduced flinching by 48.9% in the absence of naloxone and by only 31.6% in the presence of naloxone. The decreases in lifting/licking were 41.7 and 31%, respectively, under the corresponding conditions. The ratio of the efficacy of BAM22 in the presence of naloxone over that in the absence of naloxone was 0.65 for flinching and 0.74 for lifting/licking.
Table 1.
Comparison of antinociceptive effects of BAM22 (5 nmol, i.t.) in the absence and presence of naloxone
| Saline (A) | BAM22 (B) | Effect (1-B/A) × 100% (S) | Naloxone (C) | Naloxone−BAM22 (D) | Effect (1−D/C) × 100% (N) | Comparison of Efficiency (N/S) | |
|---|---|---|---|---|---|---|---|
| Flinches First Phase | 70±5.4 | 35.5±9.8 | 49.5 | 92±12.7 | 42±10.6 | 54.5 | 1.11 |
| Flinches Second Phase | 457±39 | 234±22 | 48.9 | 551±36 | 377±26 | 31.6 | 0.65 |
| Lifting+licking Second Phase | 27.8±1.1 | 16.2±2.0 | 41.7 | 34.1±1.2 | 23.5±3.2 | 31 | 0.74 |
TW response
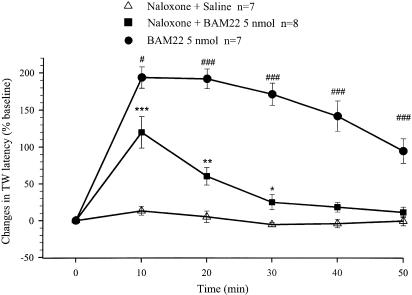
To minimize any adverse and sustained effects of the heat stimulus in this test, the TW response was recorded every 10 min following drug administration, for 50–90 min. I.t. administration of 5 nmol of BAM22 potently and persistently increased the TW latency. Most rats responded to the heat stimulus with a TW latency longer than the cutoff time, even at 10 min, the earliest recording time following drug administration (Figure 4, n=7). The increase in latency lasted up to 90 min.
Figure 4.
Effects of i.t. administration of BAM22 or saline on the TW latency in the absence and presence of naloxone. Naloxone (1 mg kg−1, i.p.) was injected 2 min before i.t. BAM22 (5 nmol) or saline, which was the start of latency measurements (0 min). Data are presented as percentage of increases in the baseline value of TW latency. Each data point and error bar represents the group mean±s.e.m. of 6–8 rats. Administration of BAM22 produced large long-duration increases in the TW latency. These increases were attenuated by systemic injection of naloxone, but were still significantly different from the effects in the vehicle group. The TW latency baselines in these three groups were 6.7±0.3, 6.1±0.3 and 5.8±0.3 s, respectively. **P<0.01, compared with the responses in the saline-treated groups (t-test); ##P<0.01 and ###P<0.001, compared with the response of the BAM22 groups in the presence of naloxone (t-test).
Rats were injected intraperitoneally with naloxone (1 mg kg−1) 2 min before i.t. BAM22 (5 nmol). The drug produced an increase in the TW latency and the time course of the effect is illustrated in Figure 4 (n=8). The drug effect peaked at 10 min, with an increase in TW latency of 120±21%, and lasted approximately 30 min. As the TW latency was not recorded as real time for some rats in the group treated with BAM22 alone, the ratio of the effect of BAM22 in the absence and presence of naloxone was not analyzed.
Another group of rats was treated with naloxone (1 mg kg−1, i.p.) 2 min before i.t. saline (n=7; Figure 4). This treatment did not generate any significant effect on the TW latency compared to the pretreatment baseline. The TW latency baselines in these three groups were not significantly different (6.7±0.3, 6.1±0.3 and 5.8±0.3 s, respectively).
Discussion
The present study examined the effects of i.t. administration of BAM22 on acute and persistent nociception. We found that BAM22 suppressed pain-related behaviors in the formalin test, and this suppression occurred in both the first and second phases. Systemic injection of naloxone at a dose that completely abolished the analgesic effect of morphine partially attenuated the antinociceptive action of BAM22. In the presence of naloxone, BAM22 dose-dependently inhibited nocifensive behaviors in the formalin test; the efficacy of the peptide was reduced by approximately one-third in the second phase compared to the group without naloxone. BAM22 also increased the TW latency in the absence and presence of naloxone with different efficacies. Naloxone alone enhanced formalin-induced behaviors in the second phase.
The biphasic behavioral responses evoked by injection of formalin into the hindpaw (Dubuisson & Dennis, 1977; Tjolsen et al., 1992) are paralleled by increased blood pressure and heart rate (Taylor et al., 1995) and a biphasic discharge of dorsal horn nociceptive neurons (Dickenson & Sullivan, 1987). Indeed, the formalin-induced response characterizes some of the clinical features of pain in humans following injury (McQuay et al., 1988). The behaviors of flinching (Ossipov et al., 1996; Hao et al., 2002; Yamamoto et al., 2002) and lifting/licking (Hong & Abbott, 1996; Zeitz et al., 2001; Wu et al., 2002; Yamada et al., 2002) and both behaviors together (Taylor et al., 2000; Oliva et al., 2002; Shannon & Lutz, 2002) have been reported in the literature. The fact that differential effects on flinching and lifting/licking have been observed with some agents, such as naloxone, neurotoxin, pentobarbital, ketamine and amitriptyline (Tjolsen et al., 1991; Wheeler-Aceto & Cowan, 1993; Abbott et al., 1995; Davidson & Carlton, 1998; Sawynok & Reid, 2001) indicates that information may be missed if only one of the behaviors is monitored. It is apparent that the onset of a flinch interferes with the occurrence of licking because flinching and licking cannot occur at the same time. Flinching and licking behaviors have been reported to be due to different mechanisms (Coderre et al., 1994) and therefore multiple end points for both behaviors were assessed in the present study.
The change in flinching induced by BAM22 did not seem to be consistent with the change in lifting/licking in our study. At 5 nmol, BAM22 decreased the number of flinches but did not alter the duration of lifting/licking in the first phase. This phenomenon was repeatedly seen in both the absence and presence of naloxone. It may be that flinching behavior is more sensitive to BAM22, if not to all analgesics. Alternatively, a spinal mechanism of action of the drug may be indicated, as flinching is more likely to be expressed at the spinal cord level (Coderre et al., 1994). However, BAM22 suppressed both flinching and lifting/licking in the second phase. It has been documented that the first phase evoked by formalin is an acute pain response due to activation of primary afferents and the second phase reflects an ongoing low level of C-fiber input together with a facilitatory process at the spinal level (Dickenson & Sullivan, 1987; Hunskaar & Hole, 1987; Tjolsen et al., 1992; Dallel, Raboisson et al., 1995; Puig & Sorkin, 1996). It can be concluded that BAM22 suppressed acute nociception and hypersensitivity of the spinal cord.
Several studies report that systemic administration of naloxone does not change the formalin-evoked response (Hao et al., 2002; Oliva et al., 2002; Shannon & Lutz, 2002; Yamada et al., 2002). We observed that flinching and lifting/flicking behaviors in the second phase were significantly enhanced after pretreatment with naloxone. Our results suggest that intraplantar injection of formalin evokes release of endomorphins from the regions in the CNS that are important for pain and/or pain control. This notion is in an agreement with previous studies demonstrating that formalin injection releases endomorphins and increases μ-, δ- and κ-opioid inhibitory tones in the CNS only during the second phase of the formalin response (Bourgoin et al., 1990; Millan & Colpaert, 1991; Murray & Cowan, 1991; Ossipov et al., 1996; Wu et al., 2002). The findings that naloxone did not alter the first phase in the formalin test or the TW latency may indicate that endogenous release of endomorphins is not involved in modulation of acute nociception.
BAM22 exhibits both opioid and non-opioid biological activity in vitro (Lembo et al., 2002). This observation is supported by our in vivo data that i.t. BAM22 inhibited formalin-evoked behaviors and increased the TW latency, and these effects were attenuated in the presence of naloxone. These results indicated that the two types of actions of BAM22 may not involve reciprocal antagonism. The roughly 30% reduction in the magnitude of the formalin-evoked response and the lack of effect in the later period of the second phase following blockade of opioid receptors suggest that the opioid and non-opioid responses to BAM22 were additive. As BAM22 dose-dependently suppressed flinching and lifting/licking in the second phase in the presence of naloxone, it appears to have been involved in modulating hypersensitivity of the spinal cord via a non-opioid mechanism, leading to relief of persistent pain. It may be surprising that this additive action was not seen in the first phase, as the efficacy of BAM22 in the absence and presence of naloxone was similar. However, after pretreatment with naloxone, BAM22 produced less of an increase in the TW latency, and it lasted a shorter time. Therefore, it can be concluded that the non-opioid activity of BAM22 also suppressed the acute nociception.
Activation of the newly discovered SNSR receptor (Lembo et al., 2002) may mediate the non-opioid activity of BAM22. The name sensory neuron-specific receptor may not in fact be appropriate, because this receptor is not expressed in the large- and medium-sized sensory neurons in the DRG or in sensory neurons in other structures of the CNS such as spinal dorsal horn, brainstem, thalamus and cerebral cortex. Another reason why the name sensory neuron-specific receptor does not accurately describe this novel receptor is that P2X3 purinoceptors (Burnstock, 2000), tetrodotoxin-resistant voltage-gated sodium channels (Akopian et al., 1999; Waxman et al., 1999) and mrgs (Mas-related genes) (Dong et al., 2001) are also solely expressed in a subpopulation of DRG sensory neurons. Based on the fact that peptide E, BAM22 and BAM20, the naturally cleaved products of proenkephalin A, all bind to both opioid receptors and this novel receptor (Lembo et al., 2002), and have the same source as opioid peptides (Khachaturian et al., 1983; Pittius et al., 1984), we recommend naming it opioid receptor-type receptor (ORT) to distinguish it from ORL1, or BAM peptide-activated receptor with non-opioid activity (BPAR).
It has been proposed that BAM8-22, a highly selective BPAR agonist (Lembo et al., 2002), is an endogenous ligand for BPAR, but this peptide has not been proven to be a naturally metabolized product of proenkephalin cleavage. However, BAM22, peptide E and BAM20, which are metabolized fragments of proenkephalin (Mizuno et al., 1980), may be physiological ligands for BPAR since these peptides bind to BPAR receptors (Lembo et al., 2002). BAM22, in particular, possesses a high affinity for BPAR and is concentrated in the superficial laminae of the spinal dorsal horn (Garzon et al., 1983; Iadarola et al., 1985). Therefore, it is likely that BAM22 is a major physiological ligand for BPAR. Binding of BAM22 on one receptor (opioid or BPAR) may create background excitation for activation of the other receptor. The role of BPAR in pain modulation may be to presynaptically inhibit the release of excitatory neurotransmitters from small-sized neurons in the DRG, in the same way as other inhibitory receptors (Suarez-Roca & Maixner, 1995; Li et al., 2002) that are also expressed in the small-sized neurons in the DRG, including opioid (Wang & Wessendorf, 2001), acetylcholine (Genzen et al., 2001) and GABAB (Stoyanova et al., 1998) receptors.
In summary, the functional role of BPAR is an interesting issue, because this receptor is associated preferentially with the IB4 class of nociceptors in the DRG, the function of which has not been clarified. However, as a specific antagonist of BPAR has not yet been found, it is difficult to precisely determine the function of the receptor. We used naloxone to eliminate the opioid activity of BAM22 and thus demonstrated the non-opioid analgesic effects of the peptide.
The non-opioid effects of BAM22 may be mediated via BPAR, and therefore our results suggest a role for this novel receptor in nociceptive modulation. As BPAR is distributed uniquely in a subpopulation of small-sized DRG neurons, it will be important to develop analgesics targeting this receptor.
Acknowledgments
This study was supported by grants from the Fujian Normal University and Nature Sciences Foundation of China (30340077). We thank Mr Hao Huang and Miss Wei Lin for their valuable technical assistance.
Abbreviations
- ANOVA
one-way analysis of variance
- BAM22
bovine adrenal medulla 22
- BPAR
BAM peptide-activated receptor with non-opioid activity
- CNS
central nervous system
- DRG
dorsal root ganglion
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- L
lumbar
- ORT
opioid receptor type
- SNSR
the sensory neuron-specific receptor
- TW
tail withdrawal
References
- ABBOTT F.V., FRANKLIN K.B., WESTBROOK R.F. The formalin test: scoring properties of the first and second phases of the pain response in rats. Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]
- AKOPIAN A.N., SOUSLOVA V., ENGLAND S., OKUSE K., OGATA N., URE J., SMITH A., KERR B.J., MCMAHON S.B., BOYCE S., HILL R., STANFA L.C., DICKENSON A.H., WOOD J.N. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 1999;2:541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- BLOCH B., BAIRD A., LING N., BENOIT R., GUILLEMIN R. Immunohistochemical evidence that brain enkephalins arise from a precursor similar to adrenal preproenkephalin. Brain Res. 1983;263:251–257. doi: 10.1016/0006-8993(83)90317-7. [DOI] [PubMed] [Google Scholar]
- BOERSMA C.J., POOL C.W., VAN HEERIKHUIZE J.J., VAN LEEUWEN F.W. Characterization of opioid binding sites in the neural and intermediate lobe of the rat pituitary gland by quantitative receptor autoradiography. J. Neuroendocrinol. 1994;6:47–56. doi: 10.1111/j.1365-2826.1994.tb00554.x. [DOI] [PubMed] [Google Scholar]
- BOURGOIN S., LE BARS D., CLOT A.M., HAMON M., CESSELIN F. Subcutaneous formalin induces a segmental release of Met-enkephalin-like material from the rat spinal cord. Pain. 1990;41:323–329. doi: 10.1016/0304-3959(90)90009-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. P2X receptors in sensory neurones. Br. J. Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- CODERRE T.J., YASHPAL K., HENRY J.L. Specific contribution of lumbar spinal mechanisms to persistent nociceptive responses in the formalin test. Neuroreport. 1994;5:1337–1340. [PubMed] [Google Scholar]
- DALLEL R., RABOISSON P., CLAVELOU P., SAADE M., WODA A. Evidence for a peripheral origin of the tonic nociceptive response to subcutaneous formalin. Pain. 1995;61:11–16. doi: 10.1016/0304-3959(94)00212-W. [DOI] [PubMed] [Google Scholar]
- DAVIDSON E.M., CARLTON S.M. Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res. 1998;785:136–142. doi: 10.1016/s0006-8993(97)01396-6. [DOI] [PubMed] [Google Scholar]
- DAVIS T.P., HOYER G.L., DAVIS P., BURKS T.F. Proenkephalin A-derived peptide E and its fragments alter opioid contractility in the small intestine. Eur. J. Pharmacol. 1990;191:253–261. doi: 10.1016/0014-2999(90)94157-s. [DOI] [PubMed] [Google Scholar]
- DE LA CALLE J.L., PAINO C.L. A procedure for direct lumbar puncture in rats. Brain Res. Bull. 2002;59:245–250. doi: 10.1016/s0361-9230(02)00866-3. [DOI] [PubMed] [Google Scholar]
- DICKENSON A.H., SULLIVAN A.F. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- DONG X., HAN S., ZYLKA M.J., SIMON M.I., ANDERSON D.J. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- DORES R.M., MCDONALD L.K., STEVESON T.C., SEI C.A. The molecular evolution of neuropeptides: prospects for the ‘90s. Brain Behav. Evol. 1990;36:80–99. doi: 10.1159/000115300. [DOI] [PubMed] [Google Scholar]
- DRAY A., NUNAN L., WIRE W. Proenkephalin A fragments exhibit spinal and supraspinal opioid activity in vivo. J. Pharmacol. Exp. Ther. 1985;235:670–676. [PubMed] [Google Scholar]
- DUBUISSON D., DENNIS S.G. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- FANG F.G., FIELDS H.L., LEE N.M. Action at the mu receptor is sufficient to explain the supraspinal analgesic effect of opiates. J. Pharmacol. Exp. Ther. 1986;238:1039–1044. [PubMed] [Google Scholar]
- GARZON J., SANCHEZ-BLAZQUEZ P., HOLLT V., LEE N.M., LOH H.H. Endogenous opioid peptides: comparative evaluation of their receptor affinities in the mouse brain. Life Sci. 1983;33 Suppl 1:291–294. doi: 10.1016/0024-3205(83)90500-3. [DOI] [PubMed] [Google Scholar]
- GENZEN J.R., VAN CLEVE W., MCGEHEE D.S. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J. Neurophysiol. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- HAO S., TAKAHATA O., MAMIYA K., IWASAKI H. Sevoflurance suppresses noxious stimulus-evoked expression of Fos-like immunoreactivity in the rat spinal cord via activation of endogenous opioid systems. Life Set. 2002;71:571–580. doi: 10.1016/s0024-3205(02)01704-6. [DOI] [PubMed] [Google Scholar]
- HOLLT V., HAARMANN I., GRIMM C., HERZ A., TULUNAY F.C., LOH H.H. Pro-enkephalin intermediates in bovine brain and adrenal medulla: characterization of immunoreactive peptides related to BAM-22P and peptide F. Life Sci. 1982;31:1883–1886. doi: 10.1016/0024-3205(82)90234-x. [DOI] [PubMed] [Google Scholar]
- HONG Y., ABBOTT F.V. Contribution of peripheral alpha IA-adrenoceptors to pain induced by formalin or by alpha-methyl-5-hydroxytryptamine plus noradrenaline. Eur. J. Pharmacol. 1996;301:41–48. doi: 10.1016/0014-2999(96)00009-x. [DOI] [PubMed] [Google Scholar]
- HUNSKAAR S., HOLE K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30:103–114. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- HYLDEN J.L., WILCOX G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- JADAROLA M.J., PANULA P., MAJANE E.A., YANG Y.T. The opioid octapeptide Met5-enkephalin-Arg6-Gly7-Leu8: characterization and distribution in rat spinal cord. Brain Res. 1985;330:127–134. doi: 10.1016/0006-8993(85)90013-7. [DOI] [PubMed] [Google Scholar]
- KHACHATURIAN H., LEWIS M.E., WATSON S.J. Colocalization of proenkephalin peptides in rat brain neurons. Brain Res. 1983;279:369–373. doi: 10.1016/0006-8993(83)90212-3. [DOI] [PubMed] [Google Scholar]
- LEMBO P.M., GRAZZINI E., GROBLEWSKI T., O'DONNELL D., ROY M.O., ZHANG J., HOFFERT C., CAO J., SCHMIDT R., PELLETIER M., LABARRE M., GOSSELIN M., FORTIN Y., BANVILLE D., SHEN S.H., STROM P., PAYZA K., DRAY A., WALKER P., AHMAD S. Proenkephalin A gene products activate a new family of sensory neuron-specific GPCRs. Nat. Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- LI D.P., CHEN S.R., PAN Y.Z., LEVEY A.I., PAN H.L. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J. Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADERDRUT J.L., MERCHENTHALER I., SUNDBERG D.K., OKADO N., OPPENHEIM R.W. Distribution and development of proenkephalin-like immunoreactivity in the lumbar spinal cord of the chicken. Brain Res. 1986;377:29–40. doi: 10.1016/0006-8993(86)91187-x. [DOI] [PubMed] [Google Scholar]
- MCGINTY J.F. Prodynorphin immunoreactivity is located in different neurons than proenkephalin immunoreactivity in the cerebral cortex of rats. Neuropeptides. 1985;5:465–468. doi: 10.1016/0143-4179(85)90055-1. [DOI] [PubMed] [Google Scholar]
- MCQUAY H.J., CARROLL D., MOORE R.A. Postoperative orthopaedic pain–the effect of opiate premedication and local anaesthetic blocks. Pain. 1988;33:291–295. doi: 10.1016/0304-3959(88)90287-4. [DOI] [PubMed] [Google Scholar]
- MERCHENTHALER I., MADERDRUT J.L., ALTSCHULER R.A., PETRUSZ P. Immunocytochemical localization of proenkephalin-derived peptides in the central nervous system of the rat. Neuroscience. 1986;17:325–348. doi: 10.1016/0306-4522(86)90250-2. [DOI] [PubMed] [Google Scholar]
- MESTRE C., PELISSIER T., FIALIP J., WILCOX G., ESCHALIER A. A method to perform direct transcutaneous intrathecal injection in rats. J. Pharmacol. Toxicol. Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., COLPAERT F.C. Opioid systems in the response to inflammatory pain: sustained blockade suggests role of kappa- but not mu-opioid receptors in the modulation of nociception, behaviour and pathology. Neuroscience. 1991;42:541–553. doi: 10.1016/0306-4522(91)90396-6. [DOI] [PubMed] [Google Scholar]
- MIZUNO K., MINAMINO N., KANGAWA K., MATSUO H. A new family of endogenous “big” Met-enkephalins from bovine adrenal medulla: purification and structure of docosa- (BAM-22P) and eicosapeptide (BAM-20P) with very potent opiate activity. Biochem. Biophys. Res. Commun. 1980;97:1283–1290. doi: 10.1016/s0006-291x(80)80005-2. [DOI] [PubMed] [Google Scholar]
- MURRAY C.W., COWAN A. Tonic pain perception in the mouse: differential modulation by three receptor-selective opioid agonists. J. Pharmacol. Exp. Ther. 1991;257:335–341. [PubMed] [Google Scholar]
- NOZAKI-TAGUCHI N., YAKSH T.L. Characterization of the antihyperalgesic action of a novel peripheral mu-opioid receptor agonist–loperamide. Anesthesiology. 1999;90:225–234. doi: 10.1097/00000542-199901000-00029. [DOI] [PubMed] [Google Scholar]
- OLIVA P., AURILIO C., MASSIMO F., GRELLA A., MAIONE S., GRELLA E., SCAFURO M., ROSSI F., BERRINO L. The antinociceptive effect of tramadol in the formalin test is mediated by the serotonergic component. Eur. J. Pharmacol. 2002;445:179–185. doi: 10.1016/s0014-2999(02)01647-3. [DOI] [PubMed] [Google Scholar]
- ORII R., OHASHI Y., GUO T., NELSON L.E., HASHIMOTO T., MAZE M., FUJINAGA M. Evidence for the involvement of spinal cord alphal adrenoceptors in nitrous oxide-induced antinociceptive effects in Fischer rats. Anesthesiology. 2002;97:1458–1465. doi: 10.1097/00000542-200212000-00018. [DOI] [PubMed] [Google Scholar]
- OSSIPOV M.H., KOVELOWSKI C.J., WHEELER-ACETO H., COWAN A., HUNTER J.C., LAI J., MALAN JR T.P., PORRECA F. Opioid antagonists and antisera to endogenous opioids increase the nociceptive response to formalin: demonstration of an opioid kappa and delta inhibitory tone. J. Pharmacol. Exp. Ther. 1996;277:784–788. [PubMed] [Google Scholar]
- PITTIUS C.W., SEIZINGER B.R., PASI A., MEHRAEIN P., HERZ A. Distribution and characterization of opioid peptides derived from proenkephalin A in human and rat central nervous system. Brain Res. 1984;304:127–136. doi: 10.1016/0006-8993(84)90868-0. [DOI] [PubMed] [Google Scholar]
- PUIG S., SORKIN L.S. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- QUIRION R., WEISS A.S. Peptide E and other proenkephalin-derived peptides are potent kappa opiate receptor agonists. Peptides. 1983;4:445–449. doi: 10.1016/0196-9781(83)90047-5. [DOI] [PubMed] [Google Scholar]
- REINER A. The distribution of proenkephalin-derived peptides in the central nervous system of turtles. J. Comp Neural. 1987;259:65–91. doi: 10.1002/cne.902590106. [DOI] [PubMed] [Google Scholar]
- SANCHEZ-BLAZQUEZ P., GARZON J. Opioid activity of pro-enkephalin-derived peptides in mouse vas deferens and guinea pig ileum. Neurosci. Lett. 1985;61:267–271. doi: 10.1016/0304-3940(85)90475-6. [DOI] [PubMed] [Google Scholar]
- SAWYNOK J., REID A. Antinociception by tricyclic antidepressants in the rat formalin test: differential effects on different behaviours following systemic and spinal administration. Pain. 2001;93:51–59. doi: 10.1016/S0304-3959(01)00291-3. [DOI] [PubMed] [Google Scholar]
- SHANNON H.E., LUTZ E.A. Comparison of the peripheral and central effects of the opioid agonists loperamide and morphine in the formalin test in rats. Neuropharmacology. 2002;42:253–261. doi: 10.1016/s0028-3908(01)00173-3. [DOI] [PubMed] [Google Scholar]
- SNIDER W.D., MCMAHON S.B. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- STOYANOVA I., DANDOV A., LAZAROV N., CHOUCHKOV C. GABA- and glutamate-immunoreactivity in sensory ganglia of cat: a quantitative analysis. Arch. Physiol Biochem. 1998;106:362–369. doi: 10.1076/apab.106.5.362.4360. [DOI] [PubMed] [Google Scholar]
- SUAREZ-ROCA H., MAIXNER W. Morphine produces a biphasic modulation of substance P release from cultured dorsal root ganglion neurons. Neurosci. Lett. 1995;194:41–44. doi: 10.1016/0304-3940(95)11721-8. [DOI] [PubMed] [Google Scholar]
- SWAIN M.G., MACARTHUR L., VERGALLA J., JONES E.A. Adrenal secretion of BAM-22P, a potent opioid peptide, is enhanced in rats with acute cholestasis. Am. J. Physiol. 1994;266:G201–G205. doi: 10.1152/ajpgi.1994.266.2.G201. [DOI] [PubMed] [Google Scholar]
- TAYLOR B.K., PETERSON M.A., BASBAUM A.I. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J. Neurosci. 1995;15:7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR B.K., PETERSON M.A., RODERICK R.E., TATE J., GREEN P.G., LEVINE J.O., BASBAUM A.I. Opioid inhibition of formalin-induced changes in plasma extravasation and local blood flow in rats. Pain. 2000;84:263–270. doi: 10.1016/s0304-3959(99)00212-2. [DOI] [PubMed] [Google Scholar]
- TJOLSEN A., BERGE O.G., HOLE K. Lesions of bulbo-spinal serotonergic or noradrenergic pathways reduce nociception as measured by the formalin test. Acta Physiol Scand. 1991;142:229–236. doi: 10.1111/j.1748-1716.1991.tb09151.x. [DOI] [PubMed] [Google Scholar]
- TJOLSEN A., BERGE O.G., HUNSKAAR S., ROSLAND J.H., HOLE K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- WANG H., WESSENDORF M.W. Equal proportions of small and large DRG neurons express opioid receptor mRNAs. J. Comp. Neurol. 2001;429:590–600. doi: 10.1002/1096-9861(20010122)429:4<590::aid-cne6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- WAXMAN S.G., CUMMINS T.R., DIB-HAJJ S., FJELL J., BLACK J.A. Sodium channels, excitability of primary sensory neurons, and the molecular basis of pain. Muscle Nerve. 1999;22:1177–1187. doi: 10.1002/(sici)1097-4598(199909)22:9<1177::aid-mus3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- WHEELER-ACETO H., COWAN A. Naloxone causes apparent antinociception and pronociception simultaneously in the rat paw formalin test. Eur. J. Pharmacol. 1993;236:193–199. doi: 10.1016/0014-2999(93)90589-a. [DOI] [PubMed] [Google Scholar]
- WU H.E., HUNG K.C., MIZOGUCHI H., NAGASE H., TSENG L.F. Roles of endogenous opioid peptides in modulation of nocifensive response to formalin. J. Pharmacol. Exp. Ther. 2002;300:647–654. doi: 10.1124/jpet.300.2.647. [DOI] [PubMed] [Google Scholar]
- YAMADA H., NAKAMOTO H., SUZUKI Y., ITO T., AISAKA K. Pharmacological profiles of a novel opioid receptor-like 1 (ORL(1)) receptor antagonist, JTC-801. Br. J. Pharmacol. 2002;135:323–332. doi: 10.1038/sj.bjp.0704478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO T., NOZAKI-TAGUCHI N., CHIBA T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br. J. Pharmacol. 2002;137:170–176. doi: 10.1038/sj.bjp.0704851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEITZ K.P., MALMBERG A.B., GILBERT H., BASBAUM A.I. Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain. 2001;94:245–253. doi: 10.1016/S0304-3959(01)00353-0. [DOI] [PubMed] [Google Scholar]