Abstract
Activation of rat adenosine 2A receptors (A2A R) dilates preglomerular microvessels (PGMV), an effect mediated by epoxyeicosatrienoic acids (EETs).
Incubation of PGMV with a selective A2A R agonist, 2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS 21680; 100 μM), increased isolated PGMV EET levels to 7.57±1.53 ng mg−1 protein from 1.06±0.22 ng mg−1 protein in controls (P<0.05), without affecting hydroxyeicosatetraenoic acid (HETE) levels (10.8±0.69 vs 11.02±0.74 ng mg−1 protein).
CGS 21680-stimulated EETs was abolished by preincubation with an A2A R antagonist, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) (100 μM). A selective epoxygenase inhibitor, methylsulfonyl-propargyloxyphenylhexanamide (MS-PPOH; 12 μM) prevented CGS 21680-induced increase in EETs, indicating inhibition of de novo synthesis of EETs.
In pressurized (80 mmHg) renal arcuate arteries (110–130 μm) preconstricted with phenylephrine (20 nM), superfusion with CGS 21680 (0.01–10 μM) increased the internal diameter (i.d.) concentration-dependently; vasodilation was independent of nitric oxide and cyclooxygenase activity. CGS 21680 (10 μM) increased i.d. by 32±6 μm; vasodilation was prevented by inhibition of EET synthesis with MS-PPOH.
Addition of 3 nM 5,6-EET, 8,9-EET and 11,12-EET increased i.d. by 53±9, 17±4 and 53±5 μm, respectively, whereas 14,15-EET was inactive. The responses to 5,6-EET were, however, significantly inhibited by indomethacin.
We conclude that 11,12-EET is the likely mediator of A2A R-induced dilation of rat PGMV. Activation of A2A R coupled to de novo EET stimulation may represent an important mechanism in regulating preglomerular microvascular tone.
Keywords: Adenosine, A1 receptors, A2A receptors, cytochrome P450, renal EETs, rat renal artery
Introduction
The kidney plays a decisive role in regulating blood pressure (Guyton & Coleman, 1969). The complex control mechanisms governing this renal function are orchestrated within the preglomerular microvessels (PGMV) (Navar, 1998). These microvessels, particularly afferent arterioles, maintain a constant renal blood flow and glomerular filtration rate (GFR) by adjusting their resistance in response to perfusion pressure fluctuations, that is, autoregulation (Navar et al., 1966).
A variety of autocrine, endocrine and paracrine signals arising from diverse sources – PGMV, autonomic nervous system, circulating hormones and the nephron – act on PGMV, where they generate a variety of mediators/modulators (Navar et al., 1996). Adenosine, a metabolite of ATP, modulates cellular and organ function by binding to specific cell-surface P1 purinergic receptors, of which there are four known subtypes (A1, A2A, A2B and A3) (Burnstock, 1980). These receptors are members of the large family of seven-transmembrane spanning heterotrimeric G protein-coupled receptors (Olah & Stiles, 2000). The physiological effects of adenosine are observed in nearly every tissue and organ, and are expressed in PGMV (Jackson et al., 2002).
In the kidney, activation of adenosine1 receptors (A1 R) and A2 R participate in the regulation of renal vascular tone and tubular function (Jackson & Dubey, 2001). Stimulation of A1 R constricts the renal vasculature (PGMV, efferent arterioles and outer medullary descending vasa recta) (Holz & Steinhausen, 1987), decreases tubuloglomerular feedback (TGF) (Sun et al., 2001), inhibits renin release and enhances proximal tubular NaCl reabsorption (Brown et al., 2001), whereas stimulation of A2A R increases renal blood flow (Levens et al., 1991), and reduces NaCl reabsorption (Zou et al., 1999) and decreases blood pressure (Ledent et al., 1997). Activation of A2B R dilates renal arteries in a nitric oxide-dependent manner (Martin & Potts, 1994) and inhibits cellular proliferation of extracellular matrix production by vascular smooth muscle cells (Dubey et al., 1996).
Cytochrome P450 (CYP)-derived arachidonic acid (AA) metabolites, 20-hydroxyeicosatetraenoic acids (20-HETE) and four regioisomeric cis-epoxyeicosatrienoic acids (EETs), 5,6-, 8,9-, 11,12- and 14,15-EETs, generated by hydroxylases and epoxygenases, respectively, occupy a key position in the regulation of renal vascular tone (Carroll et al., 1996; Harder et al., 1996; Imig et al., 1996b). The constrictor effect of 20-HETE on PGMV established its importance in mediating TGF and renal autoregulation. In contrast, EETs, prime candidates for endothelium-derived hyperpolarizing factors (EDHF) (Campbell et al., 1996; Fisslthaler et al., 1999), exhibit dilator activity on the renal vasculature of rats (Imig et al., 1996a) by increasing the open-state probability of calcium-dependent potassium (KCa+) channels of vascular smooth muscle cells (Campbell et al., 1996; Dumoulin et al., 1998). These lipid-derived metabolites mediate/modulate the vascular responses of many vasoactive peptides, for example, angiotensin II (AII) (Carroll et al., 1996; Croft et al., 2000), endothelin-1 (Hercule & Oyekan, 2000b) and bradykinin (Fulton et al., 1992; Imig et al., 2001). Recently, Zhao et al. (2001) demonstrated that 20-HETE also contributes to the ATP-induced constriction of afferent arterioles by activating P2X receptors. This observation suggests an interdependency involving the purinergic system and CYP-derived AA metabolites in renal microcirculatory regulation.
Activation of A2A R by a selective agonist, CGS 21680 (Levens et al., 1991), dilated PGMV (Rump et al., 1999) by acting on K+ channels independently of a nitric oxide (NO) component (Prior et al., 1999); these findings are consistent with an EET acting as a second messenger. Our study was designed to answer whether an EET mediated the renal vasodilator response to stimulating the A2A R. The A2A R selective agonist, CGS 21680, was demonstrated to stimulate EETs release from PGMV. Further, the dilator response of a PGMV, the arcuate artery, to CGS 21680 was prevented by inhibition of epoxygenases. Stimulation of EET synthesis by PGMV in response to adenosine activation of A2A R is suggested to represent a vascular mechanism that participates in the regulation of PGMV tone and reactivity and, thereby, GFR and salt and water excretion.
Methods
In order to isolate PGMV for CYP-derived AA metabolite measurements, the kidneys of anesthetized (pentobarbital; 100 mg kg−1 bw) male Sprague–Dawley rats (8–10-weeks old, Charles River, U.S.A.) were flushed, via the abdominal aorta, with 30 ml of saline to remove blood elements, followed immediately with 20 ml of a 5% iron oxide solution, as previously described (Chatziantoniou & Arendshorst, 1993; Croft et al., 2000). The minced cortex was homogenized in approximately 5 ml of PBS. The iron laden PGMV were separated from other cortical tissues, for example, tubules, by magnetic separation (Advanced Magnetics Inc.).
PGMV were washed three times in Tyrode's solution, containing indomethacin (10 μM) and L-NAME (200 μM), gassed with 95% O2–5% CO2. The composition of the Tyrode's buffer solution (mM) was 138.0 NaCl, 2.7 KCl, 1.8 CaCl2, 1.0 MgCl2, 11.9 NaHCO3, 0.42 NaHPO4 and 5.6 D-glucose. We included L-NAME, an NO synthesis inhibitor, and indomethacin, a cyclooxygenase (COX) inhibitor, in order to avoid any potential interaction of either NO (Oyekan et al., 1999) or COX (Cheng et al., 2003) with CYP-derived AA metabolite levels.
The purity of each microvascular preparation was examined using light microscopy (× 400 magnification). Only preparations that had minimal proximal tubular contamination (less than 5%) were used for the experiments. Protein concentration was determined using the Bradford (1976) method after vessels were suspended in 1N NaOH for 2–3 days and homogenized.
To quantitate basal and stimulated CYP-derived AA metabolites released by PGMV, suspensions of PGMV (∼0.5 mg protein ml−1) were incubated with NADPH (1 mM) in the presence or absence of agonists and/or antagonists at 37°C for 15 min. This time was chosen based on preliminary experiments. Enzyme inhibitors and receptor antagonists were added to the incubates for a 10–15 min preincubation at 4°C prior to the addition of NADPH. The concentrations of agonists used in this study were chosen based on preliminary experiments (data not shown). Stock solutions of CGS 21680, CPA and CHA (Sigma-Aldrich Inc., St Louis, MO, U.S.A.) were made up in Tyrode's buffer solution. The selective A2A R antagonist ZM 241385 (Poucher et al., 1995; Tocris Cookson Inc., Ellisville, MO, U.S.A.) and MS-PPOH, a selective epoxygenase inhibitor (Brand-Schieber et al., 2000, synthesized by J.R. Falck) were dissolved in ethanol. Further dilutions were made up in buffer solution, so that the ethanol concentration was less than 0.01%.
The PGMV and media eicosanoids were acidified to pH 4.0 with 9% formic acid. After addition of internal standards, 1 ng of deuterated (D2) 20-HETE (J.R. Falck), 2 ng of D8 8,9/11, 12-EET, 1 ng of D8 14,15-EET, (Biomol) samples were extracted twice with 2 × vol. ethyl acetate and evaporated to dryness.
The samples were purified by reverse phase (RP)-HPLC on a C18 μBondapak column (4.6 × 24 mm2) by using a linear gradient from acetonitrile : water : acetic acid (62.5 : 37.5: 0.05%) to acetonitrile (100%) over 20 min at a flow rate of 1 ml min−1. Fractions containing HETEs and those containing 8,9/11,12-EETs and 14,15-EET were collected on the basis of the elution profile of standards monitored by ultraviolet absorbance (205 nm). The fractions were evaporated to dryness and resuspended in 100 μl of acetonitrile.
HPLC fractions containing HETEs and EETs were derivatized as described earlier (Croft et al., 2000). The derivatized HETEs and EETs were dried with nitrogen and resuspended in 50 μl of iso-octane until gas chromatography-mass spectrometry (GC-MS) analyses. A 1 μl aliquot of derivatized CYP-derived AA metabolites, dissolved in iso-octane, was injected into a GC (Hewlett-Packard 5890) column (DB-1; 10.0 m, 0.25 mm inner diameter, 0.25 μm film thickness, Agilent). We used temperature programs ranging from 180 to 300°C (HETEs) and 150 to 300°C (EETs) at rates of 25 and 30°C min−1, respectively (Macica et al., 1993). These temperature gradients separate individual HETEs; however, they could not resolve EET regioisomers. Methane was used as a reagent gas at a flow resulting in a source pressure of 1.3 Torr and the MS (Hewlett-Packard 5989A) was operated in electron capture chemical ionization mode. The endogenous HETEs (ion m/z 391) and EETs (ion m/z 319) were identified by comparison of GC retention times with authentic HETE and EET standards . Quantitation of 20-HETE was performed by calculating the ratio of abundance with D2 20-HETE (m/z 393). The endogenous EETs (ion m/z 319) were identified by comparison of GC retention times with authentic D8 8,9-, 11,12- and 14,15-EET (m/z 327) standards. The highly labile 5,6-EET was not measured.
To isolate and pressurize renal arcuate arteries, we used previously described methods (Kauser et al., 1991; Hercule & Oyekan, 2000a). Briefly, male Sprague–Dawley rats (8–10-weeks old; Charles River, U.S.A.) were anesthetized with pentobarbital (100 mg kg−1 bw). After laparotomy, the kidneys were removed and placed in ice-cold PBS. The kidneys were decapsulated and cut in half along the corticopapillary axis. The renal papilla was removed to expose PGMV. Segments of arcuate artery were microdissected at 4°C and carefully cleared of adherent connective tissue and tubules.
The arcuate arteries were mounted on glass micropipettes, with monofilament silk, in a l ml water-jacketed perfusion vessel chamber (Instrument Department, New York Medical College). The glass micropipettes were filled with room temperature oxygenated Krebs' solution through the proximal glass micropipette prior to mounting of vessels, as well as throughout the experiments. The distal glass micropipette was closed, while the proximal micropipette was connected to a pressure-servo unit (Living System Instrumentation). Pressure was gradually increased in 20 mmHg increments to 80 mmHg, and maintained throughout all experiments. The microvessel chamber was placed on the stage of an inverted microscope (Nikon) with attached video camera (CCD). The change of internal diameters of the arcuate arteries was continuously measured with a video dimension analyzer (Living System Instrumentation), and recorded on a pen recorder.
Isolated arteries were continuously superfused with Krebs' solution containing indomethacin (10 μM), equilibrated with 95% O2/5% CO2 at 37°C, at the rate of 1 ml min−1. The composition of the Krebs'–Henseleit solution was (in mM): 118 NaCl, 5.4 KCl, 0.57; MgSO4·7H20, 2.5 CaCl2, 1.2 NaH2PO4, 2.6 NaHCO2, 11.1 glucose, pH 7.4. All arteries were equilibrated in Krebs' solution for 1 h.
Vascular responses to CGS 21680 (10 nM–10 μM) and 2-CA (1 fM–100 nM; Sigma-Aldrich Inc.) were recorded. Internal diameters were measured 1–2 min after administration of each agonist, with 10 min washing periods in between. After obtaining control responses, MS-PPOH (12 μM), a selective epoxygenase inhibitor, was added to the superfusate and concentration–response curves to agonists were repeated. In addition, the concentration–response curves to EETs (5,6-, 8,9-, 11,12- and 14,15-EETs; 10 pM–100 nM) were also constructed. Authentic EETs dissolved in ethanol were evaporated to dryness with nitrogen and reconstituted with oxygenated Krebs' solution at the time of administration to arcuate arteries.
Comparisons among several groups were made by analysis of variance, followed by a modified t-test. Paired analyses were used when comparisons were made of data obtained from the same experimental preparation (i.e., basal and stimulated levels). Data are expressed as mean±standard error and a P-value of <0.05 was considered significant.
Results
We examined the effects of A1 R agonists on CYP-derived AA metabolite levels of isolated PGMV consisting of contiguous segments of interlobar, arcuate, interlobular and afferent arteries, devoid of glomeruli and with little or no proximal tubular contamination.
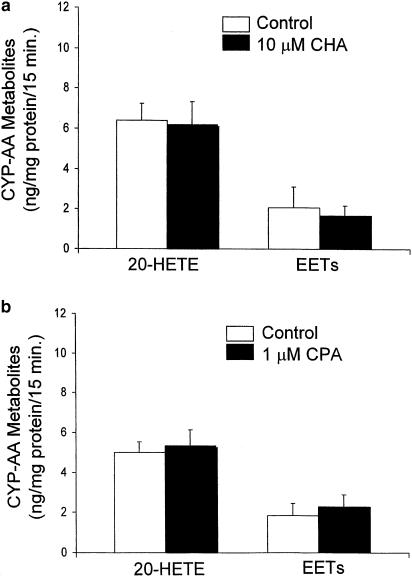
Under basal conditions of incubation of PGMV (approx. 0.5 mg protein) in 1 ml Tyrode's solution containing L-NAME (200 μM) and indomethacin (10 μM) for 15 min at 37°C, samples showed the presence of 20-HETE and EETs (8,9-, 11,12- and 14,15-EETs). The ratio of 20-HETE to EETs under basal conditions was approximately 3 : 1 or greater (Figures 1 & 2).
Figure 1.
Effect of adenosine1 receptor (A1 R) agonists on CYP-derived AA metabolites released from PGMV. PGMV, isolated by a magnetic separation method, were incubated with Tyrode's solution containing indomethacin (10 μM) and L-NAME (200 μM). The effects of: (a) CHA (10 μM) and (b) CPA (1 μM) on 20-HETE and EETs were compared to control (mean±s.e.m.; n=6).
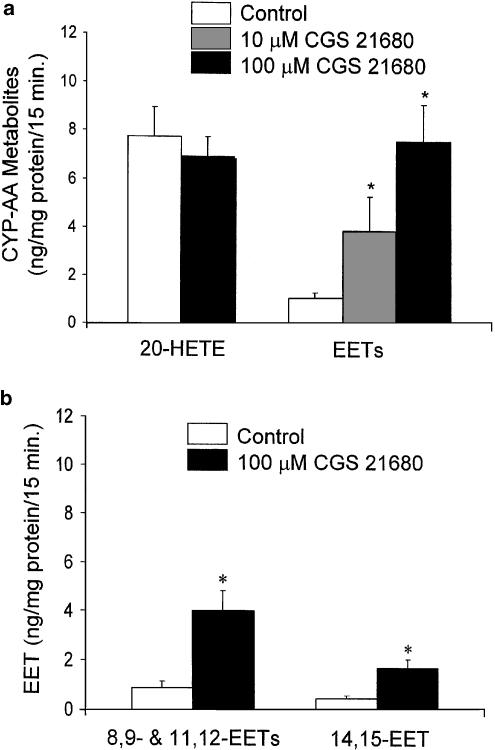
Figure 2.
Effect of A2A R agonist on CYP-derived AA metabolites of PGMV. (a) The A2A R agonist CGS 21680 significantly increased EET levels of PGMV in a concentration-dependent manner, at 10 and 100 μM (*P<0.05 vs control; n=6). CGS 21680 did not alter 20-HETE levels. (b) EETs released by PGMV were further separated by RP-HPLC into two groups: 8,9/11,12-EETs and 14,15-EET. The control level of each group of EET was compared to those in the presence of CGS 21680 (*P<0.05 vs control; n=6).
An A1 R agonist, CHA (10 μM; n=6; Figure 1a), did not affect either 20-HETE or EET levels. Similarly, a more selective, but structurally different, A1 R agonist, CPA, also did not affect the CYP-derived AA metabolite levels of PGMV (1 μM; n=6; Figure 1b), suggesting that neither 20-HETE nor an EET regioisomer contributed to the renal vasoconstrictor response to A1 R stimulation.
We determined the effects of A2A R activation on CYP-derived AA metabolite levels of PGMV with an A2A R agonist, CGS 21680 (10–100 μM; n=6). CGS 21680 significantly increased EET levels in a concentration-dependent manner (Figure 2a). CGS 21680, 10 and 100 μM, increased EET levels from 1.06±0.22 to 3.86±1.43 and to 7.57±1.53 ng mg−1 protein per 15 min, respectively. Moreover, neither concentrations of CGS 21680 altered the levels of 20-HETE. With the aim of determining whether the stimulatory effect of CGS 21680 on EET release was regiospecific, we separated EETs by using RP-HPLC: 8,9-/11,12-EETs and 14,15-EET. CGS 21680 (100 μM) increased both the paired EETs and the individual EET, indicating that the CGS 21680-induced EET release from PGMV was nonselective (Figure 2b).
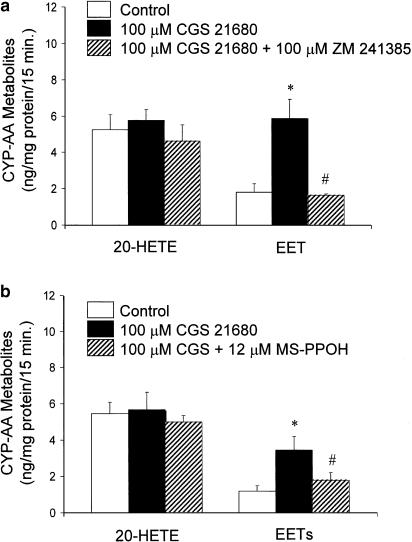
In order to verify that the stimulatory effect of CGS 21680 (100 μM) on EET release from PGMV was, indeed, an A2A R-mediated effect, we determined the EET levels in response to CGS 21680 in the presence and absence of a selective A2A R antagonist, ZM 241385 (100 μM; n=4) (Figure 3a). The stimulatory effect of CGS 21680 on EET levels was abolished by ZM 241385. Further, ZM 241385 did not affect the 20-HETE levels of PGMV. These findings indicated that CGS 21680 stimulated EET release by activating A2A R. In addition, a selective epoxygenase inhibitor, MS-PPOH (12 μM), inhibited the CGS 21680-induced EET stimulation (1.80±0.41 vs 3.45±0.73 ng mg−1 protein; P<0.05; n=4) (Figure 3b), signifying that CGS stimulated de novo synthesis of EETs rather than release of preformed EETs from storage in phospholipids (Carroll et al., 1996). The levels of 20-HETE were unaffected by both CGS 21680, ZM 241385 and MSPPOH (Figure 3), suggesting that EET stimulation is selectively coupled to A2A R.
Figure 3.
Stimulatory effect of CGS 21680 (100 μM) on EET levels of PGMV (n=6) was abolished by; (a) a selective A2A R antagonist, ZM 241385 (100 μM; *P<0.05 vs control; #P<0.05 vs CGS 21680) and (b) an epoxygenase inhibitor, MS-PPOH (12 μM; *P<0.05 vs control; #P<0.05 vs CGS 21680; n=4). Neither ZM 241385 nor MS-PPOH altered the levels of 20-HETE.
Pressurized (80 mmHg) arcuate arteries (i.d.: 100–130 μm) were preconstricted with phenylephrine (20–30 nM) in order to decrease i.d. to 60–90 μm prior to the administration of our test agents. Addition of CGS 21680 (0.01–10 μM) to the superfusate, containing L-NAME (200 μM), resulted in concentration-dependent increases in i.d. (Figure 4a). Inhibition of COX activity with indomethacin (10 μM) did not alter the concentration–response curve to CGS 21680. At 10 μM, CGS 21680 increased i.d. by 32±6 μm, responses that were prevented by preincubation with MS-PPOH (Figure 4b). The dilator responses to sodium nitroprusside (SNP; 1 μM) were, however, unaffected by MS-PPOH, indicating that inhibition of CGS 21680-induced dilation was not related to the nonselective actions of MS-PPOH. Time control studies, without addition of MS-PPOH, demonstrated that the responses to CGS 21680 (10 μM) administered at intervals of 10 min, were not diminished over a 2-h experimental time period.
Figure 4.
Arcuate renal arteries with i.d.'s of 110–130 μm were pressurized (80 mmHg) and superfused with Krebs' solution that contained L-NAME (200 μM). (a) The effect of CGS 21860 was measured in the presence and absence of a cyclooxygenase inhibitor, indomethacin (10 μM; *P<0.05 vs baseline; n=5). (b) The effect of CGS 21680 (10 μM) on the diameters of these arteries was also measured in the presence and absence of an epoxygenase inhibitor, MS-PPOH (12 μM; *P<0.05; n=8).
As the dilator response to CGS 21680 appears to be mediated by one or more EETs, the effects of EETs on pressurized arcuate arteries were studied (Figure 5). Concentration–response curves (3 × 10−11 to 3 × 10−8 M) to each regioisomer demonstrated that 3 × 10−9 M of 5,6-EET and 11,12-EET, which increased i.d. by 53±5 and 53±9 μm, respectively, were equipotent dilators and were more active than 8,9-EET; 14,15-EET was inactive. The dilator effect of 5,6-EET on arcuate arteries was abolished by inhibition of COX with indomethacin.
Figure 5.
Activity of individual EETs on isolated, pressurized arcuate arteries in the presence of L-NAME (200 μM). Vascular effects of 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET were determined in the presence and absence of indomethacin (10 μM; *P<0.05 vs baseline; #P<0.05 vs indomethacin; n=4).
We next tested the vascular effects of a stable adenosine analogue, 2-CA, on isolated arcuate arteries. As NO has been shown to contribute to adenosine-induced dilation in other vascular beds (Abebe et al., 1994; Lewis et al., 1994; Hein et al., 1999), we constructed concentration–response curves to 2-CA (1 fM–100 nM) in the presence and absence of L-NAME (200 μM). Indomethacin (10 μM) was also added to the superfusate to inhibit COX. Under control conditions, 2-CA dilated arcuate arteries at concentrations as low as 100 fM; the maximum response was observed at 1 nM. L-NAME did not affect 2-CA-induced vasodilation (Figure 6a).
Figure 6.
Pressurized arcuate renal arteries were superfused with Krebs' solution containing indomethacin (10 μM). The effects of 2-CA, a stable adenosine analogue, were measured (a) in the presence and absence of L-NAME (200 μM; n=4) and (b) in the presence and absence of MS-PPOH (12 μM). In the presence of L-NAME and MS-PPOH, the vasodilator effects of 2-CA, at higher concentrations (1 and 100 nM), were attenuated (*P<0.05 vs control; n=4–6).
In order to determine the contribution of CYP-derived AA metabolites to the vasoactive responses to adenosine, we also tested the vascular responses of arcuate arteries to 2-CA in the presence and absence of an epoxygenase inhibitor, MS-PPOH (12 μM). The vasodilator effects of 2-CA, at higher concentrations (1 and 100 nM), were significantly attenuated by epoxygenase inhibition (Figure 6b). The ED50's of 2-CA were 8 × 10−12 and 9 × 10−12 M for control and MS-PPOH, respectively; however, the maximum response was inhibited by 40% with MS-PPOH.
Discussion
The contribution of CYP-derived AA metabolite levels to activation of A1 R and A2A R was examined in isolated PGMV. In order to define the vascular responses to adenosine, we initially used a stable adenosine analogue, 2-CA, that is not subject to inactivation by adenosine deaminase/kinase or rapid removal by nucleoside carriers. 2-CA dilated isolated arcuate arteries, an effect diminished by epoxygenase inhibition (Figure 6b), indicating that EETs mediate, at least in part, adenosine-induced dilation. The concentration–response curve of 2-CA was not altered by NO synthase inhibition (Figure 6a), indicating that NO does not contribute to adenosine-induced dilation of PGMV at least under our experimental conditions of no flow. However, 2-CA is nonselective; it is capable of stimulating all the four adenosine receptors.
Both 2-CA and adenosine were shown to be capable of constricting renal microvessels via activation of A1 R in the blood-perfused juxtamedullary nephron preparation (Inscho et al., 1991; Nishiyama et al., 2001). However, this constrictor response to 2-CA via A1 R, was not observed in our microvessel preparation, the renal arcuate artery. Activation of A1 R may require the presence of AII (Munger & Jackson, 1994; Aki et al., 2002). As we used isolated PGMV, the major sources of AII – blood elements and renal tubules – were eliminated, perhaps accounting for the differences between our observations and those of Inscho et al. (1991) and Nishiyama et al. (2001).
As adenosine modulates renal vascular tone and reactivity by activating A1 R and A2 R (Holz & Steinhausen, 1987), the individual effects of these adenosine receptors on CYP-AA metabolite syntheses were studied. The effect of A1 R activation on the profile of CYP-derived AA metabolites of PGMV was defined by using CHA, a specific A1 R agonist; CHA affected neither 20-HETE nor EET levels in PGMV. The absence of an effect of A1 R stimulation on CYP-derived AA metabolite levels of PGMV was confirmed with a highly selective A1 R agonist, CPA.
The observation that 2-CA, nonselective of all the four adenosine receptors, produced epoxygenase-dependent dilation of arcuate arteries (Figure 6b), prompted us to investigate further the relationship between A2A R activation and the profile of EET synthesis by PGMV. The selective A2A R agonist CGS 21680 increased the levels of EETs of PGMV in a concentration-dependent manner, an effect involving the three measured EETs, 8,9-, 11,12- and 14,15-EET. Our HPLC method did not separate 8,9- and 11,12-EETs; whether CGS 21680 stimulated one or both still needs to be determined. The labile nature of 5,6-EET prevented us from measuring this epoxide (Fulton et al., 1998). The 5,6-EET with a short half-life in physiological buffer does not easily lend itself to direct chemical measurement, as it spontaneously converts to its DHET and δ lactone (Carroll et al., 1990).
Stimulation of epoxygenase activity by activation of A2A R was further verified by the abolition of CGS 21680-induced EET stimulation with a selective A2A R antagonist, ZM 241385. The lack of effect of CGS 21680 and ZM 241385 on 20-HETE levels in PGMV indicated their specificity relative to EET synthesis/release. In addition, the inhibitory influence of an epoxygenase inhibitor, MS-PPOH, on CGS 21680-induced EET stimulation, suggested that CGS 21680 increased EET levels by stimulating their de novo synthesis, rather than stimulating their release from storage in phospholipids (Karara et al., 1991; Croft et al., 2000). We also determined the vascular effect of the selective A2A R agonist CGS 21860 on arcuate arteries. Similar to 2-CA, CGS 21680 also dilated arcuate arteries in a concentration-dependent manner, suggesting that A2A R activation is responsible for adenosine-induced dilation. Addition of a selective A2 receptor agonist has been reported to release an endothelial-derived relaxing factor (EDRF) from rabbit arcuate arteries, an effect inhibited by the KCa+ channel blocker iberiotoxin (Rump et al., 1999).
Identification of the EET(s) that mediate the CGS 21680-induced dilation of arcuate arteries was addressed by investigating the vasodilator effects of individual EETs. Since CGS 21680-induced renal vascular effects are COX-independent, the identification of COX-independent EET activity would limit the candidate mediators of A2A R-induced dilation. The maximum responses and EC50 of 5,6- and 11,12-EETs-induced dilation in arcuate arteries were significantly higher than those of 8,9- and 14,15-EETs, suggesting that 5,6- and/or 11,12-EETs are the most likely EET regioisomers that mediate the CGS 21680-induced dilation of arcuate arteries. However, the vasodilator response of the arcuate artery to 5,6-EET was abolished by inhibition of COX, a finding which eliminated 5,6-EET as a putative mediator of the vasodilator response to A2A R activation. In our previous study, we had demonstrated that, of the EETs, the 5,6-EET was the most potent renal vasodilator, but it required transformation by COX to dilate the rabbit renal vasculature (Carroll et al., 1992). Based on these observations, 11,12-EET is the most likely candidate for mediating arcuate arterial dilation in response to activating A2A R with CGS 21680.
In conclusion, we have established that A2A R-induced dilation of renal PGMV is mediated by EETs; 11,12-EET is the most likely candidate mediator. Activation of A2A R stimulates EET levels of PGMV that may affect major indices of renal function: renal vascular resistance, GFR, renal interstitial pressure and medullary blood flow. These effects may influence sodium reabsorption by altering peritubular pressure and tubular fluid volume and flow, thereby promoting natriuresis. Since the tone and reactivity of PGMV are key components in renal autoregulation and TGF, A2A R activation can also serve in mechanisms that contribute to the regulation of blood pressure. In addition, adenosine levels surge when cellular energy demand is high, as in pathophysiological conditions such as renal ischemia (Berne, 1980). Selective A2A R agonists may provide a novel therapeutic approach to controlling blood pressure and limiting ischemic injury to the kidney.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health HL-34300, HL-25394 and GM31278. We wish to thank Melody Steinberg for editorial assistance in preparing this manuscript.
Abbreviations
- 2-CA
2-chloroadenosine
- CGS 21680 21680
2-[4-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamide-adenosine
- CHA
2-chloro-N6-cyclohexyladenosine
- CPA
2-chloro-N6-cyclopentyladenosine
- L-NAME
L-nitro-L-arginine methylester
- MS-PPOH
methylsulfonyl-propargyloxyphenylhexanamide
- PGMV
preglomerular microvessels
- ZM 241385
4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
- ABEBE W., MAKUJINA S.R., MUSTAFA S.J. Adenosine receptor-mediated relaxation of porcine coronary artery in presence and absence of endothelium. Am. J. Physiol. 1994;266:H2018–H2025. doi: 10.1152/ajpheart.1994.266.5.H2018. [DOI] [PubMed] [Google Scholar]
- AKI Y., NISHIYAMA A., MIYATAKE A., KIMURA S., KOHNO M., ABE Y. Role of adenosine A(1) receptor in angiotensin II- and norepinephrine-induced renal vasoconstriction. J. Pharmacol. Exp. Ther. 2002;303:117–123. doi: 10.1124/jpet.102.037010. [DOI] [PubMed] [Google Scholar]
- BERNE R.M. The role of adenosine in the regulation of coronary blood flow. Circ. Res. 1980;47:807–813. doi: 10.1161/01.res.47.6.807. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BRAND-SCHIEBER E., FALCK J.F., SCHWARTZMAN M. Selective inhibition of arachidonic acid epoxidation in vivo. J. Physiol. Pharmacol. 2000;51:655–672. [PubMed] [Google Scholar]
- BROWN R., OLLERSTAM A., JOHANSSON B., SKOTT O., GEBRE-MEDHIN S., FREDHOLM B., PERSSON A.E. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic receptors in the heart. Circ. Res. 1980;46:I175–I182. [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CARROLL M.A., BALAZY M., MARGIOTTA P., HUANG D.D., FALCK J.R., MCGIFF J.C. Cytochrome P-450-dependent HETEs: profile of biological activity and stimulation by vasoactive peptides. Am. J. Physiol. 1996;271:R863–R869. doi: 10.1152/ajpregu.1996.271.4.R863. [DOI] [PubMed] [Google Scholar]
- CARROLL M.A., GARCIA M.P., FALCK J.R., MCGIFF J.C. 5,6-epoxyeicosatrienoic acid, a novel arachidonate metabolite. Mechanism of vasoactivity in the rat. Circ. Res. 1990;67:1082–1088. doi: 10.1161/01.res.67.5.1082. [DOI] [PubMed] [Google Scholar]
- CARROLL M.A., GARCIA M.P., FALCK J.R., MCGIFF J.C. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J. Pharmacol. Exp. Ther. 1992;260:104–109. [PubMed] [Google Scholar]
- CHATZIANTONIOU C., ARENDSHORST W.J. Angiotensin receptor sites in renal vasculature of rats developing genetic hypertension. Am. J. Physiol. 1993;265:F853–F862. doi: 10.1152/ajprenal.1993.265.6.F853. [DOI] [PubMed] [Google Scholar]
- CHENG M.K., MCGIFF J.C., CARROLL M.A. Renal arterial 20-hydroxyeicosatetraenoic acid levels: regulation by cyclooxygenase. Am. J. Physiol. Renal Physiol. 2003;284:F474–F479. doi: 10.1152/ajprenal.00239.2002. [DOI] [PubMed] [Google Scholar]
- CROFT K.D., MCGIFF J.C., SANCHEZ-MENDOZA A., CARROLL M.A. Angiotensin II releases 20-HETE from rat renal microvessels. Am. J. Physiol. Renal Physiol. 2000;279:F544–F551. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- DUBEY R.K., GILLESPIE D.G., OSAKA K., SUZUKI F., JACKSON E.K. Adenosine inhibits growth of rat aortic smooth muscle cells. Possible role of A2b receptor. Hypertension. 1996;27:786–793. doi: 10.1161/01.hyp.27.3.786. [DOI] [PubMed] [Google Scholar]
- DUMOULIN M., SALVAIL D., GAUDREAULT S.B., CADIEUX A., ROUSSEAU E. Epoxyeicosatrienoic acids relax airway smooth muscles and directly activate reconstituted KCa channels. Am. J. Physiol. 1998;275:L423–L431. doi: 10.1152/ajplung.1998.275.3.L423. [DOI] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FULTON D., FALCK J.R., MCGIFF J.C., CARROLL M.A., QUILLEY J. A method for the determination of 5,6-EET using the lactone as an intermediate in the formation of the diol. J. Lipid Res. 1998;39:1713–1721. [PubMed] [Google Scholar]
- FULTON D., MCGIFF J.C., QUILLEY J. Contribution of NO and cytochrome P450 to the vasodilator effect of bradykinin in the rat kidney. Br. J. Pharmacol. 1992;107:722–725. doi: 10.1111/j.1476-5381.1992.tb14513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUYTON A.C., COLEMAN T.G. Quantitative analysis of the pathophysiology of hypertension. Circ. Res. 1969;24:1–19. [PubMed] [Google Scholar]
- HARDER D.R., NARAYANAN J., BIRKS E.K., LIARD J.F., IMIG J.D., LOMBARD J.H., LANGE A.R., ROMAN R.J. Identification of a putative microvascular oxygen sensor. Circ. Res. 1996;79:54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- HEIN T.W., BELARDINELLI L., KUO L. Adenosine A(2A) receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 1999;291:655–664. [PubMed] [Google Scholar]
- HERCULE H.C., OYEKAN A.O. Cytochrome P450 omega/omega-1 hydroxylase-derived eicosanoids contribute to endothelin(A) and endothelin(B) receptor-mediated vasoconstriction to endothelin-1 in the rat preglomerular arteriole. J. Pharmacol. Exp. Ther. 2000a;292:1153–1160. [PubMed] [Google Scholar]
- HERCULE H.C., OYEKAN A.O. Role of NO and cytochrome P-450-derived eicosanoids in ET-1-induced changes in intrarenal hemodynamics in rats. Am. J. Physiol Regul. Integr. Comp. Physiol. 2000b;279:R2132–R2141. doi: 10.1152/ajpregu.2000.279.6.R2132. [DOI] [PubMed] [Google Scholar]
- HOLZ F.G., STEINHAUSEN M. Renovascular effects of adenosine receptor agonists. Renal Physiol. 1987;10:272–282. doi: 10.1159/000173135. [DOI] [PubMed] [Google Scholar]
- IMIG J.D., FALCK J.R., WEI S., CAPDEVILA J.H. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J. Vasc. Res. 2001;38:247–255. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- IMIG J.D., NAVAR L.G., ROMAN R.J., REDDY K.K., FALCK J.R. Actions of epoxygenase metabolites on the preglomerular vasculature. J. Am. Soc. Nephrol. 1996a;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- IMIG J.D., ZOU A.P., STEC D.E., HARDER D.R., FALCK J.R., ROMAN R.J. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am. J. Physiol. 1996b;270:R217–R227. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- INSCHO E.W., CARMINES P.K., NAVAR L.G. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension. 1991;17:1033–1037. doi: 10.1161/01.hyp.17.6.1033. [DOI] [PubMed] [Google Scholar]
- JACKSON E.K., DUBEY R.K. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am. J. Physiol. Renal Physiol. 2001;281:F597–F612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- JACKSON E.K., ZHU C., TOFOVIC S.P. Expression of adenosine receptors in the preglomerular microcirculation. Am. J. Physiol Renal Physiol. 2002;283:F41–F51. doi: 10.1152/ajprenal.00232.2001. [DOI] [PubMed] [Google Scholar]
- KARARA A., DISHMAN E., FALCK J.R., CAPDEVILA J.H. Endogenous epoxyeicosatrienoyl-phospholipids. A novel class of cellular glycerolipids containing epoxidized arachidonate moieties. J. Biol. Chem. 1991;266:7561–7569. [PubMed] [Google Scholar]
- KAUSER K., CLARK J.E., MASTERS B.S., ORTIZ DE MONTELLANO P.R., MA Y.H., HARDER D.R., ROMAN R.J. Inhibitors of cytochrome P-450 attenuate the myogenic response of dog renal arcuate arteries. Circ. Res. 1991;68:1154–1163. doi: 10.1161/01.res.68.4.1154. [DOI] [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.M., SCHIFFMANN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- LEVENS N., BEIL M., JARVIS M. Renal actions of a new adenosine agonist, CGS 21680A selective for the A2 receptor. J. Pharmacol. Exp. Ther. 1991;257:1005–1012. [PubMed] [Google Scholar]
- LEWIS C.D., HOURANI S.M., LONG C.J., COLLIS M.G. Characterization of adenosine receptors in the rat isolated aorta. Gen. Pharmacol. 1994;25:1381–1387. doi: 10.1016/0306-3623(94)90162-7. [DOI] [PubMed] [Google Scholar]
- MACICA C., BALAZY M., FALCK J.R., MIOSKOWSKI C., CARROLL M.A. Characterization of cytochrome P-450-dependent arachidonic acid metabolism in rabbit intestine. Am. J. Physiol. 1993;265:G735–G741. doi: 10.1152/ajpgi.1993.265.4.G735. [DOI] [PubMed] [Google Scholar]
- MARTIN P.L., POTTS A.A. The endothelium of the rat renal artery plays an obligatory role in A2 adenosine receptor-mediated relaxation induced by 5′-N-ethylcarboxamidoadenosine and N6-cyclopentyladenosine. J. Pharmacol. Exp. Ther. 1994;270:893–899. [PubMed] [Google Scholar]
- MUNGER K.A., JACKSON E.K. Effects of selective A1 receptor blockade on glomerular hemodynamics: involvement of renin–angiotensin system. Am. J. Physiol. 1994;267:F783–F790. doi: 10.1152/ajprenal.1994.267.5.F783. [DOI] [PubMed] [Google Scholar]
- NAVAR L.G. Integrating multiple paracrine regulators of renal microvascular dynamics. Am. J. Physiol. 1998;274:F433–F444. doi: 10.1152/ajprenal.1998.274.3.F433. [DOI] [PubMed] [Google Scholar]
- NAVAR L.G., GUYTON A.C., LANGSTON J.B. Effect of alterations in plasma osmolality on renal blood flow autoregulation. Am. J. Physiol. 1966;211:1387–1392. doi: 10.1152/ajplegacy.1966.211.6.1387. [DOI] [PubMed] [Google Scholar]
- NAVAR L.G., INSCHO E.W., MAJID S.A., IMIG J.D., HARRISON-BERNARD L.M., MITCHELL K.D. Paracrine regulation of the renal microcirculation. Physiol. Rev. 1996;76:425–536. doi: 10.1152/physrev.1996.76.2.425. [DOI] [PubMed] [Google Scholar]
- NISHIYAMA A., INSCHO E.W., NAVAR L.G. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am. J. Physiol. Renal Physiol. 2001;280:F406–F414. doi: 10.1152/ajprenal.2001.280.3.F406. [DOI] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. The role of receptor structure in determining adenosine receptor activity. Pharmacol. Ther. 2000;85:55–75. doi: 10.1016/s0163-7258(99)00051-0. [DOI] [PubMed] [Google Scholar]
- OYEKAN A.O., YOUSEFF T., FULTON D., QUILLEY J., MCGIFF J.C. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J. Clin. Invest. 1999;104:1131–1137. doi: 10.1172/JCI6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POUCHER S.M., KEDDIE J.R., SINGH P., STOGGALL S.M., CAULKETT P.W., JONES G., COLL M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR H.M., YATES M.S., BEECH D.J. Role of K+ channels in A2A adenosine receptor-mediated dilation of the pressurized renal arcuate artery. Br. J. Pharmacol. 1999;126:494–500. doi: 10.1038/sj.bjp.0702310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUMP L.C., JABBARI-T J., VON KUGELGEN I., OBERHAUSER V. Adenosine mediates nitric-oxide-independent renal vasodilation by activation of A2A receptors. J. Hypertens. 1999;17:1987–1993. doi: 10.1097/00004872-199917121-00032. [DOI] [PubMed] [Google Scholar]
- SUN D., SAMUELSON L.C., YANG T., HUANG Y., PALIEGE A., SAUNDERS T., BRIGGS J., SCHNERMANN J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO X., INSCHO E.W., BONDLELA M., FALCK J.R., IMIG J.D. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2089–H2096. doi: 10.1152/ajpheart.2001.281.5.H2089. [DOI] [PubMed] [Google Scholar]
- ZOU A.P., NITHIPATIKOM K., LI P.L., COWLEY A.W., JR. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am. J. Physiol. 1999;276:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]