Abstract
Sepsis is associated with leukocyte activation and recruitment in the liver. We investigated the role of lymphocyte function antigen-1 (LFA-1) in endotoxin-induced leukocyte–endothelium interactions, microvascular perfusion failure, hepatocellular injury and apoptosis in the liver by use of gene-targeted mice, blocking antibodies and a synthetic inhibitor of LFA-1 (LFA703). For this purpose, mice were challenged with lipopolysaccharide (LPS)+D-galactosamine (Gal), and intravital microscopy of the liver microcirculation was conducted 6 h later.
The number of firmly adherent leukocytes in response to LPS/Gal was reduced by 48% in LFA-1-deficient mice. Moreover, endotoxin-induced increases of apoptosis and enzyme markers of hepatocellular injury were decreased by 64 and 69–90%, respectively, in LFA-1-deficient mice. Furthermore, sinusoidal perfusion was improved in endotoxemic mice lacking LFA-1.
A similar protective pattern was observed in endotoxemic mice pretreated with an antibody against LFA-1. Thus, immunoneutralization of LFA-1 reduced endotoxin-induced leukocyte adhesion by 55%, liver enzymes by 64–66% and apoptosis by 42%, in addition to the preservation of microvascular perfusion.
Administration of a novel statin-derived inhibitor of LFA-1, LFA703, significantly decreased leukocyte adhesion (more than 56%) and the subsequent liver injury in endotoxemic mice.
Thus, this study demonstrates a pivotal role of LFA-1 in supporting leukocyte adhesion in the liver. Moreover, interference with LFA-1-mediated leukocyte adhesion protects against endotoxemic liver damage, and may constitute a potential therapeutic strategy in sepsis.
Keywords: Adhesion, endotoxin, integrin, intravital microscopy, LFA703, sepsis, statins
Introduction
Leukocyte recruitment is a rate-limiting step in endotoxemic liver injury (Holman & Saba, 1988; Jaeschke et al., 1991; Marubayashi et al., 1997; Klintman et al., 2002). It has been shown that hepatic infiltration of leukocytes comprises a multistep process, in which an initial adhesive rolling interaction is a precondition for the subsequent firm adhesion of leukocytes in the liver (Klintman et al., 2002). Moreover, we have recently demonstrated that leukocyte rolling in venules is predominately mediated by the selectin family of adhesion molecules, in particular P-selectin (Klintman et al., 2003), although the α4-integrin has been reported to contribute under certain conditions (Fox-Robichaud & Kubes, 2000). Firm adhesion of leukocytes to the microvascular endothelium is dependent on the function of β2-integrins expressed on leukocytes, interacting with members of the immunoglobulin supergene family expressed on endothelial cells, such as intercellular adhesion molecule-1 (ICAM-1) (Arfors et al., 1987; Jaeschke et al., 1991; Lawrence & Springer, 1991; Klintman et al., 2002). The β2-integrins are heterodimeric molecules consisting of a common β-subunit (CD18), which is associated with one of four different α-subunits (CD11a–d) (Carlos & Harlan, 1994; Springer, 1994). The significance of β2-integrins is illustrated by the leukocyte adhesion deficiency syndrome-1, in which a mutation in the gene encoding for the common CD18 subunit results in the absence of β2-integrins (Harlan, 1993; Gahmberg, 1997). This autosomal recessive disorder is characterized by an inability to recruit neutrophils and increased susceptibility to recurrent bacterial infections (Anderson et al., 1985). However, the literature on the role of individual β2-integrins is complex and contradictory. Based on experiments using antibody (AB) treatment, it has been reported that both LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18) can mediate chemoattractant-induced leukocyte adhesion. However, the relative importance appears to be both stimulus- and organ-dependent (Argenbright et al., 1991; Morisaki et al., 1991; Issekutz & Issekutz, 1992; Tanaka et al., 1993; Rutter et al., 1994; Diacovo et al., 1996; Schmits et al., 1996; Lu et al., 1997; Ding et al., 1999; Thorlacius et al., 2000; Dunne et al., 2002). Moreover, the current complex and controversial data regarding the role of individual β2-integrins in leukocyte–endothelial cell interactions might be attributed to the use of individual monoclonal antibodies recognizing different epitopes, as well as to the use of either complete antibodies or Fab fragments. For example, two early studies reported that administration of an Ab against Mac-1 reduces leukocyte accumulation induced by endotoxins in the liver, whereas an Ab against LFA-1 was ineffective (Jaeschke et al., 1991; Morisaki et al., 1991). In contrast, Tanaka et al. (1993) observed that immunoneutralization of LFA-1 protected against hepatic damage in endotoxemic mice, and Jaeschke et al. (1996) reported that inhibition of Mac-1 had no effect on endotoxin-induced infiltration of leukocytes in the liver. The reason for these discrepant findings is not known at present. Besides inhibiting antibodies, the role of adhesion molecules may be investigated by the use of gene-targeted mice. In fact, using LFA-1-deficient mice, we and others have recently found that LFA-1 plays an important role in supporting firm leukocyte adhesion in striated muscle (Thorlacius et al., 2000; Dunne et al., 2002), the peritoneal cavity (Schmits et al., 1996; Lu et al., 1997) and the colon (Riaz et al., 2002). Nonetheless, the importance of LFA-1 for leukocyte–endothelium interactions and the relationship between leukocyte response, cellular injury and apoptosis in the liver remains controversial and elusive.
Statins are mainly used to decrease cholesterol levels, but there is a growing body of data ascribing anti-inflammatory properties to the statins. Some of these anti-inflammatory effects of statins are related to the inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase (Kwak et al., 2000; Romano et al., 2000), whereas others are independent of blocking HMG-CoA reductase activity (Weitz-Schmidt et al., 2001). One important contribution was made by Kallen et al. (1999), showing that lovostatin blocked the interaction between LFA-1 and ICAM-1 in vitro. Based on these findings and the crystal structure of the complex formed by LFA-1 and lovostatin, a new class of synthetic inhibitors of LFA-1 have been generated (Weitz-Schmidt et al., 2001; Welzenbach et al., 2002). One of these is LFA703, which inhibits LFA-1 by binding to a novel regulatory integrin site (L-site) of the I-domain distant to the metal-ion-dependent adhesion site (MIDAS) and, thus, inhibits LFA-1 function in an allosteric manner (Weitz-Schmidt et al., 2001; Welzenbach et al., 2002). A recent study showed that LFA703 was capable of inhibiting LFA-1-mediated leukocyte adhesion in colonic ischemia–reperfusion (Wan et al., 2003); however, the potential effects of LFA-703 on endotoxin-induced leukocyte–endothelium interactions and liver injury are not known.
Based on these considerations, we hypothesized that LFA-1 may mediate endotoxin-associated leukocyte–endothelium interactions in the liver. This was investigated by means of blocking antibodies, gene-targeted mice and LFA703. Another aim of this study was to determine the pathophysiological role of LFA-1 in the development of microvascular perfusion failure, hepatocellular injury and apoptosis in endotoxemic mice. For this purpose, we used intravital microscopy to visualize leukocyte–endothelium interactions in the liver microcirculation in response to endotoxin challenge.
Methods
Animals
Adult male C57/Bl6 and CD11a gene-targeted (LFA-1-deficient) mice (a gift from Dr Tak Mak, Department of Immunology, University of Toronto, Canada) weighing 23–27 g were kept on a 12–12 h light–dark cycle, with free access to food and tap water. Animals were anaesthetized by intraperitoneal (i.p.) administration of 7.5 mg ketamine hydrochloride and 2.5 mg xylazine per 100 mg body weight. The right jugular vein was cannulated with a polyethylene catheter for intravenous (i.v.) administration of test substances, fluorescent dyes and additional anesthesia. The local ethics committee approved all the experiments of this study.
Experimental protocol
At 6 h prior to surgery and intravital observation, mice were pretreated with phosphate-buffered saline (PBS, 0.2 ml), anti-LFA-1 Ab (M17/4.4.11.9, 100 μg per mouse) or a control Ab (9-A2, 100 μg per mouse) dissolved in 0.2 ml PBS, i.v., by injection into a lateral tail vein, immediately followed by i.p. administration of 0.25 ml PBS (control animals), or a combination of lipopolysaccharide (LPS; 10 μg per mouse) D-galactosamine (Gal; 18 mg per mouse) dissolved in PBS to a total volume of 0.25 ml. In separate experiments, LFA703 (0.3, 3 and 30 mg kg−1) was dissolved in a vehicle (ethanol/CremophorEL), diluted 1 : 3 in PBS, and was administered i.p. 2 h prior to LPS/Gal. A control group of animals received the vehicle (vehicle control).
Surgical procedure
In the anesthetized animals, a transverse subcostal incision was performed and the ligamentous attachments from the liver to the diaphragm and the abdominal wall were gently released. The animals were positioned on their left side and the left liver lobe was carefully exteriorized onto an adjustable stage for analysis of hepatic microcirculation by intravital fluorescence microscopy. The liver surface was covered with a circular glass to avoid tissue drying and exposure to ambient oxygen. An equilibration period of 5 min was allowed before starting microscopical observation. After intravital observations, animals were killed by exsanguination, and blood drawn from the heart was immediately centrifuged and used for analysis of plasma enzyme markers of hepatocellular injury (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)), using standard spectrophotometric procedures. Briefly, 30 μl of blood was applied onto reagent strips (Reflotron® Test Strip, Roche Diagnostics Scandinavia AB, Bromma, Sweden) designed for the specific quantitative determination of ALT and AST. These strips incorporate a plasma-separating system, which makes it possible to use whole blood. Systemic leukocyte counts, including polymorphonuclear leukocytes (PMNL) and mononuclear leukocytes (MNL), were determined using a hematocytometer.
Intravital microscopy
For observations of the liver microcirculation, we used a modified Olympus microscope (BX50WI, Olympus Optical Co. GmbH, Hamburg, Germany) equipped with different water immersion lenses (× 40 NA 0.75/ × 63 NA 0.9). The image was televised (Sony Trinitron) using a charge-coupled device video camera (FK 6990 Cohu, Pieper GmbH, Schwerte, Germany) and recorded on videotape (Panasonic SVT-S3000 S-VHS recorder) for subsequent off-line evaluation. Blood perfusion within individual microvessels was studied after contrast enhancement by FITC-dextran (0.1 ml, 0.1 mg ml−1, molecular weight 150,000). In vivo labeling of leukocytes with rhodamine-6G (0.1 ml, 0.05 mg ml−1) enabled quantitative analysis of leukocyte flow behavior in both sinusoids and postsinusoidal venules. Quantification of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Five postsinusoidal venules with connecting sinusoids were evaluated in each animal. Microcirculatory analysis included determination of the number of perfused sinusoids, given as a percentage of the total number of sinusoids observed (i.e. sinusoidal perfusion). Within postsinusoidal venules, leukocyte rolling was measured by counting the number of cells rolling in each venule during 30 s, and is expressed as cells min−1. Leukocyte rolling velocity was determined by calculating the velocity of 10 leukocytes rolling along the endothelial cell lining, and is given as μm s−1. Leukocyte adhesion was measured by counting the number of cells that adhered along the venular endothelium and remained stationary during the observation period of 30 s, and is expressed as cells mm−1 venule length. The diameter of the venules was not different between the experimental groups. Blood flow velocities were measured using CapImage software (Zeintl, Heidelberg, Germany). The velocity was determined as a mean value from 8–10 measurements per venule. Venular wall shear rate was calculated based on the Newtonian definition: Wall shear rate=8(red blood cell velocity × venular diameter−1).
Hepatocyte apoptosis was measured in the same microscopic setup as above. For this purpose, the fluorochrome Hoechst 33342 (Hoechst, 0.02 ml, 0.2 μg ml−1) was topically applied onto the liver surface for staining of hepatocyte DNA. Hoechst is a fluorescent dye that has been widely used for analysis of nuclear morphology (Kroemer et al., 1995), for example, nuclear condensation and fragmentation in cultured hepatocytes and endothelial cells (Rauen et al., 1999). In separate experiments, we have observed a strong correlation between caspase-3 and the levels of apoptosis determined by Hoechst staining in the liver (Klintman et al., 2003). After exsanguination and 10 min of incubation, six microscopical fields (using a × 63 lens) were recorded for off-line quantification of hepatocyte nuclei showing signs of apoptosis (chromatin condensation and fragmentation). Hepatocyte apoptosis is given as the percentage of the number of hepatocyte nuclei showing apoptotic features from the total number of hepatocyte nuclei observed. The results of this method correlate well with measurements of caspase-3 protease activity (Klintman et al., 2003).
Materials
FITC-dextran, Gal, LPS from E. coli, and rhodamine-6G were purchased from Sigma Chemical Co., St Louis, MO, U.S.A. Ketamine hydrochloride was from Hoffman-La Roche, Basel, Switzerland. Xylazine was from Janssen Pharmaceutica, Beerse, Belgium. The hybridoma cell line for the production of the monoclonal Ab directed against mouse LFA-1 (M17/4.4.11.9, rat IgG) was obtained from American Type Culture Collection, Rockville, MD, U.S.A., and the control Ab (9-A2, rat IgG) was from Bio-Express, West Lebanon, NH, U.S.A. Hoechst 33342 was purchased from Molecular Probes, Leiden, the Netherlands. LFA703 was from Novartis Pharma AG, Basel, Switzerland.
Statistical analyses
Data are presented as mean values+s.e.m. Statistical evaluations were performed using Kruskal–Wallis one-way analysis of variance on ranks, followed by multiple comparisons vs the control group (Dunn's method). P<0.05 was considered significant and n represents the number of animals.
Results
LFA-1 mediates leukocyte adhesion in the liver
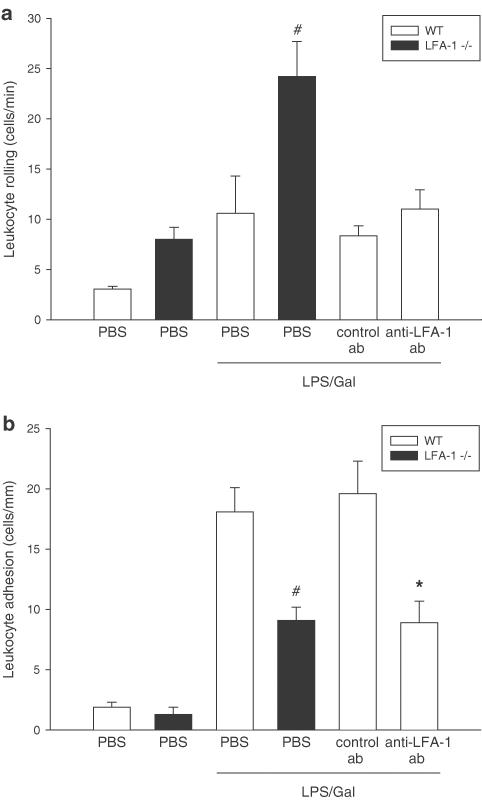
The number of rolling and adherent leukocytes was 3.0±0.3 cells min−1 and 1.6±0.3 cells mm−1, respectively, in PBS-treated control animals (Figure 1a, b, n=5). Challenge with the combination of LPS and Gal for 6 h markedly increased the leukocyte responses in the postsinusoidal venules. In fact, challenge with LPS/Gal increased venular leukocyte rolling by four-fold, that is, to 10.6±3.7 cells min−1 (Figure 1a, P<0.05 vs PBS, n=5–8). Moreover, this treatment enhanced firm leukocyte adhesion in venules by 11-fold, i.e. to 17.4±2.7 cells mm−1 (Figure 1b, P<0.05 vs PBS, n=5–8). In LFA-1-deficient mice, LPS-induced leukocyte rolling was 24.4±3.5 cells min−1 (Figure 1a, n=7). We observed that the number of adherent leukocytes was 9.1±1.1 cells mm−1 in mice lacking LFA-1 (Figure 1b, P<0.05 vs wild types, n=7) in response to endotoxin challenge, corresponding to a 48% reduction. Interestingly, LFA-1-deficient mice actually had higher total leukocyte counts than wild-type mice (Table 1, P<0.05 vs wild types). Notably, leukocyte rolling velocity was found to be 18.6±3.6 μm s−1 in wild-type mice, whereas rolling velocity was 50.5±7.6 μm s−1, that is, more than two-fold higher, in LFA-1-deficient mice (P<0.05 vs wild types).
Figure 1.
Leukocyte (a) rolling and (b) firm adhesion in hepatic postsinusoidal venules 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) in wild-type (WT) or LFA-1-deficient mice (−/−). Negative control animals received PBS. Animals were pretreated with a monoclonal Ab against LFA-1 (M17/4.4.11.9, 100 μg per mouse) and a control Ab (9-A2, 100 μg per mouse) or PBS. Data represent means±s.e.m. and n=5–8. #P<0.05 vs LPS-treated wild-type mice and *P<0.05 vs control Ab.
Table 1.
Systemic leukocyte counts
| MNL | PMNL | Total | |
|---|---|---|---|
| Control | 2.4±0.4 | 0.7±0.1 | 3.1±0.4 |
| PBS+LPS/Gal | 3.1±0.4 | 1.6±0.2 | 4.8±0.6 |
| LFA-1−/−+LPS/Gal | 4.5±1.5 | 1.7±0.3 | 6.2±1.6 |
| Control ab+LPS/Gal | 3.0±0.4 | 1.2±0.4 | 4.2±0.6 |
| Anti-LFA-1 ab+LPS/Gal | 4.6±1.0 | 1.0±0.2 | 5.6±1.2 |
Leukocytes were counted in a hematocytometer and defined as mononuclear (MNL) or polymorphonuclear (PMNL). Animals received a combination of LPS (10 μg) and D-galactosamine (Gal, 18 mg) in wild-type or LFA-1-deficient mice (−/−). Negative control animals received PBS (Control). Animals were pretreated with a monoclonal antibody against LFA-1 (M17/4.4.11.9, 100 μg per mouse) and a control antibody (9-A2, 100 μg per mouse) or PBS. Data represent means±s.e.m., and n=5–8.
Next, we evaluated the effect of immunoneutralization of LFA-1 on leukocyte–endothelium interactions in wild-type animals. We found that leukocyte rolling and adhesion was 8.4±1.0 cells min−1 and 19.6±2.7 cells mm−1, respectively, in control Ab-treated endotoxemic mice (Figure 1a, b, n=5). The pretreatment with the Ab directed against LFA-1 significantly reduced LPS/Gal-induced leukocyte adhesion by 55%, that is, down to 8.9±1.8 cells mm−1 (Figure 1b, P<0.05 vs control Ab, n=5), whereas the number of rolling leukocytes was not changed (Figure 1a, P>0.05 vs control Ab, n=5).
Microvascular perfusion and blood flow velocity
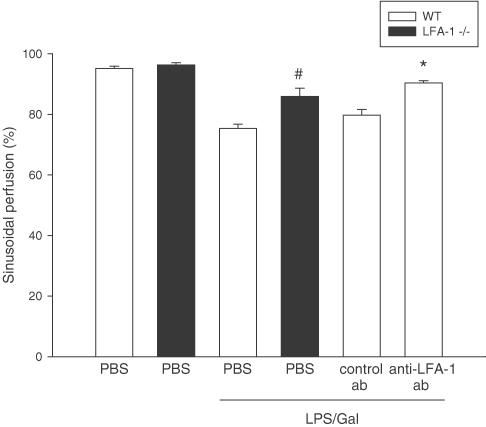
LPS induced a hepatic damage that markedly reduced sinusoidal perfusion from 96.3±0.8 to 75.4±1.4% (Figure 2, P<0.05 vs control, n=5–8). In both LFA-1-deficient and in wild-type mice receiving the Ab directed against LFA-1, it was found that this endotoxin-mediated sinusoidal perfusion was significantly improved (Figure 2, P<0.05 vs LPS/Gal, n=5–8). Microvascular blood flow velocity and wall shear rate were significantly reduced in endotoxemic mice, and inhibition of LFA-1 function did not alter these changes (Table 2, P>0.05 vs LPS/Gal, n=5–8).
Figure 2.
Sinusoidal perfusion in the murine liver 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) in wild-type (WT) or LFA-1-deficient mice (−/−). Negative control animals received PBS. Animals were pretreated with a monoclonal Ab against LFA-1 (M17/4.4.11.9, 100 μg per mouse) and a control Ab (9A-2, 100 μg per mouse) or PBS. Sinusoidal perfusion is given as the percentage of observed sinusoids with functional perfusion. Data represent means±s.e.m. and n=5–8. #P<0.05 vs LPS-treated wild-type mice and *P<0.05 vs control Ab.
Table 2.
Hemodynamics
| Diameter (μm) | Blood flow velocity (mm s−1) | Wall shear rate (s−1) | |
|---|---|---|---|
| Control | 27.4±1.2 | 1.9±0.3 | 355±62 |
| LFA-1−/−+PBS | 29.0±2.5 | 1.6±0.2 | 261±27 |
| PBS+LPS/Gal | 29.1±2.5 | 0.7±0.1* | 125±20* |
| LFA-1−/−+LPS/Gal | 32.2±1.4 | 0.8±0.1* | 134±19* |
| Control ab+LPS/Gal | 31.6±2.0 | 0.8±0.1* | 125±6* |
| Anti-LFA-1 ab+LPS/Gal | 31.5±2.4 | 0.9±0.1* | 151±12* |
Blood flow velocities were measured off-line by frame-to-frame analysis of the videotaped images using CapImage software. LPS (10 μg) and D-galactosamine (Gal, 18 mg) or PBS alone were administered to wild-type or LFA-1-deficient mice (−/−). Negative control animals received PBS (Control). Wild-type animals were pretreated with a monoclonal antibody against LFA-1 (anti-LFA-1, M17/4.4.11.9, 100 μg per mouse), a control antibody (9-A2, 100 μg per mouse) or PBS. Data represent means±s.e.m. and n=5–8.
P<0.05 vs PBS+LPS/Gal.
Inhibition of LFA-1 protects against hepatic damage in endotoxemia
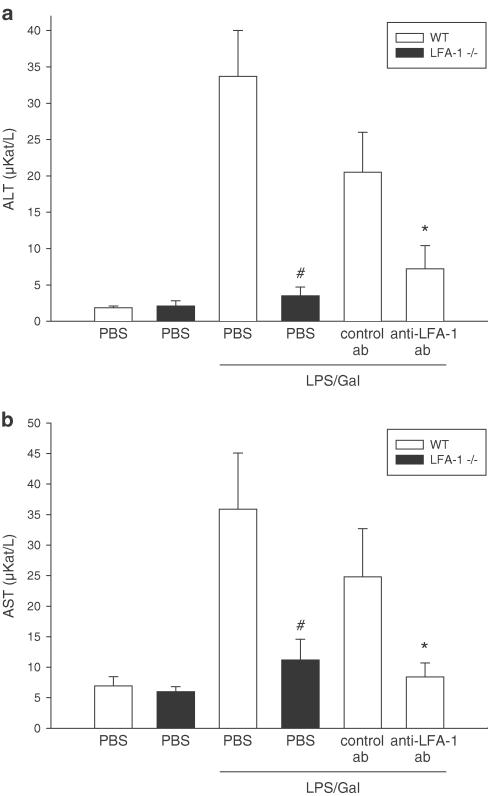
Administration of LPS/Gal provoked an extensive damage to the liver, demonstrated by the increase in liver enzymes and apoptosis (Figures 3 and 4). It was found that ALT and AST were increased by 15- and 5-fold, respectively (Figure 3). Interestingly, LFA-1-deficient mice were protected against the increase in liver enzymes induced by LPS/Gal, that is, ALT was reduced by 90% (from 33.7±6.3 to 3.5±1.2 μKat l−1) and AST by 69% (from 35.9±9.2 to 11.2±3.4 μKat l−1) (Figure 3a, b, P<0.05 vs wild types, n=7–15). Similarly, pretreatment with the anti-LFA-1 Ab markedly reduced liver enzymes compared to administration of the control Ab in endotoxemic mice, that is, ALT was significantly reduced by 65% (from 20.5±5.5 to 7.2±3.2 μKat l−1) and AST by 66% (from 24.8±7.9 to 8.4±2.3 μKat l−1) (Figure 3a, b, P<0.05 vs control Ab, n=5).
Figure 3.
Liver enzymes 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) in wild-type (WT) or LFA-1-deficient mice (−/−). Negative control animals received PBS. Animals were pretreated with a monoclonal Ab against LFA-1 (M17/4.4.11.9, 100 μg per mouse) and a control Ab (9-A2, 100 μg per mouse) or PBS. The levels of: (a) ALT and (b) AST were determined spectrophotometrically. Data represent mean±s.e.m. and n=5–8. #P<0.05 vs LPS-treated wild-type mice and *P<0.05 vs control Ab.
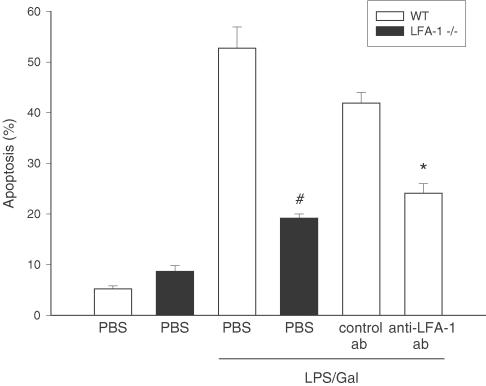
Figure 4.
Apoptosis of hepatocytes 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) in wild-type (WT) or LFA-1-deficient mice (−/−). Negative control animals received PBS. Animals were pretreated with a monoclonal Ab against LFA-1 (M17/4.4.11.9, 100 μg per mouse) and a control Ab (9-A2, 100 μg per mouse) or PBS. Hepatocyte apoptosis is given as the percentage of observed hepatocyte nuclei with morphological signs of apoptosis, that is, chromatin condensation and fragmentation, after administration of the fluorochrome Hoechst 33342. Data represent means± s.e.m. and n=5–8. #P<0.05 vs LPS-treated wild-type mice and *P<0.05 vs control Ab.
Hepatocyte apoptosis is a key feature in liver endotoxemia. Herein, we evaluated apoptosis using the DNA-binding fluorescent dye Hoechst 33342, which stains the nuclei of hepatocytes and permits quantification of the percentage of cells with nuclear condensation and fragmentation. Indeed, we found that the percentage of apoptotic hepatocytes increased to 52.7±4.2%, that is, by 10-fold, in animals treated with LPS (Figure 4, P<0.05 vs control, n=5–8). Interestingly, this increase in apoptosis was reduced by 64% in LFA-1-deficient animals (Figure 4, P<0.05 vs wild types, n=7–8). Moreover, pretreatment with the Ab directed against LFA-1 significantly decreased the percentage of apoptotic hepatocytes by 42%, that is, down to 24.1±1.9% from 41.9±2.1% in control Ab-treated mice (Figure 4, P<0.05 vs control Ab, n=5).
LFA703 protects against endotoxemic liver injury
We found that administration of LFA703 dose-dependently reduced endotoxin-induced leukocyte adhesion in the liver (Table 3). For example, 3 mg kg−1 of LFA703 significantly reduced leukocyte adhesion by more than 56% (Table 3, P<0.05 vs vehicle, n=5–12). On the other hand, leukocyte rolling was insensitive to LFA703 treatment in endotoxemic mice (data not shown). Moreover, LFA703 improved the microvascular perfusion in the liver of endotoxin-exposed mice (Table 3). Indeed, we found that LFA703 significantly protected against septic liver injury. At 3 mg kg−1 of LFA703, the LPS-induced increases in ALT and AST were reduced by 72 and 68%, respectively (Table 3, P<0.05 vs vehicle, n=5–12). Notably, endotoxin-induced apoptosis was decreased by 64% in animals receiving 3 mg kg−1 of LFA703 (Table 3, P<0.05 vs vehicle, n=5–12). Administration of LFA703 had no significant effect on systemic leukocyte counts or on blood flow velocities in the liver (data not shown).
Table 3.
Effect of LFA 703 on endotoxin-induced leukocyte recruitment and liver injury
| Leukocyte adhesion (cells mm−1) | Sinusoidal perfusion (%) | ALT (μKat l−1) | AST (μKat l−1) | Apoptosis (%) | |
|---|---|---|---|---|---|
| Control | 1.6±0.9 | 95±1 | 1.9±0.3 | 7.0±1.6 | 5.2±0.6 |
| Vehicle+LPS/Gal | 16.3±2.9# | 76.6±1.6# | 31.8±4.4# | 37.6±5.5# | 44.7±6.2# |
| 0.3+LPS/Gal | 11.4±2.0 | 80.0±2.9 | 35.4±9.6 | 26.7±6.0 | 38.4±4.6 |
| 3+LPS/Gal | 7.6±1.2* | 90.9±0.7* | 9.9±4.0* | 11.6±2.2* | 18.9±0.9* |
| 30+LPS/Gal | 5.6±0.9* | 89.6±0.7* | 10.1±2.8* | 14.6±3.0* | 23.9±1.6* |
Wild-type mice received a combination of LPS (10 μg) and D-galactosamine (Gal, 18 mg). Negative control animals received PBS (control) and positive controls received LFA703 vehicle (Vehicle). Animals were pretreated for 2 h with LFA 703 (0.3, 3 and 30 mg kg−1, i.p., n=6–12). The number of adherent leukocytes was determined in postsinusoidal venules, using intravital fluorescence microscopy. Sinusoidal perfusion is given as the percentage of observed sinusoids with functional perfusion. The levels of ALT and AST were determined spectrophotometrically. Hepatocyte apoptosis is given as the percentage of observed hepatocyte nuclei with morphological signs of apoptosis, that is, chromatin condensation and fragmentation, after administration of the fluorochrome Hoechst 33342. Data represent means±s.e.m. and n=5–12.
P<0.05 vs control
P<0.05 vs vehicle+LPS/Gal.
Discussion
This study documents a key role of LFA-1 in supporting firm adhesion in the liver during endotoxemia. Moreover, we provide evidence showing that inhibition of LFA-1 function markedly abrogates septic liver damage. These findings suggest an important link between LFA-1-mediated leukocyte adhesion on one hand and hepatocellular injury, apoptosis and sinusoidal perfusion failure on the other hand. Thus, these findings suggest that LFA-1 may not only mediate firm adhesion of leukocytes, but also constitute a potential target in the development of specific therapies directed against pathological inflammation in the liver.
Leukocyte accumulation is a hallmark of endotoxin-induced liver injury (Holman & Saba, 1988; Jaeschke et al., 1991; Klintman et al., 2002). We have recently shown that leukocyte rolling is a selectin-dependent event (Klintman et al., 2002), predominately mediated by P-selectin in the liver in response to endotoxemia (Klintman et al., 2003). Moreover, we have reported that CD18 supports firm leukocyte adhesion in hepatic venules (Klintman et al., 2002). However, the individual role of β2-integrins (CD11a–d/CD18) in endotoxin-induced leukocyte–endothelium interactions and liver injury is controversial. In the present study, we observed that immunoneutralization of LFA-1 (CD11a/CD18) markedly reduced (55%) leukocyte adhesion in postsinusoidal venules of endotoxemic mice. Moreover, it was found that endotoxin-induced leukocyte adhesion was also reduced by 48% in LFA-1-deficient mice. Taken together, these observations strongly suggest that LFA-1 is an important adhesion molecule supporting stationary adhesion of leukocytes in postsinusoidal venules in septic liver injury. This notion is in line with recent studies suggesting a predominant role of LFA-1 in mediating firm adhesion of leukocytes in venules of the peritoneum, skin, striated muscle and colon (Schmits et al., 1996; Lu et al., 1997; Ding et al., 1999; Thorlacius et al., 2000; Dunne et al., 2002; Riaz et al., 2002). It should be noted that LFA-1 did not account for all adhesion of leukocytes in postsinusoidal venules. It is possible that Mac-1 (CD11b/CD18) may account for the residual LFA-1-independent leukocyte adhesion. Alternatively, a previous study reported that leukocytes use α4-integrins for adhesion in hepatic venules (Fox-Robichaud & Kubes, 2000), and a recent report suggested that blocking VCAM-1 may protect against neutrophil-mediated septic liver injury (Essani et al., 1997). Thus, it is also possible that such an α4-VCAM-1-mediated pathway may support the residual level of leukocyte adhesion remaining after inhibition of LFA-1.
It is to be noted that we observed a significantly higher number of rolling leukocytes in LFA-1-deficient mice, compared to wild-type mice. This may partly be attributable to the higher leukocyte rolling velocity observed in mice lacking LFA-1 compared to LFA-1-expressing animals. This finding may at first glance appear surprising. However, in light of two recent studies demonstrating an important role of LFA-1 in reducing leukocyte rolling velocity in venules of the mouse mesentery (Ding et al., 1999), and cremaster muscle (Dunne et al., 2002), it may be suggested that LFA-1 also controls rolling velocity in postsinusoidal venules of the liver. In this context, it should be mentioned that ICAM-1, which is one ligand for LFA-1, has been implicated in supporting leukocyte rolling in venules of both the mouse cremaster muscle and the rat liver (Vollmar et al., 1995; Kunkel et al., 1996). Furthermore, as seen in Table 1, total systemic leukocyte counts were elevated in LPS/Gal-challenged LFA-1-deficient mice, which may add to the findings that leukocyte rolling is increased in these mice.
The previous literature is complex and partly contradictory with respect to the role of individual β2-integrins in leukocyte adhesion, and the importance of LFA-1 and Mac-1 appears to vary depending on the type of inflammatory stimulus and experimental model (Argenbright et al., 1991; Issekutz & Issekutz, 1992; Tanaka et al., 1993; Rutter et al., 1994; Diacovo et al., 1996; Kunkel et al., 1996; Schmits et al., 1996; Lu et al., 1997; Ding et al., 1999; Thorlacius et al., 2000; Dunne et al., 2002; Riaz et al., 2002). These discrepancies cannot be resolved at present, but may be related to the use of different Ab preparations (e.g. Fab fragments or complete antibodies) and/or antibodies recognizing different epitopes, considering that the potential shortcomings of evaluating the in vivo function of an adhesion molecule with inhibitory antibodies include the possibility that different antibodies can have additional effects, such as immune-mediated damage or elimination of circulating cells on which the target antigen is expressed. Moreover, it has been reported that the effectiveness of a specific Ab may be completely dependent on the type of mouse strain used (Ramos et al., 1997). Another approach to investigate the role of specific adhesion molecules in leukocyte recruitment is the use of gene-targeted mice. In fact, genetic manipulation of vascular adhesion molecules in mice has greatly contributed to the understanding of the molecular mechanisms regulating leukocyte–endothelium interactions (Ley et al., 1995; Frenette et al., 1996; Kunkel et al., 1996; Schmits et al., 1996; Lu et al., 1997; Ding et al., 1999; Thorlacius et al., 2000; Dunne et al., 2002; Gironella et al., 2002; Riaz et al., 2002). On the other hand, potential drawbacks using gene-targeted mice include the genetic compensation that has been increasingly recognized in recent years. For example, it has been observed that P-selectin-deficient mice have a compensatory upregulation of VCAM-1 and ICAM-1 (Gironella et al., 2002). Thus, one powerful impact of our present study is that we demonstrate that LFA-1 is a key molecule in mediating leukocyte adhesion and hepatic damage by using both immunoneutralization and gene-targeted mice.
Tissue recruitment of leukocytes is a key component in immune surveillance and host defense reactions (Carlos & Harlan, 1994; Springer, 1994). However, under various circumstances, the activation and infiltration of leukocytes result in tissue damage in certain diseases, including ischemia–reperfusion injury, graft rejection and endotoxemia. Herein, we observed that both immunoneutralization and gene deletion of LFA-1 reduced hepatocellular injury by more than 64–90% and apoptosis by more than 42–64%, suggesting that LFA-1-mediated leukocyte adhesion constitutes a fundamental role in septic liver injury. This notion is in line with a previous study showing that administration of a monoclonal Ab against LFA-1 protects against liver injury in a model based on Propionibacterium and endotoxin (Tanaka et al., 1993). Indeed, a key role of leukocytes has previously been forwarded by Hewett et al. (1993), demonstrating that neutrophil depletion markedly attenuates LPS-provoked liver injury.
Numerous studies have shown that statins exert potent anti-inflammatory effects besides reducing cholesterol levels. The anti-inflammatory effects of statins have been ascribed to both HMG-CoA reductase-dependent and independent mechanisms. Weitz-Schmidt et al. (2001) have developed a statin-based inhibitor, LFA703, that effectively and specifically inhibits the function of LFA-1 and lacks activity on HMG-CoA reductase. We wanted to evaluate the potential effect of LFA703 on endotoxemic liver injury and, at the same time, this compound would provide a third way, besides the use of gene-deficient mice and blocking antibodies, to determine the role of LFA-1 in endotoxin-induced leukocyte adhesion in the liver. Herein, we found that LFA703 dose-dependently decreased adhesion of leukocytes to the venular endothelium in the liver, suggesting that LFA703 is an effective inhibitor of LFA-1-dependent interactions in vivo. This notion is also corroborated by a recent study showing that LFA703 inhibits reperfusion-induced leukocyte adhesion in colonic ischemia–reperfusion injury (Wan et al., 2003). Moreover, in parallel to the inhibition of leukocyte adhesion, it was observed that LFA703 significantly decreased hepatocellular injury and apoptosis in endotoxemic mice. This is the first study showing that a statin-based molecule has the capacity to inhibit hepatic leukocyte adhesion and control pathological inflammation in the liver. In addition, these present findings also lend further support to our concept that leukocyte adhesion in the liver microcirculation is indeed supported by LFA-1. Thus, by use of a monoclonal Ab, gene-targeted mice and a synthetic inhibitor, our data document that LFA-1 is a key adhesion molecule in mediating stationary leukocyte adhesion and subsequent liver injury in endotoxemia. In this context, it is also worthwhile to note that LFA703 does not interfere with the function of human Mac-1 (CD11b/CD18) or very late antigen-4, indicating a high selectivity of LFA703 to LFA-1 (Weitz-Schmidt et al., 2001). These novel findings suggest that statin-based inhibitors of LFA-1, such as LFA703, may be useful to pharmacologically control pathological inflammation in the liver.
In conclusion, our present investigation demonstrates for the first time that LFA-1 is a dominant molecule mediating firm leukocyte adhesion in the liver in endotoxemia. In addition, our data show that inhibition of LFA-1-dependent leukocyte adhesion attenuates endotoxin-induced hepatocellular injury, apoptosis and reductions in sinusoidal perfusion. Based on these findings, it may be suggested that LFA-1 may be a potential target in the protection against septic liver damage.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (K2000-04P-13411-01A, K2002-73-X-14273-01A), Crafoordska stiftelsen, Blanceflors stiftelse, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke och Margareta Bergqvists stiftelse för främjande av cancerforskning, Magnus Bergvalls stiftelse, Mossfelts stiftelse, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet (2001-907), Teggers stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital and Lund University.
Abbreviations
- Ab
antibody
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- FITC
fluorescein isothiocyanate
- Gal
D-galactosamine
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-coenzyme A
- I
inserted
- ICAM-1
intercellular adhesion molecule-1
- i.p.
intraperitoneally
- i.v.
intravenously
- LFA-1
lymphocyte function antigen-1
- LFA-703
lymphocyte function antigen-703
- LPS
lipopolysaccharide
- MIDAS
metal-ion-dependent adhesion site
- MNL
monomorphonuclear leukocytes
- PBS
phosphate-buffered saline
- PMNL
polymorphonuclear leukocytes
- VCAM-1
vascular cell adhesion molecule-1
References
- ANDERSON D.C., SCHMALSTEIG F.C., FINEGOLD M.J., HUGHES B.J., ROTHLEIN R., MILLER L.J., KOHL S., TOSI M.F., JACOBS R.L., WALDROP T.C., GOLDMAN A.S., SHEARER W.T., SPRINGER T.A. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J. Infect. Dis. 1985;152:668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- ARFORS K.E., LUNDBERG C., LINDBOM L., LUNDBERG K., BEATTY P.G., HARLAN J.M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. [PubMed] [Google Scholar]
- ARGENBRIGHT L.W., LETTS L.G., ROTHLEIN R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte–endothelial adhesion in rabbits. J. Leukoc. Biol. 1991;49:253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- CARLOS T.M., HARLAN J.M. Leukocyte–endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- DIACOVO T.G., ROTH S.J., BUCCOLA J.M., BAINTON D.F., SPRINGER T.A. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- DING Z.M., BABENSEE J.E., SIMON S.I., LU H., PERRARD J.L., BULLARD D.C., DAI X.Y., BROMLEY S.K., DUSTIN M.L., ENTMAN M.L., SMITH C.W., BALLANTYNE C.M. Relative contribution of LFA-1 and Mac-1 to neutrophil adhesion and migration. J. Immunol. 1999;163:5029–5038. [PubMed] [Google Scholar]
- DUNNE J.L., BALLANTYNE C.M., BEAUDET A.L., LEY K. Control of leukocyte rolling velocity in TNF-alpha-induced inflammation by LFA-1 and Mac-1. Blood. 2002;99:336–341. doi: 10.1182/blood.v99.1.336. [DOI] [PubMed] [Google Scholar]
- ESSANI N.A., BAJT M.L., FARHOOD A., VONDERFECHT S.L., JAESCHKE H. Transcriptional activation of vascular cell adhesion molecule-1 gene in vivo and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxin shock. J. Immunol. 1997;158:5941–5948. [PubMed] [Google Scholar]
- FOX-ROBICHAUD A., KUBES P. Molecular mechanisms of tumor necrosis factor alpha-stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology. 2000;31:1123–1127. doi: 10.1053/he.2000.6961. [DOI] [PubMed] [Google Scholar]
- FRENETTE P.S., MAYADAS T.N., RAYBURN H., HYNES R.O., WAGNER D.D. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- GAHMBERG C.G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 1997;9:643–650. doi: 10.1016/s0955-0674(97)80117-2. [DOI] [PubMed] [Google Scholar]
- GIRONELLA M., MOLLA M., SALAS A., SORIANO A., SANS M., CLOSA D., ENGEL P., SALAS A., PIQUE J.M., PANES J. The role of P-selectin in experimental colitis as determined by antibody immunoblockade and genetically deficient mice. J. Leukoc. Biol. 2002;72:56–64. [PubMed] [Google Scholar]
- HARLAN J.M. Leukocyte adhesion deficiency syndrome: insights into the molecular basis of leukocyte emigration. Clin. Immunol. Immunopathol. 1993;67:S16–S24. doi: 10.1006/clin.1993.1079. [DOI] [PubMed] [Google Scholar]
- HEWETT J.A., JEAN P.A., KUNKEL S.L., ROTH R.A. Relationship between tumor necrosis factor-alpha and neutrophils in endotoxin-induced liver injury. Am. J. Physiol. 1993;265:G1011–G1015. doi: 10.1152/ajpgi.1993.265.6.G1011. [DOI] [PubMed] [Google Scholar]
- HOLMAN J.M., JR, SABA T.M. Hepatocyte injury during post-operative sepsis: activated neutrophils as potential mediators. J. Leukoc. Biol. 1988;43:193–203. doi: 10.1002/jlb.43.3.193. [DOI] [PubMed] [Google Scholar]
- ISSEKUTZ A.C., ISSEKUTZ T.B. The contribution of LFA-1 (CD11a/CD18) and MAC-1 (CD11b/CD18) to the in vivo migration of polymorphonuclear leucocytes to inflammatory reactions in the rat. Immunology. 1992;76:655–661. [PMC free article] [PubMed] [Google Scholar]
- JAESCHKE H., FARHOOD A., FISHER M.A., SMITH C.W. Sequestration of neutrophils in the hepatic vasculature during endotoxemia is independent of beta 2 integrins and intercellular adhesion molecule-1. Shock. 1996;6:351–356. doi: 10.1097/00024382-199611000-00009. [DOI] [PubMed] [Google Scholar]
- JAESCHKE H., FARHOOD A., SMITH C.W. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am. J. Physiol. 1991;261:G1051–G1056. doi: 10.1152/ajpgi.1991.261.6.G1051. [DOI] [PubMed] [Google Scholar]
- KALLEN J., WELZENBACH K., RAMAGE P., GEYL D., KRIWACKI R., LEGGE G., COTTENS S., WEITZ-SCHMIDT G., HOMMEL U. Structural basis for LFA-1 inhibition upon lovastatin binding to the CD11a I-domain. J. Mol. Biol. 1999;292:1–9. doi: 10.1006/jmbi.1999.3047. [DOI] [PubMed] [Google Scholar]
- KLINTMAN D., SCHRAMM R., MENGER M.D., THORLACIUS H. Leukocyte recruitment in hepatic injury: selectin-mediated leukocyte rolling is a prerequisite for CD18-dependent firm adhesion. J. Hepatol. 2002;36:53–59. doi: 10.1016/s0168-8278(01)00226-4. [DOI] [PubMed] [Google Scholar]
- KLINTMAN D., XIANG L., THORLACIUS H.Important role of P-selectin in leukocyte recruitment, hepatocellular injury and apoptosis in endotoxemic mice Clin. Diagn. Lab. Immunol. 2004(in press) [DOI] [PMC free article] [PubMed]
- KROEMER G., PETIT P., ZAMZAMI N., VAYSSIERE J.L., MIGNOTTE B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- KUNKEL E.J., JUNG U., BULLARD D.C., NORMAN K.E., WOLITZKY B.A., VESTWEBER D., BEAUDET A.L., LEY K. Absence of trauma-induced leukocyte rolling in mice deficient in both P-selectin and intercellular adhesion molecule 1. J. Exp. Med. 1996;183:57–65. doi: 10.1084/jem.183.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAK B., MULHAUPT F., MYIT S., MACH F. Statins as a newly recognized type of immunomodulator. Nat. Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- LAWRENCE M.B., SPRINGER T.A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- LEY K., BULLARD D.C., ARBONES M.L., BOSSE R., VESTWEBER D., TEDDER T.F., BEAUDET A.L. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LU H., SMITH C.W., PERRARD J., BULLARD D., TANG L., SHAPPELL S.B., ENTMAN M.L., BEAUDET A.L., BALLANTYNE C.M. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J. Clin. Invest. 1997;99:1340–1350. doi: 10.1172/JCI119293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUBAYASHI S., OSHIRO Y., MAEDA T., FUKUMA K., OKADA K., HINOI T., IKEDA M., YAMADA K., ITOH H., DOHI K. Protective effect of monoclonal antibodies to adhesion molecules on rat liver ischemia–reperfusion injury. Surgery. 1997;122:45–52. doi: 10.1016/s0039-6060(97)90263-4. [DOI] [PubMed] [Google Scholar]
- MORISAKI T., GOYA T., TOH H., NISHIHARA K., TORISU M. The anti Mac-1 monoclonal antibody inhibits neutrophil sequestration in lung and liver in a septic murine model. Clin. Immunol. Immunopathol. 1991;61:365–375. doi: 10.1016/s0090-1229(05)80008-x. [DOI] [PubMed] [Google Scholar]
- RAMOS C.L., KUNKEL E.J., LAWRENCE M.B., JUNG U., VESTWEBER D., BOSSE R., MCINTYRE K.W., GILLOOLY K.M., NORTON C.R., WOLITZKY B.A., LEY K. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–3018. [PubMed] [Google Scholar]
- RAUEN U., POLZAR B., STEPHAN H., MANNHERZ H.G., DE GROOT H. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 1999;13:155–168. doi: 10.1096/fasebj.13.1.155. [DOI] [PubMed] [Google Scholar]
- RIAZ A.A., WAN M.X., SCHAFER T., SCHRAMM R., EKBERG H., MENGER MD JEPPSSON B., THORLACIUS H. Fundamental and distinct roles of P-selectin and LFA-1 in ischemia/reperfusion-induced leukocyte-endothelium interactions in the colon. Ann. Surg. 2002;236:777–784. doi: 10.1097/00000658-200212000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANO M., DIOMEDE L., SIRONI M., MASSIMILIANO L., SOTTOCORNO M., POLENTARUTTI N., GUGLIELMOTTI A., ALBANI D., BRUNO A., FRUSCELLA P., SALMONA M., VECCHI A., PINZA M., MANTOVANI A. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab. Invest. 2000;80:1095–1100. doi: 10.1038/labinvest.3780115. [DOI] [PubMed] [Google Scholar]
- RUTTER J., JAMES T.J., HOWAT D., SHOCK A., ANDREW D., DE BAETSELIER P., BLACKFORD J., WILKINSON J.M., HIGGS G., HUGHES B., ROBINSON M.K. The in vivo and in vitro effects of antibodies against rabbit beta 2-integrins. J. Immunol. 1994;153:3724–3733. [PubMed] [Google Scholar]
- SCHMITS R., KUNDIG T.M., BAKER D.M., SHUMAKER G., SIMARD J.J., DUNCAN G., WAKEHAM A., SHAHINIAN A., VAN DER HEINDEN A., BACHMANN M.F., OHASHI P.S., MAK T.W., HICKSTEIN D.D. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J. Exp. Med. 1996;183:1415–1426. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., KOBAYASHI K., TAKAHASHI A., ARAI I., HIGUCHI S., OTOMO S., HABU S., NISHIMURA T. Inhibition of inflammatory liver injury by a monoclonal antibody against lymphocyte function-associated antigen-1. J. Immunol. 1993;151:5088–5095. [PubMed] [Google Scholar]
- THORLACIUS H., VOLLMAR B., GUO Y., MAK T.W., PFREUNDSCHUH M.M., MENGER M.D., SCHMITS R. Lymphocyte function antigen 1 (LFA-1) mediates early tumour necrosis factor alpha-induced leucocyte adhesion in venules. Br. J. Haematol. 2000;110:424–429. doi: 10.1046/j.1365-2141.2000.02162.x. [DOI] [PubMed] [Google Scholar]
- VOLLMAR B., GLASZ J., MENGER M.D., MESSMER K. Leukocytes contribute to hepatic ischemia/reperfusion injury via intercellular adhesion molecule-1-mediated venular adherence. Surgery. 1995;117:195–200. doi: 10.1016/s0039-6060(05)80085-6. [DOI] [PubMed] [Google Scholar]
- WAN M.X., SCHRAMM R., KLINTMAN D., WELZENBACH K., WEITZ-SCHMIDT G., THORLACIUS H. A statin-based inhibitor of lymphocyte function antigen-1 protects against ischemia/reperfusion-induced leukocyte adhesion in the colon. Br. J. Pharmacol. 2003;140:395–401. doi: 10.1038/sj.bjp.0705432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEITZ-SCHMIDT G., WELZENBACH K., BRINKMANN V., KAMATA T., KALLEN J., BRUNS C., COTTENS S., TAKADA Y., HOMMEL U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- WELZENBACH K., HOMMEL U., WEITZ-SCHMIDT G. Small molecule inhibitors induce conformational changes in the I domain and the I-like domain of lymphocyte function-associated antigen-1. Molecular insights into integrin inhibition. J. Biol. Chem. 2002;277:10590–10598. doi: 10.1074/jbc.M110521200. [DOI] [PubMed] [Google Scholar]