Abstract
Dilatation of the cerebral vasculature is recognised to be involved in the pathophysiology of migraine. Furthermore, elevated levels of prostaglandin E2 (PGE2) occur in the blood, plasma and saliva of migraineurs during an attack, suggestive of a contributory role. In the present study, we have characterised the prostanoid receptors involved in the relaxation and contraction of human middle cerebral arteries in vitro.
In the presence of indomethacin (3 μM) and the TP receptor antagonist GR32191 (1 μM), PGE2 was found to relax phenylephrine precontracted cerebral arterial rings in a concentration-dependent manner (mean pEC50 8.0±0.1, n=5).
Establishment of a rank order of potency using the EP4>EP2 agonist 11-deoxy PGE1, and the EP2>EP4 agonist PGE1-OH (mean pEC50 of 7.6±0.1 (n=6) and 6.4±0.1 (n=4), respectively), suggested the presence of functional EP4 receptors. Furthermore, the selective EP2 receptor agonist butaprost at concentrations <1 μM failed to relax the tissues.
Blockade of EP4 receptors with the EP4 receptor antagonists AH23848 and EP4A caused significant rightward displacements in PGE2 concentration–response curves, exhibiting pA2 and pKB values of 5.7±0.1, n=3, and 8.4, n=3, respectively.
The IP receptor agonists iloprost and cicaprost relaxed phenylephrine precontracted cerebral arterial rings (mean pEC50 values 8.3±0.1 (n=4) and 8.1±0.1 (n=9), respectively). In contrast, the DP and FP receptor agonists PGD2 and PGF2α failed to cause appreciable relaxation or contraction at concentrations of up to 30 μM. In the absence of phenylephrine contraction and GR32191, the TP receptor agonist U46619 caused concentration-dependent contraction of cerebral artery (mean pEC50 7.4±0.3, n=3).
These data demonstrate the presence of prostanoid EP4 receptors mediating PGE2 vasodilatation of human middle cerebral artery. IP receptors mediating relaxation and TP receptors mediating contraction were also functionally demonstrated.
Keywords: Prostaglandin E2, EP4 receptor, human middle cerebral artery, migraine
Introduction
The actions of the five naturally occurring prostanoid metabolites of arachidonic acid (PGD2, PGE2, PGF2α, PGI2 and thromboxane A2) are mediated via interaction with specific plasma membrane, G-protein-coupled receptors. Five major subdivisions of the prostanoid receptor family, termed DP, EP, FP, IP and TP, have been defined on the basis of their pharmacological sensitivity and molecular identity (Coleman et al., 1994a). In smooth muscle, FP and TP receptors are functionally associated with contractile responses, while DP and IP receptors mediate relaxation. EP receptors have been pharmacologically classified further into EP1, EP2, EP3 and EP4 subtypes, on the basis of their relative sensitivities to a range of naturally occurring and synthetic agonists and antagonists. While EP1 and EP3 receptors are coupled to Ca2+ mobilisation and the inhibition of cAMP via Gq/Gi G-proteins and mediate smooth muscle contraction, EP2 and EP4 receptors are coupled to the stimulation of adenylyl cyclase via Gs G-proteins, and have previously been shown to exert relaxant effects on vascular smooth muscle (Negishi et al., 1995).
Prostaglandins are important mediators of pain and inflammation, and considerable evidence implicates their involvement in the pathogenesis of migraine headache. Clinically, intravenous administration of the cyclooxygenase inhibitor aspirin is effective in treating acute migraine (Diener, 1999). Furthermore, non-steroidal anti-inflammatory drugs such as paracetamol, ibuprofen, ketoprofen and diclofenac, which can inhibit prostaglandin synthesis, have been shown to be 2–3-fold more effective than placebo in treating migraine and tension headache (The Diclofenac-K/Sumatriptan Migraine Study Group, 1999; Kellstein et al., 2000; Codispoti et al., 2001; Dib et al., 2002; Prior et al., 2002). Levels of PGE2 are elevated in the plasma and saliva of migrainers during an attack (Nattero et al., 1989; Obach Tuca et al., 1989), and in venous blood it reaches a sustained peak response within 2 h of migraine onset (Sarchielli et al., 2000). Prostaglandins have also been implicated in alcohol-induced migraine attacks and hangover, where low concentrations of ethanol can enhance PGE2-stimulated cAMP formation, stimulate prostaglandin biosynthesis and block prostaglandin metabolism (Parantainen, 1983). Furthermore, migraine-like symptoms can be induced in migraineurs by the exogenous administration of prostaglandins of the E series (Carlson et al., 1968; Peatfield et al., 1981). In addition, a well-observed, dominant adverse side effect seen on oral administration of the IP receptor agonist iloprost is headache (Hildebrand, 1997; Gao et al., 2002).
Clinically effective treatments such as the 5-HT1B/1D receptor agonists, known as triptans, are supposed to derive their antimigraine benefit via inhibition of neuropeptide-mediated activation of trigeminovascular afferents (Goadsby & Edvinsson, 1993) and vasoconstriction of the cerebral and meningeal vasculature (Humphrey & Feniuk, 1991; Moskowitz, 1993), but not the cerebral microcirculation (Kobari et al., 1993). Of these two interlinked components, electrical or inflammatory mediated stimulation of rat trigeminal ganglia in vitro has been demonstrated to cause a significant release of the neuropeptide calcitonin gene-related peptide (CGRP) from sensory nerve fibres, and a delayed synthesis and release of PGE2 from dura mater (Rich et al., 1996; Ebersberger et al., 1999). This, in turn, can lead to the activation of pain-stimulating trigeminovascular afferents that innervate and cause vasodilatation of the cranial and cerebral vasculature (Williamson et al., 1997). cAMP-coupled, functional EP prostanoid receptors have recently been demonstrated to be present on cultured rat trigeminal neurones, where stimulation mediates Ca2+-dependent CGRP release (Jenkins et al., 2001).
The effects of prostanoids on isolated cerebral blood vessels have been examined previously, but considerable species differences have been reported. PGE2 has been demonstrated to weakly relax 5-HT precontracted feline basilar and middle cerebral arteries (Whalley et al., 1989), but to contract canine, rabbit and human basilar arteries (Nakagomi et al., 1988; Parsons & Whalley, 1989). Despite potential experimental protocol and species differences, these data may suggest that distinct populations of prostanoid receptors are found in different vascular regions, as illustrated by differences in vasoconstrictor 5-HT receptors present in the macro and microcirculation. Given the potential involvement of PGE2 in the pathophysiology of migraine, in the present study, we have pharmacologically classified prostanoid receptors mediating responses to PGE2 on human middle cerebral artery.
Methods
Human tissues
All samples of human tissue were obtained through medically qualified intermediaries at the Netherlands Brain Bank with the informed consent of the donor or donor's next of kin, and with approval of the local research ethics committee. Tissues were rapidly removed at autopsy, transported on wet ice in phosphate-buffered saline (PBS), and stored in Krebs buffer at 4°C until the experiment, which was carried out within 72 h of tissue removal from the patient. Viable cerebral arteries were obtained from 19 donors (11 male, eight female,), age range 59–88 (mean age±s.e.m. of 76±4 years).
Pharmacology
Sections of middle cerebral artery were carefully removed from samples of human cerebral vasculature containing an intact circle of Willis using sharp dissection scissors. Visibly, atherosclerotic regions of tissue were not used. Intact rings of middle cerebral arteries, ∼2–3 mm in length and 1–2 mm internal diameter, were suspended between stainless steel hooks in 10 ml organ baths containing oxygenated (95% O2/5% CO2) Krebs' buffer containing (in mM); NaCl 118.2, KCl 4.69, MgSO4·7H2O 1.18, KH2PO4 1.19, glucose 11.1, NaHCO325.0, CaCl2·6H2O 2.5, indomethacin 0.003, pH 7.4 at 37°C. Tissues were placed under a tension equivalent to 5 mN and left to equilibrate for a period of at least 60 min. Responses were recorded using isometric transducers coupled to an Apple Macintosh computer via a MacLab interface. Following the equilibration period, a cumulative contractile concentration–response curve to phenylephrine (minimum of 5 min contact time of each concentration of agonist) was constructed in all tissues. After washout, an approximate EC50 concentration of phenylephrine (1 μM) was added to obtain a stable contraction. Tissues were subsequently exposed to increasing concentrations of a single prostanoid receptor agonist, in the absence or presence of receptor antagonists (incubated for 60 min prior to exposure to agonist), to pharmacologically characterise and functionally determine the role of prostanoid receptors in controlling arterial tone. Untreated tissues were run in parallel to agonist-treated tissue, to serve as time-matched controls. Experiments on the relaxant effects of prostanoids were performed in the presence of the TP receptor antagonist GR32191 (1 μM) (Lumley et al., 1989); as in the absence of GR32191, PGE2 caused vasodilatation at low concentrations, but vasoconstriction at concentrations greater than 0.1 μM (n=3 data not shown). The maximal relaxatory responses of tissues were determined at the end of each experiment, by the addition of prostacyclin (1 μM).
Materials
The following compounds were used in this study: PGE2, PG12, PGD2, PGE1-OH and 11-deoxy PGE1, PGF2α (Alexis Corp, U.K.), [1R-[1α(Z),2β,3β,5α]]-(+)-7-[5-([1,1′-biphenyl]-4-ylmethoxy)-3-hydroxy-2-(1-piperidinyl)cyclopentyl]-4-heptonic acid (GR32191) was a kind gift from Glaxo SmithKline, U.K., iloprost and cicaprost were gifts from Schering AG, Germany, 1α(Z),2β,5α]-(+/−)-7-[5-[[(1,1′-biphenyl)-4-yl]methoxy]-2-(4-morpholinyl)-3-oxocyclopentyl]-4-heptenoic acid (AH-23848) (Coleman et al., 1994b) and 4′-[3-butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5-dihydro-[1,2,4]triazol-4-ylmethyl] biphenyl-2-sulfonic acid (3-methyl-thiophene-2-carbonyl)-amide (EP4A) (Machwate et al., 2001,2003) were synthesised in-house.
Data analysis
To calculate a pKB value for EP4A, the mean CR was plotted as log (CR-1) against log antagonist molar concentration, according to the method of Arunlakshana & Schild (1959). If the slope of the plot did not differ significantly from unity, it was constrained to unity to calculate an apparent pKB value. As only one concentration of AH23848 was tested, a slope of unity was assumed and pA2 estimated using the Gaddum–Schild equation (where pA2=log[concentration ratio−1] −log[antagonist]).
Results
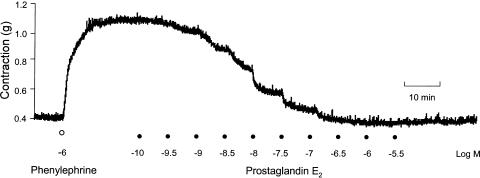
Addition of phenylephrine (10−8–10−4 M at half-log increments) caused reproducible, concentration-dependent contractile responses of isolated preparations of the human middle cerebral artery, exhibiting a mean pEC50 of 5.8±0.1 (n=7). Contractions observed at all phenylephrine concentrations were well maintained. Application of increasing concentrations of PGE2 to cerebral artery rings precontracted with an approximate pEC50 concentration of phenylephrine (1 μM) produced concentration-dependent relaxations to basal levels (Figure 1), exhibiting a mean pEC50 for PGE2 of 8.0±0.1 (n=5) (Figure 2). Mechanical denudation of the arterial endothelium or the addition of the nitric oxide synthase inhibitor L-NAME (100 μM) had no effect on PGE2 relaxations (n=3, data not shown), suggestive that these responses are mediated via direct action on the vascular smooth muscle and not via nitric oxide generation in the endothelium.
Figure 1.
Typical response to PGE2 on phenylephrine precontracted human middle cerebral artery. The figure shows concentration-dependent relaxation in response to increasing concentrations of PGE2 (0.1 nM–3 μM in half-log increments).
Figure 2.
Characterisation of PGE2 relaxatory response using a range of prostanoid ligands on human middle cerebral artery. (a) The EP4>EP2 agonist 11-deoxy PGE1 and the EP2>EP4 agonist PGE1-OH (1 nM–10 μM), caused relaxation of cerebral artery rings, with a rank order suggestive of the presence of functional EP4 receptors. Butaprost was without significant effect at concentrations below 1 μM. (b) The IP receptor agonists iloprost and cicaprost (0.3 nM–10 μM) equipotently caused concentration-dependent relaxations of phenylephrine precontracted cerebral rings. PGD2 (1 nM–100 μM) produced only weak relaxatory responses at high concentrations. Data are expressed as mean±s.e.m.% of phenylephrine contraction.
Of the four known members of the EP prostanoid receptor family, only EP2 and EP4 receptors potently mediate relaxatory responses of smooth muscle to PGE2. PGE2-induced relaxant responses were characterised in three ways: by establishment of a rank order of agonist potencies of prostanoid ligands, via the use of a selective EP2 agonist, and finally with the use of two known EP4 receptor antagonists.
A range of PGE2-related analogues was shown to cause relaxations of phenylephrine precontracted preparations (Figure 2). Establishment of a rank order of potency using the EP4>EP2 agonist 11-deoxy PGE1, and the EP2>EP4 agonist PGE1-OH, suggested the presence of functional EP4 receptors. The mean pEC50 values for 11-deoxy PGE1 and PGE1-OH were 7.6±0.1 (n=6) and 6.4±0.1 (n=4), respectively. The selective EP2 agonist butaprost was without effect at concentrations below 1 μM, suggestive of the absence of functional EP2 receptors in human middle cerebral arteries (n=3).
In human middle cerebral arterial rings, the combined TP/EP4 receptor antagonist AH23848 (10 μM) caused a surmountable, rightward shift in the PGE2 concentration–response curve (pA2 5.7±0.1; n=3; Figure 3a). The high-affinity, selective EP4 antagonist EP4A caused rightward, surmountable displacement of PGE2 concentration–effect curves (Figure 3b). Schild analysis generated a plot with slope not significantly different from unity (1.2±0.2) and a pKB of 8.4 (n=3/4, at each concentration, Figure 3c).
Figure 3.
Effects of EP4 receptor antagonists on PGE2-mediated relaxation of phenylephrine pre-contracted human middle cerebral artery. Cerebral artery rings were preincubated for 60 min with (a) AH23848 (10 μM) or (b) different concentrations of EP4A (30, 300, 1000 and 3000 nm), and then cumulatively concentration-dependently relaxed with PGE2 in the presence of GR32191 (1 μM). Data are expressed as percentage of the phenylephrine contraction, and are given as mean±s.e.m. for n=3/4 donors. (c) Schild plot of the antagonist effect of EP4A on responses to PGE2 in human middle cerebral artery.
Further characterisation of relaxatory prostanoid receptors demonstrated that the stable IP /receptor agonists iloprost and cicaprost potently relaxed phenylephrine precontracted human middle cerebral arteries, exhibiting mean pEC50 values of 8.3±0.1 (n=4) and 8.1±0.1 (n=9), respectively (Figure 2b). The DP receptor agonist PGD2 produced only weak relaxations at high concentrations (n=5). PGF2α was without appreciable effect at concentrations up to 100 μM (n=5). In the absence of phenylephrine precontraction and GR32191, the TP receptor agonist U-46619 caused concentration-dependent contractions, exhibiting a mean pEC50 value of 7.5±0.1 (n=5, data not shown).
Discussion
In the present study, we have demonstrated that PGE2 is a potent vasodilator of human precontracted middle cerebral artery, and have characterised the receptor mediating this response. The EP receptor family consists of four members that exhibit nanomolar affinity for the endogenous prostanoid PGE2 (Coleman et al., 1994a). Human middle cerebral arteries were found to express specific mRNA for each of the prostanoid receptors (unpublished observations, R. Davis). Pharmacological classification using endogenous and synthetic ligands demonstrated that the EP4>EP2 agonist 11-deoxy PGE1 and the EP2>EP4 agonist PGE1-OH were both full agonists, exhibiting approximately two-fold and 40-fold lower potency than PGE2, respectively. These data are consistent with the relative binding affinities reported for these agonists at cloned human, rat, rabbit and mouse EP4 receptors (Breyer et al., 1996; Boie et al., 1997; Kiriyama et al., 1997; Davis & Sharif, 2000). Furthermore, butaprost, which exhibits nanomolar affinity for the EP2 receptor (Gardiner, 1986) but micromolar affinity for the EP4 receptor, was without effect at concentrations below 1 μM, suggestive of the absence of functional EP2 receptors. Characterisation of the presence of other prostanoid receptors functionally expressed in human middle cerebral arteries demonstrated contractile TP and relaxatory IP receptors, in good agreement with previous studies (Uski et al., 1983), but the absence of EP1, EP2, EP3, FP and DP receptors.
Although rank orders of agonist potency pointed to a role for EP4 receptors in the cerebral vasodilator response to PGE2, an evaluation of various EP4 receptor antagonists was performed to consolidate this preliminary conclusion. The weak EP4 antagonist activity of the TP antagonist AH23848 was first described on PGE2-mediated relaxation of phenylephrine precontracted isolated smooth muscle rings of pig saphenous vein (Coleman et al., 1994b). Although described as a low affinity, competitive antagonist at the EP4 receptor in this tissue, there are reports suggesting that its functional antagonism is not truly surmountable (Blaschke et al., 1996). In the present study, AH23848 (10 μM) was found to cause a significant rightward shift in the PGE2-mediated vasodilatation of human middle cerebral artery, exhibiting a pA2 value (5.7) consistent with that previously reported (pA2 5.4; Coleman et al., 1994b). Further support for the functional expression of vasodilatory EP4 receptors was demonstrated with the recently reported potent and selective EP4 antagonist EP4A (Machwate et al., 2001,2003). At human recombinant EP receptors, EP4A exhibits high affinity for the EP4 receptor (pKi=7.6), and approximately 80-, 800-, 280- and 30-fold selectivity over EP3, EP2/EP1, IP and TP receptors, respectively. EP4A caused concentration-related rightward, surmountable displacement of PGE2 concentration–effect curves in the human middle cerebral artery, with a pKB consistent with PGE2 activity via EP4 receptors. While the highest concentrations of EP4A utilised may exhibit some weak antagonism at relaxatory IP receptors present in the human middle cerebral artery, it is unlikely that this contributes to its inhibition of PGE2-mediated relaxation, given the low affinity of PGE2 for IP receptors. The potency of EP4A on IP receptor-mediated functions has not been reported.
The timing of the cerebral vascular component and augmentation of blood flow involved in the pathophysiology of migraine has been open to speculation. While the triptans (5-HT1B/1D receptor agonists) can mediate vasoconstriction of the cerebral vasculature (Humphrey & Feniuk, 1991), their clinical efficacy may derive from inhibition of vasodilatation caused by neuropeptide or other inflammatory mediators released from trigeminal neurones (Goadsby & Edvinsson, 1993). Trigeminal sensory and visceral projections innervate the middle cerebral artery (Arbab et al., 1988), and stimulation of these afferents in healthy volunteers with capsaisin can mediate pain and cranial vasodilatation (May et al., 2001). Cortical spreading depression (CSD), a putative trigger for migraine with aura, has been shown experimentally to increase meningeal blood flow via vasodilatation associated with the stimulation of trigeminovascular afferents and trigeminal-dependent parasympathetic activation. However, the reverse is not true, such that sustained vasodilatation does not trigger trigeminal activation after CSD (Bolay et al., 2002), and demonstrating that the vasodilatation component is secondary to afferent stimulation.
While much emphasis has focused on the neuropeptide CGRP as the agent mediating vasodilatation following trigeminal activation, a role for prostaglandins in migraine has been implicated. Elevated levels of PGE2 in the plasma, saliva and venous blood have been reported in migraineurs during an attack (Nattero et al., 1989; Obach Tuca et al., 1989; Sarchielli et al., 2000), and inhibitors of prostaglandin synthesis have shown some clinical efficacy. Although CGRP and PGE2 are not released in an in vitro model of CSD, (Ebersberger et al., 2001), electrical or inflammatory mediated stimulation of rat trigeminal ganglia in vitro does cause a significant release of the CGRP from sensory nerve fibres, and a delayed synthesis and release of PGE2 from dura mater (Rich et al., 1996; Ebersberger et al., 1999), an effect which can be inhibited by dihydroergotamide or sumatriptan (Buzzi et al., 1991). Moreover, PGE2-induced activation of EP2 and possibly other EP receptors can stimulate the release of CGRP from rat cultured trigeminal neurones (Jenkins et al., 2001) and sensory afferents in vivo following irritation-induced visceral pain (Friese et al., 1997; Boku et al., 2001). In rat dura (Zimmermann et al., 2002) and rat tracheal afferent nerves (Hua et al., 1994), PGE2 released secondary to agonist stimulation can mediate CGRP release. Taken together, these data potentially suggest an early involvement of PGE2 in the development and prolongation of migraine, potentially upstream of CGRP release.
In conclusion, accumulating evidence suggests that endogenously produced prostaglandins, most notably PGE2, play a direct role in the pathophysiology of migraine by stimulating CGRP release from trigeminal afferents and thus mediating vasodilatation. In the present study, we have demonstrated for the first time that PGE2 can also directly mediate vasodilatation of human cerebral arteries via EP4 receptors. These data are hypothesised to be of clinical significance with reference to the aetiology of the vasodilator component of migraine. In addition, antagonism of PGE2-induced cerebral vascular dilatation may be therapeutically beneficial. The characterisation of prostanoid receptors mediating CGRP release from human trigeminal afferents and vasodilatation in human meningeal and pial vessels is now the subject of on-going studies.
Abbreviations
- AH23848
[1α(Z),2β,5α]-(+/−)-7-[5-[[(1,1′-biphenyl)-4-yl]methoxy]-2-(4-morpholinyl)-3-oxocyclopentyl]-4-heptenoic acid
- GR32191
1,11α,15S-trihydroxy-prost-13E-en-9-one;9-oxo-15S-hydroxy-prost-13E-en-1-oic acid
- EP4A
(4′-[3-butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5-dihydro-[1,2,4]triazol-4-ylmethyl]-biphenyl-2-sulfonic acid (3-methyl-thiophene-2-carbonyl)-amide]
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
- PGE1-OH
prostaglandin E1 alcohol
- PGF2α
prostaglandin F2α
- PGI2
prostaglandin I2
References
- ARBAB M.A., DELGADO T., WIKLUND L., SVENDGAARD N.A. Brain stem terminations of the trigeminal and upper spinal ganglia innervation of the cerebrovascular system: WGA-HRP transganglionic study. J. Cereb. Blood Flow Metab. 1988;8:54–63. doi: 10.1038/jcbfm.1988.8. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative use of drug antagonists. Br. J. Pharmacol. Chemother. 1959;257:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLASCHKE V., JUNGERMANN K., PUSCHEL G.P. Exclusive expression of the Gs-linked prostaglandin E2 receptor subtype 4 mRNA in human mononuclear Jurkat and KM-3 cells and coexpression of subtype 4 and 2 mRNA in U-937 cells. FEBS Lett. 1996;394:39–43. doi: 10.1016/0014-5793(96)00928-3. [DOI] [PubMed] [Google Scholar]
- BOIE Y., STOCCO R., SAWYER N., SLIPETZ D.M., UNGRIN M.D., NEUSCHAFER-RUBE F., PUSCHEL G.P., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterisation of the four rat prostaglandin EP receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- BOKU K., OHNO T., SAEKI T., HAYASHI H., HAYASHI I., KATORI M., MURATA T., NARUMIYA S., SAIGENJI K., MAJIMA M. Adaptive cytoprotection mediated by prostaglandin I2 is attributed to sensitisation of CGRP-containing sensory nerves. Gastroenterology. 2001;120:134–143. doi: 10.1053/gast.2001.20916. [DOI] [PubMed] [Google Scholar]
- BOLAY H., REUTER U., DUNN A.K., HUANG Z., BOAS D.A., MOSKOWITZ M.A. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat. Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- BREYER R.M., DAVIS L.S., NIAN C., REHDA R., STILLMAN B., JACOBSON H.R., BREYER M.D. Cloning and expression of the rabbit prostaglandin EP4 receptor. Am. J. Physiol. 1996;270:F485–F493. doi: 10.1152/ajprenal.1996.270.3.F485. [DOI] [PubMed] [Google Scholar]
- BUZZI M.G., CARTER W.B., SHIMIZU T., HEATH H., MOSKOWITZ M.A. Dihydroergotamine and sumatriptan attenuate levels of CGRP in plasma in rat superior sagittal sinus during electrical stimulation of the trigeminal ganglion. Neuropharmacology. 1991;30:1193–1200. doi: 10.1016/0028-3908(91)90165-8. [DOI] [PubMed] [Google Scholar]
- CARLSON L.A., EKELUND L.G., ORÖ L. Clinical and metabolic effects of different doses of prostaglandin E1 in man. Prostaglandin and related factors. Acta Med. Scand. 1968;83:423–430. doi: 10.1111/j.0954-6820.1968.tb10502.x. [DOI] [PubMed] [Google Scholar]
- CODISPOTI J.R., PRIOR M.J., FU M., HARTE C.M., NELSON E.B. Efficacy of non-prescription doses of ibuprofen for treating migraine headache. A randomised controlled trial. Headache. 2001;41:665–679. doi: 10.1046/j.1526-4610.2001.041007665.x. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLET A., SHELDRICK R.L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994b;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. VIII. Internation Union of Pharmacology Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994a;46:205–229. [PubMed] [Google Scholar]
- DAVIS T.L., SHARIF N.A. Pharmacological characterisation of [3H]-prostaglandin E2 binding to the cloned human EP4 receptor. Br. J. Pharmacol. 2000;130:1919–1926. doi: 10.1038/sj.bjp.0703525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIB M., MASSIOU H., WEBER M., HENRY P., GARCIA-ACOSTA S., BOUSSER M.G. Efficacy of oral ketoprofen in acute migraine: a double-blind randomised clinical trial. Neurology. 2002;58:1660–1665. doi: 10.1212/wnl.58.11.1660. [DOI] [PubMed] [Google Scholar]
- DIENER H.C. Efficacy and safety of intravenous acetylsalicylic acid lysinate compared to subcutaneous sumatriptan and parenteral placebo in the acute treatment of migraine. A double-blind, double-dummy, randomised, multicenter, parallel group study. Cephalagia. 1999;19:581–588. doi: 10.1046/j.1468-2982.1999.019006581.x. [DOI] [PubMed] [Google Scholar]
- EBERSBERGER A., AVERBECK B., MESSLINGER K., REEH P.W. Release of substance P, calcitonin gene-related peptide and prostaglandin E2 following electrical and chemical stimulation in vitro. Neuroscience. 1999;89:901–907. doi: 10.1016/s0306-4522(98)00366-2. [DOI] [PubMed] [Google Scholar]
- EBERSBERGER A., SCHAIBLE H.G., AVERBECK B., RICHTER F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache. Ann. Neurol. 2001;49:7–13. [PubMed] [Google Scholar]
- FRIESE N., DIOP L., CHEVALIER E., ANGEL F., RIVIERE P.J., DAHL S.G. Involvement of prostaglandins and CGRP-dependent sensory afferents in peritoneal irritation-induced visceral pain. Regul. Pept. 1997;70:1–7. doi: 10.1016/s0167-0115(97)02141-1. [DOI] [PubMed] [Google Scholar]
- GAO I.K., SCHOLZ P., BOEHME M.W.J., NORDEN C., LEMMEL E.-M. A 7-day oral treatment of patients with active rheumatoid arthritis using the prostacyclin analog iloprost: cytokine modulation, safety and clinical effects. Rheumatol. Int. 2002;22:45–51. doi: 10.1007/s00296-002-0187-x. [DOI] [PubMed] [Google Scholar]
- GARDINER P.J. Characterisation of prostanoid relaxant/inhibitory receptor using a highly selective agonist, TR4979. Br. J. Pharmacol. 1986;87:45–56. doi: 10.1111/j.1476-5381.1986.tb10155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. The trigeminal system and migraine: studies characterising cerebrovascular and neuropeptide changes seen in man and cat. Ann. Neurol. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- HILDEBRAND M. Pharmacokinetics and tolerability of oral iloprost in thromboangiitis obliterans patients. Eur. J. Pharmacol. 1997;53:51–56. doi: 10.1007/s002280050336. [DOI] [PubMed] [Google Scholar]
- HUA X.Y., JINNO S., BACK S.M., TAM E.K., YAKSH T.L. Multiple mechanisms for the effects of capsaicin, bradykinin and nicotine on CGRP release from tracheal afferent nerves: role of prostaglandins, sympathetic nerves and mast cells. Neuropharmacology. 1994;33:1147–1154. doi: 10.1016/s0028-3908(05)80004-8. [DOI] [PubMed] [Google Scholar]
- HUMPHREY P.P., FENIUK W. Mode of action of the anti-migraine drug sumatriptan. Trends Pharmacol. Sci. 1991;12:444–446. doi: 10.1016/0165-6147(91)90630-b. [DOI] [PubMed] [Google Scholar]
- JENKINS D.W., FENIUK W., HUMPHREY P.P.A. Characterisation of the prostanoid receptor types involved in mediating calcitonin gene-related peptide release from cultured rat trigeminal neurones. Br. J. Pharmacol. 2001;134:1296–1302. doi: 10.1038/sj.bjp.0704357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLSTEIN D.E., LIPTON R.B., GEETHA R., KORONKIEWICZ K., EVANS F.T., STEWART W.F., WILKES K., FUREY S.A., SUBRAMANIAN T., COOPER S.A. Evaluation of a novel solubilised formulation of ibuprofen in the treatment of migraine headache: a randomised, double-blind, placebo-controlled, dose-ranging study. Cephalagia. 2000;20:223–243. doi: 10.1046/j.1468-2982.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., MOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–224. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBARI M., FUKUUCHI Y., TOMITA M., TANAHASHI N., KONNO S., TAKEDA H. Effects of sumatriptan on the cerebral intraparenchymal microcirculation in the cat. Br. J. Pharmacol. 1993;110:1445–1448. doi: 10.1111/j.1476-5381.1993.tb13983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUMLEY P., WHITE B.P., HUMPHREY P.P. GR32191, a highly potent and specific thromboxane A2 receptor blocking drug on platelets and vascular and airways smooth muscle, in vitro. Br. J. Pharmacol. 1989;97:783–794. doi: 10.1111/j.1476-5381.1989.tb12017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHWATE M., HARADA S., LEU C.T., SEEDOR G., LABELLE M., GALLANT M., HUTCHIN S., LACHANCE SAWYER N., SLIPETZ D., METTERS K.M., RODAN S.B., YOUNG R., RODAN G.A. Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol. Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- MACHWATE M., HARADA S., LEU C.T., SEEDOR G., LABELLE M., GALLANT M., HUTCHIN S., LACHANCE SAWYER N., SLIPETZ D., METTERS K.M., RODAN S.B., YOUNG R., RODAN G.A. Correction to: Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol. Pharmacol. 2003;64:192. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- MAY A., BUCHEL C., TURNER R., GOADSBY P.J. Magnetic resonance angiography in facial and other pain: neurovascular mechanisms of trigeminal sensation. J. Cereb. Blood Flow Metab. 2001;21:1171–1176. doi: 10.1097/00004647-200110000-00005. [DOI] [PubMed] [Google Scholar]
- MOSKOWITZ M.A. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol. Sci. 1993;13:307–377. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- NAKAGOMI T., KASSELL N.F., SASAKI T., LEHMAN R.M., TORNER J.C., HONGO K., LEE J.H. Effects of removal of the endothelium on vasoconstriction in canine and rabbit basilar arteries. J. Neurosurg. 1988;68:757–766. doi: 10.3171/jns.1988.68.5.0757. [DOI] [PubMed] [Google Scholar]
- NATTERO G., ALLAIS G., DE LORENZO C., BENEDETTO C., ZONCA M., MELZI E., MASSOBRIO M. Relevance of prostaglandins in true menstrual migraine. Headache. 1989;29:232–237. doi: 10.1111/j.1526-4610.1989.hed22904233.x. [DOI] [PubMed] [Google Scholar]
- NEGISHI M., SUGIMOTO Y., ICHIKAWA A. Molecular mechanisms of diverse actions of prostanoid receptors. Biochem. Biophys. Acta. 1995;1259:109–120. doi: 10.1016/0005-2760(95)00146-4. [DOI] [PubMed] [Google Scholar]
- OBACH TUCA J., PLANAS J.M., PARELLADA P.P. Increase in PGE2 and TXA2 in the saliva of common migraine patients. Actions of calcium channel blockers. Headache. 1989;29:498–501. doi: 10.1111/j.1526-4610.1989.hed2908498.x. [DOI] [PubMed] [Google Scholar]
- PARANTAINEN J. Prostaglandin in alcohol intolerance and hangover. Drug Alcohol Depend. 1983;11:239–248. doi: 10.1016/0376-8716(83)90016-9. [DOI] [PubMed] [Google Scholar]
- PARSONS A.A., WHALLEY E.T. Effects of prostanoids on human and rabbit basilar arteries pre-contracted in vitro. Cephalalgia. 1989;9:165–171. doi: 10.1046/j.1468-2982.1989.0903165.x. [DOI] [PubMed] [Google Scholar]
- PEATFIELD R.C., GAVEL M.J., ROSE F.C. The effect of infused prostacyclin in migraine and cluster headache. Headache. 1981;21:190–195. doi: 10.1111/j.1526-4610.1981.hed2105190.x. [DOI] [PubMed] [Google Scholar]
- PRIOR M.J., COOPER K.M., MAY L.G., BOWEN D.L. Efficacy and safety of acetaminophen and naproxen in the treatment of tension-type headache. A randomised, double blind, placebo-controlled trial. Cephalalgia. 2002;22:232–240. doi: 10.1046/j.1468-2982.2002.00419.x. [DOI] [PubMed] [Google Scholar]
- RICH G., YOBER E.J., PROKUSKI L., MOORE S.A. Prostaglandin production in cultured cerebral microvasculature smooth muscle is serum dependent. Am. J. Physiol. 1996;270:C1379–C1387. doi: 10.1152/ajpcell.1996.270.5.C1379. [DOI] [PubMed] [Google Scholar]
- SARCHIELLI P., ALBERTI A., CODINI M., FLORIDI A., GALLAI V. Nitric oxide metabolites, prostaglandin and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia. 2000;20:907–918. doi: 10.1046/j.1468-2982.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- THE DICLOFENAC-K/SUMATRIPTAN MIGRAINE STUDY GROUP Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti-inflammatory drug, diclofenac-potassium, in comparison to oral sumatriptan and placebo. Cephalalgia. 1999;19:232–240. doi: 10.1046/j.1468-2982.1999.019004232.x. [DOI] [PubMed] [Google Scholar]
- USKI T., ANDERSSON K.E., BRANDT L., EDVINSSON L., LIUNOGREN B. Responses of isolated feline and human cerebral arteries to prostacyclin and some of its metabolites. J. Cereb. Blood Flow Metab. 1983;3:238–245. doi: 10.1038/jcbfm.1983.32. [DOI] [PubMed] [Google Scholar]
- WHALLEY E.T., SCHILLING L., WAHL M. Cerebrovascular effects of prostanoids: in vitro studies in feline middle cerebral and basilar artery. Prostaglandins. 1989;38:625–634. doi: 10.1016/0090-6980(89)90045-2. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetised rat-intravital microscope studies. Cephalalgia. 1997;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN K., REEH P.W., AVERBECK B. ATP can enhance the proton-induced CGRP release through P2Y receptors and secondary PGE2 release in isolated rat dura. Pain. 2002;97:259–265. doi: 10.1016/S0304-3959(02)00027-1. [DOI] [PubMed] [Google Scholar]