Abstract
In pressurised rat mesenteric small arteries (50 mmHg), we examined the effects of stimulation with U46619, endothelin-1 (ET-1) or phenylephrine (PE) on changes in vessel diameter, global [Ca2+]i, individual smooth muscle cell [Ca2+]i and Ca2+-sensitisation of contraction.
U46619 or ET-1 gave tonic diameter reductions, whereas PE-stimulated vessels gave tonic contractions or initial vasoconstrictions followed by diameter oscillations. Global [Ca2+]i changes were transient for each agonist, with tonic constrictions being accompanied by maintained submaximal global [Ca2+]i levels.
U46619, ET-1 or PE tonic constrictions were accompanied by apparently asynchronous [Ca2+]i waves in individual smooth muscle cells of the vessel wall, as examined by confocal fluorescent microscopy. In vessels exhibiting vasomotion to PE, some apparent synchrony of activation of individual cells was evident; however, this was incomplete with many cells responding out of phase with their neighbours.
In α-toxin-permeabilised preparations, agonist-induced Ca2+-sensitisation of constriction at submaximal Ca2+ (pCa6.7) in the presence of GTP was greater with U46619 or ET than PE.
We conclude that, in pressurised mesenteric arteries, (i) a general feature of receptor-coupled constriction is the generation of periodic smooth muscle [Ca2+]i waves; (ii) complete synchrony of Ca2+ oscillations between smooth muscle cells is not a prerequisite for receptor-coupled vasomotion; (iii) varied Ca2+-sensitising actions of agonists may partly determine tonic or phasic vessel responses to different stimuli.
Keywords: U46619, endothelin, phenylephrine, Ca2+ waves, Ca2+ sensitisation, smooth muscle
Introduction
Receptor-coupled stimulation of vascular contractility involves elevating smooth muscle intracellular Ca levels ([Ca2+]i) and also sensitising the myofilaments to the [Ca2+]i increase (Somlyo & Somlyo, 2000). In the former, the major source of [Ca2+]i elevation is transplasmalemmal Ca2+ influx, although the sarcoplasmic reticulum (SR) is also a prominent intracellular store of releasable Ca2+ used for smooth muscle contractile activation. The Ca2+ released from the SR, via inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ release and/or transsarcolemmal calcium-induced calcium release (CICR), can either directly activate the contractile filaments or indirectly alter cell excitability by affecting ion channel activity in the plasma membrane (Taggart, 2001).
Recent attention has focussed on two modes of Ca2+ release from the SR: elementary spontaneous releases of Ca2+ within a confined region of the cell (Ca2+ sparks) and propagated Ca2+ waves; the latter are thought to be initiated at sites of Ca2+ spark activity in single isolated portal venous smooth muscle cells (Mironneau et al., 1996; Gordienko & Bolton, 2002). Ca2+ waves in isolated cells and intact preparations have been found to be dependent upon voltage-gated Ca2+ entry and ryanodine- and IP3-sensitive Ca2+ release from the SR (Miriel et al., 1999; Peng et al., 2001; Gordienko & Bolton, 2002; White & McGeown, 2002). In general, Ca2+ sparks have been thought to oppose contraction by activating transient outward potassium current in a restricted region of the plasma membrane, and contribute little to global [Ca2+]i elevations (Heppner et al., 2002). On the other hand, Ca2+ waves may activate transient inward currents (Mironneau et al., 1996) and spread a considerable distance (tens of microns) – thereby having the potential to contribute to global [Ca2+]i and activate contraction – although the exact contribution of waves to microvessel constriction is a matter of debate (Lee et al., 2001). In isometrically held preparations, a close interplay between SR Ca2+ release and membrane potential alteration has been suggested to result in synchronisation of Ca2+ transients in neighbouring cells and contribute to periods of phasic alterations in tension (Peng et al., 2001). In nonisometric preparations, oscillations in vessel diameter have been associated with alterations in membrane potential (Bartlett et al., 2000; Haddock et al., 2002; Oishi et al., 2002).
We and others have shown that α-adrenergic stimulation induces Ca2+ wave activity in pressurised arteries (Miriel et al., 1999; Taggart et al., 2002). However, the effects of other potent vasoconstrictors such as thromboxanes and endothelin on smooth muscle cell Ca2+ dynamics of pressurised vessels are not known. Consequently, the functional impact of Ca2+ responses in individual smooth muscle cells to varied receptor-coupled stimuli on vasoreactivity in these preparations remains to be established. It is thus important to examine the functional relationship between agonist-dependent changes in [Ca2+]i in individual smooth muscle cells and vessel diameter. Resistance arteries examined under isobaric conditions retain their physiological shape and experience true intraluminal pressures, longitudinal and axial stresses. In addition, pressurised arteries exhibit altered sensitivity to vasoconstrictor and vasodilator agents compared to vessels examined under isometric conditions (e.g. Dunn et al., 1994; Falloon et al., 1995). Consideration must, therefore, also be given to the Ca2+-sensitising actions of different agonists in determining their overall contribution to the Ca2+-dependent vasomotor tone of small arteries. For example, in rabbit pulmonary artery and rat uterus, thromboxane or endothelin receptor stimulation appears to be a more potent Ca2+-sensitising stimulant than other G-protein-coupled agents (Himpens et al., 1990; Somlyo & Somlyo, 1998).
In the present study, therefore, we have monitored the diameter responsiveness of pressurised rat mesenteric arteries to thromboxane receptor or endothelin receptor stimulation, in combination with examining the patterns of global vessel [Ca2+]i, dynamic changes of [Ca2+]i in individual smooth muscle cells and magnitude of Ca2+ sensitisation of tone. Results were compared to those occurring during α-adrenergic stimulation with phenylephrine (PE), or a depolarising stimulus with high K solution (Mauban et al., 2001). The implications of the findings for receptor-coupled regulation of pressurised arterial vasoreactivity are discussed.
Methods
Rat mesenteric resistance artery isolation and cannulation
Male Wistar rats (250–300 g) were killed according to national guidelines by stunning, followed by cervical dislocation. The mesentery was removed and placed in ice-cold physiological salt solution (PSS) of composition (mM): NaCl 119, KCl 4.7, MgSO4·7H2O 1.2, NaHCO3 25, KH2PO4 1.17, K2EDTA 0.03, glucose 5.5, CaCl2·2H2O 1.6 at pH 7.4. A fourth-order mesenteric artery (2–3 mm in length) was dissected from each animal and cleaned of adherent adipose and connective tissue. Arteries were placed in PSS, within the bath chamber of a pressure myograph (model CH/1, Living Systems Instrumentation, U.S.A.) positioned atop an inverted microscope (Nikon diaphot 200). Each vessel was cannulated onto two glass micropipettes (tip diameters 30–50 μm), as described previously (Shaw et al., 2000), and pressurised to 50 mmHg using a pressure servo-control unit. The cannulae were held firmly in place between securable jaws at a slight angle (∼10°), to allow positioning of the vessel wall close to the bottom of the microscope slide. The bath was perfused with PSS at 37°C and gassed with 95% air/5% CO2. Lumen diameters were continuously measured using a video image analyser (LSI). Following an equilibration period of 30 min, each vessel was subjected to a ‘run-up' procedure consisting of three separate 2-min stimulations with 60 mM KCl (isosmotically substituted for NaCl), as detailed previously (Shaw et al., 2001; 2003).
Experimental protocol for receptor agonists
Arteries were exposed to 10 μM of the thromboxane mimetic 9,11-dideoxy-11α,9α-epoxy methanoprostaglandin (U46619, a thromboxane analogue), 100 nM of the receptor agonist endothelin-1 (ET-1), 10 μM of the α-adrenergic receptor agonist PE or 60 mM KCl (high K+ solution; isosmotically substituted for NaCl) for up to 5 min to observe the effects of each stimulus on vascular tone. In vessels where more than one stimulus was applied, the order of addition was swapped.
Measurement of arterial [Ca2+]i signals
For measurements of global vessel wall [Ca2+]i, pressurised arteries were loaded with 20 μM of the membrane-permeant acetoxymethylester form of the ratiometric calcium-sensitive dye Indo-1 (Molecular Probes) for 3 h in HEPES-buffered salt solution (in mM: NaCl 154, KCl 5.4,. MgSO4 1.2, glucose 10, CaCl2 10, HEPES 10, pH 7.4 with NaOH) at room temperature. The ratiometric nature of the dye allows for assessments of [Ca2+]i that are independent of diameter changes. Following washing with PSS, the vessels were mounted on the inverted microscope and viewed with a × 10 Fluor objective. Tissues were excited at 340 nm and emissions at 400 and 500 nm recorded. Vessels were constricted with 10 μM U46619, 100 nM ET-1, 10 μM PE or 60 mM KCl (in the absence of inhibitors of noradrenaline release from nerve terminals). In previous studies, the Indo-1 ratio has been successfully calibrated in terms of absolute Ca2+ values in rat mesenteric arteries (Austin & Wray, 1995; Ahmed et al., 2000; Shaw et al., 2003) and indicated that the emission ratio (400 : 500 nm) is an appropriate monitor of [Ca2+]i in response to exogenous stimuli. A 600 nm filter placed in front of the microscope incident light was used to weakly illuminate arteries in order for vascular diameter and Indo-1 Ca2+ ratio to be continuously and simultaneously measured.
Measurement of [Ca2+]i in individual smooth muscle cells of the vessel wall was accomplished by laser-scanning confocal microscopy. Pressurised arteries were incubated with the membrane-permeant acetoxymethylester form of the calcium-sensitive dye Fluo-3 (15 μM, Molecular Probes) for 3 h in HEPES-buffered salt solution. Following washing with PSS, the vessel chamber was placed on a Nikon Diaphot 300 inverted microscope attached to a Bio-Rad MRC 1024 confocal laser-scanning head, and viewed with a × 60 Fluor water objective (NA 1.2). As per vessels in which global [Ca2+]i was measured, tissues were constricted at 25°C with 10 μM U46619, 100 nM ET-1, 10 μM PE or 60 mM KCl (in the absence of inhibitors of noradrenaline release from nerve terminals) and, if the constriction was maintained, changes in individual cellular [Ca2+]i measured as described below. Experiments examining single cellular [Ca2+]i changes were, in common with other workers (Mauban et al., 2001; Zhang et al., 2001), performed at 25°C in order to limit dye leakage from the tissue, which is rapid at 37°C. In some experiments, the effects of submaximal doses of U46619 or ET-1 that resulted in constriction, 25–50% of the maximal, on subcellular Ca2+ changes were compared to those induced by maximal doses. Vessels that exhibited vasomotion to 10 μM PE were returned to PSS and incubated with 5 μM of the myosin light chain kinase/PI3 kinase inhibitor wortmannin for 30 min. Wortmannin inhibits smooth muscle contraction without altering the membrane potential or global [Ca2+]i changes (Burke et al., 1996; Longbottom et al., 2000; Ahmed, 2001), and has been applied by others in the measurement of smooth muscle [Ca2+]i dynamics (Pucovsky et al., 2002; Yamazawa & Iino, 2002). The tissue was again exposed to 10 μM PE and, at the appropriate time span of exposure that induced vasomotion in these arteries before wortmannin exposure, examined by confocal microscopy. A small amount of vasomotion (approximately 10% of initial magnitude) remained in response to 10 μM PE following wortmannin treatment. The remaining small amount of movement in the x–y plane – no greater than 8 μm – was detected at the edge of the image. This meant that identification of regions of interest (ROI, see below) in individual smooth muscle cells, visible for lengths of 50–100 μm, between consecutive frames was not impaired by gross cellular movements. Fluo-3 fluorescence was excited at 488 nm with a Kr/Ar laser source (100 mW). Fluo-3 is a more appropriate indicator of [Ca2+]i in these circumstances than Indo-1, because it (a) is excitable in the visible light range of the laser light source (488 nm); (b) the dye exhibits greater increases in fluorescence intensity upon binding of Ca2+. The tissue fluorescence emitted in the confocal plane of focus at 515 nm was collected by a photomultiplier tube and digitally recorded with Bio-RAD Lasersharp software. Longitudinal optical sections of the lower to mid-vessel wall were obtained, typically encompassing between eight and 20 smooth muscle cells. Whole-field optical scans in the same z-plane of focus were taken every 0.5–1 s. Off-line analysis of stored images was performed with Image J software (Version 1.27z). An ROI was described within individual cells in confocal images, positioned such that the ROI always remained within an identified cell. The software was able to calculate the average fluorescence value within the ROI to allow us to follow how Ca2+ altered with time in individual cells of the vessel.
α-toxin permeabilisation
In this set of experiments, pressurised arteries (50 mmHg) were equilibrated in PSS at room temperature and stimulated with two separate exposures to 60 mM KCl. Intraluminal solution was the Relaxing solution of composition (in mM): sodium creatine phosphate 10, Na2ATP 5.2, magnesium methanesulphonate 7.3, potassium methane sulphonate 74, K2EGTA 1, PIPES 30, pH 7.1 with KOH. Vessels were then equilibrated for 30–45 min in external Relaxing solution and, subsequently, a droplet of activating solution pCa6.7 containing 500 U ml−1 α-toxin plus 10 μM A23187 carefully placed over the vessel. Permeabilisation was assumed to be complete after 10–15 min when the ensuing constriction had reached a plateau; the artery was then returned to Relaxing solution. A reference constriction to activating solution pCa4.5 was performed, at room temperature, in agreement with other studies (Hill et al., 2000; Ayman et al., 2003) to limit the contractile run-down of permeabilised preparations, and returned to the Relaxing solution. Agonist-induced Ca2+-sensitisation of constriction was then examined as follows: vessels were exposed, in turn, to activating solution pCa6.7, solution pCa6.7 plus 10 μM GTP and GTP pCa6.7 solution plus either 10 μM PE or 10 μM U46619 or 100 nM ET-1. Following return to Relaxing solution, another reference constriction to pCa4.5 solution was performed, before the agonist Ca2+-sensitisation process was repeated with the alternative agonist. The order of agonist addition was reversed between experiments, except for ET-1, which was always applied last due to the long time for washout of the agent. Ca2+-sensitisation of constriction was calculated as the change in diameter observed by addition of agonist to GTP pCa6.7 solution as a percentage of the change in diameter observed to pCa4.5 solution. Activating solutions pCa6.7 and pCa4.5 contained 10 mM EGTA, and were prepared by addition of the appropriate amount of CaEGTA (Horiuti, 1988).
Drugs and chemicals
All drugs and chemicals, with the exception of Indo-1(AM), Fluo 3(AM, both Molecular Probes, The Netherlands), A23187 and α-toxin (Calbiochem, U.K.), were obtained from Sigma (U.K.). Indo-1(AM) and Fluo-3(AM) were dissolved in Pluronic F-127 20% solution in dimethylsulphoxide (DMSO, obtained from Molecular Probes) to give 1 mM stock solutions. A 10 mM stock solution of U46619 was made by dissolving in a mixture of 100% ethanol and 1 mg kg−1 sodium carbonate (1 : 2). ET-1 and PE and were dissolved in distilled water. Wortmannin was dissolved in DMSO to a 5 mM stock solution.
Statistics
All results are expressed as mean±standard error of the mean, with n representing the number of animals. Differences between groups were compared by ANOVA, differences between data sets by paired Student's t-test, with significance taken as P<0.05.
Results
Patterns of tonic and oscillatory diameter and global [Ca2+]i changes with agonist stimulation
The mean resting diameter of rat mesenteric arteries pressurised to 50 mmHg used in agonist-induced experiments was 212±4. 2 μm (n=108). Superfusion of rat mesenteric arteries with 10 μM U46619 (n=20) induced large vascular constrictions with a change in diameter of 144±7.0 μm. Constrictions to U46619 were not accompanied by oscillations in vessel diameter and remained tonic in nature over the time of exposure to the vasoconstrictive stimulus (4–5 min; Figure 1a). Similarly, constrictions to ET-1 (100 nM; change in diameter of 151+13 μm) were always tonic in nature (n=5; Figure 1b). PE (10 μM) caused a large constriction of mesenteric arteries (the change in diameter was 141±3.7 μm (n=50)). In 28% of cases, a tonic constriction was maintained with 10 μM PE (Figure 1d). In the remainder of cases, unlike U46619 stimulation, tissues developed regular oscillations in vessel diameter of mean amplitude 28.9±3.0 μm (22±2.0% of initial change in diameter observed to 10 μM PE). These oscillations were of high frequency (8.0±0.4 oscillations min−1), took 27±2 s to begin following the addition of PE, and were maintained throughout the period of PE exposure (up to 5 min; Figure 1e). KCl (60 mM; n=27), like U46619 and ET, induced tonic constrictions in diameter of 141±6.5 μm, respectively, not significantly different from the maximum change in diameter observed to 10 μM U46619, 100 nM ET or 10 μM PE (Figure 1c). Incubation under resting conditions with 10 μM Nw-nitro-L-arginine (L-NNA), an inhibitor of nitric oxide synthase and endothelial-mediated relaxations of high K+-treated pressurised mesenteric arteries (Shaw et al., 2000), for 30 min had no effect on the change in diameter to subsequent exposure of U46619 (constrictions of 124±8.3 and 138±14 μm before and after L-NNA, respectively, n=6) or PE (constrictions of 119±15 and 138±23 μm before and after L-NNA, respectively, n=3). In the case of PE, all the three vessels exhibited rhythmic contractions to the agonist the frequency or amplitude of which were not significantly altered by L-NNA.
Figure 1.
Simultaneous measurement of global [Ca2+]i and diameter pressurised arteries. Vessel wall [Ca2+]i and intraluminal diameter were continuously recorded as described in the Methods section. Panels (a–d) illustrate tonic changes in [Ca2+]i and diameter to U46619, ET, KCl or PE, respectively. Panel (e) demonstrates an example of rapid phasic changes in both these parameters with 10 μM PE.
Agonist-induced arterial constrictions were accompanied by elevations of global vessel [Ca2+]i (Figure 1). The elevation in [Ca2+]i was transient in all ET-1- or U46619-stimulated vessels and in three out of five PE-stimulated vessels examined. Therefore, the maintained diameter reductions were accompanied by suprabasal, but submaximal, levels of global [Ca2+]i (29±7.5, 69±2.4 or 81±10.2% of peak for ET-1, U46619 or PE stimulations, respectively; Figure 2). In vessels that, following the initial vasoconstriction, showed vasomotion to PE, the diameter oscillations (of amplitude 40±7.1 μm or 28±3.1% of maximum change in diameter to PE) were also accompanied by oscillations in global [Ca2+]i (52±8.2% of the maximum change in [Ca2+]i).
Figure 2.
Summary of global [Ca2+]i and diameter measurements in pressurised arteries. Following stimulation with each receptor agonist ET-1 (panel (a)), U46619 (panel (b)) or PE (panel (c)), the peak [Ca2+]i levels and corresponding diameters were noted (the initial phase) and compared with the [Ca2+]i and diameter levels during tonic vessel activation during 3–5 min stimulation (tonic phase).
Effects of external stimuli on vessel smooth muscle cellular[Ca2+]i profiles
In a separate series of experiments, we used laser-scanning confocal fluorescent microscopy to examine the nature of [Ca2+]i changes in individual smooth muscle cells of pressurised vessels in response to agonist stimulation. Intracellular [Ca2+] levels in individual neighbouring smooth muscle cells were monitored. In all vessels stimulated with U46619 (n=9) or ET-1 (n=5), there was an initial elevation of [Ca2+]i in smooth muscle cells, that was associated with the initial vasoconstriction. This was followed, during tonic diameter reductions, by the onset of periodic cyclical changes of [Ca2+]i in individual smooth muscle cells (n=9 vessels), evident as propagated waves of [Ca2+]i (Figure 3). In vessels stimulated with high K+ solution, there was no evidence of [Ca2+]i wave propagation; [Ca2+]i appeared elevated in all smooth muscle cells throughout the period of exposure to high K+ solution (n=5 vessels) with relatively little fluctuation in steady state. Propagated [Ca2+]i waves were also present in these vessels when tonically activated by PE. A montage of five consecutive images illustrating changes in [Ca2+]i of adjacent individual smooth muscle cells in the mid-wall of a pressurised artery during exposure to 10 μM PE is shown in Figure 3b. Also shown in Figure 3c is a line plot of the [Ca2+]i response of the same individual smooth muscle cell to 10 μM PE and 10 μM U46619. This illustrates similar oscillatory [Ca2+]i responses during continued stimulation with U46619 or PE. Upon examination of at least three individual cells per vessel, the frequency of cellular [Ca2+]i oscillations when exposed to maximal doses of agonist were 3.58+0.41 min−1 for U46619-stimulated vessels (n=9), 5.66+0.94 min−1 for ET-1-stimulated arteries (n=5) and 4.92+0.49 min−1 for PE-stimulated arteries (n=18). In tonically constricted vessels, individual cells exhibited little or no synchronicity in response to U46619, ET-1 or PE, as illustrated in the continuous line plots of cells from one vessel in Figure 3e – individual cell [Ca2+]i changes occurred out of phase with each other. In 40% of vessels (scanned continuously for at least 1 min), there was evidence of [Ca2+]i wave events originating from more than one point in individual cells.
Figure 3.
Cellular [Ca2+]i responses of a pressurised mesenteric artery during tonic constrictions. (a) Confocal images of [Ca2+]i levels in individual smooth muscle cells of a pressurised artery at rest and immediately following agonist stimulation. (b) Consecutive confocal images showing the [Ca2+]i response during continued stimulation with PE (10 μM), images are separated by 1.02 s. Several cells can be seen responding out of phase with each other. (c) Traces from one cell responding to PE (10 μM) or U46619 (10 μM). The cell from which measurements were taken is marked by the white circle in the second image of panel A. (d) Montage of six consecutive frames (1 s apart) in one smooth muscle cell of an intact artery exposed to 10 μM PE. A wave is initiated in a confined region of the cell and then spreads throughout the length of the cell. (e) Continuous line plots of [Ca2+]i changes in 13 individual smooth muscle cells of a pressurised artery on exposure to PE (10 μM); the [Ca2+]i changes appear asynchronous. The averaged signal from all 13 cells is indicated by the thicker black line.
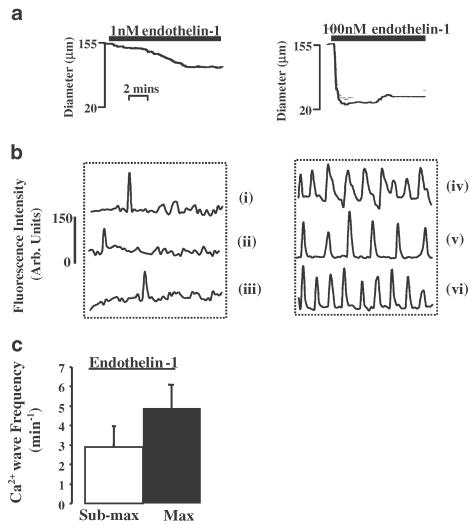
In all of the above experiments, we utilised concentrations of agonist that produced maximum constrictions of vessels. In a separate set of experiments, we examined the vessel diameters, and patterns of [Ca2+]i oscillations in individual smooth muscle cells, when exposed to submaximal doses of U46619 or ET-1. Measures of 10−8 M–3 × 10−6 M U46619- or 10−9 M–3 × 10−9 M ET-1-induced vessel constrictions that were 44±6.8% (n=7) or 35±14% (n=6) of the maximum, respectively. Constrictions to these submaximal doses of U46619 or ET-1 resulted in cellular [Ca2+]i oscillations of lower frequency, 1.57±0.62 and 2.91±1.20 min−1, respectively, than compared to maximal doses of agonist, 3.53±1.16 and 6.86±1.52 min−1, respectively (two-way ANOVA, P<0.01). An example of vessel constrictions, and the accompanying line plots of [Ca2+]i oscillations from individual cells of the artery, to these submaximal concentrations or maximal doses of ET-1 is shown in Figure 4.
Figure 4.
Dose-dependent changes in vessel diameter and in smooth muscle cell [Ca2+]i oscillations. Vessels were constricted with submaximal doses of ET-1 (10−9–3 × 10−9 M) and alterations in diameter and smooth muscle cell [Ca2+]i oscillation frequency noted. These were compared to changes in these parameters in the same vessels, on exposure to maximal doses of ET-1 (10−8 M). Representative tracings from one vessel illustrating the effects of submaximal or maximal ET-1 on changes of diameter (panel (a)) and [Ca2+]i oscillations in three individual cells (panel (b) continuous line plots, cells (i)–(iii) submaximal ET-1, cells (iv)–(vi) maximal ET-1). The average changes in smooth muscle cell [Ca2+]i oscillations to submaximal or maximal doses of ET-1 are illustrated in panel (c).
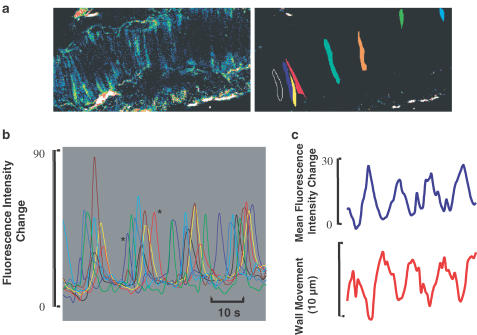
In pressurised arteries showing oscillations in diameter, there were also periodic [Ca2+]i oscillations noted in individual smooth muscle cells. It has previously been shown that during vasomotion individual cellular [Ca2+]i transients may become synchronised between groups of cells to produce phasic contractions of the whole vessel (Mauban et al., 2001; Peng et al., 2001). Therefore, we examined in detail in Figure 5 how a group of several smooth muscle cells behaved in an artery exhibiting vasomotion. In panel (a), there are two images of the artery showing the positions of the vessel (left) and the cells from which measurements were taken (right). Measurements of fluorescence were taken from ROI in each of the cells as indicated, the [Ca2+]i transients from each were normalised for amplitude and are superimposed in panel (b) (the colour of the trace refers to an individual cell). This normalisation procedure assumes that the rise in [Ca2+]i in each cell is of a similar magnitude. Panel (b) shows that the Ca transients are not synchronous in the different cells, for example, between the two [Ca2+]i transients of adjacent cells marked *, there is a time lapse of 7 s even though they are part of the same ‘cycle' of vasomotion. In the upper part of panel (c), we show the mean of the [Ca2+]i signals from the cells in (b). This averaged [Ca2+]i trace shows phasic increases and decreases that are in phase with the movements of the vessel wall, as shown in the lower trace. Wall movement was tracked using a nonmuscle fluorescent element of the adventitia of the vessel. Panel (c), therefore, illustrates that, during vasomotion of the vessel wall, the average Ca signal from the smooth muscle cells changed in parallel without absolute synchronisation of the [Ca2+]i signals between individual cells. Similar results were seen in another three vessels.
Figure 5.
Smooth muscle subcellular Ca changes in a pressurised artery exhibiting vasomotion to stimulation with PE (10 μM). Panel (a), an illustration of [Ca2+]i signals in smooth muscle cells with PE stimulation (left) and outlines of the smooth muscle cells selected for analysis (right). Panel (b), a continuous plot of the [Ca2+]i changes of the smooth muscle cells in (a). The amplitudes of the [Ca2+]i changes have been normalised such that each cell undergoes a similar change. Panel (c), the mean [Ca2+]i (top panel) from all cells selected in (a) and displayed in (b), and the accompanying wall movement (lower panel). Note, that wortmannin treatment (see Methods section) has resulted in vasomotion being associated with only a small amount of wall movement, thus minimising the movement artefact.
Agonist-dependent Ca2+ sensitisation of vessel constrictions
The functional response of arteries to [Ca2+]i elevations in individual smooth muscle cells will be determined not only by the nature of those [Ca2+]i oscillations, but also by any agonist-mediated sensitisation to the activating [Ca2+]i. As indicated in the global [Ca2+]i measurements above, tonic diameter reductions to each agonist were associated with [Ca2+]i levels maintained below the initial peak value indicative of sensitisation of tone. We, therefore, examined further the degree of Ca2+ sensitisation of contractility induced by each agonist in arteries permeabilised with α-toxin as, in this situation, the [Ca2+]i of the bathing medium surrounding the myofilaments can be clamped while other receptor-coupled signalling events remain intact. In these tissues, exposure to a pCa6.7 GTP-containing solution gave submaximal constrictions 49±6.4% of those to pCa4.5 (n=12). Subsequent exposure to 10 μM U46619 further promoted vessel constriction such that U46619-induced Ca2+-sensitisation was 63±10% of constrictions to pCa4.5 (n=6). This was significantly greater than the corresponding Ca2+-sensitisation induced by PE (36±6.8% magnitude of constrictions to pCa4.5; Figure 6a). In a separate set of experiments, we also compared the ET-1-induced Ca2+-sensitisation to that of PE. In this case, ET-1-induced Ca2+-sensitisations of amplitude 52±6.8% of constrictions to pCa4.5, again, significantly greater than those to PE (11±3.1%; n=6; Figure 6b).
Figure 6.
Comparison of the Ca2+-sensitising actions of U46619 or ET-1 to PE in pressurised mesenteric arteries. (a) Following α-toxin permeabilisation, submaximal force to pCa6.7, in the presence of GTP, was substantially augmented by 10 μM U46619 – agonist-induced Ca2+-sensitisation of force reached 90% of that to maximal pCa4.5 activating solution. Following return to baseline with pCa9 relaxing solution, submaximal pCa6.7-GTP force was increased to 56% of pCa 4.5 solution by 10 μM PE. (b) In a separate experiment, PE induced a Ca2+ sensitisation of force reached 86% of pCa4.5 solution. Following washout with pCa9 relaxing solution, however, 100 nM ET-1 application resulted in promotion of pCa6.7-GTP force to 120% of the pCa4.5 solution.
Discussion
In this study, we have observed the effects of three receptor-coupled stimuli – U46619, ET-1 and PE – on the patterns of global vessel wall [Ca2+]i, individual cellular [Ca2+]i dynamics, Ca2+ sensitisation of contractility and diameter changes in pressurised arteries. We find that all the three stimuli result in the generation of periodic waves of [Ca2+]i in individual smooth muscle cells. Both U46619 and ET-1 elicit maintained constrictions, whereas PE produced constrictions that were either tonic or followed by rapid diameter oscillations. Changes in global vessel [Ca2+]i mirrored those of the diameter. Agonist-induced Ca2+-sensitisation of tone was significantly greater with U46619 or ET-1 than PE.
Single-cell Ca2+ oscillations in pressurised arteries
A marked feature of the responses of pressurised mesenteric arteries to agonist stimulation was the generation of periodic [Ca2+]i waves in individual smooth muscle cells that persisted for the duration of the stimulus. In this study, such [Ca2+]i waves were infrequently observed under resting conditions, as has been reported in a previous study where vessels exhibited myogenic tone (Miriel et al., 1999). In vessels tonically constricted with U46619, ET-1 or PE, there appeared to be little, if any, synchronisation of [Ca2+]i oscillations between smooth muscle cells – periodic [Ca2+]i oscillations in individual cells occurred out of phase with one another. In previous studies of different isometric and isobaric preparations that were tonically responsive to α-adrenergic agonists, a similar pattern was found (Miriel et al., 1999; Ruehlmann et al., 2000; Lee et al., 2001; Mauban et al., 2001; Peng et al., 2001). While it is difficult to conclusively exclude synchronisation, however weak, between the cells visualised – or even to exclude the possibility that the visualised cells are influenced by nonvisualised cells of the preparations – a consensus between different laboratories appears to be that maintained constrictions of vascular preparations to varied receptor-coupled agonists are accompanied by apparently asynchronous smooth muscle [Ca2+]i oscillations.
PE has been reported to dose-dependently increase the frequency of [Ca2+]i oscillations in rat mesenteric artery (Mauban et al., 2001; Zhang et al., 2001) and rabbit inferior vena cava (Ruehlmann et al., 2000), the latter study correlating this to concentration-dependent isometric contractions. We found similar dose-dependent effects of U46619 and ET-1. Tonic constriction of vessels to 25–50% of minimum diameter by submaximal doses of U46619 or ET-1 resulted in a lower frequency of cellular [Ca2+]i oscillations compared to that when stimulated maximally with these agonists. Therefore, our data, together with that reviewed by Lee et al. (2002), suggests that one mechanism by which multiple receptor-coupled agonists can increase blood vessel constriction is to dose-dependently enhance the frequency of smooth muscle cell [Ca2+]i oscillations.
In a proportion of vessels stimulated maximally with PE, but not U46619 or ET-1, the initial vasoconstrictions were followed by small amplitude oscillations in diameter. Surprisingly, the frequency of [Ca2+]i oscillations in individual cells in these situations was not significantly different from those stimuli resulting in tonic constrictions. The peaks of [Ca2+]i. transients in adjacent cells also remained separated by as much as 7 s, suggesting a lack of complete synchronisation between neighbouring cells. A similar finding has been reported in pairs of smooth muscle cells freshly isolated from guinea-pig mesentery (Pucovsky et al., 2002). This appears quite different from the circumstances in which oscillations in tension (Peng et al., 2001) or diameter (Mauban et al., 2001) have been shown to occur with completely synchronised changes in smooth muscle cell [Ca2+]i, with all cells responding simultaneously. Nonetheless, in the present experiments, the averaged cellular [Ca2+]i signals oscillated in common with the vasomotion. It is feasible that, in a group of oscillating cells apparently behaving independently from each other, there may be periods in which some cells operate in the same time phase as their neighbours. This may occur by chance or by the influence of a weak modulatory mechanism. The lack of complete synchrony explains our observations of a higher frequency and smaller, irregular amplitude of oscillations in either tension or diameter compared to those occurring with completely synchronised smooth muscle cell [Ca2+]i changes (Mauban et al., 2001; Peng et al., 2001). The frequency of vascular diameter oscillations to PE in the pressurised vessels herein are also similar to those recorded in vivo (Bartlett et al., 2000). Intercellular electrical coupling between myo-endothelial cells or pairs of smooth muscle cells in blood vessels has been observed following current injection to one impaled cell (Emerson & Segal, 2000; Yashiro & Duling, 2000; Peng et al., 2001; Yamazaki & Kitamura, 2003), and has been suggested to form the basis of regular vasomotion (Bartlett et al., 2000; Peng et al., 2001) – myoendothelial gap junctions in distal mesenteric arteries (Sandow & Hill, 2000) offer one possible route for such intercellular communication. We suggest, therefore, that, while complete synchronisation of [Ca2+]i signals between neighbouring smooth muscle cells can occur (Mauban et al., 2001; Peng et al., 2001), it appears not to be a prerequisite for the genesis of rapid irregular vasomotion to PE in pressurised arteries.
Agonist-induced Ca2+ sensitisation of vessel constrictions
In α-toxin permeabilised tissues, the promotion of vessel submaximal Ca2+ constriction (pCa6.7) by U46619 or ET-1 was approximately double that of PE. In accordance with many publications in the field (Himpens et al., 1990; Somlyo & Somlyo, 1998; Hill et al., 2000; Ayman et al., 2003), we utilised the concentrations of each agonist (0.1 μM ET-1, 10 μM U46619 or 10 μM PE) that produces maximal constriction of intact vessels. Such doses of U46619 and ET-1 thus induce potent Ca2+-sensitisation in pressurised mesenteric vessels as, indeed, occurs with these agents in other smooth muscle tissues (Himpens et al., 1990; Somlyo & Somlyo, 1998). In addition, in rabbit pulmonary artery, a similar increased potency of Ca2+-sensitisation by U46619 over α-adrenergic stimulation was found (Himpens et al., 1990). This may reflect an increased activation of rho-associated kinase by U46619 or ET-1 (the Ca2+-sensitisation to U46619 or ET-1 was abolished by the rho kinase inhibitor Y-27632 – data not shown) than that invoked by PE in these arteries; α-adrenergic Ca2+-sensitisation of force in isometric mesenteric arteries has been attributed to the activation of PKC (Buus et al., 1998). Although not examined in the present study, it will be of interest to determine whether the reported difference in Ca2+-sensitisation between maximal concentrations of agonists persists throughout complete pCa- and agonist dose–response curves.
The overall contractile response of a vessel to a G-protein receptor-coupled agonist will depend upon the influence of the ensuing Ca2+ transients and any alterations in myofilament sensitivity to those Ca2+ elevations. Although the three agonists used in this study produce similar [Ca2+]i transient profiles in individual cells, the vessel myofilament Ca2+-sensitivity is enhanced more by U46619 or ET-1 than PE. This increased myofilament Ca2+-sensitivity will have the effect of promoting maintenance of tone. This may, at least partly, contribute to the finding that contractile responses to maximal stimulation with U46619 or ET-1 are maintained, whereas those to PE often exhibit oscillations in diameter.
Ca2+ waves and smooth muscle sarcoplasmic reticulum
Smooth muscle [Ca2+]i wave events have been found to be altered by modulators of SR Ca2+ homeostasis including inhibitors of CICR, IP3-induced Ca2+ release and SR Ca2+-ATPase (Lee et al., 2001; Mauban et al., 2001; Peng et al., 2001; Gordienko & Bolton, 2002; White & McGeown, 2002; Yamazawa & Iino, 2002), suggesting that they involve regenerative release of Ca2+ from the SR. In our live tissue-imaging experiments, the speed of image acquisition in the whole-field view (0.5–1 s) was insufficient to allow accurate discrimination of Ca2+ wave velocities to different agonists or of faster subcellular events such as Ca2+ sparks. These sparks arise from elementary Ca2+ release events from the SR and, in isolated smooth muscle cells, often occur at a discrete focal discharge site(s) (Gordienko et al., 2001). Summation of Ca2+ spark events, either spontaneously or with exogenous stimulation (Gordienko et al., 2001; Heppner et al., 2002), can result in the generation of Ca2+ waves. All of the above illustrates that an important question for future consideration is to what extent the SR morphological arrangement determines the characteristics of [Ca2+]i waves in smooth muscle cells of the vessel wall. A preliminary, qualitative electron microscopic study of the ultrastructure of smooth muscle cells in pressurised vessels reported a prominent SR distribution in the cell centre, with noticeable SR appearance close to the plasma membrane too (Jones et al., 2003). A similar SR distribution has been suggested by light and electron microscopic studies of aortic, vas deferens, and portal venous smooth muscle (Nixon et al., 1994; Gordienko et al., 2001; Taggart, 2001).
Potential significance of receptor-coupled [Ca2+]i waves and Ca2+ sensitisation of force in pressurised small arteries
It is evident from the data herein, and that of others, that receptor-coupled alterations in global [Ca2+]i and the ensuing reductions in vessel diameter are the result of spatial averaging of dynamic periodic [Ca2+]i oscillations in individual smooth muscle cells. Thromboxane, endothelin or α-adrenergic receptor stimulation, all result in the generation of [Ca2+]i waves in individual smooth muscle cells. The relevance of this prominent [Ca2+]i wave activity in response to different agonists remains to be fully determined. It may offer a means of selectively activating the myofilaments separate from other Ca2+-sensitive pathways (Lee et al., 2001). Indeed, a recent study has reported that a portion of cellular calmodulin, a Ca2+-binding protein essential for Ca2+ activation of myosin light chain kinase and subsequent contraction, is tightly bound to the myofilaments (Wilson et al., 2002). Also, periodic [Ca2+]i oscillations of neighbouring smooth muscle cells may offer an energetically favourable means of maintaining vessel reactivity to receptor-coupled stimuli, without the need for each individual cell to retain elevated [Ca2+]i levels for prolonged periods – a multitude of biomechanical steps link [Ca2+]i elevations to altered contraction such that the force duty cycle of each individual smooth muscle cell in response to a [Ca2+]i transient may last considerably longer than the oscillation itself; when averaged across all cells of the vessel, this, in turn, allows periodic [Ca2+]i oscillations to promote maintained tone. The differing Ca2+-sensitising actions of G-protein-coupled receptors may offer an additional level of tone regulation at the individual smooth muscle level and, at least partly, explain the tonic or phasic receptor-coupled responsiveness of the whole vessel.
Acknowledgments
This work was supported by the British Heart Foundation. We are grateful to Dr Rob Hinch (University of Oxford) for assistance with the analysis of data.
Abbreviations
- A23187
antibiotic A23187
- [Ca2+]i
intracellular calcium
- CICR
calcium-induced calcium release
- DMSO
dimethylsulphoxide
- EGTA
ethylene glycol-bis (β-amino-ethylether)-N,N,N′,N′-tetraacetic acid
- ET-1
endothelin-1
- GTP
guanosine triphosphate
- IP3
D-myo-inositol 1,4,5-triphosphate
- L-NNA
Nw-nitro-L-arginine
- PE
phenylephrine
- PSS
physiological salt solution
- ROI
regions of interest
- SR
sarcoplasmic reticulum
- U46619
9,11-dideoxy-11α,9α-epoxy methanoprostaglandin
References
- AHMED S.Interactions between intracellular pH, calcium and force in isolated rat portal venous smooth muscle 2001University of Manchester; PhD Thesis [Google Scholar]
- AHMED S., SHAW L., AUSTIN C., TAGGART M.J. Indo-1 calibration in isolated rat smooth muscle: an in situ method without the use of ionophores. J. Physiol. 2000;528:11–12. [Google Scholar]
- AUSTIN C., WRAY S. The effects of extracellular pH and calcium change on force and intracellular calcium in rat vascular smooth muscle. J. Physiol. 1995;488:281–291. doi: 10.1113/jphysiol.1995.sp020966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYMAN S., WALLACE P., WAYMAN C.P., GIBSON A., MCFADZEAN I. Receptor-independent activation of Rho-kinase-mediated calcium sensitisation in smooth muscle. Br. J. Pharmacol. 2003;139:1532–1538. doi: 10.1038/sj.bjp.0705394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT I.S., CRANE G.J., NEILD T.O., SEGAL S.S. Electrophysiological basis of arteriolar vasomotion in vivo. J. Vasc. Res. 2000;37:568–575. doi: 10.1159/000054090. [DOI] [PubMed] [Google Scholar]
- BURKE E.P., GERTHOFFER W.T., SANDERS K.M., PUBLICOVER N.G. Wortmannin inhibits contraction without altering electrical activity in canine gastric smooth muscle. Am. J. Physiol. 1996;270:C1405–C1412. doi: 10.1152/ajpcell.1996.270.5.C1405. [DOI] [PubMed] [Google Scholar]
- BUUS C.L., AALKJAER C., NILSSON H., JUUL B., MOLLER J.V., MULVANY M.J. Mechanisms of Ca2+ sensitization of force production by noradrenaline in rat mesenteric small arteries. J. Physiol. 1998;510:577–590. doi: 10.1111/j.1469-7793.1998.577bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN W.R., WELLMAN G.C., BEVAN JA. Enhanced resistance artery sensitivity to agonists under isobaric compared with isometric conditions. Am. J. Physiol. 1994;266:H147–H155. doi: 10.1152/ajpheart.1994.266.1.H147. [DOI] [PubMed] [Google Scholar]
- EMERSON G.G., SEGAL S.S. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ. Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- FALLOON B.J., STEPHENS N., TULIP J.R., HEAGERTY A.M. Comparison of small artery sensitivity and morphology in pressurised and wire-mounted preparations. Am. J. Physiol. 1995;268:H670–H678. doi: 10.1152/ajpheart.1995.268.2.H670. [DOI] [PubMed] [Google Scholar]
- GORDIENKO D.V., GREENWOOD I.A., BOLTON T.B. Direct visualisation of sarcoplasmic reticulum regions discharging Ca2+ sparks in vascular myocytes. Cell Calcium. 2001;29:13–28. doi: 10.1054/ceca.2000.0180. [DOI] [PubMed] [Google Scholar]
- GORDIENKO D.V., BOLTON T.B. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+ release events in rabbit portal vein myocytes. J. Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDOCK R.E., HIRST G.D.S., HILL C.E. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J. Physiol. 2002;540:219–229. doi: 10.1113/jphysiol.2001.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., SANTANA L.F., NELSON M.T. Alkaline pH shifts Ca2+ sparks to Ca2+ waves in smooth muscle cells of pressurised cerebral arteries. Am. J. Physiol. 2002;283:H2169–H2176. doi: 10.1152/ajpheart.00603.2002. [DOI] [PubMed] [Google Scholar]
- HILL P.B., DORA K.A., HUGHES A.D., GARLAND C.J. The involvement of intracellular Ca2+ in 5-HT1B/1D receptor-mediated contraction of the rabbit isolated renal artery. Brit. J. Pharmacol. 2000;130:835–842. doi: 10.1038/sj.bjp.0703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIMPENS B., KITIZAWA T., SOMLYO A.P. Agonist-dependent modulation of Ca2+-sensitivity in rabbit pulmonary artery smooth muscle. Eur. J. Physiol. 1990;417:21–28. doi: 10.1007/BF00370764. [DOI] [PubMed] [Google Scholar]
- HORIUTI K. Mechanism of contracture on cooling of caffeine-treated frog skeletal muscle fibres. J. Physiol. 1988;398:131–148. doi: 10.1113/jphysiol.1988.sp017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES C.J.P., SHAW L., O'NEILL S.C., AUSTIN C., TAGGART M.J. Ultrastructural examination of the sarcoplasmic reticulum of snmooth muscle cells in pressurised rat resistance arteries. J. Vasc. Res. 2003;40:297. [Google Scholar]
- LEE C.H., POBURKO D., SAHOTA P., SANDHU J., RUEHLMANN D.O., VAN BREEMAN C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001;534:641–650. doi: 10.1111/j.1469-7793.2001.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE C.H., POBURKO D., KUO K., SEOW C.Y., VAN BREEMAN C. Ca2+ oscillations, gradients, and homeostasis in vascular smooth muscle. Am. J. Physiol. 2002;282:H1571–H1583. doi: 10.1152/ajpheart.01035.2001. [DOI] [PubMed] [Google Scholar]
- LONGBOTTOM E.R., LUCKAS M.J., KUPITTIYANANT S., BADRICK E., SHMIGOL T., WRAY S. The effects of inhibiting myosin light chain kinase on contraction and calcium signalling in human and rat myometrium. Pflügers Arch. 2000;440:315–321. doi: 10.1007/s004240000305. [DOI] [PubMed] [Google Scholar]
- MAUBAN J.R.H., LAMONT C., BALKE W., WIER G.W. Adrenergic stimulation of rat resistance arteries affects Ca2+ sparks, Ca2+ waves, and Ca2+ oscillations. Am. J. Physiol. 2001;280:H2399–H2405. doi: 10.1152/ajpheart.2001.280.5.H2399. [DOI] [PubMed] [Google Scholar]
- MIRIEL V.A., MAUBAN J.R.H., BLAUSTEIN M.P., WIER G.W. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J. Physiol. 1999;518:815–824. doi: 10.1111/j.1469-7793.1999.0815p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRONNEAU J., ARNAUDEAU S., MACREZ-LEPRETRE N., BOITTIN F.X. Ca sparks and Ca waves activate different Ca-dependent ion channels in single myocytes from rat portal vein. Cell Calcium. 1996;20:153–160. doi: 10.1016/s0143-4160(96)90104-9. [DOI] [PubMed] [Google Scholar]
- NIXON G.F., MIGNERY G.A., SOMLYO A.V. Immunogold localization of inositol 1,4,5-trisphosphate receptors and characterization of ultrastructural features of the sarcoplasmic reticulum in phasic and tonic smooth muscle. J. Muscle Res. Cell Motil. 1994;15:682–700. doi: 10.1007/BF00121075. [DOI] [PubMed] [Google Scholar]
- OISHI H., SCHUSTER A., LAMBOLEY M., STERIOPULOS N., MESITER J., BENY J.L. Role of membrane potential in vasomotion of isolated pressurized rat arteries. Life Sci. 2002;71:2239–2248. doi: 10.1016/s0024-3205(02)02014-3. [DOI] [PubMed] [Google Scholar]
- PENG H., MATCHKOV V., IVARSEN A., AALKJAER C., NILSSON H. Hypothesis for the initiation of vasomotion. Circ. Res. 2001;88:810–815. doi: 10.1161/hh0801.089603. [DOI] [PubMed] [Google Scholar]
- PUCOVSKY V., GORDIENKO D.V., BOLTON T.B. Effect of nitric oxide donors and noradrenaline on Ca2+ release sites and global intracellular Ca2+ in myocytes from guinea-pig small mesenteric arteries. J. Physiol. 2002;539:25–39. doi: 10.1113/jphysiol.2001.012978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUEHLMANN D.O., LEE C.H., POBURKO D., VAN BREEMAN C. Asynchronous Ca2+ waves in intact venous smooth muscle. Circ. Res. 2000;86:e72–e79. doi: 10.1161/01.res.86.4.e72. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., Hill C.E. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ. Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- SHAW L., TAGGART M.J., AUSTIN C. Mechanisms of 17β-oestradiol-induced vasodilation in isolated pressurised rat small arteries. Br. J. Pharm. 2000;129:555–565. doi: 10.1038/sj.bjp.0703084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW L., TAGGART M.J., AUSTIN C. Effects of oestrous cycle and gender on acute vasodilatory responses of isolated pressurised rat mesenteric arteries to 17 β-oestradiol. Br. J. Pharm. 2001;132:1055–1062. doi: 10.1038/sj.bjp.0703908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW L., AHMED A., AUSTIN C., TAGGART M.J. Inhibitors of actin filament polymerisation attenuate force but not global [Ca2+]i in isolated pressurised resistance arteries. J. Vasc. Res. 2003;40:1–10. doi: 10.1159/000068940. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol. Scand. 1998;164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGGART M.J. Excitation–contraction coupling in smooth muscle: a role for caveolae and caveolins. News Physiol. Sci. 2001;16:60–65. doi: 10.1152/physiologyonline.2001.16.2.61. [DOI] [PubMed] [Google Scholar]
- TAGGART M.J., SHAW L., O'NEILL S.C., AUSTIN C. Sub-cellular Ca dynamics in smooth muscle cells of intact pressurised arteries. J. Vasc. Res. 2002;39 S1:77. [Google Scholar]
- WHITE C., MCGEOWN J.G. Carbachol triggers RyR-dependent Ca2+ release via activation of IP3 receptors in isolated rat gastric myocytes. J. Physiol. 2002;542:725–733. doi: 10.1113/jphysiol.2002.020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D.P., SUTHERLAND C., WALSH M.P. Ca2+ activation of smooth muscle contraction: evidence for the involvement of calmodulin that is bound to the triton insoluble fraction even in the absence of Ca2+ J. Biol. Chem. 2002;277:2186–2192. doi: 10.1074/jbc.M110056200. [DOI] [PubMed] [Google Scholar]
- YAMAZAKI J., KITAMURA K. Intercellular electrical coupling in vascular cells pressurised in rat intact cerebral arterioles. J. Vasc. Res. 2003;40:11–27. doi: 10.1159/000068934. [DOI] [PubMed] [Google Scholar]
- YAMAZAWA T., IINO M. Simultaneous imaging of Ca signals in interstitial cells of Cajal and longitudinal smooth muscle cells during rhythmic activity in mouse ileum. J. Physiol. 2002;538:823–835. doi: 10.1113/jphysiol.2001.013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YASHIRO Y., DULING B.R. Integrated Ca2+ signalling between smooth muscle and endothelium of resistance vessels. Circ. Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- ZHANG W.J., BALKE C.W., WIER W.G. Graded α1-adrenoceptor activation of arteries involves recruitment of smooth muscle cells to produce ‘all or none' Ca2+ signals. Cell Calcium. 2001;29:327–334. doi: 10.1054/ceca.2000.0193. [DOI] [PubMed] [Google Scholar]