Abstract
In the present study, we examined the effect of E-ring 8-isoprostanes on cholinergic neurotransmission in guinea-pig trachea and identified the receptor(s) involved. As isoprostanes are isomeric with prostaglandins, PGE2 and sulprostone (a selective EP3-receptor agonist) were examined in parallel.
8-Iso-PGE1, 8-iso-PGE2 (0.1 nM–1 μM), sulprostone (1 nM–1 μM) and PGE2 (1 μM) suppressed EFS-evoked [3H]ACh release from guinea-pig trachea in a concentration-dependent manner, producing 39.5, 53.9, 61.2 and 59.9% inhibition, respectively, at 1 μM. It should be noted that an established maximum effective concentration was not determined.
Neither SQ 29,548 (1 μM; a TP-receptor antagonist) nor AH 6809 (10 μM; an EP1-/EP2-/DP-receptor antagonist) reversed the inhibitory effect of these compounds.
L-798,106, a novel and highly selective EP3-receptor antagonist, produced a parallel shift to the right of the concentration–response curves that described the inhibitory action of sulprostone on EFS-evoked contractile responses in guinea-pig vas deferens (an established EP3-receptor-expressing tissue), from which a mean pA2 of 7.48 was derived. On guinea-pig trachea, L-798,106 also antagonised sulprostone-induced inhibition of EFS-induced twitch responses, with similar potency (mean pA2=7.82).
The inhibitory effects of 8-iso-PGE1, 8-iso-PGE2, sulprostone and PGE2 on EFS-induced [3H]ACh release was blocked by L-798,106 at a concentration (10 μM) that binds only weakly to human recombinant EP1-, EP2- and EP4-receptor subtypes expressed in HEK 293 cells.
These data suggest that E-ring 8-isoprostanes, PGE2 and sulprostone inhibit EFS-evoked [3H]ACh release from cholinergic nerves innervating guinea-pig trachea, by interacting with prejunctional prostanoid receptors of the EP3-subtype.
Keywords: Cholinergic neurotransmission, 8-isoprostanes, EP3-receptors, acetylcholine release, trachea
Introduction
Stimulation of airway parasympathetic nerves leads to muscarinic receptor-mediated bronchoconstriction, mucus secretion and dilatation of bronchial blood vessels (Barnes, 1992; Belvisi, 2002), and provides the primary means by which airways tone is regulated in guinea-pigs and humans (Taylor et al., 1984; Barnes, 1993). A number of prejunctional receptors are present on autonomic nerve terminals that can regulate neurotransmitter output. We have reported previously that 8-Br-cAMP and cAMP-elevating drugs such as β2-adrenoceptor agonists facilitate cholinergic neurotransmission to guinea-pig trachea (Belvisi et al., 1996), which is consistent with the finding that cAMP enhances the exocytosis of acetylcholine (ACh) from nerve terminals in general (Wilson, 1974; Standaert & Dretchen, 1979; Wessler & Anschutz, 1988). However, PGE2, which is also known to elevate cAMP in many tissues, suppresses electrical field stimulation (EFS)-evoked [3H]ACh release from parasympathetic nerves innervating the airways of a number of species including the guinea-pig (Spicuzza et al., 1998), dog (Deckers et al., 1989) and humans (Reinheimer et al., 1998). As there are multiple molecularly distinct EP-receptors (see below for classification; Coleman et al., 1994a; Breyer et al., 2001), it was concluded that PGE2 inhibits cholinergic transmission to the airways by interacting with a subtype that does not couple positively to adenylyl cyclase. This idea is supported by the finding that the EP3-selective agonists GR 63799X, M & B 28,767 and sulprostone mimic the effect of PGE2 on ACh output from guinea-pig and human airways, being more potent than agonists that have selectivity for the other EP-receptor variants (Spicuzza et al., 1998). The ability of PGE2 to attenuate transmitter output is not peculiar to the parasympathetic nervous system. Indeed, several studies have documented that the release of noradrenaline (Stjarne, 1973; Hedqvist, 1974) and the cotransmitter ATP (Driessen & Starke, 1994), measured indirectly from sympathetic nerve terminals, is also suppressed following agonism of EP3-receptors (see Coleman et al., 1987,1994a; Lawrence et al., 1992).
Generically, the isoprostanes constitute a large family of novel prostaglandin (PG)-like lipids that are formed by the nonenzymatic peroxidation of arachidonic acid by free radicals and reactive oxygen species (Morrow et al., 1990). Structurally, isoprostanes are isomeric with PGs, and have been given the prefixes D-, E- and Fα- to denote the prostane ring shared with PGD2, PGE2 and PGF2α, respectively (Janssen, 2001). An additional level of complexity is that peroxidation of AA can occur at one of four carbon atoms producing four regioisomers – the so-called 5-, 8-, 12- and 15-series isoprostanes – each consisting of eight racemic diastereomers (Taber et al., 1997).
8-Isoprostanes are present in urine and plasma at significantly elevated levels in subjects with respiratory diseases associated with oxidative stress such as asthma (Montuschi et al., 1999), COPD (Montuschi et al., 1998) and cystic fibrosis (Montuschi et al., 2000), when compared to normal healthy individuals. In fact, it has been proposed that isoprostanes can be used as biomarkers of airway inflammation and that the level of expression reflects disease severity (Kharitonov & Barnes, 2001). However, these novel lipids also have direct effects in the lung. For example, 8-isoprostanes can contract and relax human airways smooth muscle (Kawikova et al., 1996; Janssen et al., 2000), enhance neutrophil function (Zahler & Becker, 1999) and promote transepithelial anion secretion (Cowley, 2003). Thus, isoprostanes constitute yet another group of mediators that can regulate, positively or negatively, smooth muscle contractility, and potentially, inflammatory responses in the airways.
Many of the effects of 8-isoprostanes are known to be mediated through prostanoid receptors, although evidence for a specific isoprostane-binding site(s) has also been presented (see Janssen, 2001). Five classes of G-protein-coupled receptor for the naturally occurring prostanoid agonists have been defined and given the prefix DP, EP, FP, IP and TP, where the first letter refers to the natural ligand most selective for that receptor. Molecular biological techniques subsequently confirmed this pharmacological classification with the cloning and expression of cDNAs for representatives of the five prostanoid receptors in a number of species including humans (Coleman et al., 1994a; Breyer et al., 2001). In addition, four subtypes of the EP-receptor, denoted EP1−4, and multiple spliced variants of the EP3-subtype can also be derived (Breyer et al., 2001). Despite being isomeric with the PGs, D-, E- and F-ring 8-isoprostanes are not invariably agonists at prostanoid receptors of the DP-, EP- or FP-receptor subtypes, respectively.
To date, most research with the isoprostanes has focused on their ability to directly control smooth muscle tone. In this paper, we report the novel ability of 8-iso-PGE1 and 8-iso-PGE2 to negatively regulate the release of ACh from cholinergic nerves supplying the guinea-pig trachea following EFS, and have classified the prejunctional receptor involved. As isoprostanes and PGs are isomeric, we also compared, in parallel, the effect of PGE2 and sulprostone, which are known to suppress cholinergic transmission in this preparation. Since quantitative measurement of ACh overflow from nerve endings is the only direct method to demonstrate unequivocally the occurrence of a prejunctional modulation of cholinergic neurotransmission (Patel et al., 1995), the effects of the isoprostanes and PGs on EFS-induced [3H] ACh release from isolated guinea-pig trachea was investigated.
Methods
ACh release
Preparation of guinea-pig trachea
Male Dunkin–Hartley guinea-pigs (Harlan-Olac) (300–500 g) were killed by cervical dislocation and the tracheal tissue prepared, as previously described (Patel et al., 1995; Belvisi et al., 1996). The lungs, with trachea and bronchi attached, were rapidly removed and placed in oxygenated Krebs–Henseleit solution (KHS) of the following composition (in mM): NaCl 118, KCl 3.5; MgSO4 1.2; NaH2PO4 1.13; CaCl2 2.5; glucose 6 and NaHCO3 25.5. The trachea was dissected away from the lungs and main bronchi, and opened longitudinally by cutting through the cartilage; the epithelium was subsequently removed by careful dissection and with minimal damage to the smooth muscle. Indomethacin (10 μM) was present in the KHS throughout to prevent the formation of endogenous PGs, which have been demonstrated to affect cholinergic neurotransmission and [3H]ACh release per se (Walters et al., 1984; Deckers et al., 1989; Wessler et al., 1994; Belvisi et al., 1996).
Measurement of [3H]ACh release
Neuronal release of [3H]ACh was determined as described previously (Patel et al., 1995; Belvisi et al., 1996; Spicuzza et al., 1998). Briefly, eight strips of epithelium-denuded trachea were studied in parallel. Each tissue was connected on the top and bottom with a silver wire and mounted longitudinally in a jacketed chamber. The tissues were superfused (Watson-Marlow model 503S; Smith & Nephew, Falmouth) at a rate of 1 ml min−1 throughout the experiment, with oxygenated KHS (pH 7.4) maintained at 37°C. The tissues were allowed to equilibrate for 30 min, during which time they were continuously superfused with KHS. EFS (40 V, 0.5 ms, 4 Hz) was applied continuously for the last 10 min via the silver wire electrodes. Tissues were then placed into vials containing 1.5 ml of oxygenated KHS supplemented with [3H]choline (67 nM; specific radioactivity 2.78 TBq mmol−1) and EFS was applied (40 V, 0.5 ms, 4 Hz) for 45 min in order to facilitate the uptake of [3H]choline into cholinergic nerve terminals. At the end of this period, tissues were superfused with KHS containing hemicholinium-3 (10 μM) to prevent the re-uptake of unlabelled choline into the nerves. Preparations were washed for 2 h before the beginning of the experiment to ensure a stable baseline of tritium release. During this period, the superfusate was discarded. It has been shown previously that most of the tritium outflow evoked by EFS of epithelium-containing trachea is [3H]phosphorylcholine in addition to [3H]ACh, whereas EFS of epithelium-denuded tracheal preparations releases the neurotransmitter only (Wessler et al., 1990). Furthermore, the release of [3H]ACh following EFS of guinea-pig trachea is better maintained when cyclooxygenase enzymes are inhibited (D'Agostino et al., 1990). Accordingly, in the studies described herein, epithelium-free preparations were used and indomethacin (10 μM) was present throughout (Ward et al., 1993; Patel et al., 1995; Spicuzza et al., 1998).
Following the wash period, samples of superfusate were collected directly into scintillation vials at 1 min intervals for 3 min before, 1 min during and 3 min after EFS (40 V, 0.5 ms pulse width, 4 Hz for 1 min), and at 5-min intervals outside these times. Previous studies from this laboratory have confirmed that the tritium released under the aforementioned conditions is neuronal in origin, and is not the result of tissue contractions (Ward et al., 1993). Drugs were added to the KHS superfusing each tissue after one control EFS, as detailed in the text and figure legends. A test EFS was then applied approximately 15 and 30 min after addition of the drugs, as indicated. In some experiments, an EP3-receptor antagonist, L-798,106 (10 μM), was added to the superfusing KHS 30 min before a third stimulus and, in these studies, the vehicle or agonist was also present prior to the second stimulus and then throughout the duration of the experiment. At the end of each experiment, tissues were solubilised with 1 ml Soluene (Canberra Packard, Pangbourne), and were counted together with aliquots of each fraction in 4 ml scintillant (Pico-Fluor 40; Canberra Packard). After determination of radioactivity, the fractional release of tritium from each preparation was calculated as a rate coefficient of each collection period at the midpoint time (Patel et al., 1995; Spicuzza et al., 1998). The increase in tritium overflow evoked by EFS was expressed as a percentage increase in the rate coefficient during the period of EFS over the average release for the preceding 3 min control period.
Contractile studies – vas deferens
Tissue preparation
Male Dunkin–Hartley guinea-pigs (Harlan-Olac) (300–500 g) were killed by cervical dislocation. Vasa deferentia were excised and the loose connective tissue removed. Each vas deferens (approximately 15 mm in length) was suspended by steel hooks between two platinum wire electrodes in 10 ml organ baths at 37°C containing KHS, and connected via silk threads to Grass FT-03 force–displacement transducers (Quincy, MA, U.S.A. to detect the changes in isometric tension, which were recorded on a Grass 7D polygraph (Grass Instruments).
Experimental protocol
Vasa deferentia were set to an initial tension of 1.5 g and left to equilibrate for 30 min before application of EFS (1 s; 1 ms; 60 V; 10 Hz) supplied via a Digitimer D343 stimulator (Digitimer Ltd, Welwyn Garden City, Hertfordshire) every 60 s for 30 min (Tam et al., 1997; Jones et al., 1998). Sulprostone (1 pM–1 μM) was added cumulatively to the bath after stable EFS responses were achieved. In some experiments, the EP3-receptor antagonist L-798,106 (200 nM; Juteau et al., 2001) was added to the bath 30 min prior to sulprostone.
Contractile studies – trachea
Measurement of contractile responses
Transverse segments of guinea-pig trachea, each containing three to four cartilaginous rings, were prepared and suspended between two parallel platinum-wire field electrodes (10 mm apart) in 10 ml organ baths containing KHS at 37°C, which was continuously gassed with a 95% O2/5% CO2 mixture, as described previously (Takahashi et al., 1994). The tissues were allowed to equilibrate for 1 h with frequent washing under a resting tension of 1g. Isometric contractile responses were measured with force–displacement transducers (model FT-03; Grass Instruments) connected to a polygraph (Model 7D; Grass Instruments).
EFS-induced cholinergic contractile response
For experiments involving cholinergic contractile responses, EFSs were applied for 15 s every 120 s at a frequency of 4 Hz at 40 V, which causes approximately 50% of the maximal neuronally induced contraction (Takahashi et al., 1994). After at least four stable responses of equal magnitude were recorded, sulprostone (0.1 nM–3 μM) was introduced cumulatively to the bath. In some experiments, tissues were incubated with both the EP1- and TP-receptor antagonists SC-51089 (10 μM) and SQ 29,548 (1 μM), respectively, in the absence and presence of L-798,106 (200 nM), a selective EP3-receptor antagonist, 30 min before the addition of sulprostone.
Effect of sulprostone on smooth muscle tone
High concentrations of sulprostone are known to contract guinea-pig trachea by activating receptors of the EP1- and/or TP-receptor subtypes (Coleman & Kennedy, 1985; McKenniff et al., 1988; Featherstone et al., 1990). To allow the role of EP3-receptors to be assessed pharmacologically in the suppression of cholinergic transmission, concentration–response curves were constructed to sulprostone in the presence of SC 51089 (10 μM; EP1-receptor antagonist) and SQ 29,548 (1 μM; TP-receptor antagonist), to determine the highest concentration that did not increase smooth muscle tone.
Drugs, chemicals and analytical reagents
Indomethacin, PGE2 and hemicholinium-3 were obtained from Sigma-Aldrich (Poole, Dorset). 8-Isoprostanes, 6-isopropoxy-9-oxoxanthine-2-carboxylic acid (AH 6809), 1S-[1α,2α(Z),3α,4α]]-7-[3-[[2-[(phenylamino)carbonyl]hydrazino]methyl]-7-oxabicyclo [2.2.1] hept-2-y1]-5-heptenoic acid (SQ 29,548) and sulprostone were obtained from Cayman Chemicals (Ann Arbor, Michigan, U.S.A.). 5-Bromo-2-methoxy-N-[3-(2-naphthalen-2-yl-methylphenyl)-acryloyl]-benzenesulphonamide (L-798,106) was a kind gift from Merck Frost (Quebec, Canada) and 8-chlorodibenz[b,f][1,4]oxazepine-10(11H)-carboxylic acid, 2-[1-oxo-3-(4-pyridinylpropyl] hydrazide monohydrochloride (SC-51089) was obtained from Biomol (via Affiniti Research Products Ltd., Exeter). All drugs were made up daily and dissolved in DMSO, except indomethacin, PGE2 and SC 51089, which were dissolved in 5% NaHCO3, ethanol and distilled H2O, respectively.
Data and statistical analyses
Data points, and values in the text and figure legends, represent the mean±s.e.m. of ‘n' independent determinations using tissues from different animals. In all [3H]ACh release experiments, each tissue acted as its own control and results obtained before and after drug treatment were compared by Student's t-test for paired data. P-values less than 0.05 were considered to be statistically significant.
Concentration–response curves were analysed by least-squares, nonlinear iterative regression with the ‘PRISM' curve-fitting program (GraphPad software, San Diego, U.S.A.) and pECX/pICX values were subsequently interpolated from curves of best fit. Estimates of antagonist affinity were calculated using the equation pKB=log(CR−1)−log[B] (Schild, 1949), where CR is the concentration ratio calculated from the EC50 of the agonist in the presence of the antagonist divided by the EC50 of the agonist alone, KB is the equilibrium dissociation constant and [B] is the concentration of the antagonist. In the experiments described herein, the term pA2 is substituted for pKB as antagonists were used at one concentration only, which precludes the assumptions being made about the nature of the antagonism.
Results
Effect of E-ring 8-isoprostanes, PGE2 and sulprostone on EFS-induced [3H]ACh release from guinea-pig trachea
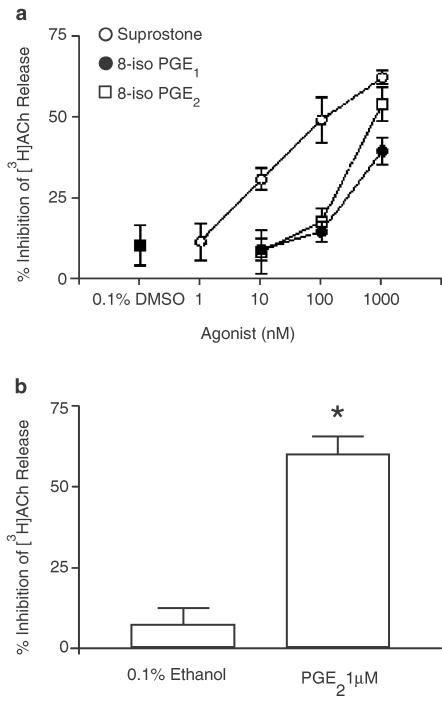
8-Iso-PGE1, 8-iso-PGE2 (both 0.01–1 μM) and the selective EP3-receptor agonist sulprostone (1 nM–1 μM) suppressed the release of [3H]ACh from guinea-pig trachea following EFS, in a concentration-dependent manner. At the highest concentration of prostanoid or isoprostane tested (1 μM), ACh output was reduced by 39.5±4.1, 53.0±5.2 and 62.1±2.2%, respectively (Figures 1a, 2). It should be noted that this was not an established maximum effective concentration. Sulprostone (pIC25=8.34±0.11) was ∼45- and ∼33-fold more potent than 8-iso-PGE1 (pIC25=6.64±0.14) and 8-iso-PGE2 (pIC25=6.79±0.12), respectively. DMSO (0.1% v v−1), the vehicle for the isoprostanes and sulprostone, had no significant effect on EFS-induced [3H]ACh (10.3±6.3% inhibition, P>0.05; Figure 1a).
Figure 1.
Effect of E-series 8-isoprostanes, sulprostone and PGE2 on EFS-induced [3H]ACh release from guinea-pig trachea. Panel (a) shows the mean concentration–response curves constructed for the inhibition of ACh release evoked by 8-iso-PGE1, 8-iso-PGE2 (both 10 nM–1 μM) and sulprostone (1 nM–1 μM). In panel (b), the effect of PGE2 (1 μM) on EFS-induced [3H]ACh is shown. Each tissue acted as its own control such that data points/bars reflect the percentage change in EFS-induced [3H]ACh output after drug administration, relative to the first control stimulation. Data points and bars represent the mean±s.e.m. of five to eight independent observations. *P<0.05 – significant inhibition of EFS-induced [3H]ACh release.
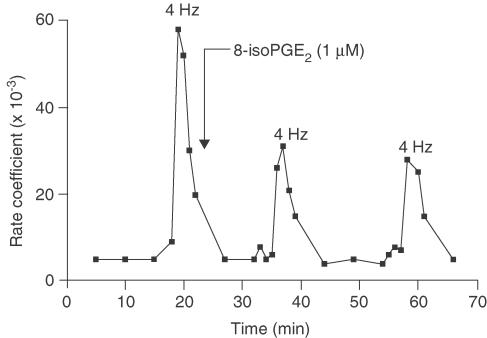
Figure 2.
Representative data for the inhibition of EFS-induced [3H]ACh release from an individual guinea-pig tracheal strip by 8-iso-PGE2. Each point represents the rate coefficient of ACh output, which is a measure of the fractional quantity of tritium released per unit time.
We have previously reported that PGE2 inhibits cholinergic transmission to guinea-pig trachea in a concentration-related fashion (Spicuzza et al., 1998). Consequently, only a single concentration (1 μM) of PGE2 was used in the present experiments, which blocked EFS-evoked [3H]ACh release by 59.9±5.9% (Figure 1b). Ethanol (0.1% v v−1, the vehicle for PGE2) failed to significantly modify cholinergic transmission (7.1±3.9% inhibition, P>0.05; Figure 1b).
Effect of a TP-receptor antagonist on the inhibition of EFS-induced [3H]ACh release evoked by E-ring 8-isoprostanes
To determine whether TP-receptors mediated the inhibitory effect of 8-iso-PGE1 and/or 8-iso-PGE2 on EFS-evoked [3H]ACh release, tracheae were superfused with KHS containing the TP-receptor antagonist SQ 29,548 (1 μM) for 30 min prior to the third EFS. SQ 29,548 per se had no significant effect on cholinergic transmission (7.2±3.0% inhibition, n=6, P>0.05) and did not antagonise the inhibitory action of either 8-iso-PGE1 or 8-iso-PGE2 (each at 1 μM; Table 1). The vehicle for SQ 29,548 (0.1% v v−1 DMSO) did not significantly modify EFS-induced [3H]ACh release (6.3±1.6% inhibition, n=6, P>0.05).
Table 1.
Effect of a selective TP-receptor antagonist, SQ 29,548 (1 μM), and the EP1-/DP-receptor antagonist, AH 6809 (10 μM), on the inhibitory effects of 8-iso-PGE1 (1 μM) and 8-iso-PGE2 (1 μM) on EFS-induced [3H]ACh release from guinea-pig trachea
| Inhibition of EFS-induced [3H]ACh release (%) | |||||
|---|---|---|---|---|---|
| Isoprostane | n | −SQ 29,548 | +SQ 29,548 | −AH 6809 | +AH 6809 |
| 8-iso-PGE1 | 5 | 27.6±2.2 | 24.6±1.7 | 38.7±3.0 | 35.5±4.2 |
| 8-iso-PGE2 | 6 | 38.4±4.8 | 37.1±3.0 | 42.0±6.8 | 47.8±4.6 |
Each tissue acted as its own control, such that the data reflect the percentage change in EFS-induced [3H]ACh output after drug administration relative to the first control stimulation.
Effect of AH 6809 on the inhibition of EFS-induced [3H]ACh release evoked by E-ring 8-isoprostanes
AH 6809 is an antagonist at the EP1- and DP-receptor subtypes (Coleman et al., 1987; Keery & Lumley, 1988; Woodward et al., 1995). To determine whether these prostanoid receptors mediated the inhibitory effect of 8-iso-PGE1 and/or 8-iso-PGE2 on EFS-evoked [3H]ACh release, tracheae were superfused with KHS containing AH 6809 (10 μM) for 30 min prior to the third EFS. AH 6809 by itself had no significant inhibitory effect on cholinergic transmission (6.7±4.3% inhibition, n=6, P>0.05), and did not antagonise the inhibitory actions of 8-iso-PGE1 or 8-iso-PGE2 (Table 1). The vehicle for AH 6809 (0.1% v v−1 DMSO) did not significantly modify EFS-induced [3H]ACh release (5.6±2.6% inhibition, n=5, P>0.05).
Effect of a novel and selective EP3-receptor antagonist, L-798,106, on EFS-induced contractile responses of guinea-pig vasa deferentia
To determine whether EP3-receptors mediated the inhibitory effect of 8-iso-PGE1 and/or 8-iso-PGE2 on EFS-evoked [3H]ACh release in guinea-pig trachea, a novel and a highly selective EP3-receptor antagonist, L-798,106, was employed. As the affinity estimates of this antagonist have not been reported in isolated tissues, the pA2 of L-798,106 at EP3-receptors was first determined by Schild (1949) analysis in the guinea-pig vas deferens, an established EP3-receptor-expressing tissue (Lawrence et al., 1992; Coleman et al., 1994b; Tam et al., 1997; Jones et al., 1998). In these experiments, sulprostone was used as the agonist due to its high potency and selectivity for the EP3-receptor subtype (Tam et al., 1997; Jones et al., 1998).
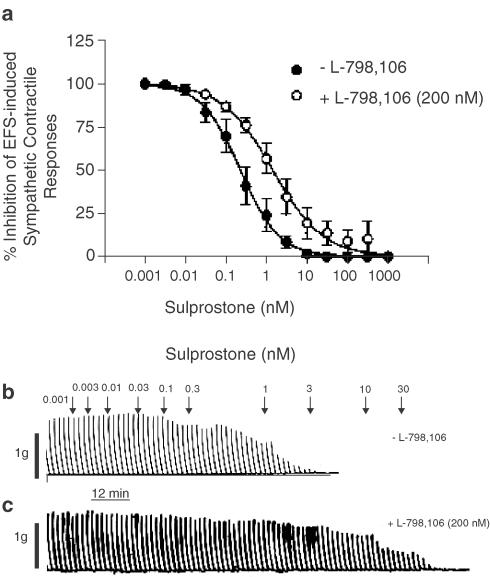
On the guinea-pig isolated vas deferens, sulprostone (0.001 nM–1 μM) inhibited the contractile responses elicited by EFS in a concentration-dependent manner (1 s, 1 ms, 60 V, 10 Hz), with a pIC50 of 9.54±0.12 and complete inhibition achieved at 10–30 nM (Figure 3a, b). In the presence of L-798,106 (200 nM; ∼670 × Ki at human recombinant EP3-receptors; Juteau et al., 2001), the sulprostone concentration–response curve was displaced 6.5-fold to the right and in a parallel fashion, from which an apparent pA2 of 7.48±0.25 (n=5) was calculated (Figure 3a–c).
Figure 3.
Inhibition by sulprostone of EFS-induced contractile responses in guinea-pig vas deferens and the effect of L-798,106. Panel (a) shows the mean concentration–response curves of the effect of sulprostone (1 pM–1 μM) on EFS-induced contractile responses in the absence and presence of the EP3-receptor antagonist L-798,106 (200 nM). Panels (b) and (c) show representative traces of these results. Each data point in (a) represents the mean±s.e.m. of five independent observations.
Effect of EP1- and TP-receptor blockade on sulprostone-induced contractions of guinea-pig tracheal smooth muscle
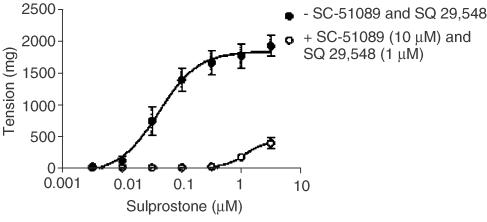
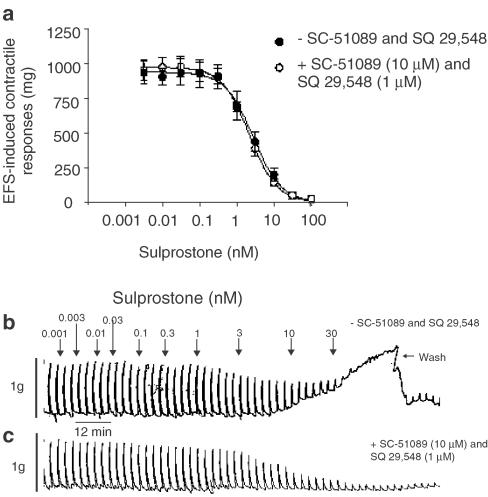
To accurately determine the affinity of L-798,106 at sulprostone-sensitive prejunctional receptors on cholinergic nerves that supply the guinea-pig trachea, it was necessary in the first instance to determine the highest concentration of sulprostone that could be studied on cholinergic neurotransmission without increasing the smooth muscle tone, which is known to be mediated by both EP1- and TP-receptors (Coleman & Kennedy, 1985; McKenniff et al., 1988; Featherstone et al., 1990). The cumulative addition of sulprostone to guinea-pig trachea evoked concentration-related contractions from which a pEC50 of −7.38±0.09 (M) was derived (Figure 4). In the presence of SC-51089 (10 μM) and SQ 29,548 (1 μM), concentrations that selectively block EP1- and TP-receptors, respectively, the mean sulprostone concentration–response curve was displaced ∼100-fold to the right, such that, at the highest concentration (3 μM) examined, contractions reached only 20% of the maximum response seen in the absence of the antagonists (Figure 4). Under these experimental conditions, sulprostone could be used at concentrations up to 300 nM before the effects on smooth muscle tone became evident (Figure 4).
Figure 4.
Antagonism of sulprostone-induced contraction of guinea-pig trachea by combined EP1- and TP-receptor blockade. Cumulative concentration–response curves were constructed to sulprostone in the absence and presence of the EP1- and TP-receptor antagonists SC-51089 (10 μM) and SQ 29,548 (1 μM), respectively. Each data point represents the mean±s.e.m. of four independent observations.
Effect of a novel and selective EP3-receptor antagonist, L-798,106, on EFS-induced cholinergic contractile responses of guinea-pig tracheal smooth muscle
On the guinea-pig isolated trachea sulprostone (0.003–100 nM) inhibited in a concentration-dependent manner contractile responses elicited by EFS (15 s, 1 ms, 60 V, 4 Hz) with a pIC50 of 8.58±0.07. However, consistent with the data shown in Figure 4, a complete concentration-response relationship could not be constructed with certainty as sulprostone also contracted the trachea at concentrations above 3 nM (Figure 5a,b). In the presence of SC-51089 (10 μM) and SQ 29,548 (1 μM), which abolished the ability of sulprostone to increase tracheal tone, EFS-induced contractile responses were also inhibited by sulprostone with a pIC50 (8.65±0.07) that was not significantly different (P>0.05) from that derived in the absence of antagonists. Indeed, Figure 5a convincingly shows that EP1- and TP-receptor blockade did not alter the position of the mean sulprostone concentration-response curves that described the inhibition of EFS-induced cholinergic contractile responses.
Figure 5.
Inhibition by sulprostone of EFS-induced cholinergic contractile responses in guinea-pig trachea and the effect of SC-51089 and SQ 29,548. Panel (a) shows the mean concentration–response curves of the effect of sulprostone (1 pM–1 μM) on EFS-induced contractile responses in the absence and presence of the EP1- and TP-receptor antagonists SC-51089 (10 μM) and SQ 29,548 (1 μM), respectively. Panels (b) and (c) show representative traces of these results. Each data point in (a) represents the mean±s.e.m. of four independent observations.
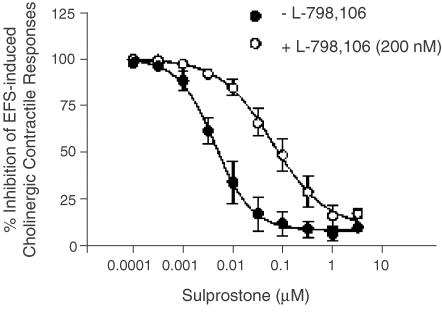
On the guinea-pig isolated trachea, sulprostone (0.001 nM–3 μM), in the presence of SC-51089 (10 μM) and SQ,29-548 (1 μM) inhibited in a concentration-dependent manner cholinergic contractile responses elicited by EFS (15 s, 1 ms, 40 V, 4 Hz) with a pIC50 and maximum inhibition of 8.32±0.16 and 90.3±3.863% respectively (Figure 6). In the presence of L-798,106 (200 nM) the mean sulprostone concentration-response curve was displaced 14-fold to the right and in a parallel fashion from which an apparent pA2 of 7.82±0.07 (n=7) was calculated (Figure 6). This affinity estimate was not significantly different from that derived for L-798,106 in the vas deferens (P>0.05).
Figure 6.
Inhibition by sulprostone of EFS-induced cholinergic contractile responses in guinea-pig trachea and the effect of L-798,106. Cumulative concentration–response curves were constructed to sulprostone (100 pM–3 μM) for the inhibition of EFS-induced contractile responses in the absence and presence of the EP3-receptor antagonist L-798,106 (200 nM). The EP1- and TP-receptor antagonists SC-51089 (10 μM) and SQ 29,548 (1 μM) were present throughout to prevent contraction of the smooth muscle at high concentrations of sulprostone. Each data point represents the mean±s.e.m. of seven independent observations.
Effect of L-798,106 on the inhibition of EFS-evoked [3H]ACh release from guinea-pig trachea evoked by 8-iso-PGE1, 8-iso-PGE2, PGE2 and sulprostone
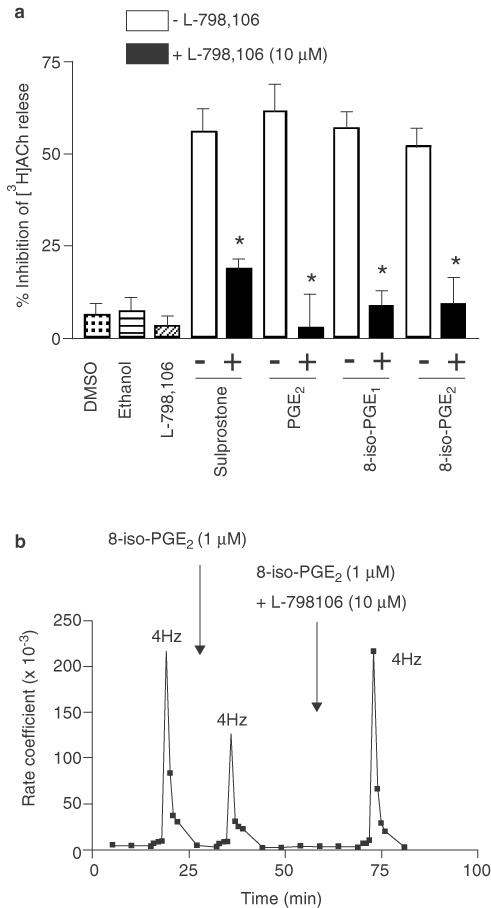
Having determined the affinity of L-798,106 at EP3-receptors in two guinea-pig tissues where transmitter output was measured indirectly, the same antagonist was tested for its ability to attenuate 8-iso-PGE1-, 8-iso-PGE2-, PGE2- and sulprostone-induced inhibition of [3H]ACh from guinea-pig trachea. In these experiments, L-798,106 was used at 10 μM (∼667 times its pA2 at EP3-receptors), a concentration that has little or no activity at the EP1-, EP2- or EP4-receptor subtypes (Juteau et al., 2001). Under these conditions, L-798,106 significantly attenuated the inhibitory effect of all agents tested (in % inhibition of EFS-induced release: 8-iso-PGE1 from 56.9±4.6 to 8.6±4.2; 8-iso-PGE2 from 51.6±5.1 to 9.2±7.1; PGE2 from 61.2±7.9 to 2.9±8.9; sulprostone from 55.9±6.2 to 18.8±2.8; Figure 7a). The inhibition of [3H]ACh release was observed 15 min after the application of each test agonist and reversal was complete 30 min following the addition of L-798,106 (see Figure 7b for 8-iso-PGE2 results).
Figure 7.
Effect of a selective EP3-receptor antagonist on the inhibition by E-ring 8-isoprostanes, sulprostone and PGE2 of EFS-induced [3H]ACh release from guinea-pig trachea. In panel (a), the ability of L-798,106 (10 μM) to antagonise the inhibitory effect of 8-iso-PGE1 (1 μM), 8-iso-PGE2 (1 μM), sulprostone (100 nM) and PGE2 (1 μM) on EFS-induced [3H]ACh output is shown. The unfilled and black bars denote inhibition of [3H]ACh release evoked by the agonist indicated, in the absence and presence of L-798,106, respectively. Each tissue acted as its own control such that bars reflect the percentage change in EFS-induced [3H]ACh output after drug administration relative to the first control stimulation. The effects of 0.1% v v−1 DMSO (vehicle for 8-iso-PGE1, 8-iso-PGE2 and sulprostone), 0.1% v v−1 ethanol (vehicle for PGE2) and L-798,106 (10 μM) are also shown. Panel (b) shows representative data for the inhibition and reversal by 8-iso-PGE2 and L-798,106, respectively, of EFS-induced [3H]ACh release from an individual guinea-pig tracheal strip. In (a), each data point represents the rate coefficient of ACh output, which is a measure of the fractional quantity of tritium release per unit time. Bars represent the mean±s.e.m. of five to six independent observations. *P<0.05 – significant reversal by L-798,106 of agonist-induced inhibition of [3H]ACh output.
Discussion
The aim of the present study was to determine if 8-iso-PGE1 and 8-iso-PGE2 regulate ACh release from parasympathetic nerves innervating guinea-pig trachea, and to identify the prejunctional receptor(s) involved in any effect. As isoprostanes are isomeric with PGs, the effects of PGE2 and the selective EP3-receptor agonist sulprostone were re-assessed concurrently in this system, as they are known to inhibit EFS-evoked [3H]ACh release from tracheal smooth muscle, with a pharmacology consistent with the agonism of prejunctional EP3-receptors (Belvisi et al., 1996; Spicuzza et al., 1998).
Four distinct receptor subtypes for E-series PGs have been cloned, and these can be distinguished pharmacologically with selective agonists and antagonists (see Coleman et al., 1994a; Breyer et al., 2001). Studies using naturally occurring and synthetic prostanoid receptor ligands have attempted to classify the prejunctional EP-receptor subtype(s) on cholinergic nerve terminals that innervate guinea-pig trachea. Spicuzza et al. (1998) demonstrated that 17-phenyl-ω-trinor PGE2 (selective EP1-receptor agonist) and AH 13205 (selective EP2-receptor agonist; Nials et al., 1993) were greater than 12- and 100-fold less potent, respectively, than PGE2 at inhibiting [3H]ACh release, suggesting that neither the EP1- nor EP2-receptor subtype mediates this response. In addition, Spicuzza et al. (1998) found that the inhibition of [3H]ACh release by PGE2 was insensitive to AH 6809 when used at a concentration 10–100 times higher than its affinity for the EP1- and DP-receptor subtypes (Spicuzza et al., 1998). Indeed, this latter finding is confirmed here. Collectively, these historical data and the insensitivity of the response to the TP-receptor antagonist SQ 29,548, documented herein, suggest that PGE2, sulprostone and, potentially, E-ring 8-isoprostanes suppress cholinergic neurotransmission to guinea-pig trachea by activating prejunctional prostanoid receptors of the EP3-subtype. This conclusion is supported by the finding that the highly selective EP3-receptor agonists GR 63799X and M & B 28,767 were respectively seven times more potent than, and equipotent with, PGE2 at suppressing [3H]ACh release from guinea-pig trachea (Spicuzza et al., 1998). Although the selective agonists or antagonists at EP4-receptors have not been used within this study, this subtype is positively coupled to adenylyl cyclase and hence would elevate cAMP, a process that is thought to enhance rather than inhibit ACh release from nerve terminals (Wilson, 1974; Standaert & Dretchen, 1979; Wessler & Anschutz, 1988; Belvisi et al., 1996).
It is well recognised that establishing the rank orders of agonist potencies in isolated cells and tissues is not a robust method for classifying receptors, and that unequivocal receptor classification can only be determined with selective antagonists. Thus, in the present study, we performed additional experiments to identify the receptor that mediates the inhibition by sulprostone of cholinergic neurotransmission using a novel and recently described EP3-receptor antagonist, L-798,106 (Juteau et al., 2001). In radioligand-binding studies, L-798,106 has a Ki of 300 pM for the human recombinant EP3-receptor expressed in HEK 293 cells, which is >16,000, >3000 and >16,000 times higher than its affinity at human recombinant EP1-, EP2- and EP4-receptor subtypes, respectively (Juteau et al., 2001). As an antagonist's affinity for a specific receptor can vary between species and by the way it is determined (e.g. radioligand binding vs Schild (1949) analysis), the pA2 of L-798,106 for antagonising the inhibition by sulprostone of EFS-evoked contractile responses in the guinea-pig vas deferens, an established EP3-receptor-expressing tissue (Lawrence et al., 1992; Coleman et al., 1994b; Tam et al., 1997; Jones et al., 1998) was determined and compared with the guinea-pig trachea under comparable experimental conditions. From these experiments, pA2 values of 7.48 and 7.82 were calculated for L-798,106 on the vas deferens and trachea, respectively. These affinity estimates were not significantly different from one another, providing compelling evidence that the inhibition by sulprostone of EFS-induced contractile responses of the guinea-pig vas deferens and trachea are mediated by the same prejunctional prostanoid receptor, and that this receptor is of the EP3-subtype. To the authors' knowledge, this is the first report documenting the affinity of an EP3-receptor antagonist in isolated tissues. Clearly, L-798,106 provides an invaluable research tool to aid the classification of EP-receptor-mediated events in other cells and tissues where a role for the EP3-subtype is equivocal.
One striking discrepancy of the present study that merits highlighting was that the affinity of L-798,106 determined by Schild (1949) analysis for the prejunctional EP3-receptor expressed on guinea-pig nerve varicosities supplying the vas deferens (33 nM) and trachea (15 nM) was 50 and 110 times lower than its Ki at the human recombinant EP3-subtype determined by radioligand binding (300 pM; Juteau et al., 2001). Equilibrium dissociation rate constants for reversible drug–receptor interactions are assumed to follow the law of mass action, and should be the same, irrespective of whether they are determined operationally or by ligand binding. To account for the anomalous behaviour of L-798,106, we suggest that the most likely explanation is a difference between the human and guinea-pig EP3-receptor subtype. Indeed, there are many examples in the literature where an antagonist's affinity for a G-protein-coupled receptor varies considerably across species. For example, the Ki for bradyzide, a nonpeptide antagonist at the bradykinin B2-receptor, is 800 times lower at the human recombinant B2-subtype expressed in COS-7 cells relative to the rodent homologue (Burgess et al., 2000). Similar species-related differences in the affinity of adenosine receptor antagonists have also been noted. This is most conspicuous for the A3-adenosine receptor where the affinity of many xanthine-based antagonists is highly species-dependent and is typically ∼100-fold higher for the human subtype when compared to the rat variant (see Fredholm et al., 2001). Clearly, additional studies with L-798,106 in EP3-receptor-expressing human tissues could help resolve this inconsistency. An alternative explanation may relate to the method used to determine affinity. However, while discrepancies between pA2 and pKi values are not uncommon, the authors are unaware of any examples where differences of two orders of magnitude have been authenticated.
L-798,106 also reversed the inhibitory effect of E-ring 8-isoprostanes, PGE2 and sulprostone on EFS-induced [3H]ACh release. Although this smooth muscle preparation is not suited for the construction of response curves from which concentration ratios can be calculated reproducibly, L-798,106 was used at a concentration (10 μM) that is reportedly selective for the EP3-receptor subtype (Juteau et al., 2001). Thus, we conclude that, irrespective of whether cholinergic transmission is measured indirectly (EFS-induced contractile responses) or directly ([3H]ACh release), the inhibitory effect of 8-iso-PGE1, 8-iso-PGE2, PGE2 and sulprostone is mediated through prejunctional receptors on cholinergic nerve terminals that have the characteristics of the EP3-subtype.
There are few publications on the regulation by 8-isoprostanes of autonomic neurotransmission. In the present study, we have added to the literature, by presenting persuasive evidence using L-798,106, that 8-iso-PGE1 and 8-iso-PGE2 activate the same receptor as PGE2 to temper cholinergic transmission to guinea-pig airways. These results are consistent with the ability of 8-iso-PGE2 to suppress EFS-induced noradrenaline release from bovine isolated irides and the rat isolated stomach (Awe et al., 2000; Opere et al., 2001; Nakamura et al., 2003; Yokotani et al., 2003). However, isoprostanes do not behave predictably. For example, the Fα-ring 8-isoprostane, 8-iso-PGF2α, enhances and inhibits, respectively, noradrenergic transmission to the irides and stomach by activating prejunctional TP-receptors (Awe et al., 2000; Opere et al., 2001; Nakamura et al., 2003; Yokotani et al., 2003). It is of interest that we have reported previously that 8-iso-PGF2α reduces EFS-evoked [3H]ACh release from guinea-pig trachea, although the participation of TP-receptors was not determined (Spicuzza et al., 2001). It is clear from these limited studies that, despite being isomeric with the PGs, D-, E- and Fα-ring 8-isoprostanes are not invariably agonists at prostanoid receptor of the DP-, EP- or FP-receptor subtypes, respectively.
PGE2 is known to modulate noradrenergic and cholinergic neurotransmission from central and peripheral nerves in several species. Indeed, the existence of prejunctional EP-receptors on postganglionic sympathetic nerve terminals has been widely reported. In rat vena cava (Molderings et al., 1992), human saphenous vein and human pulmonary artery (Molderings et al., 1994), rat trachea (Racke et al., 1992) and guinea-pig atrium (Mantelli et al., 1991), EFS-evoked [3H]noradrenaline release is inhibited by PGE2 through, what is believed to be, a prejunctional EP3-receptor. Taken together, these observations strongly suggest that prejunctional EP3-receptors, that can be activated by PGE2 and more novel ligands like the E-ring 8-isoprostanes, are ubiquitously expressed on autonomic nerve varicosities and, when activated, suppress neurotransmitter output.
In conclusion, the results of the present study demonstrate that E-ring 8-isoprostanes and PGE2 inhibit EFS-evoked [3H]ACh release from cholinergic nerve endings through an interaction with prejunctional prostanoid receptors of the EP3-subtype. Recently, it was published that isoprostanes are elevated in the airways of patients with several respiratory disorders (Montuschi et al., 1999,2000), and it is possible that the negative regulation of ACh output by these novel lipids from parasympathetic nerves may be of clinical relevance. Indeed, inhibition by 8-isoprostanes and PGE2 of cholinergic transmission could be beneficial in airway inflammatory diseases where vagal tone may be increased such as in COPD and nocturnal asthma. Thus, these novel lipids constitute yet another group of mediators that can regulate, positively or negatively, smooth muscle tone, neurotransmission and, potentially, inflammatory responses in the airways.
Acknowledgments
DLC was supported by a MRC (U.K.)/Aventis collaborative studentship. MAG is an Alberta Heritage Senior Medical Scholar and is funded by the Canadian Institutes of Health Research. We would like to thank Merck Frosst (Quebec, Canada) for supplying L-798,106, and Sissie Wong and Kerryn McCluskie for their help in the laboratory.
Abbreviations
- ACh
acetylcholine
- EFS
electrical field stimulation
- KHS
Krebs–Henseleit solution
- PG
prostaglandin
References
- AWE S.O., OPERE C.A., HARRIS L.C., UKETUI A.J., OHIA S.E. Effect of isoprostanes on sympathetic neurotransmission in the human isolated iris-ciliary body. Neurochem. Res. 2000;25:491–496. doi: 10.1023/a:1007560025570. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Modulation of neurotransmission in airways. Physiol. Rev. 1992;72:699–729. doi: 10.1152/physrev.1992.72.3.699. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Muscarinic receptor subtypes in airways. Eur. Respir. J. 1993;6:328–331. [PubMed] [Google Scholar]
- BELVISI M.G. Overview of the innervation of the lung. Curr. Opin. Pharmacol. 2002;2:211–215. doi: 10.1016/s1471-4892(02)00145-5. [DOI] [PubMed] [Google Scholar]
- BELVISI M.G., PATEL H.J., TAKAHASHI T., BARNES P.J., GIEMBYCZ M.A. Paradoxical facilitation of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by isoprenaline. Br. J. Pharmacol. 1996;117:1413–1420. doi: 10.1111/j.1476-5381.1996.tb15300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREYER R.M., BAGDASSARIAN C.K., MYERS S.A., BREYER M.D. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- BURGESS G.M., PERKINS RANG H.P., CAMPBELL E.A., BROWN M.C., MCINTYRE P., URBAN L., DZIADULEWICZ E.K., RITCHIE T.J., HALLETT A., SNELL C.R., WRIGGLESWORTH R., LEE W., DAVIS C., PHAGOO S.B., DAVIS A.J., PHILLIPS E., DRAKE G.S., HUGHES G.A., DUNSTAN A., BLOOMFIELD G.C. Bradyzide, a potent non-peptide B2 bradykinin receptor antagonist with long-lasting oral activity in animal models of inflammatory hyperalgesia. Br. J. Pharmacol. 2000;129:77–86. doi: 10.1038/sj.bjp.0703012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN R.A., GRIX S.P., HEAD S.A., LOUTTIT J.B., MALLETT A., SHELDRICK R.L. A novel inhibitory prostanoid receptor in piglet saphenous vein. Prostaglandins. 1994b;47:151–168. doi: 10.1016/0090-6980(94)90084-1. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I. Characterisation of the prostanoid receptors mediating contraction of guinea-pig isolated trachea. Prostaglandins. 1985;29:363–375. doi: 10.1016/0090-6980(85)90096-6. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., KENNEDY I., SHELDRICK R.L. New evidence with selective agonists and antagonists for the subclassification of PGE2-sensitive (EP) receptors. Adv. Prostagland. Thromboxane Leukotr. Res. 1987;17:467–470. [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994a;46:205–229. [PubMed] [Google Scholar]
- COWLEY E.A. Isoprostane-mediated secretion from human airway epithelial cells. Mol. Pharmacol. 2003;64:298–307. doi: 10.1124/mol.64.2.298. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO G., CHIARI M.C., GRANA E., SUBISSI A., KILBINGER H. Muscarinic inhibition of acetylcholine release from a novel in vitro preparation of the guinea-pig trachea. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:141–145. doi: 10.1007/BF00166956. [DOI] [PubMed] [Google Scholar]
- DECKERS I.A., RAMPART M., BULT H., HERMAN A.G. Evidence for the involvement of prostaglandins in modulation of acetylcholine release from canine bronchial tissue. Eur. J. Pharmacol. 1989;167:415–418. doi: 10.1016/0014-2999(89)90451-2. [DOI] [PubMed] [Google Scholar]
- DRIESSEN B., STARKE K. Modulation of neural noradrenaline and ATP release by angiotensin II and prostaglandin E2 in guinea-pig vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:618–625. doi: 10.1007/BF00169366. [DOI] [PubMed] [Google Scholar]
- FEATHERSTONE R.L., ROBINSON C., HOLGATE S.T., CHURCH M.K. Evidence for thromboxane receptor mediated contraction of guinea-pig and human airways in vitro by prostaglandin (PG) D2, 9α,11β-PGF2 and PGF2α. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;341:439–443. doi: 10.1007/BF00176337. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., KLOTZ K.N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- HEDQVIST P. Prostaglandin action on noradrenaline release and mechanical responses in the stimulated guinea pig vas deferens. Acta Physiol. Scand. 1974;90:86–93. doi: 10.1111/j.1748-1716.1974.tb05566.x. [DOI] [PubMed] [Google Scholar]
- JANSSEN L.J. Isoprostanes: an overview and putative roles in pulmonary pathophysiology. Am. J. Physiol. 2001;280:L1067–L1082. doi: 10.1152/ajplung.2001.280.6.L1067. [DOI] [PubMed] [Google Scholar]
- JANSSEN L.J., PREMJI M., NETHERTON S., CATALLI A., COX G., KESHAVJEE S., CRANKSHAW D.J. Excitatory and inhibitory actions of isoprostanes in human and canine airway smooth muscle. J. Pharmacol. Exp. Ther. 2000;295:506–511. [PubMed] [Google Scholar]
- JONES R.L., QIAN Y.M., CHAN K.M., YIM A.P. Characterization of a prostanoid EP3-receptor in guinea-pig aorta: partial agonist action of the non-prostanoid ONO-AP-324. Br. J. Pharmacol. 1998;125:1288–1296. doi: 10.1038/sj.bjp.0702189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUTEAU H., GAREAU Y., LABELLE M., STURINO C.F., SAWYER N., TREMBLAY N., LAMONTAGNE S., CARRIERE M.C., DENIS D., METTERS K.M. Structure–activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg. Med. Chem. 2001;9:1977–1984. doi: 10.1016/s0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]
- KAWIKOVA I., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. 8-Epi-PGF2α, a novel noncyclooxygenase-derived prostaglandin, constricts airways in vitro. Am. J. Respir. Crit. Care Med. 1996;153:590–596. doi: 10.1164/ajrccm.153.2.8564103. [DOI] [PubMed] [Google Scholar]
- KEERY R.J., LUMLEY P. AH6809, a prostaglandin DP-receptor blocking drug on human platelets. Br. J. Pharmacol. 1988;94:745–754. doi: 10.1111/j.1476-5381.1988.tb11584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHARITONOV S.A., BARNES P.J. Exhaled markers of pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;163:1693–1722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- LAWRENCE R.A., JONES R.L., WILSON N.H. Characterization of receptors involved in the direct and indirect actions of prostaglandins E and I on the guinea-pig ileum. Br. J. Pharmacol. 1992;105:271–278. doi: 10.1111/j.1476-5381.1992.tb14245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTELLI L., AMERINI S., RUBINO A., LEDDA F. Prejunctional prostanoid receptors on cardiac adrenergic terminals belong to the EP3 subtype. Br. J. Pharmacol. 1991;102:573–576. doi: 10.1111/j.1476-5381.1991.tb12214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENNIFF M., RODGER I.W., NORMAN P., GARDINER P.J. Characterisation of receptors mediating the contractile effects of prostanoids in guinea-pig and human airways. Eur. J. Pharmacol. 1988;153:149–159. doi: 10.1016/0014-2999(88)90601-2. [DOI] [PubMed] [Google Scholar]
- MOLDERINGS G., MALINOWSKA B., SCHLICKER E. Inhibition of noradrenaline release in the rat vena cava via prostanoid receptors of the EP3-subtype. Br. J. Pharmacol. 1992;107:352–355. doi: 10.1111/j.1476-5381.1992.tb12750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLDERINGS G.J., COLLING E., LIKUNGU J., JAKSCHIK J., GOTHERT M. Modulation of noradrenaline release from the sympathetic nerves of the human saphenous vein and pulmonary artery by presynaptic EP3- and DP-receptors. Br. J. Pharmacol. 1994;111:733–738. doi: 10.1111/j.1476-5381.1994.tb14799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTUSCHI P., CIABATTONI G., PAREDI P., PANTELIDIS P., DU BOIS R., KHARITONOV S.A., BARNES P.J. 8-Isoprostane as a biomarker of oxidative stress in interstitial lung diseases. Am. J. Respir. Crit. Care Med. 1998;158:1524–1527. doi: 10.1164/ajrccm.158.5.9803102. [DOI] [PubMed] [Google Scholar]
- MONTUSCHI P., COLLINS J.V., CIABATTONI G., LAZZERI N., CORRADI M., KHARITONOV S.A., BARNES P.J. Exhaled 8-isoprostane as an in vivo biomarker of lung oxidative stress in patients with COPD and healthy smokers. Am. J. Respir. Crit. Care Med. 2000;162:1175–1177. doi: 10.1164/ajrccm.162.3.2001063. [DOI] [PubMed] [Google Scholar]
- MONTUSCHI P., CORRADI M., CIABATTONI G., NIGHTINGALE J., KHARITONOV S.A., BARNES P.J. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am. J. Respir. Crit. Care Med. 1999;160:216–220. doi: 10.1164/ajrccm.160.1.9809140. [DOI] [PubMed] [Google Scholar]
- MORROW J.D., HARRIS T.M., ROBERTS L.J. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990;184:1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- NAKAMURA K., OKADA S., ONO K., YOKOTANI K. Effects of 8-iso-prostaglandin E2 and 8-iso-prostaglandin F2α on the release of noradrenaline from the isolated rat stomach. Eur. J. Pharmacol. 2003;470:73–78. doi: 10.1016/s0014-2999(03)01756-4. [DOI] [PubMed] [Google Scholar]
- NIALS A.T., VARDEY C.J., DENYER L.H., THOMAS M., SPARROW S.J., SHEPHERD G.D., COLEMAN R.A. AH13205, a selective prostanoid EP2-receptor agonist. Cardiovasc. Drug Rev. 1993;11:165–179. [Google Scholar]
- OPERE C.A., AWE S.O., HARRIS L.C., LEDAY A.M., OHIA S.E. Potentiation of sympathetic neurotransmission in bovine isolated irides by isoprostanes. Free Radic. Res. 2001;35:257–264. doi: 10.1080/10715760100300791. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. Evidence for prejunctional muscarinic autoreceptors in human and guinea pig trachea. Am. J. Respir. Crit. Care Med. 1995;152:872–878. doi: 10.1164/ajrccm.152.3.7663798. [DOI] [PubMed] [Google Scholar]
- RACKE K., BAHRING J., LANGER C., BRAUTIGAM M., WESSLER I. Prostanoids inhibit release of endogenous norepinephrine from rat isolated trachea. Am. Rev. Respir. Dis. 1992;146:1182–1186. doi: 10.1164/ajrccm/146.5_Pt_1.1182. [DOI] [PubMed] [Google Scholar]
- REINHEIMER T., HARNACK E., RACKE K., WESSLER I. Prostanoid receptors of the EP3 subtype mediate inhibition of evoked [3H]acetylcholine release from isolated human bronchi. Br. J. Pharmacol. 1998;125:271–276. doi: 10.1038/sj.bjp.0702057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILD H.O. pAx and competitive drug antagonism. Br. J. Pharmacol. Chemother. 1949;4:277–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPICUZZA L., BARNES P.J., DI MARIA G.U., BELVISI M.G. Effect of 8-iso-prostaglandin F2α on acetylcholine release from parasympathetic nerves in guinea pig airways. Eur. J. Pharmacol. 2001;416:231–234. doi: 10.1016/s0014-2999(01)00882-2. [DOI] [PubMed] [Google Scholar]
- SPICUZZA L., GIEMBYCZ M.A., BARNES P.J., BELVISI M.G. Prostaglandin E2 suppression of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by interacting with prostanoid receptors of the EP3-subtype. Br. J. Pharmacol. 1998;123:1246–1252. doi: 10.1038/sj.bjp.0701720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANDAERT F.G., DRETCHEN K.L. Cyclic nucleotides and neuromuscular transmission. Fed. Proc. 1979;38:2183–2192. [PubMed] [Google Scholar]
- STJARNE L. Kinetics of secretion of sympathetic neurotransmitter as a function of external calcium: mechanism of inhibitory effect of prostaglandin E. Acta Physiol. Scand. 1973;87:428–430. doi: 10.1111/j.1748-1716.1973.tb05408.x. [DOI] [PubMed] [Google Scholar]
- TABER D.F., MORROW J.D., ROBERTS L.J. A nomenclature system for the isoprostanes. Prostaglandins. 1997;53:63–67. doi: 10.1016/s0090-6980(97)00005-1. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., BELVISI M.G., PATEL H., WARD J.K., TADJKARIMI S., YACOUB M.H., BARNES P.J. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am. J. Respir. Crit. Care Med. 1994;150:1640–1645. doi: 10.1164/ajrccm.150.6.7952627. [DOI] [PubMed] [Google Scholar]
- TAM F.S., CHAN K., BORREAU J.P., JONES R.L. The mechanisms of enhancement and inhibition of field stimulation responses of guinea-pig vas deferens by prostacyclin analogues. Br. J. Pharmacol. 1997;121:1413–1421. doi: 10.1038/sj.bjp.0701275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR S.M., PARE P.D., SCHELLENBERG R.R. Cholinergic and nonadrenergic mechanisms in human and guinea pig airways. J. Appl. Physiol. 1984;56:958–965. doi: 10.1152/jappl.1984.56.4.958. [DOI] [PubMed] [Google Scholar]
- WALTERS E.H., O'BYRNE P.M., FABBRI L.M., GRAF P.D., HOLTZMAN M.J., NADEL J.A. Control of neurotransmission by prostaglandins in canine trachealis smooth muscle. J. Appl. Physiol. 1984;57:129–134. doi: 10.1152/jappl.1984.57.1.129. [DOI] [PubMed] [Google Scholar]
- WARD J.K., BELVISI M.G., FOX A.J., MIURA M., TADJKARIMI S., YACOUB M.H., BARNES P.J. Modulation of cholinergic neural bronchoconstriction by endogenous nitric oxide and vasoactive intestinal peptide in human airways in vitro. J. Clin. Invest. 1993;92:736–742. doi: 10.1172/JCI116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSLER I., ANSCHUTZ S. Adrenoceptor stimulation enhances transmitter output from the rat phrenic nerve. Br. J. Pharmacol. 1988;94:669–674. doi: 10.1111/j.1476-5381.1988.tb11574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESSLER I., HELLWIG D., RACKE K. Epithelium-derived inhibition of [3H]acetylcholine release from the isolated guinea-pig trachea. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:387–393. doi: 10.1007/BF00169454. [DOI] [PubMed] [Google Scholar]
- WESSLER I., REINHEIMER T., BRUNN G., ANDERSON G.P., MACLAGAN J., RACKE K. Adrenoceptors mediate inhibition of [3H]-acetylcholine release from the isolated rat and guinea-pig trachea: role of the airway mucosa and prostaglandins. Br. J. Pharmacol. 1994;113:1221–1230. doi: 10.1111/j.1476-5381.1994.tb17128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON D.F. The effects of dibutyryl cyclic adenosine 3′,5′-monophosphate, theophylline and aminophylline on neuromuscular transmission in the rat. J. Pharmacol. Exp. Ther. 1974;188:447–452. [PubMed] [Google Scholar]
- WOODWARD D.F., PEPPERL D.J., BURKEY T.H., REGAN J.W. 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH 6809), a human EP2-receptor antagonist. Biochem. Pharmacol. 1995;50:1731–1733. doi: 10.1016/0006-2952(95)02035-7. [DOI] [PubMed] [Google Scholar]
- YOKOTANI K., NAKAMURA K., OKADA S. Prostanoid EP3- and TP-receptor-mediated inhibition of noradrenaline release from the isolated rat stomach. Eur. J. Pharmacol. 2003;459:187–193. doi: 10.1016/s0014-2999(02)02857-1. [DOI] [PubMed] [Google Scholar]
- ZAHLER S., BECKER B.F. Indirect enhancement of neutrophil activity and adhesion to cultured human umbilical vein endothelial cells by isoprostanes (iPF2α-III and iPE2-III) Prostagland. Other Lipid Mediat. 1999;57:319–331. doi: 10.1016/s0090-6980(98)00079-3. [DOI] [PubMed] [Google Scholar]