Abstract
A voltage-dependent sodium current has been described in the highly invasive breast cancer cell line MDA-MB-231. Its activity is associated with the invasive properties of the cells. The aim of our study was to test whether this current (INa) is sensitive to three representative calcium channel blockers: verapamil, diltiazem and nifedipine. INa was studied in patch-clamp conditions.
INa was sensitive to verapamil (IC50=37.6±2.5 μM) and diltiazem (53.2±3.6 μM), while it was weakly sensitive to nifedipine.
The tetrodotoxin (TTX) concentration, which fully blocks INa (30 μM), did not affect cell proliferation. Diltiazem and verapamil, at concentrations that do not fully block INa, strongly reduced cell proliferation, suggesting, regarding proliferation, that these molecules act on targets distinct from sodium channels. These targets are probably not other ionic channels, since the current measured at the end of a 500 ms long pulse in the voltage range between −60 and +40 mV was unaffected by verapamil and diltiazem.
We conclude that the sodium channel expressed in MDA-MB-231 cells is sensitive to several calcium channel blockers. The present study also underlines the danger of concluding to the possible involvement of membrane channel proteins in any phenomenon on the sole basis of pharmacology, and without an electrophysiological confirmation.
Keywords: Breast cancer, invasivity, MDA-MB-231, electrophysiology, calcium channel blockers
Introduction
Among women, breast cancer is the commonest cancer and the first cause of death. Death occurs primarily after the development of metastasis. One of the problems engendered by chemotherapy is the occurrence of a multidrug resistance phenotype (MDR), which impairs the efficiency of the treatment (Moscow & Cowan, 1988). MDR is mainly associated to the presence of the drug carrier called P-glycoprotein (or P-gp) in the plasmalemma, which extrudes drugs from the cytoplasm (Kartner et al., 1983). Interestingly, this P-gp is blocked by molecules such as verapamil and diltiazem (Cornwell et al., 1987). Thus, in some drug-resistant metastatic cancers, the efficacy of chemotherapy was improved when verapamil was given in association with the usual drugs combination (Timcheva & Todorov, 1996; Belpomme et al., 2000).
In breast cancer, the role of ionic channels has been known since the work of Marino et al. (1994). Since this pioneer work, the role of ionic channels in breast cancer cell lines focused mainly on potassium channels (Strobl et al., 1995; Wonderlin et al., 1995; Woodfork et al., 1995; Ouadid-Ahidouch et al., 2000, 2001). The expression and the role of these channels in cell proliferation have been studied, and at least four types of potassium channels exist in the MCF-7 cell line (Wegman et al., 1991; Woodfork et al., 1995; Klimatcheva & Wonderlin, 1999; Ouadid-Ahidouch et al., 2001). The presence of a sodium channel protein has been observed in biopsies of tumours, which developed lymph node metastasis, and in a highly metastatic breast cancer cell line, MDA-MB-231 (Fraser et al., 2002). The current described in the MDA-MB-231 cells is involved in the invasiveness process (Roger et al., 2003). The sodium channel protein found in this cell line is the main cardiac isoform NaV1.5 (Fraser et al., 2002). In cardiac preparations, verapamil and D600 (two phenylalkylamine analogues) have been shown to block INa (McLean et al., 1974; Galper & Catterall, 1978, 1979).
In the present study, we investigated if the fast inward sodium current found in MDA-MB-231 cells was sensitive to L-type calcium channel blockers.
Methods
Cell culture
The breast cancer cell line MDA-MB-231 was purchased from the American Type Culture Collection (Rockville, MD, U.S.A.) at passage 28. All experiments were carried out within 20 additional passages. Cells were grown in Dulbecco's modified Eagle's medium (DMEM, 4.5 g l−1 glucose, 584 mg l−1 glutamine and 3.7 g l−1 NaHCO3), supplemented with 5% foetal bovine serum (FBS) and with 1% antibiotics (mixture of 5000 U ml−1 penicillin and 5000 μg ml−1 streptomycin). Cells were grown in an atmosphere saturated with humidity at 37°C and 5% CO2.
Solutions
The physiological saline solution (PSS) had the following composition (in mM): NaCl 140, KCl 4, MgCl2 1, CaCl2 2, D-glucose 11.1 and HEPES 10, adjusted to pH 7.4 with 1 M NaOH. The intrapipette solution had the following composition (in mM): K-aspartate 110, KCl 10, NaCl 10, MgCl2 8, K2-ATP 8, EGTA 10, HEPES 10, adjusted to pH 7.2 with 1 M KOH. Tetrodotoxin (TTX), verapamil, diltiazem and nifedipine were added to the PSS at the concentrations indicated in the figure legends. All drugs and chemicals were purchased from Sigma-Aldrich (St Quentin, France).
Electrophysiology
For electrophysiological analyses, cells were seeded into 35-mm Petri dishes at 2500 cells cm−2. Before patch-clamping, the growth medium was rinsed and replaced with PSS. Patch pipettes were pulled from nonheparinised haematocrit tubes to a resistance of 3–5 MΩ. Currents were recorded under the voltage-clamp mode at room temperature, using an Axopatch 200 B patch-clamp amplifier (Axon Instrument, Burlingame, CA, U.S.A.). When studying INa, analogue signals were filtered at 5 kHz, using a five-pole low-pass Bessel filter, and sampled at 10 kHz using a 1322A Digidata converter. When we studied the effects of blockers on the end-pulse current, analogue signals were filtered at 2 kHz and sampled at 4 kHz. PClamp software (v8.1, Axon Instrument) was used for generation of voltage commands, acquisition and analysis of whole-cell currents. Cells were studied in the ruptured patch configuration. Cell capacitance and series resistances were electronically compensated by about 60%. The P/2 subpulse correction of cell leakage and capacitance was used to study INa. The cell under investigation was continuously superfused with PSS or test solutions. The superfusion was performed by positioning the cell under study at the tip of a conical microcapillary that received the outlet of six microcapillaries connected to 20-ml syringes. Solutions were selected with solenoids allowing flowing or not (flow rate 500 μl min−1).
Inward sodium currents were recorded by depolarising the cells from a holding potential of −100 mV to a maximal test pulse of −5 mV (corresponding to the maximal inward current) for 30 ms every 500 ms. End-pulse currents, which contain background currents and voltage-gated currents that do not inactivate were studied by depolarising the cells from a holding potential of −100 mV to a test pulse of +40 mV for 500 ms every 5 s. The protocol to build the end-pulse current–voltage (Iep–V) curve was as follows: from a holding potential of −100 mV, the membrane was stepped to potentials between −60 and +40 mV, with 10-mV increments, for 500 ms, at a stimulation frequency of 0.5 Hz. INa amplitude was determined as the difference between the inward current maximum peak and the current amplitude at the end of the depolarising pulse. The end-pulse current was measured as the mean current amplitude during the last 20 ms of a 500 ms long pulse. Current amplitudes, normalised on cell capacitance so that differences in cell sizes were taken into account, were expressed as current density (pA pF−1). A mean cell capacitance of 32.9±0.8 pF (n=182 cells) has been found in our experiments.
The percentage block or reduction of Na+ current (INa) was calculated from the difference in peak current generated by a depolarisation to −5 mV with and without the drug. The drug dose–response curves were fitted to a sigmoidal logistic function of the following form:
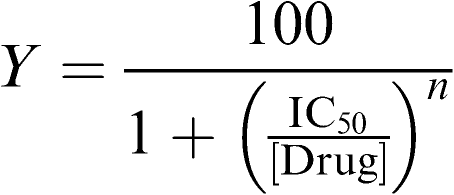 |
where Y is the percent block at a given concentration of drug ([drug]), IC50 is the concentration of drug at which 50% of the current was blocked, and n is the Hill coefficient giving the slope.
Cell survival and proliferation
Cells were seeded at 4 × 104 cells per well in six wells of a 24-well plate for a given condition on three separate experiments. Cells were grown for a total of 5 days. The medium and the different substances tested were changed every other day. Growth and viability of cells were measured as a whole by the tetrazolium salt assay (Mosmann, 1983). Cells were incubated at 37°C with the tetrazolium salt (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) and metabolically active cells reduced the dye to purple formazan. Formazan crystals were dissolved with DMSO. The absorbance was measured at 570 nm. Identical numbers of living cells of the different cell lines yielded weaker or stronger formazan absorbances, indicating different reducing capacities among the different cell lines. Cell proliferation was expressed as formazan 570 nm absorbance and was not converted to cell numbers, since each cell line had its own control condition on the same day of the experiment.
For easier comparison between conditions, the results obtained for proliferation were normalised: cells measured in wells, for a given condition, were added and the ratio of these sums (total number of cells in the presence of the drug/total number of cells in control experiments) calculated for each experiment. The means were then calculated on these daily calculated ratios.
Statistics
Data are described as mean±standard error of the mean (n=number of cells). One-way ANOVA on ranks followed by a Student–Neumann–Keuls test were used. P<0.05 was considered as significant. The mean of each triplicate was used to create data points for comparing cell growth in different conditions.
Results
Previously, we described a novel fast inward sodium current in the human metastatic breast cancer cell line MDA-MB-231 (Roger et al., 2003). This current, which is involved in the invasive properties of MDA-MB-231 cells, is a high-threshold current. Its sensitivity to TTX is quite weak against those of the neuronal and skeletal muscle sodium currents. It is thus classified as TTX-resistant. It is of interest to notice that the selective blockade of INa by TTX does not unmask the presence of a fast transient outward current or of an inward TTX-insensitive current. The cardiac NaV1.5 sodium channel isoform has been described in this cell line (Fraser et al., 2002). The presence of this isoform has also been found in biopsies of metastatic breast cancer (Fraser et al., 2002). The cardiac INa is sensitive to several calcium channel blockers. Thus, we examined whether L-type calcium channel blockers belonging to the three families of blockers: dihydropyridine (nifedipine), benzothiazepine (diltiazem) and phenylalkylamine (verapamil) are blockers of INa in MDA-MB-231 cells.
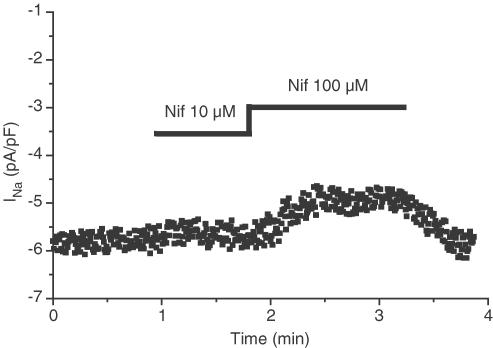
Figure 1 shows that the sodium current is weakly sensitive to the dihydropyridine nifedipine. We found a mean inhibition of 8.7±2.2% at 10 μM and 28.0±3.2% at 100 μM nifedipine (n=4).
Figure 1.
Representative effect of nifedipine on INa. INa was elicited by a 30 ms long depolarisation to −5 mV from a holding potential of −100 mV every 500 ms. INa amplitude was measured during each depolarisation and plotted as a function of time. Nifedipine was externally applied to the cell at the times and concentrations indicated at the top of the graph. The same results were obtained with three other MDA-MB-231 cells.
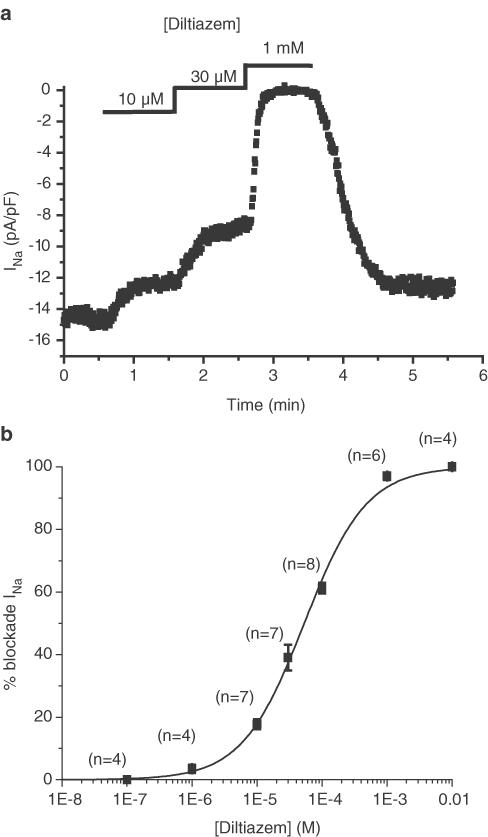
Diltiazem, a benzothiazepine known for its ability to block cardiac L-type calcium channels, dose-dependently and reversibly blocked INa with an IC50 of 53.2±3.6 μM and a Hill coefficient of 0.91±0.06, indicating a 1 : 1 ratiometric interaction between diltiazem and channel proteins (Figure 2). This sensitivity to diltiazem is in the same range as the sensitivity of the L-type cardiac calcium current (Koidl et al., 1997).
Figure 2.
Effect of diltiazem on INa. (a) Representative example of the effect of three different diltiazem concentrations on the amplitude of INa, elicited by a 30 ms long depolarisation to −5 mV from a holding potential of −100 mV every 500 ms. INa amplitude was measured during each depolarisation and plotted as a function of time. Diltiazem was externally applied to the cell at the times and concentrations indicated at the top of the graph. (b) Dose–response curve of diltiazem inhibition of INa. Numbers above each point give the number of cells used at the given diltiazem concentration. The logistic fit gives an IC50 of 53.2±3.6 μM and a Hill coefficient of 0.91±0.06.
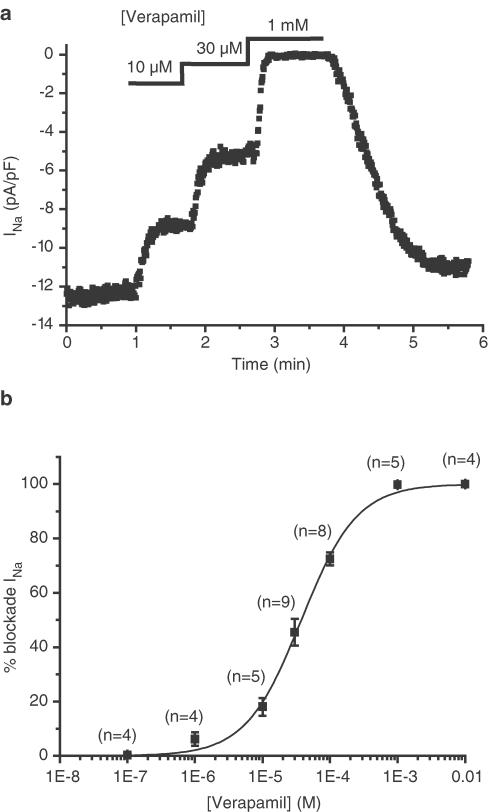
INa is also blocked by the third blocker of L-type calcium channel blocker that we tested, verapamil (Figure 3). Verapamil dose-dependently and reversibly blocked INa. As shown in Figure 3, the sensitivity of INa to verapamil is comparable to that of diltiazem. The IC50 is 37.6±2.5 μM and the Hill coefficient is 1.05±0.08.
Figure 3.
Effect of verapamil on INa. (a) Representative example of the effect of three different verapamil concentrations on the amplitude of INa, elicited by a 30 ms long depolarisation to −5 mV from a holding potential of −100 mV every 500 ms. INa amplitude was measured during each depolarisation and plotted as a function of time. Verapamil was externally applied to the cell at the times and concentrations indicated at the top of the graph. (b) Dose–response curve of verapamil inhibition of INa. Numbers above each point indicate the number of cells used at the given verapamil concentration. The logistic fit gives an IC50 of 37.6±2.5 μM and a Hill coefficient of 1.05±0.08.
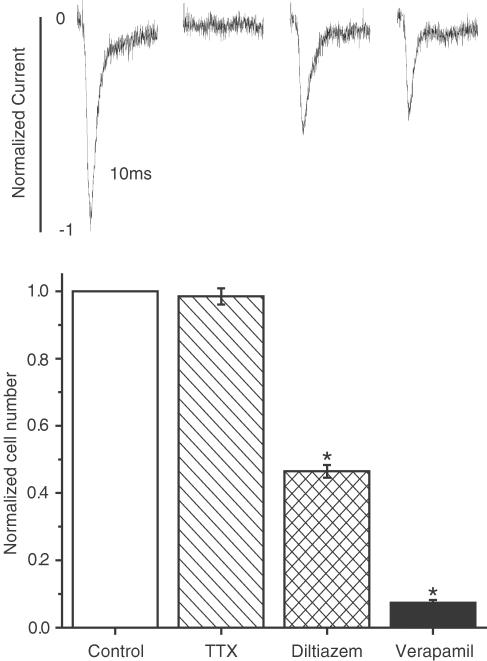
From these experiments, it appears that INa is sensitive to verapamil and diltiazem, but not to nifedipine. The next experiments were performed to evaluate how these blockers affect a specific property of cancer cells, proliferation. We previously reported that TTX, at a concentration which fully blocks INa, had no effect on cell proliferation and migration, but was effective in reducing cell invasion through a Matrigel® layer (Roger et al., 2003). To verify if the same applies to all blockers, we evaluated the effects of 30 μM TTX, 100 μM diltiazem and 100 μM verapamil on cell proliferation. As shown in Figure 4, diltiazem and verapamil, although applied at a concentration that does not fully block INa, significantly reduced cell proliferation, while TTX, at a concentration that fully blocks the current, had no effect.
Figure 4.
Relative effects of INa blockers on proliferation of MDA-MB-231 cells. The concentrations of the blockers were 30 μM TTX, 100 μM diltiazem and 100 μM verapamil. Results are normalised as explained in Methods section. *P<0.05 when comparing blocker vs control. Above each bar is shown a representative normalised INa obtained with the same drug concentration as the respective proliferation test.
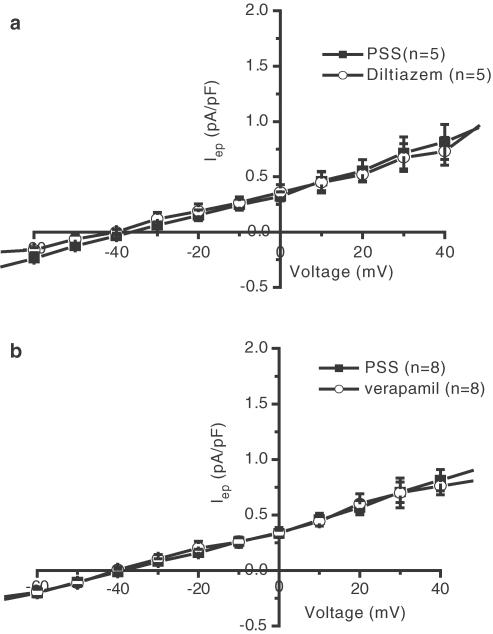
Calcium channel blockers like verapamil are known to block some cardiac potassium channels as efficiently as they block INa (Zhang et al., 1997). In addition, blockade of potassium channels has been shown to interfere with the proliferation of human cancer cells (Rybalchenko et al., 2001). Therefore, we evaluated the effect of 100 μM verapamil and 100 μM diltiazem on the end-pulse current of a 500 ms depolarisation at different voltages. After such a long depolarisation, INa is completely inactivated and the remaining currents are background and time/voltage-dependent currents (mainly carried by chloride and potassium ions). As shown in Figure 5, the drugs had no effect on the overall remaining current, suggesting a lack of effect of these molecules on ionic channels, except INa.
Figure 5.
Effect of diltiazem and verapamil on end-pulse current (Iep). (a) Mean current–voltage relationship obtained from five cells before (PSS) and after application of 100 μM diltiazem. (b) Mean current–voltage relationship obtained from eight cells before (PSS) and after application of 100 μM verapamil. Cells were depolarised from −60 to +40 mV for 500 ms by 10-mV increments from a holding potential of −100 mV.
Discussion
The main result of this study is that INa, which has been previously shown to be involved in the invasive process (Roger et al., 2003), is blocked by L-type calcium channel blockers and P-gp blockers. We also show that verapamil and diltiazem reduce cell proliferation through mechanisms that do not involve inhibition of ionic channels.
A correlation has been found between the metastatic potency of human breast cancer tumours and the expression of the NaV1.5 sodium channel protein, which has also been found in MDA-MB-231 cells (Fraser et al., 2002). This isoform is the one mainly found in cardiac preparations and involved in the propagation of the action potential. In cancer cells, there is no evidence of elicitation of action potentials that could explain its role in the invasive process. Roger et al. (2003) proposed that INa participates in the regulation of the intracellular sodium homeostasis, thanks to a window current present at the membrane potential. Cardiac sodium current has been shown to be blocked by verapamil and D600, but this block is mainly obtained in embryonic heart cells (McLean et al., 1974; Galper & Catterall, 1978). The sodium channel protein found in MDA-MB-231 cells is the neonatal form (Fraser et al., 2002). Thus, the particular sensitivity of INa to calcium channel blockers in breast cancer cells could be explained by the expression of this particular fœtal isoform, which differs from the adult isoform by seven amino acids in the IS3–S4 linker (Fraser et al., 2002).
There are two main consequences to this particular sensitivity. Verapamil is often used as a P-gp blocker. In human therapy, verapamil has been used, in association with conventional chemotherapy, in order to reduce the chemoresistance of metastatic breast cancer (Timcheva & Todorov, 1996; Belpomme et al., 2000). Since it has been shown that the expression of the NaV1.5 is associated with lymph node invasion on the one hand, and that, on the other hand, verapamil blocks the NaV1.5 expressed in breast cancer cells, it will be interesting to determine by which mechanism verapamil exerts its beneficial effect: blockade of the sodium current and/or reversion of the MDR phenotype. It is of interest to note that the presence of a voltage-gated sodium current has been linked with the development of a MDR in a human leukaemia cell line (Yamashita et al., 1987). However, this link is not mechanistically clarified. When INa was blocked (TTX) or missing (no sodium in the PSS), we never observed the presence of an L-type calcium current or of a transient outward current (Roger et al., 2003). Moreover, we did not find any effect of verapamil and diltiazem on the end-pulse currents between −60 and +40 mV (background- and voltage-dependent current which does not inactivate, and is carried mainly by chloride and potassium ions). This strongly suggests that these molecules have no effect on currents other than INa in the MDA-MB-231 cells.
It has been suggested that potassium channels are involved in the proliferation of human breast cancer cells, since blocking these channels with TEA (Ouadid-Ahidouch et al., 2001) or verapamil (Rybalchenko et al., 2001) leads to a dose-dependent decrease in proliferation (Woodfork et al., 1995). Potassium currents are not active in MDA-MB-231 cells, since we did not find any effect on the membrane potential when we changed the external potassium concentration (data not shown). Thus, since potassium channels are not open in our conditions, since L-type calcium channels are not functionally expressed and since INa is not involved in the proliferation process, the antiproliferative effects of verapamil and diltiazem are not mediated through a block of ionic channels altogether.
This underlines the danger in interpreting experiments in which ionic channel blockers are used without the knowledge of the functional electrophysiology of the studied cells. For example, it has been shown that verapamil was able to inhibit tumour protease production and metastasis development in the murine mammary carcinoma cells F3II-Adr (Farias et al., 1998). This result was attributed to the ability of verapamil to interfere with calcium-dependent signalling pathways that regulate the production of proteases and, as a consequence, the appearance of metastases. Up to now, it has never been possible to demonstrate the presence of voltage-gated calcium channels in breast cancer cell lines. This strongly suggests that the effects of verapamil can be attributed to targets other than calcium channels. These targets could be sodium channels, if they exist in the F3II-Adr cells. But, it can also be due to an effect on targets distinct from ionic channels.
In conclusion, our results clearly illustrate two points. First, the sodium channels expressed in metastatic breast cancer are sensitive to calcium channel blockers like verapamil and diltiazem, but not to nifedipine. Such a blockade is not related to proliferation inhibition, but could be clinically exploited, since it has been shown to impede cell invasion (Roger et al., 2003). This particular sensitivity, probably due to the particular isoform of the channel (cardiac neonatal isoform), could be exploited to develop new molecules, which do not interfere with adult cardiac ionic channels. Indeed, the expression of a cardiac sodium channel in metastatic breast cancer cells makes it difficult to act on the channel without affecting the cardiac function. However, if blockers, which can selectively block the neonate isoform vs adult isoform, are developed, we can fairly expect effects on metastasis occurrence without cardiac side effects. Second, this study underlines the danger of concluding about pathways involved in processes like proliferation or invasion on the sole basis of pharmacological experiments without performing a functional ionic channel study.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- FBS
foetal bovine serum
- IC50
concentration of drug blocking 50% of the current
- INa
fast sodium current
- MDR
multidrug resistance phenotype
- P-gp
P-glycoprotein
- PSS
physiological saline solution
- TEA
tetraethylammonium
- TTX
tetrodotoxin
References
- BELPOMME D., GAUTHIER S., PUJADE-LAURAINE E., FACCHINI T., GOUDIER M.J., KRAKOWSKI I., NETTER-PINON G., FRENAY M., GOUSSET C., MARIE F.N., BENMILOUD M., STURTZ F. Verapamil increases the survival of patients with anthracycline-resistant metastatic breast carcinoma. Ann. Oncol. 2000;11:1471–1476. doi: 10.1023/a:1026556119020. [DOI] [PubMed] [Google Scholar]
- CORNWELL M.M., PASTAN I., GOTTESMAN M.M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J. Biol. Chem. 1987;262:2166–2170. [PubMed] [Google Scholar]
- FARIAS E.F., AGUIRRE GHISO J.A., LADEDA V., BAL DE KIER JOFFE E. Verapamil inhibits tumor protease production, local invasion and metastasis development in murine carcinoma cells. Int. J. Cancer. 1998;78:727–734. doi: 10.1002/(sici)1097-0215(19981209)78:6<727::aid-ijc10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- FRASER S.P., DISS J.K.J., MYCIELSKA M.E., COOMBES R.C., DJAMGOZ M.B.A. Voltage-gated sodium channel expression in human breast cancer cells: Possible functional role in metastasis. Breast Cancer Res. Treat. 2002;76 Suppl. 1:S142. [Google Scholar]
- GALPER J.B., CATTERALL W.A. Developmental changes in the sensitivity of embryonic heart cells to tetrodotoxin and D600. Dev. Biol. 1978;65:216–227. doi: 10.1016/0012-1606(78)90191-4. [DOI] [PubMed] [Google Scholar]
- GALPER J.B., CATTERALL W.A. Inhibition of sodium channels by D600. Mol. Pharmacol. 1979;15:174–178. [PubMed] [Google Scholar]
- KARTNER N., RIORDAN J.R., LING V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- KLIMATCHEVA E., WONDERLIN W.F. An ATP-sensitive K(+) current that regulates progression through early G1 phase of the cell cycle in MCF-7 human breast cancer cells. J. Membr. Biol. 1999;171:35–46. doi: 10.1007/s002329900556. [DOI] [PubMed] [Google Scholar]
- KOIDL B., MIYAWAKI N., TRITTHART H.A. A novel benzothiazine Ca2+ channel antagonist, semotiadil, inhibits cardiac L-type Ca2+ currents. Eur. J. Pharmacol. 1997;322:243–247. doi: 10.1016/s0014-2999(96)00995-8. [DOI] [PubMed] [Google Scholar]
- MARINO A.A., ILIEV I.G., SCHWALKE M.A., GONZALEZ E., MARLER K.C., FLANAGAN C.A. Association between cell membrane potential and breast cancer. Tumour Biol. 1994;15:82–89. doi: 10.1159/000217878. [DOI] [PubMed] [Google Scholar]
- MCLEAN M.J., SHIGENOBU K., SPERELAKIS N. Two pharmacological types of cardiac slow Na+ channels as distinguished by verapamil. Eur. J. Pharmacol. 1974;26:379–382. doi: 10.1016/0014-2999(74)90250-7. [DOI] [PubMed] [Google Scholar]
- MOSCOW J.A., COWAN K.H. Multidrug resistance. J. Natl. Cancer Inst. 1988;80:14–20. doi: 10.1093/jnci/80.1.14. [DOI] [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- OUADID-AHIDOUCH H., CHAUSSADE F., ROUDBARAKI M., SLOMIANNY C., DEWAILLY E., DELCOURT P., PREVARSKAYA N. KV1.1 K(+) channels identification in human breast carcinoma cells: involvement in cell proliferation. Biochem. Biophys. Res. Commun. 2000;278:272–277. doi: 10.1006/bbrc.2000.3790. [DOI] [PubMed] [Google Scholar]
- OUADID-AHIDOUCH H., LE BOURHIS X., ROUDBARAKI M., TOILLON R.A., DELCOURT P., PREVARSKAYA N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether a-gogo K+ channel. Receptors Channels. 2001;7:345–356. [PubMed] [Google Scholar]
- ROGER S., BESSON P., LE GUENNEC J.Y. Involvement of a novel fast inward sodium current in the invasion capacity of a breast cancer cell line. Biochim. Biophys. Acta. 2003;1616:107–111. doi: 10.1016/j.bbamem.2003.07.001. [DOI] [PubMed] [Google Scholar]
- RYBALCHENKO V., PREVARSKAYA N., VAN COPPENOLLE F., LEGRAND G., LEMONNIER L., LE BOURHIS X., SKRYMA R. Verapamil inhibits proliferation of LNCaP human prostate cancer cells influencing K+ channel gating. Mol. Pharmacol. 2001;59:1376–1387. doi: 10.1124/mol.59.6.1376. [DOI] [PubMed] [Google Scholar]
- STROBL J.S., WONDERLIN W.F., FLYNN D.C. Mitogenic signal transduction in human breast cancer cells. Gen. Pharmacol. 1995;26:1643–1649. doi: 10.1016/0306-3623(95)00062-3. [DOI] [PubMed] [Google Scholar]
- TIMCHEVA C.V., TODOROV D.K. Does verapamil help overcome multidrug resistance in tumor cell lines and cancer patients. J. Chemother. 1996;8:295–299. doi: 10.1179/joc.1996.8.4.295. [DOI] [PubMed] [Google Scholar]
- WEGMAN E.A., YOUNG J.A., COOK D.I. A 23-pS Ca2(+)-activated K+ channel in MCF-7 human breast carcinoma cells: an apparent correlation of channel incidence with the rate of cell proliferation. Pflugers Arch. 1991;417:562–570. doi: 10.1007/BF00372952. [DOI] [PubMed] [Google Scholar]
- WONDERLIN W.F., WOODFORK K.A., STROBL J.S. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J. Cell. Physiol. 1995;165:177–185. doi: 10.1002/jcp.1041650121. [DOI] [PubMed] [Google Scholar]
- WOODFORK K.A., WONDERLIN W.F., PETERSON V.A., STROBL J.S. Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J. Cell. Physiol. 1995;162:163–171. doi: 10.1002/jcp.1041620202. [DOI] [PubMed] [Google Scholar]
- YAMASHITA N., HAMADA H., TSURUO T., OGATA E. Enhancement of voltage-gated Na+ channel current associated with multidrug resistance in human leukemia cells. Cancer Res. 1987;47:3736–3741. [PubMed] [Google Scholar]
- ZHANG S., SAWANOBORI T., HIRANO Y., HIRAOKA M. Multiple modulations of action potential duration by different calcium channel blocking agents in guinea pig ventricular myocytes. J. Cardiovasc. Pharmacol. 1997;30:489–496. doi: 10.1097/00005344-199710000-00013. [DOI] [PubMed] [Google Scholar]