Abstract
TRPM8 (CMR1) is a Ca2+-permeable channel, which can be activated by low temperatures, menthol, eucalyptol and icilin. It belongs to the transient receptor potential (TRP) family, and therefore is related to vanilloid receptor type-1 (VR1, TRPV1). We tested whether substances which are structurally related to menthol, or which produce a cooling sensation, could activate TRPM8, and compared the responses of TRPM8 and VR1 to these ligands.
The effects of 70 odorants and menthol-related substances on recombinant mouse TRPM8 (mTRPM8), expressed in HEK293 cells, were examined using a FLIPR® assay. In all, 10 substances (linalool, geraniol, hydroxycitronellal, WS-3, WS-23, FrescolatMGA, FrescolatML, PMD38, CoolactP and Cooling Agent 10) were found to be agonists.
The EC50 values of the agonists defined their relative potencies: icilin (0.2±0.1 μM)>FrescolatML (3.3±1.5 μM) > WS-3 (3.7±1.7 μM) >(−)menthol (4.1±1.3 μM) >frescolatMAG (4.8±1.1 μM) > cooling agent 10 (6±2.2 μM) >(+)menthol (14.4±1.3 μM) > PMD38 (31±1.1 μM) > WS-23 (44±7.3 μM) > Coolact P (66±20 μM) > geraniol (5.9±1.6 mM) > linalool (6.7±2.0 mM) > eucalyptol (7.7±2.0 mM) > hydroxycitronellal (19.6±2.2 mM).
Known VR1 antagonists (BCTC, thio-BCTC and capsazepine) were also able to block the response of TRPM8 to menthol (IC50: 0.8±1.0, 3.5±1.1 and 18±1.1 μM, respectively).
The Ca2+ response of hVR1-transfected HEK293 cells to the endogenous VR1 agonist N-arachidonoyl-dopamine was potentiated by low pH. In contrast, menthol- and icilin-activated TRPM8 currents were suppressed by low pH.
In conclusion, in the present study, we identified 10 new agonists and three antagonists of TRPM8. We found that, in contrast to VR1, TRPM8 is inhibited rather than potentiated by protons.
Keywords: TRPM8, CMR1, cold, menthol, VR1, odorants, proton activation, FLIPR, pain
Introduction
The recent cloning and characterization of the cold-menthol receptor (TRPM8; CMR1) (McKemy et al., 2002; Peier et al., 2002) was a major breakthrough in the study of thermosensation. TRPM8 is activated by menthol, eucalyptol and icilin, and by temperatures below ∼25°C. It belongs to the ‘long', or melastatin, subfamily of the transient receptor potential (TRP) family of ion channels (Montell et al., 2002), and shows pronounced outward rectification with a relatively high permeability for Ca2+ ions, and little selectivity between monovalent cations. The TRPM8 channel is expressed specifically in a subset of temperature-sensing trigeminal and dorsal root ganglion neurones (Peier et al., 2002; Reid et al., 2002a, 2002b; Nealen et al., 2003). Recently, a second cold receptor, ANKTM1, has been identified (Story et al., 2003), which, in contrast to TRPM8, is coexpressed with VR1 in a different subset of pain- and temperature-sensing trigeminal and dorsal root ganglion neurones. ANKTM1 is activated by icilin, but not menthol. These TRP channels play a major role in thermosensation (McKemy et al., 2002; Patapoutian et al., 2003).
Although treatment with menthol or eucalyptol, or with cold temperatures, is a traditional method of pain relief (Wright, 1870; Green & Mcanliffe, 2000; Davies et al., 2002; Galeotti et al., 2002; Shanghai Medicinal Herbs, Essential Balm), little is known about the underlying analgesic mechanisms. It has been demonstrated that menthol blocks Na+ and Ca2+ channels in dorsal root ganglion cells (Swandulla et al., 1987; Haeseler et al., 2002). Others have postulated that the analgesic activity of (–)menthol is mediated by selective activation of κ-opioid receptors (Galeotti et al., 2002).
The cold receptor TRPM8 is distantly related to the well-characterized heat-sensitive vanilloid receptor VR1 (or TRPV1). VR1 also belongs to the TRP channel family, but is activated by temperatures >42°C, or by ligands such as capsaicin and resiniferatoxin (RTX). Two endogenous VR1 agonists have been identified, anandamide (ANA) and N-arachidonoyl-dopamine (NADA) (Zygmunt et al., 1999; Di Marzo et al., 2001; Huang et al., 2002). Various VR1 antagonists have also been reported, for example, capsazepine, iodo-resiniferatoxin (I-RTX) and N-(4-tert.butyl-phenyl)-4-(3-chloropyridin-2-yl) tetrahydro-pyrazine-1(2H)-carboxamide (BCTC). These have analgesic effects in vivo (Bevan et al., 1992; Walpole et al., 1994; Catarina et al., 1997; 2000; Tominaga et al., 1998; Wahl et al., 2001; Pomonis et al., 2003; Rigoni et al., 2003).
Protons act as endogenous activators and modulators of VR1 responses. Low pH enhances the apparent VR1-binding affinity of capsaicin, and potentiates the channel gating of VR1 receptors (Caterina et al., 1997; 2000; Tominaga et al., 1998; Olah et al., 2001; Ryu et al., 2003). Since inflammation leads to acidification of the inflamed tissue, VR1 is thought to play a major role in the transduction of inflammatory pain.
As VR1 and TRPM8 are distantly related, and no antagonists have been described for TRPM8, we tested the effects of VR1 antagonists on TRPM8. Further, we investigated whether the responses of VR1 and TRPM8 towards agonists are influenced by pH.
Methods
Materials
Hank's balanced salt solution (HBSS), phosphate-buffered saline (PBS) and all cell culture reagents were obtained from Invitrogen (Karlsruhe, Germany). (−)Menthol, capsaicin, capsazepine, ruthenium red, eugenol, 4α-phorbol 12,13-didecanoate (4α-PDD) and probenecid were obtained from Sigma-Aldrich (Taufkirchen, Germany). (+)Menthol was purchased from Fluka-Sigma-Aldrich (Taufkirchen, Germany). Linalool, hydrocitronellal and citronellal were obtained from Henkel (Düsseldorf, Germany). WS-3 was obtained from Givaudan (Dubendorf, Switzerland). Icilin was purchased from Tocris (Ellisville, MO, U.S.A.). Frescolat ML and MGA were obtained from Haarmann & Reimer GmbH (Holzminden, Germany). WS-23 was obtained from Millennium Chemicals (Jacksonville, FL, U.S.A.). Cooling Agent 10, Coolact P and PMD38 were obtained from Takasago (Paris, France). BCTC and thio-BCTC (N-(4-tert.-butyl-phenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-(thio) carboxamide) were synthesized according to published methods, and tested as free bases (Pomonis et al., 2003).
Cloning and expression of mTRPM8 and hVR1 receptors in HEK293 cells
mTRPM8 cDNA (GenBank accession NM_134252) was a generous gift of Ardem Patapoutian, Scripps Institute, La Jolla, U.S.A. This was subcloned into the NheI and KpnI sites of the pcDNA5-Vector (Invitrogen, Karlsruhe, Germany), as described previously (Peier et al., 2002). The hVR1 cDNA (GenBank accession AJ272063) was cloned in pcDNA3.1 in a manner similar to that described previously (Hayes et al., 2000; Smart et al., 2000). HEK 293 cells were transiently transfected with mTRPM8 and hVR1 using Lipofectamine 2000 (Invitrogen, Karlsruhe, Germany) according to the manufacturer's instructions.
Cell culture
HEK293 cells were routinely grown as monolayers in minimum essential medium (MEM) supplemented with nonessential amino acids, 10% fetal calf serum and 0.2 mM L-glutamine, and maintained under 95% O2 / 5% CO2 at 37°C.
Measurement of [Ca2+]i using the FLIPR ® assay
mTRPM8- and hVR1-transfected HEK293 cells were seeded into black-walled clear-base poly-D-lysine-coated 96-well plates (Becton Dickinson, Meylan Cedex, France) at a density of 25,000 cells per well in MEM, supplemented as described above, and cultured overnight. The cells were then incubated with MEM containing the cytoplasmic calcium indicator Fluo-4AM (4 μM; Molecular Probes, Eugene, Oregon, U.S.A.) at 37°C for 30 min. The cells were washed twice with HBSS supplemented with 2.5 mM probenecid and 20 mM HEPES, resuspended in the same buffer, and incubated for 15 min at 37°C. Subsequently, the plates were inserted into a fluorometric imaging plate reader (FLIPR®; Molecular Devices, Sunnyvale, CA, U.S.A.), and the fluorescence (λex=488 nM, λem=510–570 nM) from [Ca2+]i was determined before and after the addition of various concentrations of test compounds (Sullivan et al., 1999; Jerman et al., 2000).
In experiments designed to define the influence of low pH on mTRPM8 and hVR1 currents in HEK293 cells, contributions from the endogenous hASICa (acid-activated channel) are conceivable. This channel is desensitized by short exposures to low pH (Gunthorpe et al., 2001). Consequently, transfected cells were incubated at pH 6.3 for at least 1 min prior to measurements.
Data analysis
EC50 values were determined as the concentration of test substance required to produce half-maximal increases in [Ca2+]i. Maximal [Ca2+]i responses were measured as peak fluorescence intensity (FI) minus basal FI, and expressed as percentages of the maximum response to icilin. Data are given as means±s.e.m., unless otherwise stated. Curve fitting and parameter estimations were performed with Microsoft Excel 97 and Graph Pad Prism 3.01 (GraphPad Software Inc., CA, U.S.A.).
Results
Identification of TRPM8 agonists
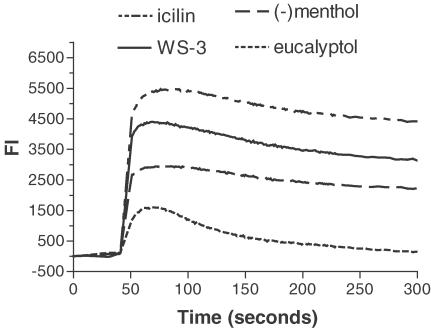
mTRPM8 cDNA was cloned into a mammalian expression vector as described in ‘Methods', and this was used to transfect HEK293 cells transiently with mTRPM8 for functional studies. Ca2+ fluorescence was measured using the FLIPR® assay. The known agonists (−)menthol, icilin and eucalyptol caused increases in [Ca2+]i in mTRPM8-transfected HEK293 cells (Figure 1).
Figure 1.
[Ca2+]i responses to TRPM8 agonists. [Ca2+]i fluxes induced by icilin (5 μM), WS-3 (30 μM), (−)menthol (30 μM) and eucalyptol (5 mM) were monitored using the FLIPR® assay in mTRPM8-transfected HEK293 cells. [Ca2+]i responses were measured as changes in fluorescence intensity (FI) before and after the addition of agonists. The data shown are representative plots of the fluorescence signals against time during assays.
Compounds from a library of odorants, or which were chemically related to menthol, were screened. In addition, the compound libraries of the fragrance industries were searched for compounds that produce cooling sensations. In all, 70 compounds were investigated at two concentrations (50 μM and 10 mM). Of these, 10 (linalool, geraniol, hydroxycitronellal, WS-3, WS-23, Frescolat MGA, Frescolat ML, PMD 38, Coolact P and Cooling Agent 10) produced increases in [Ca2+]i in mTRPM8-transfected HEK293 cells, and were studied in more detail.
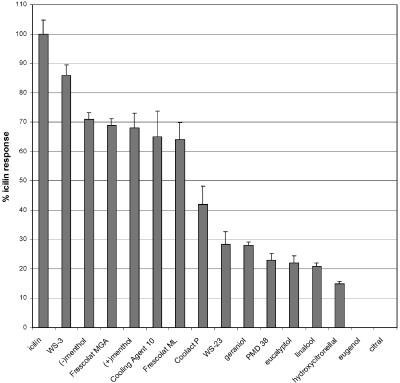
Various concentrations of these agonists were tested on mTRPM8-transfected, nontransfected and hVR1-transfected HEK293 cells. All of the identified TRPM8 agonists led to concentration-dependent increases in [Ca2+]i in mTRPM8-transfected HEK293 cells, but not in nontransfected or hVR1-transfected HEK293 cells (data not shown), proving that the compounds are specific agonists of mouse TRPM8. The efficacies and potencies of linalool, geraniol, hydroxycitronellal, WS-3, WS-23, Frescolat MGA, Frescolat ML, PMD 38, Coolact P and Cooling Agent 10 are shown in Figure 2 and in Table 1.
Figure 2.
Efficacy of TRPM8 agonists. [Ca2+]i responses were measured as maximal increases in fluorescence, expressed as percentages of the maximum icilin response. They are given as means±s.e.m. (n=4–8).
Table 1.
Chemical structures, efficacies and potencies of mTRPM8 antagonists and agonists
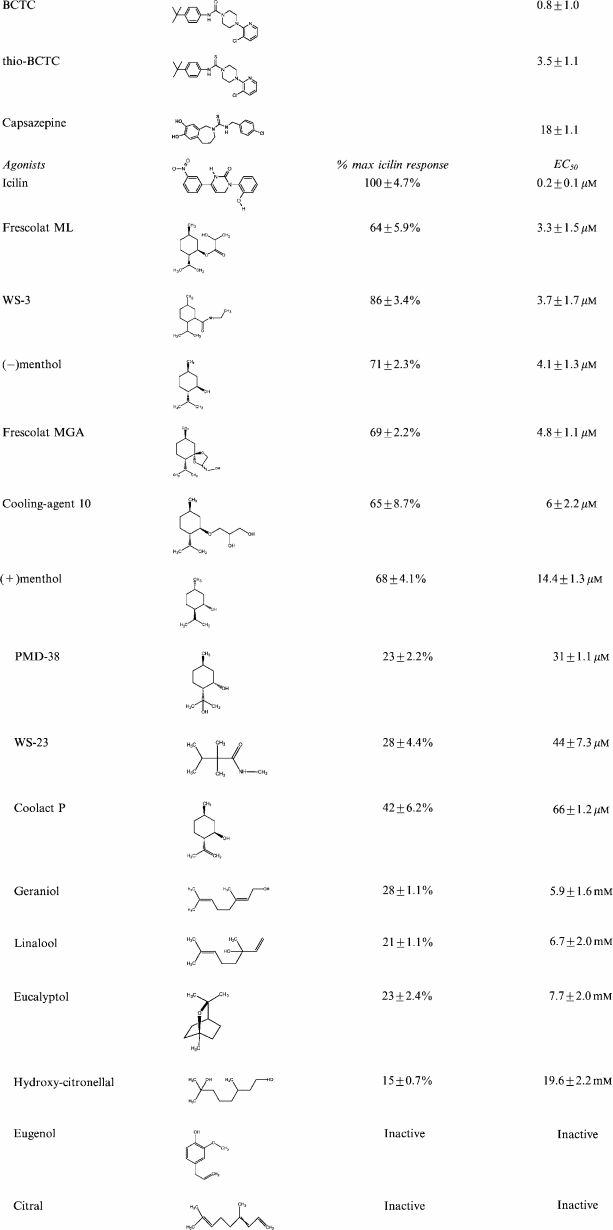 |
Linalool, geraniol, hydroxycitronellal, WS-3, WS-23, FrescolatMGA, FrescolatML, PMD38, CoolactP and Cooling Agent 10 were identified as novel partial TRPM8 agonists. The efficacies and potencies of mTRPM8 antagonists and agonists are given. Changes in [Ca2+]i were measured using the FLIPR® described in Methods (n=4–8).
Analysis of the [Ca2+]i response curves showed that application of the agonists led to one of two different types of Ca2+ influx kinetics in mTRPM8-transfected HEK293 cells (Figure 1). For the first group of agonists (icilin, menthol, WS-3, WS-23, Frescolat MGA, Frescolat ML, PMD 38, Coolact P and Cooling Agent 10), the [Ca2+]i response was typified by an initial very rapid onset (within ca. 1 s) and fast rate of increase. This reached a peak value after ca. 20–30 s, followed by a gradual slight decline over the course of the assay (Figure 1). The high [Ca2+]i level was maintained for at least 4 min in the continued presence of the agonists.
The second type of agonist effect was produced by the odorants linalool, geraniol, hydroxycitronellal and eucalyptol (Figure 1). These induced a slower initial increase in [Ca2+]i, which peaked at roughly the same time as the response to the first type of agonist, but declined much more rapidly. Indeed, the increase in [Ca2+]i above pre-test levels was negligible at the end of the assay period.
TRPM8 agonist potency and efficacy
The EC50 values of the agonists are listed in Table 1, ranked by potency. The order of potency, from the most to least potent was: icilin (0.2±0.1 μM) > frescolatML (3.3±1.5 μM) > WS-3 (3.7±1.7 μM) > (−)menthol (4.1±1.3 μM) > frescolatMAG (4.8±1.1 μM) > Cooling Agent 10 (6±2.2 μM) > (+)menthol (14.4±1.3 μM) > PMD38 (31±1.1 μM) > WS-23 (44±7.3 μM) > Coolact P (66±20 μM) > geraniol (5.9±1.6 mM) > linalool (6.7±2.0 mM) > eucalyptol (7.7±2.0 mM) > hydroxycitronellal (19.6±2.2 mM).
The efficacies of the TRPM8 agonists are shown in Figure 2 as percentages of the maximal response to the most potent agonist, icilin (Wei & Seid, 1983).
The rank order of efficacy for the agonists was: icilin > WS-3 > (−)menthol > FrescolatMAG > (+)menthol > Cooling Agent 10 > FrescolatML > CoolactP > WS-23 > geraniol > PMD38 > eucalyptol > linalool > hydroxycitronellal.
The efficacies of WS-3, both menthol isomers, Frescolat ML, Frescolat MGA and Cooling Agent 10 were slightly lower than that of icilin, but they were markedly less potent. Coolact P, PMD38 and WS-23 were rather weak agonists, with substantially reduced efficacies. The odorants linalool, geraniol and hydroxycitronellal were extremely weak agonists with rather low efficacies (Table 1, Figure 2).
Partial overlap of ligands for TRPM8 and VR1
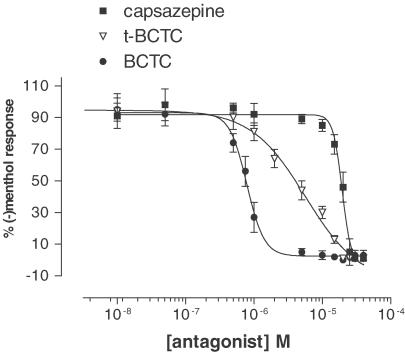
The heat receptor VR1 is distantly related to TRPM8, and is well characterized as a pain target (Caterina et al., 1997; 2000). For this reason, we investigated whether TRPM8 and VR1 have common pharmacological aspects. It was shown that the VR1 antagonists capsazepine, BCTC and thio-BCTC inhibited the [Ca2+]i response of mTRPM8 to 20 μM menthol in a concentration-dependent manner. IC50 values for this inhibition were 18±1.1 μM for capsazepine, 0.8±1.0 μM for BCTC and 3.5±1.1 μM for thio-BCTC, as shown in Figure 3.
Figure 3.
Antagonists of TRPM8. The VR1 antagonists (capsazepine, thio-BCTC and BCTC) inhibited the [Ca2+]i increases induced by 20 μM (−)menthol via mTRPM8 channels in a concentration-dependent manner. [Ca2+]i was monitored as described above. Responses were measured as peak increases in fluorescence, and expressed as percentages of the uninhibited response (mean±s.e.m., n=4).
Although these antagonists displayed the same potency ranking for both hVR1 and mTRPM8 (BCTC>thio-BCTC>capsazepine), their antagonistic potencies for mTRPM8 were much lower than for the hVR1 receptor (IC50 mTRPM8 vs hVR1: capsazepine, 18±1.1 vs 2.6±1.2 μM (Smart et al., 2001); thio-BCTC, 3.5±1.1 μM vs 54.3±21.8 nM (data not shown); BCTC 0.8±1.0 μM vs 34.9±19.4 nM (Valenzano et al., 2003)).
BCTC (10 μM) and thio-BCTC (10 μM) completely blocked the mTRPM8 response to 0.5 μM icilin, whereas capsazepine (30 μM) only blocked 40% of the response. Like Valenzano et al. (2003), we were not able to detect any quenching of Fluo-4 fluorescence by either BCTC or thio-BCTC. In contrast to their antagonism of icilin, neither BCTC nor thio-BCTC were able to block the [Ca2+]i increase induced by 4α-phorbol 12,13-didecanoate (4α-PDD) in hTRPV4-transfected HEK293 cells; neither did they inhibit the ATP-induced [Ca2+]i increase in CHO K1 cells (data not shown). This demonstrates that BCTC and thio-BCTC are selective antagonists for certain TRP channels.
Interestingly, the VR1 antagonist I-RTX had no influence on mTRPM8 currents (data not shown), and neither did the channel blocker ruthenium red (Peier et al., 2002) nor the VR1 agonists capsaicin and RTX (data not shown).
pH sensitivity of TRPM8 and VR1
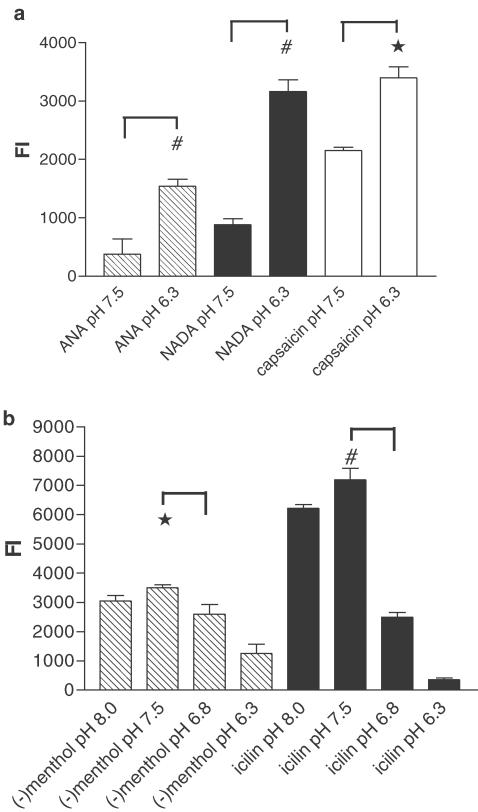
Protons act as endogenous activators or modulators of VR1 (Caterina et al., 1997; Ryu et al., 2003). When capsaicin (0.1 μM) was added to hVR1-transfected HEK293 cells at pH 6.3, a 1.6-fold increase in Ca2+ flux compared to that at pH 7.5 (Figure 4a) was observed. At pH 6.3, the VR1 response to the endogenous VR1 agonists ANA and NADA was even more markedly increased, by ca.3.5- to 4.0-fold (Figure 4a).
Figure 4.
pH sensitivity of hVR1 and mTRPM8. (a) [Ca2+]i was monitored in hVR1-transfected HEK293 cells before and after the addition of the agonists ANA (2 μM), NADA (2 μM), or capsaicin (0.1 μM). Responses were measured as increases in peak fluorescence intensity. Agonists were applied in buffers at either pH 7.5 or pH 6.3 (n=3). *P<0.01 and P<0.005, unpaired t-test. (b) [Ca2+]i was monitored in mTRPM8-transfected HEK293 cells before and after the addition of (−)menthol (20 μM) or icilin (0.5 μM). Agonist responses were measured as increases in peak fluorescence intensity. Agonists were applied at pH 8.0, pH 7.5, pH 6.8, or pH 6.3 (n=3). *P<0.05 and P<0.005, unpaired t-test.
In contrast, the Ca2+ influxes induced by menthol and icilin through mTRPM8 channels were almost completely inhibited at pH 6.3 (Figure 4b); Ca2+ influx was also reduced when the agonists were applied at pH 8.0 rather than at pH 7.5. Thus, hVR1 and mTRPM8 channels react oppositely to acidic conditions: VR1 is potentiated, and TRPM8 is inhibited.
Discussion and conclusion
TRPM8 agonist potency and efficacy
The TRPM8 receptor is a transducer of cold stimuli in the somatosensory system (McKemy et al., 2002; Peier et al., 2002). However, due to the lack of specific and water-soluble ligands, only a limited pharmacological characterization has been possible to date. After screening a small library of 70 odorants, and other substances related to menthol (Eccles, 1994), we were able to identify 10 novel TRPM8 agonists. Consistent with the reported molecular pharmacology of TRPM8 (McKemy et al., 2002; Peier et al., 2002), menthol, eucalyptol and icilin increased [Ca2+]i in mTRPM8-transfected HEK293 cells in the present study (Figure 1; Table 1). McKemy et al. did not specify the optical purity of the menthol they used; in our studies, the 1R,3R,4S form of (−)menthol was ca. 3.5-fold more effective than that of (+)menthol (Table 1), indicating that there is a slight preference for the (−) enantiomeric form.
Some of the identified TRPM8 agonists, namely WS-3, Coolact P, Cooling Agent 10, and PMD38, are used as cooling agents in the food and cosmetics industry. This cooling sensation may in part be mediated by TRPM8. Manufacturers report the cooling strength of WS-3 to be ca. five-fold, Coolact P ca. four-fold, Cooling Agent 10 ca. 4.5-fold and PMD38 ca. 9.5-fold greater than that of (−)menthol. These estimates do not correlate with our results (Table 1). As an agonist for mTRPM8, WS-3 displayed a slightly higher efficacy and a similar potency to (−)menthol, but Coolact P and PMD38 displayed equal or lower efficacies and potencies. It is not unusual for in vitro and in vivo data to be discrepant. Probably, additional factors such as membrane permeability, metabolism, chemical stability, solubility, subjectivity and volatility of the tested compounds have also to be taken into account (Watson et al., 1978). There may also be differences between species in the ligand specificity of receptors (here, mouse vs human). Conceivably, these substances also activate other cold-transducing receptors such as ANKTM1, which is also activated by icilin (Story et al., 2003). Future studies will address the selectivity of the identified novel TRPM8 agonists.
Besides menthol and eucalyptol, we were able to identify three novel natural odorants (linalool, geraniol and hydroxycitronellal) that activate the mTRPM8 receptor. These natural odorants are found in formulations used in aroma therapies, for example, against headaches (Shanghai Medicinal Herbs, Essential Balm).
It is worth noting that an analgesic effect was reported recently for the novel TRPM8 ligand linalool, and also for menthol (Peana et al., 2003). Linalool is a fresh, pungent and flowery odorant found in plants such as Convallaria majalis (lily of the valley) and Zingiber officinale (ginger). We also found agonistic effects on mTRPM8 for geraniol, the main odorant component of roses, and hydroxycitronellal, a fresh citrus odorant.
The weak potency and efficacy of these odorants in our in vitro assays could be partially explained by their hydrophobicity and poor aqueous solubility. The observed concentration dependance of the responses to geraniol, linalool, eucalyptol and hydrocitronellal (Table 1) was in the same range as that reported for eucalyptol with TRPM8 (EC50: 3.4±0.4 mM; McKemy et al., 2002).
The evidence thus shows that menthol, eucalyptol, icilin and all of the newly identified agonists can produce cooling sensations, which may, at least in part, be explained by the activation of the TRPM8 cold receptor. Additionally, some of the compounds described here as agonists for TRPM8 have analgesic effects in vivo, suggesting a role for TRPM8 in pain relief.
Partial overlap of ligands for TRPM8 and VR1
The VR1 antagonists capsazepine, BCTC and thio-BCTC, though not I-RTX, inhibited the Ca2+ influx induced through TRPM8 by 20 μM menthol in a concentration-dependent manner. The channel blocker ruthenium red, and the VR1 agonists capsaicin and RTX, had no effect on TRPM8. Capsaicin, capsazepine, BCTC, RTX and I-RTX are supposed to share the same binding pocket at transmembrane domains (TM) 2–3 of the VR1 receptor (Jordt & Julius, 2002; Valenzano et al., 2003). At the predicted capsaicin-binding region of VR1 (TM2 and TM3, Jordt & Julius, 2002), the amino-acid sequence identity of VR1 and TRPM8 was 36%, compared to an overall amino-acid sequence identity of 21%. Future studies will have to ascertain whether capsazepine, BCTC and thio-BCTC interact at a TRPM8 site corresponding to the capsaicin-binding site of VR1.
We have shown here for the first time that capsazepine is an antagonist of recombinant mTRPM8. Interestingly, Reid et al. (2002a) demonstrated that capsazepine is able to block native cold- and menthol-induced Ca2+ currents in rat dorsal root ganglion. This observation might be explained by the fact that capsazepine inhibits TRPM8. Our results extend the range of receptors, such as voltage-gated Ca2+-channels (Docherty et al., 1997) and nicotinic acetylcholine receptors (Liu & Simon, 1997; Wardle et al., 1997), that are known to interact with capsazepine.
BCTC has until now been regarded as a highly specific VR1 antagonist, since no interactions with 60 other receptors were observed in a study by Valenzano et al. (2003). However, our results indicate that BCTC also antagonizes TRPM8 at submicromolar concentrations, which has implications for its use as a specific VR1 antagonist in vivo. BCTC seemed to be a more specific VR1 antagonist than capsazepine, as no interactions with TRP channels other than VR1 have been published to date. Our results show that BCTC acts as an antagonist for TRPM8, which may indicate that BCTC could be an inhibitor for other related TRP channels.
Looking at the chemical structures and potential pharmacophore, the similarities and differences between BCTC and icilin are obvious. Both molecules have two aromatic rings and a urea moiety in common. The distances between these pharmacophore elements are clearly different, which may partly explain why icilin acts as an agonist and BCTC as an antagonist. This has to be confirmed with studies using structural analogues of BCTC and icilin.
pH sensitivity of TRPM8 and VR1
The activation of VR1 by capsaicin was potentiated by low pH in this study. Caterina et al. (1997) reported that capsaicin-induced currents were ca. five-fold greater at pH 6.3 than at pH 7.6 in a Xenopus oocyte expression system. However, we only observed a 1.6-fold increase in Ca2+ flux compared to that at pH 7.5 (Figure 4a).
The effect of ANA, an endogenous CB1 and VR1 agonist (Di Marzo et al., 2001), was also strongly potentiated by low pH in this study (Figure 4a). Olah et al. (2001) also reported that acidification potentiates the activity of ANA, whereas others observed no potentiation (Smart et al., 2000; for review Ralevic et al., 2002). The difference may be due to methodological discrepancies. We also observed that another endogenous CB1 and VR1 agonist, NADA (Bisogno et al., 2000), was even more strongly potentiated by acid pH than ANA (Figure 4a).
In contrast to VR1, the TRPM8-mediated Ca2+ response to menthol and icilin was inhibited by low pH (Figure 4b). Thus, TRPM8 and VR1 are oppositely modulated by low pH. Under inflammatory conditions, when acidification of inflamed tissue occurs, both mechanisms may play a role in the development of hyperalgesia. The reduced pH could sensitize VR1 and thereby make the tissue more susceptible to pain stimuli, and increasing heat sensations; the same acidic conditions would inhibit TRPM8, and reduce ‘pleasant cool' sensations. Thus, VR1 and TRPM8 may act in concert under inflammatory conditions, and cause an aggravation of thermal hyperalgesia.
In conclusion, we have identified 10 novel TRPM8 agonists. The identification of three natural odorants that activate TRPM8, together with the fact that TRPM8 is expressed in the trigeminus, which belongs to the sensory system of the olfactory epithelium, suggests that TRPM8 could be an important ‘Chemosensory Trigeminal Nerve Receptor'. Agonistic TRPM8 responses to (−)menthol and icilin were inhibited dose-dependently by three well-known VR1 antagonists (capsazepine, thio-BCTC and BCTC). This suggests a partial overlap between the ligand specificities of TRPM8 and VR1, whereas the VR1 response to endogenous agonists was strongly potentiated by low pH, the TRPM8 response was inhibited.
Acknowledgments
We would like to thank Elke Janocha, Tanja Waldmann, Thomas Krüger and Ingrid Wetzels for technical support. Professor Dr Wolfgang Strassburger supported us with an expert structural comparison of BCTC and icilin. We also thank Dr Derek Saunders, Dr Gregor Bahrenberg and Dr Erik Wade for valuable contributions and editing. This work was supported by the Bundesministerium für Bildung und Forschung (01 GG 9818/0). We would also like to thank Givaudan, Haarmann & Reimer, Takasago and Millennium Chemicals for substance samples.
Abbreviations
- ANA
anandamide
- BCTC
N-(4-tert.butyl-phenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carboxamide
- [Ca2+]i
intracellular calcium concentration
- cDNA
complementary DNA
- CMR1
cold-menthol receptor 1
- FLIPR®
fluorometric imaging plate reader
- HEK293
human embryonic kidney cells
- NADA
N-arachidonoyl-dopamine
- thio-BCTC
N-(4-tert.butyl-phenyl)-4-(3-chloropyridin-2-yl) tetrahydro-pyrazine-1(2H)-(thio) carboxamide
- TRPM8
transient receptor potential melastatin subfamily channel 8
- VR1
vanilloid receptor type-1
References
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISOGNO T., MELCK D., BOBROV M.YU., GRETSKAYA N.M., BEZUGLOV V.V., DE PETROCELLIS L., DI MARZO V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DAVIES J.S., HARDING L.M., BARANOWSKI A.P. A novel treatment of postherpetic neuralgia using peppermint oil. Clin. J. Pain. 2002;8:200–202. doi: 10.1097/00002508-200205000-00011. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L. Anandamide: some like it hot. Trends Pharmacol. Sci. 2001;227:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. Menthol and related cooling compounds. J. Pharm. Pharmacol. 1994;46:618–630. doi: 10.1111/j.2042-7158.1994.tb03871.x. [DOI] [PubMed] [Google Scholar]
- GALEOTTI N., DI CESARE MANNELLI L., MAZZANTI G., BARTOLINI A., GHELARDINI C. Menthol: a natural analgesic compound. Neurosci. Lett. 2002;322:145–148. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- GARCIA-MARTINEZ C., MORENILLA-PALAO C., PLANELLS-CASES R., MERINO J.M., FERRER-MONTIEL A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J. Biol. Chem. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- GREEN B.G., MCAULIFFE B.L. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol. Behav. 2000;68:631–639. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., SMITH G.D., DAVIS J.B., RANDALL A.D. Characterisation of a human acid-sensing ion channel (hASIC1a) endogenously expressed in HEK293 cells. Pflugers Arch. 2001;442:668–674. doi: 10.1007/s004240100584. [DOI] [PubMed] [Google Scholar]
- HAESELER G., MAUE D., GROSSKREUTZ J., BUFLER J., NENTWIG B., PIEPENBROCK S., DENGLER R., LEUWER M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2002;19:571–579. doi: 10.1017/s0265021502000923. [DOI] [PubMed] [Google Scholar]
- HAYES P., MEADOWS H.J., GUNTHORPE M.J., HARRIES M.H., DUCKWORTH D.M., CAIRNS W., HARRISON D.C., CLARKE C.E., ELLINGTON K., PRINJHA R.K., BARTON A.J., MEDHURST A.D., SMITH G.D., TOPP S., MURDOCK P., SANGER G.J., TERRETT J., JENKINS O., BENHAM C.D., RANDALL A.D., GLOGER I.S., DAVIS J.B. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERMAN J.C., BROUGH S.J., PRINJHA R., HARRIES M.H., DAVIS J.B., SMART D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br. J. Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci. Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- MCKEMY D.D., NEUHAUSSER W.M., JULIUS D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V., BINDELS R.J., BRUFORD E.A., CATERINA M.J., CLAPHAM D.E., HARTENECK C., HELLER S., JULIUS D., KOJIMA I., MORI Y., PENNER R., PRAWITT D., SCHARENBERG A.M., SCHULTZ G., SHIMIZU N., ZHU M.X. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- NEALEN M.L., GOLD M.S., THUT P.D., CATERINA M.J. TRPM8 mRNA is expressed in a subset of cold-responsive trigeminal neurons from rat. J. Neurophysiol. 2003;90:515–520. doi: 10.1152/jn.00843.2002. [DOI] [PubMed] [Google Scholar]
- OLAH Z., KARAI L., IADAROLA M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- PATAPOUTIAN A., PEIER A.M., STORY G.M., VISWANATH V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat. Rev. Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- PEANA A.T., D'AQUILA P.S., CHESSA M.L., MORETTI M.D., SERRA G., PIPPIA P. (−)-Linalool produces antinociception in two experimental models of pain. Eur. J. Pharmacol. 2003;460:37–41. doi: 10.1016/s0014-2999(02)02856-x. [DOI] [PubMed] [Google Scholar]
- PEIER A.M., MOQRICH A., HERGARDEN A.C., REEVE A.J., ANDERSSON D.A., STORY G.M., EARLEY T.J., DRAGONI I., MCINTYRE P., BEVAN S., PATAPOUTIAN A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- POMONIS J.D., HARRISON J.E., MARK L., WALIGORA D.T., BRISTOL D.R., VALENZANO K.J., WALKER K. BCTC (N-(4-tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl) tetrahydropryazine-1(2H)-carbox-amide), a novel, orally-effective vanilloid receptor 1 antagonist with analgesic properties: II. In vivo characterization in rat Models of Inflammatory and Neuropathic Pain. J. Pharmacol. Ex. Ther. 2003;306:387–393. doi: 10.1124/jpet.102.046268. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., SMART D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- REID G., BABES A., PLUTEANU F. A cold- and menthol-activated current in rat dorsal root ganglion neurones: properties and role in cold transduction. J. Physiol. 2002a;545:595–614. doi: 10.1113/jphysiol.2002.024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REID G., FLONTA M.L. Ion channels activated by cold and menthol in cultured rat dorsal root ganglion neurones. Neurosci. Lett. 2002b;324:164–168. doi: 10.1016/s0304-3940(02)00181-7. [DOI] [PubMed] [Google Scholar]
- RIGONI M., TREVISANI M., GAZZIERI D., NADALETTO R., TOGNETTO M., CREMINON C., DAVIS J.B., CAMPI B., AMADESI S., GEPPETTI P., HARRISON S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br. J. Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYU S., LIU B., QIN F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J. Gen. Physiol. 2003;122:45–61. doi: 10.1085/jgp.200308847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., GUNTHORPE M.J., BROUGH S.J., RANSON J., CAIRNS W., HAYES P.D., RANDALL A.D., DAVIS J.B. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur. J. Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- STORY G.M., PEIER A.M., REEVE A.J., EID S.R., MOSBACHER J., HRICIK T.R., EARLEY T.J., HERGARDEN A.C., ANDERSSON D.A., HWANG S.W., MCINTYRE P., JEGLA T., BEVAN S., PATAPOUTIAN A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- SULLIVAN E., TUCKER E.M., DALE I.L. Measurement of [Ca2+] using the fluorometric imaging plate reader (FLIPR) Methods Mol. Biol. 1999;114:125–133. doi: 10.1385/1-59259-250-3:125. [DOI] [PubMed] [Google Scholar]
- SWANDULLA D., CARBONE E., SCHAFER K., LUX H.D. Effect of menthol on two types of Ca currents in cultured sensory neurons of vertebrates. Pflugers Arch. 1987;409:52–59. doi: 10.1007/BF00584749. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- VALENZANO K.J., GRANT E., HACHICHA M., HODGES D., LIMBERIS J., MALIK S., ROTSHTEYN Y., SCHMID L., SUN Q., TAFESSE L., WHITTEMORE E. BCTC (N-(4-tertiarybutylphenyl)-4-(3–chloropyridin-2-yl)tetrahydro-pyrazine–1(2H)-carbox–amide), a novel, orally- effective vanilloid receptor 1 antagonist with analgesic properties: I. In vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- WAHL P., FOGED C., TULLIN S., THOMSEN C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- WALPOLE C.S., BEVAN S., BOVERMANN G., BOELSTERLI J.J., BRECKENRIDGE R., DAVIES J.W., HUGHES G.A., JAMES I., OBERER L., WINTER J. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 1994;24:1942–1954. doi: 10.1021/jm00039a006. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J., SANGER G.J. Pharmacological characterization of the vanilloid receptor in the rat dorsal spinal cord. Br. J. Pharmacol. 1997;121:1012–1016. doi: 10.1038/sj.bjp.0701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON H.R., HEMS R., ROWSELL D.G., SPRING D.J. New compounds with the menthol cooling effect. J. Soc. Cosmet. Chem. 1978;29:185–200. [Google Scholar]
- WEI E.T. Pharmacological aspects of shaking behavior produced by TRH, AG-3-5, and morphine withdrawal. Fed. Proc. 1981;40:1491–1496. [PubMed] [Google Scholar]
- WEI E.T., SEID D.A.J. AG-3–5: a chemical producing sensations of cold. Pharm. Pharmacol. 1983;35:110–112. doi: 10.1111/j.2042-7158.1983.tb04279.x. [DOI] [PubMed] [Google Scholar]
- WRIGHT A. Oil of peppermint as a local anaesthetic. Lancet. 1870;2464:726. [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]