Abstract
Histamine is generally regarded as a pro-inflammatory mediator in diseases such as allergy and asthma. A growing number of studies, however, suggest that this autacoid is also involved in the downregulation of human polymorphonuclear leukocyte (PMN) functions and inflammatory responses through activation of the Gs-coupled histamine H2 receptor.
We report here that histamine inhibits thapsigargin- and ligand (PAF and fMLP)-induced leukotriene (LT) biosynthesis in human PMN in a dose-dependent manner.
The suppressive effect of histamine on LT biosynthesis was abrogated by the histamine H2 receptor antagonists cimetidine, ranitidine, and tiotidine. In contrast, the histamine H1, H3, and H4 receptor antagonists used in this study were ineffective in counteracting the inhibitory effect of histamine on the biosynthesis of LT in activated human PMN.
The inhibition of LT biosynthesis by histamine was characterized by decreased arachidonic acid release and 5-lipoxygenase translocation to the nuclear membrane.
Incubation of PMN with the cAMP-dependent protein kinase (PKA) inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide prevented the inhibitory effect of histamine on LT biosynthesis, suggesting an important role for PKA in this effect of histamine on LT biosynthesis in PMN.
These data provide the first evidences that, similarly to adenosine and prostaglandin E2, histamine is a potent suppressor of LT biosynthesis, and support the concept that histamine may play a dual role in the regulation of inflammation.
Keywords: 5-Lipoxygenase, arachidonic acid, autacoid, cAMP, histamine, leukocyte, leukotriene, lipid mediator, PKA
Introduction
Leukotrienes (LTs) are lipid mediators of inflammation derived from the oxygenation of arachidonic acid (AA) by the 5-lipoxygenase (5-LO). LTB4 is a potent chemoattractant and activates polymorphonuclear leukocytes (PMN) (Ford-Hutchinson et al., 1980; Palmblad et al., 1981; Dewald & Baggiolini, 1985). Moreover, de novo LTB4 biosynthesis has been shown to play a role in the rabbit blood PMN transmigration elicited by a wide variety of pro-inflammatory mediators (Marleau et al., 1999). The cysteinyl-LT (LTC4, D4 and E4) induce bronchoconstriction (Dahlén et al., 1980), increase vascular permeability (Dahlén et al., 1981), and smooth muscle cell proliferation (Cohen et al., 1995; Rajah et al., 1996). In human PMN, the biosynthesis of LTB4 is initiated by a rise of intracellular calcium concentration ([Ca2+]i), followed by the translocations of the type IVA cytosolic phospholipase A2 (cPLA2) and 5-LO to the nuclear envelope (Woods et al., 1993; Pouliot et al., 1996), where LT biosynthesis likely occurs.
cAMP-elevating agents have been reported to be potent inhibitors of 5-LO product generation. Adenosine and the A2A receptor agonist CGS-21680 have indeed been shown to inhibit LTB4 generation in human whole blood (Krump et al., 1996) and in human PMN (Krump et al., 1997; Krump & Borgeat, 1999). The inhibition of LT biosynthesis in activated PMN by other cAMP-elevating, namely prostaglandin (PG) E2, the β-adrenergic agonist isoproterenol, and type IV phosphodiesterase, inhibitors has also been reported (Ham et al., 1983; Schudt et al., 1991; Fonteh et al., 1993; Dennis & Riendeau, 1999; Flamand et al., 2000). Although the cAMP-mediated inhibition of LT biosynthesis in human PMN clearly implicates the inhibition of AA release (Fonteh et al., 1993; Flamand et al., 2000) and 5-LO translocation (Flamand et al., 2002), the molecular mechanisms involved in these events remain poorly understood.
Histamine is a well-known pro-inflammatory autacoid associated with diseases such as allergy and asthma. The biological effects of histamine implicate different receptor subtypes; pro-inflammatory actions of histamine including vasodilatation and increased vascular permeability are mainly mediated through H1 receptor (H1R) occupancy. The symptom attenuation of allergic diseases is indeed achieved using H1R antagonists. Other studies have shown that histamines participate in the regulation of leukocyte adhesion to the endothelial wall through stimulation of adhesion molecule expression by endothelial cells in an H1R-dependent manner (Miki et al., 1996; Saito et al., 1996; Burns et al., 1999). Interestingly, a growing number of studies also support an opposite role of this autacoid in the downregulation of PMN functional responses. Indeed, histamine inhibits lysosomial enzyme release, respiratory burst, adhesion, chemotaxis, and calcium influx in agonist-stimulated human PMN (Busse & Sosman, 1976; Busse et al., 1980; Radermecker & Maldague, 1981; Seligmann et al., 1983; Burde et al., 1989; Zimmerman & Millard, 1989; Francis et al., 1991; Hirasawa et al., 1991; Bury & Radermecker, 1992; Bury et al., 1992; Leino et al., 1993). All of these inhibitory effects of histamine on human PMN are the consequence of H2R activation, which causes the elevation of intracellular cAMP concentrations ([cAMP]i) (Gespach & Abita, 1982).
Since PMN possesses the H2R, we investigated the putative impact of histamine on the ability of PMN to generate LTB4. We report here that stimulation of the H2R inhibits LTB4 biosynthesis by activated human PMN in vitro. The observed inhibition of LT biosynthesis by histamine was only mimicked by the specific H2R agonist amthamine, and prevented by the H2R antagonists cimetidine, ranitidine, and tiotidine, but not by H1R, H3R, or H4R antagonists. The inhibition of LT biosynthesis by histamine correlated with decreased AA release, and 5-LO translocation to the nuclear membranes. Moreover, the specific cAMP-dependent protein kinase (PKA) inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide (H-89) efficiently prevented the inhibitory effect of histamine on LTB4 biosynthesis.
Methods
Materials
19-OH-PGB1, 19-OH-PGB2, A23187, adenosine deaminase (ADA), cytochalasin B, diamine oxydase, dimethyl sulfoxide (DMSO), fMLP, histamine, β-mercaptoethanol, nonidet P-40 (NP-40), PAF, PGB1, PGB2, PMSF, and Triton X-100 were obtained from Sigma Chemical (St Louis, MI, U.S.A.). Amthamine dihydrobromide, imetit dihydrobromide, thioperamide maleate, clobenpropit dihydrobromide, histamine trifluoromethyl toluidide (HTMT), tiotidine, mepyramine maleate, and trans-tripolidine HCl were puchased from Tocris Cookson Inc. (Ballwin, MO, U.S.A.). H-89 and KT-5720 were obtained from Biomol Research Laboratories (Plymouth Meeting, PA, U.S.A.). Fura-2-AM was obtained from Molecular Probes (Eugene, OR, U.S.A.). The cPLA2 antiserum MF-142 was kindly provided by Dr Denis Riendeau (Merck Frosst, Kirkland, Québec, Canada). Monoclonal 5-LO antibody was purchased from Research Diagnostics (Flanders, NJ, U.S.A.). The ECL Detection Kit was purchased from Perkin-Elmer Life Sciences (Boston, MA, U.S.A.). Hank's Balanced Salt Solution (HBSS) and Hepes, Ficoll–Paque, and Trypan blue were purchased from Wisent Laboratories (St-Bruno, Québec, Canada). Thapsigargin, cimetidine, dimaprit, and ranitidine-HCl were from RBI (Natick, MA, U.S.A.).
Isolation of human neutrophils
Venous blood from healthy volunteers was collected in heparinized tubes and PMN were isolated as previously described (Boyum, 1968). Briefly, after discarding the platelet-rich plasma, erythocytes were removed by dextran sedimentation. Mononuclear cells were then separated from the granulocytes by centrifugation on Ficoll–Paque cushions, and a hypotonic lysis was performed on the granulocyte cell pellet to remove the remaining erythrocytes. The granulocyte suspension contained mainly PMN (⩾95%) with eosinophils as the major contaminant, and cell viability was always greater than 98%, as measured by trypan blue exclusion. PMN were finally re-suspended in HBSS containing 1.6 mM CaCl2 at 5 or 10 × 106 cells ml−1, as indicated.
Cell stimulations
In experiments with human blood, freshly drawn heparinized blood samples were pre-incubated (30 min, 37°C) in the presence of 6 nM TNF-α and 1 μg ml−1 LPS, then stimulated with 1 μM fMLP for 20 min. In experiments with isolated PMN, pre-warmed cells (5 × 106 ml−1, 37°C) were primed with 700 pM GM-CSF, 1.2 nM TNF-α, and 10 μM cytochalasin B for 30 min. Cells were then stimulated with 300 nM of either PAF or fMLP for 5 min in the presence of the priming agents. In experiments where thapsigargin was used as the stimulus, PMN were not previously exposed to priming agents and unprimed pre-warmed PMN (5 × 106 ml−1, 37°C) were stimulated with 100 nM thapsigargin for 10 min. In order to eliminate the inhibitory constraint of endogenous adenosine on LT biosynthesis in PMN suspensions, 0.1 U ml−1 ADA was added 10 min before cell stimulation in all experimental settings, as described previously (Krump et al., 1997). Histamine and amthamine were added 5 min before stimulation of PMN with agonists; PKA inhibitors and histamine receptor antagonists were added 20 and 10 min, respectively, before stimulation. In the experiments where chelation of extracellular Ca2+ was performed, EGTA was added to a final concentration of 2 mM, simultaneously with thapsigargin, PAF, or fMLP.
5-LO product and AA analysis
For the determination of 5-LO products, cell incubations were terminated by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH : MeCN, 1 : 1 (v : v)) containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards. The denatured cell suspensions were centrifuged (600 × g, 10 min) to eliminate the precipitated material, and the supernatants were collected and analyzed by reversed phase (RP)-HPLC using an online extraction procedure, as described previously (Borgeat et al., 1990). The sum of LTB4, its ω-oxidation products 20-OH- and 20-COOH-LTB4, LTB4 isomers 6-(E)- and 6-(E)-12-epi-LTB4, and 5-hydroxyeicosatetraenoic acid (5-HETE) was compiled, and are referred to as 5-LO products. In experiments with human whole human blood, incubations were stopped by placing the samples in an ice-water bath. The plasma obtained by centrifugation (300 × g, 20 min) was denatured with 10 volumes of a cold stop solution (4°C, MeOH : MeCN, 1 : 1 (v : v)) containing 12.5 ng of both 19-OH-PGB1 and PGB1 as internal standards. The denatured samples were centrifuged (600 × g, 20 min) and the supernatants were then evaporated (in a water bath at 22°C) to a volume of ∼1 ml using a stream of nitrogen, and analyzed by RP-HPLC, as described previously (Surette et al., 1993). For the analysis of AA release, PMN incubations were stopped by the addition of 0.5 volume of a cold (4°C) stop solution (MeOH : MeCN, 1 : 1 (v : v)) containing 12.5 ng of both 19-OH-PGB2 and PGB2, and 20 ng of 2H8-AA. The denatured incubation media were centrifuged and the supernatants were analyzed by RP-HPLC. The AA-containing fraction was collected, evaporated to dryness under reduced pressure using a Speed-Vac evaporator and re-dissolved in 50 μl MeCN for analysis by LC-MS using electrospray ionization in the negative mode, as described previously (Borgeat et al., 1998).
Analysis of cPLA2 and 5-LO in nuclear membrane fractions
For the preparation of the nuclear membrane fractions, PMN suspensions were incubated under the conditions described previously, but at the concentration of 107 cells ml−1. Incubations were stopped with 1 volume of cold (4°C) incubation media, and cell suspensions were then quickly centrifuged (600 × g, 90 s). PMN pellets (2 × 107 cells) were suspended in 500 μl of cold (4°C) sonication buffer (250 mM sucrose, 1 mM EGTA, 10 mM HEPES, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, 2 mM DFP, and 1 mM PMSF). Cell disruption was performed at 4°C using a Branson sonifier #450 (20 s at a power setting of 1.5 and 100% duty cycle). Sonicates were centrifuged at 12,000 × g for 15 min and the supernatants were centrifuged at 180,000 × g for 45 min. The supernatants (referred to as the cytosolic fractions) and pellets (referred to as membranes) were immediately solubilized in an electrophoresis sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 100 mM dithiothreitol, 10% glycerol, 0.01% bromophenol blue, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin, and 1 mM PMSF) and boiled for 10 min. Samples were analyzed by SDS–PAGE (Laemmli, 1970) using 10% polyacrylamide gels. Proteins were then transferred at 0.5 A for 3 h at 4°C onto Immobilon-P PVDF membranes. Transfer efficiency as well as equal loading was visualized by Ponceau red staining. For the determination of cPLA2 and 5-LO, the PVDF membranes were soaked for 30 min at 25°C in Tris-buffered saline (25 mM Tris-HCl, pH 7.6, 200 mM NaCl, 0.15% Tween 20) containing 5% dried milk (w : v), blotted with the 5-LO and the cPLA2 antisera, and revealed using a horseradish peroxidase-coupled monoclonal antibody and the ECL detection kit.
Measurement of [Ca2+]i
PMN (107 cells ml−1) were incubated for 30 min at 37°C with 1 μM Fura-2-AM in HBSS containing 1.6 mM CaCl2. Cells were then washed twice, re-suspended at 107 cells ml−1, transferred into the thermally controlled (37°C) and magnetically stirred cuvette of the spectrofluorometer (Aminco-Bowman series 2, SLM-Aminco, Urbana, IL, U.S.A.). Histamine (10 μM) was added 5 min before stimulation of PMN with 100 nM PAF. In the experiments where the PKA inhibitor H-89 was used, PMN were incubated with the latter for 10 min before the addition of histamine. PAF was always added 10 s after the beginning of data acquisition. In the experiments where chelation of extracellular Ca2+ was performed, EGTA (final concentration of 2 mM) was added simultaneously with PAF. Fluorescence was monitored at excitation and emission wavelengths of 340 and 510 nm, respectively. Raw data were transformed using the following formula: 224((y−Fmin)(Fmax−y)−1). Fmax was obtained by disrupting the cells with 1% Triton X-100 and Fmin was obtained by adding 5 mM of both EGTA and NaOH. In experiments where chelation of extracellular Ca2+ was performed with 2 mM EGTA, the average Fmax and Fmin of the tests preformed without EGTA were used, in order to transform the raw data.
Results
Inhibition of LTB4 biosynthesis by histamine
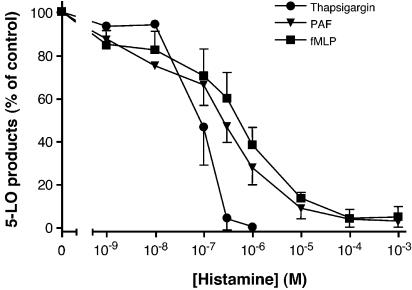
Given the previous observations that autacoids such as adenosine and PGE2 are potent activators of adenylate cyclase and inhibit LT biosynthesis in human PMN, and since the activation of H2R also rises [cAMP]i in PMN (Gespach & Abita, 1982), a first series of experiments was performed to evaluate whether histamine inhibits LT biosynthesis in activated human PMN. As shown in Figure 1, incubation of GM-CSF/TNF-α/cytochalasin B-treated human PMN with increasing concentrations of histamine progressively leads to the inhibition of LT biosynthesis induced by PAF and fMLP with an IC50 of ∼300 nM, and 90% inhibition at 10 μM histamine. The fMLP-induced LT biosynthesis in LPS/TNF-α-treated human whole blood was also investigated. The observed inhibition of LT biosynthesis by histamine in whole blood was similar to that observed in fMLP-stimulated PMN (data not shown). Interestingly, the biosynthesis of LT in unprimed, thapsigargin-activated PMN was more sensitive to the inhibitory effect of histamine with an IC50 of ∼30 nM, and an almost complete inhibition of LT biosynthesis in the presence of 1 μM histamine. In Figure 1, the average amount (±s.e.m.) of the 5-LO products obtained for the control values in thapsigargin-, PAF-, and fMLP-activated PMN were 97(±12), 53(±4), and 45(±5) pmol million−1 cells, respectively. DMSO-stimulated PMN did not produce detectable levels of LT (detection limit of 2.5 pmol) for either unprimed or primed cells.
Figure 1.
Effect of histamine on LT biosynthesis in activated human PMN. Pre-warmed PMN suspensions (5 × 106 cells ml−1, 37°C) were stimulated with PAF, fMLP, or thapsigargin, as described in Methods. Incubations were terminated by adding 0.5 volume of a cold (4°C) stop solution containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards, and 5-LO products were analyzed as described in Methods. Histamine was added to the cell suspensions 5 min before the addition of the stimuli. The data shown are the mean (±s.e.m.) of three experiments, each performed in triplicates.
We previously reported that endogenous adenosine present in PMN suspensions exerted an important inhibitory constraint on LT biosynthesis upon activation with agonists (Krump et al., 1996; 1997; Flamand et al., 2000). In the same studies, we also demonstrated that removal of endogenous adenosine with ADA before stimulation of the cells with either physiological agonists or thapsigargin strongly enhances LT biosynthesis. Since endogenous autacoids (such as adenosine) can suppress eicosanoid generation, experiments were undertaken to evaluate the putative inhibitory constraint of endogenous histamine on LT biosynthesis in our PMN suspensions. Incubation of PMN suspensions with increasing concentrations of diamine oxidase (which converts histamine to the inactive metabolite imidazole acetic acid) did not affect the biosynthesis of LT induced by thapsigargin and fMLP (data not shown), demonstrating that, in the experimental conditions tested, basophils (the cellular source of histamine among PMN) were in insufficient number or did not release histamine in an amount resulting in a significant reduction of LT biosynthesis.
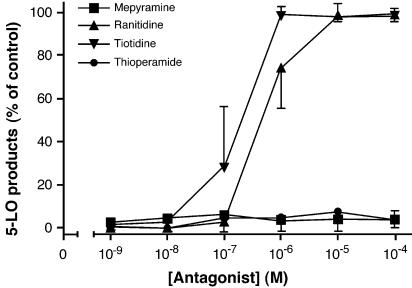
In order to confirm the involvement of the H2R in the inhibitory effect of histamine on LT biosynthesis in activated PMN, a series of experiments was performed with several histamine receptor antagonists. As shown in Figure 2 only tiotidine and ranitidine, two H2R antagonists, blocked the inhibitory effect of 1 μM histamine on thapsigargin-induced LT biosynthesis. Neither the H1R antagonist mepyramine nor the H3R/H4R antagonist thioperamide could relieve the inhibitory effect of histamine in these experimental conditions. The inhibitory effect of histamine on LT biosynthesis was also reversed by cimetidine, another H2R antagonist, while the H1R antagonist tripolidine and the H3R antagonist clobenpropit remained ineffective (data not shown). Moreover, experiments undertaken with the H1R agonist HTMT or the H3R agonist imetit did not enhance (nor inhibit) the biosynthesis of LT in primed PMN and in thapsigargin-activated PMN, while the H2R agonists dimaprit and amthamine resulted in an inhibition of LT biosyntesis in thapsigargin-activated PMN, similar to the one observed with histamine itself (data not shown). Altogether, these results provide strong pharmacological evidences for the implication of the H2R in this experimental model.
Figure 2.
Effect of histamine receptor antagonists on the histamine-induced inhibition of LT biosynthesis. Pre-warmed PMN suspensions (5 × 106 cells ml−1, 37°C) were stimulated with 100 nM thapsigargin, as described in Methods. Incubations were terminated by adding 0.5 volume of a cold (4°C) stop solution containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards and analyzed for 5-LO products, as described in Methods. The histamine receptor antagonists and histamine (1 μM) were always added to the cell suspensions 10 and 5 min, respectively, before the addition of thapsigargin. Data shown are the mean (±s.e.m.) of at least three experiments, each performed in triplicates.
Inhibition of AA release by histamine
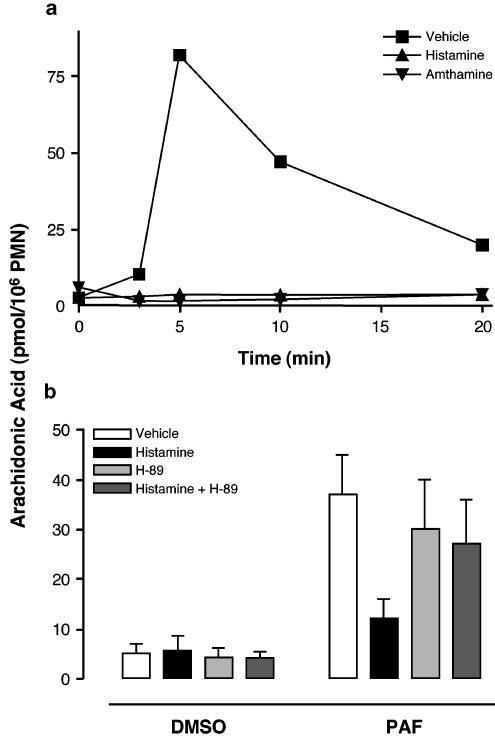
It is well established that substrate availability is a limiting factor in eicosanoid biosynthesis. Furthermore, several studies support that inhibition of LT biosynthesis by elevated [cAMP]i involves a decrease in AA release (Fonteh et al., 1993; Hichami et al., 1995; Flamand et al., 2000; Grenier et al., 2003). Given that the H2R is positively coupled to the adenylate cyclase, we investigated the putative effect of histamine on the release of AA in stimulated PMN. Following exposure to PAF or fMLP, GM-CSF/TNF-α-primed PMN rapidly release AA through the action of cPLA2 (Syrbu et al., 1999; Marshall et al., 2000; Degousee et al., 2002). Both histamine and the H2R agonist amthamine completely blocked the thapsigargin-induced AA release (Figure 3a). Similar results were observed in PMN stimulated with PAF (Figure 3b) or fMLP (not shown). Moreover, the inhibitory effect of histamine on ligand-induced AA release was prevented by the PKA inhibitor H-89. Interestingly, the inhibitory effect of histamine on ligand-induced AA release was not due to a decrease in cPLA2 phosphorylation on Ser-505 (visualized by bandshift) nor to an inhibition of cPLA2 translocation to the membranes in fMLP-activated human PMN (Figure 4).
Figure 3.
Effect of histamine on AA release in stimulated human PMN. (a) Pre-warmed PMN suspensions (107 cells ml−1; 37°C) were pre-incubated for 5 min in the presence of either 1 μM histamine or the H2R agonist amthamine (or diluent), then stimulated with 100 nM thapsigargin for the indicated times. (b) Pre-warmed PMN suspensions (107 cells ml−1; 37°C) were pre-incubated for 15 min in the presence of 10 μM H-89 (or diluent), followed by a further 5 min pre-incubation with 1 mM histamine. PMN were then stimulated with 300 nM PAF (or diluent) for 2 min. All incubations (a, b) were stopped by adding 1 volume of an ice-cold (4°C) stop solution containing 12.5 ng of both 19-OH-PGB2 and PGB2, and 20 ng 2H8-AA. AA was extracted and purified from the denatured incubation media by RP-HPLC using an on-line extraction procedure, and analyzed by LC-MS, as described in Methods. The data shown (a, b) are the mean (±s.e.m.) of duplicate incubations from single experiments representative of three.
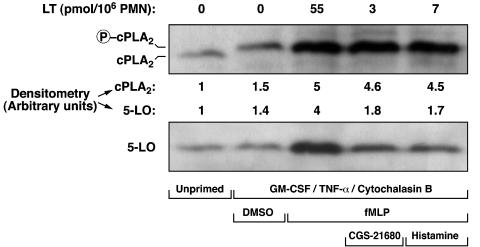
Figure 4.
Effect of CGS-21680 and histamine on cPLA2 and 5-LO translocation. Pre-warmed GMCSF/TNF-α/cytochalasin B-treated PMN suspensions (107 cells ml−1, 37°C) were incubated with 10 μM CGS-21680 or 1 mM histamine, and then stimulated with fMLP, as described in Methods. Incubations were stopped by the addition of 1 volume of cold (4°C) incubation buffer, and the cell suspensions were immediately centrifuged (4°C). Fractionation of the cell pellets was performed as described in Methods, and membrane fractions were analyzed by SDS–PAGE and immunoblotted with the cPLA2 and the 5-LO antibodies. Data shown are from one experiment representative of two.
Inhibition of 5-LO translocation by histamine
It is also clearly established that activation of the 5-LO in human PMN involves a translocation of the enzyme from cytosolic to nuclear structures (Woods et al., 1993; Pouliot et al., 1996), a process previously demonstrated to be inhibited by elevated [cAMP]i (Flamand et al., 2002). In the present study, we investigated the effect of histamine on this key cellular event in LT biosynthesis. Figure 4 clearly shows that activation of GM-CSF/TNF-α/Cytochalasin B-treated PMN with fMLP induced a translocation of both cPLA2 and 5-LO to the nuclei. Interestingly, histamine and the adenosine A2A receptor agonist CGS-21680 strongly inhibited 5-LO translocation to the FLAP-containing membranes, in sharp contrast to the observed cPLA2 translocation and phosphorylation at Ser-505, which were unaltered (Figure 4). In these experimental settings, the translocation of 5-LO to the membrane fractions was totally abolished by the LT biosynthesis inhibitor and 5-LO-activating protein (FLAP) antagonist MK-0591 (data not shown), confirming that the observed 5-LO translocations were the result of an interaction with FLAP-containing nuclear membranes, rather than the result of an unspecific interaction of 5-LO with membranes.
Modulation of Ca2+ mobilization by histamine in PMN
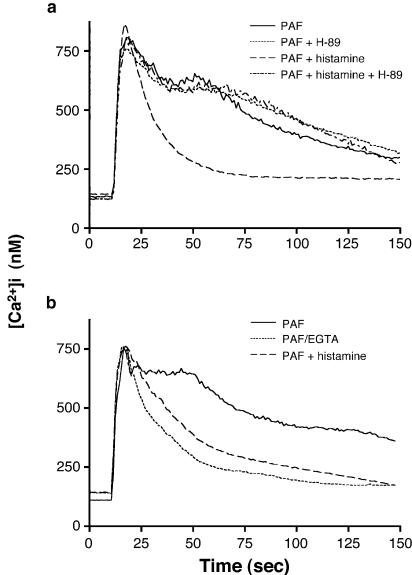
It is well documented that Ca2+ is required for LT biosynthesis. Since it has previously been demonstrated that histamine inhibits Ca2+ influx in agonist-stimulated PMN (Leino et al., 1993), we investigated herein the putative causal relationship between the inhibitory effect of histamine on Ca2+ influx and LT biosynthesis. In human PMN, PAF- and fMLP-induced rise of [Ca2+]i is the consequence of a rapid and transient release of Ca2+ from intracellular stores, followed by an influx of extracellular Ca2+. In a first series of experiments, we confirmed that, in our experimental conditions, histamine inhibits Ca2+ influx in ligand-activated PMN, but does not affect the release of Ca2+ from intracellular stores (Figure 5a), in perfect agreement with Leino et al. (1993). PMN suspensions were also pre-incubated with or without the PKA inhibitor H-89 to confirm the role of PKA (and cAMP) on the inhibitory effect of histamine on Ca2+ influx. Figure 5a clearly shows that H-89 prevented the inhibitory effect of histamine on Ca2+ influx. Figure 5b shows that the inhibitory effect of histamine on Ca2+ influx can be mimicked by EGTA; the Ca2+ influx observed in PAF-stimulated PMN is indeed effectively inhibited when EGTA is simultaneously added with PAF.
Figure 5.
Effect of histamine and EGTA on Ca2+ mobilization in human PMN. Fura-2-loaded PMN (107 cells ml−1) were pre-incubated at 37°C for 15 min, and then stimulated with 100 nM PAF (a, b). The PKA inhibitor H-89 (10 μM) and histamine (10 μM) were added 10 and 5 min, respectively, before PAF stimulation and histamine. Additions of PAF were performed 10 s after the beginning of data acquisition. Fluorescence was measured at excitation and emission wavelengths of 340 and 510 nm, respectively, as described in Methods. In experiments where chelation of extracellular Ca2+ was performed (b), EGTA (2 mM) was added simultaneously with PAF. Data shown are from single experiments representative of at least three.
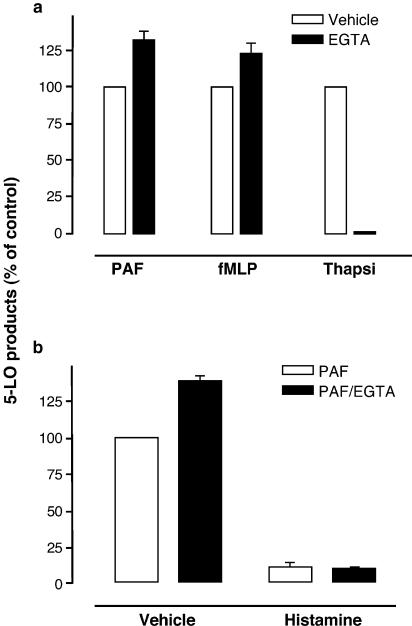
Another series of experiments was then performed to directly assess the relevance of Ca2+ influx blockade to the inhibitory effect of histamine on LT biosynthesis. Human PMN were incubated in HBSS containing 1.6 mM CaCl2 in the presence or absence of histamine. Addition of the Ca2+ chelator EGTA simultaneously with PAF, fMLP, and thapsigargin (Figure 6a) shows that, in our experimental setting, EGTA does not inhibit (but slightly enhances) LT biosynthesis induced by the physiological agonists. In sharp contrast, the addition of EGTA completely inhibited LT biosynthesis induced by the pharmacological agonist thapsigargin. In PMN stimulated with PAF in the presence of EGTA, the inhibitory effect of histamine on PAF-induced LT biosynthesis was still observed (Figure 6b), clearly demonstrating that histamine inhibition of Ca2+ influx is unrelated to histamine inhibition of LT biosynthesis LT. It is noteworthy that, in these experimental settings, EGTA was added simultaneously with the agonists (PAF, fMLP, or thapsigargin) to mimic the inhibitory effect of histamine (blockade of Ca2+ influx) in agonist-stimulated PMN. The addition of EGTA before stimulation (5 min or more) with PAF, fMLP, or thapsigargin results in the depletion of intracellular Ca2+ pools and, consequently, in the inhibition of Ca2+ mobilization and LT biosynthesis (data not shown).
Figure 6.
(a) Effect of EGTA on agonist-induced LT biosynthesis in human PMN. Pre-warmed PMN suspensions (5 × 106 cells ml−1, 37°C) were stimulated in the presence or absence of 2 mM EGTA, as described in Methods. (b) Inhibitory effect of histamine on LT biosynthesis in the presence of EGTA. Pre-warmed PMN suspensions (5 × 106 cells ml−1, 37°C) were stimulated as described in Methods, in the presence or absence of 2 mM EGTA. Histamine (1 mM) was added to the cell suspensions 5 min before the addition of PAF. All incubations were terminated by adding 0.5 volume of a cold (4°C) stop solution containing 12.5 ng of both 19-OH-PGB2 and PGB2 as internal standards and 5-LO products were analyzed, as described in Methods. In incubations where chelation of extracellular Ca2+ was performed, EGTA was added simultaneously with either PAF, fMLP, or thapsigargin. Data represent the mean (±s.e.m.) of triplicate incubations from a single experiment representative of three.
Discussion
The present study was performed to investigate the putative inhibitory effect of histamine on LT biosynthesis in activated human PMN and to understand the mechanisms involved. Histamine actions are regulated by receptor subtypes, and several suppressive effects of this autacoid on human PMN functions have already been reported (Busse & Sosman, 1976; Busse et al., 1980; Radermecker & Maldague, 1981; Seligmann et al., 1983; Burde et al., 1989; Zimmerman & Millard, 1989; Francis et al., 1991; Hirasawa et al., 1991; Bury & Radermecker, 1992; Bury et al., 1992; Leino et al., 1993). The inhibitory effects of histamine on PMN functional responses described so far are related to the activation of the H2R present on these cells. In the present study, we show that histamine dose-dependently inhibits LT biosynthesis in thapsigargin- and agonist-stimulated PMN.
Our data also clearly demonstrate that the inhibitory effect of histamine on LT biosynthesis is solely dependent upon H2R activation. Only the H2R antagonists used in this study were indeed capable of preventing the inhibitory effect of 1 μM histamine on thapsigargin-induced LT biosynthesis. Interestingly, some earlier studies have reported an inhibitory effect of H1R antagonists on LT biosynthesis and other PMN functions (Taniguchi et al., 1991; Cheria-Sammari et al., 1995; Amsellem et al., 1998), suggesting that activation of this receptor could stimulate PMN functional responses. However, there is so far no report of H1R expression on peripheral blood PMN (Petty & Francis, 1986), indicating that the inhibitory effect of H1R antagonists on LT biosynthesis in activated PMN is likely the consequence of an unspecific effect of the antagonists (Baroody & Naclerio, 2000).
The mechanism of the histamine-induced inhibition of LT biosynthesis was then addressed; given that the H2R is positively coupled to the adenylate cyclase (Gespach & Abita, 1982), the implication of cAMP-regulated events was specifically investigated. While the inhibition of LT biosynthesis by cAMP-enhancing agents has been observed repeatedly, the mechanism involved is yet incompletely understood. However, we and others have previously reported that cAMP-elevating agents such as adenosine, PGE2, type IV phosphodiesterase inhibitors, and isoproterenol cause a decrease of AA release in stimulated leukocytes (Fonteh et al., 1993; Hichami et al., 1995; Flamand et al., 2000; Grenier et al., 2003). Such a blockade of AA release was also observed with histamine. This observation is in agreement with an earlier study reporting the H2R-dependent decrease (by histamine) of AA release in A23187- and ATP-stimulated CHO cells (Traiffort et al., 1992). As expected, the PKA inhibitor H-89, which did not alter AA release in unstimulated (DMSO-treated) PMN, abolished the inhibitory effect of histamine on thapsigargin- and PAF-induced AA release and LT biosynthesis (not shown). Similar results were obtained with the structurally distinct PKA inhibitor KT-5720 in thapsigargin-stimulated PMN (data not shown), supporting an important role of cAMP and PKA in the inhibitory effect of histamine on AA release and LT biosynthesis in activated PMN.
The cPLA2 mediates AA release in human PMN (Syrbu et al., 1999; Marshall et al., 2000; Degousee et al., 2002) and experiments with cPLA2-deficient mice confirmed the central role of this enzyme for eicosanoid biosynthesis by inflammatory cells (Bonventre et al., 1997; Uozumi et al., 1997). In the present study, histamine and other cAMP agents did not alter the translocation or phosphorylation of cPLA2 at Ser-505 (two molecular events involved in its activation) in fMLP-activated PMN. However, a cAMP/PKA-dependent phosphorylation of cPLA2 might explain the inhibition of AA release in PMN exposed to cAMP-elevating agents. Murthy and Macklouf indeed showed a downregulation of cPLA2 activity by elevated [cAMP]i in rabbit smooth muscle cells, involving a PKA-dependent phosphorylation of the enzyme (Murthy & Makhlouf, 1998). The authors could not define whether this inhibitory phosphorylation event was the consequence of a direct phosphorylation of cPLA2 by PKA or an indirect, PKA-dependent effect. The primary structure analysis of cPLA2 reveals three putative PKA phosphorylation sites at amino acids 57–60 (RKRT), 281–284 (KKKS), and 282–285 (KKSS). Additional experiments are, however, required to assess this putative inhibitory mechanism of cPLA2 and define its involvement in the cAMP-mediated inhibition of AA release in activated human PMN.
The possibility that histamine and other cAMP-elevating agents act by inhibiting the priming effect of GM-CSF, TNF-α, and cytochalasin B is unlikely. Indeed, histamine was added 25 min after the priming agents (see Methods). Moreover, histamine and other cAMP-elevating agents also block AA release and LT biosynthesis in thapsigargin-activated PMN, an experimental condition where human PMN are not exposed to GM-CSF, TNF-α, and cytochalasin B.
LT biosynthesis and 5-LO translocation were previously shown to be inhibited by cAMP-elevating agents (Flamand et al., 2002). In the present study, ligand-induced PMN activation caused a translocation of 5-LO to the nuclear compartment and the H2R agonist amthamine or histamine itself inhibited 5-LO translocation, in full agreement with our previous observations using a variety of cAMP-elevating agents. The persistence of cPLA2 translocation while 5-LO translocation is strongly inhibited by histamine and CGS-21680 is intriguing, and clearly emphasizes the involvement of distinct regulatory mechanism(s) in the translocation of both proteins to the FLAP-containing membranes. The hypothesis currently investigated in our laboratory is that AA is an important regulator of 5-LO translocation, and that inhibition of cPLA2 and AA release by cAMP-elevating agents results in the inhibition of 5-LO translocation (data not shown). However, the elucidation of the mechanisms involved in the cAMP-mediated inhibition of AA release and 5-LO translocation in activated PMN requires further investigations. Taken together, our data demonstrated that the inhibition of LT biosynthesis in ligand-activated PMN by histamine implicates cAMP-mediated inhibition of AA release and 5-LO translocation, but not the histamine-induced inhibition of Ca2+.
Exposure of human PMN to CGS-21680 (an adenosine A2A receptor agonist) leads to the inhibition of agonist-induced Ca2+ influx, but not release from internal stores (Tsuruta et al., 1992; Flamand et al., 2000). More recently, we confirmed these observations (Flamand et al., 2000) by the selective measurement of Ca2+ influx using Fura-2 fluorescence quenching with exogenously added Mn2+(Merritt et al., 1989). In the present study, histamine also inhibited Ca2+ influx in PAF-activated PMN, as observed previously with the A2A receptor agonist CGS-21680. This inhibition of Ca2+ influx by histamine was reversed by the PKA inhibitor H-89, suggesting a cAMP/PKA-dependent mechanism.
All the PMN agonists used in this study elevate the [Ca2+]i. Moreover, AA release, 5-LO translocation, and LT biosynthesis are three Ca2+-regulated events. It was therefore tempting to link the histamine-induced inhibition of agonist-mediated Ca2+ influx to the inhibition of LT biosynthesis; this hypothesis was carefully assessed. Chelation of extracellular Ca2+ with EGTA effectively inhibited the PAF-induced Ca2+ influx, as expected. Such treatment of PMN with EGTA led to the inhibition of thapsigargin-induced LT biosynthesis, while, interestingly, PAF- and fMLP-induced LT biosynthesis were unchanged (or slightly enhanced), demonstrating that extracellular Ca2+ was essential for thapsigargin- but not ligand-induced LT biosynthesis. The inhibitory effect of histamine on PAF-stimulated human PMN was still observed in the presence of the Ca2+ chelator, in perfect analogy with our previous observations using CGS-21680 as the inhibitory agent (Flamand et al., 2000), clearly demonstrating that the blockade of Ca2+ influx is not the mechanism by which histamine (and other cAMP elevating agents) inhibits the PAF- and fMLP-induced LT biosynthesis. In contrast, the more potent inhibition of thapsigargin-induced LT biosynthesis by cAMP-elevating agents observed in PMN is likely related, at least in part, to the blockade of Ca2+ influx, since extracellular Ca2+ is essential for cPLA2 activation and LT biosynthesis in PMN activated with this pharmacological agent (Reddy et al., 1995).
The observation that, in contrast to agonist-induced LT biosynthesis, thapsigargin-induced LT biosynthesis shows an absolute requirement for extracellular Ca2+ (Figure 5) is intriguing. One explanation for this phenomenon could be the differences in the kinetics of Ca2+ mobilization (and AA release and LT biosynthesis), and the contribution of intracellular Ca2+ release and Ca2+ influx to the build-up of [Ca2+]i in both experimental conditions. Indeed, in agonist-stimulated PMN, [Ca2+]i reaches its maximal level within 2–3 s of stimulation; this rise of [Ca2+]i is essentially dependent on the agonist-induced inositol-trisphosphate-mediated Ca2+ release from internal stores, and Ca2+ influx clearly occurs after this initial burst of [Ca2+]i (the relative contribution of Ca2+ release and influx to [Ca2+]i and the difference in their time courses can be seen in Figure 4). In contrast, the rise of [Ca2+]i in thapsigargin-activated PMN is slower (maximal at 30–60 s) and the contribution of Ca2+ influx to the maximal [Ca2+]i is very significant (40–60%) (data not shown). It is therefore conceivable that, in the absence of extracellular Ca2+, the [Ca2+]i does not reach the level required for cPLA2 and 5-LO activation.
The biological significance of the inhibitory effects of histamine (and other autacoids) on PMN functional responses is intriguing. Histamine is mainly reputed to be a potent pro-inflammatory agent. However, the data presented herein as well as several published studies strongly support its involvement as an anti-inflammatory agent also capable of downregulating immune responses. It is possible that such dual pro- and anti-inflammatory roles of histamine may be expressed at different times in the course of an inflammatory response. While histamine will, for example, promote vasodilatation, it may, at a later stage, downregulate inflammation and even contribute to resolution by decreasing the functional responses of leukocytes accumulating at inflammatory sites. In this regard, inhibition of LTB4 biosynthesis by histamine would contribute to slow down PMN recruitment. The biological significance of the anti-inflammatory effects of autacoids such as adenosine has been demonstrated in elegant studies involving mice lacking the A2A receptor, a receptor positively coupled to the adenylyl cyclase and implicated in the anti-inflammatory effects of endogenous adenosine (Ohta & Sitkovsky, 2001). It was shown that the A2A receptor deficiency results in marked increase in the severity of the inflammatory response. In analogy, the downregulatory role of histamine on leukocyte functions and immune process has also been assessed in an in vivo setting using H2R-deficient mice which showed upregulation of both Th1 and Th2 cytokines (Jutel et al., 2001). Studies in models of inflammatory diseases using H2R-deficient mice should allow to delineate the role of histamine and its H2R in the pathophysiological regulation of inflammatory and immune responses.
Acknowledgments
This work was supported by grants of the Canadian Institutes of Health Research (CIHR) and The Arthritis Society of Canada (TAS). Nicolas Flamand is the recipient of a doctoral award from the CIHR.
Abbreviations
- AA
arachidonic acid
- ADA
adenosine deaminase
- [Ca2+]i
intracellular calcium concentration
- [cAMP]i
intracellular cAMP concentration
- CGS-21680
2-[p-(2-carboxyethyl)]phenylethyl-amino-5-N-ethylcarboxy-amidoadenosine
- cPLA2
type IVA phospholipase A2
- DMSO
dimethyl sulfoxide
- H-89
N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinoline-sulfonamide
- 5-HETE
5-hydroxyeicosatetraenoic acid
- H1R
histamine H1 receptor
- H2R
histamine H2R
- HTMT
histamine trifluoromethyl toluidide
- 5-LO
5-lipoxygenase
- LT
leukotriene
- PG
prostaglandin
References
- AMSELLEM C., CZARLEWSKI W., LAGARDE M., PACHECO Y. Inhibitory effect of loratadine on leukotriene B4 production by neutrophils either alone or during interaction with human airway epithelial cells. Pulmon. Pharmacol. Ther. 1998;11:245–252. doi: 10.1006/pupt.1998.0151. [DOI] [PubMed] [Google Scholar]
- BAROODY F.M., NACLERIO R.M. Antiallergic effects of H1-receptor antagonists. Allergy. 2000;55:17–27. doi: 10.1034/j.1398-9995.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- BONVENTRE J.V., HUANG Z., TAHERI M.R., O'LEARY E., LI E., MOSKOWITZ M.A., SAPIRSTEIN A. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–625. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- BORGEAT P., PICARD S., DALLAIRE N., POULIOT M., SURETTE M.E.Quantitative studies of the lipid mediators of inflammation using liquid chromatography-electrospray mass spectrometry Molecular and Cellular Basis of Inflammation 1998Totowa, NJ: Humana Press Inc; 275–288.ed. Serhan C.N. & Ward, P.A. pp [Google Scholar]
- BORGEAT P., PICARD S., VALLERAND P., BOURGOIN S., ODEIMAT A., SIROIS P., POUBELLE P.E. Automated on-line extraction and profiling of lipoxygenase products of arachidonic acid by high-performance liquid chromatography. Methods Enzymol. 1990;187:98–116. doi: 10.1016/0076-6879(90)87014-t. [DOI] [PubMed] [Google Scholar]
- BOYUM A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of mononuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- BURDE R., SEIFERT R., BUSCHAUER A., SCHULTZ G. Histamine inhibits activation of human neutrophils and HL-60 leukemic cells via H2-receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;340:671–678. doi: 10.1007/BF00717743. [DOI] [PubMed] [Google Scholar]
- BURNS A.R., BOWDEN R.A., ABE Y., WALKER D.C., SIMON S.I., ENTMAN M.L., SMITH C.W. P-selectin mediates neutrophil adhesion to endothelial cell borders. J. Leukocyte Biol. 1999;65:299–306. doi: 10.1002/jlb.65.3.299. [DOI] [PubMed] [Google Scholar]
- BURY T., RADERMECKER M.F. Role of histamine in the chemotactic deactivation of polymorphonuclear leukocytes following incubation with formylmethionyl peptides. Int. Arch. Allergy Appl. Immunol. 1992;97:109–114. doi: 10.1159/000236105. [DOI] [PubMed] [Google Scholar]
- BURY T.B., CORHAY J.L., RADERMECKER M.F. Histamine-induced inhibition of neutrophil chemotaxis and T-lymphocyte proliferation in man. Allergy. 1992;47:624–629. doi: 10.1111/j.1398-9995.1992.tb02385.x. [DOI] [PubMed] [Google Scholar]
- BUSSE W.W., COOPER W., ANDERSON C. Dimaprit inhibition of zymosan-stimulated β-glucuronidase release from human granulocytes. Agents Actions. 1980;10:15–18. doi: 10.1007/BF02024173. [DOI] [PubMed] [Google Scholar]
- BUSSE W.W., SOSMAN J. Histamine inhibition of neutrophil lysosomal enzyme release: an H2 histamine receptor response. Science. 1976;194:737–738. doi: 10.1126/science.185696. [DOI] [PubMed] [Google Scholar]
- CHERIA-SAMMARI S., ALOUI R., GORMAND F., CHABANNES B., GALLET H., GROSCLAUDE M., MELAC M., RIHOUX J.P., PERRIN-FAYOLLE M., LAGARDE M., PACHECO Y. Leukotriene B4 production by blood neutrophils in allergic rhinitis. Effects of cetirizine. Clin. Exp. Allergy. 1995;25:729–736. doi: 10.1111/j.1365-2222.1995.tb00010.x. [DOI] [PubMed] [Google Scholar]
- COHEN P., NOVERAL J.P., BHALA A., NONN S.E., HERRICK D.J., GRUNSTEIN M.M. Leukotriene D4 falicitates airway smooth muscle cell proliferation via modulation of the IGF axis. Am. J. Physiol. 1995;269:L151–L157. doi: 10.1152/ajplung.1995.269.2.L151. [DOI] [PubMed] [Google Scholar]
- DAHLÉN S.E., BJORK J., HEDQVIST P., ARFORS K.E., HAMMARSTROM S., LINDGREN J.A., SAMUELSSON B. Leukotrienes promote plasma leakage and leukocytes adhesion in post capillary venules: in vivo effects with relevance to the acute inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLÉN S.E., HEDQVIST P., HAMMARSTROM S., SAMUELSSON B. Leukotrienes are potent constrictors of human bronchi. Nature. 1980;288:484–486. doi: 10.1038/288484a0. [DOI] [PubMed] [Google Scholar]
- DEGOUSEE N., GHOMASHCHI F., STEFANSKI E., SINGER A., SMART B.P., BORREGAARD N., REITHMEIER R., LINDSAY T.F., LICHTENBERGER C., REINISCH W., LAMBEAU G., ARM J., TISCHFIELD J., GELB M.H., RUBIN B.B. Groups IV, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 2002;277:5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- DENNIS D., RIENDEAU D. Phosphodiesterase 4-dependent regulation of cyclic AMP levels and leukotriene B4 biosynthesis in human polymorphonuclear leukocytes. Eur. J. Pharmacol. 1999;367:343–350. doi: 10.1016/s0014-2999(98)00987-x. [DOI] [PubMed] [Google Scholar]
- DEWALD B., BAGGIOLINI M. Activation of NADPH oxidase in human neutrophils. Synergism between fMLP and the neutrophil products PAF and LTB4. Biochem. Biophys. Res. Commun. 1985;128:297–304. doi: 10.1016/0006-291x(85)91678-x. [DOI] [PubMed] [Google Scholar]
- FLAMAND N., BOUDREAULT S., PICARD S., AUSTIN M., SURETTE M.E., PLANTE H., VALLÉE M.-J., KRUMP E., GILBERT C., NACCACHE P.H., LAVIOLETTE M., BORGEAT P. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am. J. Respir. Crit. Care Med. 2000;161:S88–S94. doi: 10.1164/ajrccm.161.supplement_1.ltta-18. [DOI] [PubMed] [Google Scholar]
- FLAMAND N., SURETTE M.E., PICARD S., BOURGOIN S.G., BORGEAT P. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol. Pharmacol. 2002;62:250–256. doi: 10.1124/mol.62.2.250. [DOI] [PubMed] [Google Scholar]
- FONTEH A.N., WINKLER J.D., TORPHY T.J., HERAVI J., UNDEM B.J., CHILTON F.H. Influence of isoproterenol and phosphodiesterase inhibitors on platelet-activating factor in the human neutrophil. J. Immunol. 1993;151:339–350. [PubMed] [Google Scholar]
- FORD-HUTCHINSON A.W., BRAY M.A., SHIPLEY M.E., SMITH M.J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980;286:264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- FRANCIS J.W., TODD R.F., BOXER L.A., PETTY H.R. Histamine inhibits cell spreading and C3bi receptor clustering and diminishes hydrogen peroxide production by adherent human neutrophils. J. Cell. Physiol. 1991;147:128–137. doi: 10.1002/jcp.1041470117. [DOI] [PubMed] [Google Scholar]
- GESPACH C., ABITA J.P. Human polymorphonuclear neutrophils. Pharmacological characterization of histamine receptor mediating the elevation of cyclic AMP. Mol. Pharmacol. 1982;21:78–85. [PubMed] [Google Scholar]
- GRENIER S., FLAMAND N., PELLETIER J., NACCACHE P.H., BORGEAT P., BOURGOIN S.G. Arachidonic acid activates phospholipase D in human neutrophils. Essential role of endogenous leukotriene B4 and inhibition by adenosine A2A receptor engagement. J. Leukocyte Biol. 2003;73:111–222. doi: 10.1189/jlb.0702371. [DOI] [PubMed] [Google Scholar]
- HAM E.A., SODERMAN D.D., ZANETTI M.E., DOUGHERTY H.W., MCCAULEY E., KUEHL F.A. Inhibition by prostaglandins of leukotriene B4 release from activated neutrophils. Proc. Natl. Acad. Sci. U.S.A. 1983;80:4349–4353. doi: 10.1073/pnas.80.14.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICHAMI A., BIOCHOT E., GERMAIN N., LEGRAND A., MOODLEY I., LAGENTE V. Involvement of cyclic AMP in the effects of phosphodiesterase IV inhibitors on arachidonate release from mononuclear cells. Eur. J. Pharmacol. 1995;291:91–97. doi: 10.1016/0922-4106(95)90129-9. [DOI] [PubMed] [Google Scholar]
- HIRASAWA N., WATANABE M., MUE S., TSURUFUJI S., OHUCHI K. Downward regulation of neutrophil infiltration by endogenous histamine without affecting vascular permeability responses in air-pouch-type carrageenin inflammation in rats. Inflammation. 1991;15:117–126. doi: 10.1007/BF00917506. [DOI] [PubMed] [Google Scholar]
- JUTEL M., WATANABE T., KLUNKER S., AKDIS M., THOMET O.A.R., MALOLEPSZY J., ZAK-NEJMARK T., KOGA R., TAKASHI K., BLASER K., AKDIS C.A. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- KRUMP E., BORGEAT P. Adenosine, an endogenous inhibitor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Adv. Exp. Med. Biol. 1999;447:107–115. [PubMed] [Google Scholar]
- KRUMP E., LEMAY G., BORGEAT P. Adenosine A2 receptor-induced inhibition of leukotriene B4 synthesis in whole blood ex vivo. Br. J. Pharmacol. 1996;117:1639–1644. doi: 10.1111/j.1476-5381.1996.tb15334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRUMP E., PICARD S., MANCINI J.A., BORGEAT P. Suppression of leukotriene B4 biosynthesis by endogenous adenosine in ligand-activated human neutrophils. J. Exp. Med. 1997;186:1401–1406. doi: 10.1084/jem.186.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LEINO L., TUOMINEN H.B., AKERMAN K.E.O. Histamine modulation of Ca2+ homeostasis in human neutrophils. J. Leukocyte Biol. 1993;54:584–589. doi: 10.1002/jlb.54.6.584. [DOI] [PubMed] [Google Scholar]
- MARLEAU S., FRUTEAU DE LACLOS B., SANCHEZ A.B., POUBELLE P.E., BORGEAT P. Role of 5-lipoxygenase products in the local accumulation of neutrophils in dermal inflammation in the rabbit. J. Immunol. 1999;163:3449–3458. [PubMed] [Google Scholar]
- MARSHALL J., KRUMP E., LINDSAY T., DOWNEY G.P., FORD D.A., ZHU P., WALKER P., RUBIN B. Involvement of cytosolic phospholipase A2 and secretory phospholipase A2 in arachidonic acid release from human neutrophils. J. Immunol. 2000;164:2084–2091. doi: 10.4049/jimmunol.164.4.2084. [DOI] [PubMed] [Google Scholar]
- MERRITT J.E., JACOB R., HALLAM T.J. Use of manganese to discriminate between calcium influx and mobilization from internal stores in stimulated human neutrophils. J. Biol. Chem. 1989;264:1522–1527. [PubMed] [Google Scholar]
- MIKI I., KUSANO A., OTHA S., HANAI N., OTOSHI M., MASAKI S., SATO S., OHMORI K. Histamine enhanced the TNF-α-induced expression of E-selectin and ICAM-1 on vascular endothelial cells. Cell Immunol. 1996;171:285–288. doi: 10.1006/cimm.1996.0205. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Differential regulation of phospholipase A2 (PLA2)-dependent Ca2+ signaling in smooth muscle by cAMP- and cGMP-dependent protein kinases. Inhibitory phosphorylation of cPLA2 by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 1998;273:34519–34526. doi: 10.1074/jbc.273.51.34519. [DOI] [PubMed] [Google Scholar]
- OHTA A., SITKOVSKY M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- PALMBLAD J., MALMSTEN C.L., UDEN A.M., RÅDMARK O., ENGSTEDT L., SAMUELSSON B. Leukotriene B4 is a potent and sterospecific stimulator of neutrophil chemotaxis and adherence. Blood. 1981;58:658–661. [PubMed] [Google Scholar]
- PETTY H.R., FRANCIS J.W. Polymorphonuclear leukocyte histamine receptors: occurrence in cell surface clusters and their redistribution during locomotion. Proc. Natl. Acad. Sci. U.S.A. 1986;83:4332–4335. doi: 10.1073/pnas.83.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POULIOT M., MCDONALD P.P., KRUMP E., MANCINI J.A., MCCOLL S.R., WEECH P.K., BORGEAT P. Colocalization of cytosolic phospholipase A2, 5-lipoxygenase, 5-lipoxygenase-activating protein at the nuclear membrane of A23187-stimulated human neutrophils. Eur. J. Biochem. 1996;238:250–258. doi: 10.1111/j.1432-1033.1996.0250q.x. [DOI] [PubMed] [Google Scholar]
- RADERMECKER M.F., MALDAGUE M.P. Depression of neutrophil chemotaxis in atopic individuals. An H2 histamine receptor response. Int. Arch. Allergy Appl. Immunol. 1981;65:144–152. doi: 10.1159/000232750. [DOI] [PubMed] [Google Scholar]
- RAJAH R., NUNN S.E., HERRICK D.J., GRUNSTEIN M.M., COHEN P. Leukotriene D4 induces MMP-1, which functions as an IGFBP protease in human airway smooth muscle cells. Am. J. Physiol. 1996;271:L1014–L1022. doi: 10.1152/ajplung.1996.271.6.L1014. [DOI] [PubMed] [Google Scholar]
- REDDY S., BOSE R., RAO G.H., MURTHY M. Phospholipase A2 activation in human neutrophil requires influx of extracellular Ca2+ and leukotriene B4. Am. J. Physiol. 1995;268:C138–C146. doi: 10.1152/ajpcell.1995.268.1.C138. [DOI] [PubMed] [Google Scholar]
- SAITO H., SHIMIZU H., MITA H., MAEDA Y., AKIYAMA K. Histamine augments VCAM-1 expression on IL-4- and TNF-α-stimulated human umbilical vein endothelial cells. Int. Arch. Allergy Immunol. 1996;111:126–132. doi: 10.1159/000237357. [DOI] [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., FORDERKUNZ S., HATZELMANN A., ULLRICH V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and calcium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- SELIGMANN B.E., FLETCHER M.P., GALLIN J.I. Histamine modulation of human neutrophil oxidative metabolism, locomotion, degranulation, and membrane potential changes. J. Immunol. 1983;130:1902–1909. [PubMed] [Google Scholar]
- SURETTE M.E., PALMANTIER R., GOSSELIN J., BORGEAT P. Lipopolysaccharides prime whole human blood and isolated neutrophils for the increased synthesis of 5-lipoxygenase products by enhancing arachidonic acid availability: involvement of the CD14 antigen. J. Exp. Med. 1993;178:1234–1355. doi: 10.1084/jem.178.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYRBU S.I., WATERMAN W.H., MOLSKI T.F., NAGARKATTI D., HAJJAR J.J., SHA'AFI R.I. Phosphorylation of cytosolic phospholipase A2 and the release of arachidonic acid in human neutrophils. J. Immunol. 1999;162:2334–2340. [PubMed] [Google Scholar]
- TANIGUCHI K., MASUDA Y., TAKANAKA K. Inhibitory effects of histamine H1 receptor blocking drugs on metabolic activations of neutrophils. J. Pharmacobiodyn. 1991;14:87–93. doi: 10.1248/bpb1978.14.87. [DOI] [PubMed] [Google Scholar]
- TRAIFFORT E., RUAT M., ARRANG J.-M., LEURS R., PIOMELLI D., SCHWARTZ J.-C. Expression of a cloned rat histamine H2 receptor mediating inhibition of arachidonate release and activation of cAMP accumulation. Proc. Natl. Acad. Sci. U.S.A. 1992;89:2649–2653. doi: 10.1073/pnas.89.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUTA S., ITO S., MIKAWA H. Adenosine inhibits divalent cation influx across human neutrophil plasma membrane via surface adenosine A2 receptors. Cell Signal. 1992;4:543–551. doi: 10.1016/0898-6568(92)90023-2. [DOI] [PubMed] [Google Scholar]
- UOZUMI N., KUME K., NAGASE T., NAKATANI N., ISHII S., TASHIRO F., KOMAGATA Y., MAKI K., IKUTA K., OUCHI Y., MIYAZAKI J.-I., SHIMIZU T. Role of cytosolic phospholipase A2 in allergic response and parturition. Nature. 1997;390:618–622. doi: 10.1038/37622. [DOI] [PubMed] [Google Scholar]
- WOODS J.W., EVANS J.F., ETHIER D., SCOTT S.R., VICKERS P.J., HEARN J.A., HEIBEIN S., CHARLESON S., SINGER I.I. 5-Lipoxygenase and 5-lipoxygenase activating protein are localized in the nuclear enveloppe of activated human leukocytes. J. Exp. Med. 1993;178:1935–1946. doi: 10.1084/jem.178.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN J., MILLARD J. H2-antagonist inhibition of human neutrophil superoxide anion synthesis. Clin. Pharmacol. Ther. 1989;45:487–494. doi: 10.1038/clpt.1989.62. [DOI] [PubMed] [Google Scholar]