Abstract
Single mechanically skinned fibres from the rat extensor digitorum longus muscle, which allow access to intracellular compartments, were used to examine the effects of 0.5–100 μM chlorpromazine hydrochloride (CPZ) on the major steps of the excitation–contraction (E–C) coupling to elucidate the involvement of skeletal muscle in the neuroleptic malignant syndrome (NMS).
At 1 μM, CPZ caused a 20–30% increase in the force response induced by t-system depolarisation and a marked increase in the rate of caffeine-induced SR Ca2+ release. At [CPZ]⩾2.5 μM, there was an initial increase followed by a marked decrease of the t-system depolarisation-induced force responses, while the potentiating effect on the caffeine-induced SR Ca2+ release remained. These effects were reversible.
CPZ had no effect on the maximum Ca2+-activated force, but caused reversible, concentration-dependent increases in the Ca2+ sensitivity of the contractile apparatus at [CPZ] ⩾10 μM, with a 50% predicted shift of 0.11 pCa (−log [Ca2+]) units at 82.3 μM CPZ.
CPZ did not alter the rate of SR-Ca2+ loading at 1 and 10 μM, but reversibly reduced it by ∼40% at 100 μM by reducing the SR Ca2+ pump. Nevertheless, the SR Ca2+ content was greater when fibres became unresponsive to t-system-induced depolarisation in the presence than in the absence of 100 μM CPZ.
The results show that CPZ has concentration-dependent stimulatory and inhibitory effects on various steps of the E–C coupling, which can explain the involvement of skeletal muscle in NMS and reconcile previous divergent data on CPZ effects on muscle.
Keywords: Chlorpromazine and sarcoplasmic reticulum, chlorpromazine and the t-system, chlorpromazine and the contractile apparatus, mechanically skinned muscle fibre
Introduction
A potentially fatal condition known as the neuroleptic malignant syndrome (NMS) is associated with the administration of antipsychotic drugs used in the treatment of schizophrenia, mania or psychotic depression (incidence 0.2%; mortality 4–30% (Andreassen & Pedersen, 2000)). The syndrome is characterised by muscular rigidity, fever, hyperthermia, altered consciousness and autonomic nervous system instability (e.g. tachycardia, profuse sweating, dyspnoea). The immediate discontinuation of the antipsychotic drugs is necessary to manage NMS (Andreassen & Pedersen, 2000).
There has been evidence of a direct muscle involvement in NMS such as IIB fibre atrophy and necrosis (Araki et al., 1988) and alteration in SR Ca2+-handling properties (Takagi, 1981; Lòpez et al., 1989). A commonly used antipsychotic drug is chlorpromazine hydrochloride (CPZ). It reaches a serum level between 0.3 μM (Curry, 1970) and 2.5 μM, where it begins to have toxic effects (Baldessarini, 1990). Studies by different groups of investigators have shown that CPZ has complex effects on skeletal muscle with sites of action at the level of the surface membrane (Andersson, 1973; Buttar & Frank, 1977), sarcoplasmic reticulum (SR) (Balzer et al., 1968; Bindoli & Fleischer, 1983; Lòpez et al., 1989; Vale, 1985; Volpe et al., 1984; Yoshida, 2000) and myofibrils (Takagi, 1981; Yoshida, 2000). Some of the results are, however, often controversial because of different muscle preparations used and different experimental conditions. Here we present results showing the effects of a broad range of [CPZ] (0.5–100 μM) on the major functional systems involved in the excitation–contraction coupling (E–C) of fast-twitch rat skeletal muscle studied under the same conditions. The results were obtained with the mechanically skinned fibre preparation that allows direct access to the intracellular environment to probe specific events in the E–C coupling (Stephenson et al., 1998). In this preparation, fibre activation can be achieved either by the normal sequence of events starting with the depolarisation of the sealed t-system and activation of the voltage sensors (Lamb & Stephenson, 1990a, 1990b; Launikonis & Stephenson, 2001), by direct activation of the ryanodine receptors (RyRs)/SR Ca2+ release channels (Endo & Iino, 1980; Fink & Stephenson, 1987; Lamb & Stephenson 1990a; Launikonis & Stephenson, 2001), thus bypassing the voltage sensor activation, or by direct activation of the contractile machinery in strongly Ca2+-buffered solutions (Fink et al., 1986). The results show that CPZ affects the function of the mammalian skeletal muscle at doses that are measured in the blood serum of patients treated with CPZ and help explain why skeletal muscle plays an important role in NMS.
Methods
Animals and the skinned fibre preparation
Male rats (Long Evans hooded, 3 months old) were killed by halothane overdose (2% v v−1) in accordance with permits granted by the La Trobe University Animal Ethics Committee. The extensor digitorum longus muscles were dissected, blotted on filter paper and pinned out on a layer of Sylgard 184 (Dow Chemicals, Midland, MI, U.S.A.) in a Petri dish containing paraffin oil (Ajax Chemicals, Sydney, Australia). Single fibres were then isolated, mechanically skinned with fine forceps under a dissecting microscope and attached to a sensitive force transducer (AME875 SensoNor, Horton, Norway) while immersed in oil, as described by Fink et al. (1986). The length and diameter were measured as previously described (Lamb & Stephenson, 1990b). The fibre was stretched by 20% above the resting level to increase the sensitivity for detecting the force produced in any segment of the preparation, and was then immersed in a relaxing solution, mimicking the myoplasmic environment with respect to [ATP], pH, [Na+]+[K+], [Mg2+], [Ca2+], ionic strength, osmolality and creatine phosphate (K+-repriming solution; see Table 1). Force was continually recorded on a chart recorder (Linear, U.S.A.).
Table 1.
Composition of solutions (mM)
| Solutions | HDTA | EGTAtotal | Na+ | K+ | Mg2+ | pCa |
|---|---|---|---|---|---|---|
| K+-repriming | 49.95 | 0.05 | 37 | 125 | 1 | 7.1 |
| Na+-depolarising | 49.95 | 0.05 | 162 | 0 | 1 | 7.1 |
| High-relaxing | 0 | 50 | 37 | 125 | 1 | >9 |
| Maximum-Ca2+-activating | 0 | 50 | 37 | 125 | 1 | 4.5 |
| SR-Ca2+-loading | 49.5 | 0.5 | 37 | 125 | 1 | 6.7 |
| SR-Ca2+-washing | 49.4 | 0.6 | 37 | 125 | 1 | >9 |
| SR-Ca2+-releasea | 49.5 | 0.5 | 37 | 125 | 0.015 | >9 |
All solutions had a pH of 7.10±0.01 at room temperature, final osmolalities of 290±10 mosmol kg−1 and contained (mM): HEPES, 90; ATP, 8; CP, 10; NaN3, 1. Various amounts of Ca2+ were present in solutions to produce the stated pCa (−log [Ca2+]), which was verified with a Ca2+-electrode (Orion, Boston, U.S.A.).
Caffeine (30 mM) was added as solid to the SR-Ca2+-release solution.
Solutions
The composition of solutions used in this study is shown in Table 1. The solutions were prepared as described in Stephenson & Williams (1981). Stock solutions of CPZ (1–100 mM CPZ) were freshly prepared in Na+-depolarising solution. The stock solutions were then diluted by a factor of at least 1000 by adding a small volume to ‘test solutions' as required and the equivalent amount of Na+-depolarising solution (without CPZ) was added to the ‘control solutions'. All chemicals were obtained from Sigma (St Louis, MO, U.S.A.) except for hexamethylene-diamine tetraacetic acid (HDTA), which was obtained from Fluka Basel (Switzerland).
Experimental protocols
The effects of CPZ on muscle contraction were examined after stimulation of the skinned fibre preparation by (1) depolarisation of the sealed transverse tubular (t-) system, (2) direct activation of the contractile apparatus and (3) direct activation of SR Ca2+ release. All experiments were performed at 22±2°C.
Contractions induced by t-system depolarisation were achieved by first polarising the sealed t-system by equilibrating the skinned fibre preparations in a K+-repriming solution for 1 min, followed by rapid immersion in a Na+-depolarising solution (see Table 1) (Lamb & Stephenson, 1990a; Launikonis & Stephenson, 2001). After reaching a steady response (three consecutive responses not differing by more than 10% in amplitude between each other), the preparations were exposed to [CPZ] (0.5–100 μM) in Na+-depolarising and K+-repriming solutions for 5 min and, unless otherwise stated, the preparations were then returned to CPZ-free solutions to test for reversibility of the CPZ-induced effects.
Direct activation of the contractile apparatus was performed by equilibrating the skinned fibres in well Ca2+-buffered solutions of different pCa between 9 and 4.5, which were obtained by mixing in different proportions high-relaxing and maximum Ca2+-activating solutions (Table 1). Preparations were activated in identical Ca2+-activating solutions without CPZ, with CPZ (1, 10, 100 μM) and again without CPZ. After exposure to CPZ, the preparations were washed for 2 min in high-relaxing solution. The steady-state force responses in the various solutions were then expressed relative to the corresponding value for the maximum force response P0i for each steady-state value, which was calculated using Eq. (1) (see Rees & Stephenson, 1987), to correct for the small decrease in the maximum response with repeated activations (<5% between consecutive maximum activations):
where P0 and P′0 represent two consecutive values for the maximum force responses, m is the total number of intervening submaximal activations between P0 and P′0, and i is the number corresponding to the respective submaximal activation. The relative steady-state force-pCa data were then fitted by Hill curves with GraphPad Software (Prism, San Diego, CA, U.S.A.), which are characterised by two parameters: the Hill coefficient h, and the Ca2+ concentration corresponding to 50% maximum Ca2+-activated force, [Ca2+50], in Equation (2):
Direct activation of SR Ca2+ release was achieved by exposing the skinned fibre preparation for 1 min to 30 mM caffeine and 0.015 mM Mg2+ (SR Ca2+-release solution in Table 1), which thoroughly depletes the SR Ca2+ by activating the RyRs/SR Ca2+-release channels (Fryer & Stephenson, 1996). Note that no detectable releasable Ca2+ remained in the SR after 1 min exposure to the SR Ca2+-release solution in the presence of 1–100 μM CPZ. The areas under the caffeine and low Mg2+-induced responses can be used to estimate the relative amount of Ca2+ in the SR (Endo & Iino, 1980; Fink & Stephenson, 1987; Launikonis & Stephenson, 1997) making appropriate corrections where necessary, as described in Results. Direct activation of the SR Ca2+ release was performed in the presence or in the absence of CPZ (1, 10, 100 μM) after the SR was either loaded with Ca2+ in the SR Ca2+-loading solution (Table 1), in the absence or in the presence of CPZ. Prior to the release of Ca2+ from the SR, the preparations were equilibrated in the SR-Ca2+-washing solution, with or without CPZ, depending on the type of experiment performed.
Analyses of results
Data are given as means±s.e.m.'s. GraphPad Software (Prism, San Diego, CA, U.S.A.) was used for statistical analyses and curve fitting. Two-way ANOVA, Bonferroni post-test and Student's t-test were used to examine statistical significance.
Results
Effect of CPZ on t-system depolarisation-induced force responses
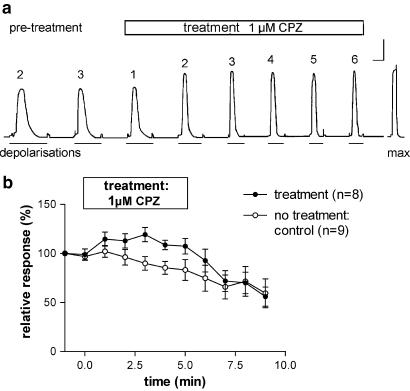
Transfer of a mechanically skinned fibre preparation from the K+-repriming solution to the Na+-depolarising solution causes depolarisation of the sealed t-system and activation of the voltage sensors, which, in turn, activate the RyRs and cause Ca2+ release from the SR. The released Ca2+ ions activate the contractile apparatus and the SR Ca2+ pump that facilitates their own re-accumulation (Lamb & Stephenson, 1990a). Upon maintained depolarisation, the voltage sensors inactivate and, when this occurs, Ca2+ release from the SR stops, most of the released Ca2+ is returned to the SR and the fibre relaxes (Lamb & Stephenson, 1990a). On average, the t-system depolarisation-induced force response in the absence of CPZ (see pretreatment responses in Figure 1a and Lamb & Stephenson, 1994) reaches about 75% of the maximum Ca2+-activated force, and, with time, there is a slow and gradual decline in the amplitude of depolarisation-induced force responses (see controls in Figures 1 and 2) due to an inherent run-down to depolarisation-induced force activation, which is both time- and use-dependent (Lamb & Stephenson, 1990a; 1994).
Figure 1.
Effect of 1 μM CPZ on depolarisation-induced force responses. (a) Representative depolarisation-induced force responses under control conditions (pretreatment) and when preparations were exposed to 1 μM CPZ for up to 5 min (responses 1–6; numbers above traces represent the response number under the pretreatment (control) and test conditions). The last response in (a) represents the maximum Ca2+-activated force in this preparation. Fibre dimensions: length=0.8 mm, diameter=48 μm. Calibration bars: horizontal: 2 s during depolarisation-induced force responses, 30 s elsewhere; vertical: 0.1 mN. (b) Depolarisation-induced force responses for control conditions and before, during and after exposure to 1 μM CPZ. The horizontal bar indicates the time of exposure to CPZ. Results are expressed in % relative to the size of pretreatment responses. Number of preparations (n)=9 for controls and 8 for 1 μM CPZ.
Figure 2.
Effects of 10 and 100 μM CPZ on depolarisation-induced force responses. (a) Representative traces showing depolarisation-induced force responses following exposure to 10 μM CPZ and recovery after washout of the drug. Note that there is a sharp rise in the response immediately upon exposure to CPZ in the Na+-depolarisation solution and a full recovery after washout of the drug. The last force response is the maximum Ca2+-activated force produced in the preparation. Fibre dimensions: length=1.4 mm, diameter=48 μm. Calibration bars: horizontal: 2 s during depolarisation-induced force responses, 30 s elsewhere; vertical: 0.1 mN. (b, c) Depolarisation-induced force responses for controls (n=9) and when preparations were exposed for 5 min to 10 μM (n=5) and 100 μM (n=3) CPZ. The horizontal bars indicate the times of exposure to CPZ.
When the pretreatment force responses had stabilised (usually after 3–4 depolarisation–repolarisation cycles; see Figures 1a, 2a), the fibres were exposed to different [CPZ] (0.5–100 μM) added to both the Na+-depolarising and the K+-repriming solutions. In the presence of 1 μM CPZ, there was a 20–30% increase in the force response induced by t-system depolarisation, raising the peak of the force response to a value close to the maximum Ca2+-activated force level (Figure 1a). The rate of force development expressed as the ratio between peak force and time to peak was also higher by about 20% after exposure to 1 μM CPZ. At [CPZ]⩾2 μM, there was an initial increase in the force response immediately after exposure to CPZ in the Na+-depolarising solution, and this increase was followed by a marked decrease. Representative responses for 1 and 10 μM CPZ are shown in Figures 1a and 2a, respectively, and results for 1, 10 and 100 μM CPZ are summarised in Figures 1b and 2b,c together with control data. The results in the presence and absence of CPZ are statistically different (two-way ANOVA; P<0.0001). For [CPZ] ⩽10 μM, the effects were also fully reversible after washing off the drug in SR Ca2+-washing solution for several minutes (see Figures 1b and 2b). Figure 3a shows the concentration–response curves for depolarisation-induced force responses immediately after exposure to CPZ and Figure 3b displays the data after 5 min exposure to the drug. Both curves show a peak increase, one occurring at 7.5 μM and the other at 1 μM CPZ.
Figure 3.
Dose–response curves for depolarisation-induced force responses immediately (a) and 5 min after exposure to CPZ (b). Results are expressed as means±s.e.m.'s relative to values for control fibres and the curves were fitted to the data assuming drug binding to both activation and inhibition sites.
The biphasic nature of the curve in Figure 3b suggests that CPZ may have at least two sites of action on skeletal muscle fibres at [CPZ] ⩾1 μM. One of these sites of action could be associated with the dihydropyridine receptor (DHPR)/voltage sensor, which can exist in either, primed, activated and inactivated states (Melzer et al., 1995). Since these states depend on the level of t-system polarisation, CPZ effects were measured when the t-system was normally polarised (and the DHPRs were mainly primed) or chronically depolarised (and the DHPRs were inactivated) at the time of CPZ application. For this purpose, preparations were either exposed to CPZ (1 and 10 μM) in the K+-repriming solution for 5 min (polarised) or exposed to CPZ in the Na+-depolarising solution (chronically depolarised) for 4 min and 15 s (and 45 s in the K+-repriming solution) before they were depolarised in the Na+-depolarising solution (with CPZ). Since there is no significant difference between the two treatments (Figure 4), the results indicate that the CPZ effects do not depend on whether the DHPRs/voltage sensors were primed or inactivated when exposed to the drug. Note that exposure to 10 μM CPZ for 5 min without activation (Figure 4) completely abolished the ability of the preparation to respond to t-system depolarisation, while the response was depressed by about 80% when the preparations had undergone five depolarisation-repriming cycles during exposure to 10 μM CPZ for 5 min (Figure 3b). This may suggest that the inhibitory action of CPZ on the depolarisation-induced responses is not as marked when DHPRs are being activated.
Figure 4.
CPZ effects at 1 and 10 μM do not depend on t-system state of polarisation. Fibres were first exposed for 4 min 15 s to CPZ either when the t-system was depolarised (1 μM-Na, n=4; 10 μM-Na, n=3) or when the t-system was normally polarised (1 μM-K, n=3; 10 μM-K, n=3), and then were kept for 45 s in a normally polarising solution with CPZ before activation in the Na+-depolarising solution with CPZ. Responses in the presence of CPZ are expressed as percentages of pretreatment responses.
Further experiments were performed on the contractile apparatus and the SR to identify specific steps in the E–C coupling between voltage sensor activation and the contractile response, which are affected by CPZ.
Effects of CPZ on the contractile apparatus
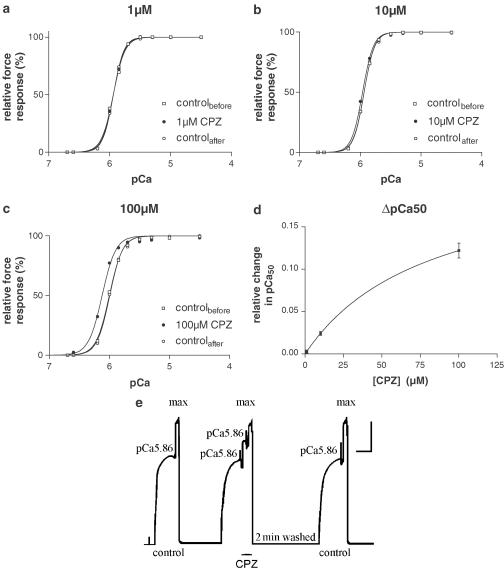
Heavily Ca2+-buffered solutions with 50 mM EGTA were used to directly activate the contractile apparatus in the presence and absence of CPZ (Stephenson & Williams, 1981; Fink et al., 1986). Representative Ca2+-activation data points with 1, 10 and 100 μM CPZ fitted with Hill curves (see Methods) are shown in Figure 5a–c. There was no statistically significant change in the value of the Hill coefficient in the presence of 1–100 μM CPZ (P>0.5). The sensitivity to Ca2+ expressed as pCa50 (=−log10[Ca502+]) also did not change when 1 μM CPZ was present in solutions (ΔpCa50=0.003±0.005; t-test: P<0.56, n=5), but increased significantly in the presence of 10 μM (ΔpCa50=0.0241±0.005, t-test: P<0.008, n=5) and 100 μM CPZ (ΔpCa50=0.122±0.022, t-test: P<0.002, n=6). The effect of CPZ on ΔpCa50 is shown in Figure 5d. As shown in Figure 5e, the CPZ effects on pCa50 were fully reversible and there was no effect on maximum Ca2+-activated force.
Figure 5.
CPZ effects on steady-state Ca2+-activated force responses. (a–c) Representative force–pCa curves for individual fibres exposed to paired heavily Ca2+-buffered solutions with and without 1 μM (a), 10 μM (b) and 100 μM (c) CPZ. Results with CPZ are compared to control runs before and after exposure to the drug. Graphs are fitted to sigmoidal (Hill) curves by nonlinear regression (see Methods). (d) CPZ-induced shift of pCa50 (ΔpCa50=difference between pCa50 in the presence of CPZ and the mean of the pCa50 control values before and after the drug treatment) in 16 skinned fibres. Results (means±s.e.m.'s, n=5–6) were fitted by nonlinear regression to a one-site binding curve. The maximum predicted shift value was 0.22 pCa units at high [CPZ] with 50% of the pCa50 shift occurring at 82.3 μM CPZ. (e) Representative force responses at pCa 5.86 and pCa 4.5 (maximum activation) in the absence and presence of 100 μM CPZ. Upon exposure to CPZ at constant pCa (5.86), force increased rapidly and markedly to a new level and this effect was completely reversed after 2 min in high-relaxing solution. Fibre dimensions: length=1.4 mm, diameter=50 μm. Calibration bars: horizontal: 30 s; vertical: 0.1 mN. Similar responses were obtained with three other preparations.
Thus, the CPZ effects on depolarisation-induced force responses for [CPZ] ⩽1 μM cannot be due to CPZ effects on the contractile apparatus. The slight increase in Ca2+ sensitivity at 10 μM could only explain a potentiation effect of <5%, while the more marked effect at 100 μM CPZ could explain 15–20% of the potentiation, when the preparations were first depolarised in the presence of [CPZ] (see Figure 3a). These estimates were made from the data shown in Figure 5b and c, assuming that the pCa value in myofibrils at the peak of the depolarisation-induced force responses under control conditions would have been close to 5.86 to produce about 75% of the maximum Ca2+-activated response (Figure 1a). Then, the responses would have increased from 75 to 78% and from 75 to 90% in the presence of 10 and 100 μM CPZ, respectively.
CPZ effects on the SR
Direct activation of the RyRs/SR Ca2+-release channels by 30 mM caffeine and low [Mg2+] comprehensively releases Ca2+ from the SR in the absence or presence of 1–100 μM CPZ (see Methods). Therefore, when transferring the skinned fibre preparation from the SR Ca2+-washing solution to the SR-Ca2+-release solution, the contractile apparatus becomes transiently activated by Ca2+ released from the SR. These caffeine-induced force responses were used to determine (i) direct CPZ effects on RyRs/SR Ca2+-release channels, (ii) the relative amount of SR Ca2+ and (iii) the rates of Ca2+ loading in the SR in the presence and absence of CPZ in SR Ca2+-loading solution (see Methods).
Loading the SR with Ca2+ to a certain level (20 s loading in SR Ca2+-loading solution) in the absence of CPZ and then releasing it with caffeine produced force responses that were consistently faster and larger when the SR Ca2+ was released in the presence than in the absence of CPZ at all CPZ concentrations. Thus, the rate of caffeine-induced force development (expressed as the ratio between peak caffeine-induced force and time to peak) in the presence of CPZ was greater by 49.0±12.9% (n=3) in 1 μM, 55.0±11.0% (n=3) in 10 μM and 84.7±5.2% (n=3) in 100 μM CPZ compared with controls. Similarly, the areas under the caffeine-induced responses were increased in the presence of CPZ compared to controls by 1.20±0.05 for 1 μM (n=3), 1.26±0.03 (n=3) for 10 μM and 1.07±0.06 (n=3) for 100 μM CPZ.
The significant increase in the rate of caffeine-induced responses observed at 1 μM CPZ, when the sensitivity to Ca2+ of the contractile apparatus was not altered, provides clear-cut evidence that the rate of RyR-dependent Ca2+-release from the SR must be markedly potentiated in the presence of 1 μM [CPZ]. The increased Ca2+ sensitivity in the presence of 10 and 100 μM CPZ (Figure 5) can account for about 10 and 45% of the increase in the rate of caffeine-induced responses at 10 and 100 μM CPZ, considering that the control caffeine-induced force response was about 60% of the maximum force response (Figure 6). Therefore, the rate of RyR-dependent Ca2+ release from the SR may not increase further as [CPZ] is raised above 1 μM.
Figure 6.
Effect of CPZ on caffeine-induced SR Ca2+ release. Representative caffeine-induced force responses for two fibres in the absence and in the presence of 1 μM CPZ (a) or 100 μM CPZ (b) after the preparations were loaded with Ca2+ at pCa 6.7 without and with CPZ (SR-Ca2+ solution) for 20 s and washed for 30 s in washing solution (without and with CPZ) before Ca2+ was released with caffeine in the release solution (without and with CPZ). Responses in the presence of CPZ (after 5 min continuous exposure to the drug) are compared to responses before incubation with CPZ and after washing out the drug for 2 min. The last responses in panels (a) and (b) represent the maximum Ca2+-activated force. Calibration bars: horizontal: 2 s during caffeine-induced force responses; 30 s during exposure to maximum-activating solution; vertical: 0.1 mN. Fibre dimensions: (a) length=1.2 mm, diameter=60 μm; (b) length= 1.0 mm, diameter=48 μm. (c) The relative rate of caffeine-induced force development for three preparations (ratio between peak force and time to peak) in the presence of 1, 10 and 100 μM CPZ (dark bars) and after the drug was washed out for 2 min (white bars). The data are expressed as % of the rate of caffeine-induced force development prior to CPZ exposure.
The ratio between areas under caffeine-induced force responses is normally used as a reliable parameter to estimate the relative amounts of Ca2+ in the SR (Endo & Iino, 1980; Fink & Stephenson, 1987; Launikonis & Stephenson, 1997), and, for our control conditions (no CPZ), there was direct proportionality between the areas under caffeine-induced force responses and loading time (10–40 s) in the SR Ca2+-loading solution (data not shown). Since the areas under the caffeine-induced force responses were larger in the presence of CPZ than in the absence of the drug for the same level of Ca2+ loading, the ratios between the corresponding areas under the caffeine-induced responses in the presence and absence of CPZ can be used to correct for the caffeine-induced force responses obtained in the presence of CPZ to enable making comparisons between results (Herrmann-Frank et al., 1999).
Figure 6a and b shows representative caffeine-induced force responses in the absence and in the presence of CPZ when preparations were loaded for the same length of time (20 s) in the SR Ca2+-loading solution in the absence and the presence of CPZ, respectively, and the rates of caffeine-induced force development in the presence of CPZ compared with controls are summarised in Figure 6c. The relative areas under the caffeine-force responses after correction for the presence of CPZ in the SR Ca2+-release solution using the factors indicated above are shown in Figure 7. From these results (Figure 7), one can conclude that the SR Ca2+ loaded into the SR after 20 s was significantly depressed in the presence of 100 μM CPZ (P<0.007), but not in the presence of 1 and 10 μM CPZ (P>0.05). The decreased Ca2+-loading ability in the presence of 100 μM CPZ (Figure 7) can be due to a reduced SR Ca2+-pump activity and/or an increased rate of Ca2+ leak from the SR during loading. To distinguish between these possibilities, Ca2+-leak experiments were performed in the presence and absence of 100 μM CPZ. In these experiments, the preparations were loaded with Ca2+ to the same level and then transferred for 15 or 45 s to the SR-Ca2+-washing solution, where the SR could not load Ca2+ but Ca2+ could leak out from the SR. The fraction of Ca2+ lost from the SR over a 30 s period was then assessed from the ratio (A15−A45)/A15, where A15 and A45 are the areas under the caffeine-induced force responses after 15 and 45 s in the SR-Ca2+-washing solution, respectively. As there was no significant difference in the fraction of Ca2+ lost in the absence or presence of 100 μM CPZ (paired t-test, P>0.1), one can conclude that 100 μM CPZ depresses primarily the SR-Ca2+ pump. Note that all CPZ effects on the SR described above were fully reversible after washing the preparation in K+-repriming solution for 2 min.
Figure 7.
Effect of CPZ on SR Ca2+-loading ability in mechanically skinned EDL fibres of the rat. Relative areas under the caffeine-induced force responses in the presence of 1, 10 and 100 μM CPZ after being reduced by a factor of 1.20 for 1 μM CPZ, 1.26 for 10 μM CPZ and 1.07 for 100 μM CPZ to take into consideration the larger areas in the presence of CPZ compared with controls for the same amount of Ca2+ present in the SR (see text) following 20 s SR Ca2+ loading in the presence of CPZ. These areas are compared to the appropriate (control) areas under the caffeine responses prior to CPZ exposure (n=6 for 1 μM; n=3 for 10 μM and n=3 for 100 μM). The corrected areas are proportional to the SR Ca2+ content. The areas for 100 μM CPZ are statistically significantly smaller than the areas for controls (paired t-test: P<0.01; n=6).
Thus far, the SR experiments showed that ⩽10 μM CPZ facilitates RyR opening in the presence of caffeine without affecting SR-Ca2+ loading, which explains the potentiation of depolarisation-induced force responses in the presence of 1 and 10 μM CPZ. At 100 μM CPZ, the SR-Ca2+ loading after 20 s was reduced due to inhibition of the SR-Ca2+ pump. Therefore, the depression of depolarisation-induced force responses with 100 μM CPZ could be explained under these conditions if the SR becomes depleted of Ca2+. However, this was not the case since the relative SR Ca2+ content measured when depolarisation-induced force responses were reduced to less than 3% of their maximum response in the presence of 100 μM CPZ was actually markedly greater than when the depolarisation-induced responses were run down to less than 3% of their maximum response in the absence of the drug (Figure 8) (Note that run down only pertains to depolarisation-induced responses and not to either Ca2+-activated or caffeine-induced force responses.) Consequently, the inhibition of the SR-Ca2+ pump in the presence of 100 μM CPZ is not sufficient to explain the depression of depolarisation-induced force responses at [CPZ] ⩾10 μM, suggesting that CPZ at concentrations ⩾10 μM must also exert an inhibitory effect on E–C coupling at the level of the DHPR-RyR complex.
Figure 8.
SR Ca2+ content after abolition of depolarisation-induced force responses in the presence of 100 μM CPZ compared to SR Ca2+ content after complete run down of depolarisation-induced force responses in the absence of CPZ (control). Relative areas under caffeine-induced force responses after 2 min wash in CPZ-free K+-repriming solution and 15 s in SR-Ca2+-washing solution, following complete abolition of voltage sensor-induced force responses. Results are expressed as % of areas after 20 s loading and 15 s wash of the same preparation in the absence of CPZ (n=3 for 100 μM CPZ and n=8 for control fibres). The areas are proportional to the SR Ca2+ content, which in control preparations was close to the endogenous level of Ca2+ loading.
Discussion
CPZ, E–C coupling and NMS
CPZ is a highly lipid soluble molecule, able to cross the blood–brain barrier (Cassidy et al., 1988; Baldessarini, 1990), and therefore able to equilibrate across cellular membranes. The pharmacological serum level of CPZ given as antipsychotic medication is between 0.3 and 2.5 μM (Curry, 1970; Baldessarini, 1990), which, even when considering that the largest proportion of the CPZ in the blood serum is bound to serum proteins (Baldessarini, 1990), should be relatively close to the level of 1 μM CPZ where CPZ has been found in this study to markedly and reversibly potentiate the voltage sensor-induced SR Ca2+ release and increase the rate of Ca2+ release induced by caffeine and low Mg2+. At 1 μM, CPZ does not alter the Ca2+ sensitivity of the contractile apparatus, the maximum Ca2+-activated force, the unidirectional Ca2+ leak from the SR, or the net Ca2+ uptake by the SR. Considering that skeletal muscle fibres in patients afflicted by the NMS have higher [Ca2+] at rest compared with controls (pCa 6.3, Lòpez et al., 1989), approaching the threshold for Ca2+ activation of the contractile apparatus (Fink et al., 1990; Figure 5), and that the SR Ca2+ can be more easily released in the presence of CPZ in patients with NMS than in controls (Takagi, 1976), it follows that, at therapeutic levels, individuals prone to NMS will be more susceptible to the synergistic effect of CPZ on SR Ca2+ release when the RyRs are activated by voltage sensors or by specific agonists such as caffeine. Although 1 μM CPZ does not affect the resting membrane potential in skeletal muscle fibres (Andersson, 1973, Buttar & Frank, 1977), it is known that the action potential is reduced in amplitude and becomes more prolonged in the presence of 1 μM CPZ due to its inhibitory action on the Na+- (Buttar & Frank, 1977) and delayed rectifier K+-channels (Andersson, 1973). Thus, by increasing the level of inactivation of the Na+ channels, without altering the membrane potential, one would expect that 1 μM CPZ would also increase the threshold for initiating action potentials and this would have an overall stabilising effect on the sarcolemma. However, if the threshold for the action potential was reached, then CPZ at 1 μM would synergistically potentiate the activation of the RyRs/SR Ca2+-release channels, causing greater and more prolonged Ca2+ release from the SR. This would lead to even more prolonged contractions and activation of the SR-Ca2+ pump in patients susceptible to NMS. Both these processes are exothermic, producing a considerable amount of heat associated with the continuous hydrolysis of ATP in the muscle cell. This sequence of events would explain the onset of muscular rigidity due to the sustained contraction of muscles, fever and hyperthermia, which are hallmarks of the NMS, thus explaining the role of the skeletal muscle in NMS.
CPZ effects at concentrations >1 μM
This study has shown that as the [CPZ] increases above the pharmacological level, it has multifaceted, concentration-dependent actions on E–C coupling in skeletal muscle. Thus, at concentrations greater than 1 μM but lower than 10 μM, CPZ exerts strong potentiating effects on the height and rate of force development in caffeine- and low Mg2+-induced responses and on voltage sensor-induced force responses immediately after exposure to the drug. However, within minutes, the response to voltage sensor activation is markedly depressed (Figures 2 and 3b), even though the SR is not depleted of Ca2+ (Figure 8). Thus, in this concentration range, the CPZ effects are clearly biphasic (Figure 2b). Since neither the membrane potential (Andersson, 1973; Buttar & Frank, 1977) nor the SR-Ca2+ pump function (Worsfold & Peter, 1970; Volpe et al., 1984) are being affected in this [CPZ] range, and since the potentiating effect of CPZ on the contractile apparatus is also small, one can conclude that the synergistic action of CPZ on activating the RyR/SR Ca2+-release channels described above for 1 μM [CPZ] is maintained over this [CPZ] range. The potentiation of the twitch force response (Andersson, 1973) and the shift of the voltage sensor activation curve to more negative membrane potentials in frog muscle fibres (Andersson, 1972) in the presence of 5–10 μM CPZ can also be fully explained by a synergistic effect of CPZ on activating the RyR/SR Ca2+-release channels without acting on other steps in the E–C coupling downstream from voltage sensor activation.
Let us now consider the inhibitory effect of CPZ on depolarisation-induced force responses at concentrations greater than 1 μM (Figure 3b). This effect cannot be caused by CPZ action on the contractile apparatus, which becomes slightly more rather than less sensitive to Ca2+ (Figure 5b). Inhibition of the SR Ca2+ pump must also be discarded as an explanation because even a small reduction in the SR Ca2+ pump activity would markedly slow down force relaxation following depolarisation-induced force responses (Bakker et al., 1996). This was not the case (Figures 1a and 2a). The SR Ca2+ content was also not depleted at [CPZ] ⩽10 μM (Figures 7 and 8) and the inhibitory action is unlikely to be on the RyR/SR Ca2+-release channel because the increased rate of force development in the presence of caffeine and low Mg2+ is maintained long after the preparations no longer respond to t-system depolarisation (see Figures 2 and 7).
Since CPZ is known to inhibit L-type Ca2+ channels in non-muscle cells with IC50 values between 2 and 10 μM (Ito et al., 1996; Ueda et al., 1997; Lee et al., 1999), a very likely explanation for this inhibitory effect is direct or indirect action of the drug on the DHPR/voltage sensor, which is an L-type Ca2+ channel (Melzer et al., 1995). With this interpretation, one can also explain previously reported inhibitory effects induced by CPZ on twitch force responses of the gastrocnemius muscle of the cat muscle (Kopera & Armitage, 1954), because an effect on the DHPRs would affect the responses to either action potential-induced or t-system depolarisation by ion substitution-induced force responses in the same way.
At 100 μM, CPZ had a clear effect on increasing the sensitivity to Ca2+ of the contractile apparatus. Such an effect was previously reported not only with CPZ (Takagi, 1981; Yoshida, 2000), but also with trifluoperazine (Kurebayashi & Ogawa, 1988), and it is likely to be due to the binding of phenothiazines to troponin C. This increase in Ca2+ sensitivity was fully reversible, and explains in part the increased rate of force development in the presence of caffeine and low Mg2+. At this concentration, there was also a clear depression of the rate of SR Ca2+ accumulation, suggesting a decreased rate of the SR Ca2+ pump, as was also found by others (Balzer et al., 1968, Takagi, 1981; Vale, 1985; Volpe et al., 1984; Yoshida, 2000). Interestingly, however, when the responses to the Na+-depolarising solution were abolished in the presence of 100 μM CPZ, the SR Ca2+ content was significantly greater than in the absence of the drug. This does only apparently contradict the previous statement, because what was observed here was an increased level of Ca2+ loading, while before we did consider a decreased rate of Ca2+ loading in the presence of CPZ. Earlier observations have shown that, at higher concentrations, CPZ can have opposite effects on the rate of Ca2+ accumulated in the SR and the level of SR Ca2+ loading (Vale, 1985). The observation reported here is therefore important, because it shows that a decreased level of pump activity does not always lead to a decreased SR Ca2+ content.
CPZ has been extensively used as a calmodulin (CaM) antagonist (see, for example, Ballejo et al., 1986). However, it is unlikely that any of the CPZ effects reported here are related to the inhibition of CaM function following binding of CPZ to CaM, because if this were the case and CPZ had bound to CaM and displaced it from its binding sites, then it would be expected that the CPZ–CaM complex would have washed off rapidly from the skinned fibre preparation (Stephenson & Stephenson, 1993), and therefore it would not have been possible to fully reverse these CPZ-induced effects by simply washing off the drug.
Conclusions
In conclusion, the results obtained from this study on mechanically skinned muscle fibres show that CPZ has multiple sites of action on the contractile process affecting DHPRs in the t-system, the SR Ca2+-release channels, the SR Ca2+ pump and the Ca2+-activation properties of the contractile apparatus. The results help reconcile previous apparently divergent observations, and provide the basis for understanding why skeletal muscle plays an important role in CPZ-induced NMS.
Acknowledgments
We thank Mrs Aida Yousef for technical support and the National Health and Medical Research Council of Australia for financial support.
Abbreviations
- CaM
calmodulin
- CPZ
chlorpromazine hydrochloride
- DHPR
dihydropyridine receptor
- E–C coupling
excitation–contraction coupling
- HDTA
hexamethylene diamine tetraacetate
- NMS
neuroleptic malignant syndrome
- pCa
−log [Ca2+]
- RyR
ryanodine receptor/SR Ca2+-release channel
- SR
sarcoplasmic reticulum
References
- ANDERSSON K.E. Effects of chlorpromazine, imipramine and quinidine on the mechanical activity of single skeletal muscle fibres of the frog. Acta Physiol. Scand. 1972;85:532–546. doi: 10.1111/j.1748-1716.1971.tb05292.x. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E. Effects of chlorpromazine, imipramine and quinidine on action potential and tension development in single skeletal muscle fibres of the frog. Acta Physiol. Scand. 1973;88:330–341. doi: 10.1111/j.1748-1716.1973.tb05461.x. [DOI] [PubMed] [Google Scholar]
- ANDREASSEN M.D., PEDERSEN S. Malignant neuroleptic syndrome. A review of epidemiology, risk factors, diagnosis, differential diagnosis and pathogenesis of MNS. Ugeskr Laeger. 2000;162:1366–1370. [PubMed] [Google Scholar]
- ARAKI M., TAKAGI A., HIGUCHI I., SUGITA H. Neuroleptic malignant syndrome: caffeine contracture of single muscle fibres and muscle pathology. Neurology. 1988;38:297–301. doi: 10.1212/wnl.38.2.297. [DOI] [PubMed] [Google Scholar]
- BAKKER A.J., LAMB G.D., STEPHENSON D.G. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J. Muscle Res. Cell Motil. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- BALDESSARINI R.J.Drugs and the treatment of psychiatric disorders Goodman's and Gilman's The Pharmacological Basis of Therapeutics 1990New York: Pergamon Press, Inc; 383–405.ed. Goodman, L. & Gilman, A. pp [Google Scholar]
- BALLEJO G., CALIXTO J.B., MEDEIROS Y.S. In vitro effects of calcium entry blockers, chlorpromazine and fenoterol upon human pregnant myometrium contractility. Br. J. Pharmacol. 1986;89:515–523. doi: 10.1111/j.1476-5381.1986.tb11151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALZER H., MAKINOSE M., HASSELBACH W. The inhibition of the sarcoplasmic calcium pump by prenylamine, reserpine, chlorpromazine and imipramine. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmacol. 1968;260:444–455. doi: 10.1007/BF00537359. [DOI] [PubMed] [Google Scholar]
- BINDOLI A., FLEISCHER S. Induced Ca2+ release in skeletal muscle sarcoplasmic reticulum by sulfhydryl reagents and chlorpromazine. Arch. Biochem. Biophys. 1983;221:458–466. doi: 10.1016/0003-9861(83)90164-9. [DOI] [PubMed] [Google Scholar]
- BUTTAR H.S., FRANK G.B. Effects of antipsychotic drugs on action potential production in skeletal muscle I. Chlorpromazine and promethazine. Can. J. Physiol. Pharmacol. 1977;55:452–461. doi: 10.1139/y77-065. [DOI] [PubMed] [Google Scholar]
- CASSIDY S.L., LYMPANY P.A., HENRY J.A. Lipid solubility of a series of drugs and its relevance to fatal poisoning. J. Pharm. Pharmacol. 1988;40:130–132. doi: 10.1111/j.2042-7158.1988.tb05197.x. [DOI] [PubMed] [Google Scholar]
- CURRY S.H. Plasma protein binding of chlorpromazine. J. Pharm. Pharmacol. 1970;22:193–197. doi: 10.1111/j.2042-7158.1970.tb08496.x. [DOI] [PubMed] [Google Scholar]
- ENDO M., IINO M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J. Muscle Res. Cell Motil. 1980;1:89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- FINK R.H.A., STEPHENSON D.G. Ca2+-movements in muscle modulated by the state of K+-channels in the sarcoplasmic reticulum membranes. Pflügers Arch. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- FINK R.H.A., STEPHENSON D.G., WILLIAMS D.A. Potassium and ionic strength effects the isometric force of skinned twitch muscle fibres of the rat and toad. J. Physiol. 1986;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK R.H.A., STEPHENSON D.G., WILLIAMS D.A. Physiological properties of skinned fibres from normal and dystrophic (Duchenne) human muscle activated by Ca2+ and Sr2+ J. Physiol. 1990;420:337–353. doi: 10.1113/jphysiol.1990.sp017916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRYER M.W., STEPHENSON D.G. Total and sarcoplasmic reticulum calcium contents of skinned fibres from the rat skeletal muscle. J. Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRMANN-FRANK A., LÜTTGAU H.C., STEPHENSON D.G. Caffeine and excitation–contraction coupling in skeletal muscle: a stimulating story. J. Muscle Res. Cell Motil. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- ITO K., NAKAZAWA K., KOIZUMI S., LIU M., TAKEUCHI K., HASHIMOTO T., OHNO Y., INOUE K. Inhibition by antipsychotic drugs of L-type Ca2+ channel current in PC12 cells. Eur. J. Pharmacol. 1996;314:143–150. doi: 10.1016/s0014-2999(96)00500-6. [DOI] [PubMed] [Google Scholar]
- KOPERA J., ARMITAGE A.K. Comparison of some pharmacological properties of chlorpromazine, promethazine and pethidine. Br. J. Pharmacol. 1954;9:392–401. doi: 10.1111/j.1476-5381.1954.tb00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUREBAYASHI N., OGAWA Y. Increase by trifluoperazine in calcium sensitivity of myofibrils in a skinned fibre from frog skeletal muscle. J. Physiol. 1988;403:407–424. doi: 10.1113/jphysiol.1988.sp017256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB G.D., STEPHENSON D.G. Calcium release in skinned muscle fibres of the toad by transverse tubule depolarisation or by direct stimulation. J. Physiol. 1990a;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB G.D., STEPHENSON D.G. Control of calcium release and the effect of ryanodine in skeletal muscle fibres of the toad. J. Physiol. 1990b;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMB G.D., STEPHENSON D.G. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J. Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUNIKONIS B.S., STEPHENSON D.G. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. J. Physiol. 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUNIKONIS B.S., STEPHENSON D.G. Effects of membrane cholesterol manipulation on excitation–contraction coupling in skeletal muscle of the toad. J. Physiol. 2001;534:71–85. doi: 10.1111/j.1469-7793.2001.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE I.S., PARK T.J., SUH B.C., KIM Y.S., RHEE I.J., KIM K.T. Chlorpromazine-induced inhibition of catecholamine secretion by a differential blockade of nicotinic receptors and L-type Ca2+ channels in rat pheochromocytoma cells. Biochem. Pharmacol. 1999;58:1017–1024. doi: 10.1016/s0006-2952(99)00181-1. [DOI] [PubMed] [Google Scholar]
- LÒPEZ J.R., SÀNCHEZ V., LÒPEZ M.J. Sarcoplasmic ionic calcium concentration in neuroleptic malignant syndrome. Cell Calcium. 1989;10:223–233. doi: 10.1016/0143-4160(89)90005-5. [DOI] [PubMed] [Google Scholar]
- MELZER W., HERRMANN-FRANK A., LÜTTGAU H.C. The role of Ca2+ ions in excitation–contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- REES B.B., STEPHENSON D.G. Thermal dependence of maximum Ca2+-activated force in skinned muscle fibres of the toad Bufo marinus acclimated at different temperatures. J. Exp. Biol. 1987;129:309–327. doi: 10.1242/jeb.129.1.309. [DOI] [PubMed] [Google Scholar]
- STEPHENSON D.G., LAMB G.D., STEPHENSON G.M. Events of the excitation–contraction–relaxation (E–C–R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol. Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- STEPHENSON D.G., WILLIAMS D.A. Calcium-activated force response in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J. Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPHENSON G.M., STEPHENSON D.G. Endogenous MLC2 phosphorylation and Ca2+-activated force in mechanically skinned skeletal muscle fibres of the rat. Pflügers Arch. 1993;424:30–38. doi: 10.1007/BF00375099. [DOI] [PubMed] [Google Scholar]
- TAKAGI A. Abnormality of sarcoplasmic reticulum in malignant hyperpyrexia. Shinkei Kenkyu no Shimpo (Tokyo) 1976;20:109–113. [Google Scholar]
- TAKAGI A. Chlorpromazine and skeletal muscle: a study of skinned single fibres of the guinea pig. Exp. Neurol. 1981;73:477–486. doi: 10.1016/0014-4886(81)90281-8. [DOI] [PubMed] [Google Scholar]
- UEDA K., YAGAMI T., ASAKURA K., KAWASAKI K. Chlorpromazine reduces toxicity and Ca2+ uptake induced by amyloid beta protein (25–35) in vitro. Brain Res. 1997;748:184–188. doi: 10.1016/s0006-8993(96)01300-5. [DOI] [PubMed] [Google Scholar]
- VALE M.G.P. Effects of phenothiazine drugs on the active Ca2+ transport by sarcoplasmic reticulum. Biochem. Pharmacol. 1985;34:4245–4249. doi: 10.1016/0006-2952(85)90279-5. [DOI] [PubMed] [Google Scholar]
- VOLPE P., COSTELLO B., CHU A., FLEISCHER S. The effect of phenothiazines on Ca2+ fluxes in skeletal muscle sarcoplasmic reticulum. Arch. Biochem. Biophys. 1984;233:174–179. doi: 10.1016/0003-9861(84)90614-3. [DOI] [PubMed] [Google Scholar]
- WORSFOLD M., PETER J.B. Kinetics of calcium transport by fragmented sarcoplasmic reticulum. J. Biol. Chem. 1970;245:5545–5552. [PubMed] [Google Scholar]
- YOSHIDA K. Effects of chlorpromazine on the skeletal muscle – a study using skinned single fibres of the guinea pig. Masui. 2000;49:484–490. [PubMed] [Google Scholar]