Abstract
The mechanisms of action of antagonists of the γ-aminobutyric acid C (GABAC) receptor picrotoxin, quercetin and pregnanolone were studied.
Ionic currents (chloride), mediated through human homomeric GABAρ1 receptors expressed in Xenopus oocytes, were recorded by two-electrode voltage clamp.
Dose–response (D–R) curves and kinetic measurements of GABAρ1 currents were carried out in the presence or absence of antagonists. Use-dependent actions were also evaluated.
Picrotoxin, quercetin and pregnanolone exerted noncompetitive actions.
IC50 values measured at the EC50 for GABA (1 μM) were as follows: picrotoxin 0.6±0.1 μM (Hill coefficient n=1.0±0.2); quercetin 4.4±0.4 μM (n=1.5±0.2); pregnanolone 2.1±0.5 μM (n=0.8±0.1).
These antagonists produced changes only in the slope of the linear current–voltage relationships, which was indicative of voltage-independent effects.
The effect of picrotoxin on GABAρ1 currents was use-dependent, strongly relied on agonist concentration and showed a slow onset and offset. The mechanism was compatible with an allosteric inhibition and receptor activation was a prerequisite for antagonism.
The effect of quercetin was use-independent, showed relatively fast onset and offset, and resulted in a slowed time course of the GABA-evoked currents.
The effect of pregnanolone was use-independent, presented fast onset and a very slow washout, and did not affect current activation.
All the antagonists accelerated the time course of deactivation of the GABAρ1 currents.
Keywords: GABA, GABAC receptors, chloride channels, picrotoxin, flavonoids, steroids, retina, Xenopus oocytes
Introduction
Three classes of γ-aminobutyric acid (GABA) receptors have been characterized based on their pharmacology, namely GABAA, GABAB and GABAC receptors (Macdonald & Olsen, 1994; Chebib & Johnston, 1999; Bowery et al., 2002). GABAA and GABAC receptors are ligand-gated chloride (Cl−) channels, while GABAB receptors are metabotropic receptors coupled to G proteins (Bormann & Feigenspan, 1995; Johnston, 1996; Mckernan & Whiting, 1996; Barnard et al., 1998; Moss & Smart, 2001; Bowery et al., 2002). GABAA receptors are heteromeric proteins formed by different subunits: α1–6, β1–4, γ1–3, δ, ɛ, π, θ (Cherubini & Conti, 2001), whereas GABAC receptors can be homomeric or heteromeric, and might be exclusively composed by ρ subunits (ρ1, ρ2 and ρ3) (Cutting et al., 1991; Polenzani et al., 1991; Enz et al., 1995; Ogurusu et al., 1997; 1999; Enz & Cutting, 1998; Zhang et al., 2001). A number of physiological roles have been proposed for GABAC receptors, mainly at vertebrate visual areas (Dong & Werblin, 1998; Schmidt et al., 2001; Shen & Slaughter, 2001; McCall et al., 2002).
GABAC receptors exhibit a distinctive pharmacological profile including resistance to the competitive GABAA antagonist bicuculline (Cutting et al., 1991; Polenzani et al., 1991; Sivilotti & Nistri, 1991; Shimada et al., 1992; Kusama et al., 1993; Wang et al., 1994; Ogurusu et al., 1999) and differences in their modulation by steroids, benzodiazepines and barbiturates (Harrison et al., 1987; Polenzani et al., 1991; Majewska, 1992; Prince & Simmonds, 1992; Woodward et al., 1992a, 1992b; Feigenspan et al., 1993; Amin & Weiss, 1994; Feigenspan & Bormann, 1994; Qian & Dowling, 1994; Calvo & Miledi, 1997; Morris et al., 1999). Meanwhile, picrotoxin and other Cl− channel inhibitors show clear differences in affinity, but produce similar effects on GABAA and GABAC receptors (Newland & Cull-candy, 1992; Woodward et al., 1992a; Feigenspan et al., 1993; Yoon et al., 1993; Qian & Dowling, 1994; Dong & Werblin, 1995; Gurley et al., 1995; Wang et al., 1995; Zhang et al., 1995; Dong & Werblin, 1996; Wotring et al., 1999). The mechanisms of picrotoxin antagonism of ionotropic GABA receptors are still controversial.
In addition, it has been demonstrated that quercetin and other flavonoids antagonize the responses mediated by GABAA and GABAC receptors (Goutman et al., 2003). It is also known that steroids can modulate ionotropic GABA receptors (Majewska, 1992). For example, pregnanolone potentiates GABAA receptor-mediated currents, but antagonizes GABAC receptor responses (Majewska, 1992; Calvo & Miledi, 1997; Morris et al., 1999). The precise mechanisms underlying the actions of flavonoids and steroids on the GABAC receptors were not studied before.
We study here the mechanisms of action of picrotoxin (an alkaloid), quercetin (a flavonoid) and pregnanolone (a steroid) on recombinant homomeric GABAρ1 receptors, expressed in Xenopus laevis oocytes. The effects of antagonists on dose–response (D–R) curves for GABA and on the kinetics of GABA-evoked currents were evaluated in two-electrode voltage-clamp electrophysiological experiments.
Methods
RNA preparation, oocyte isolation and cell injection
A human cDNA encoding the ρ1 GABAC receptor subunit, cloned in pBS (SK-), a vector suitable for in vitro transcription, was used as a template to synthesize cRNAs in vitro. cRNA solutions (0.1–0.3 ng nl−1) were prepared in RNase-free H2O and stored at −70°C.
Xenopus laevis (Nasco, Modesto, CA, U.S.A.) oocytes at stages V and VI were used for expression of exogenous cRNAs. Isolation and maintenance of cells were carried out as previously described (Miledi et al., 1989). Briefly, frogs were anaesthetized with 3-aminobenzoic-acid ethylester (∼1 g ml−1) and ovaries surgically removed. Ovaries were incubated with 200 U ml−1 collagenase for 50 min at room temperature (RT), and isolated oocytes were maintained in an incubator at 17°C in Barth's medium (in mM: 88 NaCl; 0.33 Ca(NO3)2; 0.41 CaCl2; 1 KCl; 0.82 MgSO4; 2.4 NaHCO3; 10 HEPES and 0.1 mg ml−1 gentamycin; pH adjusted to 7.4 with NaOH). After 1 day, each oocyte was manually microinjected (microinjector Drummond Sci. Co., Broomall, PA, U.S.A.) with 50 nl of a solution containing 5–15 ng of cRNA.
Electrophysiological recordings
Two-electrode voltage-clamp current recordings were performed 3–7 days after oocyte injection with an Axoclamp 2B amplifier (Axon Instruments, Union City, CA, U.S.A.). Standard glass recording electrodes were made in a puller Narishige PB-7 (Narishige Scientific Instrument Lab., Tokyo, Japan) and filled with 3 M KCl. Resistance values were approximately 1 MΩ. The holding potential was set to –70 mV and current traces acquired in a PC through a Labmaster TL-1 DMA interface (Scientific solutions Inc, Solon, OH, U.S.A.) using AXOTAPE software (Axon Instruments).
Cells were placed in a chamber (volume 100 μl) continuously superfused (12 ml min−1) with frog Ringer's solution (in mM: 115 NaCl; 2 KCl; 1.8 CaCl2; 5 HEPES; pH 7.0). The agonist and other drugs were applied through the perfusion system. The speed of solution exchange (dead time plus equilibration time in the chamber) was measured as follows: an oocyte expressing GABAρ1 receptors was placed in the recording chamber and a response evoked with a low concentration of agonist. Once the current reached the maximum, a low Cl− Ringer's solution (in mM: 65 NaCl; 2 KCl; 1.8 CaCl2; 1.8 MgCl2; 50 mM methane sulphonic acid; 5 HEPES; pH 7.0) was applied through the superfusion system, without changing the GABA concentration. The time required for equilibration, after the change in the current driving force, was measured. Complete solution exchange (≈95%) was achieved in less than 4 s.
Picrotoxin, quercetin and steroid stocks were made up freshly each day, and solutions prepared in normal Ringer's solution containing DMSO to a maximal concentration of 0.3%. This concentration of DMSO did not produce either alterations in the oocyte properties, or direct actions on responses to the drugs tested. The pH was always adjusted to 7.0. Unless stated, antagonists were applied 30 s before their co-application with GABA, and both washed at the same time after responses reached a plateau. All the experiments were carried out at RT (23–24°C).
Materials
The transcription kit mMessage mMachine was purchased from Ambion (Austin, TX, U.S.A.) and Type I or Type II collagenase from Worthington (Freehold, NJ, U.S.A.). Picrotoxin, quercetin (3,3′,4′,5′,7-pentahydroxyflavone), pregnanolone (5β-pregnan-3α-ol-20 one), isopregnanolone (5α-pregnan-3α-ol-20 one), epipregnanolone (5β-pregnan-3β-ol-20 one), allopregnanolone (5α-pregnan-3β-ol-20 one), 1,2,5,6-tetrahydropyridine-4-yl methylphosphinic acid (TPMPA), all the salts, HEPES, 3-aminobenzoic-acid ethylester, RNase-free H2O and dimethyl sulphoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.).
Data analysis
Data were analysed with Origin v. 6.0 (MicroCal, Northampton, MA, U.S.A.). Statistical analysis was performed using Student's t-test (two-tail). Kinetic studies were performed in two ways, giving equivalent results. In order to avoid a direct or indirect reference to microscopic rate constants of gating schemes, we avoided the use of fittings to exponential equations and calculations of the characteristic τ. The first method consisted of the calculation of the corresponding activation (tact 10–90%) and de-activation constants (tdeact 10–90%), performed over current records ensembles (averaged traces). tact 10–90% represents the time required to go from 10 to 90% of the final current amplitude, and tdeact 10–90% represents the time required to go from 10 to 90% of the whole current-deactivation process. The second method consisted of the calculation of the tact 10–90% and tdeact 10–90% from individual current traces, and then the average of those values.
Inhibition curves for antagonists were fit with the logistic equation: I=100[1/1+(B/IC50)n], where B is the concentration of antagonist used, IC50 is the concentration of antagonist that produces half-maximal inhibition and n is the Hill coefficient. Dose–response curves (D–R) for GABA were fit with another expression of the mentioned logistic equation: Imax=100{1−1/[1+(A/EC50)n]}, where A is the agonist concentration used, EC50 is the concentration of agonist that elicits half maximal responses and n is the Hill coefficient.
Results
Characterization of the actions of picrotoxin on homomeric GABAρ1 receptors
Antagonism of GABAρ1 receptors by picrotoxin
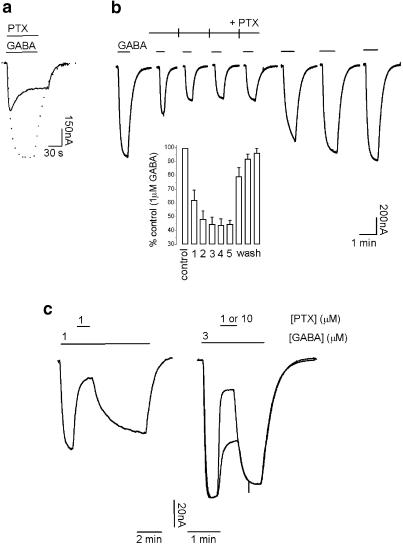
The first trace in Figure 1a illustrates a typical whole-cell response to 1 μM GABA recorded from an oocyte expressing GABAρ1 receptors at a membrane potential of −70 mV. GABA produced large current responses ranging from 300 to 500 nA approximately. The amplitude of the GABAρ1 responses recorded in the same cell was markedly reduced in the presence of 0.1–30 μM picrotoxin, as observed in the following traces. A transient peak current was usually observed during the onset of the response to the agonist, when picrotoxin was simultaneously applied with GABA (Figure 1a). This can be noticed, for example, in the presence of 0.1, 0.3 or 1 μM picrotoxin, where a stable decay in the maximal current amplitude took place. These transients were also observed if picrotoxin was applied before rather than simultaneously with GABA (see Discussion below).
Figure 1.
Inhibition of GABAρ1 responses by picrotoxin: (a) Representative recordings illustrating the effect of increasing concentrations of picrotoxin on GABAρ1 currents elicited by 1 μM GABA. GABA applications (indicated by bars) were spaced by 3-min washings and currents recorded in the presence of picrotoxin (pre-applied during 40s) were flanked by control responses (not shown for simplicity). For this and subsequent figures, oocytes were voltage-clamped at −70 mV, unless stated. (b) Inhibition curve for picrotoxin. Inhibition was expressed as a fraction of the control values obtained for 1 μM GABA. (c) Dose–response curves for GABA in the presence of 1, 10 and 100 μM picrotoxin. Response amplitudes were expressed as a fraction of 30 μM GABA-evoked currents. (d) Current–voltage relationship of the 1 μM GABA-evoked responses with or without 10 μM picrotoxin. The holding potential (Vh) ranged from −120 to +40 mV.
In order to evaluate the potency of picrotoxin, inhibition curves were carried out. Due to the delay found in the blockage produced by picrotoxin, only steady-state inhibition values were used. Figure 1b illustrates the averaged data from 10 different experiments, where increasing concentrations of picrotoxin were tested at the GABA EC50 (1 μM). Full inhibition of the GABAρ1 receptor responses was observed only for values higher than 30 μM. The IC50 for picrotoxin was 0.6±0.1 μM with a Hill coefficient of 1.0±0.2. Lower degrees of inhibition were obtained for higher (10 and 30 μM) GABA concentrations (Figure 1b).
Dose–response analysis
Figure 1c illustrates D–R curves for GABA, either under control conditions or performed in the presence of 1, 10 and 100 μM picrotoxin. Picrotoxin produced rightward shifts inducing significant changes in the GABA EC50. The values were as follows: control, EC50=1.0±0.1 μM (Hill n=1.8±0.2); 1 μM picrotoxin, EC50=2.1±0.3 μM (Hill n=2.0±0.3); 10 μM picrotoxin, EC50=6.8±0.1 μM (Hill n=1.6±0.3) and 100 μM picrotoxin, EC50=8.6±1.0 μM (Hill n=0.5±0.2) (P<0.05). These experiments also showed that 100 μM picrotoxin caused significant reductions in the GABA maximal efficacy. Shifts to the right in the D–R curve with concomitant insurmountable antagonistic effects at high doses of GABA were indicative of a noncompetitive allosteric effect.
Voltage-independent effects of picrotoxin on GABAρ1 receptors
The effect of picrotoxin was independent of the membrane potential. A significant change in the slope without alteration in the linearity of the I–V relationship was observed in the presence of 10 μM picrotoxin in the range between −120 and +40 mV. Figure 1d illustrates a representative experiment (out of five).
Use-dependent actions of picrotoxin on GABAρ1 receptors
Figure 2a shows GABAρ1 responses elicited by 1 μM GABA alone (control, dashed trace) or by co-application of 1 μM GABA and 1 μM picrotoxin (solid trace). As mentioned before, ionic currents in the presence of picrotoxin transiently peaked and then slowly decreased due to a continuous increment in the effect of the antagonist. The percentage of inhibition measured at the peak of the GABAρ1 currents was 45.7±2.5% (n=6), while the magnitude of inhibition calculated at the steady state was 64.0±4.5% (n=6). This delay for reaching a steady-state level of inhibition could be simply attributed to the slow developing action of picrotoxin on GABAρ1 receptors. However, identical results were obtained if picrotoxin was delivered by superfusion 3 min before GABA (not shown). Pretreatment with picrotoxin should allow inhibition to reach a steady state in advance of agonist application if the antagonist's binding site could be accessed at rest. These results are in agreement with previous evidences (Woodward et al., 1992a; Qian & Dowling, 1994), and suggest that the slow onset observed for picrotoxin-mediated inhibition was due to a use-dependent mechanism, implying that activation of the channel was a prerequisite for inhibitory interactions.
Figure 2.
Weak use dependency and slow kinetics characterizing the inhibition of GABAρ1 currents by picrotoxin: (a) representative current trace obtained by co-application of 1 μM GABA and 1 μM picrotoxin (solid trace; dashed trace represents control response to 1 μM GABA). (b) Characteristic responses elicited by consecutive applications of 1 μM GABA. Picrotoxin application (1 μM) started 40 s before the second application of GABA and extended to the fifth one (ticks represent 3-min intervals separating GABA applications). Histogram (inset) summarizing these experiments (n=4). (c) Wash-out of picrotoxin inhibition by different concentrations of GABA (1 μM, left trace and 3 μM, right trace). Picrotoxin was removed after steady-state inhibition was achieved. Picrotoxin (1 μM) was applied on top of a response to 1 μM GABA (left trace) and 1 or 10 μM picrotoxin was applied on a response to 3 μM GABA (superimposed right traces).
Use dependency of the actions of picrotoxin on GABAρ1 currents was also revealed by consecutive applications of 1 μM GABA during extended incubations with 1 μM picrotoxin (Figure 2b). Under these conditions, the responses evoked by the first application of GABA were blocked in 37.7±7.1% (n=4), whereas, for the next three successive GABA responses, slight but steadily increases in the levels of inhibition were observed, reaching a maximum value of 55.8±4.4% (n=4). These inhibition values did not significantly differ from those obtained in experiments like the one illustrated in Figure 2a (see above). Consistently, the wash-out of picrotoxin was also facilitated by channel activity. After a sustained picrotoxin inhibition, long washings (45 min) with frog Ringer's solution were required, in order to recover the GABA-evoked control currents (not shown). In contrast to this slow apparent picrotoxin dissociation, three successive applications of GABA (Figure 2b) accelerated picrotoxin wash-out, reducing the time needed for a complete recovery of the responses to about 15 min. The first current recorded during picrotoxin wash-out (sixth record, Figure 2b) had a very slow activation rate, and did not reach a plateau in the period of agonist application (50 s), due to the partial washing of the drug.
In addition, we observed that the recovery of GABAρ1 receptors from picrotoxin inhibition showed dependency on the agonist concentration. This dependency was revealed by applications of picrotoxin performed on top of the responses (once the current plateau was reached) elicited by different concentrations of GABA (Figure 2c). Wash-out of picrotoxin was faster when GABA concentration was increased. At 1 μM GABA, recovery time (measured as the 10–90% rise interval) from the effect caused by 1 μM picrotoxin was 128±13 s and the inhibition produced was 64.7±4.5% (n=7) (left trace). A three-fold increment in the concentration of GABA (3 μM) yielded 10–90% rise intervals of about six times faster: 20±2 s, at the same picrotoxin concentration with an inhibition of 38.9±7.8% (n=5). At 3 μM GABA, wash-out rates were faster (21±1 s), even for higher concentrations of picrotoxin (e.g. 10 μM) that induced more inhibition (77.6±4.0%; n=4).
Effects of picrotoxin on GABAρ1 current-deactivation kinetics
Time courses for activation and deactivation of GABAC receptors are relatively slow; a long-lasting relaxation agrees with data showing that GABAC receptors lock the agonist on its binding site (Chang & Weiss, 1999). In agreement with these previous evidences, we observed that ionic currents reached the baseline approximately 1 min after the agonist (GABA 1 μM) was removed from the chamber. Deactivation was well fit to a single exponential function with a characteristic τdeact=16±2 s (n=17) (see Methods), and this value was similar to previously published data (Chang & Weiss, 1999). In order to quantify the effects of picrotoxin on GABAρ1 current-deactivation kinetics, relaxation constants instead of τ (see Methods) were measured in the presence or absence of this antagonist. Control responses evoked by 1 μM GABA had a tdeact 10–90% of 34±3 s (n=17). The application of picrotoxin during agonist wash-out significantly accelerated GABAρ1 receptor deactivation (Figure 3). In the presence of 1 μM picrotoxin, tdeact 10–90% was 18±4 s (n=5, P<0.05), and for 10 μM picrotoxin t10–90% was 6±1 s (n=3, P<0.05).
Figure 3.
GABAρ1 current-deactivation kinetics accelerated by picrotoxin: representative current traces (superimposed and scaled) show the washing out of 1 μM GABA responses in control conditions or in the presence of 1 and 10 μM picrotoxin. The time to baseline was accelerated by picrotoxin applications upon GABA removal. The inset summarizes the t10–90% calculated for control responses or currents recorded in the presence of picrotoxin or 10 μM TPMPA (see text).
It has been demonstrated for GABAA receptors that competitive antagonists do not accelerate receptor deactivation (Bianchi & Macdonald, 2001). In contrast, noncompetitive GABAA antagonists including picrotoxin, applied during the entire interval of current relaxation, produce an increase in the deactivation rates (Bianchi & Macdonald, 2001). In order to confirm if this was also the case for GABAC receptors, we studied the effect of the competitive GABAC specific antagonist TPMPA on GABAρ1 current relaxation. We observed that, up to 10 μM, TPMPA had no effects on channel deactivation. Thus, tdeact 10–90% in the presence of 10 μM TPMPA was 36±7 (n=4) (Figure 3). These results confirmed the competitive nature of TPMPA (Ragozzino et al., 1996) and a noncompetitive antagonism of picrotoxin over GABAρ1 receptors.
Characterization of the actions of quercetin on homomeric GABAρ1 receptors
Antagonism of GABAρ1 receptors by quercetin
It is known that several flavonoids modulate GABAC receptors. For example, quercetin antagonizes GABAρ1 currents with an IC50 of 4.4 μM and a Hill coefficient of 1.5 in a dose-dependent and reversible manner, and produces similar actions on GABAA receptors (Goutman et al., 2003). We now analysed the mechanism of action of quercetin on GABAρ1 receptors more in detail.
Figure 4a illustrates the effect of increasing concentrations of quercetin on GABAρ1 currents elicited by 1 μM GABA. Figure 4b shows that (10 and 30 μM) quercetin produced rightward shifts, and depression of the maximal responses of D–R curves for GABA. Significant increases (P<0.05) in the GABA EC50 were observed after quercetin treatment: control EC50=1.0±0.1 μM (Hill n=1.8±0.2), quercetin 10 μM EC50=2.7±1.4 μM (Hill n=1.5±1.4) and quercetin 30 μM EC50=3.2±0.5 μM (Hill n=3.2±3.0), concomitantly with reductions in the maximal efficacy (see Figure 4b). Consequently, the antagonism exerted by quercetin at the GABAρ1 receptor was noncompetitive.
Figure 4.
Inhibition of GABAρ1 responses by quercetin: (a) representative traces illustrating the effect of increasing concentrations of quercetin on GABAρ1 currents elicited by 1 μM GABA. (b) Dose–response curves for GABA in the presence of 10 and 30 μM quercetin. Response amplitudes were expressed as fraction of 30 μM GABA-evoked currents. (c) Current–voltage relationship of 1 μM GABA-evoked responses with or without 10 μM quercetin. The holding potential (Vh) ranged from −120 to +40 mV.
Actions of quercetin were independent of the membrane voltage. Changes were only observed in the slope, but not the shape of the linear I–V relationship (Figure 4c).
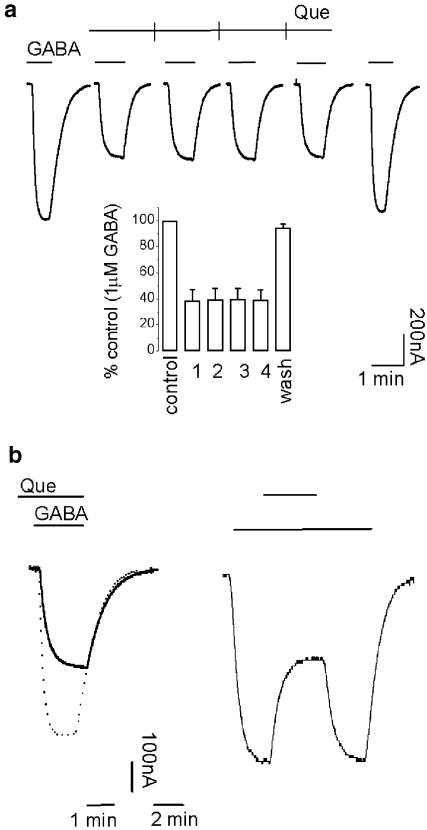
In contrast to picrotoxin actions, the effects of quercetin were use-independent. Repetitive applications of 4 μM quercetin produced similar values of inhibition (Figure 5a). The application of quercetin before, together or on top of the GABA-evoked current produced identical results (Figure 5b). Taken together, these results suggested that the effects did not depend on channel activation. Wash-out of quercetin showed no dependency either on channel activity (Figure 5a, b). Responses recovered very fast from quercetin antagonism; a 3 min wash in normal frog Ringer's solution was enough to return to the control values of amplitude and current kinetics.
Figure 5.
Use independency and fast kinetics characterizing the inhibition of GABAρ1 currents by quercetin: (a) same protocol as in Figure 2b. Histogram (inset) summarizing these experiments (n=4). GABA concentration was 1 μM and quercetin 4 μM. (b) Effects of 4 μM quercetin on GABAρ1 responses to 1 μM GABA applied before (left trace) or on top (right trace) of receptor activation. Quercetin was removed once inhibition reached a steady state. The superimposed dashed trace represents a control response to 1 μM GABA.
Effects of quercetin on GABAρ1 current kinetics
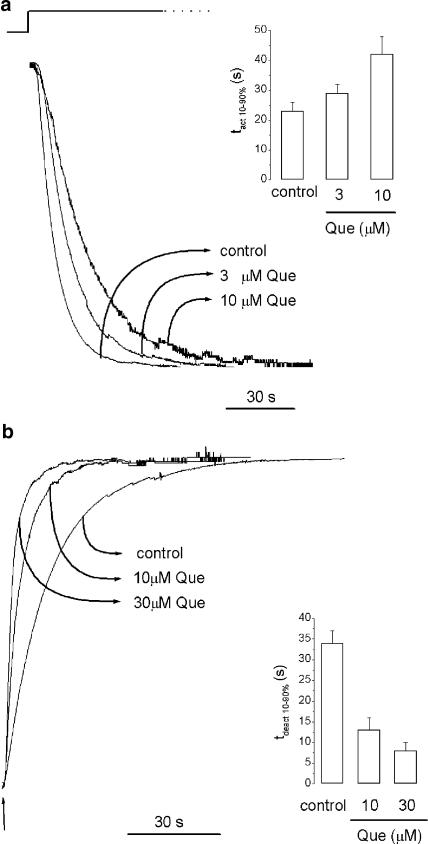
Changes in both activation and deactivation of the GABAρ1 currents were observed in the presence of this flavonoid. Quercetin slowed activation of GABAρ1 currents elicited by 1 μM GABA (Figure 6a). The time required to reach maximum amplitude was markedly different in the presence of quercetin and changes in the tact 10–90% were observed. The control activation constant was tact 10–90%=23±3 s (n=7), and rose to tact 10–90%=30±3 s (n=6) or tact 10–90%=42±6 s (n=4) in the presence of either 3 or 10 μM quercetin, respectively (P<0.05).
Figure 6.
Quercetin slows activation and accelerates the deactivation of the GABAρ1 currents: (a) representative current traces (superimposed and scaled) illustrating the effect of 3 and 10 μM quercetin on current activation. The inset summarizes the tact 10–90% calculated for control responses to 1 μM GABA or in the presence of quercetin. (b) Representative current traces (superimposed and scaled) showing the wash-out of control responses to 1 μM GABA or in the presence of 10 and 30 μM quercetin. The time to baseline was accelerated by quercetin application upon GABA removal. The inset summarizes the tdeact 10–90% calculated for control responses to 1 μM GABA or in the presence of quercetin.
Quercetin produced a more rapid deactivation of GABAρ1 currents as well (Figure 6b). In the presence of 10 μM quercetin, tdeact 10–90%=13±3 s (n=5, P<0.05), 30 μM quercetin tdeact 10–90%=8±2 s (n=6, P<0.05). These results suggest that quercetin antagonism was also noncompetitive (Bianchi & Macdonald, 2001), but differed from that observed for picrotoxin.
Characterization of the actions of pregnanolone on homomeric GABAρ1 receptors
Antagonism of GABAρ1 receptors by pregnanolone
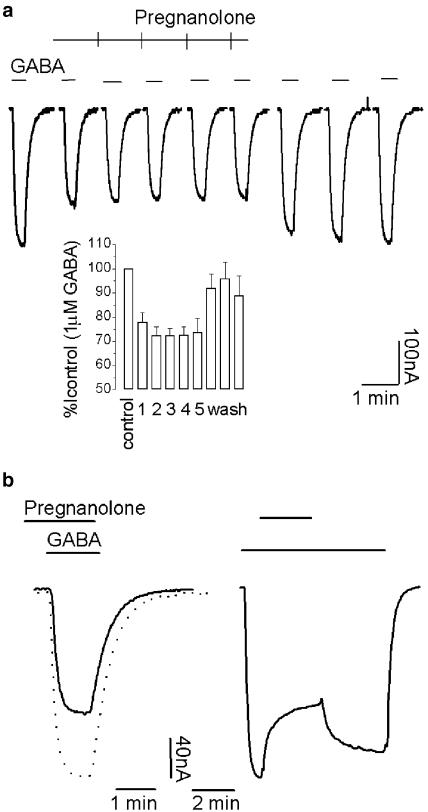
The effects of pregnanolone sulphate on GABAρ1 currents were in agreement with previous reports (Calvo & Miledi, 1997; Morris et al., 1999). Pregnanolone 10 μM inhibited responses elicited by 1 μM GABA by 63.2±3.5% (n=5) (Figure 7a). The maximal concentration of pregnanolone tested was 100 μM, due to its solubility limit. The effect was stereoselective, since under similar conditions (10 μM steroid and 1 μM GABA) none of the pregnanolone isomers were capable to antagonize GABA responses. The percentage of inhibition for isopregnanolone was 1.5±0.7%, while for epipregnanolone 3.6±1.8% and allopregnanolone 8.34±4.2% (n=4).
Figure 7.
Inhibition of GABAρ1 responses by pregnanolone: (a) representative traces illustrating the effect of increasing concentrations of pregnanolone on GABAρ1 currents elicited by 1 μM GABA. (b) Inhibition curves for pregnanolone. The effect of pregnanolone was evaluated at three different concentrations of GABA: 0.4, 1 and 30 μM. Inhibition was expressed as a fraction of the control values obtained at each agonist concentration. (c) D–R curves for GABA in the presence of 1 and 10 μM pregnanolone. Response amplitudes were expressed as a fraction of 30 μM GABA-evoked currents. (d) Degree of antagonism produced by pregnanolone at different GABA concentrations. The abscissa represents a logarithmic scale. (e) Current–voltage relationship of 1 μM GABA-evoked responses with or without 10 μM pregnanolone. The holding potential (Vh) ranged from −120 to +40 mV.
Inhibition curves for pregnanolone were carried out at different concentrations of GABA. IC50's were not significantly different: 1.1±0.1 μM at 0.4 μM GABA; 1.9±1.3 μM at GABA 1 μM; 1.7±0.3 μM at GABA 30 μM (Figure 7b). Maximal values of inhibition, produced by pregnanolone, decreased if higher concentrations of agonist were used. Pregnanolone was inhibited in approximately 84% responses elicited by GABA 0.4 μM, while 70 or 39% of inhibition was obtained using 1 or 30 μM GABA, respectively (Figure 7b).
Figure 7c illustrates D–R curves for GABA performed either alone or in the presence of pregnanolone. EC50's were as follows: 1 μM pregnanolone EC50: 1.2±0.1 μM (Hill n=2.0±0.2) and 10 μM pregnanolone EC50: 1.2±0.1 μM (Hill n=2.3±0.6). These values did not differ significantly from the control value: EC50=1.0±0.1 μM (Hill n=1.8±0.2). These results suggest that the sensitivity for GABA did not change in the presence or the absence of this steroid.
An insurmountable inhibitory effect for pregnanolone was also seen in D–R curves (Figure 7c) at saturating concentrations of GABA (30 μM). Maximal responses were inhibited approximately by 13% in the presence of 1 μM pregnanolone and 40% at 10 μM. Lack of changes in sensitivity together with a decrease in maximal efficacy would be compatible with an uncompetitive mechanism of action (Chen & Lipton, 1997). However, this was not the case for pregnanolone, because we did not observe increases in blockage efficacy at higher levels of activation (i.e. using higher concentrations of agonist) (Figure 7d), which is typical for an uncompetitive blockage. In contrast, pregnanolone blockage appeared to be a little more pronounced at low concentrations of GABA, which is compatible with a noncompetitive mechanism of action.
The action of pregnanolone was independent of the membrane voltage. Changes were only observed in the slope, but not the shape of the linear I–V relationship (Figure 7e).
In agreement with previous observations, the action of pregnanolone was persistent (Morris et al., 1999). For example, for 10 μM pregnanolone, the inhibitory effects were still apparent even after a 40 min wash with Ringer's solution, and wash-out was not facilitated by channel activity (not shown). Use-dependent effects were not observed for pregnanolone inhibition (Figure 8a), and on top applications of this steroid gave similar degrees of inhibition (Figure 8b).
Figure 8.
Use independency and slow washing kinetics characterizing the inhibition of GABAρ1 currents by pregnanolone: (a) same protocol as in Figure 2b. Inset: histogram summarizing these experiments (n=4). GABA concentration was 1 μM and pregnanolone 1 μM. (b) Effect of 1 μM pregnanolone on GABAρ1 responses applied before (left trace) or on top (right trace) of receptor activation by 1 μM GABA. Pregnanolone was removed once inhibition reached the steady state. The superimposed dashed trace represents a control response to 1 μM GABA.
Effects of pregnanolone on GABAρ1 current kinetics
Pregnanolone did not affect the time course of activation of the GABAρ1 currents, and accelerated its deactivation (Figure 9). The activation of GABAρ1 currents evoked by 1 μM GABA in the presence of 1 and 10 μM pregnanolone showed no differences with respect to control values: control tact 10–90%=17±2 s (n=4), while in the presence of 10 μM pregnanolone tact 10–90%=16±2 s (n=4). Meanwhile, deactivation of GABAρ1 currents was accelerated in the presence of 2 or 10 μM pregnanolone. The values measured were: tdeact 10–90%=29±1 s (n=6) and tdeact 10–90%=23±1 s (n=8, P<0.05), respectively. These results suggested a noncompetitive mechanism for pregnanolone antagonism.
Figure 9.
GABAρ1 current-deactivation kinetics accelerated by pregnanolone: representative current traces (superimposed and scaled) show the wash-out of 1 μM GABA responses, control or in the presence of 2 and 10 μM pregnanolone. The time to baseline was accelerated by pregnanolone application upon GABA removal. The inset summarizes the t10–90% calculated for control responses or currents recorded in the presence of pregnanolone.
Discussion
The mechanisms of action of three different GABAρ1 receptor antagonists were studied through D–R analysis and kinetic experiments. Our results suggest that picrotoxin, pregnanolone and quercetin antagonize GABAρ1 receptors via different mechanisms.
Picrotoxin
Direct evidences for an allosteric noncompetitive mechanism underlying picrotoxin inhibition of GABAC receptors are provided. We studied the mechanism of action of picrotoxin at the human homomeric GABAρ1 receptors expressed in Xenopus oocytes. D–R curves for GABA were shifted to the right in the presence of 1–100 μM picrotoxin (Figure 1a). The inhibition was surmounted by saturating concentrations of GABA at low concentrations of picrotoxin, but not fully overcome at higher concentrations of this antagonist. It is known that pure noncompetitive antagonists show a constant potency of inhibition all over the range of agonist concentration, but inhibition curves showed that picrotoxin instead acted more strongly on GABAρ1 responses elicited by lower doses of GABA (Figure 1b). These data would suggest a mixed or complex type of antagonism and are in agreement with previous reports on native GABAA (Smart & Constanti, 1986) and GABAC receptors (Woodward et al., 1993; Qian & Dowling, 1994; Wang et al., 1994).
The IC50 of picrotoxin found under the present conditions is consistent with data reported before (Wang et al., 1994) and similar to the value reported for bovine receptors (Woodward et al., 1992a). Some variation can be observed in IC50's among native GABAC receptors from diverse cold-blooded animals (Qian & Dowling, 1994; Takahashi et al., 1995; Dong & Werblin, 1996), indicating that many receptor variants could exist.
Compelling evidences have pointed out against an open-channel blocking mechanism (pore blocking) for picrotoxin inhibition of ionotropic GABA receptors (Smart & Constanti, 1986; Newland & Cull-candy, 1992; Yoon et al., 1993). An allosteric action of mixed characteristics, competitive and noncompetitive, was alternatively suggested (Smart & Constanti, 1986; Qian & Dowling, 1994). More recently, site-directed mutagenesis studies have pinpointed a single amino-acid residue in the second membrane-spanning region as involved in determining picrotoxin sensitivity at GABAA and GABAC receptors (Gurley et al., 1995; Xu et al., 1995; Wang et al., 1995; Zhang et al., 1995; Pan et al., 1997; Chang & Weiss, 1998). It has also been demonstrated that, in GABAC receptors, both competitive and noncompetitive components of inhibition are determined by the same amino acid (Wang et al., 1995). We did not observe here an uncompetitive inhibition for picrotoxin acting on GABAρ1 receptors, as expected for an open-hannel blocker (Chen & Lipton, 1997). Our results agree with data from site-specific fluorescence studies supporting a noncompetitive mechanism as the more realistic model for the action of picrotoxin (Chang & Weiss, 2002), and we will see below that kinetic experiments strengthen this hypothesis.
It has been demonstrated that the deactivation of GABAA receptors is accelerated by noncompetitive, but not by competitive, antagonists (Bianchi & Macdonald, 2001), a property that is attributed to a strong increase in affinity for the agonist when the receptor channel is in the open state. So, while bicuculline does not change GABAA current deactivation because of its inability to displace the agonist from its binding site, picrotoxin accelerates deactivation due to the fact that its action is not dependent on agonist binding (Bianchi & Macdonald, 2001). In addition, GABAC receptors show a very slow deactivation kinetics, which has been related to a mechanism consisting of agonist locking in its binding site during channel openings (Chang & Weiss, 1999). Based on all these previous evidences, we decided to study the mechanism of action of picrotoxin analysing the effect of the toxin on the kinetics of GABA-induced ionic currents. Strong increases in the deactivation parameters of the GABAρ1 currents were observed in the presence of picrotoxin. This effect was observed even at a 1 μM concentration that only produced a shift to the right in the D–R curve, but not a significant insurmountable blockage. Under similar conditions, TPMPA, the specific competitive antagonist of GABAC receptors, did not change this relaxation time. Thus, taken together, all our results were consistent with an allosteric noncompetitive mechanism of inhibition.
On the other hand, use-dependent actions have been described for picrotoxin on ionotropic GABA receptors (Newland & Cull-candy, 1992; Woodward et al., 1992a; Yoon et al., 1993). Thus, GABA binding might induce a conformational change in the structure of the picrotoxin-binding site that accounts for the described use-dependent picrotoxin blockage (Newland & Cull-candy, 1992; Chang & Weiss, 2002). The effects of picrotoxin on GABAC receptors have a weak use-dependent component compared to GABAA receptors (Woodward et al., 1992a; Dong & Werblin, 1996). Thus, in the continuous presence of picrotoxin, an increase in the level of inhibition developed during repeated applications of GABA (Figure 2b) that can be attributed to a use-dependent action. As GABAρ1 receptors do not desensitize, or desensitize very little at moderate concentrations of agonist, this kind of changes could be accurately evaluated during sustained current responses (Figure 2a). Similar results were previously observed for the actions of picrotoxin on GABAC receptors from catfish retina (Dong & Werblin, 1996).
At high concentrations of GABA, early transients induced by picrotoxin were prevented, indicating that steady-state blockage and steady-state activation were attained, at least, simultaneously. From this observation, we can also conclude that the time course of the action of picrotoxin depended on the agonist concentration. On the other hand, increases in picrotoxin concentration also eliminated transient effects, suggesting that the rate of picrotoxin blockage was dose-dependent.
Recovery from picrotoxin blockage occurred in the absence of GABA after long washings with Ringer's solution. However, it was clearly facilitated by receptor activation (Figure 2b). Similarly, GABAC receptors from bovine retina and catfish also show use-dependent effects during recovery from inhibition (Woodward et al., 1992a; Dong & Werblin, 1996), and this is also true for GABAA receptors (Newland & Cull-candy, 1992).
A clear agonist concentration dependency was observed during the washout of picrotoxin in the presence of GABA. High concentrations of agonist facilitated recovery of the responses, independently of the concentration of picrotoxin (Figure 2c). Recent studies have demonstrated structural rearrangements induced by picrotoxin at a site near the agonist-binding pocket of GABAρ1 receptors (Chang & Weiss, 2002). The authors proposed that this antagonist could not reach its binding site unless the channel is open, and that picrotoxin produces a conformational change that closes the channel. We think that this interpretation can account for the D–R curve profile and use-dependent effects observed in our experiments.
Quercetin
We have recently characterized the actions of a group of flavonoids on GABAA and GABAC receptors, describing the overall inhibiting effect of quercetin and related compounds. Quercetin showed to be not only active at ionotropic GABA receptors, but also to modulate other ligand-gated ion channels (Goutman et al., 2003). Now, we propose a noncompetitive mechanism for the action of quercetin on GABAρ1 receptors, based on the increase observed in the EC50's in the D–R curves for GABA, with a concomitant reduction in the maximal efficacy. A slight dependency on agonist dose was also found for the inhibition of quercetin, with lower potency at higher doses of the agonist. The action of quercetin was easily washable and showed a short onset time. Deactivation kinetics indicated that a decrease in relaxation time was seen in the presence of 10 and 30 μM quercetin, confirming a noncompetitive mechanism of action. In addition, a delay in the activation time course was observed. No findings of a similar effect have been reported for other GABA receptor modulators, and additional experiments will be necessary in order to postulate a more detailed mechanism of action.
The effect of quercetin was clearly use-independent, since no changes in blockage efficacy were seen during repetitive activation of currents in the presence of this flavonoid, and a very fast unblockage process was always observed. Flavonoids could cause a rebound on the responses during washing (Goutman et al., 2003), which was more prominent when higher doses of GABA were used (Figure 4a). Quercetin unbinding and GABA trapping during channel opening could be the major causes for this phenomenon.
Preliminary observations show that quercetin effects on GABAA receptors share many of the features described here for its actions on GABAC receptors (data not show). However, additional studies will be required to determine if a common mechanism underlies the quercetin antagonism of GABAA receptors and other members of the ligand-gated ion channels superfamily.
Pregnanolone
Modulation of GABAC receptors by neurosteroids, including pregnanolone, have been previously demonstrated (Calvo & Miledi, 1997; Morris et al., 1999). Pregnanolone is part of a group of naturally occurring progesterone metabolites, which differ in the stereochemistry of a pair of carbon atoms (Majewska, 1992). We observed that pregnanolone was the sole isomer showing a clear inhibitory effect on GABAρ1 currents, suggesting that inhibition could not be attributed to a nonspecific disturbance of the lipid milieu around the channel. Bidirectional effects were also reported for some steroids acting on GABAρ1 receptors, like, for example, alphaxolone or allopregnanolone (Morris et al., 1999). Pregnanolone, instead, showed a simple antagonism with no contrasting actions regarding the dose of agonist used.
No significant differences in the sensitivity to pregnanolone were evidenced at different concentrations of GABA, yielding all IC50 values between 1 and 2 μM. This is consistent with a noncompetitive inhibition, which was also confirmed by the reduction in maximal efficacy at D–R curves and increases in GABAρ1 current-deactivation velocity, similar to that observed for picrotoxin and quercetin. Conversely, no significant increase in EC50 for GABA was found in the presence of 1 and 10 μM pregnanolone, whereas equivalent concentrations of picrotoxin (1 and 10 μM) or quercetin (10 and 30 μM) produced marked shifts to the right in D–R curves (10-fold for picrotoxin and 7.5 for quercetin). Higher steroid concentrations were not tested because of its poor solubility in water.
Recovery from inhibition by pregnanolone required a long wash with Ringer's solution, confirming previous results (Woodward et al., 1992a; Morris et al., 1999; Calvo & Miledi, 1997), and was unaltered by receptor activation even at saturating concentrations of GABA. Given the hydrophobic nature of steroids, we suggest that pregnanolone unbinding was highly limited because of the strongly favoured partitioning in the lipid–receptor interface compared to the aqueous milieu, and that this restriction could not be overcome by channel opening. The onset for pregnanolone inhibition was tested using repetitive activations, as previously described for picrotoxin or quercetin. No significant changes were found in the levels of inhibition; thus, no changes in the percentage of blockage over successive applications indicated no use dependency.
Taken together, the present results show that picrotoxin, quercetin and pregnanolone all noncompetitively antagonized homomeric GABAρ1 receptors, each showing different allosteric mechanisms.
Acknowledgments
We thank Dr Ana Belén Elgoyhen for comments on the manuscript, Drs A. Martínez-Torres and R. Miledi for ρ1 cDNA, Dr Alejandro Paladini and Mr P. Szkarlatiuk for technical assistance and Pew Foundation and IBRO for support. This work was funded by grants from ANPCyT (FONCyT PICT 99 No 5-6800 BID 1201) and CONICET (PIP 98).
Abbreviations
- Cl−
chloride
- GABA
γ-aminobutyric acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- I–V
current–voltage
- tact and tdeact
activation and de-activation constants
References
- AMIN J., WEISS D.S. Homomeric rho 1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- BIANCHI M.T., MACDONALD R.L. Agonist trapping by GABAA receptor channels. J. Neurosci. 2001;21:9083–9091. doi: 10.1523/JNEUROSCI.21-23-09083.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMANN J., FEIGENSPAN A. GABAC receptors. Trends Neurosci. 1995;18:515–519. doi: 10.1016/0166-2236(95)98370-e. [DOI] [PubMed] [Google Scholar]
- BOWERY N.G., BETTLER B., FROESTL W., GALLAGHER J.P., MARSHALL F., RAITERI M., BONNER T.I., ENNA S.J. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acid (B) receptors: structure and function. Pharmacol. Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- CALVO D.J., MILEDI R.Antagonism of human homomeric GABAr1 receptors by steroids 1997Society for Neuroscience; (abstract) [Google Scholar]
- CHANG Y., WEISS D.S. Substitutions of the highly conserved M2 leucine create spontaneously opening rho1 gamma-aminobutyric acid receptors. Mol. Pharmacol. 1998;53:511–523. doi: 10.1124/mol.53.3.511. [DOI] [PubMed] [Google Scholar]
- CHANG Y., WEISS D.S. Channel opening locks agonist onto the GABAC receptor. Nat. Neurosci. 1999;2:219–225. doi: 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- CHANG Y., WEISS D.S. Site-specific fluorescence reveals distinct structural changes with GABA receptor activation and antagonism. Nat. Neurosci. 2002;5:1163–1168. doi: 10.1038/nn926. [DOI] [PubMed] [Google Scholar]
- CHEBIB M., JOHNSTON G.A. The ‘ABC' of GABA receptors: a brief review. Clin. Exp. Pharmacol. Physiol. 1999;26:937–940. doi: 10.1046/j.1440-1681.1999.03151.x. [DOI] [PubMed] [Google Scholar]
- CHEN H.S., LIPTON S.A.Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism J. Physiol. 199749927–46.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERUBINI E., CONTI F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- CUTTING G.R., LU L., O'HARA B.F., KASCH L.M., MONTROSE-RAFIZADEH C., DONOVAN D.M., SHIMADA S., ANTONARAKIS S.E., GUGGINO W.B., UHL G.R., et al. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONG C.J., WERBLIN F.S. Zinc downmodulates the GABAc receptor current in cone horizontal cells acutely isolated from the catfish retina. J. Neurophysiol. 1995;73:916–919. doi: 10.1152/jn.1995.73.2.916. [DOI] [PubMed] [Google Scholar]
- DONG C.J., WERBLIN F.S. Use-dependent and use-independent blocking actions of picrotoxin and zinc at the GABAC receptor in retinal horizontal cells. Vision Res. 1996;36:3997–4005. doi: 10.1016/s0042-6989(96)00141-1. [DOI] [PubMed] [Google Scholar]
- DONG C.J., WERBLIN F.S. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J. Neurophysiol. 1998;79:2171–2180. doi: 10.1152/jn.1998.79.4.2171. [DOI] [PubMed] [Google Scholar]
- ENZ R., BRANDSTATTER J.H., HARTVEIT E., WASSLE H., BORMANN J. Expression of GABA receptor rho 1 and rho 2 subunits in the retina and brain of the rat. Eur. J. Neurosci. 1995;7:1495–1501. doi: 10.1111/j.1460-9568.1995.tb01144.x. [DOI] [PubMed] [Google Scholar]
- ENZ R., CUTTING G.R. Molecular composition of GABAC receptors. Vision Res. 1998;38:1431–1441. doi: 10.1016/s0042-6989(97)00277-0. [DOI] [PubMed] [Google Scholar]
- FEIGENSPAN A., BORMANN J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur. J. Pharmacol. 1994;288:97–104. doi: 10.1016/0922-4106(94)90014-0. [DOI] [PubMed] [Google Scholar]
- FEIGENSPAN A., WASSLE H., BORMANN J. Pharmacology of GABA receptor Cl− channels in rat retinal bipolar cells. Nature. 1993;361:159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- GOUTMAN J.D., WAXEMBERG M.D., DONATE-OLIVER F., POMATA P.E., CALVO D.J. Flavonoid modulation of ionic currents mediated by GABA(A) and GABA(C) receptors. Eur. J. Pharmacol. 2003;461:79–87. doi: 10.1016/s0014-2999(03)01309-8. [DOI] [PubMed] [Google Scholar]
- GURLEY D., AMIN J., ROSS P.C., WEISS D.S., WHITE G. Point mutations in the M2 region of the alpha, beta, or gamma subunit of the GABAA channel that abolish block by picrotoxin. Receptors Channels. 1995;3:13–20. [PubMed] [Google Scholar]
- HARRISON N.L., MAJEWSKA M.D., HARRINGTON J.W., BARKER J.L. Structure–activity relationships for steroid interaction with the gamma-aminobutyric acid A receptor complex. J. Pharmacol. Exp. Ther. 1987;241:346–353. [PubMed] [Google Scholar]
- JOHNSTON G.A. GABAc receptors: relatively simple transmitter-gated ion channels. Trends Pharmacol. Sci. 1996;17:319–323. [PubMed] [Google Scholar]
- KUSAMA T., WANG T.L., GUGGINO W.B., CUTTING G.R., UHL G.R. GABA rho 2 receptor pharmacological profile: GABA recognition site similarities to rho 1. Eur. J. Pharmacol. 1993;245:83–84. doi: 10.1016/0922-4106(93)90174-8. [DOI] [PubMed] [Google Scholar]
- MACDONALD R.L., OLSEN R.W. GABAA receptor channels. Annu. Rev. Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- MAJEWSKA M.D. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog. Neurobiol. 1992;38:379–395. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- MCCALL M.A., LUKASIEWICZ P.D., GREGG R.G., PEACHEY N.S. Elimination of the rho1 subunit abolishes GABA(C) receptor expression and alters visual processing in the mouse retina. J. Neurosci. 2002;22:4163–4174. doi: 10.1523/JNEUROSCI.22-10-04163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKERNAN R.M., WHITING P.J. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- MILEDI R., PARKER I., SUMIKAWA K.Transplanting receptors from brain into oocytes Fidia Research Foundation Neuroscience Award Lecture 1989New York: Raven Press; 57–89.ed. Smith, J. pp [Google Scholar]
- MORRIS K.D., MOOREFIELD C.N., AMIN J. Differential modulation of the gamma-aminobutyric acid type C receptor by neuroactive steroids. Mol. Pharmacol. 1999;56:752–759. [PubMed] [Google Scholar]
- MOSS S.J., SMART T.G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- NEWLAND C.F., CULL-CANDY S.G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J. Physiol. 1992;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OGURUSU T., EGUCHI G., SHINGAI R. Localization of gamma-aminobutyric acid (GABA) receptor rho 3 subunit in rat retina. Neuroreport. 1997;8:925–927. doi: 10.1097/00001756-199703030-00022. [DOI] [PubMed] [Google Scholar]
- OGURUSU T., YANAGI K., WATANABE M., FUKAYA M., SHINGAI R. Localization of GABA receptor rho 2 and rho 3 subunits in rat brain and functional expression of homooligomeric rho 3 receptors and heterooligomeric rho 2 rho 3 receptors. Receptors Channels. 1999;6:463–475. [PubMed] [Google Scholar]
- PAN Z.H., ZHANG D., ZHANG X., LIPTON S.A. Agonist-induced closure of constitutively open gamma-aminobutyric acid channels with mutated M2 domains. Proc. Natl. Acad. Sci. U.S.A. 1997;94:6490–6495. doi: 10.1073/pnas.94.12.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLENZANI L., WOODWARD R.M., MILEDI R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINCE R.J., SIMMONDS M.A. 5 Beta-pregnan-3 beta-ol-20-one, a specific antagonist at the neurosteroid site of the GABAA receptor-complex. Neurosci. Lett. 1992;135:273–275. doi: 10.1016/0304-3940(92)90454-f. [DOI] [PubMed] [Google Scholar]
- QIAN H., DOWLING J.E. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J. Neurosci. 1994;14:4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGOZZINO D., WOODWARD R.M., MURATA Y., EUSEBI F., OVERMAN L.E., MILEDI R. Design and in vitro pharmacology of a selective gamma-aminobutyric acid C receptor antagonist. Mol. Pharmacol. 1996;50:1024–1030. [PubMed] [Google Scholar]
- SCHMIDT M., BOLLER M., OZEN G., HALL W.C. Disinhibition in rat superior colliculus mediated by GABAc receptors. J. Neurosci. 2001;21:691–699. doi: 10.1523/JNEUROSCI.21-02-00691.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN W., SLAUGHTER M.M. Multireceptor GABAergic regulation of synaptic communication in amphibian retina. J. Physiol. 2001;530:55–67. doi: 10.1111/j.1469-7793.2001.0055m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMADA S., CUTTING G., UHL G.R. Gamma-aminobutyric acid A or C receptor? Gamma-aminobutyric acid rho 1 receptor RNA induces bicuculline-, barbiturate-, and benzodiazepine-insensitive gamma-aminobutyric acid responses in Xenopus oocytes. Mol. Pharmacol. 1992;41:683–687. [PubMed] [Google Scholar]
- SIVILOTTI L., NISTRI A. GABA receptor mechanisms in the central nervous system. Prog. Neurobiol. 1991;36:35–92. doi: 10.1016/0301-0082(91)90036-z. [DOI] [PubMed] [Google Scholar]
- SMART T., CONSTANTI A. Studies on the mechanism of action of picrotoxinin and other convulsants at the crustacean muscle GABA receptor. Proc. R. Soc. Lond. 1986;227:191–216. doi: 10.1098/rspb.1986.0019. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI K., MIYOSHI S., KANEKO A. GABA-induced chloride current in catfish horizontal cells mediated by non-GABAA receptor channels. Jpn. J. Physiol. 1995;45:437–456. doi: 10.2170/jjphysiol.45.437. [DOI] [PubMed] [Google Scholar]
- WANG T.L., GUGGINO W.B., CUTTING G.R. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J. Neurosci. 1994;14:6524–6531. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG T.L., HACKAM A.S., GUGGINO W.B., CUTTING G.R. A single amino acid in gamma-aminobutyric acid rho 1 receptors affects competitive and noncompetitive components of picrotoxin inhibition. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11751–11755. doi: 10.1073/pnas.92.25.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Characterization of bicuculline/baclofen-insensitive gamma-aminobutyric acid receptors expressed in Xenopus oocytes. I. Effects of Cl− channel inhibitors. Mol. Pharmacol. 1992a;42:165–173. [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Effects of steroids on gamma-aminobutyric acid receptors expressed in Xenopus oocytes by poly(A)+ RNA from mammalian brain and retina. Mol. Pharmacol. 1992b;41:89–103. [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acid B receptor agonists and antagonists. Mol. Pharmacol. 1993;43:609–625. [PubMed] [Google Scholar]
- WOTRING V.E., CHANG Y., WEISS D.S.Permeability and single channel conductance of human homomeric rho1 GABAC receptors J. Physiol. 1999521327–336.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU M., COVEY D.F., AKABAS M.H. Interaction of picrotoxin with GABAA receptor channel-lining residues probed in cysteine mutants. Biophys. J. 1995;69:1858–1867. doi: 10.1016/S0006-3495(95)80056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOON K.W., COVEY D.F., ROTHMAN S.M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J. Physiol. 1993;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG D., PAN Z.H., AWOBULUYI M., LIPTON S.A. Structure and function of GABA(C) receptors: a comparison of native versus recombinant receptors. Trends Pharmacol. Sci. 2001;22:121–132. doi: 10.1016/s0165-6147(00)01625-4. [DOI] [PubMed] [Google Scholar]
- ZHANG D., PAN Z.H., ZHANG X., BRIDEAU A.D., LIPTON S.A. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc. Natl. Acad. Sci. U.S.A. 1995;92:11756–11760. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]