Abstract
The observation that the immunosuppressants, cyclosporine A (CsA) and tacrolimus, have pressor effects, but sirolimus does not, has led to an hypothesis that generalised sympathoexcitation, resulting from inhibition of calcineurin by CsA and tacrolimus underlies their pressor effects, because sirolimus does not inhibit calcineurin. It is unknown if sirolimus has haemodynamic actions not accompanied by a pressor effect, and whether or not the pressor effects of CsA and tacrolimus are accompanied by similar haemodynamic changes. Therefore, the first aim of our studies was to investigate these possibilities in conscious, chronically-instrumented, male, Sprague-Dawley rats.
CsA (5.9 mg kg−1 bolus i.v.) caused rapid-onset, prolonged hypertension, tachycardia and mesenteric vasoconstriction. There was a slower onset renal vasoconstriction, but no significant change in hindquarters vascular conductance; all the effects of CsA were significantly greater than those of vehicle. CsA given by infusion (over 30 min or 2 h) caused changes qualitatively similar to those above. Repeated administration of CsA over 4 days did not enhance its cardiovascular effects.
Pretreatment with the angiotensin (AT1) receptor antagonist, losartan, and the endothelin (ETA and ETB) receptor antagonist, SB 209670, reduced the pressor and mesenteric vasoconstrictor effects of CsA. Additional administration of the α-adrenoceptor antagonist, phentolamine, completely inhibited the cardiovascular effects of CsA.
Tacrolimus (450 μg kg−1 bolus i.v.) caused similar peak pressor and tachycardic effects to CsA, but these were much slower in onset, and were maximal when there were no significant regional vasoconstrictions, indicating that the pressor effect was probably due to a rise in cardiac output. However, although propranolol reversed the tachycardic effect of tacrolimus, it did not influence the pressor response.
Sirolimus (450 μg kg−1 bolus i.v.) had no tachycardic action, and only a modest, transient pressor effect, accompanied by equally brief reductions in renal, mesenteric, and hindquarters vascular conductances.
The differences between the regional haemodynamic profiles of equipressor doses of CsA and tacrolimus, and the finding that sirolimus has significant cardiovascular actions, indicate that generalised sympathoexcitation, resulting from calcineurin inhibition (with CsA and tacrolimus), is unlikely to be the sole explanation of their pressor effects.
Keywords: Cyclosporine A, tacrolimus, sirolimus, haemodynamics
Introduction
The widespread use of cyclosporine A (CsA) as a major, post-transplant immunosuppressive agent has revealed its substantial side effects on renal function and blood pressure (Borel, 1989; Sander et al., 1996). There is much discussion about the mechanisms underlying the disruption of renal physiology by CsA, with some experiments indicating that acute administration of CsA causes selective renal vasoconstriction (e.g., Abraham et al., 1991), possibly due, in part, to increased renal vascular sensitivity to noradrenaline, angiotensin II and/or vasopressin (Garr & Paller, 1990). However, Garr & Paller (1990) also found that CsA (20 mg kg−1) had no effect on systemic arterial blood pressure in anaesthetised rats, whereas others (e.g., Moss et al., 1985; Abraham et al., 1991; Morgan et al., 1991; Chiu et al., 1992; Lyson et al., 1993; 1994; Zhang & Victor, 2000) have observed acute pressor effects of CsA (5 – 40 mg kg−1). Although the majority of these studies used lower doses of CsA than Garr & Paller (1990), it is notable that Chiu et al. (1992) used the same and higher (40 mg kg−1) doses, and also observed clear increases in blood pressure.
Any pressor effects of CsA are likely an amalgam of all the putative actions of the drug, including sympathetic activation (Sander et al., 1996; but see Kaye et al., 1993), stimulation of the renin – angiotensin system, endothelin (ET) release and modulation of prostanoid/thromboxane and nitric oxide (NO) production (see Carrier et al., 1991; Lo Russo et al., 1996; Stroes et al., 1997; Oriji & Keiser, 1998), at least. In such a multifactorial system, the apparent primacy of the contribution of sympathetic activation to the pressor effect of CsA (Sander et al., 1996) may be due to its being the major determinant of vascular tone, against which the effects of the other factors are expressed. From this perspective, it would appear that a detailed analysis of the concurrent regional haemodynamic effects of CsA is the only way in which to delineate any regional selectivity in its vasoconstrictor action. However, to our knowledge, few studies have assessed the regional haemodynamic effects of CsA (e.g., Abraham et al., 1991; Morgan et al., 1991; Davis et al., 1994). Moreover, the experiments in the papers cited were carried out in acutely prepared rats, and in none were changes in mesenteric haemodynamics measured, in spite of the major contribution of this vascular bed to total peripheral conductance. Thus, our main objective was to extend the studies of Morgan et al. (1991), Abraham et al. (1991) and Davis et al. (1994) by monitoring, simultaneously, renal, mesenteric and hindquarters vascular responses to CsA, and to do so in the absence of the confounding effects of anaesthesia. Those experiments showed CsA to cause pressor and regionally selective vasoconstrictor effects. Therefore, in additional experiments, the possible involvement of endothelin-, angiotensin II- and α-adrenoceptor-mediated mechanisms in the effects of CsA was investigated.
An apparent advance in understanding the mechanisms underlying CsA-induced hypertension came from the finding that tacrolimus (FK 506) also had pressor effects, but sirolimus (rapamycin) had not (Lyson et al., 1993; Zhang & Victor, 2000), leading to the hypothesis that inhibition of neuronal calcineurin (which occurs with CsA and tacrolimus, but not with sirolimus) triggers renal afferent nerve activation to produce generalised, sympathetically-mediated vasoconstriction (Zhang & Victor, 2000). However, in those studies (Lyson et al., 1993; Zhang & Victor, 2000), no assessment was made of the regional haemodynamic effects of tacrolimus or sirolimus. This is an important point because, although the pressor effects of CsA and tacrolimus were similar, their effects on renal sympathetic nerve activity appeared different (Lyson et al., 1993). Moreover, the absence of an effect of sirolimus on blood pressure occurred in spite of a reduction in renal sympathetic nerve activity (Lyson et al., 1993). Hence, these could be examples of situations in which the measurement of mean arterial blood pressure, alone, fails to detect the underlying differences in regional haemodynamic changes (Bennett & Gardiner, 2003). Therefore, our second main objective was to provide the comparative data for the regional haemodynamic effects of CsA, tacrolimus and sirolimus in our conscious rat model.
Since the experiments with tacrolimus showed that its maximal pressor effect was not associated with vasoconstriction, but occurred at the peak of a tachycardic effect (see Results), we went on to explore the possible involvement of β-adrenoceptor-mediated cardiac effects in the response to tacrolimus with the use of propranolol.
Some of the results herein have been presented to the British Pharmacological Society (Bennett et al., 2004).
Methods
All experiments were carried out on male, Sprague – Dawley rats (Charles River, U.K., body weights 380 – 420 g), with approval from the Local Ethical Review Committee and under licence from the Home Office. Animals were housed in the Biomedical Services Unit at Nottingham for at least 2 weeks before undergoing any procedure; during this time, they were kept in groups of three or four and given free access to food and water. Thereafter, under anaesthesia (fentanyl and medetomidine; 300 μg kg−1 of each i.p.), miniature, pulsed Doppler probes were implanted around the left renal and superior mesenteric arteries, and the distal abdominal aorta (to monitor hindquarters flow), using procedures previously described in detail (Gardiner et al., 1990b, 1990c). Anaesthesia was reversed with atipamezole, and analgesia provided by nalbuphine (both 1 mg kg−1 s.c.); animals were subsequently housed individually. At least 7 days later, under anaesthesia (as above), a catheter was implanted in the distal abdominal aorta (via the ventral caudal artery), and catheters implanted in the right jugular vein. The former was used for monitoring intra-arterial blood pressure and (from it) heart rate, and the latter for drug administration. All experiments were conducted with animals in their home cages, with free access to food and water, at least 24 h after catheter implantation. During the cardiovascular monitoring periods, animals were connected (via a tether system) to a custom-designed data-acquisition system (see below), and recordings were made for 20 – 30 min before any intervention, and continuously for 6 h thereafter. Data were analysed off-line at time periods chosen on the basis of the interventions (see below), so as to allow close monitoring of the haemodynamic responses to the different dosing regimes.
Experiment 1: effects of CsA administered as a rapid bolus or slow infusion
In order to maximise the likelihood of obtaining data relevant to the majority of published findings, CsA at a dose of 5.9 mg kg−1 (Kawai et al., 1998) was used. The protocol on any experimental day ran for 6 h since there is evidence for prolonged effects of CsA on sympathetic nerve activity (Lyson et al., 1993). Data from pilot experiments indicated there were no carry-over effects of CsA, when a 3-day protocol was employed. Therefore, in the present study, animals (n=9) were randomised to receive CsA (5.9 mg kg−1 in 0.26 ml) as a rapid bolus (given over 15 s) or slow infusion (given over 2 h) on the first experimental day, and the other CsA treatment on the third experimental day, with vehicle (cremophor (∼50 μl) diluted in saline to 260 μl as above) administered as a bolus on the second experimental day.
Since all the studies from Victor's group (e.g., Morgan et al., 1991; Lyson et al., 1993; 1994; Zhang & Victor, 2000) have involved infusion of CsA over 30 min, this protocol was adopted in a separate group of animals (n=8).
Experiment 2: effects of repeated administrations of CsA over 4 days
One aspect of clinical importance with regard to CsA is the development of enhanced adverse effects with repeated administration (Sander et al., 1996). Therefore, responses to repeated bolus injections (n=4) or infusions (over 2 h) (n=4) of CsA (5.9 mg kg−1) on 4 consecutive experimental days were assessed, starting at 07:00 h on each day.
Experiment 3: effects of losartan, SB 209670 and phentolamine on responses to CsA
We found that the effects of repeated bolus doses of CsA over 4 consecutive days were reproducible (see Experiment 2, Results). Therefore, in a group of eight animals, CsA (5.9 mg kg−1 bolus) was administered in the presence of saline (0.1 ml bolus, 0.4 ml h−1 infusion) on day 1, SB 209670 (600 μg kg−1 bolus, 600 μg kg−1 h−1 infusion, n=4) or losartan (10 mg kg−1 bolus, n=4) on day 2, SB 209670 together with losartan (doses as above) on day 3 and SB 209670, losartan plus phentolamine (1 mg kg−1, 1 mg kg−1 h−1) on day 4. The pretreatments were started 60 min before administration of CsA.
Experiment 4: effects of tacrolimus administered as a rapid bolus or slow infusion
Pilot experiments showed that, at the doses used, tacrolimus and sirolimus were soluble in 5% propylene glycol+2% Tween 80 in sterile saline, and that this vehicle did not have a pressor effect (unlike cremophor – see Results). Therefore, this was the vehicle used in the experiments with tacrolimus and sirolimus.
Lyson et al. (1993) chose a dose of 150 μg kg−1 tacrolimus, on the basis of its having about the same immunosuppressive action as CsA at a dose of 5 mg kg−1, and found that it caused a substantial pressor effect in anaesthetised rats. Therefore, responses to bolus injection and 2 h infusion of tacrolimus at a dose of 150 μg kg−1 were initially assessed (n=4). However, in conscious rats, we found that this dose of tacrolimus was without consistent cardiovascular actions, so additional experiments were performed with tacrolimus at a dose of 450 μg kg−1 (i.e., the lowest dose producing consistent cardiovascular effects), either as a bolus (over 15 s) or infusion (over 2 h).
Experiment 5: effects of propranolol on responses to tacrolimus
Experiment 4 showed that tacrolimus had tachycardic and pressor effects that were maximal when there was no regional vasoconstriction (see Results), consistent with the effect being due to an increase in cardiac output. Therefore, in a separate group of animals (n=4), propranolol (1 mg kg−1, 0.5 mg kg−1 h−1) was administered 5 min after bolus injection of tacrolimus (450 μg kg−1).
Experiment 6: effects of rapid bolus or slow infusion of sirolimus
In order to obtain data that could be compared with those for tacrolimus, effects of bolus injection (over 15 s) and infusion (over 2 h) of sirolimus at 450 μg kg−1 were assessed.
In pilot experiments, it was established that administration of tacrolimus or sirolimus on the first experimental day had no carry-over effects on the next day. Therefore, animals (n=4) were randomised to receive bolus injection of tacrolimus or sirolimus on the first experimental day, and the remaining active treatment on the third experimental day, with vehicle being given on the second day. Infusions of tacrolimus or sirolimus were given to a separate group of rats (n=4) following a similar protocol to that above.
Data analysis
Data were sampled every 2 ms, averaged each cardiac cycle and stored to disc every 5 s, using the haemodynamics data-acquisition system designed and constructed at the Instrumentation Laboratories, University of Maastricht. Off-line, data were analysed (Datview, University of Maastricht) using electronically derived averages across times selected by the analyst, on the basis of the profile of response to CsA. Hence, measurements were made across a 2-min epoch prior to administration of the drug, across a 10 s epoch about 30 s (i.e., 25 – 35 s) after administration, across 20 s epochs around 1, 2, 3, 4 and 5 min after drug administration, and thereafter across 1-min epochs around 10, 20, 30 and 60 min, and every hour until 6 h after drug administration. None of the drugs administered had overt behavioural effects, that is, all the data were collected in the resting state.
These data were compiled (for all animals in the group) in a spreadsheet and exported into a custom-designed statistical analysis package. Within-group analyses were carried out by a nonparametric equivalent of ANOVA (Friedman's test; Theodorsson-Norheim, 1987). Between-group analyses were by Wilcoxon's test, or Mann – Whitney U-test, as appropriate; P⩽0.05 was taken as significant.
Drugs
Sandimmun (Novartis, obtained from Hospital Pharmacy, Queen's Medical Centre, Nottingham NG7 2UH, U.K.), that is, CsA, was dissolved at a concentration of 50 mg ml−1 in absolute ethanol and polyethoxylated castor oil (cremophor). Solutions for administration were obtained by diluting an appropriate volume (∼50 μl) of Sandimmun with sterile isotonic saline, to a total volume of 260 μl. Cremophor (mixture of absolute ethanol and polyethoxylated castor oil) was prepared by Camurus (Science Park Ideon, Lund, Sweden) (batch number 211Cy-0010-2005/BN). Tacrolimus (FK506) was purchased from Calbiochem (through CN Biosciences, Nottingham, Boulevard Industrial Park, NG9 2JR, U.K.), and sirolimus (rapamycin) was obtained from Tocris Cookson (Avonmouth, BS11 8TA, U.K.).
SB 209670 ([(+)-(1S, 2R, 3S)-3-(2-carboxymethoxy-4-methoxyphenyl)-1-13,4-methylenedioxyphenyl)-5-(prop-1-yloxy)indane-2-carboxylic acid]) was a gift from Dr E. Ohlstein (SKB, U.S.A.). Losartan potassium was a gift from Dr R.D. Smith (DuPont, U.S.A.). Phentolamine mesylate was purchased from Sigma, U.K.
Fentanyl citrate was obtained from Janssen-Cilag (High-Wycombe, U.K.); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were obtained from Pfizer (Sandwich, Kent, U.K.); nalbuphine hydrochloride (Nubain) was obtained from Bristol-Myers-Squibb (Houslow, U.K.).
Results
Table 1 summarises the data for resting cardiovascular variables in all groups of animals used in the studies.
Table 1.
Resting cardiovascular variables in the experimental groups described in the Methods
| Experiment 1 (n=9) | Experiment 2 (n=8) | Experiment 3 (n=8) | Experiment 4 & 6 (n=8) | Experiment 5 (n=4) | |
|---|---|---|---|---|---|
| Heart rate (beats min−1) | 339±8 | 328±3 | 350±9 | 334±7 | 338±12 |
| Mean BP (mmHg) | 103±3 | 103±2 | 103±2 | 107±3 | 103±1 |
| Renal DS (kHz) | 10.2±1.0 | 8.7±0.5 | 7.7±0.6 | 7.3±0.7 | 8.3±1.2 |
| Mesenteric DS (kHz) | 11.8±0.7 | 11.9±1.0 | 9.1±0.7 | 10.3±0.5 | 9.1±0.2 |
| Hindquarters DS (kHz) | 3.7±0.5 | 4.2±0.5 | 4.2±0.3 | 4.2±0.4 | 4.0±0.6 |
| Renal VC ((kHz mmHg−1) 103) | 98±9 | 86±6 | 76±6 | 69±8 | 80±12 |
| Mesenteric VC ((kHz mmHg−1) 103) | 115±8 | 117±11 | 89±7 | 97±7 | 88±2 |
| Hindquarters VC ((kHz mmHg−1) 103) | 36±4 | 41±5 | 41±4 | 40±4 | 38±5 |
All values are mean±s.e.m. for pretreatment recordings. DS=Doppler shift, VC=vascular conductance. The data for Experiment 1 represent those recorded on the first day (see Methods), the data for Experiment 2 are means of the two subgroups (see Methods); the animals in experiments 4 and 6 were the same (see Methods) and are shown as a single group.
Experiment 1: effects of CsA administered as a rapid bolus or slow infusion
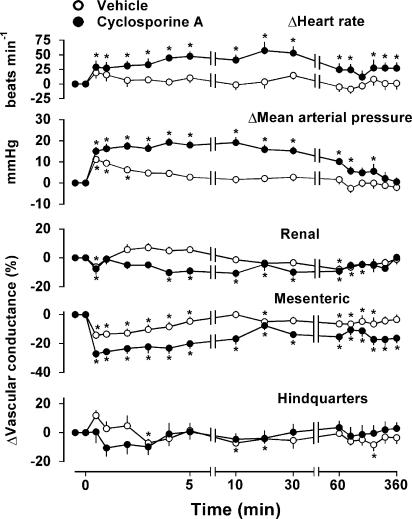
Bolus injection of cremophor (diluted with sterile saline to match CsA administration) had a slight, brief pressor effect accompanied by significant, but transient, mesenteric vasoconstriction, with more variable changes in the renal and hindquarters vascular beds (Figure 1). Hence, there were transient increases in renal and hindquarters flows, and a decrease in mesenteric flow (data not shown).
Figure 1.
Cardiovascular changes in conscious, male, Sprague – Dawley rats (n=9) given i.v. bolus injection of CsA (5.9 mg kg−1) or vehicle (cremophor). The values are means and vertical bars show s.e.m.; *P⩽0.05 versus baseline (Friedman's test).
Bolus injection of CsA had marked, and prolonged, pressor and tachycardic effects (sustained for over 1 h; Figure 1), which were accompanied by a rapid onset mesenteric vasoconstriction, and a more slowly developing, less marked, renal vasoconstriction. This difference between the mesenteric and renal vasoconstrictor effects of CsA resulted in its pressor effects being accompanied by increases in renal flow (30 s,+6±4%, 1 min, +14±2%; P⩽0.05), but decreases in mesenteric flow (30 s, −17±3%, P⩽0.05; 1 min, −15±1%; P⩽0.05). There was a significant increase in hindquarters flow (maximum+18±5% at 5 min; P⩽0.05) following CsA, because there was no significant reduction in hindquarters vascular conductance (Figure 1). The integrated (areas under or over the curves, 0 – 60 min) pressor, tachycardic and mesenteric and renal vasoconstrictor effects of CsA were significantly different from those of vehicle (Figure 1).
Infusion of CsA over 30 min caused a more slowly developing rise in mean arterial pressure, fall in heart rate and falls in mesenteric and renal vascular conductance, which reached maxima only at the end of the 30-min infusion (+18±3 mmHg, +36±8 beats min−1, −13±4%, −11±2%, respectively; all P⩽0.05). There was no significant change in hindquarters vascular conductance following 30-min infusion of CsA.
Infusion of CsA over 2 h produced even more slowly developing pressor and tachycardic responses (peak at 2 h, +13±2 mmHg, +47±10 beats min−1, respectively; both P⩽0.05), accompanied by a reduction in mesenteric vascular conductance (−15±4%; P⩽0.05), but no significant change in renal or hindquarters vascular conductances.
Experiment 2: effects of repeated administration of CsA over 4 days
The cardiovascular responses to repeated administration of CsA by bolus injection showed no signs of increasing effect over the 4 days. Thus, the maximum pressor effects of CsA were 16±6, 25±5, 21±3 and 22±5 mmHg, the maximum values of tachycardia were 82±38, 43±14, 51±18 and 68±13 beats min−1, and the maximum reductions in mesenteric vascular conductance were −30±6, −38±6, −33±5 and −26±7%, on days 1, 2, 3 and 4, respectively. Likewise, the responses to infusion of CsA were similar over the 4 days (data not shown).
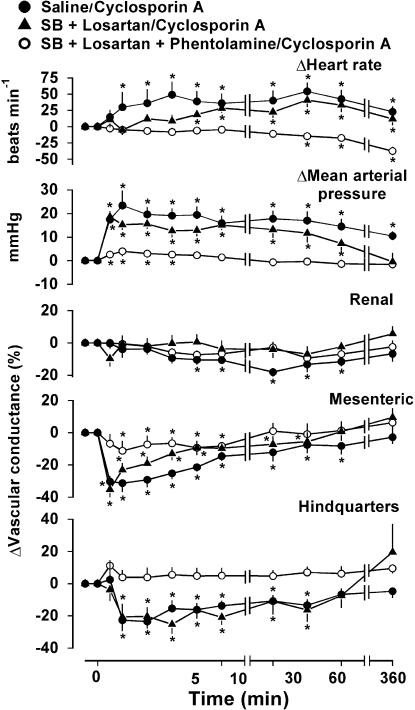
Experiment 3: effects of losartan, SB209670 and phentolamine on responses to CsA
Table 2 shows the resting cardiovascular variables 60 min after saline or the antagonists, and Figure 2 shows the responses to CsA in these conditions. Losartan and SB 209670 together caused a decrease in mean blood pressure, together with increases in heart rate, mesenteric Doppler shift, and renal, mesenteric and hindquarters vascular conductances (Table 2). In this condition, the pressor and mesenteric vasoconstrictor effects of CsA were reduced (Figure 2).
Table 2.
Resting cardiovascular variables in the same group of animals (n=8) in the presence of saline, losartan+SB 209670, and losartan+SB209670+ phentolamine
| Saline | Losartan+SB209760 | Losartan+SB209760+phentolamine | |
|---|---|---|---|
| Heart rate (beats min−1) | 340±11 | 409±15* | 494±7* |
| Mean BP (mmHg) | 103±2 | 82±5* | 48±3* |
| Renal DS (kHz) | 7.9±0.7 | 8.3±0.8 | 6.2±0.6 |
| Mesenteric DS (kHz) | 8.4±0.9 | 10.3±0.9* | 8.4±0.9 |
| Hindquarters DS (kHz) | 4.3±0.4 | 4.4±0.4 | 7.6±0.4* |
| Renal VC ((kHz mmHg −1) 103) | 77±7 | 105±14* | 130±9* |
| Mesenteric VC ((kHz mmHg−1) 103) | 82±9 | 130±16* | 175±19* |
| Hindquarters VC ((kHz mmHg−1) 103) | 42±4 | 55±6* | 159±8* |
All values are mean±s.e.m. for recordings made before administration of CsA. DS=Doppler shift, VC=vascular conductance
P⩽0.05 versus saline treatment (Friedman's test).
Figure 2.
Cardiovascular changes in the same conscious, male, Sprague – Dawley rats (n=8) given i.v. bolus injection of CsA (5.9 mg kg−1) in the presence of saline, losartan and SB 209670, or losartan, SB209670 and phentolamine. Values are mean and vertical bars show s.e.m.; P⩽0.05 versus baseline (Friedman's test). Between-experiment differences are given in the text.
Losartan, SB209670 and phentolamine together caused substantial hypotension, accompanied by tachycardia and marked increases in hindquarters Doppler shift and renal, mesenteric and hindquarters vascular conductances (Table 2). In this condition, CsA had little cardiovascular effect (Figure 2).
Experiment 4: effects of tacrolimus administered as a rapid bolus or slow infusion
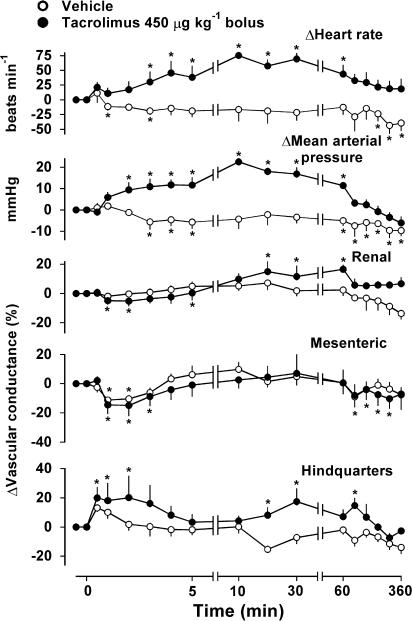
Administration of tacrolimus at a dose of 150 μg kg−1 (Lyson et al., 1993), either by bolus or infusion, caused no consistent cardiovascular changes (data not shown). However, bolus injection of tacrolimus at 450 μg kg−1 caused slow-onset, but marked, pressor and tachycardic effects that were significantly greater than those of vehicle (Figure 3), and, at their peaks, were similar to the effects of CsA. The pressor effect of tacrolimus was initially accompanied by transient renal and mesenteric vasoconstriction and hindquarters vasodilatation, although none of the regional haemodynamic changes were significantly different from those seen with vehicle, and none were present when the maximum pressor effect occurred (Figure 3).
Figure 3.
Cardiovascular changes in conscious, male, Sprague – Dawley rats (n=4) given i.v. bolus injection of tacrolimus (450 μg kg−1) or vehicle . Values are mean and vertical bars show s.e.m.; *P⩽0.05 versus baseline (Friedman's test).
Infusion of tacrolimus over 2 h produced a slow rise in mean arterial blood pressure (peak at 2 h, +18±5 mmHg; P⩽0.05) and a variable tachycardia (+21±15 beats min−1), accompanied by reductions in mesenteric and hindquarters vascular conductances (−20±9, −10±3%, respectively; both P⩽0.05).
Experiment 5: effects of propranolol on responses to tacrolimus
Administration of propranolol 5 min after tacrolimus abolished the tachycardia (+21±4 down to −7±8 beats min−1), but not the pressor effect of tacrolimus (+5±4 before and +8±5 mmHg after propranolol).
Experiment 6: effects of sirolimus administered as a rapid bolus or slow infusion
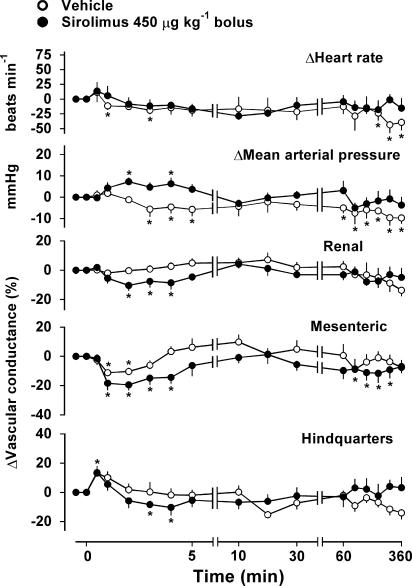
Bolus injection of sirolimus at 450 μg kg−1 caused a significant, albeit slight and transient, increase in mean arterial blood pressure, but it had no significant influence on heart rate (Figure 4). There were reductions in renal, mesenteric and hindquarters vascular conductances which, over the first 10 min following administration, were significantly greater than the integrated changes seen following vehicle (Figure 4). Infusion of sirolimus had no consistent cardiovascular effects (data not shown).
Figure 4.
Cardiovascular changes in conscious, male, Sprague – Dawley rats (n=4) given i.v. bolus injection of sirolimus (450 μg kg−1) or vehicle. Values are mean and vertical bars show s.e.m.; *P⩽0.05 versus baseline (Friedman's test).
Discussion
The major objectives of the present work were: (i) to provide a full haemodynamic profile of the effects of CsA in conscious rats, and to delineate the mechanisms involved; (ii) to compare this with that of another calcineurin inhibitor, tacrolimus and (iii) to determine the regional haemodynamic action of sirolimus in the same model.
Cardiovascular effects of CsA
There were clear effects of the vehicle (cremophor) that were directionally similar to the changes seen with CsA (see below), with the exception of the renal vascular bed, where there was a tendency towards vasodilatation with cremophor, but a slow-onset vasoconstriction with CsA.
An effect of the vehicle was not unexpected in the light of earlier findings (e.g., Yaris et al., 1992; 1994; Lodge, 1994; Yaris & Tuncer, 1995a, 1995b; Hardy et al., 2000; Sanchez et al., 2001), albeit in vitro. Thus, several of the mechanisms identified in those studies (e.g., release of thromboxane A2; increase in noradrenaline sensitivity) would be predicted to cause a rise in blood pressure due to vasoconstriction, although such an effect was not seen by Zhang & Victor (2000), possibly because they infused cremophor over 30 min. Consistent with this proposal, Abraham et al. (1991) observed renal vasoconstrictor effects of cremophor in decerebrate rats, when it was injected in a volume of 1 ml over 5 min. However, Abraham et al. (1991) also reported that this caused a substantial fall in cardiac output and mean arterial pressure, so the action of cremophor is clearly complex. The tendency we observed for cremophor to cause selective renal vasodilatation is unexplained, but it may account for the finding that CsA caused a much less marked renal than mesenteric vasoconstriction (see below).
The present results showed regionally selective haemodynamic effects of CsA (i.e., mesenteric>renal≥hindquarters vasoconstriction), which were most marked when the drug was given as a rapid bolus injection. The accompanying pressor effect was temporally associated with the mesenteric vasoconstriction, which resulted in a decrease in flow, in contrast to the slow-onset renal vasoconstriction, which was associated with an early increase in flow. A feasible interpretation of these findings is that the pressor effect of CsA was due to mesenteric vasoconstriction, whereas the reduction in renal vascular conductance was an autoregulatory response to the increase in renal flow driven by the rise in pressure. These findings differ from those of Morgan et al. (1991), who showed a close relation between the pressor effect of CsA and its ability to increase renal sympathetic nerve activity and cause renal vasoconstriction; femoral vasoconstriction and increase in lumbar sympathetic nerve activity were slower in onset and more sustained. However, Morgan et al. (1991) made no measurements of the effects of CsA on mesenteric haemodynamics, and there are several other differences between their experiments and ours. Most notably, perhaps, their experiments were in anaesthetised animals and CsA was administered by infusion over 30 min. But, even when we administered CsA in the same way to conscious rats, there was only very modest renal vasoconstriction, and no consistent vasoconstriction in the hindquarters, whereas there was a pressor effect which was apparently related to a mesenteric vasoconstriction.
Our finding that CsA caused no reduction in hindquarters flow, even when it reduced hindquarters vascular conductance (Experiment 3), is consistent with an earlier observation that CsA had no effect on skeletal muscle blood flow (McKenzie et al., 1985). However, it is at odds, not only with the experiments of Morgan et al. (1991, see above), but also those of Davis et al. (1994) in anaesthetised rats, although the latter study used a much higher dose of CsA (20 mg kg−1 over 10 min). In addition, Davis et al. (1994) observed an acute pressor effect of CsA in the presence of ganglion blockade, so this could not have been due to neurogenic vasoconstriction (see below).
A review of the literature on the effects of CsA indicates that, on the constrictor side, increased sympathetic activity (Sander et al., 1996), diminished NO production (Diederich et al., 1994; Roullet et al., 1994; Oriji & Keiser, 1998), increased ET production (Takeda et al., 1995), and renin – angiotensin activity (Lassila et al., 2000), and enhanced vascular responsiveness to vasoconstrictors (Lo Russo et al., 1996), have been described. On the dilator side, increased NO production (Stroes et al., 1997), with no involvement of ET, or the renin – angiotensin system (Sander et al., 1996), or changes in responsiveness to vasoconstrictors (Garr & Paller, 1990; Textor et al., 1990) have been reported. To some extent, these disparate findings may be a result of different dosing regimes, and acute or chronic protocols, but also likely reflect the interactive nature of the mechanisms. For example, if it is the case that CsA influences NO production, then this would be expected to modulate ET release (Boulanger & Lüscher, 1990; Gardiner et al., 1996). In our experience, inhibition of NO synthesis (Gardiner et al., 1990b, 1990c) causes marked hypertension accompanied by constriction in mesenteric, renal and hindquarters beds, and in that rank order for both magnitude and time course of development. Hence, our results with CsA could not be explained simply by inhibition of NO production. Likewise, acute release of ET would be expected to cause marked renal, as well as mesenteric vasoconstriction, although lack of early hindquarters vasoconstriction is consistent with the haemodynamic profile of ET (Gardiner et al., 1990a). However, such effects of CsA are likely superimposed on changes in constrictor responsiveness (Garr & Paller, 1990; Textor et al., 1990), as well as diminished dilator responsiveness (Stein et al., 1995), modulated by effects of superoxide radicals (Diederich et al., 1994).
In order to delineate the possible contributions from angiotensin II, endothelin and sympathoexcitation to the haemodynamic effects of CsA, we assessed the effects of losartan, SB 209670 and phentolamine on responses to the drug. We found that losartan and SB 209670 inhibited the pressor and mesenteric effects of CsA, consistent with the involvement of angiotensin II and endothelin (see above). However, the effects of losartan and SB 209670 were modest, in comparison to the additional effect of phentolamine which caused almost complete abolition of responses to CsA in the presence of the other antagonists, consistent with a major involvement of sympathoexcitation in the haemodynamic effects of CsA (see Sander et al., 1996). But, it is notable that administration of phentolamine, in the presence of losartan and SB 209670, caused a more marked increase in hindquarters than in mesenteric vascular conductance, indicating a substantially greater vasomotor tone in the former vascular bed; yet, CsA had a lesser constrictor effect in the hindquarters than in the mesenteric vascular bed under normal conditions. Hence, it is likely that opposing dilator mechanisms were activated by CsA in the hindquarters (see above).
With repeated administration of CsA (by bolus or infusion) over 4 days, there was no apparent desensitisation to its cardiovascular effects, but, equally, there were no signs of carry-over or progressive development of sustained hypertension. Thus, it is possible that, in those studies describing incremental increases in blood pressure with chronic (7+ days) administration of CsA (e.g., Diederich et al., 1994; Roullet et al., 1994; Takeda et al., 1995), the phenomenon is related to high doses causing cumulative changes in the NO/ET balance, for example, with subsequent nephrotoxicity (Sander et al., 1996). It is notable that, in our experiments, acute bolus injection of CsA caused an increase, rather than a decrease, in renal flow. Therefore, from our results, it is feasible that any renal damage caused by CsA is attributable to drug-induced renal hyperperfusion, rather than selective, persistent renal vasoconstriction (see Introduction).
Cardiovascular effects of tacrolimus
With tacrolimus at a dose of 450 μg kg−1, administered as a bolus, there were pressor and tachycardic effects that were similar to, or even somewhat greater than, those of CsA, although they took much longer to develop. Interestingly, these effects persisted long after any significant regional vasoconstriction and, moreover, the latter was no greater than that seen with vehicle. Hence, our observations indicate that the sustained pressor effect of tacrolimus must have been due to an increase in cardiac output, in line with the tachycardia, and the absence of negative inotropic effects of tacrolimus (Milting et al., 2001), in contrast to those of CsA (Janssen et al., 2000). However, our finding that the tachycardic effect of tacrolimus was reversed by propranolol, but the rise in blood pressure was not, indicates that any increase in cardiac output was likely not due to sympathoexcitation. Overall, our findings appear to be at odds with the ability of tacrolimus to increase renal sympathetic nerve activity, albeit less so than CsA (Morgan et al., 1991), but it may be that differences in effects of CsA and tacrolimus on end-organ sensitivity account for this disparity (Benigni et al., 1992; Epstein et al., 1998; De Lima et al., 1999; Takeda et al., 1999). Certainly, the absence of renal vascular effects of tacrolimus is consistent with a lesser nephrotoxic effect of this drug (e.g., Kim et al., 2003).
It seems, on the basis of our observations, that the Zhang & Victor (2000) hypothesis, that is, that calcineurin inhibitors act through a common mechanism to evoke hypertension by sympathetically mediated vasoconstriction, needs to be reconsidered in the light of the similar pressor effects of CsA and tacrolimus occurring in the presence (CsA) or absence (tacrolimus) of regional vasoconstriction. However, disparate effects of CsA and tacrolimus on many mechanisms, such as in vitro constriction (Epstein et al., 1998), and prostanoid (Benigni et al., 1992) and NO production (Dusting et al., 1999), have been described, so there is a clear precedent for differences in their pharmacodynamic actions. A recent example that may be relevant to their differential regional haemodynamic effects is the observation of Harrison et al. (1998) that CsA and tacrolimus can both release neuropeptides from sensory nerves, but by different mechanisms. Furthermore, our finding that prolonged infusion of tacrolimus caused some mesenteric and hindquarters vasoconstriction in association with a slowly developing pressor effect might be explained by pharmacokinetic differences between tacrolimus and CsA, as might the differences in the rates of onset of the pressor effects of the two drugs when given by rapid bolus injection. While it is notable that the Ki values for CsA and tacrolimus against calcineurin are very similar (Lyson et al., 1993), we have found no data about the speed of action or duration of effect of the two drugs in this regard. However, any pharmacokinetic differences between CsA and tacrolimus would not explain the dissociation between pressor and vasoconstrictor influences seen with the bolus dose of tacrolimus.
Cardiovascular effects of sirolimus
Here, we found that acute administration of sirolimus had a slight, but significant, pressor effect, accompanied by modest renal, mesenteric and hindquarters vasoconstriction. Hence, it is unlikely that the apparent lack of pressor effect of sirolimus reported by Lyson et al. (1993) was due to constriction in one vascular bed being buffered by vasodilatation in another. However, the difference in pressor effects of sirolimus and tacrolimus reported by Lyson et al. (1993) was seen at a dose of 150 μg kg−1, whereas, in the conscious rats studied here, tacrolimus was without a consistent pressor action at that dose. Nonetheless, at the higher dose, tacrolimus had a much more marked pressor effect than sirolimus, possibly due to the former having cardiac effects (see above).
Previous comparison of CsA and sirolimus showed that the latter had little effect on glomerular filtration rate, although it was not without influence on glomerular haemodynamics (Sabbatini et al., 2000). Interestingly, the action of sirolimus to increase the renal afferent and efferent resistance was reversed by L-arginine, indicating that sirolimus might act to inhibit NO production (Sabbatini et al., 2000). We saw no obvious signs of this, since the haemodynamic effects of sirolimus were only slightly greater than vehicle, whereas even low doses of NO synthase inhibitors in our model cause vasoconstriction even before a pressor effect is seen (see above; Gardiner et al., 1990b, 1990c). However, it is notable that Sabbatini et al. (2000) used sirolimus at a dose of 5 mg kg−1, which is far greater than that we employed, and much above its immunosuppressive dose (Lyson et al., 1993).
Other recent evidence consistent with sirolimus having cardiovascular actions comes from the finding that it induces heme oxygenase-1 in human pulmonary arterial endothelial and vascular smooth muscle cells (Visner et al., 2003). Such an action, if seen in vivo, would be expected to exert haemodynamic effects (e.g., Naik et al., 2003), but might not be apparent with acute administration. Although clinical data indicate that sirolimus may have advantages over calcineurin inhibitors, its use in patients is clearly not without adverse actions on renal function, and it can also cause systemic arterial hypertension (Vasquez, 2000). While the mechanisms involved in these effects remain to be established, it appears that they could be due to cardiovascular influences of sirolimus, which are, however, much less than those of CsA or tacrolimus.
In conclusion, at bolus doses which caused similar, substantial increases in mean arterial blood pressure and in heart rate, CsA evoked sustained vasoconstriction (mesenteric>renal), whereas tacrolimus did not, consistent with the pressor effect of the latter being due to an increase in cardiac output. Under the same conditions, sirolimus had slight pressor and vasoconstrictor actions. The effects of CsA involved angiotensin II and endothelin, but were largely dependent upon α-adrenoceptor activation. The pressor effects of tacrolimus did not appear to be due to β-adrenoceptor activation. Thus, our findings are not easily reconciled with the hypothesis that hypertension only occurs after calcineurin inhibition (with CsA or tacrolimus), and results from activation of renal afferent neurones causing generalised sympathoexcitation (Zhang & Victor, 2000).
Acknowledgments
This work was supported by funding from Camurus AB, Ideon, Lund, Sweden.
Abbreviations
- CsA
cyclosporine A
- ET
endothelin
- NO
nitric oxide
References
- ABRAHAM J.S., BENTLEY F.R., GARRISON N.R., CRYER H.M. The influence of the cyclosporine vehicle, cremophor EL, on renal microvascular blood flow in the rat. Transplantation. 1991;52:101–105. doi: 10.1097/00007890-199107000-00021. [DOI] [PubMed] [Google Scholar]
- BENIGNI A., MORIGI M., PERICO N., ZOJA C., AMUSCHATEGUI C.S., PICCINELLI A., DONADELLI R., REMUZZI G. The acute effect of FK 506 and cyclosporine on endothelial cell function and renal vascular resistance. Transplantation. 1992;54:775–778. doi: 10.1097/00007890-199211000-00002. [DOI] [PubMed] [Google Scholar]
- BENNETT T., GARDINER S.M. Fostering orphan receptors: an indispensable role for integrative, in vivo, haemodynamic studies. Curr. Opin. Pharmacol. 2003;3:1–6. doi: 10.1016/s1471-4892(03)00004-3. [DOI] [PubMed] [Google Scholar]
- BENNETT T., GARDINER S.M., KEMP P.A., MARCH J.E., FALLGREN B.Regional haemodynamic effects of cyclosporine A (CsA) in conscious rats Br. J. Pharmacol. 2004(in press) [DOI] [PMC free article] [PubMed]
- BOREL J.F. Pharmacology of cyclosproine (Sandimmune) IV pharmacological properties in vivo. Pharmacol. Rev. 1989;41:259–372. [PubMed] [Google Scholar]
- BOULANGER G., LÜSCHER T.F. Release of endothelin from the porcine aorta: inhibition by endothelium-derived nitric oxide. J. Clin. Invest. 1990;85:587–590. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRIER M., TRONC F., STEWART D., PELLETIER L.C. Dose-dependent effect of cyclosporin on renal arterial resistance in dogs. Am. J. Physiol. 1991;261:H1791–H1796. doi: 10.1152/ajpheart.1991.261.6.H1791. [DOI] [PubMed] [Google Scholar]
- CHIU P.J.S., VEMULAPALLI S., SABIN C., RIVELLI M., BERNARDINO V., SYBERTZ E.J. Sympathoadrenal stimulation, not endothelin, plays a role in acute pressor response to cyclosporine in anesthetized rats. J. Pharmacol. Exp. Ther. 1992;261:994–999. [PubMed] [Google Scholar]
- DAVIS L.S., HALEEN S.J., DOHERTY A.M., CODY W.L., KEISER J.A. Effects of selective endothelin antagonists on the hemodynamic response to cyclosporine A. J. Am. Soc. Nephrol. 1994;4:1448–1454. doi: 10.1681/ASN.V471448. [DOI] [PubMed] [Google Scholar]
- DE LIMA J.J.G., XUE H., COBURN L., ANDOH T.F., MCCARRON D.A., BENNETT W.M., ROULLET J.-B. Effects of FK 506 in rat and human resistance arteries. Kid. Int. 1999;55:1518–1527. doi: 10.1046/j.1523-1755.1999.00366.x. [DOI] [PubMed] [Google Scholar]
- DIEDERICH D., SKOPEC J., DIEDERICH A., DAI F.X. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994;23:957–961. doi: 10.1161/01.hyp.23.6.957. [DOI] [PubMed] [Google Scholar]
- DUSTING G.J., AKITA K., HICKEY H., SMITH M., GUREVICH V. Cyclosporin A and tacrolimus (FK506) suppress expression of inducible nitric oxide synthase in vitro by different mechanisms. Br. J. Pharmacol. 1999;128:337–344. doi: 10.1038/sj.bjp.0702782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN A., BEALL A., WYNN J., MULLOY L., BROPHY C.M. Cyclosporine, but not FK506, selectively induces renal and coronary artery smooth muscle contraction. Surgery. 1998;123:456–460. [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T. Regional haemodynamic effects of endothelin-1 and endothelin-3 in conscious Long Evans and Brattleboro rats. Br. J. Pharmacol. 1990a;99:107–112. doi: 10.1111/j.1476-5381.1990.tb14662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T., PALMER R.M.J., MONCADA S. Control of regional blood flow by endothelium-derived nitric oxide. Hypertension. 1990b;15:486–492. doi: 10.1161/01.hyp.15.5.486. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic effects of NG-nitro-L-arginine methyl ester in conscious, Long Evans rats. Br. J. Pharmacol. 1990c;101:625–631. doi: 10.1111/j.1476-5381.1990.tb14131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Effects of the non-peptide, non-selective endothelin antagonist, bosentan, on regional haemodynamic responses to NG-monomethyl-L-arginine in conscious rats. Br. J. Pharmacol. 1996;118:352–354. doi: 10.1111/j.1476-5381.1996.tb15409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARR M.D., PALLER M.S. Cyclosporine augments renal but not systemic vascular reactivity. Am. J. Physiol. 1990;258:F211–F217. doi: 10.1152/ajprenal.1990.258.1.F211. [DOI] [PubMed] [Google Scholar]
- HARDY G., STANKE-LABESQUE F., DEVEAUX G., DEVILLIER P., SESSA C., BESSARD G. Cyclosporine A and cremophor EL induce contractions of human saphenous vein: involvement of thromboxane A2 receptor-dependent pathway. J. Cardiovasc. Pharmacol. 2000;36:693–698. doi: 10.1097/00005344-200012000-00002. [DOI] [PubMed] [Google Scholar]
- HARRISON S., REDDY S., PAGE C.P., SPINA D. Stimulation of airway sensory nerves by cyclosporine A and FK506 in guinea-pig isolated bronchus. Br. J. Pharmacol. 1998;125:1405–1412. doi: 10.1038/sj.bjp.0702198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN P.M.L., ZEITZ O., KEWELOH B., SEIGEL U., MAIER L.S., BARCKHAUSEN P., PIESKE B., PRESTLE J., LEHNART S.E., HASENFUSS G. Influence of cyclosporine A on contractile function, calcium handling and energetics in isolated human and rabbit myocardium. Cardiovasc. Res. 2000;47:99–107. doi: 10.1016/s0008-6363(00)00052-3. [DOI] [PubMed] [Google Scholar]
- KAWAI R., MATHEW D., TANAKA C., ROWLAND M. Physiologically based pharmacokinetics of cyclosporine A: extension to tissue distribution kinetics in rats and scale-up to human. J. Pharmacol. Exp. Ther. 1998;287:457–468. [PubMed] [Google Scholar]
- KAYE D., THOMPSON J., JENNINGS G., ESLER M. Cyclosporine therapy after cardiac transplantation causes hypertension and renal vasoconstriction without sympathetic activation. Circulation. 1993;88:1101–1109. doi: 10.1161/01.cir.88.3.1101. [DOI] [PubMed] [Google Scholar]
- KIM H.C., PARK S.B., HAN S.Y., WHANG E.A., JEON D.S., KIM H.T., CHO W.H., PARK C.H. Primary immunosuppression with tacrolimus in renal transplantation: a single center experience. Transplant. Proc. 2003;35:217–218. doi: 10.1016/s0041-1345(02)04017-4. [DOI] [PubMed] [Google Scholar]
- LASSILA M., FINCKENBERG P., PERE A.-K., KROGERUS L., AHONEN J., VAPAATALO H., NURMINEN M.-L. Comparison of enalapril and valsartan in cyclosporine A-induced hypertension and nephrotoxicity in spontaneously hypertensive rats on high-sodium diet. Br. J. Pharmacol. 2000;130:1139–1347. doi: 10.1038/sj.bjp.0703422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LODGE N.J. Direct vasoconstrictor effects of Sandimmune (Cyclosporine A) are mediated by its vehicle cremophor EL: inhibition by the thromboxane A2/prostaglandin endoperoxide receptor antagonist ifetroban. J. Pharmacol. Exp. Ther. 1994;271:730–734. [PubMed] [Google Scholar]
- LO RUSSO A., PASSAQUIN A.-C., SKUTELLA A.M., RUEGG U.T. Effect of cyclosporin A and analogues on cytosolic calcium and vasoconstriction: possible lack of relationship to immunosuppressive activity. Br. J. Pharmacol. 1996;118:885–892. doi: 10.1111/j.1476-5381.1996.tb15482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYSON T., ERMEL L.D., BELSHAW P.J., ALBER D.G., SCHREIBER S.L., VICTOR R.G. Cyclosporine- and FK 506-induced sympathetic activation correlates with calcineurin-mediated inhibition of T-cell signalling. Circ. Res. 1993;73:596–602. doi: 10.1161/01.res.73.3.596. [DOI] [PubMed] [Google Scholar]
- LYSON T., MCMULLAN D.M., ERMEL L.D., MORGAN B.J., VICTOR R.G. Mechanism of cyclosporine-induced sympathetic activation and acute hypertension in rats. Hypertension. 1994;23:667–675. doi: 10.1161/01.hyp.23.5.667. [DOI] [PubMed] [Google Scholar]
- MCKENZIE N., DEVINENI R., VEZINA W., KEOWN P., STILLER C. The effect of cyclosporine on organ blood flow. Transplant. Proc. 1985;17:1973–1975. [PubMed] [Google Scholar]
- MILTING H., JANSSEN P.M.L., WANGENMANN T., KOGLER H., DOMEIER E., SEIDLER T., HAKIM K., GRAPOW M., ZEITZ O., PRESTLE J., ZERKOWSKI H.R. FK506 does not affect cardiac contractility and adrenergic response in vitro. Eur. J. Pharmacol. 2001;430:299–304. doi: 10.1016/s0014-2999(01)01387-5. [DOI] [PubMed] [Google Scholar]
- MORGAN B.J., LYSON T., SCHERRER U., VICTOR R.G. Cyclosporine causes sympathetically mediated elevations in arterial pressure in rats. Hypertension. 1991;18:458–466. doi: 10.1161/01.hyp.18.4.458. [DOI] [PubMed] [Google Scholar]
- MOSS N.G., POWELL S.L., FALK R.J. Intravenous cyclosporine activates afferent and efferent renal nerves and causes sodium retention in innervated kidneys in rats. Proc. Natl. Acad. Sci. U.S.A. 1985;82:8222–8226. doi: 10.1073/pnas.82.23.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAIK J.S., O'DONAUGHY T.L., WALKER B.R. Endogenous carbon monoxide is an endothelial-derived vasodilator factor in the mesenteric circulation. Am. J. Physiol. 2003;284:H838–H845. doi: 10.1152/ajpheart.00747.2002. [DOI] [PubMed] [Google Scholar]
- ORIJI G.K., KEISER H.R. Role of nitric oxide in cyclosporine A-induced hypertension. Hypertension. 1998;32:849–855. doi: 10.1161/01.hyp.32.5.849. [DOI] [PubMed] [Google Scholar]
- ROULLET J.B., XUE H., MCCARRON D.A., HOLCOMB S., BENNETT W.M. Vascular mechanisms of cyclosporine-induced hypertension in the rat. J. Clin. Invest. 1994;93:2244–2250. doi: 10.1172/JCI117222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABBATINI M., SANSONE G., UCCELLO F., DE NICOLA L., NAPPI F., ANDREUCCI V.E. Acute effects of rapamycin on glomerular dynamics: a micropuncture study in the rat. Transplantation. 2000;69:1946–1990. doi: 10.1097/00007890-200005150-00034. [DOI] [PubMed] [Google Scholar]
- SANCHEZ H., ZOLL J., BIGARD X., VEKSLER V., METTAUER B., LAMPERT E., LONSDORFER J., VENTURA-CLAPIER R. Effect of cyclosporin A and its vehicle on cardiac and skeletal muscle mitochondria: relationship to efficacy of the respiratory chain. Br. J. Pharmacol. 2001;133:781–788. doi: 10.1038/sj.bjp.0704129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDER M., LYSON T., THOMAS G.D., VICTOR R.G. Sympathetic neural mechanisms of cyclosporine-induced hypertension. Am. J. Hypertens. 1996;9:121S–138S. doi: 10.1016/0895-7061(96)00288-9. [DOI] [PubMed] [Google Scholar]
- STEIN C.M., HE H., PINCUS T., WOOD A.J.J. Cyclosporine impairs vasodilation without increased sympathetic activity in humans. Hypertension. 1995;26:705–710. doi: 10.1161/01.hyp.26.4.705. [DOI] [PubMed] [Google Scholar]
- STROES E.S.G., LUSCHER T.F., DE GROOT F.G., KOOMANS H.A., RABELINK T.J. Cyclosporin A increases nitric oxide activity in vivo. Hypertension. 1997;29:570–575. doi: 10.1161/01.hyp.29.2.570. [DOI] [PubMed] [Google Scholar]
- TAKEDA Y., MIYAMORI I., FURUKAWA K., INABA S., MABUCHI H. Mechanisms of FK 506-induced hypertension in the rat. Hypertension. 1999;33:130–136. doi: 10.1161/01.hyp.33.1.130. [DOI] [PubMed] [Google Scholar]
- TAKEDA Y., MIYAMORI I., WU P., YONEDA T., FURUKAWA K., TAKEDA R. Effects of an endothelin receptor antagonist in rats with cyclosporine-induced hypertension. Hypertension. 1995;26:932–936. doi: 10.1161/01.hyp.26.6.932. [DOI] [PubMed] [Google Scholar]
- TEXTOR S.C., SMITH-POWELL L., TELLES T. Altered pressor responses to NE and ANG II during cyclosporin A administration to conscious rats. Am. J. Physiol. 1990;258:H854–H860. doi: 10.1152/ajpheart.1990.258.3.H854. [DOI] [PubMed] [Google Scholar]
- THEODORSSON-NORHEIM E. Friedman and Quade tests: BASIC computer program to perform non-parametric two-way analysis of variance and multiple comparisons on ranks of several related samples. Comput. Biol. Med. 1987;17:85–99. doi: 10.1016/0010-4825(87)90003-5. [DOI] [PubMed] [Google Scholar]
- VASQUEZ E.M. Sirolimus, a new agent for prevention of renal allograft rejection. Am. J. Health-Syst. Pharm. 2000;57:437–448. doi: 10.1093/ajhp/57.5.437. [DOI] [PubMed] [Google Scholar]
- VISNER G.A., LU F., ZHOU H., LIU J., KAZEMFAR K., AGARWAL A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- YARIS E., TUNCER M. The effect of indomethacin on cyclosporin A- and its solvent-induced inhibition of endothelium-dependent relaxation and the drug-induced contraction of rabbit isolated arteries. Gen. Pharmacol. 1995a;26:93–97. doi: 10.1016/0306-3623(94)00168-m. [DOI] [PubMed] [Google Scholar]
- YARIS E., TUNCER M. Cyclosporin A and cremophor-EL augment renal vascular responses to various agonists and nerve stimulation. Arch. Int. Pharmacodyn. 1995b;329:405–417. [PubMed] [Google Scholar]
- YARIS E., TUNCER M., ILHAN M. Actions of cyclosporine A preparation and cremophor-EL in rabbit mesenteric artery and thoracic aorta in vitro. Clin. Sci. 1992;83:179–182. doi: 10.1042/cs0830179. [DOI] [PubMed] [Google Scholar]
- YARIS E., TUNCER M., KAYAALP S.O., ILHAN M. Direct vascular smooth muscle contractile effect of cyclosporin A and its vehicle in rabbit isolated arteries. Arch. Int. Pharmacodyn. 1994;327:166–174. [PubMed] [Google Scholar]
- ZHANG W., VICTOR R.G. Calcineurin inhibitors cause renal afferent activation in rats: a novel mechanism of cyclosporine-induced hypertension. Am. J. Hypertens. 2000;13:999–1004. doi: 10.1016/s0895-7061(00)00288-0. [DOI] [PubMed] [Google Scholar]