Abstract
This study was designed to investigate the effects of the nitric oxide (NO) donors sodium nitroprusside (SNP), 3-morpholinosydnonimine (SIN-1) and S-nitroso-N-acetylpenicillamine (SNAP) on N-formyl-L-methionyl-L-leucyl-phenylalanine (fMLP, 1 × 10−7 M)-induced human eosinophil chemotaxis, cyclic guanosine-3′,5′-monophosphate (cGMP) levels, protein nitration and cytotoxicity.
Human eosinophils were exposed to SNP, SIN-1 and SNAP (0.001–1.0 mM) for either short (10 min) or prolonged (90 min) time periods. Exposition of eosinophils with these NO donors significantly inhibited the eosinophil chemotaxis irrespective of whether cells were exposed to these agents for 10 or 90 min. No marked differences were detected among them regarding the profile of chemotaxis inhibition.
Exposition of eosinophils to SNP, SIN-1 and SNAP (0.001–1.0 mM) markedly elevated the cGMP levels above basal levels, but the 90-min exposition resulted in significantly higher levels compared with the 10-min protocols (5.3±0.6 and 2.6±0.2 nM 1.5 × 106 cells−1, respectively). The cGMP levels achieved with SNAP were greater than SNP and SIN-1.
The NO donors did not induce cell toxicity in any experimental condition used. Additionally, eosinophils exposed to SNP, SIN-1 and SNAP (1.0 mM each) either for 10 or 90 min did not show any tyrosine nitration in conditions where a strong nitration of bovine serum albumin was observed.
Our findings show that inhibitory effects of fMLP-induced human eosinophil chemotaxis by NO donors at short or prolonged exposition time were accompanied by significant elevations of cGMP levels. However, additional elevations of cGMP levels do not change the functional profile (chemotaxis inhibition) of stimulated eosinophils.
Keywords: cGMP levels, chemotaxis, cytotoxicity, eosinophil, protein nitration, nitric oxide, SIN-1, SNAP, sodium nitroprusside
Introduction
Nitric oxide (NO) is synthesized by many cell types, and participates in several physiopathological processes (Sethi & Dikshit, 2000). The action of NO is generally carried out through the activation of the soluble guanylyl cyclase, thereby increasing intracellular levels of cyclic guanosine-3′,5′-monophosphate (cGMP), which acts as its second messenger (Murad, 1986). For this reason, cGMP is frequently used as a sensitive indirect measure of NO production. NO can be exogenously supplied to tissues and cells by various NO-generating compounds, each of with its own specific properties. These compounds have been useful for studying physiopathological processes and molecular mechanisms in which NO is involved. Several types of NO-releasing agents are available, including sydnonimines (3-morpholinosydnonimine, molsidomine), organic nitrates (glyceryl trinitrate) and nitrites (amyl nitrite), inorganic nitroso compounds (sodium nitroprusside (SNP)) and S-nitrosothiols (S-nitroso-N-acetylpenicillamine (SNAP), S-nitrosoglutathione) (Feelisch & Stamler, 1996; Moncada et al., 1997). Such compounds have shown diverse and remarkable biological effects, and in some cases they appear to be opposed to one another, although differences in concentrations, incubation times or experimental conditions might explain the apparent contradictions (Ferrero et al., 1999). SNP is an agent with either a nitroso or nitrosyl functional group that spontaneously releases small amounts of NO over long periods (Rao et al., 1991; Bates et al., 1992), while SNAP releases NO by mechanisms highly dependent on the components of a given system, particularly with regard to the concentration of reduced thiol and transition metals present (Dicks et al., 1996; Gordge et al., 1996; Singh et al., 1996). The NO donor 3-morpholinosydnonimine (SIN-1) is a co-donor of NO and superoxide anion (O2−) to form the peroxynitrite anion (ONOO−; Feelisch et al., 1989).
Eosinophils play an important role in host defense mechanisms in parasitic infestation and pathogenesis of allergic, immunological and malignant disorders (Giembycz & Lindsay, 1999). Previous studies demonstrated the existence of a functional NO–cGMP pathway in rat (Zanardo et al., 1997) and human (Thomazzi et al., 2001) purified eosinophils that modulates the in vitro locomotion of this cell type. In vivo treatment with NO synthesis inhibitor selectively attenuates the eosinophil infiltration in airways of allergic mice (Feder et al., 1997; Koarai et al., 2000; Iijima et al., 2001) and rats (Ferreira et al., 1998) as well as in pleural cavity of the rat stimulated with carrageenin (Ferreira et al., 1996), thus corroborating the in vitro studies. Although cyclic nucleotides are known to modulate leukocyte activation processes, their role in cell locomotion is still controversial. In human isolated neutrophils and mononuclear cells, increase of cGMP levels has been shown to either stimulate (Kaplan et al., 1989; Belenky et al., 1993a,1993b; Wanikiat et al., 1997) or inhibit (Schröder et al., 1990; Bath et al., 1991; Moilanen et al., 1993; Kosonen et al., 1999; Conran et al., 2001) the in vitro chemotaxis and adhesion. On the other hand, the soluble guanylyl cyclase inhibitor 1H-[1,2,4] oxidiazolo[4,3-α] quinoxalin-1-one (ODQ) has been clearly shown to reduce concentration-dependently N-formyl-L-methionyl-L-leucyl-phenylalanine (fMLP)-induced eosinophil chemotaxis, suggesting a key role for cGMP in these cells (Zanardo et al., 1997; Thomazzi et al., 2001). The present study was designed to further clarify the relationship between in vitro eosinophil locomotion and intracellular concentrations of cGMP levels in this cell type. To achieve this, human isolated eosinophils were treated with varying concentrations of SNP, SNAP and SIN-1 under a short (10 min) or prolonged (90 min) exposure time, after which fMLP-induced chemotaxis, cGMP levels and cell viability were evaluated. Since protein nitration, possibly mediated by ONOO−, has been shown to mediate in vivo cGMP-independent actions (Brennan et al., 2002), we have also evaluated the tyrosine nitration in eosinophils exposed to short and prolonged time to the NO donors.
Methods
Eosinophil isolation
Blood was collected from healthy volunteers (male and female volunteers, aged 18–50 years) who were not under medication. Informed consent and approval from the local ethical committee were obtained before the study.
Human eosinophils were isolated from peripheral blood using a method adapted from that of Hansel et al. (1991). Briefly, 120 ml blood collected in 3.13% (w v−1) sodium citrate from a healthy subject was diluted 1 : 1 with phosphate-buffered saline (PBS), and 35 ml diluted blood overlaid onto a 15 ml Percoll gradient (1.082±0.005 g ml−1, pH 7.4, 340 mOs kgH2O−1). Gradients were centrifuged at 1000 × g for 20 min at 4°C (Hermle model Z360k centrifuge, Germany) and the cell pellet was collected. Red cells contained in the granulocyte pellet were lysed with lysing buffer (in mM: NH4Cl 155, KHCO3 10 and EDTA 0.1). Washed granulocytes were incubated with anti-CD16 immunomagnetic microbeads before passing on a steel-matrix column in a magnetic field, and the CD16-negative eosinophils were collected. Eosinophils (92–99% purity) were then resuspended in minimum essential medium (MEM, pH 7.2). Contaminating cells were mononuclear cells.
Experimental design: in vitro treatment of eosinophils
In order to explore the effects of NO donors on eosinophil chemotaxis, cGMP levels, protein nitration and cell toxicity, two experimental protocols varying the exposure time of NO donors with eosinophils were carried out. In the former protocols, eosinophil suspensions were exposed for 90 min to SNP, SIN-1, SNAP (0.001–1.0 mM each) or the vehicle dimethyl sulphoxide (DMSO, 0.3% for SNAP) at 37°C in 5% CO2. In these 90-min exposition protocols, eosinophils were incubated for 30 min with the NO donors and maintained in contact with these drugs for another 60 min, which corresponded to the time spent in chemotaxis assay with fMLP (1 × 10−7 M), thus performing a whole time period of 90 min of exposition to NO donors. In further experiments, eosinophil suspensions were exposed for short-term (10 min) periods to SNP, SIN-1, SNAP (0.1 mM each) or the vehicle DMSO (0.3% for SNAP) at 37°C in 5% CO2. In these 10-min exposure protocols, cells were previously maintained at 37°C in 5% CO2 for 20 min to mimic the conditions employed in 90-min exposition studies. Next, the NO donors were added to eosinophil suspension for 10 min, after which cells were washed and placed in the microchemotaxis chamber to carry out the chemotaxis assays for 60 min using fMLP (1 × 10−7 M) as a chemoattractant agent.
Chemotaxis assay
Eosinophils were resuspended at a concentration of 5 × 106 cells ml−1 in MEM/ovalbumin, and migration assays were performed using a 48-well microchemotaxis chamber (Richards & McCullough, 1984). The bottom wells of the chamber were filled with the chemoattractant agent fMLP (1 × 10−7 M) in 28 μl MEM, whereas the upper wells were filled with eosinophils (50 μl) that had been treated or not with SNP, SIN-1, SNAP or diBu-cGMP. The bottom and upper cells were separated by a polycarbonate filter of 5 μm. The chamber was then incubated for 60 min at 37°C with 5% CO2 atmosphere. At the end of the incubation period, the filter was removed, washed, fixed in methanol for 2 min, stained with Diff-Quik and mounted on a glass slide. Each incubation was carried out in triplicate and migration was determined by counting eosinophils that had migrated completely through the filter in five random high-power fields (HPF, × 1000) per well.
Extraction and measurement of cGMP from eosinophils
Eosinophils were isolated and resuspended to a concentration of 1 × 107 cells ml−1 in PBS. To achieve this number of eosinophils, a pool of three volunteers was used for each assay. Cells were incubated with the phosphodiesterase inhibitor 3-isobutyl-1-methyl-xanthine (IBMX, 2 mM) for 30 min at room temperature before adding the NO donors. In order to mimick the conditions employed in the chemotaxis assays (10- and 90-min protocols), eosinophils were incubated (37°C, humidified atmosphere) with the NO donors (0.001–1.0 mM each) for 10 or 90 min, after which the reaction was interrupted by the addition of cold acidified absolute ethanol to a final concentration of 67% (v v−1), and samples were vigorously agitated by hand for 30 s. Cell samples were then incubated on ice for 30 min before centrifuging at 4000 × g for 30 min at 4°C. The supernatants were collected and retained and the precipitates were washed with 0.5 ml 67% (v v−1) acidified ethanol before centrifuging again at 14,000 × g for 5 min at room temperature. The supernatants from these washed samples were collected and added to the first supernatants collected and dried at 55–60°C under a stream of nitrogen in a water bath and stored at −20°C until measurement of cGMP. cGMP in 1.5 × 106 cells well−1 was measured using a Cayman kit according to Pradelles & Grassi (1989) and Maxey et al. (1992).
Western blot for 3-nitrotyrosine
Eosinophils were isolated, resuspended to a concentration of 1 × 107 cells ml−1 in Tris (50 mM, pH 7.4) and incubated (37°C, humidified atmosphere) with SNP, SIN-1 and SNAP (1.0 mM each) for 10 or 90 min. A protease inhibitor cocktail (2.0 mM AEBSF, 1.6 μM aprotinin, 42 μM leupeptin, 72 μM bestatin, 30 μM pepstatin A and 28 μM E-64) and EDTA (1.0 mM) were added to each sample, and stored at −80°C until analysis. For the assay, frozen eosinophil samples were thawed, sonicated (5 min) and centrifuged (10,000 × g, 4°C) for 15 min. The eosinophil supernatant and precipitates were separated. The precipitates were resuspended in buffer (1% NP-40, 20 mM Tris-HCl pH 7.5, 400 mM NaCl, 1 mM EDTA), sonicated (5 min), centrifuged (10,000 × g, 15 min, 4°C) and pellets discarded. The samples (supernatants from either thawed cells or precipitates) were assayed for protein concentration (Lowry method) using the Bio-Rad DC protein assay. Equivalent protein amounts (40 μg) were loaded on SDS–polyacrylamide gel electrophoresis (SDS–PAGE, 7.5%). Following electrophoresis, samples, nitrated bovine serum albumin (BSA, made from the incubation of 1.0 mM peroxynitrite with 1 mg ml−1 BSA for 5 min) and molecular weight standards, were electrophoretically transferred to PVDF membranes and blocked for 30 min at room temperature in solution containing 5% nonfat milk in Tris-buffered saline-tween (TBS-T, 20 mM Tris-HCl pH 7.2, 0.3 M NaCl with 0.1% Tween-20). The membrane was incubated overnight in 5% nonfat milk containing a 1 : 2000 dilution of mouse monoclonal anti-nitrotyrosine antibodies at 4°C. Next, the blot was washed eight times (10 min each) with TBS-T and then incubated with 5% nonfat milk containing 1 : 8000 horseradish peroxidase-conjugated secondary antibodies for 1 h while shaking at room temperature. The membrane was washed another eight times (10 min each) with TBS-T. Detection of nitrated proteins was performed by enhanced chemiluminescence (ECL plus Western blotting detection system).
MTT assay
Cell toxicity was estimated using the tetrazolium salt reduction test (MTT assay) by eosinophils after exposure to NO donors (Mosmann, 1983; Ribeiro-Dias et al., 2000). Eosinophils were isolated and resuspended to a concentration of 2 × 106 cells ml−1in MEM. Cells were exposed to SNP, SIN-1 or SNAP (0.001–1.0 mM each) for either 10 or 90 min at 37°C in a humidified atmosphere. Eosinophils (100 μl well−1), treated or not, and 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT, 10 μl well−1, 5 mg ml−1 in PBS) were added in triplicate to a 96-well plate. Cells were allowed to incubate for 3 h at 37°C and 5% CO2. After incubation, 100 μl of 10% SDS in 0.01 M HCl was added to each well. Cell samples were then incubated for 18 h at 37°C, 5% CO2 and absorbance measured at 540 nm in a microplate reader (Multiscan MS, Labsystems, U.S.A.).
Materials
VarioMACS system and microbeads were purchased from Miltenyi Biotec Inc. (Auburn, CA, U.S.A.). DMSO, MTT, fMLP, IBMX, MEM, 3-morpholinosydnonimine (SIN-1), SNAP, Percoll, protease inhibitor cocktail and SNP were purchased from Sigma (St Louis, MO, U.S.A.). Polycarbonate filter (5 μm) was obtained from Nuclepore (Pleasanton, CA, U.S.A.). Diff-Quik was obtained from Baxter Healthcare Corp. (DE, U.S.A.). Kit for measurement of cGMP was obtained from Cayman Chemical Co. (Ann Arbor, MI, U.S.A.). PVDF membranes and Bio-Rad DC protein assay kit were purchased from Bio-Rad Laboratories (CA, U.S.A.). Mouse monoclonal anti-nitrotyrosine antibodies were purchased from Upstate Biotechnology (MA, U.S.A.). Horseradish peroxidase-conjugated secondary antibodies, molecular weight standards and ECL plus Western blotting detection system were purchased from Amersham Biosciences Corp. (NJ, U.S.A.).
Statistical analysis
Data are expressed as the mean values±s.e.m. of at least three separate experiments carried out in triplicate. Data were analysed by analysis of variance (ANOVA) for multiple comparisons followed by Tukey's test, or unpaired Student t-test when appropriate. A value of P<0.05 was taken as significant.
Results
Effect of NO donors on the fMLP-stimulated eosinophil chemotaxis
Activation of human eosinophils (37°C, 5% CO2) with fMLP (1 × 10−7 M) induced a significant cell chemotaxis compared to random migration (P<0.001). However, chemotactic responses to fMLP were about 1.5 times higher (P<0.05) in the 90-min in comparison with the 10-min exposure protocols (Figure 1).
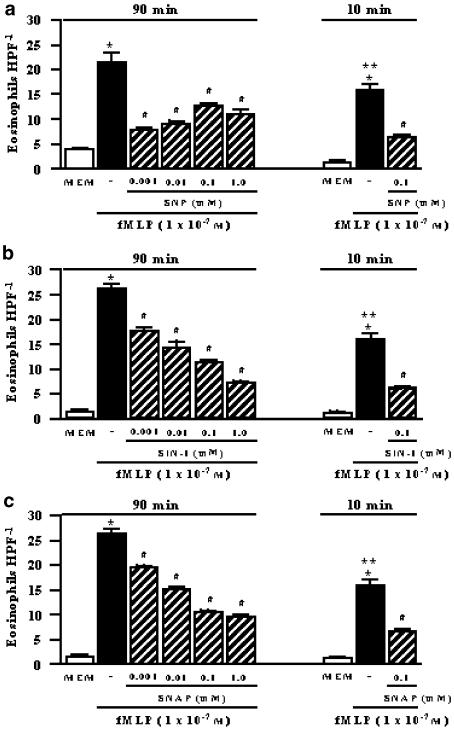
Figure 1.
Inhibitory effects of NO donors on fMLP (1 × 10−7 M)-stimulated eosinophil chemotaxis. The human eosinophil suspension (5 × 106 cells ml−1) was exposed for 90 or 10 min with the NO donors SNP (panel a), SIN-1 (panel b) or SNAP (panel c) in concentrations of 0.001–1.0 mM each. Control migration (fMLP) is represented by the solid column, whereas random chemotaxis (MEM, in the absence of fMLP) is represented by the open column in both conditions. Each experiment was carried out in triplicate (n=3). Eosinophil migration is expressed as the mean number of migrated cells per HPF. The results are shown as the mean values±s.e.m. *P<0.001 compared to respective MEM, **P<0.05 compared to fMLP alone at 90 min, #P<0.001 compared to respective fMLP.
Figure 1 shows that exposure of human eosinophils for 90 min (37°C, 5% CO2) to 0.001–1.0 mM of NO donors (n=3 individuals, each in triplicate) significantly inhibited (P<0.001) the fMLP-induced chemotaxis in all concentrations used. The chemotaxis inhibition by SIN-1 and SNAP followed a clear concentration-dependent response, whereas inhibition by SNP reached maximum response with the first concentration used (0.001 mM). The vehicle DMSO (0.3% for SNAP) had no significant effect on fMLP-induced eosinophil chemotaxis compared to untreated cells (26.2±0.9 and 24.2±0.6 eosinophils HPF−1, untreated cells and DMSO, respectively).
In separate experiments, 10-min exposure protocols (see Methods) were carried out using eosinophils that have been preincubated for 20 min at 37°C in the absence of drugs prior to the addition of the NO donors. Using this protocol, a marked reduction (P<0.001) of fMLP-induced chemotaxis by the NO donors SNP, SIN-1 and SNAP (0.1 mM each) was also observed (Figure 1).
Effect of NO donors on intracellular levels of cGMP in eosinophils
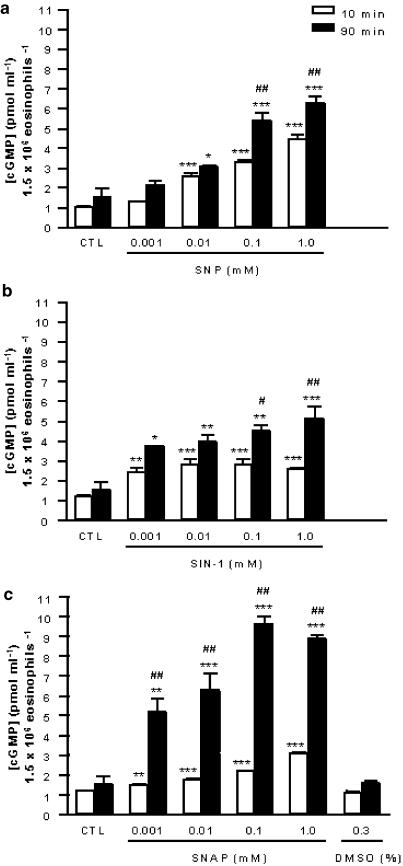
Treatment of eosinophils with SNP (0.001–1.0 mM; n=3 individuals, each in triplicate) for 10 min (37°C, 5% CO2) concentration-dependently increased the cGMP levels (Figure 2a). A concentration-dependent increase in cGMP levels was also observed when eosinophils were exposed for long time periods (90 min) to this NO donor (P<0.05; Figure 2a). In addition, the levels of this cyclic nucleotide in this latter protocol (90-min treatment) were 62 and 41% higher (P<0.001) compared to 10-min incubation at concentrations of 0.1 and 1.0 mM, respectively.
Figure 2.
Effect of NO donors on intracellular levels of cGMP in eosinophils after 10 min (open columns) or 90 min (closed columns) of exposition. The human eosinophil suspension (1.5 × 106 cells well−1) was incubated (37°C) with the NO donors SNP (panel a), SIN-1 (panel b), SNAP (panel c) or DMSO (panel c). Unstimulated cells are represented by CTL (control). Each experiment was carried out in triplicate (n=3). The levels of cGMP are expressed in pmol ml−1 1.5 × 106 eosinophils−1. The results are shown as the mean values±s.e.m. *P<0.05, **P<0.01 and ***P<0.001 compared to respective MEM, #P<0.05 and ##P<0.001 compared to 10-min incubation.
Treatment of eosinophils with SIN-1 (0.001–1.0 mM; n=3 individuals, each in triplicate) for 10 min increased the cGMP levels by approximately 1.9- to 2.3-fold (P<0.01) irrespective of the concentration used (Figure 2b). Treatment of eosinophils with SIN-1 (0.001–1.0 mM; n=3 individuals, each in triplicate) for 90 min caused a marked and concentration-dependent increase in cGMP levels (Figure 2b). The levels of cGMP in this latter protocol were 54, 39, 58 (P<0.05) and 96% higher (P<0.001) compared to 10-min incubation at concentrations of 0.001, 0.01, 0.1 and 1.0 mM, respectively.
Treatment of eosinophils with SNAP (0.001–1.0 mM; n=3 individuals, each in triplicate) for 10 min concentration-dependently increased the cGMP levels (Figure 2c). Treatment of eosinophils with the same concentrations of SNAP (n=3) for 90 min also caused a concentration-dependent increase in cGMP levels (Figure 2c). The levels of cGMP in this latter protocol were 246, 257, 343 and 191% higher (P<0.001) compared to 10-min incubation at concentrations of 0.001, 0.01, 0.1 and 1.0 mM, respectively. The vehicle DMSO (0.3%) alone had no effect on intracellular level of cGMP (Figure 2c).
In addition, incubation of eosinophils with SNAP (0.001–1.0 mM) for 90 min generated higher cGMP levels compared to equimolar concentrations of SNP and SIN-1 using the same incubation protocols.
In separate assays, incubation of eosinophils with fMLP (1 × 10−7 M) for 10 or 90 min significantly elevated the cGMP levels (9.4±0.1 and 7.7±0.2 pmol ml−1 1.5 × 106 eosinophils−1, respectively) compared with unstimulated cells (1.4±0.03 and 1.7±0.001 pmol ml−1 1.5 × 106 eosinophils−1, respectively; n=3).
Western blot analysis of tyrosine nitration
Western blot analysis showed a very strong nitration of BSA (5 μg well−1) (data not shown); however, the eosinophil samples exposed to SNP, SIN-1 and SNAP (1.0 mM each) for 10 or 90 min (40 μg well−1) did not show any tyrosine-nitrated proteins, even when the autoradiography film was exposed as long as 30 min.
Effect of NO donors on the eosinophils cytotoxicity
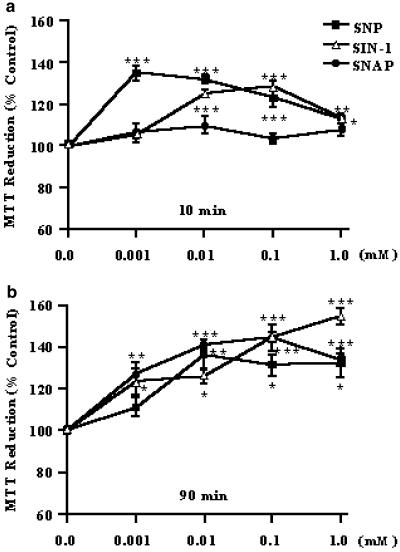
The MTT reduction assay showed that neither the short (10 min) nor the prolonged (90 min) exposure time of SNP, SIN-1 or SNAP (0.001–1.0 mM each; n=3 individuals) to human eosinophils caused any toxic effect; instead, an increase in cell activity was observed in both protocols for the NO donors (Figure 3). The vehicle used for SNAP (0.3% DMSO) also failed to affect cell toxicity (data not shown).
Figure 3.
Effect of NO donors on eosinophils by MTT assay. The human eosinophil suspension (2 × 106 cells ml−1) was incubated (37°C) for 10 min (panel a) or 90 min (panel b) with the NO donors SNP, SIN-1 or SNAP in concentrations of 0.001–1.0 mM each. Each experiment (mean values±s.e.m.) was carried out in triplicate (n=3). The result is expressed in MTT reduction (% control). *P<0.05, **P<0.01 and ***P<0.001 compared to respective untreated cells.
Discussion
We demonstrated in this study that the NO donors SNP, SIN-1 and SNAP exhibit a marked inhibitory effect on the fMLP-induced human eosinophil chemotaxis irrespective of whether eosinophils were exposed to short (10 min) or prolonged (90 min) time periods. This inhibitory effect is suggested to be unrelated to additional elevations of cGMP levels provided the prolonged exposition resulted in approximately two-fold higher levels of this cyclic nucleotide compared with the short-incubation experiments; yet the pattern of chemotaxis inhibition was basically the same.
The role of NO in cell locomotion is still controversial and opposite effects have been described depending on the experimental models and species employed. The use of different nitric oxide synthase (NOS) inhibitors or NO donors has indicated that endogenous NO may limit (Kubes et al., 1991; Gaboury et al., 1993; Kurose et al., 1994,1995) or potentiate (Ferreira et al., 1996,1998; Feder et al., 1997) the leukocyte adhesion and/or recruitment into normal or inflamed microcirculation. Stimulation or attenuation of leukocyte activity has also been described in isolated cells with the use of NOS inhibitors or NO donors (Kaplan et al., 1989; Belenky et al., 1993a,1993b; Moilanen et al., 1993; Beauvais et al., 1995; Zanardo et al., 1997; Okayama et al., 1998; Kosonen et al., 1999; Thomazzi et al., 2001). Although these discrepancies have not been clarified yet, it has been suggested that variations in cGMP levels may explain these opposite effects. Thus, high concentrations of NO donors have been shown to inhibit neutrophil chemotaxis, whereas lower NO concentrations increase this response, suggesting a biphasic regulation of chemotaxis by the NO–cGMP pathway (VanUffelen et al., 1996; Wanikiat et al., 1997). Other studies carried out with polymorphonuclear leukocytes have suggested that response to NO donors is cGMP-independent at low concentrations, and cGMP-dependent at high concentrations (VanUffelen et al., 1998; Sethi et al., 1999; Sethi & Dikshit, 2000). Recently, we have shown that eosinophils increase cGMP levels in response to SNP (Conran et al., 2001), but to our knowledge, no study until now has attempted to correlate the cGMP levels with the in vitro eosinophil chemotaxis. Our results showed that a short-time (10 min) exposure of increasing concentrations of SNP, SIN-1 or SNAP to human eosinophils elevated the cGMP levels by approximately 1.5- to 4.5-fold, and significantly reduced the fMLP-induced chemotaxis. Similarly, a prolonged exposure (90 min) of human eosinophils to these compounds increased the cGMP levels by approximately 3- to 10-fold, and such increases were also associated with significant reductions in fMLP-induced chemotaxis in the same extension as those observed in the short-exposure experiments. This indicates that additional elevations of cGMP levels do not change the functional profile (chemotaxis inhibition) of fMLP-stimulated eosinophils. Data with SNAP reinforce this concept since the cGMP levels achieved at 90 min with this agent are markedly higher than those obtained at 10 and 90 min in comparison with other NO donors, but the inhibitory concentration–response pattern of chemotaxis is about the same. In human platelets, SNAP has been shown to produce higher levels of cGMP compared with SNP due to its ability to release higher amounts of NO (Gordge et al., 1998). Therefore, it is likely that the inhibitory actions of the NO donors on eosinophil chemotaxis do not directly correlate with the intracellular cGMP elevations.
At this point, it is worth mentioning that eosinophils exposed for 90 min exhibited substantially higher (approximately 1.5-fold) chemotactic responses to fMLP than did cells exposed for 10 min. The implication of this finding is not entirely clear, but it is known that primed eosinophils obtained from atopic dermatitis (Bruijnzeel et al., 1993) and allergic rhinitis (Ferreira et al., 2002) patients show an increased migratory response to different chemotactic agents, including fMLP, platelet-activating factor and eotaxin. Priming by cytokines in neutrophils has been associated with sequential changes in fMLP receptor number and affinity, which may enhance different physiologic responses (Weisbart et al., 1986). Taken into consideration that the only difference in untreated eosinophils is that in 10-min protocols cells were washed immediately before submission to chemotaxis assay, whereas in the 90-min protocols this washing step was not performed, it is likely that during the prolonged exposition at 37°C eosinophils spontaneously release factors (e.g. platelet-activating factor, interleukin-5) that amplify their own responses to fMLP. However, our findings that NO donors inhibit the cell chemotaxis independently of the exposition time indicate that signalling mechanisms triggered by fMLP in both of the conditions used (10- and 90-min exposition) do not influence the resulting inhibition of chemotaxis. This is reinforced by data showing that incubation of eosinophils with fMLP for 10 or 90 min equally elevated the cGMP content above basal levels. It is worth pointing out that incubation of eosinophils with fMLP in order to measure the cGMP levels does not necessarily reflect the conditions of the functional assays, since in chemotaxis assays eosinophils are not in direct contact with fMLP but rather are separated by a polycarbonate filter. Measurement of cGMP levels in migrated cells into the filter has not been performed by methodological limitations.
It has been suggested that beneficial (physiological) actions of NO are mediated predominantly via activation of soluble guanylyl cyclase (i.e. cGMP-dependent) while detrimental (pathological) actions of NO are exerted primarily via direct (i.e. cGMP-independent) modifications of proteins (Hobbs, 2002). It is well established that NO can react with either superoxide or oxygen, yielding reactive nitrogen oxide species. These effects include the oxidation, nitrosation and nitration chemistry (Hanafy et al., 2001). The former includes one or two electron removal from substrate, as well as hydroxylation reactions. Nitrosation occurs when an equivalent of nitrosonium (NO+) is added to an amine, thiol, or hydroxy aromatic group. For instance, intermediates in the NO/O2 reaction convert thiol peptides to S-nitrosothiol peptides. Lastly, nitration of aromatic groups involves the addition of an equivalent of a nitrogen dioxide (NO2+), and this includes the formation of nitrotyrosine from different reactive nitrogen oxide species such as ONOO− (Wink & Mitchell, 1998). Peroxynitrite is a powerful oxidant, which can lead to the generation of other reactive radical species (Beckman & Koppenol, 1996). The eosinophilic inflammatory response in asthma is associated with protein nitration, detected as immunostaining for 3-nitrotyrosine (3NT), possibly resulting from the action of ONOO− (Saleh et al., 1998). Furthermore, ONOO− attenuates eotaxin-induced eosinophil chemotactic activity in vitro suggesting that nitration of a tyrosine residue is responsible for inhibition of this effect (Sato et al., 2000). Recent observations have also suggested that the action of peroxidases, including eosinophil peroxidase and myeloperoxidase, may be responsible for protein nitration (Duguet et al., 2001; Brennan et al., 2002). However, it is unlikely that mechanisms determining reduction of eosinophil chemotaxis by NO donors exposition in our study are consequence of cGMP-independent mechanisms, such as protein nitration, provided no tyrosine nitration could be detected in our assays that mimicked the functional assays.
A number of studies have reported that cytotoxicity as a result of long-lasting NO generation initiates apoptosis (Beauvais & Joly, 1999; Ward et al., 2000; Moulian et al., 2001). Although NO itself is not a powerful cytotoxic agent, it can render cells susceptible to other cytotoxic agents such as heavy metals, alkylating agents and radiation (Wink & Mitchell, 1998). However, the MTT assay revealed no cell toxicity with any of the concentrations of NO donors used, ruling out the possibility that decreased chemotaxis of eosinophils with the prolonged exposure to NO donors reflects cell death.
Two hypotheses could be raised to explain our results in eosinophils: (i) desensitization of the NO-cGMP signalling and/or (ii) activation of K+ channels. The first phenomenon has been demonstrated in human platelets (Mullershausen et al., 2001,2003) where incubations of these blood elements with the NO donor DEA-NO rapidly led to a reduction of the NO-induced cGMP response, as evidenced by increase in phosphodiesterase-5 activity and enzyme phosphorylation, both of which were abolished in the presence of the soluble guanylyl cyclase inhibitor ODQ. Therefore, it is plausible to speculate that increase of cGMP concentrations in human eosinophils (and/or prolonged exposure) leads to a short-term desensitization response, downregulating the cell chemotaxis. The second possibility has been evidenced in human eosinophils, where SNAP has recently been shown to induce an outflow of K+ ions, causing membrane hyperpolarization thereby reducing cytoplasmic Ca2+ concentrations (Schwingshacki et al., 2002). It is also likely therefore that this mechanism contributes to the NO-mediated reduction of eosinophil chemotaxis.
In conclusion, our study revealed that exposure to NO donors produces an inhibitory, noncytotoxic, effect on human eosinophil chemotaxis that neither depends on exposure time nor on additional elevations of cGMP levels. This inhibitory effect cannot either be attributed to tyrosine nitration in eosinophils.
Acknowledgments
Sara M. Thomazzi is supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). Edson Antunes thanks Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Abbreviations
- BSA
bovine serum albumin
- cGMP
cyclic guanosine-3′,5′-monophosphate
- DMSO
dimethyl sulphoxide
- fMLP
N-formyl-L-methionyl-L-leucyl-phenylalanine
- MEM
minimum essential medium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide
- NO
nitric oxide
- NOS
nitric oxide synthase
- 3NT
3-nitrotyrosine
- ODQ
1H-[1,2,4] oxidiazolo[4,3-α] quinoxalin-1-one
- ONOO−
peroxynitrite anion
- PBS
phosphate-buffered saline
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine
- SNP
sodium nitroprusside
- TBS-T
Tris-buffered saline-tween
References
- BATES J.N., BAKER M.T., GUERRA R., HARRISON D.G. Chemical release of nitric oxide from sodium nitroprusside to nitric oxide in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1992;262:916–922. [PubMed] [Google Scholar]
- BATH P.M.W., HASSALL D.G., GLADWIN A.-M., PALMER R.M.J., MARTIN J.F. Nitric oxide and prostacyclin. Divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler. Thromb. 1991;11:254–260. doi: 10.1161/01.atv.11.2.254. [DOI] [PubMed] [Google Scholar]
- BEAUVAIS F., JOLY F. Effects of nitric oxide on the eosinophil survival in vitro. A role for nitrosyl-heme. FEBS Lett. 1999;443:37–40. doi: 10.1016/s0014-5793(98)01673-1. [DOI] [PubMed] [Google Scholar]
- BEAUVAIS F., MICHEL L., DUBERTRET L. The nitric oxide donors, azide and hydroxylamine, inhibit the programmed cell death of cytokine-deprived human eosinophils. FEBS Lett. 1995;361:229–232. doi: 10.1016/0014-5793(95)00188-f. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BELENKY S.N., ROBBINS R.A., RENNARD S.I., GOSSMAN G.L., NELSON K.J., RUBINSTEIN I. Inhibitors of nitric oxide synthase attenuate human neutrophil chemotaxis in vitro. J. Lab. Clin. Med. 1993a;122:388–394. [PubMed] [Google Scholar]
- BELENKY S.N., ROBBINS R.A., RUBINSTEIN I. Nitric oxide synthase inhibitors attenuate human monocyte chemotaxis in vitro. J. Leukoc. Biol. 1993b;53:498–503. doi: 10.1002/jlb.53.5.498. [DOI] [PubMed] [Google Scholar]
- BRENNAN M.L., WU W., FU X., SHEN Z., SONG W., FROST H., VADSETH C., NARINE L., LENKIEWICZ E., BORCHERS M.T., LUSIS A.J., LEE J.J., LEE N.A., ABU-SOUD H.M., ISCHIROPOULOS H., HAZEN S.L. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- BRUIJNZEEL P.L.B., KUIPER P.H.M., RIHS S., BETZ S., WARRINGA R.A.J., KOENDERMAN L. Eosinophil migration in atopic dermatitis I: increased migratory response to N-formyl-methionyl-leucyl-phenylalanine, neutrophil-activating factor, platelet-activating factor and pletelet factor 4. J. Invest. Dermatol. 1993;100:137–142. doi: 10.1111/1523-1747.ep12462781. [DOI] [PubMed] [Google Scholar]
- CONRAN N., FERREIRA H.H.A., LORAND-METZE I., THOMAZZI S.M., ANTUNES E., DE NUCCI G. Nitric oxide regulates human eosinophil adhesion mechanisms in vitro by changing integrin expression and activity on the eosinophil cell surface. Br. J. Pharmacol. 2001;134:632–638. doi: 10.1038/sj.bjp.0704295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKS A.P., SWIFT H.R., WILLIAMS D.L.H., BUTLER A.R., ALSA'DONI H.H., COX B.G. Identification of Cu+ as the effective reagent in nitric oxide formation from S-nitrosothiols (RSNO) J. Chem. Soc. Perkin Trans. 1996;2:481–487. [Google Scholar]
- DUGUET A., IIJIMA H., EUM S.-Y., HAMID Q., EIDELMAN D.H. Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice. Am. J. Respir. Crit. Care Med. 2001;164:1119–1126. doi: 10.1164/ajrccm.164.7.2010085. [DOI] [PubMed] [Google Scholar]
- FEDER L.S., STELTS D., CHAPMAN R.W., MANFRA D., CRAWLEY Y., JONES H., MINNICOZZI M., FERNANDEZ X., PASTER T., EGAN R.W., KREUTNER W., KUNG T.T. Role of nitric oxide on eosinophilic lung inflammation in allergic mice. Am. J. Respir. Cell Mol. Biol. 1997;17:436–442. doi: 10.1165/ajrcmb.17.4.2845. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., OSTROWSKI J., NOACK E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989;14:S13–S22. [PubMed] [Google Scholar]
- FEELISCH M., STAMLER J.S.Donors of nitrogen oxides Methods in Nitric Oxide Research 1996New York: Wiley and Sons Ltd; 71–115.ed. Feelisch, M. & Stamler, J.S. pp [Google Scholar]
- FERREIRA H.H.A., BEVILACQUA E., GAGIOTI S.M., DE LUCA I.M.S., ZANARDO R.C.O., TEIXEIRA C.E., SANNOMIYA P., ANTUNES E., DE NUCCI G. Nitric oxide modulates eosinophil infiltration in antigen-induced airway inflammation in rats. Eur. J. Pharmacol. 1998;358:253–259. doi: 10.1016/s0014-2999(98)00575-5. [DOI] [PubMed] [Google Scholar]
- FERREIRA H.H.A., LODO M.L.S., MARTINS A.R., KANDRATAVICIUS L., SALAROLI A.F., CONRAN N., ANTUNES E., DE NUCCI G. Expression of nitric oxide synthases and in vitro migration of eosinophils from allergic rhinitis subject. Eur. J. Pharmacol. 2002;442:155–162. doi: 10.1016/s0014-2999(02)01507-8. [DOI] [PubMed] [Google Scholar]
- FERREIRA H.H.A., MEDEIROS M.V., LIMA C.S.P., FLORES C.A., SANNOMIYA P., ANTUNES E., DE NUCCI G. Inhibition of eosinophil chemotaxis by chronic blockade of nitric oxide biosynthesis. Eur. J. Pharmacol. 1996;310:201–207. doi: 10.1016/0014-2999(96)00379-2. [DOI] [PubMed] [Google Scholar]
- FERRERO R., RODRÍGUEZ-PASCUAL F., MIRAS-PORTUGAL M.T., TORRES M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. Br. J. Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABOURY J., WOODMAN R.C., GRANGER D.N., REINHARDT P., KUBES P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am. J. Physiol. 1993;265:H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., LINDSAY M.A. Pharmacology of the eosinophil. Pharmacol. Rev. 1999;51:213–339. [PubMed] [Google Scholar]
- GORDGE M.P., HOTHERSALL J.S., NEILD G.H., DUTRA A.A. Role of a copper (I)-dependent enzyme in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1996;119:533–538. doi: 10.1111/j.1476-5381.1996.tb15704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDGE M.P., HOTHERSALL J.S., NORONHA-DUTRA A.A. Evidence for a cyclic GMP-independent mechanism in the anti-platelet action of S-nitrosoglutathione. Br. J. Pharmacol. 1998;124:141–148. doi: 10.1038/sj.bjp.0701821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFY K.A., KRUMENACKER J.S., MURAD F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med. Sci. Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- HANSEL T.T., DE VRIES I.J.M., IFF T., RIHS S., WANDZILAK M., BETZ S., BLASER K., WALKER C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J. Soluble guanylate cyclase: an old therapeutic target re-visited. Br. J. Pharmacol. 2002;136:637–640. doi: 10.1038/sj.bjp.0704779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IIJIMA H., DUGUET A., EUM S.-Y., HAMID Q., EIDELMAN D.H. Nitric oxide and protein nitration are eosinophil dependent in allergen-challenged mice. Am. J. Respir. Crit. Care Med. 2001;163:1233–1240. doi: 10.1164/ajrccm.163.5.2003145. [DOI] [PubMed] [Google Scholar]
- KAPLAN S.S., BILLIAR T., CURRAN R.D., ZDZIARSKI U.E., SIMMONS R.L., BASFORD R.E. Inhibition of chemotaxis with N G-monomethyl-L-arginine: a role for cyclic GMP. Blood. 1989;74:1885–1887. [PubMed] [Google Scholar]
- KOARAI A., ICHINOSE M., SUGIURA H., YAMAGATA S., HATTORI T., SHIRATO K. Allergic airway hyperresponsiveness and eosinophil infiltration is reduced by a selective iNOS inhibitor, 1400W, in mice. Pulm. Pharmacol. Ther. 2000;13:267–275. doi: 10.1006/pupt.2000.0254. [DOI] [PubMed] [Google Scholar]
- KOSONEN O., KANKAANRANTA H., MALO-RANTA U., MOILANEN E. Nitric oxide-releasing compounds inhibit neutrophil adhesion to endothelial cells. Eur. J. Pharmacol. 1999;382:111–117. doi: 10.1016/s0014-2999(99)00581-6. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUROSE I., WOLF R., GRISHAM M.B., AW T.Y., SPECIAN R.D., GRANGER D.N. Microvascular responses to inhibition of nitric oxide production. Role of active oxidants. Circ. Res. 1995;76:30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- KUROSE I., WOLF R., GRISHAM M.B., GRANGER D.N. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ. Res. 1994;74:376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- MAXEY K.M., MADDIPATI K.R., BIRKMEIER J. Interference in immunoassay. J. Clin. Immunoassay. 1992;15:116–120. [Google Scholar]
- MOILANEN E., VUORINEN P., KANKAANRANTA H., METSÄ-KETELÄ T., VAPAATALO H. Inhibition by nitric oxide-donors of human polymorphonuclear leucocyte functions. Br. J. Pharmacol. 1993;109:852–858. doi: 10.1111/j.1476-5381.1993.tb13653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONCADA S., HIGGS A., FURCHGOTT R. International union of pharmacology nomenclature in nitric oxide research. Pharmacol. Rev. 1997;49:137–142. [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- MOULIAN N., TRUFFAULT F., GAUDRY-TALARMAIN Y.M., SERRAF A., BERRIH-AKNIN S. In vivo and in vitro apoptosis of human thymocytes are associated with nitrotyrosine formation. Blood. 2001;97:3521–3530. doi: 10.1182/blood.v97.11.3521. [DOI] [PubMed] [Google Scholar]
- MULLERSHAUSEN F., FRIEBE A., FEIL R., THOMPSON W.J., HOFMANN F., KOESLING D. Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J. Cell Biol. 2003;160:719–727. doi: 10.1083/jcb.200211041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULLERSHAUSEN F., RUSSWURM M., THOMPSON W.J., LIU L., KOESLING D., FRIEBE A. Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 2001;155:271–278. doi: 10.1083/jcb.200107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAD F. Cyclic guanosine monophosphate as a mediator of vasodilatation. J. Clin. Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAYAMA N., ICHIKAWA H., COE L., ITOH M., ALEXANDER J.S. Exogenous NO enhances hydrogen peroxide-mediated neutrophil adherence to cultured endothelial cells. Am. J. Physiol. 1998;274:L820–L826. doi: 10.1152/ajplung.1998.274.5.L820. [DOI] [PubMed] [Google Scholar]
- PRADELLES P., GRASSI J. Enzyme immunoassays of adenosine cyclic 3′, 5′-monophosphate and guanosine cyclic 3′,5′-monophosphate using acetylcholinesterase. Anal. Chem. 1989;61:447. doi: 10.1021/ac00180a014. [DOI] [PubMed] [Google Scholar]
- RAO D.N.R., ELGUINDI S., O'BRIEN P.J. Reductive metabolism of nitroprusside in rat hepatocytes and human erythrocytes. Arch. Biochem. Biophys. 1991;286:30–37. doi: 10.1016/0003-9861(91)90005-4. [DOI] [PubMed] [Google Scholar]
- RIBEIRO-DIAS F., BARBUTO J.A.M., TSUJITA M., JANCAR S. Discrimination between NK and LAK cytotoxic activities of murine splen cells by MTT assay: differential inhibition by PGE2 and EDTA. J. Immunol. Methods. 2000;241:121–129. doi: 10.1016/s0022-1759(00)00206-4. [DOI] [PubMed] [Google Scholar]
- RICHARDS K.L., MCCULLOUGH J. A modified microchamber method for chemotaxis and chemokinesis. Immunol. Commun. 1984;13:49–62. doi: 10.3109/08820138409025449. [DOI] [PubMed] [Google Scholar]
- SALEH D., ERNST P., LIM S., BARNES P.J., GIAID A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- SATO E., SIMPSON K.L., GRISHAM M.B., KOYAMA S., ROBBINS R.A. Effects of reactive oxygen and nitrogen metabolites on eotaxin-induced eosinophil chemotactic activity in vitro. Am. J. Respir. Cell Mol. Biol. 2000;22:61–67. doi: 10.1165/ajrcmb.22.1.3644. [DOI] [PubMed] [Google Scholar]
- SCHRÖDER H., NEY P., WODITSCH I., SCHRÖR K. Cyclic GMP mediates SIN-1-induced inhibition of human polymorphonuclear leukocytes. Eur. J. Pharmacol. 1990;182:211–218. doi: 10.1016/0014-2999(90)90279-f. [DOI] [PubMed] [Google Scholar]
- SCHWINGSHACKI A., MOQBEL R., DUSZYK M. Nitric oxide activates ATP-dependent K+ channels in human eosinophils. J. Leukoc. Biol. 2002;71:807–812. [PubMed] [Google Scholar]
- SETHI S., DIKSHIT M. Modulation of polymorphonuclear leukocytes function by nitric oxide. Thromb. Res. 2000;100:223–247. doi: 10.1016/s0049-3848(00)00320-0. [DOI] [PubMed] [Google Scholar]
- SETHI S., SINGH M.P., DIKSHIT M. Nitric oxide-mediated augmentation of polymorphonuclear free radical generation after hypoxia–reoxygenation. Blood. 1999;93:333–340. [PubMed] [Google Scholar]
- SINGH R.J., HOGG N., JOSEPH J., KALYANARAMAN B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- THOMAZZI S.M., FERREIRA H.H.A., CONRAN N., DE NUCCI G., ANTUNES E. Role of nitric oxide on in vitro human eosinophil migration. Biochem. Pharmacol. 2001;62:1417–1421. doi: 10.1016/s0006-2952(01)00782-1. [DOI] [PubMed] [Google Scholar]
- VANUFFELEN B.E., DE KOSTER B.M., VAN DEN BROEK P.J.A., VANSTEVENINCK J., ELFERINK J.G.R. Modulation of neutrophil migration by exogenous gaseous nitric oxide. J. Leuk. Biol. 1996;60:94–100. doi: 10.1002/jlb.60.1.94. [DOI] [PubMed] [Google Scholar]
- VANUFFELEN B.E., VAN DER ZEE J., DE KOSTER B.M., VANSTEVENINCK J., ELFERINK J.G.R. Sodium azide enhances neutrophil migration and exocytosis: involvement of nitric oxide, cyclic GMP and calcium. Life Sci. 1998;63:645–657. doi: 10.1016/s0024-3205(98)00316-6. [DOI] [PubMed] [Google Scholar]
- WANIKIAT P., WOODWARD D.F., ARMSTRONG R.A. Investigation of the role of nitric oxide and cyclic GMP in both the activation and inhibition of human neutrophils. Br. J. Pharmacol. 1997;122:1135–1145. doi: 10.1038/sj.bjp.0701477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD C., WONG T.H., MURRAY J., RAHMAN I., HASLETT C., CHILVERS E.R., ROSSI A.G. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem. Pharmacol. 2000;59:305–314. doi: 10.1016/s0006-2952(99)00329-9. [DOI] [PubMed] [Google Scholar]
- WEISBART R.H., GOLDE D.W., GASSON J.C. Biosynthetic human GM-CSF modulates the number and affinity of neutrophil f-Met-Leu-Phe receptors. J. Immunol. 1986;137:3584–3587. [PubMed] [Google Scholar]
- WINK D.A., MITCHELL J.B. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998;25:434–456. doi: 10.1016/s0891-5849(98)00092-6. [DOI] [PubMed] [Google Scholar]
- ZANARDO R.C.O., COSTA E., FERREIRA H.H.A., ANTUNES E., MARTINS A.R., MURAD F., DE NUCCI G. Pharmacological and immunohistochemical evidence for a functional nitric oxide synthase system in rat peritoneal eosinophils. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14111–14114. doi: 10.1073/pnas.94.25.14111. [DOI] [PMC free article] [PubMed] [Google Scholar]