Abstract
Caspases, key enzymes in the apoptosis pathway, have been detected in the brain of HD patients and in animal models of the disease. In the present study, we investigated the neuroprotective properties of a new, reversible, caspase-3-specific inhibitor, M826 (3-({(2S)-2-[5-tert-butyl-3-{[(4-methyl-1,2,5-oxadiazol-3-yl)methyl]amino}-2-oxopyrazin-1(2H)-yl]butanoyl}amino)-5-[hexyl(methyl)amino]-4-oxopentanoic acid), in a rat malonate model of HD.
Pharmacokinetic and autoradiography studies after intrastriatal (i.str.) injection of 1.5 nmol of M826 or its tritiated analogue [3H]M826 indicated that the compound diffused within the entire striatum. The elimination half-life (T1/2) of M826 in the rat striatum was 3 h.
I.str. injection of 1.5 nmol of M826 10 min after malonate infusion induced a significant reduction (66%) in the number of neurones expressing active caspase-3 in the ipsilateral striatum.
Inhibition of active caspase-3 translated into a significant but moderate reduction (39%) of the lesion volume, and of cell death (24%), 24 h after injury. The efficacy of M826 at inhibiting cell death was comparable to that of the noncompetitive NMDA receptor antagonist MK801.
These data provide in vivo proof-of-concept of the neuroprotective effects of reversible caspase-3 inhibitors in a model of malonate-induced striatal injury in the adult rat.
Keywords: Reversible caspase-3 inhibitor, malonate, Huntington's disease, apoptosis, CNS, striatum
Introduction
HD is an autosomal dominant, neurodegenerative pathology characterized by progressive striatal atrophy, severe neuronal loss and gliosis (Albin & Tagle, 1995; Brouillet et al., 1999; Cattaneo et al., 2001). A genetic mutation in the short arm of chromosome 4 affects the gene (IT15) encoding the 350 kDa protein huntingtin, leading to the translation of an abnormal polyglutamine expansion in the N-terminal fragment of the mutated protein. Although the physiological role of huntingtin in the brain remains unclear, both the mutated and normal proteins have been shown to interact with proteins involved in energy metabolism and cellular trafficking (Walling et al., 1998).
Impaired energy metabolism has been hypothesized to play a central role in the pathogenesis of HD, by causing secondary excitotoxicity leading to neuronal necrosis (Beal, 2000). The existence of a necrotic component in the development of HD-associated neurodegeneration has been well documented in several animal models of the disease (Brouillet et al., 1999; Brouillet, 2000; Cattaneo et al., 2001). More recently, evidence for an apoptotic component has also emerged: mice homozygous for the disruption of HD gene exhibit embryonic lethality that is associated with increased apoptosis (Zeitlin et al., 1995), and elevated expression and enzymatic activity of caspases, key enzymes in the apoptosis pathway, have been detected in the brain of HD patients and in animal models of this disease (Schulz et al., 1998; Ona et al., 1999; Chen et al., 2000).
Intrastriatal (i.str.) infusion of malonate, a reversible inhibitor of the mitochondrial enzyme succinate dehydrogenase, has been widely used to model in rodents the pathophysiology of HD. Succinate dehydrogenase is a component of the tricarboxylic acid cycle and complex II of the mitochondrial electron transport chain. Consequently, malonate induces ATP depletion and compromises neuronal energy metabolism. Further consequences of mitochondrial dysfunction include increased generation of free radicals and oxidative damage, release of cytochrome c, activation of effector caspases (such as caspase-3) by the apoptosome and release of apoptosis-inducing factor (AIF) (Beal, 2000; Joza et al., 2002).
Malonate-induced lesions recapitulate the combination of necrotic and apoptotic cell death observed in HD. Indeed, lesions are reduced by NMDA receptor antagonists (Beal et al., 1993; Schulz et al., 1998), as well as by free radical scavengers and NO synthase inhibitors (Maragos & Silverstein, 1995; Schulz et al., 1995; 1996; Matthews et al., 1999). More recently, caspase-1 and caspase-3 activities have been found to be elevated in HD brain and in animal models of the disease (Schulz et al., 1998; Ona et al., 1999; Chen et al., 2000), and both enzymes have been shown to cleave normal and mutated huntingtin in vitro (Goldberg et al., 1996; Wellington et al., 1998; Sanchez et al., 1999; Wellington et al., 2000). Schulz et al. (1998) have reported neuroprotective efficacy of the irreversible, polycaspase inhibitor ZVAD.fmk in the malonate model, while malonate-induced lesion is reduced in caspase-1 dominant-negative mutants (Ona et al., 1999; Andreassen et al., 2000). For these reasons, caspases and particularly caspase-1 and caspase-3 have emerged as potential targets in the treatment of HD. The objective of the present study was to assess the neuroprotective effects of a new, reversible, caspase-3 inhibitor, M826 (3-({(2S)-2-[5-tert-butyl-3-{[(4-methyl-1,2,5-oxadiazol-3-yl)methyl]amino}-2-oxopyrazin-1(2H)-yl]butanoyl}amino)-5-[hexyl(methyl)amino]-4-oxopentanoic acid), in a rat model of HD. M826 inhibits recombinant human caspase-3 activity at a median inhibitory concentration (IC50) of 5 nM (Han et al., 2002) and with a Ki of 0.7 nM. M826 also inhibits DNA fragmentation in human NT2 cells and in murine cerebellar and cortical neurons, with an IC50 of 30, 50 and 120 nM, respectively (Han et al., 2002). Part of the data presented here has been published in abstract form (Toulmond et al., 2002).
Methods
Animals and surgical procedures
Adult male, 250–300 g body weight, CD (SD) IGSBR rats were housed in groups of six in a 12 h light/dark cycle at 22°C (±2°C) with free access to food and water.
CD (SD) IGSBR rats are from an outbred stock, called CD (originally Sprague–Dawley; Charles River, St Constant, Quebec, Canada). All procedures were carried out under appropriate Animal Care Committee approval with relevant personal and project licenses in strict accordance to Merck and Co animal care policies.
Rats (fasted overnight prior to surgery) were anesthetized with isoflurane 2.5% in oxygen and placed in a stereotaxic frame. Body temperature was maintained throughout anesthesia by use of a thermoregulated heated blanket. A 22 G, 3.5 mm long guide-cannula (Plastics One, Roanoke, VA, U.S.A.) was inserted into the left striatum (Bregma: anterior 0; lateral: 2.8 mm; ventral: 3.5 mm from the brain surface; Paxinos & Watson, 1986) and anchored to the skull with two small screws and dental cement. Malonate (1.33 M in sterile Dulbbeco's PBS, pH 7) was infused into the striatum via a 28 G, 5 mm long cannula (Plastics One) inserted into the preimplanted guide-cannula and connected to a microsyringe and infusion pump (delivery rate: 1 μl min−1; duration of infusion: 1.5 min). The cannula was maintained in place, and 10 min after infusion of malonate, 5% dextrose, M826 or [3H]M826 (depending on the experiment; see below) was injected in the striatum via the same cannula (delivery rate: 1 μl min−1; duration of infusion: 1.5 min). The cannula was maintained in place for a further 5 min to ensure diffusion of the vehicle, M826 or [3H]M826. After suture of the scalp, anesthetic inhalation was stopped and the animals were returned to their home cage.
Drug administration
To establish the i.str. half-life of M826, the compound was infused (1 nmol μl−1 min−1; duration of infusion: 1.5 min) in the left striatum (using the same procedure as described above for the infusion of malonate) and the animals euthanized 5, 30 min, 1, 6 or 24 h later (n=3 per time point). In a separate group of animals, to assess whether the prior infusion of malonate would influence the i.str. concentrations of M826, M826 was infused 10 min after malonate, and the animals euthanized 1 and 24 h later (n=3 per time point). Blood samples were collected by cardiac puncture in heparinized tubes, the plasma separated and flash-frozen in liquid nitrogen. The ipsilateral and contralateral striata were harvested, weighed, placed in 1.5 ml Eppendorf tubes and flash-frozen in liquid nitrogen. All samples were stored at −20°C until processing for LC/MS-MS analysis. Equal volumes of acetonitrile (w v−1, 1 : 1) were added to the preweighed striata and plasma samples. The standard curve was established using 100 μl of rat plasma mixed with 100 μl of acetonitrile. Six dilutions (0.01, 0.05, 0.1, 0.5, 1 and 10 μg M826 ml−1 plasma acetonitrile) were used to establish the standard curve. The samples were submitted to vigorous agitation for 30 s on an SDI Ultramat 2 shaker, centrifuged at 14,000 r.p.m. for 10 min, and the supernatants analyzed by LC/MS-MS analysis. LC/MS-MS analysis was performed on a Perkin-Elmer Sciex API2000 LC/MS-MS mass spectrometer under electrospray ionization conditions with a voltage of 35–45 mV monitoring ions at m/z 576.2 (M+1)+ and m/z 332.0 (predominant fragment). Separation was achieved using a Phenomenex® Luna 5 μ C18(2) reverse phase HPLC column (50 × 2 mm2, 5 T) with a 2 min gradient from 10 to 90% acetonitrile in water containing 0.1 vol% formic acid at a flow rate of 1 ml min−1. Quantification was performed with the MacQuan 1.6 software supplied by PE Sciex.
The elimination half-life (T1/2) of M826 in the ipsilateral striatum was estimated using the log-linear regression of the concentration–time curve. M826 concentrations were below the LC/MS-MS detection limits (10 nM) in the plasma and contralateral striatum, at all the assessed time points.
To assess the i.str. diffusion of [3H]M826 by autoradiography, rats were divided into two groups (n=2 per group). The first group received an i.str. infusion of [3H]M826 in 5% dextrose (1 nmol μl−1 min−1; duration of infusion: 1.5 min; the cannula was kept in place 5 min at the end of infusion to allow diffusion, then slowly removed, as described above). To assess whether the prior infusion of malonate would influence the diffusion of [3H]M826, the second group received an infusion of malonate, followed 10 min later by an infusion of [3H]M826, via the same cannula. The rats were euthanized 1 h after [3H]M826 infusion. Isoflurane anesthesia was maintained during the whole surgical procedure, but the animals were allowed to recover from anesthesia after infusion of [3H]M826 and suture of the scalp. Brains were harvested, frozen and kept at −80°C until use.
The effects of M826 on active caspase-3-positive neuronal counts and lesion volume as end points were assessed in a separate experiment. Drug-treated animals received an i.str. infusion of 1.5 nmol of M826, 10 min after malonate infusion, control animals received an equivalent volume of vehicle (5% dextrose), as above. At 24 h after malonate infusion, the animals were euthanized, whole brains were frozen and kept at −80°C.
To assess and compare the neuroprotective effects of M826 and MK801 using DNA fragmentation as the end point, rats were divided into three groups which all received i.str. injection of malonate as above: vehicle-treated: 5% dextrose (i.str.)+saline (i.p.); M826-treated: M826 (i.str.)+saline (i.p.); MK801-treated: 5% dextrose (i.str.)+MK801 (i.p.). MK801 was administered in three successive i.p. injections: first injection: 3 mg kg−1 ml−1, 30 min prior to i.str. infusion of malonate; second: 1.5 mg kg−1 ml−1, 90 min postmalonate; third: 1.5 mg kg−1 ml–1, 180 min after malonate infusion. The MK801 dosing regimen was based on previously established brain pharmacokinetics (Vezzani et al., 1989; Willis et al., 1991; and authors, unpublished data). Vehicle- and M826-treated animals received an equivalent volume of 0.9% saline i.p. at the same time points. M826-treated animals received 1.5 nmol of M826 in 1.5 μl of 5% dextrose, infused intrastriatally in a single bolus, 10 min after malonate infusion. Vehicle- and MK801-treated animals received an equivalent volume of 5% dextrose, intrastriatally. The rats were euthanized 24 h after malonate infusion, brains harvested, and the ipsilateral and contralateral striata dissected and snap-frozen in liquid nitrogen. Samples were kept at −80°C until processing for cell death ELISA.
Tissue processing and ex vivo assays
Brains harvested for autoradiography, immunohistochemistry and Cresyl violet staining were sectioned in a cryostat. A series of adjacent brain coronal sections (10 μm thick) were taken every 400 μm and collected onto chilled (−20°C) Fisherbrand Superfrost/Plus glass slides, to yield sets of adjacent sections at six different levels throughout the striatum (B+1.70; B+1.20; B+0.7; B+0.2; B−0.3; B−0.8; Paxinos & Watson, 1986). Slides were stored at −80°C until use.
For autoradiography studies, cryostat sections were opposed to tritium film and intensifying screen (Amersham Biosciences Ltd, Chalfont St Giles, Bucks, U.K.) for 2 weeks. Digitalized autoradiographic images and calculated concentration values were generated using the MCID/M5 image analysis system (Imaging Research Inc., Ontario, Canada). The original values were read as nCi mg−1 taken against 3H standards (higher activity range, Amersham). The specific activity of the compound (2.22.109 Bq μmol−1) was then used to calculate the micromolar concentration at a given point. These values were obtained working on the assumption that the density of the brain is one. The detection limit for M826 by autoradiography was 0.6 μM.
Automated, active caspase immunohistochemistry was performed using a Biogenex Optimax Plus autostainer (Biogenex, San Ramon, CA, U.S.A.). Frozen sections were incubated for 5 min in 4% PFA at 4°C, washed 3 × 5 min in PBS, then transferred into the autostainer. Staining procedure included the following steps: prewash in Biogenex wash buffer (diluted 1 : 19 in distilled H2O; Biogenex), incubation for 2 h with a rabbit polyclonal antiactive caspase-3 antibody (A92790K, Merck-Frosst, Kirkland, Quebec, Canada; directed against the (KLH)C-LDCGIETD sequence of cleaved caspase-3; Davoli et al., 2002), diluted 1 : 2000 w v−1 in Biogenex wash buffer, 3 × 5 min wash, incubation for 1 h with Cy3-conjugated donkey anti-rabbit (1 : 1000 in Biogenex wash buffer; Jackson Immunoresearch, West Grove, PA, U.S.A.), and 3 × 5 min wash. All steps were performed at room temperature. Sections were mounted in Vectashield with DAPI mounting medium (Vector Laboratories, Burlingame, CA, U.S.A.). Controls were obtained by omitting the primary antibody. The sections were analyzed using a Sony analog camera (DXC 950) mounted on a Zeiss Axiophot 2 fluorescence microscope and Northern Eclipse® imaging software (Empix Imaging Inc., Mississauga, Ontario, Canada). Cells were counted according to in-house standard operating procedure: only bright red cells of 15–25 μm diameter, clearly identifiable under the following settings: 100% UV light, × 20 objective, 1–2 frame exposure, were counted as active caspase-3-positive cells. These cells have previously been demonstrated to be neurones (Ashdown et al., 2002). For each brain, active caspase-3-positive cells were counted on the first section taken at each of the six levels mentioned above. Results are expressed as total active caspase-3 cell counts per six sections striatum−1.
Quantification of lesion volume
Sections adjacent to those used for active caspase-3 cell counting were incubated in 1% Cresyl violet in 0.25% acetic acid, washed in tap water for 10 min, dehydrated in ascending alcohols, cleared in xylene and mounted in DPX mounting medium (Sigma Aldrich Canada, Oakville, Ontario, Canada). Lesion volumes were quantified using computer-assisted area measurement and volume integration software (Northern Eclipse®, Empix Imaging Inc.), as described previously (Pearson et al., 1999).
Cell death ELISA (Salgame et al., 1997; Han et al., 2002) was performed using a commercially available kit (Cell Death Detection ELISA Plus #1920685, Roche Biochemicals, Laval, Quebec, Canada). Striata were homogenized in lysis buffer containing a cocktail of protease inhibitors (Boehringer, Bedford, U.S.A.) and an in-house irreversible, polycaspase inhibitor, M033. M033 is a structural analogue of M920 (Bilsland et al., 2002) and has comparable potency in cell-free and whole-cell assays. Cell death ELISA was then performed on the striatal lysates according to the manufacturer's guidelines.
Materials
Sterile, injectable solutions of 5% dextrose and 0.9% saline were purchased from Abbott Laboratories Ltd (St Laurent, Quebec, Canada). Malonate (propanedioic acid disodium salt, C3H2O4Na2) was purchased from Sigma Aldrich. MK801 ((+)-5-methyl-10,11-dihydro-5,4-dibenzo[a,d]cyclohepten-5,10-imine maleate; dizocilpine) was synthesized in the Department of Medicinal Chemistry (MSD, Terlings Park, U.K.), M826 (3-({(2S)-2-[5-tert-butyl-3-{[(4-methyl-1,2,5-oxadiazol-3-yl)methyl]amino}-2-oxopyrazin-1(2H)-yl]butanoyl}amino)-5-[hexyl(methyl)amino]-4-oxopentanoic acid, bis-HCl salt; molecular weight: 648), and [3H]M826 (molecular weight: 652; SA: 60 mCi μmol−1) were synthesized in the Department of Medicinal Chemistry (Merck Frosst Canada and Co.). M826 inhibits recombinant human caspase-3 and caspase-7 at a median IC50 of 5 and 10 nM, respectively. The pH of 1 mM M826 in 5% dextrose solution is 3.13. M826 is stable in solutions in which pH ranges from 3 to 7. To ascertain that M826 would not precipitate in the cannula after infusion of malonate, we verified that both malonate and M826 are stable when mixed together in a v v−1 solution of 1 mM M826 5% dextrose and 1.33 M malonate in PBS.
Data analysis
All experiments were carried out in a blinded manner. Preparation and coding of solutions, surgery, drug administration, tissue processing and data analysis were each performed by different investigators.
The striatal lesion volume is expressed in mm3 and represents the computerized integration of the damage quantified in coronal sections taken at 400 μm intervals throughout the striatum. To take into account potential variations between brains due to freezing and processing, the striatal damage is also expressed as the percentage of tissue loss (lesioned volume vs total ipsilateral striatum volume) for each rat. The total active caspase-3 cell count per rat represents the sum of active caspase-3-positive cells counted in six sections taken at six different levels in the rat striatum. Active caspase-3 cell counts were analyzed with a Poisson distribution statistical model that is specifically designed to handle count data, including the existence of zero counts. The comparison of M826 vs vehicle was based on a t-test analogous to the standard t-test for normally distributed data. The lesion volume and lesion size data were analyzed via standard linear model with analysis of variance (ANOVA), which is equivalent to the standard t-test since there were two groups. Spearman's rank correlation was used to measure the strength of the relationship between lesion volume and active caspase-3-positive cell counts.
For the study comparing the effects of M826 and MK801 using cell death ELISA, statistical analysis was performed on the pooled raw data obtained from animals operated on three separate experimental days, each experimental day including the three treatment groups (saline+dextrose, M826+saline, MK801+dextrose). A two-factor linear model ANOVA was used to account for interstudy variability. Differences among the three treatment groups were found to be consistent. Consequently, data obtained over the 3 experimental days were pooled to compare treatment group means. Follow-up comparisons were carried out with Fisher least significant difference procedure.
Results are expressed as mean±s.e.m. P-values less than 0.05 for all tests and comparisons were deemed significant unless otherwise indicated. The logarithmic scale was used for all statistical analyses since underlying assumptions of equal variance and normal distributions are better satisfied. The data are jittered to alleviate overlap (Chambers et al., 1983). The R computing environment (Ihaka & Gentleman, 1996) was used for calculations and the production of graphs related to the data analysis.
Results
I.str. half-life and diffusion of M826
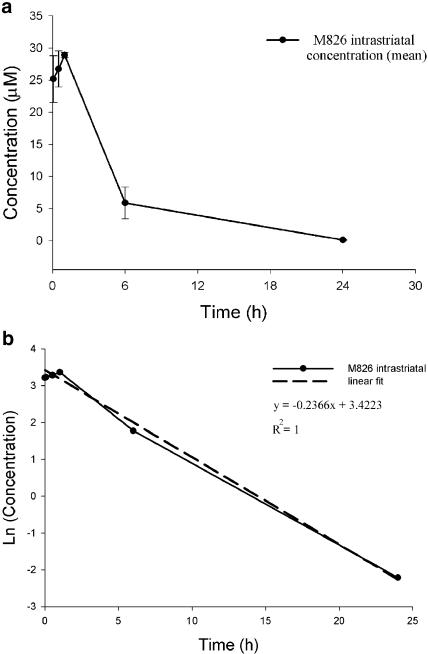
M826 (1.5 nmol) was infused into the left striatum and the concentrations of the compound in both the left (ipsilateral) and right (contralateral) striata were determined at different time points after infusion. The time course of the mean ipsilateral striatum concentrations of M826 is shown in Figure 1a. At 1 h after infusion, concentrations in the ipsilateral striatum were about 240-fold the whole cell IC50 (28.9 vs 0.12 μM in cortical neurones, Han et al., 2002). In animals having received a prior infusion of malonate, the ipsilateral i.str. concentrations (1 h: 32.3±2.9 μM; 24 h: 5.1±1.2 μM) of M826 were comparable to those observed in the absence of malonate, indicating that the presence of malonate in the striatum did not influence the chemical properties of the compound. The calculated i.str. half-life (T1/2) of M826 was 3 h (Figure 1b). The compound was undetectable by LC/MS-MS analysis in the contralateral striatum and plasma, at all time points, with or without preinfusion of malonate (not shown).
Figure 1.
Pharmacokinetics of M826. Rats received an i.str. infusion of M826 (1.5 nmol) 10 min after malonate infusion and the compound's concentrations were determined by LC-MS. (a) M826 concentrations in the ipsilateral striatum 5, 30 min, 1, 6 or 24 h after M826 infusion (n=3 rats per time point). Results are expressed as mean±s.e.m. (b) Log-linear regression of the i.str. concentration–time curve for the determination of the i.str. T1/2 of M826.
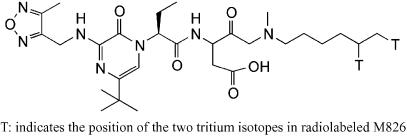
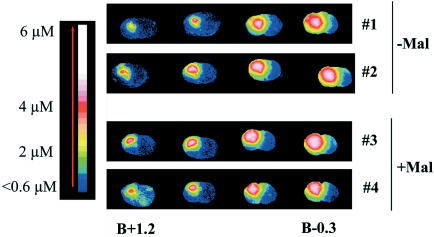
To further ascertain that the compound diffused into the striatum, tritiated M826 ([3H]M826; Figure 2) was injected intrastriatally either in the absence of malonate or 10 min after malonate infusion. The distribution of the tritiated compound in the absence of malonate, 1 h after i.str. infusion, is presented in Figure 3 (rats 1 and 2). [3H]M826 diffused throughout the entire striatum, with estimated concentrations ranging from 6 μM (‘core' of lesion injection site−1) to 0.6 μM (‘edge' of the lesion). This corresponds to five-fold the whole cell IC50 at the edge of the lesion, and 50-fold the whole cell IC50 at the core of the lesion. Some diffusion was also found in the adjacent cortex (both ipsilateral and contralateral), with concentrations below 2 μM. In accordance with the LC/MS-MS data, [3H]M826 was not detectable in the contralateral striatum.
Figure 2.
Structure of [3H]M826.
Figure 3.
I.str. distribution of [3H]M826. Rats 1 and 2 received an i.str. infusion of M826 in the absence of prior injection of malonate (Mal−). Rats 3 and 4 received an infusion of M826 10 min after injection of malonate (Mal+) in the same striatum. Both malonate and M826 were injected in the left striatum as described in Methods. All four animals were euthanized 1 h after infusion of M826. Coronal cryostat sections of the brains were processed for autoradiography. The sections were color coded from blue (weakest concentration) to white (strongest concentration).
To verify that infusion of malonate prior to that of M826 did not influence the diffusion properties of M826, [3H]M826 (n=2) was injected in the left striatum 10 min after malonate infusion in the same striatum. The data presented in Figure 3 demonstrate that the diffusion of [3H]M826 was not affected by prior injection of malonate (rats 3 and 4, malonate+[3H]M826, compared to rats 1 and 2, [3H]M826 only).
M826 inhibits caspase-3 activation and is neuroprotective
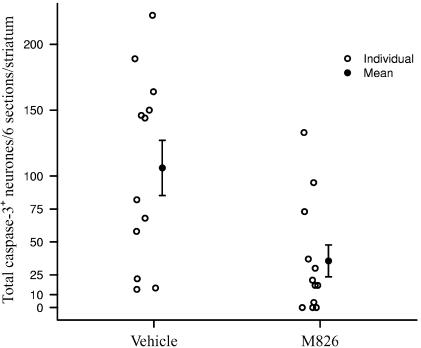
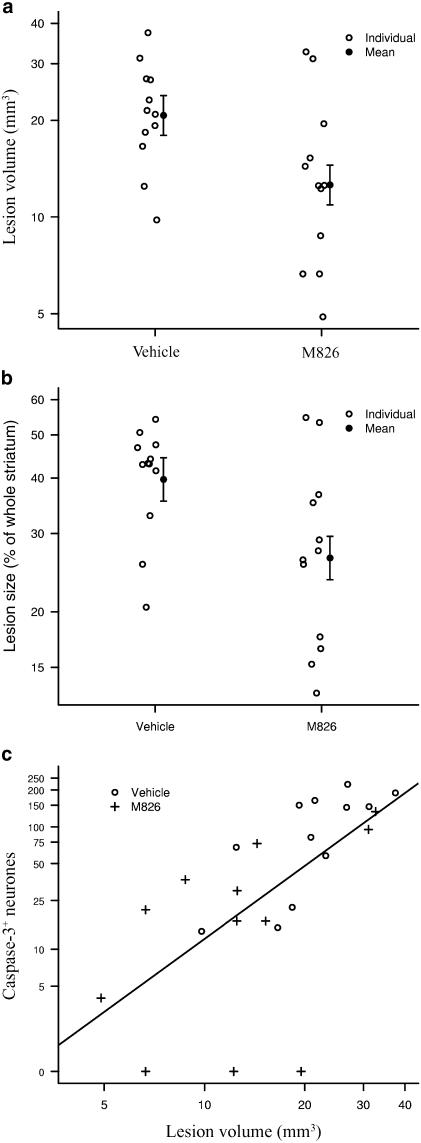
M826 induced a significant reduction (66%) in the number of neurones expressing active caspase-3 (36±12 vs 106±21 in vehicle-treated animals), counted in six sections collected throughout the striatum (Figure 4; P<0.05; P=0.011). This inhibition of caspase-3 translated into a significant but moderate reduction (39%) of the total lesion volume, 24 h after injury (12.6±1.8 mm3 vs 20.7±3.0 in vehicle-treated animals; P<0.05; P=0.022; Figure 5a). This corresponded to a 33% reduction in the proportion of the striatum that was lesioned (i.e. tissue loss), 24 h after injury (26.4±3.0 vs 39.7±4.4 in vehicle-treated animals) (Figure 5b; P<0.05; P=0.018). Overall (vehicle and M826 groups combined) correlation between lesion volume and active caspase-3-positive neurones counts is significant (R=0.77, P<0.001; Figure 5c). The correlation within the vehicle group per se is also significant (R=0.76, P<0.01; P=0.006), but the correlation within the M826 group only approaches significance (R=0.49, P=0.110), as the correlation measure is weakened by the three zero neurones count observations, which appear away from the general cluster in Figure 5c.
Figure 4.
Effect of M826 on the number of neurones expressing active caspase-3 in the injured striatum. Coronal sections taken at six levels throughout the striatum and adjacent to that used for the quantification of lesion volume were processed for the immunodetection of the active form of caspase-3. Caspase-3-positive neurones were counted at each level (see Methods and Figure 3 legend for details). M826 significantly reduced the number of cells expressing active caspase-3 (P=0.011; n=12 rats per vehicle- or M826-treated group).
Figure 5.
Effect of M826 on lesion volume. Lesion volume was assessed 24 h after malonate infusion, using Cresyl violet staining and computerized image analysis, in vehicle- or M826-treated animals (n=12 per group; see Methods for details). Treatment with M826 significantly reduced (a) the lesion volume (P=0.022) and (b) the percentage of striatal tissue loss (i.e. the proportion of the striatum lesioned; P=0.018). (c) Correlation between active caspase-3-positive neurone counts and lesion volume. Overall (combined vehicle and M826 groups) Spearman's correlation measure is significant (R=0.77, P<0.001).
In a separate experiment, the effects of M826 were compared to those of MK801, a well-described NMDA receptor antagonist that was previously shown to be neuroprotective in the malonate model (Schulz et al., 1998, and authors, unpublished data).
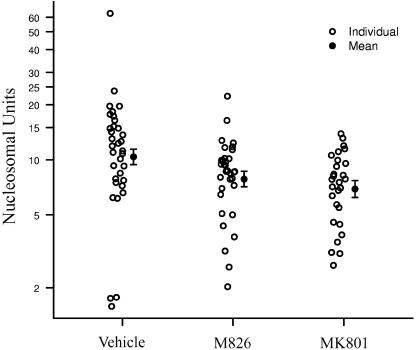
Treatment with M826 significantly reduced cell death (as measured by chromosomal DNA fragmentation) by 24% when compared to vehicle-treated animals (7.84±0.77 vs 10.37±1.00 NU; P<0.05; P=0.045). Treatment with MK801 reduced cell death by 33% when compared to vehicle-treated animals (6.92±0.73 vs 10.37±1.00 NU; P<0.01; P=0.006; Figure 6).
Figure 6.
Effect of M826 and MK801 on striatal cell death. Rats received an i.str. infusion of malonate (2 μmol) and treatment with vehicle (n=34), M826 (n=33) or MK801 (n=28). At 24 h after malonate infusion, cell death was assessed in the ipsilateral striata, using an ELISA as described in the Methods section. Both M826 (P=0.045) and MK801 (P=0.006) significantly reduced cell death. No significant difference was observed between M826- and MK801-treated animals.
Discussion
Inhibition of caspase-3 activity by the reversible, caspase-3 inhibitor, M826, resulted in a significant reduction in malonate-induced death of striatal neurones, 24 h after treatment. A 39% reduction in the total lesion volume and a 33% reduction in the proportion of the striatum that was lesioned were observed in animals treated with M826 vs vehicle alone. In addition, the number of neurones expressing active caspase-3 was significantly attenuated (66%) in the brains of animals treated with M826. Furthermore, a highly significant correlation (R=0.77) between reduction in active caspase-3 and reduction in lesion volume was demonstrated. A single i.str. dose of M826, administered 10 min after malonate infusion, reduced cell death by 24% (as measured by DNA fragmentation in tissue extracts). Taken together, these data indicate that M826 inhibits malonate-induced caspase-3 activity in the rat brain, and that inhibition of caspase-3 is neuroprotective in the malonate model of neurodegeneration.
Inhibition of apoptosis by the nonpeptidic, reversible caspase-3/7 inhibitors, isatin sulfonamides has recently been reported in whole-cell, in vitro assays (Lee et al., 2000; 2001; Concha & Abdel-Meguid, 2002). We have previously shown that M826 inhibits neuronal apoptosis in the neonatal rat brain after hypoxia–ischemia (Han et al., 2002). To our knowledge, this is the first report of the neuroprotective effects of a reversible caspase-3 inhibitor in the adult rat brain. It should be noted that while infusion of M826 in a single bolus resulted in a 66% inhibition of active caspase-3 24 h later, this only translated in a 39% reduction in lesion volume. These data indicate that, in the adult brain, an almost complete inhibition of endogenous caspase-3 might potentially be required for a more substantial reduction of the lesion. Alternatively, a more sustained coverage might be necessary. Pharmacokinetic studies indicated that the striatal concentration of M826 was still very high 1 h after injection (28.9 μM, 240-fold greater than the IC50 in a neuronal whole-cell assay; Han et al., 2002), and sufficient to inhibit caspase-3 for at least 6 h after infusion, but had fallen to the neuronal IC50 values by 24 h after injury. Finally, this might also simply indicate that caspase-3 activation is only accountable for about 30–40% of the malonate-induced lesion in the adult rat brain, and that other targets, including other proteases and/or glutamate receptors, might need to be simultaneously inactivated to reduce the lesion further (Friedlander & Yuan, 1998; Schulz et al., 1998). Furthermore, an AIF-dependent, caspase-independent apoptotic pathway could be involved (Joza et al., 2002). Indeed, Zhu et al. (2003) recently demonstrated that mitochondrial AIF release plays an important role in hypoxia–ischemia-induced neurodegeneration in the neonatal brain. The release of mitochondrial AIF was also observed in injured neurons after traumatic brain injury in the adult rat (Zhang et al., 2002). It would be of interest to examine whether AIF also plays a role in malonate-induced neurodegeneration, and if so, whether caspase-3 inhibition would induce a shift to a caspase-independent apoptotic pathway.
Our objective was solely to assess whether a reversible, highly potent and specific caspase-3 inhibitor could significantly reduce malonate-induced striatal histopathology. Owing to its poor brain barrier permeability, M826 had to be injected directly into the brain, which obviously limits its use in the clinic. Therefore, no attempts were made to determine whether the reduced malonate-induced neuronal death observed in this study translated into an improved functional outcome. The effects of M826 were only assessed at the single, i.str. dose of 1.5 nmol, because the solubility of M826 is relatively poor in aqueous vehicles, and 1 mM is the maximal concentration that can be achieved in 5% dextrose, a physiological vehicle that was therefore preferred over DMSO. Since the maximal infusable dose of M826 only resulted in an averaged 30% reduction in cell death and lesion volume, no attempt was made to assess the efficacy of the compound at lower doses and establish a dose–response curve. However, further studies examining the efficacy of M826 in a repeated dosing regimen (e.g. every 3 h to take into account the half-life of the compound), and/or after a delayed administration (⩾10 min after malonate infusion), would be of interest. In this proof-of-concept study, the 10 min delay between malonate and M826 infusions was chosen to ensure that the compound was present as soon as possible after the start of the induction of the lesion by malonate. Based on the experiments conducted to compare the striatal concentrations and diffusion of M826 in the presence or absence of malonate, and on the stability of the compound, we are confident that the presence of malonate does not modify the properties of the compound, and that it is unlikely that an interaction between malonate and M826 could explain the fact that only a moderate neuroprotection is observed.
Inhibition of caspase-3-like activity is usually assessed in vitro using the colorimetric assay of DEVD.AMC cleavage (Garcia-Calvo et al., 1998), but we did not attempt to measure the inhibition of caspase-3 activity by M826 using this method for two reasons. Firstly, M826 is a reversible inhibitor, and at steady state in vivo, unbound reversible inhibitors are present in the targeted tissue. This unbound compound reacts with caspase-3 released from cells upon tissue processing and lysing, thereby leading to an overestimation of caspase-3 inhibition in the tissue. Secondly, the antibody MF90K used in the present study specifically recognizes activated caspase-3, while DEVD.AMC lacks specificity for caspase-3 vs other caspase-3-like proteases such as caspase-7 (Thornberry et al., 1997; Garcia-Calvo et al., 1998). The quantification method that we used in this study allowed us to show fractional inhibition of caspase-3 activity in situ. It is, however, very clear that this is a labor-intensive, low-throughput method and that new, higher-throughput, biochemical methods need to be developed to assess the efficacy of future reversible caspase inhibitors. It should be noted that we are unable at this stage to conclude whether or not the reduction in malonate-induced lesion was in part due to the inhibition of caspase-7. Since caspase-3 and caspase-7 share substrate specificity, the only way to address this point would be to use an antibody that specifically recognizes activated (cleaved) caspase-7 in brain tissue. Such an antibody was not available at the time these studies were performed.
Although the irreversible polycaspase inhibitor ZVAD.fmk would have in theory been the most appropriate antiapoptotic benchmark compound, we chose not to compare the efficacy of M826 with that of ZVAD.fmk in this study. Studies assessing the neuroprotective effects of ZVAD.fmk in animal models of focal ischemia have generated both positive and negative results, depending on the model used, and on the route and timing of administration (for examples and further references see Endres et al., 1998; Gill et al., 2002). Contrary to the report by Schulz et al. (1998) that ZVAD.fmk (1 μg in 1% DMSO, in a single bolus i.str. infusion, up to 9 h after malonate injection) attenuates the size of malonate-induced lesions, in our hands, this compound did not significantly reduce the malonate-induced lesions as measured by cell death ELISA (the authors, unpublished data). Peptidyl halomethyl ketone inhibitors such as ZVAD.fmk and ZDEVD.fmk have a short half-life in vivo and are pharmacologically untractable in tissues, which prevented us from establishing their pharmacokinetics properties in the striatal parenchyma after administration. Attempts at using a radiolabeled ZVAD.fmk to follow its uptake in vivo have been unsuccessful so far due to low cellular uptake and high, nonspecific serum protein binding in vitro (Haberkorn et al., 2001). Finally, ZVAD.fmk is a general cysteine protease inhibitor (caspases, calpains and cathepsins), and its IC50 for calpains is actually lower than that for caspase-3 (Blomgren et al., 2001). The protective effects of M826 on striatal cell death were therefore compared to that of the noncompetitive NMDA receptor antagonist MK801, a well-characterized and chemically tractable compound (Foster et al., 1988; Vezzani et al., 1989; Willis et al., 1991), which has also previously been shown to be efficacious at reducing malonate-induced striatal lesion (Schulz et al., 1998; authors, unpublished data). Our data show that a single i.str. bolus of M826 10 min after malonate infusion induces a degree of neuroprotection comparable to that of repeated injections of MK801, administered prior to and after malonate infusion.
In conclusion, our results provide proof-of-concept of the neuroprotective effects of a new, reversible caspase-3 inhibitor, M826 after striatal lesion in the adult rat. Chemistry structure–activity relationship studies are underway to try and improve both the solubility and brain penetration of our inhibitors after i.v. administration.
Acknowledgments
We thank Josiane Rozon, Simon Wong and Denis Normandin, LAR, Merck Frosst, for their excellent technical assistance.
Abbreviations
- HD
Huntington's disease
- i.str.
intrastriatal
- NMDA
N-methyl-D-aspartate
- ZVAD.fmk
Cbz-Val-Ala-Asp(O-methyl)-fluoromethyl ketone
References
- ALBIN R.L., TAGLE D.A. Genetics and molecular biology of Huntington's disease. Trends Neurosci. 1995;18:11–14. doi: 10.1016/0166-2236(95)93943-r. [DOI] [PubMed] [Google Scholar]
- ANDREASSEN O.A., FERRANTE R.J., HUGHES D.B., KLIVENYI P., DEDEOGLU A., ONA V.O., FRIEDLANDER R.M., BEAL M.F. Malonate and 3-nitropropionic acid neurotoxicity are reduced in transgenic mice expressing a caspase-1 dominant-negative mutant. J. Neurochem. 2000;75:847–852. doi: 10.1046/j.1471-4159.2000.0750847.x. [DOI] [PubMed] [Google Scholar]
- ASHDOWN H.J., ROY S., ROBERTSON G.S., NICHOLSON D.W., TOULMOND S. Temporo-spatial distribution and cellular origin of caspase-3 and caspase-1, and their substrates huntingtin and IL-1, in the malonate model of neurodegeneration. Soc. Neurosci. 2002;32:198.9. [Google Scholar]
- BEAL M.F. Energetics in the pathogenesis of neurodegenerative diseases. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- BEAL M.F., BROUILLET E., JENKINS B., HENSHAW R., ROSEN B., HYAMAN B.T. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J. Neurochem. 1993;61:1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- BILSLAND J., ROY S., XANTHOUDAKIS S., NICHOLSON D.W., HAN Y., GRIMM E., HEFTI F., HARPER S.J. Caspase inhibitors attenuate 1-methyl-4-phenylpyridinium toxicity in primary cultures of mesencephalic dopaminergic neurons. J. Neurosci. 2002;22:2637–2649. doi: 10.1523/JNEUROSCI.22-07-02637.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOMGREN K., ZHU C., WANG X., KARLSSON J.-O., LEVERIN A.-L., BAHR B.A., MALLARD C., HAGBERG H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia–ischemia. J. Biol. Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- BROUILLET E. Animal models of Huntington's disease: from basic research on neuronal death to assessment of new therapeutic strategies. Funct. Neurol. 2000;15:239–251. [PubMed] [Google Scholar]
- BROUILLET E., CONDE F., BEAL M.F., HANTRAYE P. Replicating Huntington's disease phenotype in experimental animals. Prog. Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- CATTANEO E., RIGAMONTI D., GOFFREDO D., ZUCCATO C., SQUITIERI F., SIPIONE S. Loss of normal huntingtin function: new developments in Huntington's disease research. Trends Neurosci. 2001;24:182–188. doi: 10.1016/s0166-2236(00)01721-5. [DOI] [PubMed] [Google Scholar]
- CHAMBERS J.M., CLEVELAND W.S., KLEINER B., TUKEY P.A. Graphical Methods for Data Analysis. Monterey, CA: Wadsworth; 1983. [Google Scholar]
- CHEN M., ONA V.O., LI M., FERRANTE R.J., FINK K.B., ZHU S., BIAN J., GUO L., FARRELL L.A., HERSCH S.M., HOBBS W., VONSATTEL J.P., CHA J.H., FRIEDLANDER R.M. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- CONCHA N.O., ABDEL-MEGUID S.S. Controlling apoptosis by inhibition of caspases. Curr. Med. Chem. 2002;9:713–726. doi: 10.2174/0929867023370761. [DOI] [PubMed] [Google Scholar]
- DAVOLI M.A., FOURTOUNIS J., TAM J., XANTHOUDAKIS S., NICHOLSON D., ROBERTSON G., XU D. Immunohistochemical and biochemical assessment of caspase-3 activation and DNA fragmentation following transient focal ischemia in the rat. Neuroscience. 2002;115:125–136. doi: 10.1016/s0306-4522(02)00376-7. [DOI] [PubMed] [Google Scholar]
- ENDRES M., NAMURA S., SHIMIZU-SASAMATA M., WAEBER C., ZHANG L., GOMEZ-ISLA T., HYMAN B.T., MOSKOWITZ M.A. Attenuation of delayed neuronal death after mild focal ischemia in mice by inhibition of the caspase family. J. Cereb. Blood Flow Metab. 1998;18:238–247. doi: 10.1097/00004647-199803000-00002. [DOI] [PubMed] [Google Scholar]
- FOSTER A.C., GILL R., WOODRUFF G.N. Neuroprotective effects of MK-801 in vivo: selectivity and evidence for delayed neurodegeneration mediated by NMDA receptor activation. J. Neurosci. 1988;8:4745–4754. doi: 10.1523/JNEUROSCI.08-12-04745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEDLANDER R.M., YUAN J. ICE, neuronal apoptosis and neurodegeneration. Cell Death Differ. 1998;5:823–831. doi: 10.1038/sj.cdd.4400433. [DOI] [PubMed] [Google Scholar]
- GARCIA-CALVO M., PETERSON E.P., LEITING B., RUEL R., NICHOLSON D.W., THORNBERRY N.A. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 1998;273:32608–32613. doi: 10.1074/jbc.273.49.32608. [DOI] [PubMed] [Google Scholar]
- GILL R., SORIANO M., BLOMGREN K., HAGBERG H., WYBRECHT R., MISS M.-T., HOEFER S., ADAM G., NIEDERHAUSER O., KEMP J.A., LOETSCHER H. Role of caspase-3 in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J. Cereb. Blood Flow Metab. 2002;22:420–430. doi: 10.1097/00004647-200204000-00006. [DOI] [PubMed] [Google Scholar]
- GOLDBERG Y.P., NICHOLSON D.W., RASPER D.M., KALCHMAN M.A., KOIDE H.B., GRAHAM R.K., BROMM M., KASEMI-ESFARJANI P., THORNBERRY N.A., VAILLANCOURT J.P., HAYDEN M.R. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat. Genet. 1996;13:442–449. doi: 10.1038/ng0896-442. [DOI] [PubMed] [Google Scholar]
- HABERKORN U., KINSCHEF R., KRAMMER P.H., MIER W., EISENHUT M. Investigation of a potential scintigraphic marker of apoptosis: radioiodinated Z-val-Ala-DL-Asp(O-methyl)-fluoromethyl ketone. Nuclear Med. Biol. 2001;28:793–798. doi: 10.1016/s0969-8051(01)00247-5. [DOI] [PubMed] [Google Scholar]
- HAN H.B., XU D., CHOI J., HAN Y., XANTHOUDAKIS S., ROY S., TAM J., VAILLANCOURT J., COLUCCI J., SIMAN R., GIROUX A., ROBERTSON G.S., ZAMBONI R., NICHOLSON D.W., HOLTZMAN D.M. Selective, reversible caspase-3 inhibitor is neuroprotective and reveals distinct pathways of cell death after neonatal hypoxic–ischemic injury. J. Biol. Chem. 2002;277:30128–30136. doi: 10.1074/jbc.M202931200. [DOI] [PubMed] [Google Scholar]
- IHAKA R., GENTLEMAN R. R: a language for data analysis and graphics. J. Comput. Graphical Stat. 1996;5:299–314. [Google Scholar]
- JOZA N., KROEMER G., PENNINGER J.M. Genetic analysis of the mammalian machinery. Trends Genet. 2002;18:142–149. doi: 10.1016/s0168-9525(01)02618-x. [DOI] [PubMed] [Google Scholar]
- LEE D., LONG S.A., ADAMS J.L., CHAN G., VAIDYA K.S., FRANCIS T.A., KIKLY K., WINKLER J.D., SUNG C.M., DEBOUCK C., RICHARDSON S., LEVY M.A., DEWOLF W.E., JR, KELLER P.M., TOMASZEK T., HEAD M.S., RYAN M.D., HALTIWANGER R.C., LIANG P.H., JANSON C.A., MCDEVITT P.J., JOHANSON K., CONCHA N.O., CHAN W., ABDEL-MEGUID S.S., BADGER A.M., LARK M.W., NADEAU D.P., SUVA L.J., GOWEN M., NUTTALL M.E. Potent and selective nonpeptide inhibitors of caspases 3 and 7 inhibit apoptosis and maintain cell functionality. J. Biol. Chem. 2000;275:16007–16014. doi: 10.1074/jbc.275.21.16007. [DOI] [PubMed] [Google Scholar]
- LEE D., LONG S.A., MURRAY J.H., ADAMS J.L., NUTTAL M.E., NADEAU D.P., KIKLY K., WINKLER J.D., SUNG C.M., RYAN M.D., LEVY M.A., KELLER P.M., DE WOLF W.E. Potent and selective nonpeptide inhibitors of caspases 3 and 7. J. Med. Chem. 2001;44:2015–2026. doi: 10.1021/jm0100537. [DOI] [PubMed] [Google Scholar]
- MARAGOS W.F., SILVERSTEIN F.S. The mitochondrial inhibitor malonate enhances NMDA toxicity in the neonatal rat striatum. Brain Res. Dev. Brain Res. 1995;88:117–121. doi: 10.1016/0165-3806(95)00085-r. [DOI] [PubMed] [Google Scholar]
- MATTHEWS R.T., KLIVENYI P., MUELLER G., YANG L., WERMER M., THOMAS C.E., BEAL M.F. Novel free radical spin traps protect against malonate and MPTP neurotoxicity. Exp. Neurol. 1999;157:120–126. doi: 10.1006/exnr.1999.7045. [DOI] [PubMed] [Google Scholar]
- ONA V.O., LI M., VONSATTEL J.P., ANDREWS L.J., KHAN S.Q., CHUNG W.M., FREY A.S., MENON A.S., LI X.J., STIEG P.E., YUAN J., PENNEY J.B., YOUNG A.B., CHA J.H., FRIEDLANDER R.M. Inhibition of caspase-1 slows disease progression in a mouse model of Huntington's disease. Nature. 1999;399:263–267. doi: 10.1038/20446. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates. London, U.K.: Academic Press; 1986. [Google Scholar]
- PEARSON V.L., ROTHWELL N.J., TOULMOND S. Excitotoxic brain damage in the rat induces interleukin-1beta protein in microglia and astrocytes: correlation with the progression of cell death. Glia. 1999;25:311–323. [PubMed] [Google Scholar]
- SALGAME P., VARADHACHARY A.S., PRIMIANO L.L., FINCKE J.E., MULLER S., MONESTIER M. An ELISA for detection of apoptosis. Nucleic Acid Res. 1997;25:680–681. doi: 10.1093/nar/25.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANCHEZ I., XU C.J., JUO P., KAKIZAKA A., BLENIS J., YUAN J. Caspase-8 is required for cell death induced by expanded polyglutamine repeats. Neuron. 1999;22:623–633. doi: 10.1016/s0896-6273(00)80716-3. [DOI] [PubMed] [Google Scholar]
- SCHULZ J.B., HENSHAW D.R., SIWEK D., JENKINS B.G., FERRANTE R.J., CIPOLLONI P.B., KOWALL N.W., ROSEN B.R., BEAL M.F. Involvement of free radicals in excitotoxicity in vivo. J. Neurochem. 1995;64:2239–2247. doi: 10.1046/j.1471-4159.1995.64052239.x. [DOI] [PubMed] [Google Scholar]
- SCHULZ J.B., HUANG P.L., MATTHEWS R.T., PASSOV D., FISHMAN M.C., BEAL M.F. Striatal malonate lesions are attenuated in neuronal nitric oxide synthase knockout mice. J. Neurochem. 1996;67:430–433. doi: 10.1046/j.1471-4159.1996.67010430.x. [DOI] [PubMed] [Google Scholar]
- SCHULZ J.B., WELLER M., MATTHEWS R.T., HENEKA M.T., GROSCURTH P., MARTINOU J.C., LOMMATZSCH J., VON COELLN R., WULLNER U., LOSCHMANN P.A., BEAL M.F., DICHGANs J., KLOCKGETHER T. Extended therapeutic window for caspase inhibition and synergy with MK-801 in the treatment of cerebral histotoxic hypoxia. Cell Death Differ. 1998;5:847–857. doi: 10.1038/sj.cdd.4400420. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., RANO T.A., PETERSON E.P., RASPER D.M., TIMKEY T., GARCIA-CALVO M., HOUTZAGER V.M., NORDSTROM P.A., ROY S., VAILLANCOURT J.P., CHAPMAN K.T., NICHOLSON D.W. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- TOULMOND S., ASHDOWN H.J., DEGEN S., TANG K., BUREAU Y., ZHU Y., ROZON J COLUCCI J., GIROUX Y., HAN Y., ROY S., ZAMBONI R., NICHOLSON D.W., ROBERTSON G.S., FLÜCKIGER J.P. M826, a selective caspase-3 inhibitor, attenuates malonate-induced neuronal death. Soc. Neurosci. 2002;32:200.3. [Google Scholar]
- VEZZANI A., SERAFINI R., STASI M.A., CACCIA S., CONTI I., TRIDICCIO R.V., SAMANIN R. Kinetics of MK801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J. Pharmacol. Exp. Ther. 1989;249:278–290. [PubMed] [Google Scholar]
- WALLING H.W., BALDASSARE J.J., WESTFALL T.C. Molecular aspects of Huntington's disease. J. Neurosci. Res. 1998;54:301–308. doi: 10.1002/(SICI)1097-4547(19981101)54:3<301::AID-JNR1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- WELLINGTON C.L., ELLERBY L.M., HACKAM A.S., MARGOLIS R.L., TRIFIRO M.A., SINGARAJA R., MCCUTCHEON K., SALVESEN G.S., PROPP S.S., BROMM M., ROWLAND K.J., ZHANG T., RASPER D., ROY S., THORNBERRY N., PINSKY L., KAKIZUKA A., ROSS C.A., NICHOLSON D.W., BREDESEN D.E., HAYDEN M.R. Caspase cleavage of gene products associated with triplet expansion disorders generates truncated fragments containing the polyglutamine tract. J. Biol. Chem. 1998;273:9158–9167. doi: 10.1074/jbc.273.15.9158. [DOI] [PubMed] [Google Scholar]
- WELLINGTON C.L., SINGARAJA R., ELLERBY L., SAVILL J., ROY S., LEAVITT B., CATTANEO E., HACKAM A., SHARP A., THORNBERRY N., NICHOLSON D.W., BREDESEN D.E., HAYDEN M.R. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and non-neuronal cells. J. Biol. Chem. 2000;275:19831–19838. doi: 10.1074/jbc.M001475200. [DOI] [PubMed] [Google Scholar]
- WILLIS C.L., BRAZELL C., FOSTER A.C. Plasma and CSF levels of dizocilpine (MK801) required for neuroprotection in the quinolate-injected rat striatum. Eur. J. Pharmacol. 1991;196:285–290. doi: 10.1016/0014-2999(91)90441-r. [DOI] [PubMed] [Google Scholar]
- ZEITLIN S., LIU J.P., CHAPMAN D.L., PAPAIOANNOU V.E., EFSTRATIADIS A. Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington's disease gene homologue. Nat. Genet. 1995;11:155–163. doi: 10.1038/ng1095-155. [DOI] [PubMed] [Google Scholar]
- ZHANG X., CHEN J., GRAHAM S.H., DU L., KOCHANEK P.M., DRAVIAM R., GUO F., NATHANIEL P.D., SZABO C., WATKINS S.C., CLARK R.S. Intranuclear localization of apoptosis-inducing factor (AIF) and large scale nuclear fragmentation after traumatic brain injury in rats and in neuronal cultures exposed to peroxynitrite. J. Neurochem. 2002;82:181–191. doi: 10.1046/j.1471-4159.2002.00975.x. [DOI] [PubMed] [Google Scholar]
- ZHU C., QIU L., WANG X., HALLIN U., CANDE C., KROEMER G., HAGBERG H., BLOMGREN K. Involvement of apoptosis-inducing factor in neuronal death after hypoxia–ischemia in the neonatal rat brain. J. Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]