Abstract
The contribution of endogenous endothelins to nociceptive responses elicited by ovalbumin (OVA) in the hind-paw of mice sensitised to this antigen (50 μg OVA+5 mg Al(OH)3, s.c., 14 days beforehand) was investigated.
Sensitised mice exhibited greater nocifensive responsiveness to intraplantar (i.pl.) OVA (total licking time over first 30 min: 85.2±14.6 s at 0.3 μg; 152.6±35.6 s at 1 μg) than nonsensitised animals (29.3±7.4 s at 1 μg). Nocifensive responses of sensitised mice to 0.3 μg OVA were inhibited by morphine (3 mg kg−1, s.c.) or local depletion of mast cells (four daily i.pl. injections of compound 48/80).
Pretreatment with i.v. bosentan (mixed ETA/ETB receptor antagonist; 52 μmol kg−1) or A-122722.5 (selective ETA receptor antagonist; 6 μmol kg−1) reduced OVA-induced licking from 124.8±20.6 s to 45.7±13.0 s and 64.2±12.1 s, respectively, whereas A-192621.1 (selective ETB receptor antagonist; 25 μmol kg−1) enhanced them to 259.2±39.6 s.
Local i.pl. pretreatment with BQ-123 or BQ-788 (selective ETA or ETB receptor antagonists, respectively, each at 3 nmol) reduced OVA-induced licking (from 106.2±15.2 to 57.0±9.4 s and from 118.6±10.5 to 76.8±14.7 s, respectively). Sarafotoxin S6c (selective ETB receptor agonist, 30 pmol, i.pl., 30 min after OVA) induced nocifensive responses in OVA-sensitised, but not in nonsensitised, animals.
Compound 48/80 (0.3 μg, i.pl.) induced nocifensive responses per se and potentiated those induced by i.pl. capsaicin (0.1 μg). Treatment with BQ-123 (3 nmol, i.pl.) reduced only the hyperalgesic effect of compound 48/80, whereas BQ-788 (3 nmol) was ineffective.
Thus, immune-mediated Type I hypersensitivity reactions elicit mast cell- and endothelin-dependent nociception in the mouse hind-paw, which are mediated locally by both ETA and ETB receptors. The nocifensive response to antigen is amenable to blockade by systemic treatment with dual ETA/ETB or selective ETA receptor antagonists, but is sharply potentiated by systemic selective ETB receptor antagonist treatment. The apparently distinct roles played by ETB receptors in this phenomenon at local and other sites remain to be characterised.
Keywords: Endothelin, allergy, inflammation, nociception, pain, ovalbumin, antigen
Introduction
The immune system plays key roles in defending the organism against invading pathogenic microorganisms and in mechanisms leading to tissue repair. Immune responses can be either nonspecific, which operate in an innate and generalised fashion without precise recognition of the pathogen, or specific, in which the system recognises a ‘nonself' substance as an antigen which should be targeted for destruction (for reviews, see Palucka & Banchereau, 2002). Despite their usually protective functions, specific immune responses can frequently be inappropriate, inflicting inflammation and tissue injury and contributing to important pathologies, such as rheumatoid arthritis and asthma. One of the most widely recognised forms of specific immune reaction is Type I hypersensitivity (also known as immediate, allergic or anaphylactic hypersensitivity), a response triggered when the organism is re-exposed to an antigen capable of interacting with specific IgE antibodies present on mast cells and basophils, stimulating them to degranulate and promptly release many proinflammatory mediators (for reviews, see Wedemeyer et al., 2000; Benoist & Mathis, 2002). IgE antibodies are generated by B lymphocytes following antigen presentation by antigen-presenting cells such as macrophages and dendritic cells (for a review, see Gordon, 2002). Mast cells (and basophils) can also be degranulated via nonimmune mechanisms by various substances including the synthetic cationic polyamine compound 48/80, the wasp venom mastoparan and many endogenous peptides rich in cationic amino acids (for a review, see Mousli et al., 1990).
In recent years, a large body of literature has established new functions for the immune system extending well beyond the inflammatory horizon, whereby, through interactions with the nervous system, immune cells can influence many other physiological functions, leading to a variety of behavioural, endocrine and/or neural changes, including those implicated in nociceptive responsiveness (for a review, see Watkins & Maier, 2002). In this regard, it has recently been shown that mast cells and macrophages are actively engaged in activation of primary nociceptive neurons and in how these later cells process information to signal acute pain (Ribeiro et al., 2000; Parada et al., 2001).
Both mast cells (Liu et al., 1998; Koda et al., 2000) and macrophages (Ehrenreich et al., 1990) are capable of generating and releasing endothelin-1, a peptide of the endothelin family that exerts a plethora of biological actions via activation of two specific G protein-coupled receptors, named ETA and ETB receptors (for a review, see Kedzierski & Yanagisawa, 2001). Indeed, endothelin-1 mRNA expression in distinct mast cell populations are augmented in certain physiopathological conditions (Li et al., 1999; Gilbert et al., 2000). Also, extracellular big-endothelin-1(endothelin-1 precursor) can be processed by mast cell-derived chymase to yield endothelin-1(1–31) (Nakano et al., 1997), which is subsequently cleaved by endothelin-converting enzyme and/or neutral endopeptidase to originate endothelin-1 in vivo (Honoré et al., 2002). Immunoreactive endothelin-1 levels are enhanced in bronchoalveolar fluid (Sofia et al., 1993) and in the circulation (Trakada et al., 2000) of asthmatic patients, as well as in plasma (Filep et al., 1993), airway epithelial cells (Kizawa et al., 1999) and intestinal mucosal cells (Shigematsu et al., 1998) of previously sensitised guinea-pigs re-exposed to the same antigen. In addition, endothelins can cause several potential proinflammatory effects, such as mast cell degranulation (Uchida et al., 1992; Yamamura et al., 1994), enhancement of vascular permeability to cause oedema (Sirois et al., 1992; Kurose et al., 1993; Sampaio et al., 1995), upregulation of cell-adhesion molecule expression (Duperray et al., 1995; Watanabe et al., 2000) and leukocyte recruitment and activation (Gomez-Garre et al., 1992; Sampaio et al., 2000). Collectively, such findings attribute potentially significant roles to endothelins as mediators of immune reactions, including those underlying Type I hypersensitivity responses.
Endothelin-1 and other endothelin isoforms trigger pain in humans (Ferreira et al., 1989; Dahlof et al., 1990), nociceptive responses in animals (Ferreira et al., 1989; Raffa et al., 1996; Davar et al., 1998; Piovezan et al., 2000) and appear to be implicated in more long-lasting pain associated with certain experimental models of arthritis (De-Melo et al., 1998), diabetes (Jarvis et al., 2000) and bone cancer (Wacnik et al., 2001). Nevertheless, until now, very few studies have addressed the issue of pain associated with Type I hypersensitivity immune reactions (Tominaga et al., 1999; Ruggieri et al., 2000, Lavich et al., 2003) and, to the best of our knowledge, none have yet investigated if endogenous endothelins are engaged in bringing about the changes in nociceptive responsiveness which can occur in these instances.
Injection of endothelin-1 into the mouse hind-paw evokes ETA receptor-mediated nociceptive behaviour and hyperalgesia (i.e. potentiation of nociceptive responses to capsaicin and heat), in addition to paw oedema (Piovezan et al., 2000; Menéndez et al., 2003). Interestingly, selective blockade of ETA receptors inhibits oedema induced by intraplantar (i.pl.) injection of ovalbumin (OVA) into the hind-paws of mice previously sensitised to this antigen (Sampaio et al., 1995). In the light of these considerations, the current study aimed to investigate if the Type I hypersensitivity reaction triggered by OVA in the hind-paw of sensitised mice can also evoke nociceptive behaviours, and also the possible contribution of endothelins to these effects.
Methods
Animals
Male Swiss mice (18–20 g), from our own colony, were lodged in a room with controlled temperature (22±2°C) and lighting (lights on from 6:00 to 18:00 h), with free access to lab chow and tap water. All experiments were conducted between 10:00 and 17:00 h. The experimental procedures and protocols were previously approved by the committee on ethical use of laboratory animals of the Universidade Federal de Santa Catarina, where the study was conducted, and are in accordance with the ethical guidelines of the International Association for the Study of Pain (Zimmermann, 1983) and with Brazilian national legislation. Each animal was used only once and was euthanised by CO2 overdose immediately after completion of the experiment.
OVA sensitisation
Animals received a single s.c. injection of OVA (50 μg) plus Al(OH)3 (5 mg), in 200 μl of sterile saline, into the back of the neck and were returned to their home cages until use. Control non-sensitised animals were similarly treated with vehicle alone.
Assessment of nocifensive responses
At 14 days after sensitisation, animals received a 30 μl i.pl. injection of different doses of OVA (0.1 to 3.0 μg) into the right hind-paw and were placed, immediately and alone, into a glass jar (20 cm high, 20 cm wide, open top) laying over a mirror (set at an angle of about 60° relative to the table) to enable full view of the paws at all times. The amount of time each animal spent licking the injected hind-paw (in s) was recorded cumulatively, using a stopwatch chronometer, as an index of nocifensive behaviour, for up to 60 min after injection.
In another group of experiments, we investigated the influence of OVA challenge on nocifensive responsiveness to sarafotoxin S6c, a selective ETB receptor agonist (Masaki et al., 1994). To this effect, this peptide was injected in sensitised or nonsensitised mice (at 10 or 30 pmol, i.pl.) 30 min after ipsilateral i.pl. OVA (0.3 μg). An additional sensitised control group was given i.pl. phosphate-buffered saline (PBS) instead of OVA, 30 min prior to sarafotoxin S6c. All groups in this set of experiments were observed for manifestation of licking behaviour during the first 30 min following injection of the ETB selective agonist (or PBS).
In a final set of experiments, we made use of compound 48/80, a cationic polyamine that degranulates mast cells by direct activation of α-subunits of Gi/o proteins (for a review, see Mousli et al., 1990), to investigate if nonimmune mast cell activation could also elicit nocifensive behaviour. To this effect, naive nonsensitised mice received compound 48/80 (0.3 μg, i.pl.) and licking behaviour was recorded over the first 30 min following injection. At this time point, capsaicin (0.1 μg, i.pl.), the active ingredient of red chilli peppers that causes nociception by activating vanilloid VR1 receptors (Caterina et al., 1997), was injected in the same hind-paw, and licking behaviour was recorded over the next 5 min. Control mice received identical treatments with the corresponding vehicles. The capacity of compound 48/80 to potentiate capsaicin-induced nocifensive responses was taken as an index of hyperalgesia, as described for endothelin-1 by Piovezan et al. (2000).
Characterisation of nociceptive mechanisms
To assess the liability of OVA-induced nocifensive responses to inhibition by opioids, one group of sensitised animals was treated with morphine (3 mg kg−1, intraperitoneally), 60 min before antigen injection (0.3 μg, i.pl.). Another group of mice was used to evaluate the role of mast cells in OVA-induced nociceptive responses. Starting 10 days after sensitisation, these mice received daily i.pl. injections of compound 48/80 for 4 days (at 1, 3, 10 and 10 μg, respectively, injected into the same paw; as described by Parada et al., 2001), and were then challenged with OVA (0.3 μg, i.pl.) 24 h after the last injection. Injected repeatedly according to this same protocol, compound 48/80 causes long-lasting local mast cell depletion in the skin (Jaffery et al., 1994).
Specific nonpeptidic or peptidic endothelin ETA and/or ETB receptor antagonists were used to investigate the possible roles for endogenous endothelins in behavioural nocifensive and hyperalgesic responses to OVA. In these experiments, sensitised mice were treated intravenously (into a caudal vein) with either bosentan (a mixed ETA/ETB receptor antagonist; 17 or 52 μmol kg−1, corresponding to 10 or 30 mg kg−1, respectively; Clozel et al., 1994), A-122722.5 (a selective ETA receptor antagonist; 6 μmol kg–1, equivalent to 3 mg kg−1; Opgenorth et al., 1996) or A-192621.1 (a selective ETB receptor antagonist, 25 μmol kg–1, corresponding to 15 mg kg−1, Von Geldern et al., 1999), or their respective vehicles, 60 min prior to i.pl. challenge with OVA (0.3 μg). Alternatively, other groups of mice were treated locally, 15 min prior to i.pl. OVA challenge, with the selective peptidic ETA or ETB receptor antagonists BQ-123 or BQ-788 (each at 1 or 3 nmol, i.pl.; Masaki et al., 1994), respectively, or vehicle (PBS). The influence of BQ-123 and BQ-788 on nociception and hyperalgesia induced by compound 48/80 was assessed using this same i.pl. pretreatment protocol. These doses of the antagonists were selected on the basis of previous studies showing suitable in vivo efficacy (Jarvis et al., 2000; Piovezan et al., 2000; Guimarães et al., 2002), as well as the manufacturer's recommendations.
Statistical analysis
All results are expressed as the mean±s.e.m. To calculate net percentages of inhibition or potentiation of nocifensive responsiveness induced by drug treatments, the responses evoked by OVA or capsaicin in the corresponding nonsensitised control group were subtracted from the values displayed by both (drug- and vehicle-treated) sensitised groups. To evaluate statistical significance between any given sets of data, one-way ANOVA followed by Bonferroni's test were employed. The significance level was set at P<0.05.
Drugs and reagents
The following drugs or substances were used: capsaicin, grade V OVA, compound 48/80 (all from Sigma Chemical Company, St Louis, MO, U.S.A.), morphine hydrochloride (Merck A.G., Darmstadt, Germany), bosentan (a kind gift from Dr M. Clozel, Actelion Pharmaceuticals, Allschwil, Switzerland), A-127722.5 [trans-trans-2-(4-methoxyphenyl)-4-(1,3-benzodioxo-5-yl)-1((N,N-dibutylamino)carbonylmethyl)pyrrolidine-3-carboxylic acid] and A-192621.1 [[2R-(2a,3b,4a)]-4-(1,3-benzodioxol-5-yl)-1-[2-(2,6-diethylphenyl) amino]-2-oxoethyl]-2-(4-propoxyphenyl)-3-pyrrolidinecarboxylic acid] (both kindly donated by Dr T. Opgenorth, Abbott Laboratories, Abbott Park, IL, U.S.A.), BQ-123 (cyclo[DTrp-DAsp-Pro-DVal-Leu]) and sarafotoxin S6c (American Peptide Co., Sunnyvale, CA, U.S.A.) and BQ-788 (N-cis-2,6-dimethylpiperidinocarbonyl-L-g-methylleucyl-D-1-methoxycarboyl-D-norleucine; Research Biochemicals International, Natick, U.S.A.). Bosentan, A-122722.5 and A-192621.1 were initially dissolved and diluted to the desired concentrations in warm sterile water, ethanol 3% and in 100 μl of NaOH 0.1 N plus sterile water, respectively, immediately before use. Capsaicin was initially dissolved in DMSO 50% in PBS to yield a 250 μg ml−1 and then diluted to a final concentration of 5 μg ml−1 just prior to use in PBS alone. Solutions of all other substances were dissolved and diluted to the desired concentrations in PBS alone. All stock solutions were kept at −18°C as 50–100 μl aliquots and diluted to the desired concentration in the same vehicle just prior to use. The pertinent control groups were each identically treated with the corresponding vehicles alone.
Results
Behavioural responses to OVA
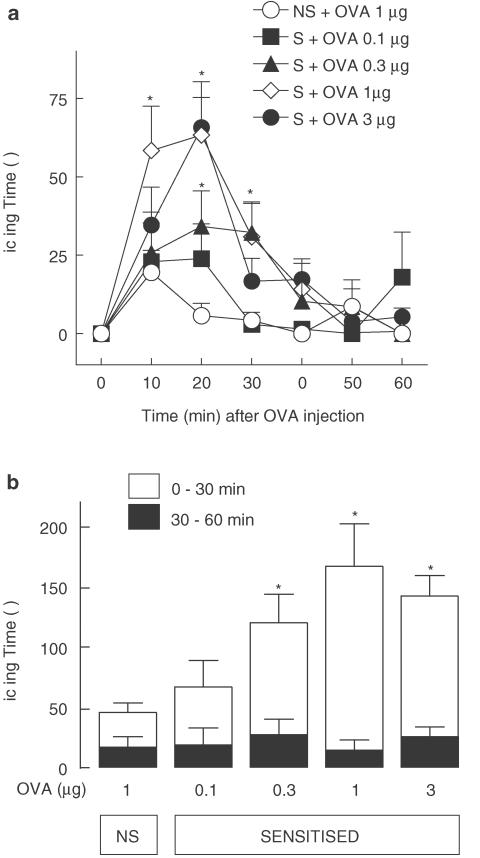
Sensitised mice displayed significantly higher levels of licking behaviour in response to i.pl. injection of OVA, at doses ⩾0.1 μg, than nonsensitised control mice. As shown in Figure 1a, this behavioural response appeared shortly after OVA injection, was dose dependent and was maximal following the dose of 1 μg. As illustrated in Figure 1b, licking responses elicited by OVA in sensitised mice were enhanced significantly, relative to those of nonsensitised animals, over the first 30 min, but not between 30 and 60 min (Figure 1b). Moreover, the response to antigen appeared to depend critically on prior sensitisation of the animal since licking responses triggered in nonsensitised animals by 1 μg OVA and PBS were similar (accumulated licking time over first 30 min: OVA: 29.3±7.4 s, PBS: 20.5±3.3 s, n=7, P>0.05). In all further experiments, we chose to use the submaximally effective 0.3 μg dose of OVA to enable demonstration of putative potentiating effects of drug treatments (i.e. to avoid ceiling effect bias).
Figure 1.
Nociceptive effects of OVA injection into the hind-paw of sensitised and nonsensitised mice, evaluated as the time spent licking the injected paw, in seconds. (a) Time course of the nocifensive licking behaviour manifested by control nonsensitised (NS) or sensitised (S) animals in response to different doses of ovalbumin (OVA). (b) Licking time accumulated over the first (0–30 min) and second (30–60 min) halves of the first hour following OVA injection. Each value represents the mean±s.e.mean of six to nine animals. Asterisks denote P<0.05 when compared to corresponding value of NS+OVA 1 μg control (ANOVA followed by Bonferroni's test).
Prior treatment with morphine hydrochloride (3 mg kg−1, i.p., 1 h beforehand) reduced OVA-induced licking in sensitised mice from 124.2±26.6 to 46.7±12.8 s (P<0.05, 84% net inhibition), a value not different from the 32.3±6.6 s displayed by nonsensitised controls (n=6, in each group). The licking responses of sensitised animals to OVA were also substantially attenuated (74% net inhibition) by prior treatment with four consecutive daily injections of the mast cell degranulator substance compound 48/80, from 186.8±23.2 to 71.9±8.9 s (P<0.05; nonsensitised control 31.7±7.1 s; n=6 in each group). Further experiments revealed that this procedure of local mast cell depletion by repeated injections of compound 48/80 selectively abolished the nocifensive response induced by the polyamine itself (10 μg, i.pl.), without modifying licking behaviour induced by capsaicin (0.1 μg, i.pl.; n=6 in each group; results not shown).
Role for endogenous endothelins in OVA-induced nociception
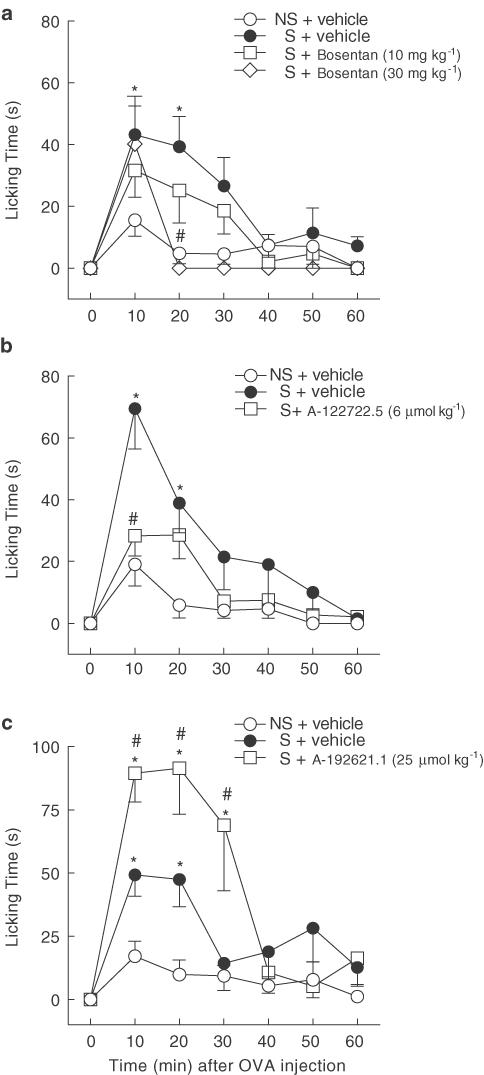
Systemic i.v. treatment of sensitised mice with a dual ETA/ETB receptor antagonist, bosentan (17 or 52 μmol kg−1), reduced OVA-induced nociception in a dose-dependent manner (Figure 2a). Nocifensive responsiveness of mice treated with the highest dose of bosentan over the first 30 min was reduced by a net 79% (from 106.3±16.4 to 45.7±13.0 s, P<0.05) to a value not statistically different from that displayed by nonsensitised control animals (29.4±7.4 s). The selective endothelin ETA receptor antagonist A-122722.5 (6 μmol kg−1, i.v.) caused an effect (65% net inhibition over first 30 min, from 124.8±20.6 to 64.2±12.1 s, P<0.05) similar to that of bosentan (Figure 2b). In sharp contrast, the selective ETB receptor antagonist A-192621.1 (25 μmol kg−1, i.v.) markedly augmented licking responses induced by OVA challenge in sensitised mice (net potentiation of 147% of licking over first 30 min, from 124.2±26.2 to 259.2±39.6 s, P<0.05; Figure 2c).
Figure 2.
Influence of systemically administered endothelin ETA and/or ETB receptor antagonists on nocifensive responses of OVA-sensitised mice to antigen. Behavioural licking responses were elicited by i.pl. injection of 0.3 μg ovalbumin (OVA) into the right hind-paw of all mice 1 h after i.v. treatment with (a) bosentan (mixed ETA/ETB receptor antagonist), (b) A-122722.5 (selective ETA receptor antagonist) or (c) A-192621.1 (selective ETB receptor antagonist), at the doses indicated, or their respective vehicles. Each value represents the mean±s.e.m. of six to nine animals. Asterisks and fences denote P<0.05 when compared to corresponding value of the vehicle-treated nonsensitised (NS) and sensitised (S) groups, respectively (ANOVA followed by Bonferroni's test).
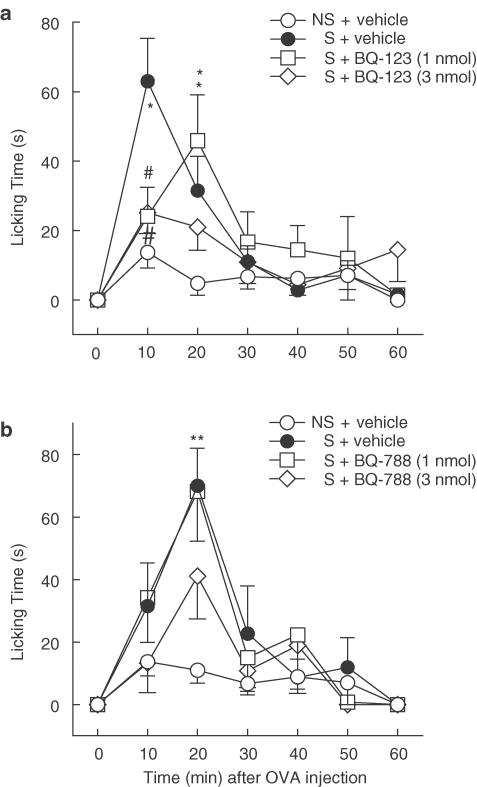
Local i.pl. treatment of sensitised mice with the selective ETA receptor antagonist BQ-123 (1 or 3 nmol) caused dose-dependent reduction of OVA-induced nocifensive responses (Figure 3a). Licking behaviour over the first 30 min was inhibited by the highest dose of BQ-123 from 106.0±15.2 to 57.1±9.4 s (P<0.05; 64% net inhibition; vehicle-treated nonsensitised control 29.4±7.2 s). Conversely, prior i.pl. treatment with the selective ETB receptor antagonist BQ-788 (1 or 3 nmol) did not significantly modify the incidence of licking behaviour elicited by OVA, as assessed over consecutive 10 min-bins, up to 60 min after antigen challenge (Figure 3b). Nonetheless, at the highest dose tested, BQ-788 significantly decreased the total licking time over the first 30 min following OVA injection, from 118.6±10.5 to 76.8±14.7 s (P<0.05; 48% net inhibition; vehicle-treated nonsensitised control 31.5±6.4 s).
Figure 3.
Influence of locally administered endothelin ETA or ETB receptor antagonists on nocifensive responses of OVA-sensitised mice to antigen. Behavioural licking responses were elicited by i.pl. injection of 0.3 μg ovalbumin (OVA) into the right hind-paw of all mice 15 min after i.pl. treatment with (a) BQ-123 (selective ETA receptor antagonist) and (b) BQ-788 (selective ETB receptor antagonist), at the doses indicated, or vehicle (PBS). Each value represents the mean±s.e.m. of six to nine animals. Asterisks and fences denote P<0.05 when compared to corresponding value of the vehicle-treated nonsensitised (NS) and sensitised (S) groups, respectively (ANOVA followed by Bonferroni's test).
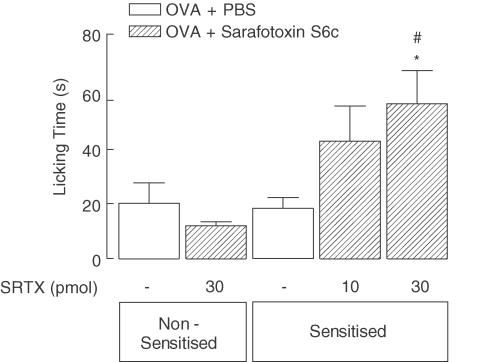
Nociceptive effect of sarafotoxin S6c in OVA-sensitised animals re-exposed to antigen
The opposing influences of systemic versus local treatments with selective ETB receptor antagonists (A-192621.1 and BQ-788, respectively) led us to assess the possible nociceptive effects of the selective ETB receptor agonist sarafotoxin S6c in sensitised mice challenged with OVA (Figure 4). Confirming our previous study (Piovezan et al., 2000), nocifensive behaviour of nonsensitised animals given sarafotoxin S6c (30 pmol, i.pl.; 30 min after OVA challenge) was not different from that elicited by PBS in either nonsensitised or sensitised mice. In sharp contrast, following OVA challenge, the selective ETB receptor agonist induced a clear graded nociceptive effect in sensitised mice, with 30 pmol inducing a three-fold greater nocifensive response than that displayed by sensitised PBS-treated mice.
Figure 4.
Influence of challenge with ovalbumin (OVA) on the nociceptive effects of sarafotoxin S6c, a selective ETB receptor agonist, in the hind-paw of nonsensitised and antigen-sensitised mice. All animals were challenged with OVA (0.3 μg, i.pl.) 30 min before ipsilateral i.pl. injection of either sarafotoxin S6c (SRTX; at the doses indicated) or PBS. Each value represents the mean±s.e.m. of six to nine animals. Asterisks and fences denote P<0.05 when compared to corresponding value of the i.pl. PBS-treated nonsensitised and sensitised groups, respectively (ANOVA followed by Bonferroni's test).
Role for endogenous endothelins in compound 48/80-induced nociception and hyperalgesia
Compound 48/80 (0.3 μg, i.pl.) induced significant nocifensive responses in nonsensitised animals throughout the first 30 min following injection, and also enhanced licking induced by subsequent capsaicin (0.1 μg, i.pl.) injection (Table 1). Prior i.pl. treatment with BQ-123 or BQ-788 (1 or 3 nmol) did not influence the nocifensive response to compound 48/80. However, the hyperalgesic effect of the polyamine towards capsaicin-induced nociception was significantly reduced by the highest dose of BQ-123, but not by BQ-788.
Table 1.
Influence of local pretreatment with BQ-123 and BQ-788 on nociception and hyperalgesia induced by i.pl. injection of compound 48/80 in the mouse hind-paw
| Treatment | Dose (i.pl.) | Nociceptiona | Hyperalgesiab |
|---|---|---|---|
| Licking (s) | Licking (s) | ||
| Saline+vehicle | — | 14.1±2.0 | 33.0±2.4 |
| +BQ-123 | 1 nmol | 11.9±1.9 | 36.7±2.9 |
| +BQ-123 | 3 nmol | 10.8±2.2 | 33.3±2.6 |
| Compound 48/80+vehicle | 0.3 μg | 38.9±3.4* | 53.7±5.0* |
| +BQ-123 | 1 nmol | 39.5±7.1* | 48.2±3.6* |
| +BQ-123 | 3 nmol | 32.6±3.8* | 37.7±2.9** |
| Saline+vehicle | — | 8.9±1.8 | 30.7±2.2 |
| +BQ-788 | 1 nmol | 11.7±1.9 | 33.1±2.7 |
| +BQ-788 | 3 nmol | 12.0±1.8 | 28.9±0.1 |
| Compound 48/80+vehicle | 0.3 μg | 48.8±7.0* | 49.7±4.9* |
| +BQ-788 | 1 nmol | 35.4±8.4* | 42.5±4.5* |
| +BQ-788 | 3 nmol | 39.1±5.2* | 44.1±4.6* |
Nociceptive effects of compound 48/80 (or saline) were assessed over the first 30 min following injection.
Hyperalgesia was evaluated as the capacity to potentiate nociceptive responses induced by capsaicin (0.1 μg; i.pl., 30 min after compound 48/80 or saline; please refer to Methods for further details).
* and ** indicate P<0.05 relative to respective saline+vehicle or compound 48/80+vehicle group, respectively (ANOVA followed by Bonferroni's test).
Discussion
The present study demonstrates that the immune-mediated Type I hypersensitivity reaction induced in OVA-sensitised mice by i.pl. challenge with this antigen evokes nocifensive behaviour. Furthermore, it provides evidence for the participation of endogenous endothelins in bringing about this effect, thus reinforcing the potential roles attributed to these peptides in nociceptive signalling mechanisms.
To date, few studies have addressed the nociceptive influences of immune-mediated reactions. To this effect, OVA has been found to inflict long-lasting articular pain in the temporomandibular and knee joints of rabbits sensitised to this antigen (Tominaga et al., 1999), and to cause shorter-lasting visceral pain when injected into the bladder of sensitised guinea-pigs (Ruggieri et al., 2000). Also, rats display a prolonged thermal hyperalgesia in response to i.pl. injection of dinitrophenylated BSA into a hind-paw passively immunised by prior i.pl. injection with IgE complexed to an antibody against the antigen (Lavich et al., 2003), but the study does not mention if antigen challenge also evokes any overt nocifensive responses indicative of spontaneous pain. Our current data shows that i.pl. OVA induced significantly more hind-paw licking in sensitised than in nonsensitised mice, whereas licking responses of nonsensitised animals receiving i.pl. OVA (1 μg) or PBS were identical. This overt behavioural response of sensitised mice to antigen was dose dependent, displayed a quick onset and was limited to the first 30 min following injection. The sensitivity of this enhanced behaviour triggered by OVA in sensitised mice to inhibition by the opioid analgesic morphine strongly suggests that it truly constitutes a cutaneous nociceptive stimulus in this condition. In addition, i.pl. OVA-induced nocifensive responses depend importantly on local IgE-mediated mast cell (and/or basophil) degranulation triggered by the antigen, as the licking behaviour was severely depressed when the antigen was injected into a hind-paw in which resident mast cells were previously depleted by repeated daily injections of compound 48/80, a nonimmune mast cell degranulating substance (Mousli et al., 1990). Together, these findings support the view that this model can be used to further explore mechanisms related to pain induced by immune reactions.
The demonstration that bosentan, a mixed ETA/ETB receptor antagonist, significantly attenuated OVA-induced nocifensive behaviour of sensitised mice was not entirely unexpected. First, i.pl. endothelin-1 induces pain, hyperalgesia and oedema in the mouse hind-paw (Piovezan et al., 1998, 2000). Moreover, i.v. OVA increases circulating endothelin-1 levels in sensitised guinea-pigs (Filep et al., 1993), perhaps in part via release from mast cells (Liu et al., 1998) and/or via conversion of extracellular big-endothelin-1 to endothelin-1(1–31) by mast cell-derived chymase (Nakano et al., 1997; Honoré et al., 2002). Also, i.pl. OVA-induced hind-paw oedema in sensitised mice is reduced by selective ETA receptor antagonists (Sampaio et al., 1995). Nonetheless, the almost complete blockade of the nocifensive response to OVA by bosentan (i.v.) indicates that endogenous endothelins play a pivotal role in its production. Prior studies have found that endothelin-1-induced nociception in the mouse hind-paw is abrogated by systemic or local treatment with selective ETA (but not ETB) receptor antagonists (Piovezan et al., 2000; Wacnik et al., 2001).
Interestingly, the antinocifensive effects afforded by the systemically administered ETA receptor antagonist, A-122722.5, have been reproduced by BQ-123 injected locally in the hind-paw of sensitised mice pretreated with OVA. This confirms that the ETA receptors, once activated, are responsible for a major portion of the nociceptive effects of OVA. Furthermore, it is suggested that these receptors are localised in the close vicinity of the site of the primary immune response (i.e. in the hind-paw). It is noteworthy that ETA receptor blockade also alleviates the mechanical allodynia associated with diabetes in rats (Jarvis et al., 2000) and pain in a model of metastatic bone cancer in mice (Wacnik et al., 2001).
In contrast, the potentiating effects of A-192621.1 on OVA-induced nociception were not reproduced by local administration of another (albeit peptidic) ETB receptor antagonist, BQ-788. Indeed, i.pl. BQ-788 was found to reduce nocifensive responses to antigen challenge, at least when values of total licking time over the first 30 min were compared. Different mechanisms may explain these opposite effects of systemically vs local administration of the ETB antagonists on OVA-induced nocifensive reponses. Firstly, the antagonist administered i.v. may displace endothelin-1 from clearance ETB receptors located on the vascular endothelium (Dupuis et al., 2000), thus rendering more of the endogeneous peptide available to further exacerbate ETA receptor-dependent nociceptive mechanisms. Secondly, by favouring ETA receptor-mediated vasoconstriction (via increased circulatory availability of endothelin-1) or blocking ongoing ETB receptor-mediated endothelium-dependent vasodilatation, systemic A-192621.1 may have direct effects on vascular resistance. The local hypoxia resulting from the reduction of blood flow in the antigen-challenged hind-paw might constitute an additional nociceptive stimulus (Graven-Nielsen et al., 2003), and hence potentiate OVA-induced nociception. These hypotheses are not mutually exclusive, but remain to be addressed in future studies.
The view that local ETB receptors play a pronociceptive role in OVA-induced nociception, suggested by our findings using local administration of BQ-788, is further strengthened by the significant licking behaviour elicited by i.pl. injection of sarafotoxin S6c following antigen challenge in sensitised, but not in nonsensitised, animals. The absence of a nociceptive effect of this selective ETB receptor agonist in the later groups fully confirms our previous findings (Piovezan et al., 2000). The same study also showed that, in naive mice, sarafotoxin S6c blocks the enhancement of nociceptive responses to i.pl. capsaicin (i.e. hyperalgesia) elicited by endothelin-1 (Piovezan et al., 2000). In addition, Khodorova et al. (2002; 2003) reported that IRL-1620 (another selective agonist of the ETB receptor) inhibits, via β-endorphin release from keratinocytes, the paw flinching behaviour elicited by exogenous endothelin-1 in the rat hind-paw.
The fact that sarafotoxin S6c only causes nociceptive responses in the mouse hind-paw following OVA challenge in sensitised mice bears resemblance with results in other nociceptive models. Thus, it does not cause articular incapacitation (pain) in the rat naive knee joint, but is very effective in doing so in a carrageenan-primed knee joint (De-Melo et al., 1998). Also, sarafotoxin S6c markedly enhances the second (inflammatory) phase of nociception induced by formalin in the mouse hind-paw, without affecting the magnitude of the first (neurogenic) phase of the response (Piovezan et al., 1997). Moreover, ETB receptor gene knockout mice display considerably less nocifensive responses (writhes) to i.p. phenylbenzoquinone than their wild-type littermates, and responses of the latter animals to this algogen are markedly reduced by systemic selective ETB receptor antagonist treatment (Griswold et al., 1999). Taken together, these findings suggest that signalling mechanisms coupled to ETB receptor activation may vary (or switch) during the course of a nocifensive response to an immune inflammatory insult.
Immune-mediated responses to antigen challenge may involve the concerted participation of various cell types, including mast cells. To explore if the nociceptive effect of OVA in sensitised mice could be mimicked by degranulation of nonsensitised mast cells via a nonimmune mechanism, we tested the effect of i.pl. compound 48/80 injection. Like OVA (in sensitised mice), the synthetic polyamine induced nocifensive licking responses per se and also enhanced licking responses to i.pl. capsaicin. At the dose tested, compound 48/80 caused nociceptive and hyperalgesic effects comparable to those induced by i.pl. injection of endothelin-1 (Piovezan et al., 2000). Although local blockade of ETA or ETB receptors (by prior i.pl. injection of BQ-123 and BQ-788, respectively) did not influence the nociceptive effect of compound 48/80, its capacity to potentiate capsaicin-induced nociception was reduced by BQ-123. Thus, non-immune activation of mast cells can also trigger an endothelin-dependent change in nocifensive responsiveness. It has been shown, however, that the array of inflammatory mediators released by skin mast cells in response to compound 48/80 is distinct from that triggered by antigen (Church et al., 1991). Perhaps this might explain the relatively smaller contribution of endothelins to the effects of compound 48/80 than of OVA in the current study.
The sources of endothelin recruited by antigen challenge and the nature of the mechanisms underlying the roles of ETA and ETB receptors, in triggering the nocifensive responses to OVA, remain to be fully characterised. However, mast cells (Liu et al., 1998; Koda et al., 2000) and macrophages (Ehrenreich et al., 1990) can produce endothelin-1. It also seems noteworthy to comment that injection of OVA into the pleural cavity of mice sensitised to this antigen induces ETA receptor-dependent local accumulation of macrophages, eosinophils and T lymphocytes, and that the CD4+ T lymphocyte sub-population display enhanced production of interleukin-4, interleukin-5 and interferon-γ (Sampaio et al., 2000). Perhaps some of these endothelin-mediated events underlie the nociceptive changes induced by antigen in the mouse hind-paw as well.
In conclusion, we propose the use of OVA-induced nocifensive behaviour in sensitised mice as a novel valid model of immune-mediated pain. In this model, challenge with the antigen mobilises endothelins to inflict ETA and ETB receptor-mediated nociceptive signals, which elicit nocifensive behaviour. In addition, our findings suggest that systemic administration of mixed ETA/ETB or selective ETA receptor antagonists (but not selective ETB receptor antagonists) might prove useful in the control of immune-mediated pain.
Acknowledgments
The kind donations of bosentan by Dr Martine Clozel (Actelion Pharmaceuticals, Allschwil, Switzerland), and A-127722-5 and A-192621.1 by Dr Terry Opgenorth (Abbott Laboratories, Abbott Park, U.S.A.) are gratefully acknowledged. The present study was supported by the Brazilian National Research Council (CNPq), Fundacao de Amparo a Pesquisa do Estado de São Paulo (Fapesp) and the Canadian Institutes of Health Research Canada. AP Piovezan and M Frighetto were the recipients of CNPq doctoral and masters scholarships respectively.
Abbreviations
- I.pl.
intraplantar
- OVA
ovalbumin
References
- BENOIST C., MATHIS D. Mast cells in autoimmune disease. Nature. 2002;420:875–887. doi: 10.1038/nature01324. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHURCH M.K., OKAYAMA Y., EL-LATI S. Mediator secretion from human skin mast cells provoked by immunological and non-immunological stimulation. Skin Pharmacol. 1991;4:15–24. doi: 10.1159/000210980. [DOI] [PubMed] [Google Scholar]
- CLOZEL M., BREU V., GRAY G.A., KALINA B., LOFFLER B.M., BURRI K., CASSAL J.M., HIRTH G., MULLER M., NEIDHART W., RAMUZ H. Pharmacological characterization of bosentan, a new potent orally active nonpeptide endothelin receptor antagonist. J. Pharmacol. Exp. Ther. 1994;270:228–235. [PubMed] [Google Scholar]
- DAHLOF B., GUSTAFSSON D., HEDNER T., JERN S., HANSSON L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J. Hypertens. 1990;8:811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- DAVAR G., HANS G., FAREED M.U., SINNOTT C., STRICHARTZ G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- DE-MELO J.D., TONUSSI C.R., D'ORLÉANS-JUSTE P., RAE G.A. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77:261–269. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- DUPERRAY A., MANTOVANI A., INTRONA M., DEJANA E. Endothelial cell regulation of leucocyte infiltration in inflammatory tissues. Mediat. Inflamm. 1995;4:322–330. doi: 10.1155/S0962935195000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPUIS J., JASMIN J.F., PRIE S., CERNACECK P. Importance of local production of endothelin-1 and of the ET(B)-receptor in the regulation of pulmonary vascular tone. Pulm. Pharmacol. Ther. 2000;13:135–140. doi: 10.1006/pupt.2000.0242. [DOI] [PubMed] [Google Scholar]
- EHRENREICH H., ANDERSON R.W., FOX C.H., RIECKMANN P., HOFFMAN G.S., TRAVIS W.D., COLIGAN J.E., KEHRL J.H., FAUCI A.S. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J. Exp. Med. 1990;172:1741–1748. doi: 10.1084/jem.172.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA S.H., ROMITELLI M., DE NUCCI G. Endothelin-1 participation in overt and inflammatory pain. J. Cardiovasc. Pharmacol. 1989;13 Suppl. 5:S220–S222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- FILEP J.G., TELEMAQUE S., BATTISTINI B., SIROIS P., D'ORLÉANS-JUSTE P. Increased plasma levels of endothelin during anaphylactic shock in the guinea-pig. Eur. J. Pharmacol. 1993;239:231–236. doi: 10.1016/0014-2999(93)91001-4. [DOI] [PubMed] [Google Scholar]
- GILBERT R.E., RUMBLE J.R., CAO Z., COX A.J., VAN EEDEN P., ALLEN T.J., KELLY D.J., COOPER M.E. Endothelin receptor antagonism ameliorates mast cell infiltration, vascular hypertrophy, and epidermal growth factor expression in experimental diabetes. Circ. Res. 2000;86:158–165. doi: 10.1161/01.res.86.2.158. [DOI] [PubMed] [Google Scholar]
- GOMEZ-GARRE D., GUERRA M., GONZALEZ E., LOPEZ-FARRE A., RIESCO A., CARAMELO C., ESCANERO J., EGIDO J. Aggregation of human polymorphonuclear leukocytes by endothelin: role of platelet-activating factor. Eur. J. Pharmacol. 1992;224:167–172. doi: 10.1016/0014-2999(92)90801-a. [DOI] [PubMed] [Google Scholar]
- GORDON S. Pattern recognition receptors: doubling up for the innate immune response. Cell. 2002;111:927–930. doi: 10.1016/s0092-8674(02)01201-1. [DOI] [PubMed] [Google Scholar]
- GRAVEN-NIELSEN T., JANSSON Y., SEGERDAHL M., KRISTENSEN J.D., MENSE S., ARENDT-NIELSEN L., SOLLEVI A. Experimental pain by ischaemic contractions compared with pain by intramuscular infusions of adenosine and hypertonic saline. Eur. J. Pain. 2003;7:93–102. doi: 10.1016/s1090-3801(02)00069-1. [DOI] [PubMed] [Google Scholar]
- GRISWOLD D.E., DOUGLAS S.A., MARTIN L.D., DAVIS T.G., DAVIS L., AO Z., LUTTMANN M.A., PULLEN M., NAMBI P., HAY D.W., OHLSTEIN E.H. Endothelin B receptor modulates inflammatory pain and cutaneous inflammation. Mol. Pharmacol. 1999;56:807–812. [PubMed] [Google Scholar]
- GUIMARÃES C.L., TRENTIN P.G., RAE G.A. Endothelin ET(B) receptor-mediated mechanisms involved in oleic acid-induced acute lung injury in mice. Clin. Sci. 2002;103 Suppl. 48:340S–344S. doi: 10.1042/CS103S340S. [DOI] [PubMed] [Google Scholar]
- HONORÉ J.C., PLANTE M., BKAILY G., RAE G.A., D'ORLÉANS-JUSTE P. Pressor and pulmonary responses to ET-1(1-31) in guinea-pigs. Br. J. Pharmacol. 2002;136:819–828. doi: 10.1038/sj.bjp.0704782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAFFERY G., COLEMAN J.W., HUNTLEY J., BELL E.B. Mast cell recovery following chronic treatment with compound 48/80. Int. Arch. Allergy Immunol. 1994;105:274–280. doi: 10.1159/000236769. [DOI] [PubMed] [Google Scholar]
- JARVIS M.F., WESSALE J.L., ZHU C.Z., LYNCH J.J., DAYTON B.D., CALZADILLA S.V., PADLEY R.J., OPGENORTH T.J., KOWALUK E.A. ABT-627, an endothelin ET(A) receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur. J. Pharmacol. 2000;388:29–35. doi: 10.1016/s0014-2999(99)00865-1. [DOI] [PubMed] [Google Scholar]
- KEDZIERSKI R.M., YANAGISAWA M. Endothelin system: the double-edged sword in health and disease. Annu. Rev. Pharmacol. Toxicol. 2001;41:851–876. doi: 10.1146/annurev.pharmtox.41.1.851. [DOI] [PubMed] [Google Scholar]
- KHODOROVA A., FAREED M.U., GOKIN A., STRICHARTZ G.R., DAVAR G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J. Neurosci. 2002;22:7788–7796. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHODOROVA A., NAVARRO B., JOUAVILLE L.S., MURPHY J.E., RICE F.L., MAZURKIEWICZ J.E., LONG-WOODWARD D., STOFFEL M., STRICHARTZ G.R., YUKHANANOV R., DAVAR G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat. Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- KIZAWA Y., KOTAKE H., KUSAMA T., SAITO K., MURAKAMI H. Antigen-induced elevation of immunoreactive endothelin-1 (ET-1) levels in ovalbumin-sensitized guinea pig airway tissue. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1999;122:239–243. doi: 10.1016/s0742-8413(98)10122-6. [DOI] [PubMed] [Google Scholar]
- KODA W., HARADA K., TSUNEYAMA K., KONO N., SASAKI M., MATSUI O., NAKANUMA Y. Evidence of the participation of peribiliary mast cells in regulation of the peribiliary vascular plexus along the intrahepatic biliary tree. Lab. Invest. 2000;80:1007–1017. doi: 10.1038/labinvest.3780106. [DOI] [PubMed] [Google Scholar]
- KUROSE I., MIURA S., FUKUMURA D., TSUCHIYA M. Mechanisms of endothelin-induced macromolecular leakage in microvascular beds of rat mesentery. Eur. J. Pharmacol. 1993;250:85–94. doi: 10.1016/0014-2999(93)90624-q. [DOI] [PubMed] [Google Scholar]
- LAVICH T.R., CORDEIRO R.S., CALIXTO J.B., SILVA P.M., MARTINS M.A. Combined action of vasoactive amines and bradykinin mediates allergen-evoked thermal hyperalgesia in rats. Eur. J. Pharmacol. 2003;462:185–192. doi: 10.1016/s0014-2999(02)02947-3. [DOI] [PubMed] [Google Scholar]
- LI Z., NIWA Y., ROKUTAN K., NAKAYA Y. Expression of endothelin-1 in macrophages and mast cells in hyperplastic human tonsils. FEBS Lett. 1999;457:381–384. doi: 10.1016/s0014-5793(99)01085-6. [DOI] [PubMed] [Google Scholar]
- LIU Y., YAMADA H., OCHI J. Immunocytochemical studies on endothelin in mast cells and macrophages in the rat gastrointestinal tract. Histochem. Cell Biol. 1998;109:301–307. doi: 10.1007/s004180050230. [DOI] [PubMed] [Google Scholar]
- MASAKI T., VANE J.R., VANHOUTTE P.M. International Union of Pharmacology nomenclature of endothelin receptors. Pharmacol. Rev. 1994;46:137–142. [PubMed] [Google Scholar]
- MENÉNDEZ L., LASTRA A., HIDALGO A., BAAMONDE A. Nociceptive reaction and thermal hyperalgesia induced by local ET-1 in mice: a behavioral and Fos study. Naunyn Schmiedbergs Arch. Pharmacol. 2003;367:28–34. doi: 10.1007/s00210-002-0655-6. [DOI] [PubMed] [Google Scholar]
- MOUSLI M., BUEB J.L., BRONNER C., ROUOT B., LANDRY Y. G protein activation: a receptor-independent mode of action for cationic amphiphilic neuropeptides and venom peptides. Trends Pharmacol. Sci. 1990;11:358–362. doi: 10.1016/0165-6147(90)90179-c. [DOI] [PubMed] [Google Scholar]
- NAKANO A., KISHI F., MINAMI K., WAKABAYASHI H., NAKAYA Y., KIDO H. Selective conversion of big endothelins to tracheal smooth muscle-constricting 31-amino acid-length endothelins by chymase from human mast cells. J. Immunol. 1997;159:1987–1992. [PubMed] [Google Scholar]
- OPGENORTH T.J., ADLER A.L., CALZADILLA S.V., CHIOU W.J., DAYTON B.D., DIXON D.B., GEHRKE L.J., HERNANDEZ L., MAGNUSON S.R., MARSH K.C., NOVOSAD E.I., VON GELDERN T.W., WESSALE J.L., WINN M., WU-WONG J.R. Pharmacological characterization of A-127722: an orally active and highly potent ETA-selective receptor antagonist. J. Pharmacol. Exp. Ther. 1996;276:473–481. [PubMed] [Google Scholar]
- PALUCKA K., BANCHEREAU J. How dendritic cells and microbes interact to elicit or subvert protective immune responses. Curr. Opin. Immunol. 2002;14:420–431. doi: 10.1016/s0952-7915(02)00365-5. [DOI] [PubMed] [Google Scholar]
- PARADA C.A., TAMBELLI C.H., CUNHA F.Q., FERREIRA S.H. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalin-induced nociception. Neuroscience. 2001;102:937–944. doi: 10.1016/s0306-4522(00)00523-6. [DOI] [PubMed] [Google Scholar]
- PIOVEZAN A.P., D'ORLÉANS-JUSTE P., SOUZA G.E., RAE G.A. Endothelin-1-induced ETA receptor-mediated nociception, hyperalgesia and oedema in the mouse hind-paw: modulation by simultaneous ETB receptor activation. Br. J. Pharmacol. 2000;129:961–968. doi: 10.1038/sj.bjp.0703154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIOVEZAN A.P., D'ORLÉANS-JUSTE P., TONUSSI C.R., RAE G.A. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can. J. Physiol. Pharmacol. 1997;75:596–600. [PubMed] [Google Scholar]
- PIOVEZAN A.P., D'ORLÉANS-JUSTE P., TONUSSI C.R., RAE G.A. Effects of endothelin-1 on capsaicin-induced nociception in mice. Eur. J. Pharmacol. 1998;351:15–22. doi: 10.1016/s0014-2999(98)00281-7. [DOI] [PubMed] [Google Scholar]
- RAFFA R.B., SCHUPSKY J.J., JACOBY H.I. Endothelin-induced nociception in mice: mediation by ETA and ETB receptors. J. Pharmacol. Exp. Ther. 1996;276:647–651. [PubMed] [Google Scholar]
- RIBEIRO R.A., VALE M.L., THOMAZZI S.M., PASCHOALATO A.B.P., POOLE S., FERREIRA S.H., CUNHA F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J.Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- RUGGIERI M.R., FILER-MAERTEN S., HIEBLE J.P., HAY D.W. Role of neurokinin receptors in the behavioral effect of intravesical antigen infusion in guinea pig bladder. J. Urol. 2000;164:197–202. [PubMed] [Google Scholar]
- SAMPAIO A.L., RAE G.A., D'ORLÉANS-JUSTE P., HENRIQUES M.G. ETA receptor antagonists inhibit allergic inflammation in the mouse. J. Cardiovasc. Pharmacol. 1995;26 Suppl. 3:S416–S418. [PubMed] [Google Scholar]
- SAMPAIO A.L., RAE G.A., HENRIQUES M.G. Participation of endogenous endothelins in delayed eosinophil and neutrophil recruitment in mouse pleurisy. Inflamm. Res. 2000;49:170–176. doi: 10.1007/s000110050577. [DOI] [PubMed] [Google Scholar]
- SHIGEMATSU T., MIURA S., HIROKAWA M., HOKARI R., HIGUCHI H., WATANABE N., TSUZUKI Y., KIMURA H., TADA S., NAKATSUMI R.C., SAITO H., ISHII H. Induction of endothelin-1 synthesis by IL-2 and its modulation of rat intestinal epithelial cell growth. Am. J. Physiol. 1998;275:G556–G563. doi: 10.1152/ajpgi.1998.275.3.G556. [DOI] [PubMed] [Google Scholar]
- SIROIS M.G., FILEP J.G., ROUSSEAU A., FOURNIER A., PLANTE G.E., SIROIS P. Endothelin-1 enhances vascular permeability in conscious rats: role of thromboxane A2. Eur. J. Pharmacol. 1992;214:119–125. doi: 10.1016/0014-2999(92)90108-g. [DOI] [PubMed] [Google Scholar]
- SOFIA M., MORMILE M., FARAONE S., ALIFANO M., ZOFRA S., ROMANO L., CARRATU L. Increased endothelin-like immunoreactive material on bronchoalveolar lavage fluid from patients with bronchial asthma and patients with interstitial lung disease. Respiration. 1993;60:89–95. doi: 10.1159/000196180. [DOI] [PubMed] [Google Scholar]
- TOMINAGA K., ALSTERGREN P., KURITA H., KOPP S. Clinical course of an antigen-induced arthritis model in the rabbit temporomandibular joint. J. Oral Pathol. Med. 1999;28:268–273. doi: 10.1111/j.1600-0714.1999.tb02037.x. [DOI] [PubMed] [Google Scholar]
- TRAKADA G., TSOURAPIS S., MARANGOS M., SPIROPOULOS K. Arterial and bronchoalveolar lavage fluid endothelin-1 concentration in asthma. Respir. Med. 2000;94:992–996. doi: 10.1053/rmed.2000.0890. [DOI] [PubMed] [Google Scholar]
- UCHIDA Y., NINOMIYA H., SAKAMOTO T., LEE J.Y., ENDO T., NOMURA A., HASEGAWA S., HIRATA F. ET-1 released histamine from guinea pig pulmonary but not peritoneal mast cells. Biochem. Biophys. Res Commun. 1992;189:1196–1201. doi: 10.1016/0006-291x(92)92331-q. [DOI] [PubMed] [Google Scholar]
- VON GELDERN T.W., TASKER A.S., SORENSEN B.K., WINN M., SZCZEPANKIEWICZ B.G., DIXON D.B., CHIOU W.J., WANG L., WESSALE J.L., ADLER A., MARSH K.C., NGUYEN B., OPGENORTH T.J. Pyrrolidine-3-carboxylic acids as endothelin antagonists. 4. Side chain conformational restriction leads to ETB selectivity. J. Med. Chem. 1999;42:3668–3678. doi: 10.1021/jm990170q. [DOI] [PubMed] [Google Scholar]
- WACNIK P.W., EIKMEIER L.J., RUGGLES T.R., RAMNARAINE M.L., WALCHECK B.K., BEITZ A.J., WILCOX G.L. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J. Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., ARAKAWA T., TOMINAGA K., FUJIWARA Y., HIGUCHI K., KUROKI T. Neutrophil accumulation in development gastric ulcer induced by submucosal injection of endothelin-1 in rats. Dig. Dis. Sci. 2000;45:880–888. doi: 10.1023/a:1005556520813. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., MAIER S.F. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- WEDEMEYER J., TSAI M., GALLI S.J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immun. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- YAMAMURA H., NABE T., KOHNO S., OHATA K. Endothelin-1, one of the most potent histamine releasers in mouse peritoneal mast cells. Eur. J. Pharmacol. 1994;265:9–15. doi: 10.1016/0014-2999(94)90217-8. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]