Abstract
Interstitial cells of Cajal (ICCs) are pacemaker cells that activate the periodic spontaneous inward currents (pacemaker currents) responsible for the production of slow waves in gastrointestinal smooth muscle. The effects of noradrenaline on the pacemaker currents in cultured ICCs from murine small intestine were investigated by using whole-cell patch-clamp techniques at 30°C.
Under current clamping, ICCs had a mean resting membrane potential of −58±5 mV and produced electrical slow waves. Under voltage clamping, ICCs produced pacemaker currents with a mean amplitude of −410±57 pA and a mean frequency of 16±2 cycles min−1.
Under voltage clamping, noradrenaline inhibited the amplitude and frequency of pacemaker currents and increased resting currents in the outward direction in a dose-dependent manner. These effects were reduced by intracellular GDPβS.
Noradrenaline-induced effects were blocked by propranolol (β-adrenoceptor antagonist). However, neither prazosin (α1-adrenoceptor antagonist) nor yohimbine (α2-adrenoceptor antagonist) blocked the noradrenaline-induced effects. Phenylephrine (α1-adrenoceptor agonist) had no effect on the pacemaker currents, whereas isoprenaline (β-adrenoceptor agonist) mimicked the effect of noradrenaline. Atenolol (β1-adrenoceptor antagonist) blocked the noradrenaline-induced effects, but butoxamine (β2-adrenoceptor antagonist) did not. In addition, BRL37344 (β3-adrenoceptor agonist) had no effect on pacemaker currents.
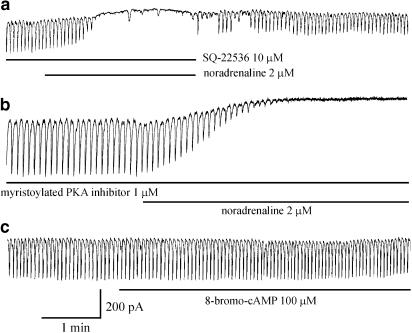
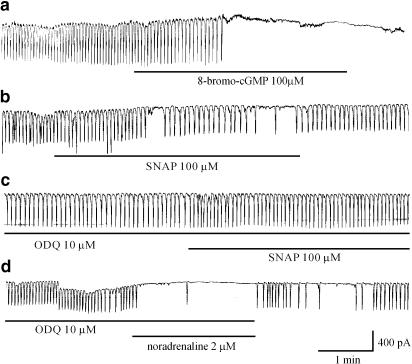
9-(Tetrahydro-2-furanyl)-9H-purine-6-amine (SQ-22536; adenylate cyclase inhibitor) and a myristoylated protein kinase A inhibitor did not inhibit the noradrenaline-induced effects and 8-bromo-cAMP had no effects on pacemaker currents. 8-Bromo-cGMP and SNAP inhibited pacemaker currents and these effects of SNAP were blocked by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ; a guanylate cyclase inhibitor). However, ODQ did not block the noradrenaline-induced effects.
Neither tetraethylammonium (a voltage-dependent K+ channel blocker), apamin (a Ca2+-dependent K+ channel blocker) nor glibenclamide (an ATP-sensitive K+ channel blocker) blocked the noradrenaline-induced effects.
The results suggest that noradrenaline-induced stimulation of β1-adrenoceptors in the ICCs inhibits pacemaker currents, and that this is mediated by the activation of G-protein. Neither adenylate cyclase, guanylate cyclase nor a K+ channel-dependent pathway are involved in this effect of noradrenaline.
Keywords: Interstitial cells of Cajal, slow waves, pacemaker currents, noradrenaline, β1-adrenoceptor
Introduction
Gastrointestinal smooth muscles exhibit spontaneous contractions. These spontaneous contractions are mediated by the periodic generation of electrical slow waves. Slow waves have a very important role in determining the activity of the muscle of the gastrointestinal system, and their impairment is likely to cause various motility disorders (Szurszewski, 1987). Gastrointestinal tract motility is regulated by these slow waves that are modulated by extrinsic and intrinsic autonomic neurones as well as by circulating hormones and drugs (Demol et al., 1989). The interstitial cells of Cajal (ICCs) are the pacemaker cells in the gastrointestinal tract. They generate slow waves and also mediate signals from enteric neurones to smooth muscle (Ward et al., 1994; Huizinga et al., 1995; Sanders, 1996). This generation of slow waves is due to the activation of spontaneous inward currents (pacemaker currents) (Koh et al., 1998).
Catecholamines have an important role in regulating smooth muscle contractility. They have both inhibitory and excitatory effects in visceral smooth muscle (Bülbring & Tomita, 1987). Stimulation of α-adrenoceptors mediates a dual (excitatory and inhibitory) effect, whereas stimulation of β-adrenoceptors mediates an inhibitory effect. The effect of activating α-adrenoceptors depends on their location and the species. For example, in the gastric antrum (Jun et al., 1993) and intestinal smooth muscle (terminal ileum) (Bauer, 1981) of guinea-pig, α-adrenoceptors mediate an excitatory effect. However, in the taenia coli of guinea-pig, α-adrenoceptors mediate an inhibitory effect (Bauer, 1982). In cat colon circular muscle stimulation of α-adrenoceptors increases the magnitude of electrical slow waves and mechanical contractions, whereas activation of β-adrenoceptors decreases them (Venkova & Krier, 1995). The relaxation of visceral smooth muscle induced by catecholamines is associated with membrane hyperpolarization mediated by α-adrenoceptors and cyclic AMP production due to the stimulation of β-adrenoceptors (Bülbring & Tomita, 1987). As these experiments were performed in intact tissues, it was difficult to differentiate between the effects on ICCs and those on smooth muscle. Therefore, in this study, we investigated the effects of noradrenaline on the electrical properties of cultured ICC cells, in order to elucidate the mechanisms of the effects of catecholamines on intestinal motility.
Methods
Preparation of cells and tissues
Balb/c mice (8–13 days old) of either sex were anaesthetized with ether and killed by cervical dislocation. The small intestines from 1 cm below the pyloric ring to the caecum were removed and opened along the mesenteric border. Luminal contents were washed away with Krebs–Ringer bicarbonate solution. The tissues were pinned to the base of a Sylgard dish and the mucosa removed by sharp dissection. Small tissue stripes of intestinal muscle (consisting of both circular and longitudinal muscles) were equilibrated in Ca2+-free Hank's solution (containing in mM: KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9 and HEPES 11) for 30 min. Then, the cells were dispersed with an enzyme solution containing collagenase (Worthington Biochemical Co, Lakewood, NJ, U.S.A.) 1.3 mg ml−1, bovine serum albumin (Sigma Chemical Co., St Louis, MO, U.S.A.) 2 mg ml−1, trypsin inhibitor (Sigma) 2 mg ml−1 and ATP 0.27 mg ml−1. Cells were plated onto sterile glass coverslips coated with murine collagen (2.5 μg ml−1, Falcon/BD) in a 35 mm culture dish. The cells were then cultured at 37°C in a 95% O2–5% CO2 incubator in a smooth muscle growth medium (SMGM, Clonetics Corp., San Diego, CA, U.S.A.) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, U.S.A.) and murine stem cell factor (SCF, 5 ng ml−1, Sigma). ICCs were identified immunologically with a monoclonal antibody for Kit protein (ACK2) labelled with Alexa Fluor 488 (molecular prove, Eugene, OR, U.S.A.) (Koh et al., 1998; 2000). The morphologies of ICCs were distinct from other cell types in the culture, so it was possible to identify the cells with phase contrast microscopy once the cells had been verified with ACK2-Alexa Fluor 488 labelling.
Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and potentials (current clamp) from cultured ICCs. Axopatch 1-D (Axon Instruments, Foster, CA, U.S.A.) amplified membrane currents and potentials. The command pulse was applied using a IBM-compatible personal computer and pClamp software (version 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on an oscilloscope, a computer monitor, and with a pen recorder (Gould 2200, Gould, Vally view, OF, U.S.A.).
Results were analysed using pClamp and Graph Pad Prism (version 2.01) software. All experiments were performed at 30°C.
Solutions and drugs
The cells were bathed in a solution containing (in mM): KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2 and HEPES 10, adjusted to pH 7.2 with tris. The pipette solution contained (in mM) KCl 140, MgCl2 5, K2ATP 2.7, Na2GTP 0.1, creatine phosphate disodium 2.5, HEPES 5 and EGTA 0.1, adjusted to pH 7.2 with tris.
Drugs used were: (−)-noradrenaline bitartrate, prazosine, yohimbine hydrochloride, phenylephrine hydrochloride, propranolol hydrochloride, isoprenaline hydrochloride, atenolol, butoxamine hydrochloride, (R*, R*)-(±)-4-[2-[(2-93-chlorophenyl)amino]propyl]phenoxy acetic acid sodium (BRL-37344), tetraethylammonium chloride, apamin, glibenclamide, guanosine 5′-O-(2-thio)diphosphate (GDPβS), 8-bromo-cAMP, myristoylated protein kinase A inhibitor (mPKAI), 8-bromo-cGMP, SNAP, 9-(tetrahydro-2-furanyl)-9H-purine-6-amine (SQ-22536) and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ). SQ-22536 and ODQ were purchased from Calbiochem Co. (San Diego, CA, U.S.A.), and the others were purchased from the Sigma Chemical Co.
All drugs were dissolved into DW or DMSO to prepare stock solutions (10 or 100 mM), and were either added to the bath solution or applied to the whole-cell preparations by superfusion. The final concentration of DMSO was less than 0.05%.
Statistical analysis
Data are expressed as means±standard errors (s.e.). Differences between the data were evaluated by Student's t-test. A P-value less than 0.05 was taken to indicate a statistically significant difference. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
Results
Noradrenaline inhibits pacemaker currents in a dose-dependent manner in ICCs
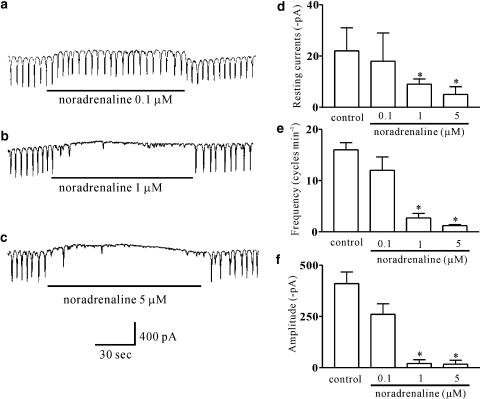
Under a voltage clamp at a holding potential of −70 mV, ICCs generated spontaneous inward currents, which are referred to as ‘pacemaker currents'. The frequency of the pacemaker currents was 16±1.4 cycles min−1 and the amplitude and resting current levels were −410±57 and −22±18 pA, respectively (n=12). The addition of noradrenaline (0.1–5 μM) decreased both the frequency and the amplitude of the pacemaker currents, and increased the resting currents in an outward direction in a concentration-dependent manner (Figure 1a–c). In the presence of noradrenaline, the resting currents were −18±11 pA at 0.1 μM (n=8), −9±2.0 pA at 1 μM (n=6) and −5±3.04 pA at 5 μM (n=5) (Figure 1d) and the corresponding frequencies and amplitudes were 12±2.6, 2.7±0.9 and 1.2±0.2 cycles min−1, and −260±52, −20.8±18 and −17±19 pA (Figure 1e and f). These values at 1 and 5 μM were significantly different from control values. These results suggest that noradrenaline inhibits pacemaker currents in a dose-dependent manner in ICCs.
Figure 1.
Concentration-dependent effect of noradrenaline on pacemaker currents in cultured ICCs of the murine small intestine. (a–c) The slow waves of ICCs exposed to noradrenaline (0.1, 1 and 5 μM) at a holding potential of −70 mV. Noradrenaline inhibited spontaneous pacemaker currents in a concentration-dependent manner in ICCs and induced increased resting currents in the outward direction. (d–f) A summary of the inhibitory effects of noradrenaline on pacemaker currents in ICCs. Each column represents the mean±s.e. (n=9–12/group). *P<0.05, significantly different from the controls.
Noradrenaline inhibits pacemaker currents through activation of β-adrenoceptors
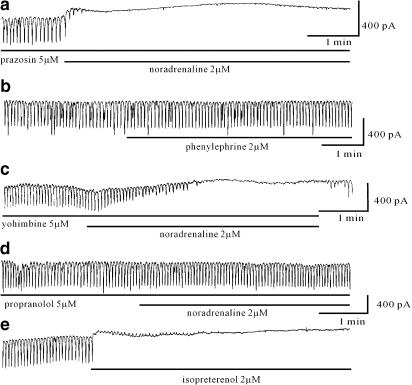
Adrenoceptor antagonists and agonists were used to identify the receptor subtype mediating the effects of noradrenaline. Prazosin, a selective α1-adrenoceptor antagonist, and yohimbine, an α2-adrenoceptor antagonist, were used. Pretreatment with prazosin (5 μM) (n=5, Figure 2a) and yohimbine (5 μM) (n=4, Figure 2c) for 10 min did not block the noradrenaline (2 μM)-induced effects. Neither prazosin nor yohimbine alone had any effect on pacemaker currents. In addition, phenylephrine (2 μM), an α1-adrenoceptor agonist, showed no significant effect on pacemaker currents (n=4, Figure 2b). These results suggest that the effects of noradrenaline are not mediated by α-adrenoceptors. In contrast, the noradrenaline-induced effects were completely blocked by pretreatment with propranolol, a nonselective β-adrenoceptor antagonist (n=5, Figure 2d). Moreover, isoprenaline, a β-adrenoceptor agonist, inhibited the pacemaker currents in a way similar to noradrenaline (n=5, Figure 2e). Isoprenaline decreased the frequency and amplitude of the pacemaker currents and increased the resting currents in an outward direction. Under control conditions at a holding potential of −70 mV, the resting current was −29±18 pA, and the frequency and amplitude of the pacemaker currents were 16±1.4 cycles min−1 and −430±48 pA, respectively. In the presence of isoprenaline (2 μM), the resting current was −2±6.5 pA, and the frequency and amplitude of the pacemaker currents were 1.1±0.4 cycles min−1 and −14±16 pA (P<0.05), respectively. This indicates that the inhibition of pacemaker currents by noradrenaline is mediated by β-adrenoceptors.
Figure 2.
Effects of α- and β-adrenoceptor antagonists on the response to noradrenaline and of α- and β-adrenoceptor agonists on pacemaker currents. (a) Prazosin (5 μM) and (c) yohimbine (5 μM), selective α1- and α2-adrenoceptor antagonists, did not block the noradrenaline-mediated inhibition of pacemaker currents. (b) Phenylephrine (2 μM), an α1-adrenoceptor agonist, had no effects on pacemaker currents. (d) Propranolol (5 μM), a nonselective β-adrenoceptor antagonist, significantly blocked the noradrenaline-induced inhibition of pacemaker currents and (e) isoprenaline (2 μM), a β-adrenoceptor agonist, mimicked the inhibitory effects of noradrenaline on pacemaker currents. Pacemaker currents were recorded from separate cells.
Involvement of β1-adrenoceptors in the effects of noradrenaline on pacemaker currents in ICCs
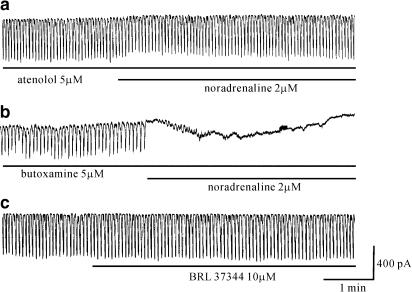
In the above results, in ICCs, noradrenaline inhibited pacemaker currents through β-adrenoceptor activation. To determine the β-adrenoceptor subtype mediating this effect, the ICCs were pretreated with either a β1-, or a β2-adrenoceptor antagonist for 10 min before the application of noradrenaline and the effect of a β3-adrenoceptor agonist was also investigated. In the presence of atenolol (5 μM), a selective β1-adrenoceptor antagonist, noradrenaline-induced effects were significantly inhibited (n=6, Figure 3a). Under control conditions at a holding potential of −70 mV, the resting currents was −33±14 pA, and the frequency and amplitude of the pacemaker currents were 16±1.2 cycles min−1 and −426±37 pA, respectively. In the presence of atenolol, the resting current was −28±15 pA, and the frequency and amplitude of the pacemaker currents induced by noradrenaline were 16±1.4 cycles min−1 and −408±24 pA, respectively. These values were not significantly different from control values. However, butoxamine (5 μM), a β2-adrenoceptor antagonist, did not block noradrenaline-induced effects (n=4, Figure 3b). In the presence of butoxamine, noradrenaline still inhibited the pacemaker currents. Neither atenolol nor butoxamine alone had any effect on pacemaker currents. In addition, BRL 37344 (5 μM), a β3-adrenoceptor agonist, had no significant effects on pacemaker currents (n=5, Figure 3c). These results suggest that β1-adrenoceptors may mediate the effects of noradrenaline on pacemaker currents in ICCs.
Figure 3.
Effects of β1- and β2-adrenoceptor antagonists on the response to noradrenaline and the effect of a β3-adrenoceptor agonist on pacemaker currents. (a) Atenolol (5 μM), a selective β1-adrenoceptor antagonist, significantly blocked the noradrenaline-mediated inhibition of pacemaker currents. (b) However, butoxamine (5 μM), a β2-adrenoceptor antagonist, did not block the noradrenaline-mediated inhibition of pacemaker currents. (c) BRL37344 (5 μM), a β3-adrenoceptor agonist, also had no effects on pacemaker currents. Pacemaker currents were recorded from separate cells.
Involvement of G-proteins in noradrenaline-induced inhibition of pacemaker currents
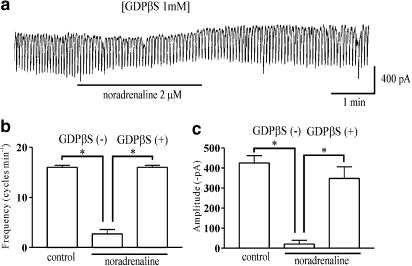
The effects of GDPβS, a nonhydrolysable guanosine 5′-di-phosphate analogue, which permanently inactivates GTP-binding proteins (Komori et al., 1993), were examined to determine whether the G-protein is involved in the effects of noradrenaline in ICCs. The inhibitory effect of noradrenaline was reduced when GDPβS (1 mM) was in the pipette (Figure 4a). Under control conditions at a holding potential of −70 mV, the frequency and amplitude were 16±0.4 cycles min−1 and −425±37 pA, respectively; the addition of noradrenaline (2 μM) decreased both the frequency and the amplitude of the pacemaker currents (frequency: 2.7±0.9 cycles min−1; amplitude: −20.8±18 pA). In the presence of GDPβS in the pipette, the effects of naradrenaline on these variables were attenuated; the frequency and amplitude were 16±0.4 cycles min−1 and −348±58 pA, respectively (n=7) (Figure 4b and c), values significantly different from those obtained with noradrenaline alone. This indicates that G-proteins have an essential role in noradrenaline-induced effects on pacemaker currents in ICCs.
Figure 4.
Effects of GDPβS on the response to noradrenaline. (a) Pacemaker currents of ICCs exposed to noradrenaline (2 μM) in the presence of GDPβS (1 mM) in the pipette. GDPβS partially blocked the noradrenaline-mediated inhibition of pacemaker currents. The effect of noradrenaline in the presence of GDPβS but not in the pipette is summarized in (b) and (c). Each column represents the mean±s.e. (n=7).
Adenylate cyclase and guanylate cyclase inhibitors and noradrenaline-induced inhibition of pacemaker currents
The effects of SQ-22536, an inhibitor of adenylate cyclase, a mPKAI and ODQ, an inhibitor of guanylate cyclase, were examined to determine whether the cyclic nucleotide-dependent pathway is involved in the effects of noradrenaline in ICCs. The ICCs were pretreated with either SQ-22536, mPKAI or ODQ for 10 min before the application of noradrenaline. In the presence of SQ-22536 (10 μM) or mPKAI (1 μM), noradrenaline (2 μM) still inhibited the pacemaker currents (n=5) (Figure 5a and b). In the presence of SQ-22536, the resting current was −4±7.3 pA, and the frequency and amplitude of the pacemaker currents were 1.2±0.4 cycles min−1 and −12±18 pA (P<0.05), respectively. In the presence of mPKAI, the resting current was −1±9.5 pA, and the frequency and amplitude of the pacemaker currents were 1±0.8 cycles min−1 and −11±16 pA (P<0.05), respectively. In addition, the cell-permeable 8-bromo-cAMP (100 μM) had no effect on the generation of pacemaker currents (n=8) (Figure 5c). In contrast, 8-bromo-cGMP inhibited the pacemaker currents. Under control conditions at a holding potential of −70 mV, the resting current was −32±19 pA, and the frequency and the amplitude of the pacemaker currents were 17.7±0.5 cycles min−1 and −412±29 pA, respectively. In the presence of 8-bromo-cGMP (100 μM), the frequency and amplitude decreased to 2.1±0.5 cycles min−1 and −13±16 pA, respectively (n=7, P<0.05) (Figure 6a). Also, SNAP, a NO donor, inhibited the generation of pacemaker currents (Figure 6b). Under control conditions at a holding potential of −70 mV, the resting current was −32±19 pA, and the frequency and the amplitude of the pacemaker currents were 16.7±1.1 cycles min−1 and −421±38 pA, respectively. In the presence of SNAP (100 μM), the frequency and amplitude decreased to 2.7±1.4 cycles min−1 and −10±11 pA, respectively (n=6, P<0.05). These effects were blocked by ODQ (Figure 6c). In the presence of ODQ (10 μM), the resting current was −28±20 pA, and the frequency and amplitude of the pacemaker currents were 17±0.6 cycles min−1 and −420±24 pA, respectively. These values were not significantly different from control values. However, ODQ did not block the inhibitory effects of noradrenaline (n=5) (Figure 6d). Under control conditions at a holding potential of −70 mV, the resting current was −28±23 pA, and the frequency and the amplitude of the pacemaker currents were 16.4±1.6 cycles min−1 and −436±27 pA, respectively. In the presence of ODQ (10 μM), the frequency and amplitude decreased to 1.1±0.7 cycles min−1 and −10±6.8 pA, respectively (n=6, P<0.05). Neither SQ-22536, mPKAI nor ODQ alone had any effect on pacemaker currents. These observations suggest that neither cyclic AMP nor cyclic GMP mediate the inhibition of pacemaker currents induced by noradrenaline.
Figure 5.
Effects of adenylate cyclase and a protein kinase A inhibitor on the noradrenaline-mediated inhibition of pacemaker currents. (a) Effect of noradrenaline on pacemaker currents in the presence of SQ-22536 (10 μM). SQ-22536 did not block the noradrenaline-mediated inhibition of pacemaker currents. (b) Effect of noradrenaline on pacemaker currents in the presence of mPKAI (1 μM). mPKAI did not block the noradrenaline-mediated inhibition of pacemaker currents. (c) Effect of 8-bromo-cAMP (100 μM) on pacemaker currents. 8-bromo-cAMP had no effect on pacemaker currents. Pacemaker currents were recorded from separate cells.
Figure 6.
Effects of a guanylate cyclase inhibitor on the noradrenaline-mediated inhibition of pacemaker currents. (a) Effect of 8-bromo-cGMP (100 μM) on pacemaker currents. 8-Bromo-cGMP inhibited pacemaker currents. (b) Effect of SNAP (100 μM) on pacemaker currents. (c) Effect of SNAP in the presence of ODQ (10 μM). SNAP also inhibited the pacemaker current and this effect was antagonized by the pretreatment with ODQ. (d) Effect of noradrenaline on pacemaker currents in the presence of ODQ (10 μM). ODQ did not block the noradrenaline-mediated inhibition of pacemaker currents. Pacemaker currents were recorded from separate cells.
Effect of K+ channel blockers on noradrenaline-induced inhibition of pacemaker currents
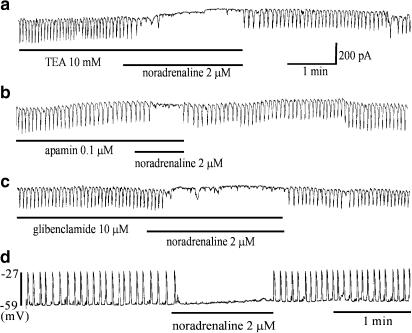
In previous reports, β-adrenoceptors have been shown to effect membrane hyperpolarization by increasing K+ conductances (Watson et al., 1996). Therefore, we tested whether the noradrenaline-induced inhibition of pacemaker currents is mediated by the activation of K+ channels by using K+ channel blockers. In the presence of either TEA (a voltage-dependent K+ channel blocker) (10 mM; n=4), apamin (a Ca2+-dependent K+ channel blocker) (0.1 μM; n=4), or glibenclamide (an ATP-sensitive K+ channel blocker) (10 μM; n=3), noradrenaline (2 μM) still inhibited the pacemaker currents (Figure 7a–c).
Figure 7.
Effects of K+ channel blockers on noradrenaline-mediated inhibition of pacemaker currents. (a) TEA (10 mM) (a voltage-dependent K+ channel blocker), (b) apamin (0.1 μM) (a Ca2+-dependnet K+ channel blocker) and (c) glibenclamide (10 μM) (an ATP-sensitive K+ channel blocker) did not block the noradrenaline-mediated inhibition of pacemaker currents. (d) Slow waves of ICCs exposed to noradrenaline (2 μM) in the current-clamping mode (I=0). Noradrenaline abolished the generation of slow waves, but did not induce the hyperpolarization of membrane potential. Pacemaker currents were recorded from separate cells.
Also, we carried out membrane potential studies to examine the effect of current clamping in ICCs. The ICCs generated slow waves under a current clamp. The resting membrane potential was −58±5 mV and amplitude 25±6 mV and slow wave frequency 16±2 cycles min−1 (n=13). The addition of noradrenaline (2 μM) completely abolished the generation of slow waves, but was unable to hyperpolarize the resting membrane potential (Figure 7d). In the presence of noradrenaline, the resting membrane potential was −62±2 mV. Thus, it is unlikely that K+ channels are associated with the inhibition of pacemaker currents in ICCs.
Discussion
ICCs regulate visceral smooth muscle activity by producing electrical slow waves. The generation of slow waves in ICCs is due to the periodic activation of spontaneous inward currents (pacemaker currents). In this study, noradrenaline was found to inhibit the pacemaker currents through activation of β1-adrenoceptors in cultured ICCs from murine small intestine.
Adrenoceptors are classified into α- and β-adrenoceptors. In our study, the noradrenaline-induced inhibition of pacemaker currents was not blocked by prazosin, an α1-adrenoceptor antagonist, and phenylephrine, an α1-adrenoceptor agonist, had no effects on pacemaker currents. Also, yohimbine, an α2-adrenoceptor antagonist, did not block the noradrenaline-induced effects. In contrast, propranolol, a nonselective β-adrenoceptor antagonist, completely blocked noradrenaline-mediated inhibition of pacemaker currents, and isoprenaline, a β-adrenoceptor agonist, inhibited pacemaker currents. In gastrointestinal tract, β-adrenoceptors are subdivided into three subtypes, β1-, β2- and β3-adrenoceptor (Manara et al., 1995). The relaxation of the guinea-pig ileum (Grassby & Broadley, 1984) and rabbit duodenum (Lands et al., 1967) by catecholamines is mediated only via the β1-adrenoceptor. However, other experiments have revealed the existence of the β2-adrenoceptor subtype. In rat colon, the β2-adrenoceptor subtype is predominant (Ek & Nahorski, 1986) and activation of β2-adrenoceptors relaxes guinea-pig taenia caecum (Akimoto et al., 2002). In addition, a typical β3-adrenoceptor subtype has been found in gastrointestinal smooth muscles of various species (Manara et al., 1995). Koike et al. (1995) reported that β-adrenoceptor-mediated relaxation induced by noradrenaline is not inhibited by propranolol, and BRL37344, a selective β3-agonist, relaxes smooth muscle in guinea-pig taenia caecum. In this study, butoxamine, a selective β2-adrenoceptor antagonist, did not block the noradrenaline-mediated inhibition of pacemaker currents, whereas atenolol, a selective β1-adrenoceptor antagonist, completely blocked these effects. In addition, BRL37344 had no effects on pacemaker currents. These results suggest that noradrenaline mediates its inhibitory action on pacemaker currents by activating β1-adrenoceptors.
Stimulating the β-adrenoceptors with catecholamines increases cyclic AMP levels by activating adenylate cyclase via the G-protein (Guan et al., 1995). In these experiments, when GDPβS was present in the pipette, the inhibition of pacemaker currents by noradrenaline was suppressed. This means that β1-adrenoceptors are coupled with G-proteins in ICCs. The relaxation of gastrointestinal smooth muscle caused by cyclic AMP is mediated through the activation of cyclic AMP-dependent protein kinase. Tsugeno et al. (1995) reported that, in guinea-pig gastric smooth muscle, an increase in cyclic AMP reduces slow-wave frequency without changing either the membrane potential or the slow-wave configuration. Dick et al. (2000) reported that SQ-22536 (0.001–1 μM) inhibited relaxations induced by isoprenaline (100 μM) in a concentration-dependent manner in isolated smooth muscle cells of guinea-pig gastric fundus. Koh et al. (2000) found that forskolin inhibited pacemaker currents, whereas 8-bromo-cAMP did not affect the generation of pacemaker currents. These forskolin-induced effects were not blocked by either SQ-22536 or a protein kinase A inhibitor in cultured ICCs from small intestine. This suggests that these forskolin-induced effects are direct and that cAMP-dependent mechanisms are not involved in the regulation of pacemaker currents. We also found that neither SQ-22536 nor mPKAI blocked the noradrenaline-induced effects and 8-bromo-cAMP had no effect on pacemaker currents. These results demonstrate that noradrenaline-mediated inhibition of pacemaker currents is not mediated by cyclic AMP production. On the contrary, pacemaker currents in cultured intestinal ICCs are modulated by cyclic GMP (Koh et al., 2000). Sodium nitroprusside, a NO donor, inhibited pacemaker currents and its effects were blocked by ODQ (10 μM), a guanylate cyclase inhibitor. In addition, 8-bromo-cyclic GMP mimicked the effect of sodium nitroprusside, a NO donor. We also found that SNAP inhibited pacemaker currents and these effects were blocked by the pretreatment with ODQ. However, ODQ did not block the noradrenaline-mediated inhibition of pacemaker currents in this study. These results suggest that noradrenaline-induced inhibition of pacemaker currents is mediated through a cyclic nucleotide-independent pathway.
β-Adrenoceptor-mediated relaxation in various smooth muscles is associated with membrane hyperpolarization caused by an increase in K+ conductance and the attenuation of spontaneous activity, resulting in relaxation (Bülbring & Tomita, 1987). In the proximal colon of the guinea-pig, β-adrenoceptor agonists hyperpolarize the smooth muscle by opening Ca2+-dependent K+ channels (Watson et al., 1996). In addition, β-adrenoceptor agonists activate Ca2+-dependent K+ channels in airway smooth muscle by a cyclic AMP-independent pathway (Kume et al., 1994) and activate ATP-dependent K+ channels in uterine smooth muscle (Harmada et al., 1994). In rat aortic smooth muscle, iberitoxin and 4-aminopyridine inhibited relaxation by activating β-adrenoceptors (Satake et al., 1996). In this study, noradrenaline did not produce hyperpolarization of the membrane in the current-clamping mode. Furthermore, neither TEA, apamin, nor glibenclamide blocked the noradrenaline-mediated inhibition of pacemaker currents. Therefore, we do not think that K+ channels are involved in the noradrenaline-mediated inhibition of pacemaker currents in ICCs.
Another possible mechanism by which noradrenaline inhibits pacemaker currents is by altering the intracellular Ca2+ kinetics. The generation of pacemaker currents of cultured ICCs of murine small intestine is dependent on the periodic oscillation of intracellular Ca2+ levels. It is abolished by cyclopiazonic acid, a Ca2+ ATPase inhibitor in the endoplasmic reticulum, and by xestospogin C, an inhibitor of IP3-receptors in endoplasmic reticulum, which suggests that IP3-mediated Ca2+ release from endoplasmic reticulum is essential for the generation of pacemaker currents (Ward et al., 2000). In addition, isoprenaline decreased intracellular Ca2+ concentrations in airway smooth muscle (Yamaguchi et al., 1995), suggesting that β-adrenoceptor stimulation reduces the availability of intracellular free Ca2+ for contraction. Therefore, noradrenaline may be able to regulate intracellular Ca2+ concentrations. However, to elucidate which of the mechanisms, involved in intracellular Ca2+ regulation, have a critical role in the noradrenaline-mediated inhibition of pacemaker currents will require further experiments.
In conclusion, the results of this study show that the inhibition of pacemaker currents by noradrenaline in the ICCs of murine small intestine is mediated by the activation of G-proteins through β1-adrenoceptors. Neither adenylate cyclase, guanylate cyclase, nor K+ channels are involved in this inhibitory effect.
Acknowledgments
This work was supported by the Korea Ministry of Science of Information and Communication (IMT-2000-C3-C5) and the Ministry of Science and Technology, Korea, and KOSEF through the Research Center for Proteinous Materials and the Korean-Japan Basic Scientific Promotion Program.
Abbreviations
- BRL-37344
(R*,R*)-(±)-4-[2-[(2-93chlorophenyl)amino]propyl]phenoxy acetic acid sodium
- GDPβS
guanosine 5′-O-(2-thio)diphosphate
- ICCs
interstitial cells of Cajal
- mPKAI
myristoylated protein kinase A inhibitor
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- SNAP
(±)-S-nitroso-N-acetylpenicillamine
- xSQ-22536
9-(tetrahydro-2-furanyl)-9H-purine-6-amine
References
- AKIMOTO Y., HORINIUCHI T., TANAKA Y., KOIKE K. The beta2- and beta3-adrenoceptor-mediated relaxation induced by fenoterol in guinea pig taenia caecum. J. Smooth Muscle Res. 2002;38:145–151. doi: 10.1540/jsmr.38.145. [DOI] [PubMed] [Google Scholar]
- BAUER V. Distribution and types of adrenoceptors in the guinea-pig ileum: the action of α- and β-adrenoceptor agonists. Br. J. Pharmacol. 1981;72:201–210. doi: 10.1111/j.1476-5381.1981.tb09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER V. Inhibition of guinea-pig taenia-coli mediated by alpha-1-adrenoceptor, beta-2-adrenoceptor, and ATP-receptor activation. Gen. Physiol. Biophys. 1982;1:175–188. [Google Scholar]
- BÜLBRING E., TOMITA T. Catecholamine action on smooth muscle. Pharmacol. Rev. 1987;39:49–96. [PubMed] [Google Scholar]
- DEMOL P., RUOFF H.J., WEITHRAUCH T.R. Rational pharmacotherapy of gastrointestinal motility disorders. Eur. J. Pediatr. 1989;148:489–495. doi: 10.1007/BF00441540. [DOI] [PubMed] [Google Scholar]
- DICK J.M., VAN GELDRE L.A., TIMMERMANS J.P., LEFEBVRE R.A. Investigation of the interaction between nitrix oxide and vasoactive intestinal polypeptide in the guinea-pig gastric fundus. Br. J. Pharmacol. 2000;129:751–763. doi: 10.1038/sj.bjp.0703089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EK B.A., NAHORSKI S.R. Beta-adrenergic control of motility in the rat colon. II. Properties of beta1-and beta2-adrenoceptors identified with 125I-(−)pindolol binding. Gastroenterology. 1986;90:408–413. doi: 10.1016/0016-5085(86)90940-6. [DOI] [PubMed] [Google Scholar]
- GUAN X.M., AMEND A., STRADER C.D. Determination of structural domains for G protein coupling and ligand binding in β3-adrenergic receptor. Mol. Pharmacol. 1995;48:492–498. [PubMed] [Google Scholar]
- GRASSBY P.F., BROADLEY K.J. Characterization of β-adrenoceptors mediating relaxation of guinea-pig ileum. J. Pharm. Pharmacol. 1984;36:602–607. doi: 10.1111/j.2042-7158.1984.tb04906.x. [DOI] [PubMed] [Google Scholar]
- HARMADA Y., NAKAYA Y., HAMADA S., KAMADA M., AONO T. Activation of K+ channels by ritodrine hydrochloride in uterine smooth muscle cells from pregnant women. Eur. J. Pharmacol. 1994;288:45–51. doi: 10.1016/0922-4106(94)90008-6. [DOI] [PubMed] [Google Scholar]
- HUIZINGA J.D., THUNBERG L., KLUPPEL M., MALYSZ J., MIKKELSEN H.B., RUSMESSEN J.J. W/kit gene requried for interstitial cells of Cajal and intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- JUN J.Y., LEE S.J., KIM S.J., SUH J.H., SO I., HWANG S.I., KIM K.W. Effects of noradrenaline on the spontaneous contraction and ionic current in the antral circular muscle of guinea-pig stomach. Kor. J. Physiol. 1993;27:115–122. [Google Scholar]
- KOH S.D., KIM T.W., JUN J.Y., WARD S.M., SANDERS K.M. Regulation of pacemaker currents in interstitial cells of Cajal by cyclic nucleotides. J. Physiol. 2000;527:149–162. doi: 10.1111/j.1469-7793.2000.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., SANDERS K.M., WARD S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J. Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIKE K., HORINOUCHI T., TAKAYANAGI I. Possible mechanisms of β-adrenoceptor-mediated relaxation induced by noradrenaline in guinea-pig taenia caecum. Eur. J. Pharmacol. 1995;279:159–163. doi: 10.1016/0014-2999(95)00147-d. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea pig ileal muscle. J. Physiol. 1993;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME H., HALL I.P., WASHABAU R.J., TAKAGI K., KOTLIKOFF M.I. Adrenergic agonists regulate Kca channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS A.M., LUDUENA F.P., BUZZO H.J. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967;6:2241–2249. doi: 10.1016/0024-3205(67)90031-8. [DOI] [PubMed] [Google Scholar]
- MANARA L., CROCI T., LANDI M. β3-adrenoceptors and intestinal motility. Fund. Clin. Pharmacol. 1995;9:332–342. doi: 10.1111/j.1472-8206.1995.tb00507.x. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M. A case of interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- SATAKE N., SHIBATA M., SHIBATA S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by β1- and β2-adrenoceptor activation in rat aortic rings. Br. J. Pharmacol. 1996;9:332–342. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZURSZEWSKI J.H.Electrical basis for gastrointestinal motility Physiology of the Gastrointestinal Tract 1987New York: Raven Press; 383–422.2nd edn., ed. Johnson L. R. pp [Google Scholar]
- TSUGENO M., HUANG S.M., PANG Y.W., CHOWDHURY J.U., TOMITA T. Effects of phosphodiesterase inhibitors on electrical activity (slow waves) in the guinea-pig gastric muscle. J. Physiol. 1995;485:493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENKOVA K., KRIER J. Postjunctional α1- and β-adrenoceptor effects of noradrenaline on electrical slow waves and phasic contractions of cat colon circular muscle. Br. J. Pharmacol. 1995;116:3265–3273. doi: 10.1111/j.1476-5381.1995.tb15134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., BURNS A.J., TORIHASHI S., SANDERS K.M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and intestinal electrical rhythmicity in murine mutants. J. Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., ORDOG T., KOH S.D., BAKER A., JUN J.Y., AMBERG G., MONAGHAN K., SANDERS K.M. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J. Physiol. 2000;525:355–361. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M.J., BYWATER R.A.R., TAYLOR G.S., LANG R.J. Effects of nitric oxide (NO) and NO donors on the membrane conductance of circular smooth muscle cells of the guinea-pig proximal colon. Br. J. Pharmacol. 1996;118:1605–1614. doi: 10.1111/j.1476-5381.1996.tb15581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAGUCHI H., KAJITA J., MADISON J.M. Isoproterenol increases peripheral [Ca2+]i and decreases inner [Ca2+]i in single airway smooth muscle cells. Am. J. Physiol. 1995;268:C771–C779. doi: 10.1152/ajpcell.1995.268.3.C771. [DOI] [PubMed] [Google Scholar]