Abstract
Methylenetetrahydrofolate reductase (MTHFR) is a regulating enzyme in folate-dependant homocysteine remethylation, because it catalyses the reduction of 5,10 methylenetetrahydrofolate to 5-methyltetrahydrofolate (5-MTHF).
Subjects homozygous for the 677C → T mutation in the MTHFR enzyme suffer from an increased cardiovascular risk. It can be speculated that the direct administration of 5-MTHF instead of folic acid can facilitate the remethylation of homocysteine in methionine.
The aim of this study was to determine the pharmacokinetic properties of orally administered 6[R,S] 5-MTHF versus folic acid in cardiovascular patients with homozygosity for 677C → T MTHFR.
This is an open-controlled, two-way, two-period randomised crossover study. Patients received a single oral dose of either 5 mg folic acid or 5 mg 5-MTHF in each period. The concentrations of the 6[S] 5-MTHF and 6[R] 5-MTHF diastereoisomers were determined in venous blood samples.
All pharmacokinetic parameters demonstrate that the bioavailability of 5-MTHF is higher compared to folic acid. The peak concentration of both isomers following the administration of 6[R,S] 5-MTHF is almost seven times higher compared to folic acid, irrespective of the patient's genotype. However, at 1 week after the administration of a single dosage 6[R,S] 5-MTHF, we detected 6[R] 5-MTHF following the administration of folic acid, indicating storage of this isomer in the body.
Our results demonstrate that oral 5-MTHF has a different pharmacokinetic profile with a higher bioavailability compared to folic acid, irrespective of the patient's genotype. Detrimental effects of the storage of high levels of the non-natural isomer 6[R] 5-MTHF cannot be excluded.
Keywords: Homocysteine, methylenetetrahydrofolate reductase, folic acid, 5-methyltetrahydrofolate
Introduction
More than 80 cross-sectional, case–control and prospective cohort studies have been published upon the relationship between homocysteine and arterial vascular disease (Refsum et al., 1998). Despite overwhelming epidemiological evidence confirming that hyperhomocysteinemia is an independent risk factor for vascular disease, the exact causal relationship remains to be proven. Methylenetetrahydrofolate reductase (MTHFR) is a regulating enzyme in folate-dependant homocysteine remethylation, because it catalyses the reduction of 5,10 methylenetetrahydrofolate to 6[S] 5-methyltetrahydrofolate (5-MTHF), the natural circulating form of folate in plasma. 6[S]5-MTHF serves as methyl donor for the conversion of homocysteine to methionine. In 1995, Frosst et al., identified a common mutation in the MTHFR gene, a transition at nucleotide 677(C → T) converting an alanine to a valine. Patients who are homozygous for this variant have an elevated total plasma homocysteine (Kluijtmans et al., 1997). Recently, it was demonstrated convincingly that these subjects, 10–20% of the population, have an increased cardiovascular risk (Klerk et al., 2002). Many studies have demonstrated that elevated homocysteine can be lowered by the intake of folic acid in doses ranging from 400 μg to 10 mg day−1 (Clarke & Armitage, 2000). Recently, we demonstrated a beneficial effect of homocysteine-lowering therapy using long-term folate therapy, 5 mg, on coronary endothelial function in hyperhomocysteinemic patients, a surrogate end point for cardiovascular events (Willems et al., 2002). In addition, Doshi et al. (2002) demonstrated that 5-MTHF had a direct beneficial effect on endothelial function, independent of plasma homocysteine levels. These results suggest that high concentrations of the natural isomer of 5-MTHF in plasma may be beneficial in cardiovascular disease. Whether these effects are related to a reduced activity of the MTHFR enzyme or due to a better bioavailability of 5-MTHF is unclear. Orally administered folic acid needs to be reduced and converted to tetrahydrofolate before it can become metabolic active. In subjects homozygous for the MTHFR 677C → T mutation, it may be speculated that the direct administration of 5-MTHF instead of folic acid can facilitate the remethylation of homocysteine into methionine. Otherwise, it is possible that the efficacy of 5-MTHF is better independently of a reduced activity of the MTHFR enzyme. 5-MTHF is available as a mixture of 6[S] and 6[R] racemates. Although it is assumed that only 6[S] 5-MTHF is bioactive, the possible biological effects of 6[R] 5-MTHF are not clear. The aim of this study was to determine the pharmacokinetic properties of a single dose of orally administered 6[R,S] 5-MTHF, a commercially available racemic mixture of the two diastereoisomers of 5-MTHF, versus folic acid in cardiovascular patients with homozygosity for the MTHFR polymorphism (TT genotype), compared to patients with the wild-type genotype (CC).
Methods
Patient selection
This study is an open-controlled, two-way, two-period randomised crossover study with a 1-week run-in and a 1-week wash-out period. Patients with established coronary heart disease were screened by DNA analysis for the presence of the MTHFR 677C → T polymorphism. Genomic DNA was extracted from peripheral blood lymphocytes by a standard procedure, and mutation analysis was performed essentially as described by Frosst et al. (1995). All patients have given informed consent. In all, 12 patients with the TT genotype MTHFR and 12 patients with wild-type MTHFR enrolled in the study.
The groups were matched for age, sex and body weight. Inclusion of patients required a body weight within 20% of normal values according to the Metropolitan Height and Weight Tables. No clinically important abnormal physical findings were tolerated. In all subjects enrolled in the study at baseline and after 4 weeks, blood samples were analysed for liver and renal function, haematology, urinalysis and vitamins. Patients with a myocardial infarction in the past 3 months were excluded, as well as patients with relevant history of liver disease, renal disease and gastrointestinal disease. Medication involved in homocysteine metabolism, the use of vitamins or blood donation in the past 3 months were not allowed. Users of significant amounts of tobacco (10 day−1), alcohol (>40 g day−1) or drugs were excluded.
Patients did not take food or drinks, apart from water, 12 h before administration of the folic acid or 5-MTHF and up to 4 h after the administration of the medication. In the period of the study, patients followed a stable diet which guaranteed a stable intake of 200 μg folate a day (the recommended daily allowance). The study medication consisted of a single oral dose of folic acid (folinaR), 5 mg or 6 [R,S] 5-MTHF (PrefolicR), 5 mg.
Study schedule
Each patient underwent a prestudy assessment within 2 weeks before the run-in period. During the first study period, the patients received a single oral dose of either 5 mg folic acid or 5 mg 6 [R,S] 5-MTHF, dissolved in 50 ml distilled water according to the randomisation list. Patients were blinded for treatment. A wash-out period of 1 week followed, after which the patients crossed over to the other treatment schedule. Administration of the folic acid or 5-MTHF to the fasting patients was performed between 0700 and 0900.
Blood samples
Venous blood samples were taken at the following times: 0 (predose), 0.5,. 1, 2, 3, 4, 5, 6, 8, 10 and 12 h following the administration of either folic acid or 6[R,S] 5-MTHF. The blood samples were collected in heparinized tubes and put on ice immediately and within 30 min centrifuged at 3000 × g for 15 min at 4°C. The supernatant plasma was transferred to a polypropylene tube containing sodium ascorbate and stored at −20°C.
Analytics
The plasma concentrations of the [6R] and [6S] 5-MTHF diastereoisomers were determined by a validated stereoselective method. After an ion-exchange step of purification from the biological matrix, 5-MTHF diastereoisomers are resolved by a stereospecific enzymatic reaction, using 5-MTHFR. At the end of the reaction, aliquots of the samples were analysed by reverse-phase high-performance liquid chromatography (HPLC) with fluorescence detection. With this method, the concentrations in plasma of the diastereoisomers in the low baseline range (3–4 ng ml−1) are determined with an interassay coefficient of variation of 10%. The limit of quantification for 6[R] 5-MTHF is kept at 5 ng ml−1 (Leeming et al., 1990). The following parameters were calculated from the plasma concentrations of [6R] and [6S] 5-MTHF acid stereoisomers obtained up to 12 h after administration of single-dose folic acid or 5-MTHF: Cmax (ng ml−1) (the highest concentration of the diastereoisomer in plasma), tmax (h) (the time when Cmax is achieved), C0–12z (ng ml−1) (the concentration of the diastereoisomer at sampling time z), AUC0–12z (the area under the curve of the plasma concentration versus time up to sampling time z), AUC∞ (the area under the curve of the plasma concentration versus time using the equation: AUC∞=AUCz+Cz λz−1) and t1/2 (h). Plasma homocysteine levels were determined at baseline, 2, 4 and 12 h after taking the vitamins, using HPLC as described by te Poele-Pothoff et al. (1995).
Statistics
Baseline characteristics are summarised by appropriate descriptive statistics. Pharmacokinetic parameters are analysed using variance analysis considering the following factors in the model: treatment period, category and sequence. The treatment effect and the treatment category interaction are tested at a 0.05 level. A log transformation is carried out on AUCz, AUC∞ and Cmax before performing ANOVA. For presentation, the point estimates (mean, s.d. and 95% confidence intervals) are converted back in the original scale.
Results
In 157 patients screened for the TT MTHFR mutation, we found 14 patients who were homozygous for this mutation. Of 27 patients initially included, 24 patients completed the study. Three patients were excluded from the study due to a protocol violation, two patients with a TT genotype and one patient with the CC genotype. Baseline characteristics of the participating patients are listed in Table 1. Mean fasting total plasma homocysteine levels (tHcy) in the patients of TT genotype was 26.8 μmol l−1 (s.d. 18.2) compared to 15.4 μmol l−1 (s.d. 3.5) in the patients with CC genotype (P<0.05). Total plasma folate was 13.5 nmol l (s.d. 5.7) in the group with TT polymorphism compared to 19.1 nmol l−1 (s.d. 17.8) in the group with CC polymorphism (P=0.35). No other significant differences were found between the two groups.
Table 1.
Baseline characteristics
| N=10(TT) | N=14(CC) | P-valuea | |
|---|---|---|---|
| Age (years) | 56.9 (range 46–64) | 57.2 (range 46–66) | 0.91 |
| Sex (male/female) | 8/2 | 11/3 | 0.55 |
| Homocysteine (μmol l−1) | 26.8 (18.1)b | 15.4 (3.5) | <0.05 |
| Folate (nmol l−1) | 13.5 (5.7) | 19.1 (17.8) | 0.35 |
| Vitamin B12 (pmol l−1) | 225 (112) | 230 (88) | 0.89 |
| Vitamin B6 (nmol l−1) | 49 (17) | 44 (12) | 0.47 |
| Total cholesterol (mmol l−1) | 5.69 (1.41) | 5.16 (0.76) | 0.25 |
| Triglycerides (mmol l−1) | 2.05 (1.1) | 2.11 (1.0) | 0.89 |
| Glucose (mmol l−1) | 5.7 (1.8) | 7.2 (2.4) | 0.11 |
| Creatinine (μmol l−1) | 101 (11.6) | 101 (39.0) | 0.99 |
Student's t-test for equality of means or χ2 test where appropriate.
s.d. within parentheses.
At 1 week after the completion of the study, plasma folate levels were increased from 13.5 to 24.5 nmol l−1 in the group with TT polymorphism compared to no change in the group with the CC polymorphism (P<0.05). Vitamin B12 and vitamin B6 did not change during follow-up. The administration of the single dosage of 5 mg folic acid or 5 mg 5-MTHF did not influence plasma homocysteine levels during the follow-up of 12 h irrespective of the patient's genotype. In both groups, homocysteine levels did not change at follow-up after 1 week.
Pharmacokinetic properties of 6[R,S] 5-MTHF versus folic acid
The main pharmacokinetic parameters following both treatment strategies for the 6[S] 5-MTHF and 6[R] 5-MTHF diastereoisomers are listed in Table 2. All pharmacokinetic parameters demonstrate that the bioavailability of 6[R,S] 5-MTHF acid is higher compared to folic acid. The peak concentration of the natural diastereoisomer 6[S] 5-MTHF following the administration of 5-MTHF is more than seven times higher compared to the peak concentration of 6[S] 5-MTHF following the administration of folic acid, 129 ng ml−1 (s.d. 42.4) versus 14.1 ng ml−1 (s.d. 9.4) (P<0.001), respectively. The peak concentration of the diastereoisomer 6[R] 5-MTHF following the administration of 5-MTHF compared to folic acid is 144 ng ml−1 (s.d. 75) versus 42 ng ml−1 (s.d. 40) (P<0.001), respectively.
Table 2.
Mean pharmacokinetic parameters for 6[S] 5-MTHF and 6[R] 5-MTHF after administration of either folk acid or 5-MTHF
| Folic acid | 6[S] 5-MTHF 5-MTHF | P-valuea | Folic acid | 6[R] 5-MTHF 5-MTHF | P-value | |
|---|---|---|---|---|---|---|
| Ke | 0.15 (0.1)b | 0.42 (0.2) | <0.001 | 0.14 (0.04) | 0.13 (0.04) | 0.66 |
| T1/2 (h) | 4.9 (2.5) | 2.7 (2.6) | <0.01 | 5.2 (1.2) | 6.0 (2.6) | <0.05 |
| Tmax (h) | 2.3 (0.8) | 1.3 (0.5) | <0.001 | 2.4 (0.7) | 1.6 (0.5) | <0.001 |
| Cmax (ng ml−1) | 14 (11) | 129 (33) | <0.001 | 42 (10) | 145 (47) | <0.001 |
| AUC 0–12 | 73 (39) | 383 (113) | <0.001 | 523 (180) | 700 (223) | <0.05 |
| AUC 0–∞ | 96 (48) | 405 (117) | <0.001 | 701 (284) | 924 (378) | <0.05 |
ANOVA one-way analysis.
s.d. within parentheses.
Influence of MTHFR 677C → T genotype on the pharmacokinetic properties of 6[R,S] 5-MTHF
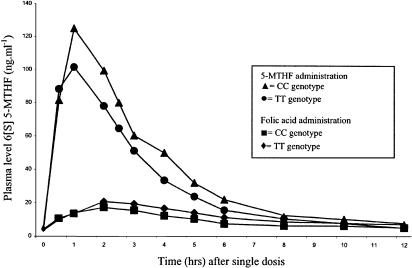
The main pharmacokinetic parameters of the biological active 6[S] 5-MTHF diastereoisomer for both treatment strategies in relationship with the patients' genotype are listed in Table 3. No significant differences in pharmacokinetic parameters exist between patients with the TT genotype and patients with CC genotype. Figure 1 shows the 12 h curve follow-up of 6[S] 5-MTHF in ng ml−1 after oral administration of folic acid and 5-MTHF in both genotype groups. Data on the 12 h curve follow-up of 6[R] 5-MTHF are essentially the same (data not shown).
Table 3.
Pharmacokinetic parameters for 6[S] 5-MTHF after administration of either folic acid or 5-MTHF in patients with (TT) and (CC) genotype
| Genotype | TT | Folic acid CC | P-valuea | TT | 5-MTHF CC | P-value |
|---|---|---|---|---|---|---|
| Ke | 0.15 (0.1)b | 0.17 (0.1) | 0.56 | 0.32 (0.1) | 0.35 (0.2) | 0.66 |
| T1/2 (h) | 5.0 (2.0) | 4.8 (2.9) | 0.83 | 2.36 (0.7) | 2.82 (3.5) | 0.69 |
| Tmax (h) | 2.4 (0.8) | 2.3 (0.8) | 0.66 | 1.3 (0.5) | 1.2 (0.5) | 0.88 |
| Cmax (ng ml−1) | 14 (11) | 14 (7) | 0.83 | 101 (29) | 107 (36) | 0.66 |
| AUC 0–12 | 72 (39) | 74 (41) | 0.90 | 303 (105) | 329 (121) | 0.58 |
| AUC 0–∞ | 99 (37) | 94 (55) | 0.82 | 312 (111) | 340 (123) | 0.35 |
ANOVA one-way analysis.
s.d. within parentheses.
Figure 1.
Genotype and treatment: 6[S] 5-MTHF plasma concentration (ng ml−1) in patients with MTHFR CC genotype or TT genotype following the administration of 6[R,S] 5-MTHF. 6[S] 5-MTHF plasma concentration (ng ml−1) in patients with MTHFR CC genotype or TT genotype following the administration of folic acid.
Pharmacokinetic properties of 6[R,S] 5-MTHF in relationship with the treatment sequence
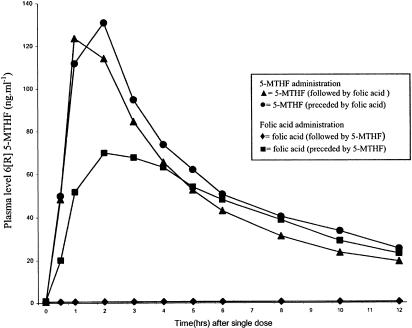
The sequence of treatment with folic acid or 5-MTHF (either folic acid or 6[R,S] 5-MTHF as the first drug) did not influence the results of the pharmacokinetic parameters for the 6[S] 5-MTHF diastereoisomer (data not shown). The washout curve for the 6[R] 5-MTHF diastereoisomer showed that in the case that folic acid was given in the first period the metabolic inactive 6[R] 5-MTHF diastereoisomer was not present in the plasma (detection limit <5 ng ml−1). However, if folic acid was given in the second period, levels of 6[R] 5-MTHF became clearly detectable in plasma. This was irrespective of the patients genotype (Figure 2).
Figure 2.
Sequence of treatment: 6[R] 5-MTHF plasma concentration (ng ml−1) in patients after folic acid intake in the first period and 6[R] 5-MTHF plasma concentration (ng ml−1) in patients after folic acid intake in the second period 6[R] 5-MTHF plasma concentration (ng ml−1) in patients after 6[R,S] 5-MTHF intake in the first period and patients after 6[R,S] 5-MTHF intake in the second period.
Discussion and conclusions
This study demonstrated a higher bioavailability of orally administered racemic 6[R,S] 5-MTHF, 5 mg compared with folic acid, 5 mg. The effects were not influenced by the MTHFR 677TT genotype of the patient. Plasma homocysteine levels did not alter following the administration of a single dosage of either folic acid or 6[R,S] 5-MTHF acid.
Pharmacokinetic properties of folic acid and 6[R,S] 5-MTHF
In our study, a prompt rise of 6[S] 5-MTHF, the natural isomer possessing biological activity (Keresztesy & Silverman, 1951), within 1–3 h following the administration of folic acid or 6[R,S] 5-MTHF was shown with peak concentrations which were seven-fold or more higher than baseline concentrations. Our data, with respect to the pharmacokinetic profile, are consistent with a previous study evaluating the effect of oral administration of folic acid in patients with the TT polymorphism compared to patients with the CC polymorphism where comparable rises of the 6[S] 5-MTHF diastereoisomer have been seen (Stern et al., 2000). It is to be expected that 5-MTHF levels are proportionally lower following the administration of folic acid compared to 6[R,S] 5-MTHF, because 6[R,S] 5-MTHF is directly available following absorption in the intestinal cell, while folic acid needs to be metabolised first into tetrahydrofolate. Depending upon folate supply, tetrahydrofolate can be transported into the portal circulation, converted to 6[S] 5-MTHF, or stored in the tissue as a tetrahydrofolate. This prolonged biochemical pathway can be a possible explanation for the difference in the pharmacokinetic parameters. Some studies used 5-MTHF as pharmacological agent to improve endothelial function is vascular patients (Doshi et al., 2002). It can be speculated that these high levels of circulating metabolic active folates may be responsible for the observed direct improvement of endothelial function.
Folates in relation to genotype
Our data demonstrate that there are no differences with respect to the pharmacokinetic properties of both folic acid and 6[R,S] 5-MTHF in relation to the patients' genotype. Mutations in MTHFR have been associated with a lower proportion of 5-MTHF and a higher proportion of formylated folates (Rosenblatt et al., 1979). This, because of a decreased MTHFR activity resulting in a lower rate of reduction of 5,10-methylene-THF to 5-MTHF, leading to increased availability of 5,10-methylene-THF for oxidation to the formylated folate forms. Our results suggest that the activity of MTHFR in vivo is not the rate-limiting step in the conversion of folic acid to 5-MTHF.
The sequence of therapy
The sequence of administration of either folic acid or 6[R,S]5-MTHF did not influence pharmacokinetic parameters with respect to the plasma level of the physiologically active form 6[S] 5-MTHF. Since oral applied 5-MTHF consists of a racemic mixture of 6[S] 5-MTHF and 6[R] 5-MTHF, it can be explained that the 6[R] 5-MTHF isomer can be detected in the plasma. Compared to plasma levels of 6[S] 5-MTHF, plasma levels of 6[R] 5-MTHF reach higher peak concentrations and still have a higher plasma concentration 12 h after the intake. These findings suggest that this isomer has a slower clearance from the plasma, possibly due to the non-natural form of 5-MTHF. Our findings are consistent with a previous study investigating the effects of high-dosage 6[R,S] 5-MTHF demonstrating a higher plasma concentration and slower plasma clearance of 6[R] 5-MTHF compared to 6[S] 5-MTHF (Mader et al., 1995). In that study, it was demonstrated that protein binding of 6[R] 5-MTHF is 88% compared to 56% of the 6[S] 5-MTHF with a decreased renal clearance as a consequence.
If folic acid was given as the first drug, 6[R] 5-MTHF could not be detected in the plasma. This is to be expected since folic acid will only be metabolised into its natural active isomer. However, when folic acid was given in the second period (1 week after the administration of the racemic 6[R,S] 5-MTHF), 6[R] 5-MTHF could be detected in the plasma, even at relative high levels (Figure 2). A previous study revealed that the clearance rate of 6[R] 5-MTHF is about four times slower than that for the 6[S] 5-MTHF (Mader et al., 1994). However, this cannot explain the fact that 1 week after a single bolus 6[R,S] 5-MTHF, we found relatively high levels of 6[R] 5-MTHF only after we supplied folic acid. The 6[R] 5-MTHF isomer was not detectable at baseline, thus before the administration of folic acid. It seems therefore less likely that the difference in plasma protein binding between 6[S] 5-MTHF and 6[R] 5-MTHF explains this phenomenon since the 6[R] 5-MTHF isomer was not detectable in plasma at baseline. Our results suggest that the 6[R] 5-MTHF isomer is stored in the body until it is released following the administration of a relatively large dosage of folic acid. Folates are stored in tissues, mainly in the liver where folates are bound tight by cytosolic and mitochondrial folate-binding proteins. This binding may not be stereospecific. The biological effects of 6[R] 5-MTHF binding are unclear. Mader et al. (1995) reported that no serious short-term side-effects were seen in patients receiving high doses of 6[R,S] 5-MTHF. Since 6[R] 5-MTHF is not metabolised, it can be speculated that it may inhibit regulatory enzymes related to folate and homocysteine metabolism. Secondly, the bioavailabity of 6[S] 5-MTHF may be reduced due to competition with the 6[R] 5-MTHF diastereoisomer.
Genotype of the patient and homocysteine levels
Several investigators have demonstrated that subjects who are homozygous for the TT polymorphism have an elevated tHcY only when plasma folate is low (Kang et al., 1987; Harmon et al., 1996; Christensen et al., 1997; Kluijtmans et al., 1997). Plasma folate levels in the group of patients with TT polymorphism were relatively low compared to folate levels in the CC group. Obviously, the administration of a single dosage of folic acid or 6[R,S] 5-MTHF did not alter homocysteine levels during the study, suggesting that long-term treatment is mandatory.
Conclusion
Our results demonstrate that 6[R,S] 5-MTHF has a different pharmacokinetic profile compared to folic acid, irrespective of the patients' MTHFR 677C → T genotype. Although the clinical significance of these differences require further investigation, the prompt seven-fold rise in the concentration of the natural isomer may be a promising feature of 5-MTHF in the treatment of vascular disease. The clinical consequences of relatively high plasma levels of the non-natural isomer, 6[R] 5-MTHF, following the administration of 6[R,S] 5-MTHF, are unknown. Since our study suggests that this isomer is stored in the body, further investigations of the clinical long-term effects of the racemic 6[R,S] 5-MTHF acid are necessary to evaluate possible detrimental effects.
Acknowledgments
Dr HJ Blom is an established investigator of the Netherlands Heart Foundation (D 97.021). This study was supported by an unrestricted grant from Knoll Pharmaceutici Spa, Italy.
Abbreviations
- AUC0–12z
The area under the curve of the plasma concentration versus time up to sampling time z
- AUC∞
the area under the curve of the plasma concentration versus time using the equation: AUC∞=AUCz+Czλz−1
- Cmax
the highest concentration in plasma
- C0-12z
the concentration at sampling time z
- 5-MTHF
5-methyltetrahydrofolate
- MTHFR
methylenetetrahydrofolate reductase
- tHcy
total plasma homocysteine
References
- CHRISTENSEN B., FROSST P., LUSSIER-CACAN S., SELHUB J., GOYETTE P., ROSENBLATT D.S., GENEST J., ROZEN R. Correlation of a common mutation in the methylenetetrahydrofolate reductase gene with plasma homocysteine in patients with premature coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1997;17:569–573. doi: 10.1161/01.atv.17.3.569. [DOI] [PubMed] [Google Scholar]
- CLARKE R., ARMITAGE J. Vitamin supplements and cardiovascular risk: review of the randomized trials of homocysteine lowering vitamin supplements. Semin. Thromb. Hemost. 2000;26:341–348. [Google Scholar]
- DOSHI S.N., MCDOWELL I.F., MOAT S.J., PAYNE N., DURRANT H.J., LEWIS M.J., GOODFELLOW J. Folic acid improves endothelial function in coronary artery disease via mechanisms largely independent of homocysteine lowering. Circulation. 2002;105:22–26. doi: 10.1161/hc0102.101388. [DOI] [PubMed] [Google Scholar]
- FROSST P., BLOM H.J., MILOS R., GOYETTE P., SHEPPARD C.A., MATTHEWS R.G., BOERS G.H.J., DEN HEIJER M., KLUIJTMANS L.A.J., VAN DEN HEUVEL L.P.W.J., ROZEN R. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- HARMON D.L., WOODSIDE J.V., YARNELL J.W.G., MCMASTER D., YOUNG I.S., MCCRUM E.E., GEY K.F., WHITEHEAD A.S., EVANS A.E. The common ‘thermolabile' variant of methylenetetrahydrofolate reductase is a major determinant of mild hyperhomocysteinemia. Q. J. Med. 1996;89:571–577. doi: 10.1093/qjmed/89.8.571. [DOI] [PubMed] [Google Scholar]
- KANG S.S., WONG P.W.K., NORUSIS M. Homocysteinemia due to folate deficiency. Metabolism. 1987;36:458–462. doi: 10.1016/0026-0495(87)90043-6. [DOI] [PubMed] [Google Scholar]
- KERESZTESY J.C., SILVERMAN M. Crystalline citrovorum factor from liver. J. Am. Chem. Soc. 1951;73:5510. [Google Scholar]
- KLERK M., VERHOEF P., CLARKE R., BLOM H.J., KOK F.J., SCHOUTEN E.G. MTHFR 677C → T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002;288:2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- KLUIJTMANS L.A.J., KASTELEIN J.P., LINDEMANS J., BOERS G.H.J., HEIL S.G., BRUSCHKE A.V.G., JUKEMA J.W., VAN DEN HEUVEL L.P.W.J., TRIJBELS F.J.M., BOERMA G.J.M., VERHEUGT F.W.A., WILLEMS F.F., BLOM H.J. Thermolabile methylene teterahydrofolate reductase in coronary artery disease. Circulation. 1997;96:2573–2577. doi: 10.1161/01.cir.96.8.2573. [DOI] [PubMed] [Google Scholar]
- LEEMING R.J., POLLOCK A., MELVILLE L.J., HAMON C.G. Measurement of 5-methyltetrahydrofolic acid in man by high-performance liquid chromatography. Metabolism. 1990;39:902–904. doi: 10.1016/0026-0495(90)90298-q. [DOI] [PubMed] [Google Scholar]
- MADER R.M., STEGER G.G., RIZOVSKI B., DJAVANMARD M.P., SCHEITHAUER W., JAKESZ R., RAINER H. Stereospeciflc pharmacokinetics of rac-5-methyltetrahydrofolic acid in patients with advanced colorectal cancer. Br. J. Clin. Pharmacol. 1995;40:209–215. [PMC free article] [PubMed] [Google Scholar]
- MADER R.M., STEGER G.G., RIZOVSKI B., JAKESZ R., RAINER H. Different stereospecific protein binding of tetrahydrofolates to human serum albumin. J. Pharm. Sci. 1994;83:1247–1249. doi: 10.1002/jps.2600830912. [DOI] [PubMed] [Google Scholar]
- TE POELE-POTHOFF M.T., VAN DEN BERG M., FRANKEN D.G., BOERS G.H., JAKOBS C., DE KROON I.F., ESKES T.K., TRIJBELS J.M., BLOM H.J. Three different methods for the determination of total homocysteine in plasma. Ann. Clin. Biochem. 1995;32:218–220. doi: 10.1177/000456329503200218. [DOI] [PubMed] [Google Scholar]
- REFSUM H., UELAND P.M., NYGÅRD O., VOLLSET S.E. Homocysteine and cardiovascular disease. Annu. Rev. Med. 1998;49:31–62. doi: 10.1146/annurev.med.49.1.31. [DOI] [PubMed] [Google Scholar]
- ROSENBLATT D.S., COOPER B.A., LUE-SHING S., WONG P.W.K., BERLOW S., NARISAWA K., BAUMGARTNER R.J. Folate distribution in cultured human cells. Studies on 5,10-CH2-H4 PteGlu reductase deficiency. Clin. Invest. 1979;63:1019–1025. doi: 10.1172/JCI109370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN L.L., BAGLEY P.J., ROSENBERG I.H., SELHUB J. Conversion of 5-formyltetrahydrofolic acid to 5-methyltetrahydrofolic acid is unimpaired in folate adequate persons homozygous for the C677 T mutation in the methylenetetrahydrofolate reductase gene. J. Nutr. 2000;130:2238–2242. doi: 10.1093/jn/130.9.2238. [DOI] [PubMed] [Google Scholar]
- WILLEMS F.F., AENGEVAEREN W.R.M., BOERS G.H.J., BLOM H.J., VERHEUGT F.W.A. Coronary endothelial function in hyperhomocysteinemia: improvement after treatment with folic acid and cobalamin in patients with coronary artery disease. J. Am. Coll. Cardiol. 2002;40:766–772. doi: 10.1016/s0735-1097(02)02016-8. [DOI] [PubMed] [Google Scholar]