Abstract
The vanilloid receptor (TRPV1) is viewed as a molecular integrator of several nociceptive stimuli. In the present study, we have investigated the role played by TRPV1 in the nociceptive response induced by the peripheral activation of kinin B2 receptor in mice.
The intraplantar (i.pl.) administration of bradykinin (BK) and the selective B2 agonist Tyr8-BK, or the vanilloid agonists resiniferatoxin and capsaicin, into the mouse paw induced a dose-related overt nociception of short duration. The B2 receptor antagonist Hoe 140 inhibited BK-induced, but not capsaicin-induced, nociceptive response. On the other hand, the TRPV1 antagonist capsazepine inhibited both capsaicin- and BK-mediated nociception.
Repeated injections of BK or capsaicin produced desensitization to their nociceptive response. Capsaicin desensitization greatly reduced BK-induced nociception, but in contrast, the desensitization to BK increased the capsaicin response.
Administration of low doses of capsaicin or acidified saline did not produce nociception when administered alone, but caused a pronounced effect when administered in association with a subthreshold dose of BK. Moreover, the degeneration of the subset of primary afferent fibers, sensitive to capsaicin, abolished both capsaicin- and BK-induced nociception.
The inhibition of phospholipase C (PLC), protein kinase C or phospholipase A2 markedly decreased the nociception caused by BK, but not that of capsaicin. BK administration increased leukotriene B4 levels in the injected paw. Likewise, BK-induced overt nociception was decreased by lipoxygenase (LOX) inhibition.
These results demonstrate that BK produces overt nociception mediated by TRPV1 receptor stimulation, via PLC pathway activation and LOX product formation.
Keywords: B2 receptor, vanilloid receptor, nociception, bradykinin, capsaicin, mice
Introduction
Pain is initiated when noxious thermal, mechanical, or chemical stimuli excite the peripheral terminals of specialized primary afferent neurons (C and Aδ fibres) called ‘nociceptors'. Tissue damage associated with infection, inflammation, or ischemia produces an array of chemical mediators that activate or sensitize nociceptor terminals to elicit pain at the site of injury (Julius & Basbaum, 2001). Vanilloid receptor 1 (TRPV1, formerly called VR1) (Montell et al., 2002) is expressed by a subset of peripheral pain-sensing neurons and may be gated by several different painful stimuli, including protons, heat, lipid mediators (such as anandamide, N-arachidonoyl-dopamine or lipoxygenase (LOX) products), and vanilloid compounds (such as capsaicin, the pungent principle of chili peppers) (Caterina et al., 1997; Zygmunt et al., 1999; Hwang et al., 2000; Huang et al., 2002). When such neurons are exposed to tissue-damaging stimuli, TRPV1 receptors become permeable to Na+ and Ca2+ ions, causing in turn neuronal depolarization. Sensory neuron firing transmits these pain signals towards the central nervous system, evoking at the same time a variety of local tissue responses (Szallasi & Blumberg, 1999; Caterina & Julius, 2001; Piomelli, 2001).
The role of endogenous TRPV1 ligands in pain processes has been affirmed on the basis that inflammation-induced nociceptive responses may be inhibited by the TRPV1 antagonist capsazepine (Santos & Calixto, 1997; Kwak et al., 1998) or following TRPV1 gene deletion (Caterina et al., 2000; Davis et al., 2000). Such findings suggest that an endogenous capsaicin-like substance seems to be produced by peripheral tissues and acts during inflammation. These and other findings indicate that TRPV1 might act as a target for the signaling pathways stimulated by inflammatory mediators, such as prostaglandins, ATP, serotonin and bradykinin (BK).
BK produces primary sensory nerve terminal depolarization and sensitization to other noxious or even innocuous stimuli through the activation of kinin B2 receptors (Calixto et al., 2000; 2001). Interestingly, there are several similarities between BK- and capsaicin-induced responses. For example, the depolarization of sensory neurons caused by BK or capsaicin is highly correlated (Martin et al., 1987) and the thermal hyperalgesia produced by BK in wild-type mice is absent in mice lacking TRPV1 (Chuang et al., 2001). Therefore, in the present study, we sought to investigate the role played by TRPV1 receptors in the nociceptive response induced by peripheral activation of kinin B2 receptor in vivo.
Methods
Animals
Male Swiss mice weighing 30–35 g, maintained at 22±2°C with free access to water and food, under a 12 : 12 h light : dark cycle, were used. Animals were acclimatized to the laboratory for at least 2 h before testing and were used once throughout the experiments. All experiments were carried out in accordance with current guidelines for the care of laboratory animals and ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983). The number of animals and the nociceptive stimulus were the minimum necessary to demonstrate the consistent effects of drug treatments.
Algogen-induced overt nociception in mice
The procedure used was similar to that described previously (Sakurada et al., 1992; De Campos et al., 1999). Volumes of 20 μl of BK (0.3–60 nmol paw−1), Tyr8-BK (1–60 nmol paw−1), des-Arg9-BK (3–30 nmol paw−1), or the vanilloids resiniferatoxin (0.005–50 fmol paw−1) or capsaicin (0.03–5.2 nmol paw−1) were injected intraplantarly (i.pl.) under the surface of the right hind paw. Separate groups of animals received an i.pl. injection of the appropriated vehicle (phosphate-buffered saline (PBS) for peptides or PBS plus ethanol 0.5% for vanilloids). Other groups of animals received an i.pl. injection of lactate (0.01 M) acidified saline (0.9% of NaCl, pH 5.5). Animals were placed individually in chambers (transparent glass cylinders of 20 cm in diameter) and were adapted for 20 min before algogen or vehicle injection. After challenge, mice were observed individually for 10 min. The amount of time spent licking the injected paw was timed with a chronometer and was considered as indicative of nociception.
Effect of treatment with drugs
To assess the involvement of kinin receptors or TRPV1 in the algogen responses induced by BK or capsaicin, animals received the selective B1 kinin receptor antagonist des-Arg9-Leu8-BK (10 nmol paw−1), the B2 kinin receptor antagonist Hoe 140 (1–10 nmol paw−1), or the TRPV1 antagonist capsazepine (1–30 nmol paw−1). To test whether the PLC–PKC or PLA2 pathways were involved in BK- or capsaicin-induced licking, we evaluated the effect of the co-administration of PLC (U73122, 1 pmol paw−1, Bleasdale et al., 1990), PKC (GF 109203X; 1 nmol paw−1, Toullec et al., 1991), PLA2 (PACOCF3; 1 nmol paw−1, Ackermann et al., 1995), LOX (baicalein, 3 nmol paw−1, Sekiya & Okuda, 1982) or cyclooxygenase (ibuprofen, 100 nmol paw−1) inhibitors in association with BK or capsaicin. Control animals received a similar volume of vehicle (20 μl paw−1).
Desensitization protocol
Repeated administration of capsaicin or BK results in a marked desensitization of their responses on sensory neurons in vitro (Mizumura et al., 1987; Dray et al., 1989). To test this hypothesis in vivo, mice received two repeated injections of the vehicle (20 μl paw−1), BK (10 nmol paw−1), or capsaicin (5.2 nmol paw−1) with a 30 min of interval between the injections. At 30 min after the last administration, vehicle, BK, or capsaicin were injected again in the previously treated mice.
Neonatal capsaicin treatment
To further explore the role of capsaicin-sensitive fibers in the nociceptive effect induced by the algogen substances, neonatal mice (on day 2 of life) were anesthetized with ether and received capsaicin (50 mg kg−1, subcutaneously) or the vehicle alone (10% ethanol, 10% Tween-80 and 80% PBS), as described previously (Ferreira et al., 1999). Animals were used at 6–7 weeks after the administration of capsaicin or vehicle (used as control).
Skin temperature measurement
BK injection produces classical inflammatory signs, including local temperature increase. Since heat is one of the TRPV1 activators, the temperature of the BK-injected paw skin was also measured using a surface radiation thermometer (Pro Check, Italy).
Leukotriene B4 (LTB4) level measurement
Separated groups of mice were killed by cervical dislocation 5 min after BK, capsaicin or vehicle i.pl. injection. The injected paw was coaxially perfused as previously described (Rocha e Silva & Antonio, 1960). A double polyethylene tube was inserted into the subcutaneous space of the paw and 400 μl of PBS was perfused at a rate of 200 μl min−1 through the inner tube, after which the perfusate was collected through the outer tube. LTB4 was measured in the collected perfusate using an Enzyme Immuno Assay kit. The assays were carried out in accordance with the manufacturer's instructions (Amersham, U.K.).
Drugs
The following drugs were used: BK, Tyr8-BK, des-Arg9-BK, des-Arg9-Leu8-BK, resiniferatoxin, baicalein, lactic acid, PBS tablets (all from Sigma Chemical Company, St Louis, MO, U.S.A.), GF109203X, U73122, PACOCF3 (Tocris, Ellisville, MO, U.S.A.), capsaicin and capsazepine (RBI, Natick, MA, U.S.A.). Hoe 140 (Icatibant) was kindly provided by Aventis (Frankfurt am Main, Germany). The stock solutions of the drugs were prepared in PBS in siliconized plastic tubes, maintained at −20°C and diluted to the desired concentration just before use. Capsaicin, capsazepine, baicalein, GF109203X, U73122, and PACOCF3 stock solutions were prepared in absolute ethanol. For i.pl. drug administration, the final concentration of ethanol did not exceed 0.5% and did not cause any detectable effect per se.
Statistical analysis
The results are presented as mean±s.e.m., except the ED50 or ID50 values (i.e. the dose of agonist necessary to produce 50% of the pain response relative to the maximum effect, or the dose of antagonists necessary to reduce agonist response by 50% relative to the control value, respectively), which are reported as geometric means accompanied by their respective 95% confidence limits. The ED50 and ID50 values were calculated using at least three doses of each drug, between the minimum and the maximum effect, using linear regression for individual experiments with the GraphPad Prism software. In order to obtain data purely derived by the treatments in algogen-induced nociception, the maximal inhibition (MI) values were represented as the difference between the licking times of the vehicle-treated and algogen-treated animals. The statistical significance between the groups was assessed by means of one-way ANOVA followed by Dunnett's or Student–Newmann–Keuls' test, as appropriate. P-values of less than 0.05 were considered as indicative of significance.
Results
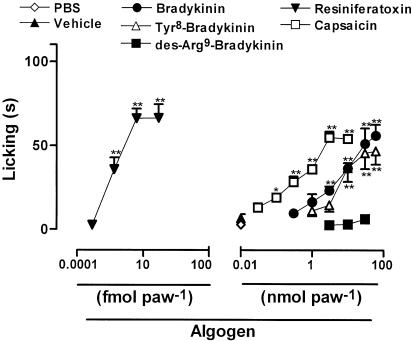
Intraplantar administration of BK or of the more selective B2 receptor agonist Tyr8-BK in mice (1–60 nmol paw−1) resulted in a dose-related overt nociceptive behavior (Figure 1). The nociception occurred quickly and did not last more than 10 min. The calculated mean ED50 values (and the 95% confidence limits) for BK- or Tyr8-BK-induced licking were 5.75 (4.85–6.82) and 7.03 (5.89–8.39) nmol paw−1, respectively. The maximal licking responses caused by BK- and Tyr8-BK were 53.9±6.5 and 41.5±8.0 s. However, the i.pl. administration of the selective B1 receptor agonist des-Arg9-BK (3–30 nmol paw−1) did not produce any detectable nociceptive behavior (Figure 1).
Figure 1.
Dose–response curves for the overt nociception caused by i.pl. injection of BK, Tyr8-BK, des-Arg9-BK, resiniferatoxin, or capsaicin in mice. The effects of the drugs are expressed as licking time (s). Each point on the curve represents the mean of 4–6 animals and vertical lines show the s.e.m. Asterisks denote the significance levels in comparison with control groups (PBS for kinins or vehicle for vanilloids; one-way ANOVA followed by Dunnett's test): *P<0.05, **P<0.01.
Similar to BK, i.pl. administration of the vanilloid agonists capsaicin (0.03–5.2 nmol paw−1) or resiniferatoxin (0.005–50 fmol paw−1) resulted in a quick and dose-related nociceptive behavior (Figure 1). The calculated mean ED50 values (and the 95% confidence limits) for these effects were 0.61 (0.32–1.14) nmol paw−1 and 0.0175 (0.0058–0.0295) fmol paw−1, and the maximal licking times were 50.3±3.6 and 63.2±8.4 s for capsaicin and resiniferatoxin, respectively. The ultrapotent TRPV1 agonist resiniferatoxin was about 300 million-fold more potent than capsaicin in the induction of nociception according to the analysis at the ED50 level. Comparing kinin- and vanilloid-induced nociceptive responses, capsaicin was about nine-fold more potent than BK in inducing nociception, although BK was as efficacious as capsaicin in the induction of licking response.
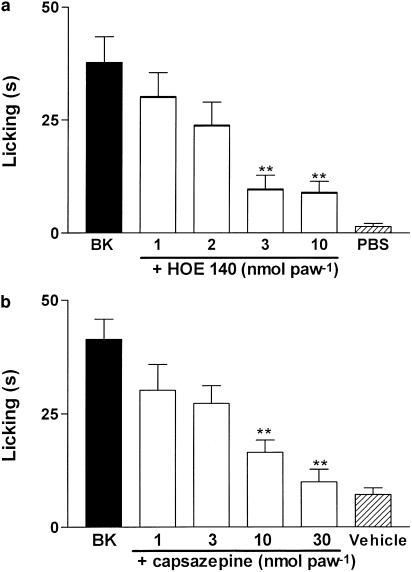
The i.pl. coadministration of the selective B2 receptor antagonist Hoe 140 (1–10 nmol paw−1) produced a dose-related inhibition of BK-induced nociception (10 nmol paw−1, Figure 2a). The calculated mean ID50 value for this effect (and its respective 95 % confidence limit) was 2.1 (2.0–2.2) nmol paw−1 and the maximal inhibition was 88±13%. On the other hand, the selective B1 receptor antagonist des-Arg9-Leu8-BK (25 nmol paw−1) completely failed to inhibit BK-induced nociception (results not shown), demonstrating that B2 receptor activation mediates BK effect. Moreover, Hoe 140 (10 nmol paw−1) was not able to affect the nociception produced by capsaicin (1 nmol paw−1, results not shown). As previously described (Santos & Calixto, 1997), the i.pl. co-administration of the selective TRPV1 antagonist capsazepine (1 nmol paw−1) completely blocked capsaicin-induced nociception (results not shown). Interestingly, the i.pl. co-administration of capsazepine (1–30 nmol paw−1) produced a dose-related and almost complete inhibition of BK-induced nociception (Figure 2b). The calculated mean ID50 value for this effect (and its respective 95% confidence limit) was 6.45 (5.09–7.81) nmol paw−1 and the maximal inhibition was 91.7±1.3%. These data suggest that the activation of TRPV1s is an important mechanism underlying BK-induced nociception in the mouse paw.
Figure 2.
Effect of i.pl. treatment with the selective B2 receptor antagonist Hoe 140 (a) or with the TRPV1 antagonist capsazepine (b) on BK-induced nociception. Each column represents the mean±s.e.m. of 4–6 mice. The asterisks denote the significance levels. **P<0.01, compared with BK-treated mice (one-way ANOVA followed by Dunnett's test).
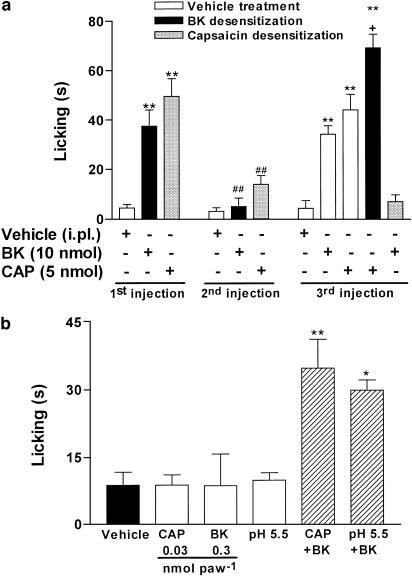
The results obtained with antagonists were confirmed using in vivo desensitization procedures. An initial i.pl. injection of BK (10 nmol paw−1) or capsaicin (5.2 nmol paw−1) produced a marked nociceptive response, as described above. However, a great reduction of the nociception was observed after a second challenge with both substances (Figure 3a). Capsaicin produced desensitization only when used at the highest dose (5 nmol paw−1), but not at a submaximal dose (1 nmol paw−1) (results not shown). Importantly, the desensitization of the paw injected with capsaicin completely abrogated the BK-induced nociceptive response. In contrast, desensitization to BK significantly increased capsaicin-induced nociceptive response (Figure 3a). These results suggest again that BK stimulates TRPV1 to produce overt nociception.
Figure 3.
BK-induced overt nociception is dependent on TRPV1 activation. (a) Desensitization to the nociceptive effect caused by the i.pl. injection of capsaicin (CPS, 5.2 nmol paw−1) or BK (10 nmol paw−1). (b) Nociceptive effect caused by the coinjection of low doses of BK (0.3 nmol paw−1) with capsaicin (CAP, 0.03 nmol paw−1) or acidified saline (pH 5.5). Each column represents the mean±s.e.m. of 4–6 mice. The asterisks denote the significance levels. (a) **P<0.01, compared with the first PBS injected mice; ##P<0.01, compared with the first BK or CPS injected mice; +P<0.01, compared with the third PBS injected mice (one-way ANOVA followed by Student–Newmann–Keuls' test). (b) *P<0.05; **P<0.01, compared with the vehicle injected mice (one-way ANOVA followed by Dunnett's test).
The results in Figure 3b suggest that BK sensitizes TRPV1 to agonist activation, as the i.pl. injection of low doses of BK (0.3 nmol paw−1), capsaicin (0.03 nmol paw−1), or acidified saline (pH 5.5) did not produce nociception when compared with the vehicle-treated animals. However, capsaicin or acidified saline administration in conjunction with a low dose of BK resulted in a significant nociceptive response (Figure 3b). Moreover, i.pl. injection of BK significantly increased the paw skin temperature (from a baseline of 27.5±0.3 to 28.1±0.2 and 28.7±0.4°C, 5 and 10 min after injection, respectively, P<0.05). Intraplantar injection of PBS did not alter the paw skin temperature (results not shown).
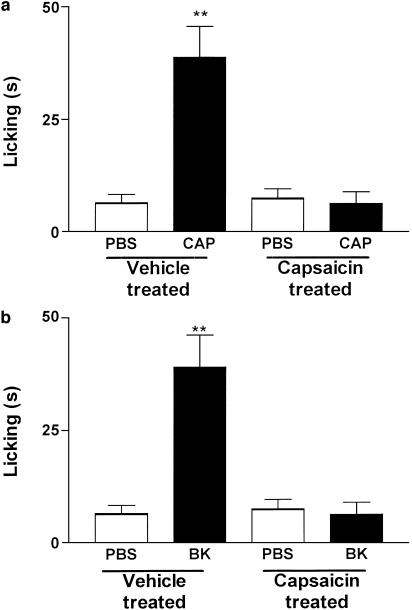
To investigate the role played by capsaicin-sensitive fibers in BK-mediated nociception, the animals were treated during the neonatal period with capsaicin (50 mg kg−1, subcutaneously), a method known to produce degeneration of the thin primary afferent fibers (Holzer, 1991) and to decrease the expression of TRPV1 at the dorsal root ganglion (Rashid et al., 2003). Present results demonstrate that the neonatal treatment with capsaicin abolished both capsaicin- and BK-induced nociception (Figure 4a and b).
Figure 4.
Disruption of the nociceptive effect caused by the i.pl. injection of (a) capsaicin (CPS, 1 nmol paw−1) or (b) BK (10 nmol paw−1) produced by treatment of neonate mice with capsaicin (50 mg kg−1, s.c.). Each column represents the mean±s.e.m. of 4–6 mice. The asterisks denote the significance levels. **P<0.01, compared with PBS injected mice (one-way ANOVA followed by Dunnett's test).
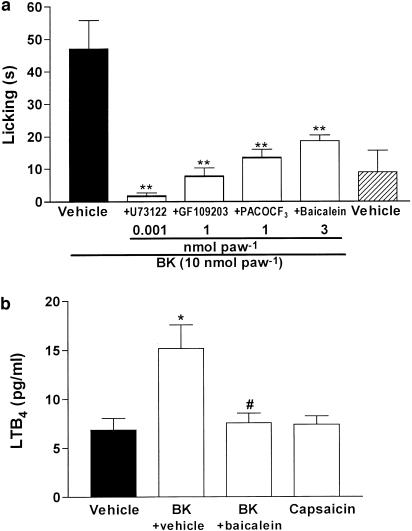
We next investigated some of the possible pathways involved in the B2 receptor-mediated activation of TRPV1. The i.pl. coadministration of the selective inhibitors of PLC U73122 (1 pmol paw−1), PLA2 PACOCF3 (0.1 nmol paw−1), or the PKC inhibitor GF 109203X (1 nmol paw−1) significantly reduced BK-induced nociception (Figure 5a). In marked contrast, these inhibitors failed to affect capsaicin-induced nociception (results not shown).
Figure 5.
Mechanisms involved in the BK-induced nociception. (a) Effect of i.pl. treatment with selective inhibitors of phospholipase C (PLC) U73122 (1 pmol paw−1), protein kinase C (PKC) GF109203X (1 nmol paw−1), PLA2 PACOCF3 (0.1 nmol paw−1) or LOX baicalein (3 nmol paw−1) on BK-induced nociception. (b) Levels of LTB4 in paw perfusate 5 min after i.pl. injection of vehicle, BK (10 nmol paw−1) or capsaicin (1 nmol paw−1). Each column represents the mean±s.e.m. of 4–6 mice. The asterisks denote the significance levels. (a) **P<0.01, compared with BK-treated mice. (b) *P<0.05, compared with vehicle-treated mice, #P<0.05, compared with BK-treated mice (one-way ANOVA followed by Dunnett's test).
Several studies suggest that some TRPV1 ligands may be generated by the degradation of the main PLA2 derivate product arachidonic acid, especially those from LOX. This pathway seems to be activated in vivo since the LOX inhibitor baicalein (3 nmol paw−1) significantly reduced BK-induced nociception (Figure 5a). Furthermore, BK was also able to increase about two times the levels of the LOX product LTB4 in the injected paw, an effect that was completely prevented by the injection of baicalein (Figure 5b). On the other hand, the cyclooxygenase inhibitor ibuprofen (100 nmol paw−1) was not able to alter BK-induced nociception (licking time of 39±4.3 and 36±5.2 s for BK or BK plus ibuprofen, respectively).
Discussion
The present study demonstrates that BK injection into the mouse paw produces a rapid and marked overt nociception. This effect seems to be related to B2 kinin receptor activation, as the selective B2 receptor agonist Tyr8-BK mimicked its response. Also, BK-induced nociception was almost abolished by the selective B2 receptor antagonist. The possible participation of kinin B1 receptor in BK action was discarded since the B1 receptor antagonist was not able to reduce BK-induced nociceptive behavior, and the injection of the B1 receptor agonist did not produce any detectable overt nociceptive response. BK-induced overt nociception was mediated, at least in part, by TRPV1 stimulation. The coadministration of the selective capsaicin receptor antagonist capsazepine with BK, at a range of dose in which it markedly inhibited capsaicin-mediated nociception, dose-dependently and completely prevented BK-mediated nociceptive response.
Another interesting and also conclusive piece of evidence indicating the involvement of TRPV1 activation in the nociception caused by BK was the complete cross-desensitization observed between capsaicin and BK. In marked contrast, the paws desensitized by repeated injection of BK exhibited a significantly higher nociceptive response to capsaicin. Similar results have been reported using an in vitro preparation of neonatal rat spinal cord with attached tail indicating that a supramaximal concentration of capsaicin to the tail reduced BK-induced ventral root depolarization (Dray et al., 1990). On the other hand, prolonged exposure to BK did not reduce capsaicin-induced depolarization (Rueff et al., 1994). TRPV1 desensitization is a complex process that seems to be mediated by calcineurin activation (Docherty et al., 1996) and by calmodulin binding (Numazaki et al., 2003). Different ligands, such as capsaicin, resiniferatoxin, and protons, produce TRPV1 desensitization with different profiles, including extracellular Ca2+-dependence and degree of efficacy (Acs et al., 1997; Mohapatra et al., 2003; Numazaki et al., 2003). Moreover, a recent study has shown that only the TRPV1 antagonist capsazepine, but not the TRPV1 channel blocker ruthenium red, inhibit capsaicin-induced desensitization to its nociceptive effect in vivo (Sakurada et al., 2003). Interestingly, TRPV1 may be resensitized since its desensitization may be reversed by PKA phosphorylation (Bhave et al., 2002). Further studies are required to answer as to why BK is able to activate, but not to desensitize, TRPV1-mediated nociceptive response.
Furthermore, the neonatal treatment of animals with capsaicin, which has been widely used to disrupt capsaicin-sensitive fibers (Holzer, 1991), also completely abrogated BK-mediated nociception. These findings provide evidence indicating that BK and capsaicin activate the same subpopulation of sensory fibers to produce overt nociception.
Recent observations furnish compelling evidence indicating that BK, acting via B2 receptors on sensory neurons, has the ability to sensitize the TRPV1 to noxious stimuli through the activation of PLC–PKC signaling pathways (Julius & Basbaum, 2001). The data shown in the present study confirm and also extend these previous in vitro observations by demonstrating that the coadministration of selective PLC antagonist, at a dose where it had no significant effect on capsaicin-mediated nociception, completely abolished BK-induced nociception. Such results suggest that also in vivo the activation of PLC pathway plays a critical role in inducing BK nociceptive action. In vitro studies carried out with native or heterologously expressed TRPV1 receptors have demonstrated that B2 kinin receptor-PLC activation might modulate TRPV1 through PKC-dependent and -independent mechanisms. The direct PLC-mediated phosphatidylinositol-4,5-bisphosphate (PIP2) hydrolysis may release TRPV1 from PIP2-mediated inhibition (Chuang et al., 2001). Moreover, TRPV1 activity may also be modulated by PKCɛ phosphorylation, increasing the gating of TRPV1 by capsaicin, anandamide, protons, and heat (Premkumar & Ahern, 2000; Vellani et al., 2001; Numazaki et al., 2002). In fact, BK has the ability to sensitize the heat response through a mechanism involving the activation of PKCɛ (Cesare et al., 1999; Sugiura et al., 2002). Our findings are in perfect agreement with such a hypothesis since they demonstrate that the coadministration of the selective PKC inhibitor GF109203X, at a dose where it failed to interfere with capsaicin-mediated algesic response, completely abolished BK-induced nociception. Results of the present study confirm and also extend these previous findings, as BK, at a dose where it did not cause nociception by itself, consistently potentiated the pronociceptive responses produced by capsaicin and low pH in vivo.
We have shown that BK produces a discrete, but significant, increase in the injected paw temperature. Added to other stimulus, this variation of temperature might contribute to TRPV1 stimulation. Under normal conditions, TRPV1 is activated by temperatures greater than 43°C (Caterina et al., 1997). However, sensitization of TRPV1 produced by phosphorylation may decrease the temperature threshold to below 35°C (Tominaga et al., 2001). Thus, when the conditions are appropriate, normal body temperature is able to act as a primary stimulus to thermoceptors (Reeh & Petho, 2000). BK action may therefore be relevant for TRPV1 activation at normal body temperature, an event that could be involved in the manifestation of burning pain or in some of the acute and chronic painful pathologies. In fact, mice lacking TRPV1 receptor have a marked reduction in thermal hyperalgesia in a model of inflammatory pain (Caterina et al., 2000; Davis et al., 2000), thus further confirming the critical role played by TRPV1 receptor in the inflammatory pain states.
Furthermore, BK may also exert indirect effects on the pain process, such as generating PLA2 lipid mediators, for example, prostaglandins and leukotrienes (Calixto et al., 2000; 2001; Shin et al., 2002). Capsaicin is a hydrophobic molecule that binds to the intracellular site of TRPV1 (Jung et al., 1999), between the second and the third transmembrane domains (Jordt & Julius, 2002). Taken together, such results suggest that TRPV1 endogenous ligand might be a lipid intracellular activator. In fact, several mediators, such as LOX products, anandamide and N-arachidonoyl-dopamine, are able to activate TRPV1 (Zygmunt et al, 1999; Hwang et al., 2000; Huang et al., 2002). There is recent in vitro evidence suggesting that BK, acting at B2 receptors, excites sensory nerve endings via activation of TRPV1 receptor through production of LOX metabolites (Hwang et al., 2000; Shin et al., 2002). The excitation of cultured sensory neurons or sensory nerve fibers of the adult rat skin by BK is greatly restricted by inhibitors of PLA2–LOX–TRPV1 pathway (McGuirk & Dolphin, 1992; Shin et al., 2002). In fact, BK stimulates LOX product formation by sensory neurones in vitro and by skin in vivo (Gammon et al., 1989; Shin et al., 2002; Wang et al., 1999). It has also been reported that BK-induced thermal hyperalgesia is blocked by LOX inhibition (Shin et al., 2002). Our results extend these previous observations and show that the PLA2 and LOX inhibitor reduce BK-induced overt nociception without interfering with capsaicin-induced nociception. Furthermore, BK injection increases the tissue levels of the 5-LOX metabolite LTB4.
Alternatively, the possibility that a cyclooxygenase-derived metabolite might also be involved in BK-mediated activation of TRPV1 channel in sensory neurons cannot be fully discarded. In fact, Pethö et al. (2001) demonstrated that BK produces both excitation and sensitization of rat C-fibres. Moreover, the BK-induced excitatory effect, in contrast to the heat sensitization, is subject to strong tachyphylaxis and is insensitive to cyclooxygenase inhibition. To explore the relationship between the in vitro and in vivo BK nociceptive effect, we have performed experiments with cyclooxygenase blocking and found that i.pl. injection of ibuprofen was not able to reduce BK-induced nociception. This result suggests that cyclooxygenase products do not seem to mediate this BK action. However, it has been reported that PGE2 causes hyperalgesia via enhancement of cAMP and activation of PKA-dependent phosphorylation of TRPV1 (Bhave et al., 2002; Hu et al., 2002). Since TRPV1 desensitization may be reversed by PKA phosphorylation (Bhave et al., 2002), prostaglandins could be involved in the lack of TRPV1 desensitization after BK repeated injections.
In summary, these results and those discussed above provide convincing evidence that BK produces nociceptive effects through its ability to sensitize the primary afferent neurons by stimulating the production of lipid-derived second messengers that in turn sensitize TRPV1 receptor (Figure 6). The nociceptive action of BK could be mediated not only by direct activation of sensory neurons but also by indirect release of lipid mediators from cells surrounding nerve terminals. Thus, following tissue injury or during certain inflammatory processes, the release of proinflammatory mediators, namely BK and arachidonic acid metabolites, causes a reduction of threshold to noxious stimuli, leading to the development of hypersensitivity states.
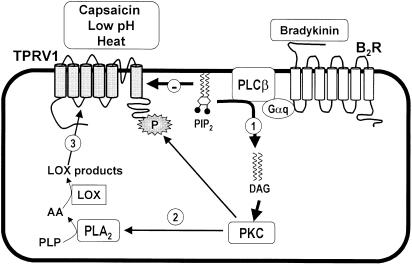
Figure 6.
Proposed mechanisms involved in BK-induced activation of TRPV1. As discussed, the current results provide consistent in vivo experimental evidence supporting the concept that, at least in part, the nociception caused by i.pl. injection of BK into the mouse paw is indirectly mediated by both activation and sensitization of TRPV1 receptor. BK, by acting at protein Gq coupled B2 receptors, is able to stimulate PLCβ that in turn produces hydrolysis of the plasma membrane phospholipid PIP2, exerting an inhibitory action on TRPV1 channel function (step 1). Furthermore, after stimulation of neurons with B2 receptor agonists such as BK, a rapid formation of diacylglycerol (DAG) occurs that in turn induces phosphorylation of key targets through PKC-mediated mechanisms (step 2). The third proposed mechanism involves the ability of BK to induce the activation of PLA2 and generation of endogenous LOX-derived products that act directly to stimulate the TRPV1 receptor (step 3).
The current results give new insights into the in vivo mechanisms through which BK causes nociception, but additionally provide promising targets for the development of new peripherally acting analgesic drugs.
Acknowledgments
This study was supported by Conselho Nacional de Desenvolvimento Científico (CNPq), Financiadora de Estudos e Projetos (FINEP) and Programa de Apoio aos Núcleos de Excelência (PRONEX) (Brazil). J.F. and G.L.S. are PhD and undergraduate students in Pharmacology, respectively, and they thank CNPq (Brazil) for the fellowship support.
Abbreviations
- BK
bradykinin
- ipl
intraplantar
- LTB4
leukotriene B4
- LOX
lipoxygenase
- PBS
phosphate-buffered saline
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PLA2
phospholipase A2
- PLC
phospholipase C
- PKC
protein kinase C
- TRPV1
vanilloid receptor
References
- ACKERMANN E.J., CONDE-FRIEBOES K., DENNIS E.A. Inhibition of macrophage Ca(2+)-independent phospholipase A2 by bromoenol lactone and trifluoromethyl ketones. J. Biol. Chem. 1995;270:445–450. doi: 10.1074/jbc.270.1.445. [DOI] [PubMed] [Google Scholar]
- ACS G., BIRO T., ACS P., MODARRES S., BLUMBERG P.M. Differential activation and desensitization of sensory neurons by resiniferatoxin. J. Neurosci. 1997;17:5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHAVE G., ZHU W., WANG H., BRASIER D.J., OXFORD G.S., GEREAU R.W. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Inflammatory pain: kinins and antagonists. Curr. Opin. Anaesthesiol. 2001;14:519–526. doi: 10.1097/00001503-200110000-00010. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., JULIUS D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CESARE P., DEKKER L.V., SARDINI A., PARKER P.J., MCNAUGHTON P.A. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- CHUANG H.H., PRESCOTT E.D., KONG H., SHIELDS S., JORDT S.E., BASBAUM A.I., CHAO M.V., JULIUS D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DE CAMPOS R.O., ALVES R.V., FERREIRA J., KYLE D.J., CHAKRAVARTY S., MAVUNKEL B.J., CALIXTO J.B. Oral antinociception and oedema inhibition produced by NPC 18884 a non-peptide bradykinin receptor antagonist. Naunyn-Schmiedberǵs Arch. Pharmacol. 1999;360:278–286. doi: 10.1007/s002109900080. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., BEVAN S., BODDEKE H.W. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- DRAY A., BETTANEY J., FORSTER P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci. Lett. 1989;91:301–307. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- DRAY A., BETTANEY J., FORSTER P. Actions of capsaicin on peripheral nociceptors of the neonatal rat spinal cord tail in vitro: dependence of extracellular ions and independence of second messengers. Br. J. Pharmacol. 1990;101:727–733. doi: 10.1111/j.1476-5381.1990.tb14148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA J., SANTOS A.R., CALIXTO J.B. Antinociception produced by systemic, spinal and supraspinal administration of amiloride in mice. Life Sci. 1999;65:1059–1066. doi: 10.1016/s0024-3205(99)00336-7. [DOI] [PubMed] [Google Scholar]
- GAMMON C.M., ALLEN A.C., MORELL P. Bradykinin stimulates phosphoinositide hydrolysis and mobilization of arachidonic acid in dorsal root ganglion neurons. J. Neurochem. 1989;53:95–101. doi: 10.1111/j.1471-4159.1989.tb07299.x. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–200. [PubMed] [Google Scholar]
- HU H.J., BHAVE G., GEREAU R.W. Prostaglandin and protein kinase A-dependent modulation of vanilloid receptor function by metabotropic glutamate receptor 5: potential mechanism for thermal hyperalgesia. J. Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species-specific sensitivity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- JULIUS D., BASBAUM A.I. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAK J.Y., JUNG J.Y., HWANG S.W., LEE W.T., OH U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in the rat: evidence for an endogenous capsaicin-like substance. Neuroscience. 1998;86:619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- MARTIN H.A., BASBAUM A.I., KWIAT G.C., GOETZL E.J., LEVINE J.D. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience. 1987;22:651–659. doi: 10.1016/0306-4522(87)90360-5. [DOI] [PubMed] [Google Scholar]
- MCGUIRK S.M., DOLPHIN A.C. G-protein mediation in nociceptive signal transduction: an investigation into the excitatory action of bradykinin in a subpopulation of cultured rat sensory neurons. Neuroscience. 1992;49:117–128. doi: 10.1016/0306-4522(92)90079-h. [DOI] [PubMed] [Google Scholar]
- MIZUMURA K., SATO J., KUMAZAWA T. Effects of prostaglandins and other putative chemical intermediaries on the activity of canine testicular polymodal receptors studied in vitro. Pflugers Arch. 1987;408:565–572. doi: 10.1007/BF00581157. [DOI] [PubMed] [Google Scholar]
- MOHAPATRA D.P., WANG S.Y., WANG G.K., NAU C. A tyrosine residue in TM6 of the vanilloid receptor TRPV1 involved in desensitization and calcium permeability of capsaicin-activated currents. Mol. Cell. Neurosci. 2003;23:314–324. doi: 10.1016/s1044-7431(03)00054-x. [DOI] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V., BINDELS R.J., BRUFORD E.A., CATERINA M.J., CLAPHAM D.E., HARTENECK C., HELLER S., JULIUS D., KOJIMA I., MORI Y., PENNER R., PRAWITT D., SCHARENBERG A.M., SCHULTZ G., SHIMIZU N., ZHU M.X. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- NUMAZAKI M., TOMINAGA T., TAKEUCHI K., MURAYAMA N., TOYOOKA H., TOMINAGA M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc. Natl. Acad. Sci. U.S.A. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUMAZAKI M., TOMINAGA T., TOYOOKA H., TOMINAGA M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase C epsilon and identification of two target serine residues. J. Biol. Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- PETHÖ G., DEROW A., REEH P.W. Bradykinin-induced nociceptor sensitization to heat is mediated by cyclooxygenase products in isolated rat skin. Eur. J. Neurosci. 2001;14:210–218. doi: 10.1046/j.0953-816x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D. The ligand that came from within. Trends Pharmacol. Sci. 2001;22:17–19. doi: 10.1016/s0165-6147(00)01602-3. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR L.S., AHERN G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- RASHID M.H., INOUE M., KONDO S., KAWASHIMA T., BAKOSHI S., UEDA H. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J. Pharmacol. Exp. Ther. 2003;304:940–948. doi: 10.1124/jpet.102.046250. [DOI] [PubMed] [Google Scholar]
- REEH P.W., PETHO G. Nociceptor excitation by thermal sensitization – a hypothesis. Prog. Brain Res. 2000;129:39–50. doi: 10.1016/S0079-6123(00)29004-3. [DOI] [PubMed] [Google Scholar]
- ROCHA E SILVA M., ANTONIO A. Release of bradykinin and the mechanism of production of a thermic edema (45°C) in the at's paw. Med. Exp. 1960;3:371. [PubMed] [Google Scholar]
- RUEFF A., PATEL I.A., URBAN L., DRAY A. Regulation of bradykinin sensitivity in peripheral sensory fibres of the neonatal rat by nitric oxide and cyclic GMP. Neuropharmacology. 1994;33:1139–1145. doi: 10.1016/s0028-3908(05)80003-6. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., KATSUMATA K., TAN-NO K., SAKURADA S., KISARA K. The capsaicin test in mice for evaluating tachykinin antagonists in the spinal cord. Neuropharmacology. 1992;31:1279–1285. doi: 10.1016/0028-3908(92)90057-v. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., MATSUMURA T., MORIYAMA T., SAKURADA C., UENO S., SAKURADA S. Differential effects of intraplantar capsazepine and ruthenium red on capsaicin-induced desensitization in mice. Pharmacol. Biochem. Behav. 2003;75:115–121. doi: 10.1016/s0091-3057(03)00066-2. [DOI] [PubMed] [Google Scholar]
- SANTOS A.R., CALIXTO J.B. Ruthenium red and capsazepine antinociceptive effect in formalin and capsaicin models of pain in mice. Neurosci. Lett. 1997;235:73–76. doi: 10.1016/s0304-3940(97)00722-2. [DOI] [PubMed] [Google Scholar]
- SEKIYA K., OKUDA H. Selective inhibition of platelet lipoxygenase by baicalein. Biochem. Biophys. Res. Commun. 1982;105:1090–1095. doi: 10.1016/0006-291x(82)91081-6. [DOI] [PubMed] [Google Scholar]
- SHIN J., CHO H., HWANG S.W., JUNG J., SHIN C.Y., LEE S.Y., KIM S.H., LEE M.G., CHOI Y.H., KIM J., HABER N.A., REICHLING D.B., KHASAR S., LEVINE J.D., OH U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGIURA T., TOMINAGA M., KATSUYA H., MIZUMURA K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J. Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- TOMINAGA M., WADA M., MASU M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOULLEC D., PIANETTI P., COSTE H., BELLEVERGUE P., GRAND-PERRET T., AJAKANE M., BAUDET V., BOISSIN P., BOURSIER E., LORIOLLE F., DUHAMELL L., CHARON D., KIRILOVSKY J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- VELLANI V., MAPPLEBECK S., MORIONDO A., DAVIS J.B., MCNAUGHTON P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG M.M., REYNAUD D., PACE-ASCIAK C.R. In vivo stimulation of 12(S)-lipoxygenase in the rat skin by bradykinin and platelet activating factor: formation of 12(S)-HETE and hepoxilins, and actions on vascular permeability. Biochim. Biophys. Acta. 1999;1436:354–362. doi: 10.1016/s0005-2760(98)00128-3. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]