Abstract
The purine nucleotide adenosine-5′-triphosphate (ATP) exerts pronounced effects on the cardiovascular system. The mechanism of action of the vasodilator response to ATP in humans has not been elucidated yet. The proposed endothelium-derived relaxing factors (EDRFs) were studied in a series of experiments, using the perfused forearm technique.
Adenosine 5′-triphosphate (0.2, 0.6, 6 and 20 nmol dl−1 forearm volume min−1) evoked a dose-dependent forearm vasodilator response, which could not be inhibited by separate infusion of the nonselective COX inhibitor indomethacin (5 μg dl−1 min−1, n=10), the blocker of Na+/K+-ATPase ouabain (0.2 μg dl−1 min−1, n=8), the blocker of KCa channels tetraethylammonium chloride (TEA, 0.1 μg dl−1 min−1, n=10), nor by the KATP-channel blocker glibenclamide (2 μg dl−1 min−1, n=10). All blockers, except glibenclamide, caused a significant increase in baseline vascular tone. The obtained results might be due to compensatory actions of unblocked EDRFs. Combined infusion of TEA, indomethacin and L-NMMA (n=6) significantly increased the baseline forearm vascular resistance. The ATP-induced relative decreases in forearm vascular resistance were 48±5, 67±3, 88±2, and 92±2% in the absence and 23±7, 62±4, 89±2, and 93±1% in the presence of the combination of TEA, indomethacin and L-NMMA (P<0.05, repeated-measures ANOVA, n=6). A similar inhibition was obtained for sodium nitroprusside (SNP, P<0.05 repeated-measures ANOVA, n=6), indicating a nonspecific interaction due to the blocker-induced vasoconstriction.
ATP-induced vasodilation in the human forearm cannot be inhibited by separate infusion of indomethacin, ouabain, glibenclamide or TEA, or by a combined infusion of TEA, indomethacin, and L-NMMA. Endothelium-independent mechanisms and involvement of unblocked EDRFs, such as CO, might play a role, and call for further studies.
Keywords: Adenosine 5′-triphosphate, KCa channels, cyclooxygenase, nitric oxide, Na+K+-ATPase, regional blood flow
Introduction
Adenosine-5′-triphosphate (ATP) is an endogenous purine nucleotide consisting of a purine base (adenine), ribose and three phosphate groups. ATP is released from aggregating thrombocytes (Holmsen et al., 1972; Meyers et al., 1982), endothelium (Schwiebert et al., 2002), sympathetic nerve endings (Burnstock & Sneddon, 1985; Satchell, 2000), and ischemic muscle cells (Gordon, 1986). Extracellular ATP exerts potent and diverse effects on the cardiovascular system via activation of P2 receptors (Gordon, 1986; Ralevic & Burnstock, 1998). In general, P2x and P2y receptors on vascular smooth muscle cells (VSMCs) mediate vasoconstriction, while stimulation of P2y receptors on endothelial cells causes vasodilation. This dual action of ATP on vascular tone may have important clinical consequences: during thrombocyte aggregation at sites of severe atherosclerosis, locally released ATP might induce vasoconstriction mediated by P2x receptors located on VSMCs, which is unopposed by P2y-receptors-mediated vasodilation because of endothelial damage (Malmsjo et al., 2000). Local vasoconstriction will then further aggrevate ischemia. Therefore, better understanding of ATP-induced vasodilation may reveal new targets for pharmacological intervention to reduce or prevent vasospasm, thrombus formation, and ischemia.
The exact mechanism of ATP-induced endothelium-dependent vasodilation in humans is still a matter of debate. In vitro studies that explored the vasomotor effect of ATP in the presence and absence of an intact endothelium revealed an important role of the endothelium in ATP-induced vasodilation.
The proposed endothelium-derived relaxing factors (EDRFs) are NO, prostacyclin, and endothelium-derived hyperpolarizing factors (EDHFs) (Brown & Burnstock, 1981; Mathie et al., 1991; Keef et al., 1992; Malmsjo et al., 1999).
The exact nature of EDHF is still uncertain, although its mechanism of action through opening of potassium channels and/or activation of Na+/K+-ATPase has been well established (Nagao & Vanhoutte, 1993; Vanhoutte et al., 1993; Levy et al., 1997; Suzuki et al., 1998). A study in isolated mesenteric rat arteries showed that the prolonged phase of vasorelaxation to ATP was attenuated by ouabain and by glibenclamide, indicating direct or indirect involvement of Na+/K+ ATPase and KATP channels (Ralevic, 2001). In vivo studies on ATP-induced vasodilation are rare. Former experiments by our group revealed that the ATP-induced vasodilation in the human forearm exceeds the vasodilation induced by equimolar adenosine infusion, which is the degradation product of ATP with the highest P1-purinergic receptor agonist activity (Rongen et al., 1994). This demonstrates that the metabolite adenosine hardly contributes to the ATP-induced vasodilation in the human forearm. This is further supported by the fact that the P1-purinoceptor antagonist theophylline did not affect the vasodilator response to ATP (Rongen et al., 1994). It was shown previously that ATP-induced vasodilation in the human forearm cannot be inhibited by the competitive NO-synthase antagonist NG-monomethyl-L-arginine (L-NMMA) (Rongen et al., 1994; Shiramoto et al., 1997). Finally, previous experiments by our group have demonstrated that the vasodilator response to intra-arterial ATP in the forearm is not limited by any vasoconstrictor action, including vasoconstriction that could theoretically have resulted from P2x receptor stimulation on vascular smooth muscle cells (Rongen et al., 1994).
The aim of this study was to identify a possible role for cyclo oxygenase products and EDHF in ATP-induced vasodilation. Cyclo oxygenase mediates the formation of the intermediate compound prostaglandin H2 (PGH2), the precursor for several prostaglandins, such as prostacyclin (PGI2) and thromboxane A2 (TXA2, an endothelium-derived contracting factor). Prostacyclin acts on receptors on VSMCs mediating vasodilation by increase of intracellular cAMP via stimulation of adenylate cyclase (Narumiya et al., 1999). In this study, cyclooxygenase activity was blocked with indomethacin.
Other arachidonic acid metabolites that might be partly responsible for EDHF activity are epoxides (EETs, formed by cytochrome P-450) and hydroxyeicosatetraenoic acid (HETE, formed by lipoxygenases). EETs and HETE mediate the relaxation of VSMCs by opening calcium-dependent potassium channels (Coats et al., 2001; Zink et al., 2001). Another compound that mediates vasodilation by opening calcium-dependent potassium channels (KCa channels) in VSMCs is endothelium-derived hydrogen peroxide (H2O2) (Barlow & White, 1998). In this study, KCa channels were blocked with tetraethylammonium (TEA). ATP-sensitive potassium channels (KATP channels) also play a role in mediating vasodilation by hyperpolarizing VSMCs (Brayden, 1996). Glibenclamide was used to block KATP channels. Finally, potassium itself acts as EDHF by inducing hyperpolarization of VSMCs by activation of Na/K-ATPase (Edwards et al., 1998). The role of Na+/K+-ATPase was studied by concomitant infusion with ouabain, a compound that inhibits Na+ /K+-ATPase and has been shown to block the relaxation and hyperpolarization caused by EDHF (Feletou & Vanhoutte, 1988). In vitro (Bauersachs et al., 1996; Lagaud et al., 1999) and in vivo studies (Taddei et al., 1999) have shown that EDRFs can compensate for the inhibition of formation or function of a single EDRF. The same might be true for ATP-induced vasodilation in the human forearm, which was studied in an additional experiment, by combined infusion with TEA, indomethacin, and L-NMMA.
Methods
Subjects
The study protocol was approved by the local ethics comittee, and all participants signed written informed consent before their participation. The investigation conforms with the principles outlined in the declaration of Helsinki. Demographic data are shown in Table 1. The experiments were performed in healthy, normotensive male and female volunteers. They did not use concomitant medication, except for oral contraceptive drugs. All participants underwent a physical examination, laboratory screening (total cholesterol, triglycerides and glucose) and electrocardiography before entering the study. Participants were asked to abstain from caffeine-containing beverages and alcohol for 24 h before the experiment, and to abstain from food intake 2 h prior to the study.
Table 1.
Baseline characteristics of the study groups (mean±s.d.)
| Indo-methacin | TEA | Gliben-clamide | Glibenclamide+low dose ATP | Ouabain | TEA+indomethacin +L-NMMA | SNP control study | |
|---|---|---|---|---|---|---|---|
| N | 12 | 10 | 10 | 6 | 8 | 6 | 6 |
| Male/female | 12/0 | 4/6 | 6/4 | 4/2 | 4/4 | 1/5 | 1/5 |
| Age (Year) | 22.9±6.0 | 22.6±3.2 | 22.5±2.2 | 21.8±2.5 | 22.3±2.1 | 20.8±0.4 | 21.2±3.6 |
| Body mass index (kg m2) | 21±1.6 | 227.7±2.1 | 23.1±2.1 | 21.5±1.4 | 23.0±2.4 | 21.6±2.9 | 22.1±1.5 |
| Systolic blood pressure (mmHG) | 129.3±9.0 | 122.5±9.3 | 122.8±7.7 | 123.5±9.5 | 120.1±8.5 | 129±10.2 | 115.3±9.3 |
| Diastolic blood pressure (mmHG) | 70.9±8.2 | 69.4±6.5 | 76.2±8.9 | 72.2±7.8 | 76.4±4.4 | 76.3±5.7 | 67.0±6.3 |
| Heart rate (bpm) | 69.1±12.3 | 60.7±11.4 | 60.8±9.5 | 62.7±15.2 | 61.5±8.0 | 68.3±7.0 | 59.8±6.3 |
| Glucose (mmol l−1) | — | 4.4±0.2 | 4.5±0.4 | 4.4±0.6 | 4.5±0.3 | 4.1±0.5 | 4.8±0.7 |
| Cholesterol (mmol l−1) | — | 4.0±0.6 | 4.0±0.4 | 3.9±0.6 | 3.8±0.5 | 4.0±0.4 | 4.1±0.8 |
| Triglycerodes (mmol l−1) | — | 1.0±0.5 | 0.8±0.5 | 0.8±0.2 | 0.9±0.4 | 1.1±0.5 | 0.8±0.1 |
General outline of the procedure
The experiments were performed in the morning in a quiet room with stable temperature (23°C), with the subjects in supine position. After local anesthesia (xylocaine 2%), the brachial artery of the nondominant arm was cannulated (Angiocath, 20 gauge, Deseret Medical, Becton Dickinson Sandy, UT, U.S.A.) for drug infusion (syringe infusion pump, type STC-521, Terumo Corp., Tokyo, Japan) and intra-arterial blood pressure measurement (Hewlett-Packard monitor, type 78353B, Hewlett-Packard GmbH, Böblingen, Germany). Drug- and volume-infusion rates were calculated per deciliter of forearm tissue, which was measured for each person by water displacement.
In protocols involving glibenclamide, a deep antecubital vein of the infused arm was cannulated for blood sampling. Bilateral forearm blood flow (FBF) was measured by ECG-triggered mercury-in-silastic strain gauge plethysmography, as described before (Rongen et al., 1995), while the hand circulation was occluded using wrist cuffs (Lenders et al., 1991). All experiments started 30 min after intra-arterial cannulation with the measurement of baseline blood flow, obtained during infusion of saline (NaCl 0.9%). Thereafter, increasing doses of ATP were coinfused with saline. Each ATP dose was infused for 5 min, together with saline or a blocker. The succeeding ATP doses were interrupted once by a 10-min drug-free interval. This was done because prolonged occlusion of the hand circulation can cause discomfort, leading to changes in blood pressure and heart rate. The rate of infused volume and the amount of connected syringes was kept constant throughout each experiment.
Effect of indomethacin on ATP-induced forearm vasodilaton (n = 12)
In this study, we used three increasing doses of ATP (0.6,. 6 and 20 nmol dl−1 FAV min−1). At 45 min after infusion of the highest dose, baseline recordings were repeated during infusion of saline followed by indomethacin (5 μg dl−1 min−1), respectively. Subsequently, ATP infusions were repeated in the presence of indomethacin.
Effect of TEA on ATP-induced forearm vasodilation (n=10)
After baseline measurements, ATP 0.2,. 0.6, 6 and 20 nmol dl−1 forearm volume (FAV) min−1 were infused. Baseline recordings were repeated after a 30-min drug-free interval. TEA (0.1 mg dl−1 min−1) was infused for 30 min, followed by coinfusion with the increasing ATP doses.
Effect of glibenclamide on ATP-induced forearm vasodilation (n=16)
In the first glibenclamide study (n=10), we infused ATP 0.2,. 0.6, 6 and 20 nmol dl−1 min−1. After 30 min, recontrol values were obtained and glibenclamide (2 μg dl−1 min−1) was subsequently infused. At 10 min after the start of glibenclamide, ATP infusions were repeated. Venous blood samples were collected from the experimental arm to measure the effect of glibenclamide on glucose-, insulin-, and C-peptide concentrations, and to determine the concentration of glibenclamide during the course of the study. To check the validity of an observed small effect of glibenclamide on ATP-induced vasodilation, the protocol was repeated, but now with ATP 0.1, 0.2, 0.4 and 0.8 nmol dl−1 min−1 (n=6).
Effect of ouabain on ATP-induced forearm vasodilation (n=8)
The outline of this study is similar to the glibenclamide study. Ouabain was infused instead of glibenclamide, in a concentration of 0.2 μg dl−1 min−1.
Influence of combined infusion of TEA, indomethacin and L-NMMA on ATP-induced forearm vasodilation (n=6)
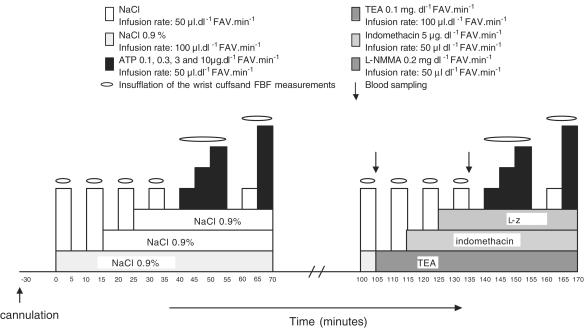
After baseline measurements, four increasing doses of ATP were infused (0.2, 2, 6 and 20 nmol dl−1 min−1). The second half of the experiment started 30 min after cessation of the highest ATP-dose. Saline infusions were subsequently replaced by infusion of TEA (0.1 mg dl−1 min−1), indomethacin (5 μg dl−1 min−1), and L-NMMA (0.2 mg dl−1 min−1). A graphic presentation of the protocol is provided in Figure 1.
Figure 1.
Infusion protocol of the ATP study with combined infusion of TEA. indomethacin, and L-NMMA.
Since this combination of antagonists increased forearm vascular resistance (FVR) significantly and reduced the % change in FVR in response to ATP, a similar protocol was used in a separate group of six volunteers, but now ATP was replaced by nitroprusside as a vasodilative control (SNP; 0.02, 0.1, 0.2, and 0.6 μg dl−1 min−1).
Blood samples were collected in three of the six participants during the second half of the infusion schedule to determine oxygen consumption before and during the combined antagonist infusion. A venous and arterial blood sample was taken at the end of placebo infusion (t=100, see Figure 1) and during antagonist infusion (t=130) for determination of oxygen saturation and hemoglobin. Oxygen consumption was calculated by measuring the arteriovenous difference in the product of saturation (%), blood flow (ml dl−1 FAV min−1) and hemoglobin (mM), expressed in arbitrary units (AUs).
Drugs and solutions
All solutions were freshly prepared. ATP (Striadyne, Wyeth Laboratories, Hoofddrop, The Netherlands) was diluted to reach the necessary concentrations. Indomethacin (GenRX-Mosby Inc., St Louis, MO, U.S.A.), TEA (Sigma Chemical Co, St Louis, MO, U.S.A.), glibenclamide (Hoechst AG, Frankfurt, Germany), L-NMMA (Sigma Chemical Co, St Louis, MO, U.S.A.) and ouabain (Pharmachemie, Haarlem, The Netherlands) were diluted in NaCl 0.9% to reach final syringe concentrations of 5 μg (indomethacin), 50 μg (TEA), 2 μg (glibenclamide), 0.2 mg (L-NMMA) and 0.2 μg (ouabain) per 50 μl, respectively. Lyophilized SNP (Nipride, Roche Nederland, Mijdrecht, The Netherlands) was diluted in glucose 5% and protected against light.
Analytical procedures
Insulin and C-peptide concentrations were determined in our laboratories using specific radioimmunoassays. In the insulin assay, standard and tracer insulin was prepared from monocomponent human insulin (Novo, Zoeterwoude, The Netherlands). Insulin concentrations below 5.0 mE l−1 remained undetected. C-peptide was measured with a standard kit (D.P.C., Los Angeles, CA, U.S.A.). The detection limit for glibenclamide was below 5.0 ng ml−1. Plasma glucose concentrations were assessed in our laboratories with a Hitachi 747 (Roche diagnostics, Indianapolis, IN, U.S.A.). Glibenclamide was measured by high-performance liquid chromatography (HPLC) (Khatri et al., 2001). Hemoglobin concentrations were assessed with the Advia 1650 (Bayer diagnostics, Leverkusen, Germany). Oxygen saturation was determined with the Rapidlab 248 (Bayer diagnostics).
Statistical analysis
Mean arterial blood pressure (MAP) was measured continuously during each recording of FBF and averaged per FBF measurement. FVR was calculated from simultaneously measured MAP and FBF (MAP/FBF) and expressed as AUs. The calculated FVRs and hemodynamic parameters obtained during the last 4 min of saline infusion or during the last 2 min of each drug infusion were averaged to one value. Drug-induced effects were expressed absolute (t-tests for the effect of an antagonist on baseline values) or as the percentage change from preceding saline infusion or antagonist infusion. All results are mean±s.e., unless indicated otherwise. Based on reproducibility data from a previous study by our group (Rongen et al., 1994), it can be estimated that for a dose of 6 nmol dl−1 FAV, a minimal difference in percentage change in FVR from baseline can be detected, of 21% (N=10) or 31% (N=6), with a power of 0.9 and an alpha of 0.05 (paired t-test). To avoid multiple comparison, the effect of antagonists on ATP and SNP-induced vasodilation were assessed with repeated-measures ANOVA. The presence of antagonists and vasodilator doses was used as within-subject factors. To explore the effect of previous vasodilator treatment on the vasoconstrictive effect of combined infusion of TEA, indomethacin, and L-NMMA, the vasodilator was used as between group factor. T-tests were applied as post hoc tests when applicable. P<0.05 (two sided) was considered statistically significant.
Results
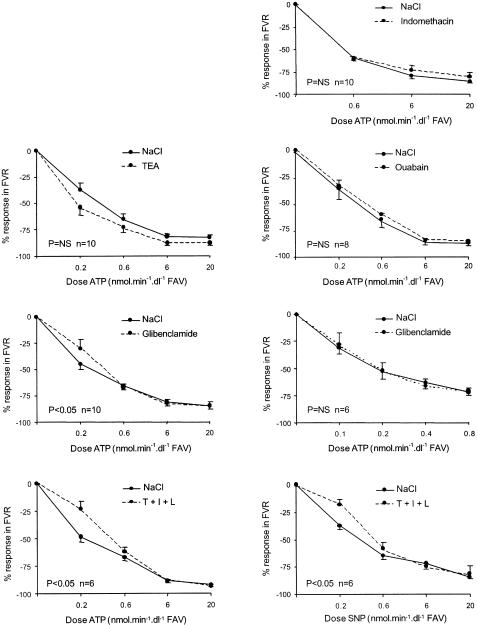
The demographic data of the participants are shown in Table 1. The course of FVR in the infused arm is shown in Figure 2 for each experiment. The course of FVR in the control arm was not significantly affected by any of the blockers or vasodilators used. Recontrol values for FVR did not differ from baseline.
Figure 2.
Relative response in FVR (infused arm) during infusion of ATP and (last graph) of SNP with and without antagonists, as indicated. T=TEA, I=indomethacin, L=L-NMMA. For doses; See text. P-values indicate ANOVA for repeated measures for the effect of the antagonists(s) on the vasodilator dose–response curve.
Effect of the antagonists on baseline FVR
Apart from glibenclamide, all used blockers induced a vasoconstrictor response. This response was most pronounced for the combined infusion of TEA, indomethacin, and L-NMMA (see Table 2). The vasoconstrictor action of TEA was only significant after previous infusion of ATP, but not after SNP (see Table 3), and significantly differed between the ATP- and SNP-pretreated group. In the ATP study with combined infusion of TEA, indomethacin, and L-NMMA, calculated values for oxygen consumption were 2.9, 7.1, and 7.2 versus 6.4, 6.1, and 6.5 AU in the absence and presence of antagonists, respectively. Forearm oxygen consumption was not affected by simultaneous infusion of TEA, L-NMMA, and indomethacin, which argues against vasoconstriction-induced ischemia.
Table 2.
Effect of the antagonists on baseline vascular tone (FVR, absolute value, mean±s.e.)
| Mean±s.e. | Saline | Antagonist |
|---|---|---|
| Indomethacin* | 53±9 | 61±9 |
| TEA* | 30±2 | 62±13 |
| Glibenclamide | 56±11 | 47±6 |
| Glibenclamide (low-dose ATP) | 44±5 | 49±9 |
| Ouabain* | 49±6 | 63±9 |
| T+l+L* | 34±8 | 92±10 |
| T+l+L* (SNP) | 48±3 | 85±10 |
T=TEA; I=indomethacin; L=L-NMMA;
:P<0.05 for baseline versus antagonist (paired t-test)
Table 3.
FVR (AU, mean±s.e.) and % change during subsequent combination of antagonists
| FVR, AU (% change in FVR) | Baseline | Recontrol | T | T+I | T+I+L |
|---|---|---|---|---|---|
| ATP (N=6) | 37±7 | 34±8 | 46±8* | 77±5† | 92±10 |
| (39±6#) | (109±57) | (21±13) | |||
| SNP (N=6) | 41±4 | 48±3 | 54±4 | 63±5 | 85±10¶ |
| (12±5) | (19±7) | (35±12) |
T=TEA; I=indomethacin; L=L-NMMA;
P<0.05 for between-group comparison of relative responses (ATP group with SNP group).
P<0.05 versus re-control (paired t-test).
P<0.05 versus T (paired t-test).
P<0.05 versus T+I (paired t-test.)
Influence of the antagonists on ATP-induced forearm vasodilation
Indomethacin, TEA, and ouabain (infused separately) did not reduce ATP-induced forearm vasodilation (see Figure 2). ATP- (0.2, 0.6, 6, and 20 nmol dl−1 min−1) induced vasodilation was significantly reduced by glibenclamide (P<0.05 for the interaction between ATP and glibenclamide). This effect was solely due to the lowest ATP dose, and could not be reproduced in an additional study with ATP infused in a lower dose range: ATP 0.1, 0.2, 0.4, and 0.8 nmol dl−1 min−1 reduced FVR by 31.1±5.9, 52.9±6.9, 62.7±5.6, and 72.2±2.6% versus 27.6±10.8, 52.1±6.8, 65.8±5.9, and 70.9±2.9% in the absence and presence of glibenclamide, respectively (P>0.1, n=6).
Glibenclamide concentrations were measured before start of the glibenclamide infusion, after the third ATP dose and after the last ATP dose. Concentrations were 1.5±0.3, 0.2±0.03, and 0.2±0.02 (ATP 0.2, 0.6, 6, and 20 nmol dl−1 min−1) and 1.2±0.1, 0.5±0.03, and 1.2±0.1 μg ml−1 (ATP 0.1, 0.2, 0.4, and 0.8 nmol dl−1 min−1), respectively. Glucose concentrations did not alter significantly: 4.5±0.1 versus 4.3±0.1 (samples taken before resp. during glibenclamide infusion) versus 4.4±0.1 versus 3.6±0.1 mmol l−1 (higher versus lower ATP dose range). Plasma insulin concentration increased significantly from 6.4±0.5 to 11.9±1.4 (higher ATP dose range, P<0.05) and 7.2±0.7 to 13.8±0.5 mE l−1 (lower dose range, P<0.05). C-peptide also increased significantly during the course of the study: from 0.4±0.1 to 0.6±0.1 (higher ATP doses, P<0.05) and 0.3±0.02 to 0.6±0.01 (lower ATP dose range, P<0.05).
Combined infusion of TEA, indomethacin, and L-NMMA inhibited ATP- as well as SNP-induced forearm vasodilation to a similar extent (Figure 2). This inhibition is therefore considered as a nonspecific effect due to the vasoconstrictive response to the infused antagonists.
Discussion
This study showed that all blockers, except glibenclamide, caused a significant increase in baseline vascular tone. ATP-induced vasodilation in the human forearm could not be inhibited by concomitant infusion of indomethacin, TEA, glibenclamide or ouabain alone, or by a combined infusion of TEA, indomethacin, and L-NMMA.
Effect of antagonists on baseline vascular tone
Human data on the influence of the used blockers on baseline vascular tone are very scarce. This is remarkable, because they have nevertheless become established and widely used compounds in pharmacological research. Data on animal experiments vary depending on species and vascular bed, as will be indicated hereafter. Indomethacin-induced increase of FVR suggests that continuous release of prostacyclin plays a role in the maintenance of resting FBF. Wilson & Kapoor (1993) and Duffy et al. (1998) previously detected that inhibition of cyclooxygenase with aspirin or indomethacin decreased the resting FBF by 20–30%. Prostacyclin also contributes to metabolic vasodilation (Kilbom & Wennmalm, 1976; Duffy et al., 1999a) as well as to resting and metabolic vasodilation in coronary arteries (Duffy et al., 1999b).
The effect of KCa channel inhibition on basal vascular tone differs depending on the experimental setting. Increase in baseline tone has been reported in cerebral arteries (Brayden & Nelson, 1992). In guinea-pig resistance arteries, no change was found (Calder et al., 1994; Pickkers & Hughes, 1995). In the current study, TEA increased the FVR of the forearm vascular bed 30 min after infusing ATP, and this vasoconstrictor response significantly differed from the effect of TEA after pretreatment with SNP. Pickkers et al. (2001) also found that TEA had no significant effect on baseline vascular tone after SNP infusion. TEA also had no influence on baseline vascular tone after infusion of hydrochlorothiazide (Pickkers et al., 1998) and C-type natriuretic peptide (CNP) (Honing et al., 2001). FVR at recontrol, just before start of TEA infusion, did not differ from baseline values, which makes it unlikely that the observed vasoconstrictor action of TEA is due to vanishing ATP-induced vasodilation by a carry-over effect, but cannot be excluded. Although the difference in TEA response between SNP- and ATP-pretreated groups was small and should not be overemphasized, this observation may indicate a pharmacodynamic carry-over effect of the previous ATP infusions on the maintenance of vascular tone, possibly by inducing the release of an alternative EDRF that could affect KCa channels at baseline.
Our finding that glibenclamide had no influence on basal vascular tone in the human forearm vascular bed is consistent with previous findings (Bijlstra et al., 1996; McAuley et al., 1997; Abbink et al., 2002). Glibenclamide had no influence on the basal vascular tone of carotid, femoral, and mesenteric endothelium-denuded strips from rats (Asano et al., 1993). However, infusion of glibenclamide into the coronary vasculature of anesthetized dogs and in isolated rabbit hearts resultated in significant increase in coronary resistance.
Ouabain infusion alone induced vasoconstriction, which has been reported before (Robinson et al., 1983; Tack et al., 1996; Dawes et al., 2002). Baseline activity of Na+K+-ATPase apparently contributes to resting vascular tone, probably by maintaining membrane polarity.
Why was ATP-induced vasodilation not inhibited by any of the antagonists we used?
First, in vivo, ATP might induce its vasodilation via an endothelium-independent, instead of the proposed endothelium-dependent, mechanism. Few in vitro studies have already shown that ATP exerts vasodilation partially via endothelium independent mechanisms (Mathieson & Burnstock, 1985; Vuorinen et al., 1992; 1994; McMillan et al., 1999). Vascular smooth muscle cells express P2y receptors which may mediate vasodilation (Wang et al., 2002).
Second, the infused concentrations of the antagonists might have been insufficient to inhibit the actions of EDRFs that are released in response to ATP. This is unlikely for any of the blockers used, however. Previously, in our laboratory, Pickkers & de Hoon showed that indomethacin at a concentration of 5 μg dl−1 FAV min−1 was able to inhibit cyclooxygenase: in a set of experiments, they confirmed adequate cyclooxygenase inhibition by the absence of thromboxane-B2 formation in blood drawn from an antecubital vein of the indomethacin infused forearm, determined by RIA (unpublished results). The observed vasoconstrictive effect of indomethacin and its clinical use as treatment for patent ductus arteriosus in preterm infants further support the blockade of vascular cyclooxygenase. Likewise, TEA 0.1 mg dl−1 FAV min−1 has been shown to inhibit vasodilation in the human forearm to the endothelium-dependent vasodilator bradykinin (Honing et al., 2000), which acts via EDHFs. TEA was also able to inhibit the CNP-induced vasodilation (Honing et al., 2001) and the vasodilation induced by acetazolamide (Pickkers et al., 2001) in the human forearm; both openers of KCa channels. The infused concentration of 2.0 μg glibenclamide min dl−1 FAV min−1 was based on a study previously performed in our laboratory, which showed that a concentration seven times lower was capable of effectively blocking KATP channels (Bijlstra et al., 1996). The significant rise in insulin and C peptide during the course of the studies with glibenclamide, indicates systemic spill of glibenclamide with subsequent stimulation of insulin secretion. However, the course in FVR in the control arm was not significantly affected by the glibenclamide infusion, indicating that the systemic changes of humoral parameters did not interfere with forearm vascular tone. Ouabain was infused at a concentration of 0.2 μg dl−1 FAV min−1, based on studies from our research group demonstrating that this concentration effectively blocked Na+K+ ATPase (Tack et al., 1996; Rongen et al., 2002). We have previously confirmed that L-NMMA 0.1 mg dl−1 FAV min−1 significantly inhibits acetylcholine-induced vasodilation (Rongen et al., 1994). As we used a dose of L-NMMA twice as high, it is unlikely that NO synthase was insufficiently inhibited. The studies regarding these references were all done under the same conditions as the currently reported experiments. We conclude that the lack of effect of the used antagonists on ATP-induced vasodilation was not due to insufficient doses.
Third, we may not have blocked the action of all EDHFs. Several EDHFs have been suggested, like metabolites of arachidonic acid produced through the cytochrome P450 (CYP 450) monooxygenase pathway and reactive oxygen species (ROS) (Pagliaro et al., 2000; Campbell & Harder, 2001). Their mechanism of action has in all cases been directly or indirectly linked to potassium channels and Na+K +ATPase. In the current study, we only blocked the KCa channels by TEA. TEA antagonizes various types of potassium channels with different degrees of potency, but the compound has been shown to block KCa channels selectively at concentrations below 1 mM (Nelson & Quayle, 1995). TEA at an infusion rate of 0.1 mg dl−1 FAV min−1 results in a local plasma concentration of approximately 0.5 mmol l−1. ATP-sensitive potassium channels (KATP channels) and voltage-dependent potassium (KV) channels can be blocked by TEA at concentrations of, respectively, 7 and 10 mM (Nelson & Quayle, 1995). Thus, the concentration we used was not sufficient to block these channels. EDHFs might, however, also exert their effects via KATP, Kv, and KIR (inwardly rectifying potassium) channels, although their role in EDHF-induced vasodilation is uncertain. Furthermore, glibenclamide did not inhibit ATP-induced vasodilation. Fourth, a redundancy of vasodilator mechanisms of ATP could potentially have prevented the inhibition of ATP- induced vasodilation by interruption of a single vasodilator pathway. However, use of a combination of substances that affect NO, COX, and EDHF still did not reveal a reduction in ATP-induced vasodilation. It is of interest for further experiments to combine the infusion of barium chloride (blocker of KIR channels) and ouabain, which might have a greater inhibitory effect on ATP-induced vasodilation than ouabain alone. Finally, there might be a different mechanism of vasodilation/EDRF besides NO, prostacyclin, and EDHFs that contributes to the ATP-induced vasodilation in vivo. For instance, evidence is accumulating that carbon monoxide (CO) can be an important vascular paracrine factor (Kozma et al., 1999).
In conclusion, the present findings do not support a role for NO, prostacyclin or EDHFs that act by opening KATP channels, KCa channels, or by activation of Na/K-ATPase in ATP-induced vasodilation. In humans, the role of endothelium-independent mechanisms and involvement of unblocked EDRFs remains to be explored.
Acknowledgments
The contribution of Dr G.A. Rongen has been made possible by a fellowship of the Royal Netherlands Academy of Arts and Sciences. The Striadyne was a generous gift of Dr K.J. Duijn, Wyeth, The Netherlands.
Abbreviations
- AU
arbitrary unit
- ATP
adenosine-5′-triphosphate
- CNP
C-type natriuretic peptide
- CO
carbon monoxide
- EDHFs
endothelium-derived hyperpolarizing factors
- EDRF
endothelium-derived relaxing factor
- EET
epoxyeicosa trienoic acid
- FAV
forearm volume
- FBF
forearm blood flow
- FVR
forearm vascular resistance
- HETE
hydroxyeicosatetraenoic acid
- H2O2
hydrogen peroxide
- KATP channels
ATP-sensitive potassium channels
- KCa channels
calcium-dependent potassium channels
- KIR channels
inwardly rectifying potassium channels
- KV channels
voltage-dependent potassium channels
- L-NMMA
NG-monomethyl-L-arginine
- MAP
mean arterial blood pressure
- PGH2
prostaglandin H2
- PGI2
prostacyclin
- SNP
sodiumnitroprusside
- TEA
tetraethylammonium chloride
- TXA2
thromboxane A2
- VSMC
vascular smooth muscle cell
References
- ABBINK E.J., WALKER A.J., VAN DER SLUIJS H.A., TACK C.J., SMITS P. No role of calcium- and ATP-dependent potassium channels in insulin-induced vasodilation in humans in vivo. Diabetes Metab. Res. Rev. 2002;18:143–148. doi: 10.1002/dmrr.269. [DOI] [PubMed] [Google Scholar]
- ASANO M., MASUZAWA-ITO K., MATSUDA T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br. J. Pharmacol. 1993;108:214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW R.S., WHITE R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am. J. Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- BAUERSACHS J., POPP R., HECKER M., SAUER E., FLEMING I., BUSSE R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- BIJLSTRA P.J., LUTTERMAN J.A., RUSSEL F.G., THIEN T., SMITS P. Interaction of sulphonylurea derivatives with vascular ATP-sensitive potassium channels in humans (published erratum appears in Diabetologia 1996;39:1414) Diabetologia. 1996;39:1083–1090. doi: 10.1007/BF00400658. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E. Potassium channels in vascular smooth muscle. Clin. Exp. Pharmacol. Physiol. 1996;23:1069–1076. doi: 10.1111/j.1440-1681.1996.tb01172.x. [DOI] [PubMed] [Google Scholar]
- BRAYDEN J.E., NELSON M.T. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- BROWN C.M., BURNSTOCK G. The structural conformation of the polyphosphate chain of the ATP molecule is critical for its promotion of prostaglandin biosynthesis. Eur. J. Pharmacol. 1981;69:81–86. doi: 10.1016/0014-2999(81)90604-x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., SNEDDON P. Evidence for ATP and noradrenaline as cotransmitters in sympathetic nerves. Clin. Sci. 1985;68 Suppl 10:89s–92s. doi: 10.1042/cs068s089. [DOI] [PubMed] [Google Scholar]
- CALDER J.A., SCHACHTER M., SEVER P.S. Potassium channel opening properties of thiazide diuretics in isolated guinea pig resistance arteries. J. Cardiovasc. Pharmacol. 1994;24:158–164. doi: 10.1097/00005344-199407000-00024. [DOI] [PubMed] [Google Scholar]
- CAMPBELL W.B., HARDER D.R. Prologue: EDHF – what is it. Am. J. Physiol Heart Circ. Physiol. 2001;280:H2413–H2416. doi: 10.1152/ajpheart.2001.280.6.H2413. [DOI] [PubMed] [Google Scholar]
- COATS P., JOHNSTON F., MACDONALD J., MCMURRAY J.J., HILLIER C. Endothelium-derived hyperpolarizing factor: identification and mechanisms of action in human subcutaneous resistance arteries. Circulation. 2001;103:1702–1708. doi: 10.1161/01.cir.103.12.1702. [DOI] [PubMed] [Google Scholar]
- DAWES M., SIENIAWSKA C., DELVES T., DWIVEDI R., CHOWIENCZYK P.J., RITTER J.M. Barium reduces resting blood flow and inhibits potassium-induced vasodilation in the human forearm. Circulation. 2002;105:1323–1328. doi: 10.1161/hc1102.105651. [DOI] [PubMed] [Google Scholar]
- DUFFY S.J., CASTLE S.F., HARPER R.W., MEREDITH I.T. Contribution of vasodilator prostanoids and nitric oxide to resting flow, metabolic vasodilation, and flow-mediated dilation in human coronary circulation. Circulation. 1999b;100:1951–1957. doi: 10.1161/01.cir.100.19.1951. [DOI] [PubMed] [Google Scholar]
- DUFFY S.J., NEW G., TRAN B.T., HARPER R.W., MEREDITH I.T. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am. J. Physiol. 1999a;276:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- DUFFY S.J., TRAN B.T., NEW G., TUDBALL R.N., ESLER M.D., HARPER R.W., MEREDITH I.T. Continuous release of vasodilator prostanoids contributes to regulation of resting forearm blood flow in humans. Am. J. Physiol. 1998;274:H1174–H1183. doi: 10.1152/ajpheart.1998.274.4.H1174. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries (see comments) Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br. J. Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON J.L. Extracellular ATP: effects, sources and fate. Biochem. J. 1986;233:309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMSEN H., STORM E., DAY H.J. Determination of ATP and ADP in blood platelets: a modification of the firefly luciferase assay for plasma. Anal. Biochem. 1972;46:489–501. doi: 10.1016/0003-2697(72)90323-5. [DOI] [PubMed] [Google Scholar]
- HONING M.L., SMITS P., MORRISON P.J., BURNETT J.C., JR, RABELINK T.J. C-type natriuretic peptide-induced vasodilation is dependent on hyperpolarization in human forearm resistance vessels. Hypertension. 2001;37:1179–1183. doi: 10.1161/01.hyp.37.4.1179. [DOI] [PubMed] [Google Scholar]
- HONING M.L., SMITS P., MORRISON P.J., RABELINK T.J. Bradykinin-induced vasodilation of human forearm resistance vessels is primarily mediated by endothelium-dependent hyperpolarization. Hypertension. 2000;35:1314–1318. doi: 10.1161/01.hyp.35.6.1314. [DOI] [PubMed] [Google Scholar]
- KEEF K.D., PASCO J.S., ECKMAN D.M. Purinergic relaxation and hyperpolarization in guinea pig and rabbit coronary artery: role of the endothelium. J. Pharmacol. Exp. Ther. 1992;260:592–600. [PubMed] [Google Scholar]
- KHATRI J., QASSIM S., ABED O., ABRAHAM B., AL LAMI A., MASOOD S. A novel extractionless hplc fluorescence method for the determination of glyburide in the human plasma: application to a bioequivalence study. J. Pharm. Pharm. Sci. 2001;4:201–206. [PubMed] [Google Scholar]
- KILBOM A., WENNMALM A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J. Physiol. 1976;257:109–121. doi: 10.1113/jphysiol.1976.sp011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZMA F., JOHNSON R.A., ZHANG F., YU C., TONG X., NASJLETTI A. Contribution of endogenous carbon monoxide to regulation of diameter in resistance vessels. Am. J. Physiol. 1999;276:R1087–R1094. doi: 10.1152/ajpregu.1999.276.4.R1087. [DOI] [PubMed] [Google Scholar]
- LAGAUD G.J., SKARSGARD P.L., LAHER I., VAN BREEMEN C. Heterogeneity of endothelium-dependent vasodilation in pressurized cerebral and small mesenteric resistance arteries of the rat. J. Pharmacol. Exp. Ther. 1999;290:832–839. [PubMed] [Google Scholar]
- LENDERS J., JANSSEN G.J., SMITS P., THIEN T. Role of the wrist cuff in forearm plethysmography. Clin. Sci. Colch. 1991;80:413–417. doi: 10.1042/cs0800413. [DOI] [PubMed] [Google Scholar]
- LEVY M., SABRY S., MERCIER J.C., DINH-XUAN A.T. Roles of vasoactive factors synthetized by endothelium in pulmonary arterial hypertension. Arch. Pediatr. 1997;4:271–277. doi: 10.1016/s0929-693x(97)87249-1. [DOI] [PubMed] [Google Scholar]
- MALMSJO M., EDVINSSON L., ERLINGE D. P2X receptors counteract the vasodilatory effects of endothelium derived hyperpolarising factor. Eur. J. Pharmacol. 2000;390:173–180. doi: 10.1016/s0014-2999(00)00010-8. [DOI] [PubMed] [Google Scholar]
- MALMSJO M., ERLINGE D., HOGESTATT E.D., ZYGMUNT P.M. Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor. Eur. J. Pharmacol. 1999;364:169–173. doi: 10.1016/s0014-2999(98)00848-6. [DOI] [PubMed] [Google Scholar]
- MATHIE R.T., RALEVIC V., ALEXANDER B., BURNSTOCK G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br. J. Pharmacol. 1991;103:1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIESON J.J., BURNSTOCK G. Purine-mediated relaxation and constriction of isolated rabbit mesenteric artery are not endothelium-dependent. Eur. J. Pharmacol. 1985;118:221–229. doi: 10.1016/0014-2999(85)90132-3. [DOI] [PubMed] [Google Scholar]
- MCAULEY D., MCGURK C., NUGENT A.G., HANRATTY C., MAGUIRE S., JOHNSTON G.D. Forearm endothelium-dependent vascular responses and the potassium-ATP channel. Br. J. Clin. Pharmacol. 1997;44:292–294. doi: 10.1046/j.1365-2125.1997.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCMILLAN M.R., BURNSTOCK G., HAWORTH S.G. Vasodilatation of intrapulmonary arteries to P2-receptor nucleotides in normal and pulmonary hypertensive newborn piglets. Br. J. Pharmacol. 1999;128:543–548. doi: 10.1038/sj.bjp.0702815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYERS K.M., HOLMSEN H., SEACHORD C.L. Comparative study of platelet dense granule constituents. Am. J. Physiol. 1982;243:R454–R461. doi: 10.1152/ajpregu.1982.243.3.R454. [DOI] [PubMed] [Google Scholar]
- NAGAO T., VANHOUTTE P.M. Endothelium-derived hyperpolarizing factor and endothelium-dependent relaxations. Am. J. Respir. Cell Mol. Biol. 1993;8:1–6. doi: 10.1165/ajrcmb/8.1.1. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S., SUGIMOTO Y., USHIKUBI F. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- PAGLIARO P., RASTALDO R., PAOLOCCI N., GATTULLO D., LOSANO G. The endothelium-derived hyperpolarizing factor: does it play a role in vivo and is it involved in the regulation of vascular tone only. Ital. Heart J. 2000;1:264–268. [PubMed] [Google Scholar]
- PICKKERS P., HUGHES A.D. Relaxation and decrease in [Ca2+]i by hydrochlorothiazide in guinea-pig isolated mesenteric arteries. Br. J. Pharmacol. 1995;114:703–707. doi: 10.1111/j.1476-5381.1995.tb17195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PICKKERS P., HUGHES A.D., RUSSEL F.G., THIEN T., SMITS P. Thiazide-induced vasodilation in humans is mediated by potassium channel activation. Hypertension. 1998;32:1071–1076. doi: 10.1161/01.hyp.32.6.1071. [DOI] [PubMed] [Google Scholar]
- PICKKERS P., HUGHES A.D., RUSSEL F.G., THIEN T., SMITS P. In vivo evidence for K(Ca) channel opening properties of acetazolamide in the human vasculature. Br. J. Pharmacol. 2001;132:443–450. doi: 10.1038/sj.bjp.0703825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V. Mechanism of prolonged vasorelaxation to ATP in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 2001;132:685–692. doi: 10.1038/sj.bjp.0703868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- ROBINSON B.F., PHILLIPS R.J., WILSON P.N., CHIODINI P.L. Effect of local infusion of ouabain on human forearm vascular resistance and on response to potassium, verapamil and sodium nitroprusside. J. Hypertens. 1983;1:165–169. doi: 10.1097/00004872-198308000-00009. [DOI] [PubMed] [Google Scholar]
- RONGEN G.A., DIJK J.P., VAN GINNEKEN E.E., STEGEMAN D.F., SMITS P., ZWARTS M.J. Repeated ischaemic isometric exercise increases muscle fibre conduction velocity in humans: involvement of Na(+)-K(+)-ATPase. J. Physiol. 2002;540:1071–1078. doi: 10.1113/jphysiol.2001.014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONGEN G.A., SMITS P., THIEN T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- RONGEN G.A., SMITS P., VERDONCK, WILLEMSEN J.J., DE ABREU R.A., VAN BELLE H., THIEN T. Hemodynamic and neurohumoral effects of various grades of selective adenosine transport inhibition in humans. Implications for its future role in cardioprotection. J. Clin. Invest. 1995;95:658–668. doi: 10.1172/JCI117711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATCHELL D. Purinergic nerves and purinoceptors: early perspectives. J. Auton. Nerv. Syst. 2000;81:212–217. doi: 10.1016/s0165-1838(00)00140-5. [DOI] [PubMed] [Google Scholar]
- SCHWIEBERT L.M., RICE W.C., KUDLOW B.A., TAYLOR A.L., SCHWIEBERT E.M. Extracellular ATP signaling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am. J. Physiol. Cell. Physiol. 2002;282:C289–C301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- SHIRAMOTO M., IMAIZUMI T., HIROOKA Y., ENDO T., NAMBA T., OYAMA J., HIRONAGA K., TAKESHITA A. Role of nitric oxide towards vasodilator effects of substance P and ATP in human forearm vessels. Clin. Sci. (Lond.) 1997;92:123–131. doi: 10.1042/cs0920123. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., YAMAMOTO Y., FUKUTA H. Endothelium-derived hyperpolarizing factor and vasodilatation. Nippon Yakurigaku Zasshi. 1998;112:195–202. doi: 10.1254/fpj.112.195. [DOI] [PubMed] [Google Scholar]
- TACK C.J., LUTTERMAN J.A., VERVOORT G., THIEN T., SMITS P. Activation of the sodium–potassium pump contributes to insulin-induced vasodilation in humans. Hypertension. 1996;28:426–432. doi: 10.1161/01.hyp.28.3.426. [DOI] [PubMed] [Google Scholar]
- TADDEI S., GHIADONI L., VIRDIS A., BURALLI S., SALVETTI A. Vasodilation to bradykinin is mediated by an ouabain-sensitive pathway as a compensatory mechanism for impaired nitric oxide availability in essential hypertensive patients. Circulation. 1999;100:1400–1405. doi: 10.1161/01.cir.100.13.1400. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., BOULANGER C.M., ILLIANO S.C., NAGAO T., VIDAL M., MOMBOULI J.V. Endothelium-dependent effects of converting-enzyme inhibitors. J. Cardiovasc. Pharmacol. 1993;22 Suppl. 5:S10–S16. doi: 10.1097/00005344-199322005-00003. [DOI] [PubMed] [Google Scholar]
- VUORINEN P., PORSTI I., METSA KETELA T., MANNINEN V., VAPAATALO H., LAUSTIOLA K.E. Endothelium-dependent and -independent effects of exogenous ATP, adenosine, GTP and guanosine on vascular tone and cyclic nucleotide accumulation of rat mesenteric artery. Br. J. Pharmacol. 1992;105:279–284. doi: 10.1111/j.1476-5381.1992.tb14246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VUORINEN P., WU X., ARVOLA P., VAPAATALO H., POSTI I. Effects of P1 and P2Y purinoceptor antagonists on endothelium-dependent and -independent relaxations of rat mesenteric artery to GTP and guanosine. Br. J. Pharmacol. 1994;112:71–74. doi: 10.1111/j.1476-5381.1994.tb13031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG L., KARLSSON L., MOSES S., HULTGARDH-NILSSON A., ANDERSSON M., BORNA C., GUDBJARTSSON T., JERN S., ERLINGE D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J. Cardiovasc. Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- WILSON J.R., KAPOOR S.C. Contribution of prostaglandins to exercise-induced vasodilation in humans. Am. J. Physiol. 1993;265:H171–H175. doi: 10.1152/ajpheart.1993.265.1.H171. [DOI] [PubMed] [Google Scholar]
- ZINK M.H., OLTMAN C.L., LU T., KATAKAM P.V., KADUCE T.L., LEE H., DELLSPERGER K.C., SPECTOR A.A., MYERS P.R., WEINTRAUB N.L. 12-Lipoxygenase in porcine coronary microcirculation: implications for coronary vasoregulation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H693–H704. doi: 10.1152/ajpheart.2001.280.2.H693. [DOI] [PubMed] [Google Scholar]