Abstract
The mechanism of toxicity of sulphur mustard was investigated by examining the biochemical effects of the analog 2-chloroethylethyl sulphide (CEES) in both human Jurkat cells as well as normal human lymphocytes.
Exposure of both types of cells to CEES resulted in a marked decrease in the intracellular concentration of the reduced form of glutathione (GSH), and CEES-induced cell death was potentiated by L-buthionine sulphoximine, an inhibitor of GSH synthesis.
CEES increased the endogenous production of reactive oxygen species (ROS) in Jurkat cells, and CEES-induced cell death was potentiated by hydrogen peroxide.
CEES induced various hallmarks of apoptosis, including collapse of the mitochondrial membrane potential, proteolytic processing and activation of procaspase-3, and cleavage of poly (ADP-ribose) polymerase.
The effects of CEES on the accumulation of ROS, the intracellular concentration of GSH, the mitochondrial membrane potential, and caspase-3 activity were all inhibited by pretreatment of cells with the GSH precursor N-acetyl cysteine or with GSH-ethyl ester. Furthermore, CEES-induced cell death was also prevented by these antioxidants.
CEES toxicity appears to be mediated, at least in part, by the generation of ROS and consequent depletion of GSH. Given that sulphur mustard is still a potential biohazard, the protective effects of antioxidants against CEES toxicity demonstrated in Jurkat cells and normal human lymphocytes may provide the basis for the development of a therapeutic strategy to counteract exposure to this chemical weapon.
Keywords: 2-Chloroethylethyl sulphide, sulphur mustard, apoptosis, N-acetyl cysteine, reactive oxygen species, mitochondrial membrane potential, poly (ADP-ribose) polymerase
Introduction
Sulphur mustard (SM) was first used as a chemical weapon on the battlefields of World War I. It has re-emerged, however, as a major concern in recent years. SM was thus used during the Iran–Iraq war of the late 1980s, and is suspected to have been deployed during the first Persian Gulf War. The vast numbers of casualties potentially inflicted by SM and the lengthy hospitalization (an average of 42 days) required for treatment of exposure to this agent render it a serious threat to military effectiveness.
The SM derivative 2-chloroethylethyl sulphide (CEES) has been studied to gain insight into the mechanism of action of SM. Like SM, CEES induces vesiculation, as well as alkylates a wide range of biological molecules. Both SM and CEES alkylate DNA strands in the 7-position of guanine, the 3-position of adenine, and the O6-position of the guanine (Ludlum et al., 1986; Zhang et al., 2002). CEES is also involved in the induction of cell death by enhancement of the DNA repair process, which activates poly (ADP-ribose) polymerase (PARP) with the subsequent NAD depletion (Papirmeister et al., 1985) and oxidative stress (Husain et al., 1996). Therefore, CEES, which is commercially available, is a valid alternative instead of SM to perform most in vitro experiments.
Although SM is absorbed into the circulation at sites of direct contact, such as the skin, eyes, and airways, these contact sites are usually most affected. If substantial amounts of the agent are absorbed, however, damage to the haematopoietic system, gastrointestinal tract, and CNS can result (Dacre & Goldman, 1996). Haematological complications of SM exposure in humans or rats include leukopenia and bone marrow depletion, indicating that this agent targets leucocytes. Exposure of the Jurkat human T-cell line to CEES has recently been shown to induce biochemical and morphological changes characteristic of apoptotic cell death. These changes include chromatin condensation, degradation of genomic DNA into both high molecular weight and oligonucleosomal fragments, upregulation of caspase-3, -4, -6, -8, and -9, as well as downregulation of Akt (protein kinase B), the latter of which inhibits apoptotic signalling (Zhang et al., 2002).
An important early event in the commitment of cells to apoptosis is loss of the mitochondrial membrane potential (ΔΨmito) (Mancini et al., 1997; Salvioli et al., 1997). The perturbation of mitochondrial function during the early stages of apoptosis is associated with an increased generation of reactive oxygen species (ROS) (Jabs, 1999), which creates an oxidative environment that contributes to the death process, at least in part, by resulting in the depletion of cellular antioxidants such as reduced glutathione (GSH) and by inducing direct damage to DNA, proteins, and lipids. Exogenous antioxidants, such as N-acetyl cysteine (NAC) and GSH, have thus been shown to protect cells against apoptosis (Hour et al., 1999; Shrivastava & Aggarwal, 1999; Li et al., 2000).
Studies with other toxic agents have implicated ROS generation and depletion of cellular antioxidants in toxicity and induced cell death (Arakaki et al., 1999; Wolfler et al., 2001; Lee et al., 2002). To provide greater insight into the mechanism of SM action in order to identify potential treatments for exposure to this agent, we have now investigated the possible contribution of ROS generation and depletion of cellular antioxidants to SM toxicity. For these studies, we examined the effects of CEES in Jurkat cells and in normal human lymphocytes. We chose these cells because of the important role played by lymphocytes in the immunological response against vesicants. Since SM and CEES are immunotoxic (Dacre & Goldman, 1996; Hur et al., 1998), in the future, antioxidants may be considered to minimize the toxicity of these vesicants and reduce immunotoxicity. In addition, many groups study the molecular mechanisms of apoptosis extensively, using Jurkat cells.
Methods
Cell culture and treatment
Jurkat T cells were bought from ATCC and were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated foetal bovine serum, penicillin (100 U ml−1), and streptomycin (100 mg ml−1). Normal human lymphocytes were derived from a normal individual and immortalized using Epstein–Barr virus at Georgetown University Shared Tissue Culture Resources. These cells were cultured in RPMI 1640 medium supplemented with sodium pyruvate (100 mg ml−1), nonessential amino acids (10 mg ml−1), glutamine (200 mg ml−1), and gentamicin (10 mg ml−1). A 14% v v−1 CEES solution was prepared in absolute ethanol, and was diluted further 1 : 2000 with complete culture medium. A 0.05% solution of ethanol in culture medium (vehicle) had no effect on cell viability (data not shown). The density of CEES is 1.07 g ml−1; a 1/14 000 dilution therefore corresponds to a CEES concentration of 600 μM.
Assay of cell viability
Cells were seeded in 24-well plates. After treatment with CEES, they were exposed to calcein-AM at a final concentration of 2.5 μM, and incubated for 30 min at 37°C. The fluorescence generated by de-esterification of calcein-AM was monitored with a CytoFluor 4000 fluorometer (PerSeptive Biosystems, Framingham, MA, U.S.A.) at excitation and emission wavelengths of 488 and 520 nm, respectively.
Assay of intracellular GSH
After exposure to CEES, cells grown in six-well plates were immediately washed twice with phosphate-buffered saline and then lysed by three cycles of freezing and thawing in 10 mM HCl. Cell lysates were depleted of protein by exposure to 5-sulphosalicylic acid at a final concentration of 10%, and subsequent centrifugation at 1000 × g for 5 min. The resulting supernatants were assayed for nonprotein sulphydryl groups by spectrophotometric determination of the reduction of 5,5′-dithio-bis-[2-nitrobenzoic acid] (DTNB) to 5-thio-2-nitrobenzoic acid, at a wavelength of 412 nm. Standard curves were constructed with known amounts of GSH in all experiments.
Assay of caspase-3 activity
Caspase-3 activity was assayed with the fluorogenic substrate Ac-DEVD-AMC, essentially as described previously (Boulares et al., 1999). In brief, cell extracts (30 μg of protein) were incubated with 40 μM Ac-DEVD-AMC in a total volume of 200 μl, and the fluorescence of free aminomethylcoumarin (AMC) generated by cleavage of the aspartate–AMC bond was measured continuously for 30 min with a CytoFluor 4000 fluorometer, at excitation and emission wavelengths of 360 and 460 nm, respectively. The fluorescence emission was plotted against time, and linear regression analysis of the initial velocity for each curve yielded the activity.
Determination of ROS concentration
The generation of ROS was monitored with the use of the cell-permeable probe dihydrodichlorofluorescein (H2DCF). Cells grown in 96-well plates were exposed to CEES and then loaded with H2DCF by incubation for 15 min in the dark with the diacetate form of the compound (10 μM). In the presence of ROS, the nonfluorescent H2DCF is oxidized to the highly fluorescent DCF. Fluorescence was measured at excitation and emission wavelengths of 485 and 530 nm, respectively.
Measurement of ΔΨmito
After treatment with CEES, cells were incubated for 10 min at 37°C with 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (15 μg ml−1), a cell-permeable dye that becomes concentrated in the mitochondria and generates red fluorescence in a manner dependent on ΔΨmito. JC-1 fluorescence was measured with a Becton Dickinson FACS flow cytometer, as described (Boulares et al., 2001).
Immunoblot analysis
Cells were harvested, washed with ice-cold phosphate-buffered saline, and lysed on ice with a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM phenylmethylsulphonyl fluoride, pepstatin (10 μg ml−1), aprotinin (10 μg ml−1), and leupeptin (20 μg ml−1). Cell lysates (30 μg of protein) were then subjected to polyacrylamide gel electrophoresis on 4–20% gradient gels in the presence of sodium dodecyl sulphate, after which the separated proteins were transferred to a nitrocellulose membrane and subjected to immunoblot analysis with various primary antibodies. Immune complexes were visualized with horseradish peroxidase-conjugated antibodies to mouse and rabbit IgG, and enhanced chemiluminescence reagents.
Materials
Fetal bovine serum was obtained from Quality Biological (Gaithersburg, MD, U.S.A.) and RPMI 1640 was from Invitrogen (Carlsbad, CA, U.S.A.). GSH-ethyl ester, NAC, L-buthionine sulphoximine (L-BSO), hydrogen peroxide, staurosporine, 3-aminobenzamide (3-AB), 5.5′-dithio-bis(2-nitrobenzoic acid), and mouse monoclonal antibodies to β-tubulin were obtained from Sigma (St Louis, MO, U.S.A.). CEES was from Aldrich (Milwaukee, WI, U.S.A.) and calcein-AM, JC-1, and H2DCF were from Molecular probes (Eugene, OR, U.S.A.). Mouse monoclonal antibodies to PARP were from BD Transduction Laboratories (San Diego, CA, U.S.A.), horseradish peroxidase-conjugated antibodies to mouse and rabbit IgG were from Amersham (Piscataway, NJ, U.S.A.), and mouse monoclonal antibodies to caspase-3 were from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Enhanced chemiluminescence reagents were obtained from Pierce (Rockford, IL, U.S.A.), and Ac-DEVD-AMC was from Biomol (Plymouth Meeting, PA, U.S.A.).
Statistical analysis
Data were analyzed by Student's t-test. A P-value of <0.05 was considered statistically significant.
Results
Role of intracellular GSH in CEES-induced death of Jurkat cells
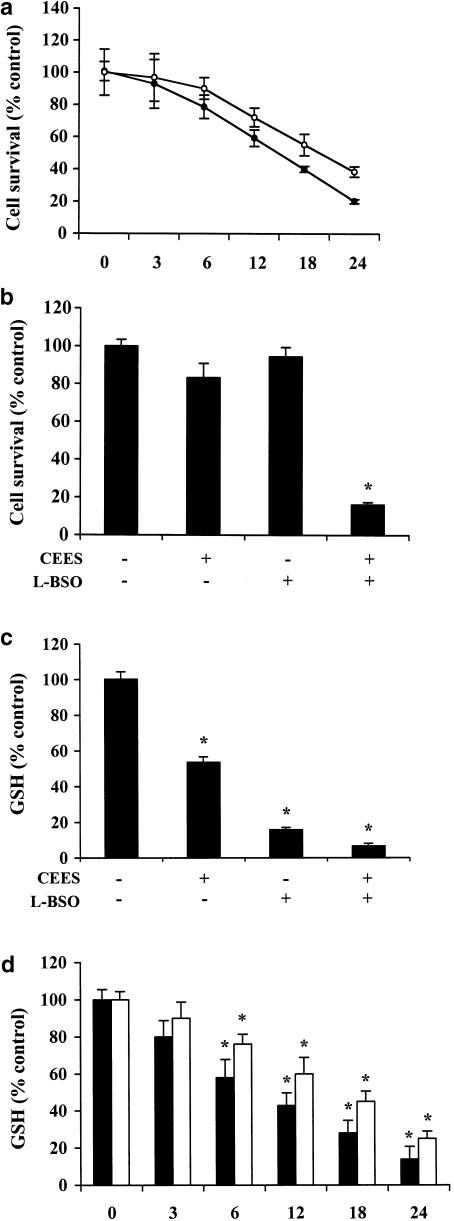
Exposure of Jurkat T cells to CEES was recently shown to induce apoptotic death (Zhang et al., 2002). In the present study, calcein-AM staining revealed that incubation of normal lymphocytes and Jurkat cells for 24 h with 600 μM CEES resulted in an approximately 60 and 80% loss of viability, respectively (Figure 1a). Given that various toxic agents affect the intracellular concentration of GSH (Hathway, 2000), we examined the possible relevance of endogenous GSH to CEES toxicity in Jurkat cells. We incubated cells for 20 h with 200 μM L-BSO, a selective inhibitor of γ-glutamylcysteine synthetase, the rate-limiting enzyme in GSH biosynthesis (Chiba et al., 1996; Oda et al., 1999), in order to deplete them of GSH. Such treatment reduced the intracellular GSH concentration by 80% (Figure 1c). The cells were then exposed to CEES for 6 h, after which the cell viability was measured by calcein-AM staining. Inhibition of GSH synthesis by L-BSO resulted in marked potentiation of CEES-induced cell death (Figure 1b), whereas CEES alone resulted in an ∼20% decrease in cell viability, the combination of the two agents resulted in >80% cell death.L-BSO per se had no significant effect on cell viability.
Figure 1.
Role of intracellular GSH in CEES-induced death in lymphocytes. (a) Jurkat cells (closed circles) and normal lymphocytes (open circles) were incubated for the indicated times with 600 μM CEES, after which cell viability was assessed by measurement of calcein-AM fluorescence. Jurkat cells were incubated first for 20 h with or without 200 μM L-BSO and then for 6 h in the additional absence or presence of CEES, after which cell viability (b) and intracellular abundance of GSH (c) was determined. (d) Jurkat cells (closed bars) and lymphocytes (open bars) were treated with 600 μM CEES at the indicated times, after which GSH content was analysed. All data are means±s.d. of triplicates from an experiment that was repeated a total of three times with similar results. (*)P<0.05 versus control value for untreated cells.
We next examined the effect of exposure to CEES for 6 h on the intracellular level of GSH in Jurkat cells. CEES induced an ∼50% decrease in the intracellular concentration of GSH (Figure 1c). Such CEES treatment also reduced the abundance of GSH further in cells that had been incubated with L-BSO for 20 h (Figure 1c). Together, these data suggest that the intracellular concentration of GSH is a determinant of CEES cytotoxicity. Therefore, in order to evaluate the kinetics of GSH reduction in normal lymphocytes and Jurkat cells exposed to CEES, we measured the levels of GSH for several time intervals from 0 to 24 h. The results (Figure 1d) indicate that CEES induces depletion of GSH content in both cells types in a time-dependent manner, as observed in the cell survival data (Figure 1a).
Role of oxidative stress in CEES-induced cell death
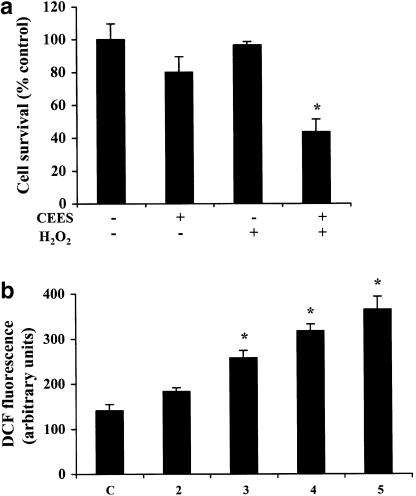
We hypothesized that the depletion of GSH in Jurkat cells induced by exposure to CEES was the result of an increased generation of ROS. To test this hypothesis, we first examined the effect of a low concentration of H2O2 on CEES-induced cell death. The cells were thus pretreated with 25 μM H2O2 for 1 h and then incubated with CEES for 6 h in the continued presence of H2O2, after which cell viability was assessed by calcein-AM staining. Whereas exposure of the cells to H2O2 alone had no effect on viability and CEES alone induced an ∼20% loss of viability, the combination of these agents resulted in >50% cell death (Figure 2a).
Figure 2.
Role of ROS in CEES-induced death of Jurkat cells. (a) Jurkat cells were incubated first for 1 h with or without 25 μM H2O2, and then for 6 h in the additional absence or presence of 600 μM CEES, after which cell viability was assessed by measurement of calcein-AM fluorescence. (b) Cells were incubated with CEES (600 μM) for the indicated times, after which ROS generation was measured by fluorometric analysis of H2DCF oxidation. (c) Cells were incubated first for 1 h with or without 1 mM GSH-ethyl ester (GSHE) or 5 mM NAC, and then for 5 h in the additional absence or presence of 600 μM CEES, after which ROS generation was determined. All data are means±s.d. of values from an experiment that was repeated three times with similar results. *P<0.05 versus control value for untreated cells.
We next examined whether the SM derivative induced the generation of ROS in Jurkat cells. Measurement of the oxidation of H2DCF to DCF by fluorometry revealed that incubation of Jurkat cells with CEES for up to 5 h induced a time-dependent increase in the endogenous production of ROS (Figure 2b). Preincubation of the cells for 1 h with the cell-permeable ethyl ester of GSH (1 mM) or with the GSH precursor NAC (5 mM) markedly inhibited the CEES-induced accumulation of ROS (Figure 2c). These results thus indeed suggested that the depletion of GSH induced by CEES in Jurkat cells occurs in response to an increased generation of ROS.
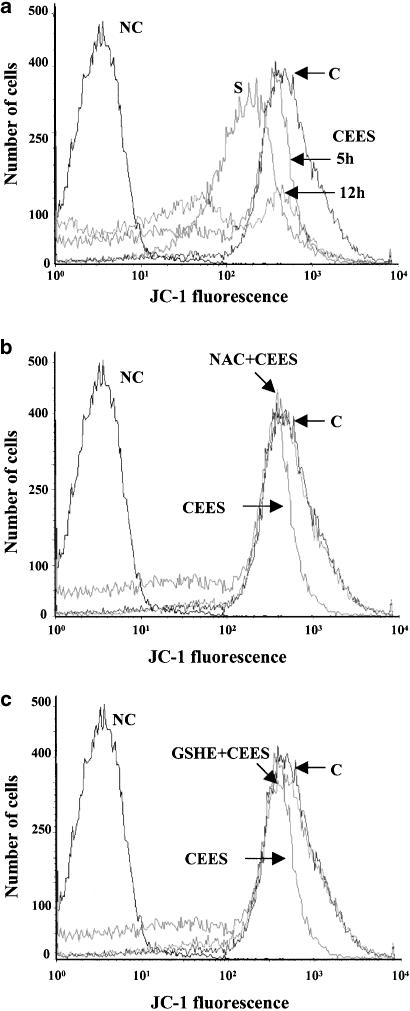
Effect of CEES on ΔΨmito in Jurkat cells
Mitochondrial dysfunction and the loss of Ψmito accompany apoptotic death in various cell types (Mancini et al., 1997; Salvioli et al., 1997). The opening of the mitochondrial permeability transition pore during the loss of ΔΨmito results in the release of several proapoptotic factors, including cytochrome c, into the cytosol, the consequent activation of caspase-9 and -3, and irreversible commitment to apoptosis (Gottlieb, 2000). We therefore examined whether CEES-induced cell death is associated with a loss of ΔΨmito. Jurkat cells were exposed to CEES for 5 or 12 h, and then ΔΨmito was examined with the specific probe JC-1 and flow cytometry. CEES indeed resulted in a time-dependent loss of ΔΨmito (Figure 3a); we exposed cells to 1 μM staurosporine, an established inducer of apoptosis, as a positive control. Pretreatment of the cells with either NAC or GSH-ethyl ester prevented the loss of ΔΨmito induced by CEES (Figure 3b and c). These data thus implicate mitochondria as a target of the SM derivative, and show that antioxidants protect against the CEES-induced loss of ΔΨmito.
Figure 3.
CEES-induced loss of ΔΨmito and protective effect of antioxidants in Jurkat cells. (a) Cells were incubated for 5 or 12 h in the presence of 600 μM CEES, or for 5 h with 1 μM staurosporine (S), after which ΔΨmito was evaluated with the use of JC-1 and flow cytometry. Traces labeled C and NC correspond to cells that were not treated with CEES or staurosporine, and that were either exposed or not, respectively, to JC-1. (b, c) Cells were incubated first for 1 h with 5 mM NAC (b) or 1 mM GSH-ethyl ester (c), and then for 5 h in the additional presence of CEES. Traces labeled C and NC were as described in (a). Panels are representative of three independent experiments.
Protective effect of thiol antioxidants against CEES-induced cell death
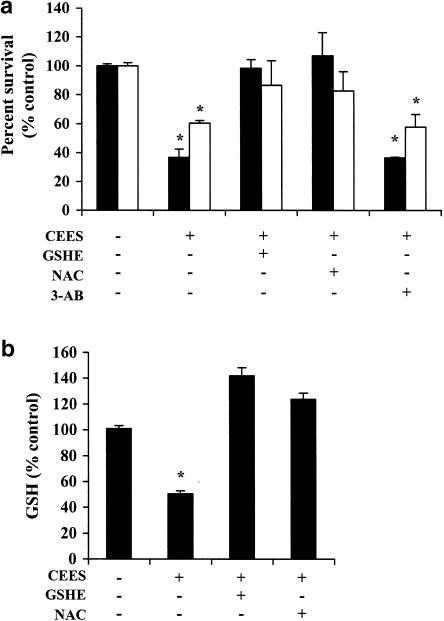
We next determined whether thiol antioxidants such as GSH and NAC are able to protect lymphocytes against CEES toxicity. Pretreatment of normal lymphocytes and Jurkat cells with NAC or GSH-ethyl ester for 1 h before exposure to the SM derivative for 18 h (in the continued presence of antioxidant) significantly inhibited cell death in both types of cells exposed to CEES alone (Figure 4a). Similar pretreatment of cells with NAC or GSH-ethyl ester also prevented the depletion of intracellular GSH in Jurkat cells induced by incubation with CEES for 6 h (Figure 4b). These data thus provided further support for the notion that CEES cytotoxicity is mediated by an increase in ROS generation and a consequent decrease in the intracellular concentration of GSH or vice versa. This is an interesting possibility and will be examined in a future manuscript.
Figure 4.
Effects of thiol antioxidants and a PARP inhibitor on CEES-induced cytotoxicity. (a) Jurkat cells (closed bars) and normal lymphocytes (open bars) were incubated first for 1 h with or without 5 mM NAC, 1 mM GSH-ethyl ester, or 5 mM 3-AB, and then for 18 h in the additional absence or presence of 600 μM CEES, after which cell viability was assessed by measurement of calcein-AM fluorescence. (b) Jurkat cells were incubated first for 1 h with or without NAC or GSH-ethyl ester, and then for 6 h in the additional absence or presence of CEES, after which the intracellular concentration of GSH was determined. All data are means±s.d. of triplicates from an experiment that was repeated a total of three times with similar results. *P<0.05 versus the control value for untreated cells.
With the use of both mouse fibroblasts stably transfected with PARP antisense cDNA and fibroblasts derived from PARP knockout mice, we have previously shown that a transient early phase of poly (ADP-ribosyl)ation is required for apoptosis triggered by antibodies to Fas or by cycloheximide (Simbulan-Rosenthal et al., 1998). We therefore examined the effect of the PARP inhibitor 3-aminobenzamide (3-AB) (5 mM) on CEES-induced death of Jurkat cells. The PARP inhibitor did not substantially affect CEES cytotoxicity (Figure 4a), suggesting that PARP is not required for cell death induced by the SM derivative.
CEES-induced activation of caspase-3
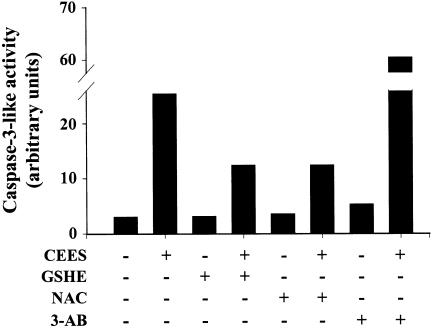
Caspase-3 is an executioner protease whose activation commits cells to apoptotic death (Nicholson et al., 1995). In addition to its other substrates, caspase-3 cleaves PARP into a 24-kDa fragment that contains the DNA-binding domain and an 89-kDa fragment that contains the automodification and catalytic domains (Tewari et al., 1995). This cleavage results in inactivation of PARP, given that its activity requires its binding to the ends of DNA strand breaks (Smulson et al., 1994). We next examined the effect of CEES on caspase-3-like activity in Jurkat cells. CEES indeed induced marked activation of caspase-3 in these cells, and this effect was inhibited by pretreatment with NAC or GSH-ethyl ester (Figure 5). When the Jurkat cells, however, are treated with the PARP inhibitor 3-AB, the CEES-induced increase in caspase-3 activation was not affected.
Figure 5.
CEES-induced activation of caspase-3 and inhibitory effects of thiol antioxidants in Jurkat cells. Cells were incubated first for 1 h with or without 5 mM NAC, 1 mM GSH-ethyl ester, or 5 mM 3-AB, and then for 12 h in the additional absence or presence of 600 μM CEES. Cell extracts were then prepared and assayed for caspase-3-like activity. Data are expressed in arbitrary units, and are from an experiment that was repeated a total of three times with similar results.
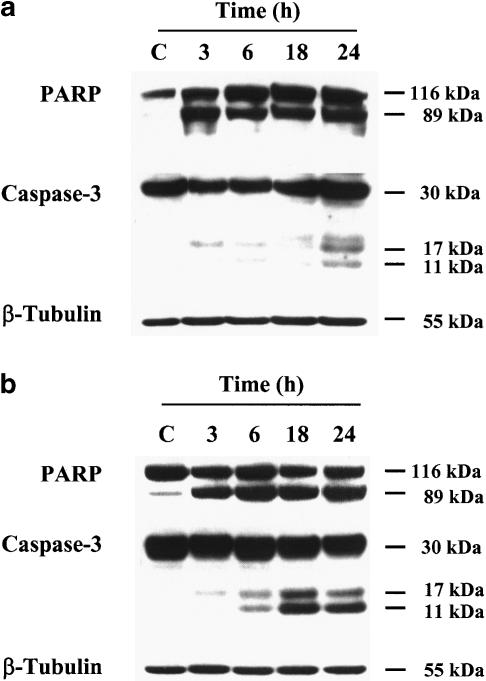
Immunoblot analysis confirmed that CEES induced the cleavage of procaspase-3 to the active form of the enzyme in a time-dependent manner (Figure 6a, b). Furthermore, the CEES-induced cleavage of procaspase-3 in normal human lymphocytes and Jurkat cells was accompanied by the cleavage of PARP, which was detected as early as 3 h after exposure to the SM derivative.
Figure 6.
CEES induces PARP cleavage and activation of procaspase-3 in lymphocytes. Jurkat cells (a) and normal lymphocytes (b) were incubated for the indicated times with CEES, after which cell lysates were subjected to immunoblot analysis with antibodies to PARP, to caspase-3, or to β-tubulin (control).
Discussion
The interaction of thiol groups with cysteine residues of intracellular proteins helps to maintain the reduced state of these residues, which is required for normal protein structure and function. Thiol compounds also function in cells to scavenge hydroxyl radicals, H2O2, singlet oxygen, as well as reactive metabolites of lipids and proteins (Jabs, 1999). NAC is a precursor of the important cellular antioxidant GSH, and incubation of cells with this compound thus increases the intracellular concentration of GSH. In contrast, incubation of cells with L-BSO, which inhibits γ-glutamylcysteine synthetase, or with H2O2 results in depletion of GSH and the creation of an oxidative intracellular environment. With the use of such compounds, we have now demonstrated that the intracellular concentration of GSH is an important determinant of CEES toxicity in both normal lymphocytes as well as Jurkat T cells.
We thus showed that CEES reduces the intracellular level of GSH, and that CEES acts synergistically with L-BSO or with H2O2 to induce cell death. The depletion of intracellular GSH induced by CEES appears to result from an increased generation of ROS. However, it is possible that CEES initially causes GSH depletion, which may consequently lead to ROS generation. This interpretation is compatible with the caspase activation event that is associated with the increase of ROS production (Chen et al., 2003). Consistent with these observations, GSH-ethyl ester and NAC each inhibited ROS accumulation and the loss of intracellular GSH induced by the SM derivative. More importantly, these exogenous antioxidants protected normal lymphocytes and Jurkat cells from CEES-induced cell death. High levels of intracellular ROS have previously been implicated in cell death induced by H2O2 (Sanij et al., 2001; Lee et al., 2002; Boulares et al., 2003). Our data thus indicate that the pro-oxidant activity of CEES and the consequent depletion of intracellular GSH are essential steps in the induction of apoptosis by this agent.
An important step in apoptosis induced by various biological and chemical agents is the loss of ΔΨmito and the consequent release into the cytosol of cytochrome c, which then interacts with Apaf-1, and the resulting complex binds to and thereby triggers the proteolytic activation of procaspase-9 (Cohen, 1997; Strasser et al., 2000). We have now shown that CEES induced a time-dependent loss of ΔΨmito in Jurkat cells, and that this effect was blocked by NAC or GSH-ethyl ester. These results suggest that the loss of ΔΨmito triggered by the SM derivative is the result of ROS generation and the depletion of intracellular GSH, and is likely to be a critical step in the induction of apoptosis. Additionally, the presence of two peaks in the measurement of ΔΨmito at longer treatments with CEES may be an indication that cell sensitivity to the toxic effects of CEES is cell cycle dependent. This interesting possibility will be the topic of future experiments.
We and others have previously shown that the proteolytic cleavage of PARP is required for the normal progression of apoptosis in human osteosarcoma cells and PARP-deficient cells (Boulares et al., 1999; Herceg & Wang, 1999). Blocking of this cleavage event thus increased both the rate of apoptotic cell death and the incidence of necrosis as a result of an excessive depletion of the PARP substrate NAD. The cleavage of PARP by caspase-3 during normal apoptosis thus preserves NAD and its precursor, ATP, for subsequent steps in the apoptotic pathway. Furthermore, we have previously shown in vivo and in vitro that when p53 becomes highly negatively charged due to poly ADP-ribosylation during apoptosis, it cannot bind to its proapoptotic DNA consensus sequences (Simbulan-Rosenthal et al., 1999, 2001). Inhibition of PARP by 3-AB did not protect Jurkat cells against CEES toxicity; instead, 3-AB significantly augmented CEES-induced increase in caspase-3 activity. We have no definitive explanation for these results. However, there is a plausible explanation for this effect, which will be tested in the future. The inhibition of PARP activity by the presence of 3-AB may affect the function of DNA-binding proteins such as p53, which would not become extensively poly ADP-ribosylated and hence negatively charged. This unmodified state of p53 promotes its binding to its DNA consensus sequence and the activation of p53-responsive proapoptotic genes such as Bax and Fas (Simbulan-Rosenthal et al., 1999, 2001). Therefore, the increased caspase-3 activity observed in Jurkat cells treated with 3-AB and CEES may be related to the induction of other proapoptotic genes that are activated presumably as a compensation for the inhibition of PARP activity.
In conclusion, our data suggest that CEES induces the generation of ROS, the depletion of intracellular GSH, collapse of ΔΨmito, activation of caspase-3, and cleavage of PARP in human lymphocytes, and that this series of events underlies the induction of apoptosis by this SM derivative. These observations may provide the basis for development of rational new strategies based on antioxidants to protect against and potentially prevent the toxic effects of SM in both cells and exposed tissues such as the skin.
Acknowledgments
This work was supported by the U.S. Army Medical Research and Material Command (contract DAMD17-02-C-0085 to M.E.S.), and by funds to A.H.B. from Louisiana State University Health Sciences Center, New Orleans, LA, U.S.A.
Abbreviations
- 3-AB
3-aminobenzamide
- AMC
aminomethylcoumarin
- CEES
2-chloroethylethyl sulphide
- ΔΨmito
mitochondrial membrane potential
- GSH
reduced glutathione
- H2DCF
dihydrodichlorofluorescein
- JC-1
5,5′,6,6′-tetrachloro-1,1′3^3′-tetraethylbenzimidazolylcarbocyanine iodide
- L-BSO
L-buthionine sulphoximine
- NAC
N-acetyl cysteine
- PARP
poly (ADP-ribose) polymerase
- ROS
reactive oxygen species
- SM
sulphur mustard
References
- ARAKAKI N., KAJIHARA T., ARAKAKI R., OHNISHI T., KAZI J.A., NAKASHIMA H., DAIKUHARA Y. Involvement of oxidative stress in tumor cytotoxic activity of hepatocyte growth factor/scatter factor. J. Biol. Chem. 1999;274:13541–13546. doi: 10.1074/jbc.274.19.13541. [DOI] [PubMed] [Google Scholar]
- BOULARES A.H., YAKOVLEV A.G., IVANOVA V., STOICA B.A., WANG G., IYER S., SMULSON M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- BOULARES A.H., ZOLTOSKI A.J., SHERIF Z.A., JOLLY P., MASSARO D., SMULSON M.E. Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2003;28:322–329. doi: 10.1165/rcmb.2001-0015OC. [DOI] [PubMed] [Google Scholar]
- BOULARES A.H., ZOLTOSKI A.J., YAKOVLEV A., XU M., SMULSON M.E. Roles of DNA fragmentation factor and poly(ADP-ribose) polymerase in an amplification phase of tumor necrosis factor-induced apoptosis. J. Biol. Chem. 2001;276:38185–38192. doi: 10.1074/jbc.M100629200. [DOI] [PubMed] [Google Scholar]
- CHEN Q., CHAI Y.C., MAZUMDER S., JIANG C., MACKLIS R.M., CHISOLM G.M., ALMASAN A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ. 2003;10:323–334. doi: 10.1038/sj.cdd.4401148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIBA T., TAKAHASHI S., SATO N., ISHII S., KIKUCHI K. Fas-mediated apoptosis is modulated by intracellular glutathione in human T cells. Eur. J. Immunol. 1996;26:1164–1169. doi: 10.1002/eji.1830260530. [DOI] [PubMed] [Google Scholar]
- COHEN G.M. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DACRE J.C., GOLDMAN M. Toxicology and pharmacology of the chemical warfare agent sulphur mustard. Pharmacol. Rev. 1996;48:289–326. [PubMed] [Google Scholar]
- GOTTLIEB R.A. Mitochondria: execution central. FEBS Lett. 2000;482:6–12. doi: 10.1016/s0014-5793(00)02010-x. [DOI] [PubMed] [Google Scholar]
- HATHWAY D.E. Toxic action/toxicity. Biol. Rev. Camb. Philos. Soc. 2000;75:95–127. doi: 10.1017/s0006323199005447. [DOI] [PubMed] [Google Scholar]
- HERCEG Z., WANG Z.Q. Failure of poly(ADP-ribose) polymerase cleavage by caspases leads to induction of necrosis and enhanced apoptosis. Mol. Cell. Biol. 1999;19:5124–5133. doi: 10.1128/mcb.19.7.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUR T.C., SHIAU S.Y., LIN J.K. Suppression of N-methyl-N′-nitro-N-nitrosoguanidine- and S-nitrosoglutathione-induced apoptosis by Bcl-2 through inhibiting glutathione-S-transferase pi in NIH3T3 cells. Toxicol. Lett. 1999;110:191–202. doi: 10.1016/s0378-4274(99)00158-7. [DOI] [PubMed] [Google Scholar]
- HUR G.H., KIM Y.B., CHOI D.S., KIM J.H., SHIN S. Apoptosis as a mechanism of 2-chloroethylethyl sulphide-induced cytotoxicity. Chem. Biol. Interact. 1998;110:57–70. doi: 10.1016/s0009-2797(97)00112-9. [DOI] [PubMed] [Google Scholar]
- HUSAIN K., DUBE S.N., SUGENDRAN K., SINGH R., DAS GUPTA S., SOMANI S.M. Effect of topically applied sulphur mustard on antioxidant enzymes in blood cells and body tissues of rats. J. Appl. Toxicol. 1996;16:245–248. doi: 10.1002/(SICI)1099-1263(199605)16:3<245::AID-JAT339>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- JABS T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem. Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- LEE M., YOU H.J., CHO S.H., WOO C.H., YOO M.H., JOE E.H., KIM J.H. Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to beta-amyloid in C6 astroglioma cells. Biochem. J. 2002;366:937–943. doi: 10.1042/BJ20020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J., HUANG C.Y., ZHENG R.L., CUI K.R., LI J.F. Hydrogen peroxide induces apoptosis in human hepatoma cells and alters cell redox status. Cell Biol. Int. 2000;24:9–23. doi: 10.1006/cbir.1999.0438. [DOI] [PubMed] [Google Scholar]
- LUDLUM D.B., KENT S., MEHTA J.R. Formation of O6-ethylthioethylguanine in DNA by reaction with the sulphur mustard, chloroethyl sulphide, and its apparent lack of repair by O6-alkylguanine-DNA alkyltransferase. Carcinogenesis. 1986;7:1203–1206. doi: 10.1093/carcin/7.7.1203. [DOI] [PubMed] [Google Scholar]
- MANCINI M., ANDERSON B.O., CALDWELL E., SEDGHINASAB M., PATY P.B., HOCKENBERY D.M. Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J. Cell Biol. 1997;138:449–469. doi: 10.1083/jcb.138.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLSON D.W., ALI A., THORNBERRY N.A., VAILLANCOURT J.P., DING C.K., GALLANT M., GAREAU Y., GRIFFIN P.R., LABELLE M., LAZEBNIK Y.A., MUNDAY N.A., RAJU S.M., SMULSON M.E., YAMIN T.T., YU V.L., MILLER D.K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- ODA T., IWAOKA J., KOMATSU N., MURAMATSU T. Involvement of N-acetylcysteine-sensitive pathways in ricin-induced apoptotic cell death in U937 cells. Biosci. Biotechnol. Biochem. 1999;63:341–348. doi: 10.1271/bbb.63.341. [DOI] [PubMed] [Google Scholar]
- PAPIRMEISTER B., GROSS C.L., MEIER H.L., PETRALI J.P., JOHNSON J.B. Molecular basis for mustard-induced vesication. Fundam. Appl. Toxicol. 1985;5:S134–S149. [PubMed] [Google Scholar]
- SALVIOLI S., ARDIZZONI A., FRANCESCHI C., COSSARIZZA A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨmito changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- SANIJ E., HATZISTAVROU T., HERTZOG P., KOLA I., WOLVETANG E.J. Ets-2 is induced by oxidative stress and sensitizes cells to H(2)O(2)-induced apoptosis: implications for Down's syndrome. Biochem. Biophys. Res. Commun. 2001;287:1003–1008. doi: 10.1006/bbrc.2001.5680. [DOI] [PubMed] [Google Scholar]
- SHRIVASTAVA A., AGGARWAL B.B. Antioxidants differentially regulate activation of nuclear factor-κB, activator protein-1, c-Jun amino-terminal kinases, and apoptosis induced by tumor necrosis factor: evidence that JNK and NF-κB activation are not linked to apoptosis. Antioxid. Redox Signal. 1999;1:181–191. doi: 10.1089/ars.1999.1.2-181. [DOI] [PubMed] [Google Scholar]
- SIMBULAN-ROSENTHAL C.M., ROSENTHAL D.S., DING R., BHATIA K., SMULSON M.E. Prolongation of the p53 response to DNA strand breaks in cells depleted of PARP by antisense RNA expression. Biochem. Biophys. Res. Commun. 1998;253:864–868. doi: 10.1006/bbrc.1998.9792. [DOI] [PubMed] [Google Scholar]
- SIMBULAN-ROSENTHAL C.M., ROSENTHAL D.S., LUO R.B., SMULSON M.E. Poly(ADP-ribosyl)ation of p53 during apoptosis in human osteosarcoma cells. Cancer Res. 1999;59:2190–2194. [PubMed] [Google Scholar]
- SIMBULAN-ROSENTHAL C.M., ROSENTHAL D.S., LUO R.B., SAMARA R., JUNG M., DRITSCHILO A., SPOONDE A., SMULSON M.E. Poly(ADP-ribosyl)ation of p53 in vitro and in vivo modulates binding to its DNA consensus sequence. Neoplasia. 2001;3:179–188. doi: 10.1038/sj.neo.7900155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMULSON M., ISTOCKM N., DING R., CHERNEY B. Deletion mutants of poly(ADP-ribose) polymerase support a model of cyclic association and dissociation of enzyme from DNA ends during DNA repair. Biochemistry. 1994;33:6186–6191. doi: 10.1021/bi00186a018. [DOI] [PubMed] [Google Scholar]
- STRASSER A., O'CONNOR L., DIXIT V.M. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- TEWARI M., QUAN L.T., O'ROURKE K., DESNOYERS S., ZENG Z., BEIDLER D.R., POIRIER G.G., SALVESEN G.S., DIXIT V.M. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- WOLFLER A., CALUBA H.C., ABUJA P.M., DOHR G., SCHAUENSTEIN K., LIEBMANN P.M. Prooxidant activity of melatonin promotes Fas-induced cell death in human leukemic Jurkat cells. FEBS Lett. 2001;502:127–131. doi: 10.1016/s0014-5793(01)02680-1. [DOI] [PubMed] [Google Scholar]
- ZHANG P., NG P., CARIDHA D., LEACH R.A., ASHER L.V., NOVAK M.J., SMITH W.J., ZEICHNER S.L., CHIANG P.K. Gene expressions in Jurkat cells poisoned by a sulphur mustard vesicant and the induction of apoptosis. Br. J. Pharmacol. 2002;137:245–252. doi: 10.1038/sj.bjp.0704856. [DOI] [PMC free article] [PubMed] [Google Scholar]