Abstract
We have investigated the vascular effects of N-arachidonoyl-dopamine (NADA), a novel endocannabinoid/vanilloid. NADA caused vasorelaxant effects comparable to those of anandamide in small mesenteric vessels (G3), the superior mesenteric artery (G0) and in the aorta.
In G3, addition of NG-nitro-L-arginine methyl ester (300 μM) or the dopamine (D1) receptor antagonist (SCH23390, 1 μM) did not affect responses to NADA. In the presence of 60 mM KCl, after de-endothelialisation, or after K+ channel inhibition with charybdotoxin (100 nM) and apamin (500 nM), relaxant responses to NADA were inhibited.
In G3, pretreatment with the vanilloid receptor (VR) agonist capsaicin (10 μM) or the VR antagonist capsazepine (10 μM) reduced vasorelaxation to NADA.
In G3, application of the CB1 antagonist SR141716A at 1 μM but not 100 nM reduced the potency of NADA. Another CB1 antagonist, AM251 (100 nM and 1 μM), did not affect vasorelaxation to NADA. After endothelial denudation, SR141716A (1 μM) did not reduce the responses further. A combination of capsaicin and SR141716A (1 μM) reduced vasorelaxation to NADA further than with capsaicin pretreatment alone. The novel endothelial cannabinoid (CB) receptor antagonist O-1918 opposed vasorelaxation to NADA in G3.
In the superior mesenteric artery (G0), vasorelaxation to NADA was not dependent on an intact endothelium and was not sensitive to O-1918, but was sensitive to capsaicin and SR141716A or AM251 (both 100 nM).
The results of the present study demonstrate for the first time that NADA is a potent vasorelaxant. In G3, the effects of NADA are mediated by stimulation of the VR and the novel endothelial CB receptor, while in G0, vasorelaxation is mediated through VR1 and CB1 receptors.
Keywords: Rat mesenteric artery, N-arachidonoyl-dopamine, anandamide, cannabinoid receptor, vanilloid receptor, vasorelaxation, endothelium, EDHF
Introduction
Since the identification of specific cannabinoid (CB) receptors (Matsuda et al., 1990), several natural ligands for the receptors have been found. One such compound is the novel endogenous substance N-arachidonoyl-dopamine (NADA), which has recently been identified in rat and bovine nervous tissues (Bisogno et al., 2000; Huang et al., 2002). Structurally, NADA has a vanillyl-amine moiety and a long-chain unsaturated fatty acid common to CBs. It is reported to bind to vanilloid receptors (VRs) with similar potency to capsaicin in human embryonic kidney cells (Huang et al., 2002), and has similar efficacy to anandamide at the CB1 receptors in rat brain cells (Bisogno et al., 2000). Very recent data have identified NADA as a central endovanilloid, which can induce hyperalgesia (Chu et al., 2003), and a peripheral endovanilloid, which can cause smooth muscle contraction in the guinea-pig bronchi and bladder (Harrison et al., 2003).
To date, the cardiovascular actions of NADA have not been examined; however, the effects of the prototypic endocannabinoid anandamide have been widely studied (for a review, see Randall et al., 2002). Several putative mechanisms have been put forward to explain the vasorelaxant effects of anandamide; however, many discrepancies still occur in the literature. Early reports indicated that anandamide acted via the release of nitric oxide (Deutsch et al., 1997). However, in rat mesenteric vessels, vasorelaxant effects of anandamide are largely insensitive to inhibition of nitric oxide synthase (Randall et al., 1996; White & Hiley, 1997; Járai et al., 1999). Although nitric oxide does not appear to mediate responses to anandamide, the vasorelaxation is partly endothelium-dependent, in some, but not all preparations (White & Hiley, 1997; Chaytor et al., 1999; Járai et al., 1999). It is therefore likely that anandamide stimulates the release of the endothelium-derived hyperpolarising factor (EDHF), which is communicated to smooth muscle cells via myoendothelial gap junctions (Chaytor et al., 1999; Harris et al., 2002).
The involvement of currently recognised CB receptors in vasorelaxation to endocannabinoids is also unclear. Some previous studies have implicated the CB1 receptor on the basis of inhibition by SR141716A, a selective CB1 receptor antagonist. However, this is complicated by the wide-ranging actions of SR141716A at various concentrations. For example, SR141716A can inhibit myoendothelial gap junctions (Chaytor et al., 1999), which themselves have been implicated in the actions of anandamide via EDHF (Chaytor et al., 1999; Harris et al., 2002). SR141716A may also antagonise the novel non-CB1 endothelial CB receptor proposed by Kunos and colleagues (Járai et al., 1999; Offertáler et al., 2003). Indeed, Offertáler et al. (2003) have reported that these responses are sensitive to a novel antagonist, O-1918. SR141716A may also have actions on the VR (De Petrocellis et al., 2001; Millns et al., unpublished data).
One of the most interesting proposals has been that anandamide stimulates VRs on perivascular sensory nerves, and acts via the release of the vasodilator neurotransmitter calcitonin gene-related peptide (CGRP; Zygmunt et al., 1999; Ralevic et al., 2000). Whether this action can account for the entire endocannabinoid vasorelaxant response has yet to be clarified, as further studies have not found that the entire response to anandamide is mediated via sensory nerves (Ralevic et al., 2000; Harris et al., 2002).
Given the agonist effects of the novel compound NADA on CB and VRs, the possibility exists that NADA may act as a vasorelaxant in a similar manner to anandamide. In this study, vasorelaxant actions of NADA have been identified and characterised in large and small rat arteries. Specifically, the roles of CB receptors, VRs and sensory nerves, the endothelium and EDHF in the vascular effects of NADA have been investigated.
Methods
Blood vessel preparation
Male Wistar rats (250–350 g) were stunned by a blow to the back of the head and killed by cervical dislocation. The aorta, superior mesenteric artery and mesenteric arterial bed were removed rapidly and placed into cold Krebs–Henseleit buffer (composition, mM: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2, D-glucose 10). From the mesenteric arterial bed, 2 mm segments of third-order branches of the superior mesenteric artery (G3) were dissected free of adherent connective and adipose tissue. G3 vessels were mounted on fine tungsten wires (40 μm diameter) on a Mulvany–Halpern myograph (Myo-Interface Model 410A, Danish Myo Technology, Denmark) (Mulvany & Halpern, 1977). The superior mesenteric artery (G0; 3–4 mm in length) was also cleaned of adherent tissue, and was mounted on fixed-segment support pins using the Multi Myograph system (Model 610M, Danish Myo Technology, Denmark). The thoracic aorta was dissected free of adherent connective and adipose tissue, and cut into rings 6–8 mm long (Tep-areenan et al., 2003). The aortic rings were placed in 50 ml organ baths containing Krebs–Henseleit buffer, mounted between two stainless steel hooks and attached by thread to an isometric force displacement transducer (LETICA 210). Tension was measured and was recorded on a MacLab 4e recording system (ADInstruments, U.K.).
Once mounted, all vessels were kept at 37°C in Krebs–Henseleit buffer and gassed with 5% CO2 in O2. The mesenteric vessels were stretched to an optimal passive tension of 5 mN and the thoracic aorta to 10 mN tension. All vessels were allowed to equilibrate, and the contractile integrity of each was tested by its ability to contract to 60 mM KCl by at least 5 mN.
In some mesenteric preparations, the endothelium was removed by abrasion with a human hair. Preparations (both G3 and G0) were considered denuded when relaxation to 10 μM carbachol after precontraction was less than 20% relaxation (see White & Hiley, 1997). All other G3 and G0 preparations were endothelium-intact.
Experimental protocol
Viable vessels were contracted with U46619 (10–300 nM), a thromboxane mimetic, to increase tension by at least 5 mN. The average increase in tension for G3 vessels was 10.4±0.4 mN (n=121), for the superior mesenteric artery it was 19.8±0.6 mN (n=60), and for the aorta it was 9.0±0.7 mN (n=16). Once a stable contraction was achieved, the vasorelaxant effects of NADA were assessed as cumulative concentration–response curves by addition of NADA to the buffer. The steady-state response to NADA was taken at each concentration and expressed as a percentage relaxation of the imposed U46619 contraction.
To assess the involvement of dopamine receptors in vasorelaxation to NADA, the dopamine receptor antagonist SCH23390 (1 μM, Martin & Broadley, 1995) was added to preparations 20 min before pre-constriction. The involvement of nitric oxide was investigated using the nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME, 300 μM, Randall & Griffith, 1991). In these experiments, L-NAME was present in the buffer throughout the whole experiment. To assess the role of K+ channels, vessels were depolarised and contracted with 60 mM KCl (Adeagbo & Triggle, 1993) and concentration-response curves constructed.
To investigate the role of the endothelium in NADA-mediated vasorelaxation, the endothelium was removed using a human hair (White & Hiley, 1997). The role of the EDHF was also assessed using a number of K+ channel inhibitors in the presence of both nitric oxide synthase and cyclooxygenase inhibitors (McCulloch et al., 1997). In these experiments, L-NAME (300 μM) and indomethacin (10 μM) were present in the KREBS solution throughout the experiments to inhibit nitric oxide and prostanoid synthesis, respectively. Preparations were then incubated with charybdotoxin (ChTX) to block large calcium-activated K+ channels and voltage-sensitive K+ channels (ChTX, 100 nM) and apamin to block small calcium-activated K+ channels (500 nM), as this combination inhibits EDHF responses (Randall & Kendall, 1998; Zygmunt et al., 1997). Concentration–response curves were then constructed to NADA.
To assess the involvement of VRs, capsazepine (10 μM, a VR antagonist) was added to the buffer 20 min before preconstriction (Zygmunt et al., 1999). Also, some vessels were incubated for 1 h with the vanilloid agonist capsaicin (10 μM) to deplete the sensory nerves of neurotransmitters. This was followed by a 20-min washout period (Zygmunt et al., 1999; Harris et al., 2002).
To assess the involvement of CB receptors, either SR141716A (100 nM and 1 μM, a CB1 receptor antagonist, Rinaldi-Carmona et al., 1994) or AM251 (100 nM and 1 μM, a CB1 receptor antagonist; Gardiner et al., 2002) were added to the preparations 20 min before preconstriction. The effects of SR141716A were also examined in conjunction with endothelial denudation and after capsaicin pretreatment to deplete sensory nerves. In addition, endothelial denudation was combined with capsaicin pretreatment alone, or in combination with SR141716A at 1 μM.
To establish whether NADA acts at the novel endothelial CB receptor, some vessels were treated with O-1918 (1 μM), a silent antagonist of the receptor at which abnormal cannabidiol acts, which is neither CB1 nor CB2. This receptor is proposed to be a novel endothelial CB receptor (Offertáler et al., 2003).
As a comparison to G3 vessels, the vascular effects of NADA were also investigated in the superior mesenteric artery (G0) to establish any possible regional differences between resistance (G3) and conduit (G0) vessels. Specifically, the effects of L-NAME, de-endothelialisation, capsaicin treatment and the antagonists SR141716A, AM251 and O-1918 were examined.
Statistical analysis
The concentration of vasorelaxant giving the half-maximal response (EC50) was obtained from the concentration–response curve fitted to a sigmoidal logistic equation, with the minimum vasorelaxation set to zero using the GraphPad Prism package (Tep-areenan et al., 2003). Maximal responses and pEC50 (negative logarithm of the EC50) values are expressed as mean±s.e.m. The number of animals in each group is represented by n. Data were compared, as appropriate, by Students's unpaired t-test or by analysis of variance (ANOVA) with statistical significance between manipulations and controls determined by Dunnett's post hoc test.
Drugs
All drugs were supplied by Sigma Chemical Co. (Poole, U.K.), except where stated. Carbachol and L-NAME were dissolved in Krebs–Henseleit solution. NADA, anandamide, SCH23390, capsaicin, capsazepine and SR141716A were dissolved in ethanol at 10 mM, with further dilutions made in distilled water. Anandamide and NADA were obtained as ethanolic solutions from Tocris (U.K.). SR141716A was supplied by Research Biochemicals International as part of the Chemical Synthesis Programme of the National Institute of Mental Health contract (NOIMH3003). SCH23390 was obtained from the Schering Corporation (U.S.A.). AM251 was a kind gift from Professor Sheila Gardiner and was initially dissolved in DMSO to 10 mM, with further dilutions in distilled water. O-1918 was generously donated by Dr George Kunos. O-1918 was dissolved in ethanol at 10 mM, with further dilutions made in distilled water.
Results
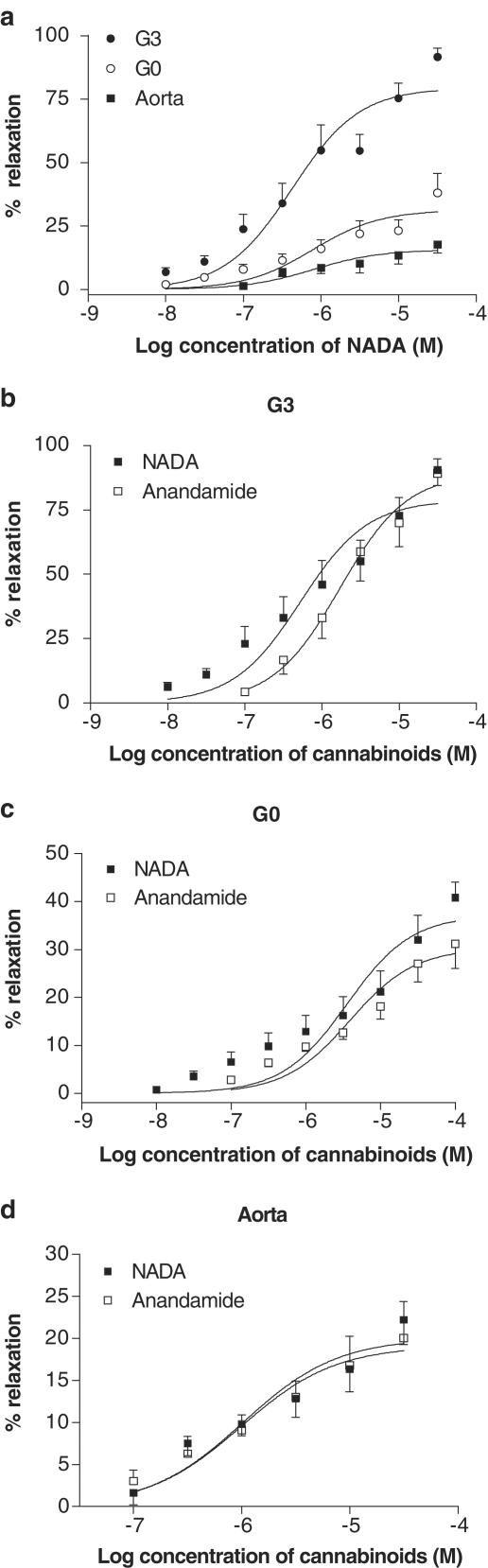
NADA caused vasorelaxation in all of the preconstricted blood vessels tested. The small resistance arteries (G3) were significantly more responsive to NADA than the superior mesenteric artery (GO) or the aorta (Figure 1a; Table 1). An original representative trace of the effects of NADA in precontracted G3 vessels is presented in Figure 2. In G3, the vasorelaxant effects of NADA were significantly more potent than anandamide (P<0.05), with a slightly lower maximal vasorelaxant effect (P<0.05). In the superior mesenteric artery, NADA was significantly more effective than anandamide (P<0.05). The vasorelaxant effects of NADA were comparable to those of anandamide in the aorta (Table 1; Figure 1b–d).
Figure 1.
Vasorelaxation to NADA in isolated segments of G0 (n=7) and G3 (n=10) of the mesenteric bed and of the aorta (n=8) compared to that of anandamide (GO n=7; G3 n=9; aorta n=8). Data are given as means, with error bars representing s.e.m.
Table 1.
Comparison of the vasorelaxant effects of NADA and anandamide in the third-order branch of the superior mesenteric artery (G3), the superior mesenteric artery (G0) and in the aorta
| NADA | Anandamide | |||||
|---|---|---|---|---|---|---|
| pEC50 | Rmax | n | pEC50 | Rmax | n | |
| G3 | 6.39±0.12* | 79.1±4.4* | 10 | 5.77±0.14 | 89.2±7.1 | 9 |
| G0 | 5.45±0.15 | 37.2±3.0* | 7 | 5.41±0.14 | 30.2±2.4 | 7 |
| Aorta | 5.99±0.17 | 20.0±1.8 | 8 | 6.00±0.21 | 19.1±2.2 | 8 |
Data are given as mean±s.e.m.
denotes a significant difference between anandamide and NADA P<0.05 versus WT.)
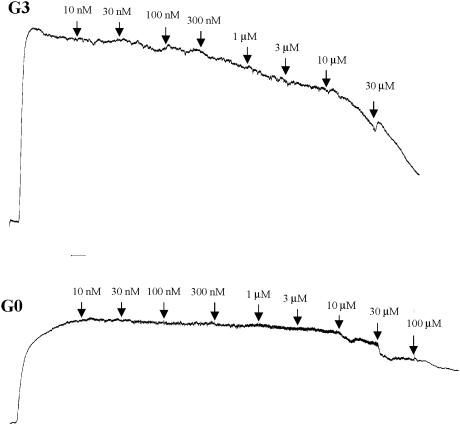
Figure 2.
A representative trace of the vasorelaxant effects of NADA in preconstricted G3 and G0 mesenteric arteries.
Isolated mesenteric resistance arteries (G3)
Characterisation of vasorelaxant response to NADA
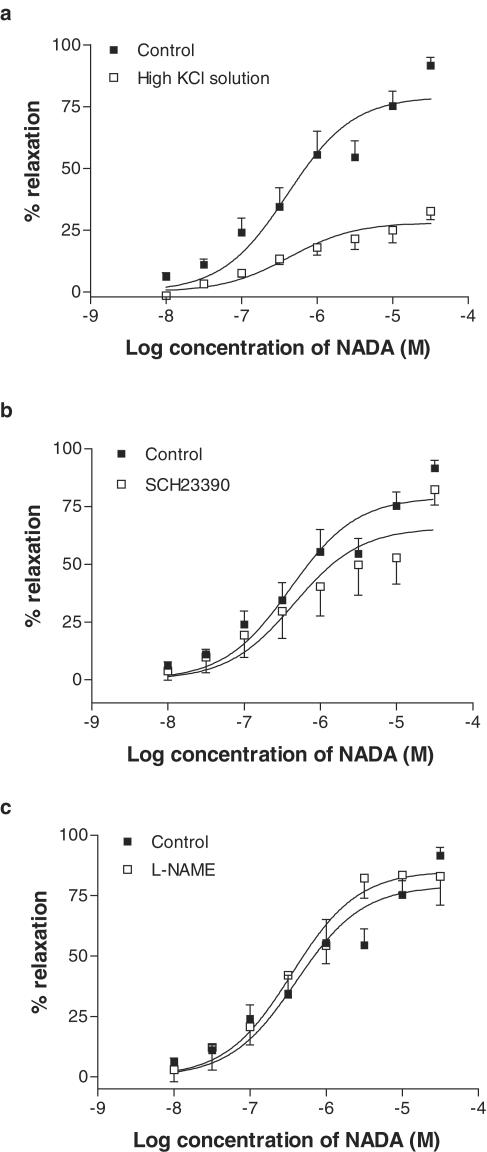
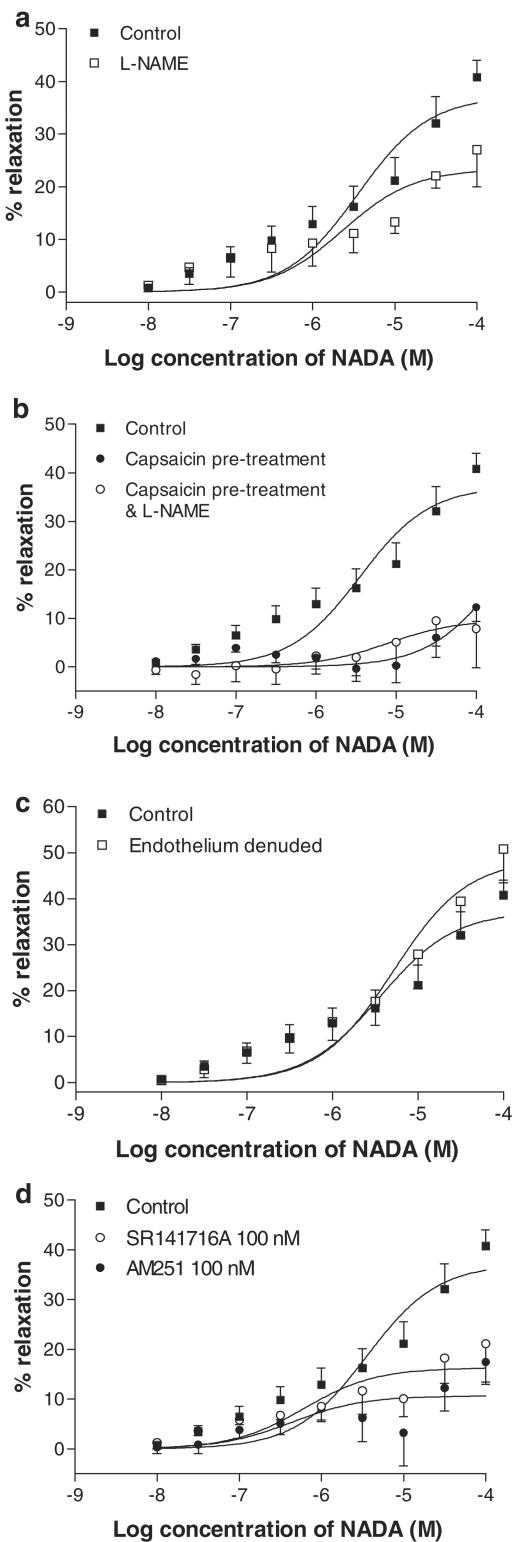
When vessels were preconstricted with 60 mM KCl, the maximal relaxant response to NADA was significantly inhibited (Table 2; Figure 3a). In preparations contracted with U46619, the dopamine receptor antagonist SCH23390 (1 μM) had a small significant inhibitory response on the fitted maximal vasorelaxation response to NADA (Table 2; Figure 3b). However, the actual mean values obtained at the maximal concentration of NADA were not different from that seen in control arteries (control, 91.6±3.5% relaxation; SCH23390, 82.3±6.7% relaxation). Addition of L-NAME (300 μM) did not affect vasorelaxation to NADA (Table 2; Figure 3c).
Table 2.
Effects of various treatments on the vasorelaxation responses to NADA in third-order branches (G3) of the rat superior mesenteric artery
| pEC50 | Rmax | n | |
|---|---|---|---|
| Control | 6.39±0.12 | 79.1±4.4 | 10 |
| KCl contracted vessels | 6.37±0.16 | 28.1±2.1 | 4 |
| SCH23390 (1 μM) | 6.34±0.22 | 65.8±6.9** | 7 |
| L-NAME (300 μM) | 6.45±0.14 | 85.3±5.4 | 6 |
| Endothelium denuded | 5.70±0.29** | 39.1±4.7** | 6 |
| ChTx (100 nM) & apamin (500 nM) with L-NAME (300 μM) & indomethacin (10 μM) | 5.73±0.29** | 40.0±7.0** | 5 |
| Capsazepine (10 μM) | 5.37±0.11** | 78.7±6.3 | 5 |
| Capsaicin pretreatment (10 μM) | 6.05±0.17** | 60.3±6.4** | 10 |
| Capsaicin pretreatment (10 μM) & L-NAME (300 μM) | 5.51±0.16** | 62.8±5.5** | 6 |
| SR141716A (100 nM) | 6.73±0.21 | 75.3±6.8 | 5 |
| SR141716A (1 μM) | 5.56±0.17** | 63.8±7.0** | 6 |
| Endothelium denuded & SR141716A (1 μM) | 5.78 ± 0.28** | 47.1±7.8** | 6 |
| Endothelium denuded & capsaicin pretreatment (10 μM) | 5.17±0.14** | 90.0±10.4 | 6 |
| Endothelium denuded & capsaicin pretreatment (10 μM) & SR141716A | 4.91±0.14** | 87.9±11.5 | 6 |
| Capsaicin pretreatment (10 μM) & SR141716A (1 μM) | 5.02±0.34** | 54.1±16.2** | 6 |
| AM251 (100 nM) | 6.86±0.30 | 65.8±8.3** | 6 |
| AM251 (1 μM) | 6.73±0.21 | 69.8±6.4 | 6 |
| O-1918 (1 μM) | 5.26±0.20** | 69.8±10.8 | 6 |
Data are given as means±s.e.m. Significant inhibitory effects compared with control vasorelaxant responses are indicated by *. * P<0.05.
P<0.05, as determined by ANOVA.
Figure 3.
Vasorelaxant responses to NADA in the presence of a high potassium solution (n=4, a), the dopamine receptor antagonist SCH23390 (1 μM, n=7, b), and after nitric oxide synthase inhibition by L-NAME (300 μM, n=6, c). Data are given as means, with error bars representing s.e.m.
Endothelium-dependence and the role of EDHF
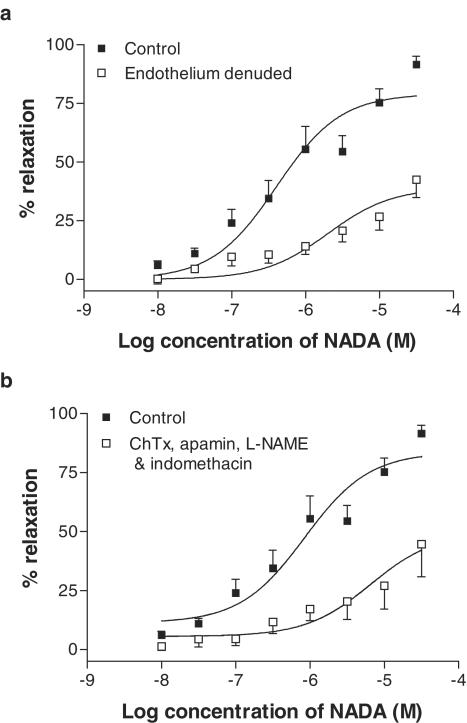
Removal of the endothelium resulted in a significant reduction in the relaxant responses to NADA (Table 2; Figure 4a). In endothelium-intact vessels, and in the presence of L-NAME (300 μM) and indomethacin (10 μM), the combination of 100 nM ChTX and 500 nM apamin produced comparable inhibition of NADA-induced vasorelaxation in G3 vessels (Table 2; Figure 4b).
Figure 4.
Vasorelaxant responses to NADA in small resistance vessels after removal of the endothelium (n=6, a), and after inhibition of EDHF activity with ChTX (100 nM) and apamin (500 nM) in the presence of L-NAME (300 μM) and indomethacin (10 μM) (n=5, b). Data are given as means, with error bars representing s.e.m.
Involvement of VRs
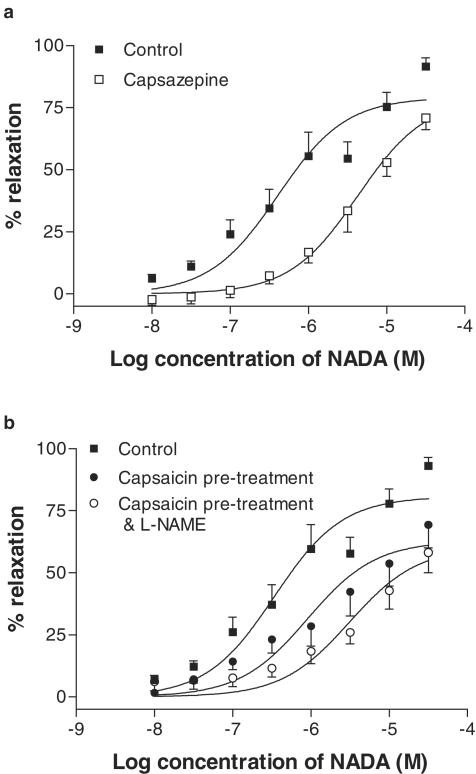
Pretreatment with capsazepine (10 μM) caused a 10.5-fold shift in the concentration–response curve to NADA, without affecting the maximal response (Table 2; Figure 5a). Similarly, pretreatment with capsaicin (10 μM) significantly reduced the relaxant effects of NADA (Figure 5b). In the presence of L-NAME (300 μM), capsaicin was still effective at reducing the effects of NADA (Figure 5b).
Figure 5.
Effects of the VR antagonist capsazepine (10 μM, n=5, a), and pretreatment with the VR agonist capsaicin (10 μM, n=10, b) on vasorelaxation to NADA in small resistance vessels. Data are given as means with error bars representing s.e.m.
Effects of CB receptor antagonism
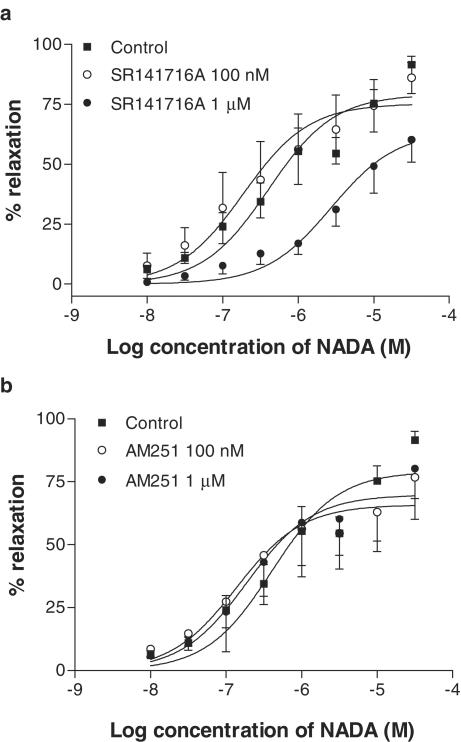
The CB1 receptor antagonist SR141716A (1 μM) caused a 6.8-fold rightward shift in the concentration–response curve to NADA (Table 2; Figure 6a). However, SR141716A at 100 nM did not reduce the vasorelaxant responses to NADA (Figure 6a). A second CB1 receptor antagonist, AM251, did not affect the responses to NADA at 1 μM (Figure 6b), but at 100 nM, AM251 had a small inhibitory effect on the maximal response to NADA (Table 2; Figure 6b).
Figure 6.
Effects of the CB1 receptor antagonists SR141716A at 100 nM (n=5) and 1 μM (n=6, a) and AM251 at 100 nM (n=6) and at 1 μM (n=6, b) on vasorelaxation to NADA in small-resistance vessels. Data are given as means, with error bars representing s.e.m.
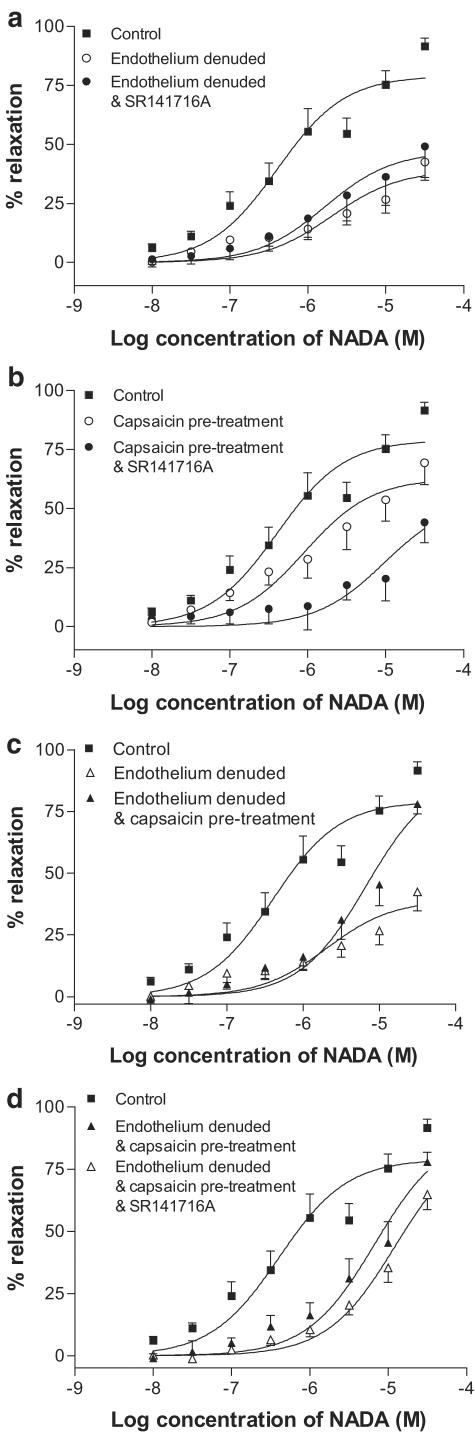
In endothelium-denuded preparations, the addition of SR141716A (1 μM) did not affect the vasorelaxation to NADA further than was seen following endothelial denudation alone (Table 2; Figure 7a). In endothelium-intact preparations, capsaicin treatment for 1 h was combined with incubation with SR141716A (1 μM). In this experiment, the potency of NADA in G3 vessels was reduced further than that with capsaicin treatment alone (Figure 7b). When capsaicin treatment was combined with endothelial denudation, it was found that, while the potency of NADA was reduced, there was no reduction in the maximum vasorelaxant effects (Figure 7c; Table 2). With the further addition of SR141716A at 1 μM, the potency of NADA was reduced somewhat further, with no change in the maximal vasorelaxant response to NADA (Figure 7d; Table 2).
Figure 7.
Effects of the CB1 receptor antagonist SR141716A (1 μM) on vasorelaxation to NADA in small resistance vessels after endothelial denudation (n=6, a) and after pretreatment of vessels with capsaicin (10 μM, n=6, b) and after all the three manipulations (n=6, d). Data are given as means, with error bars representing s.e.m.
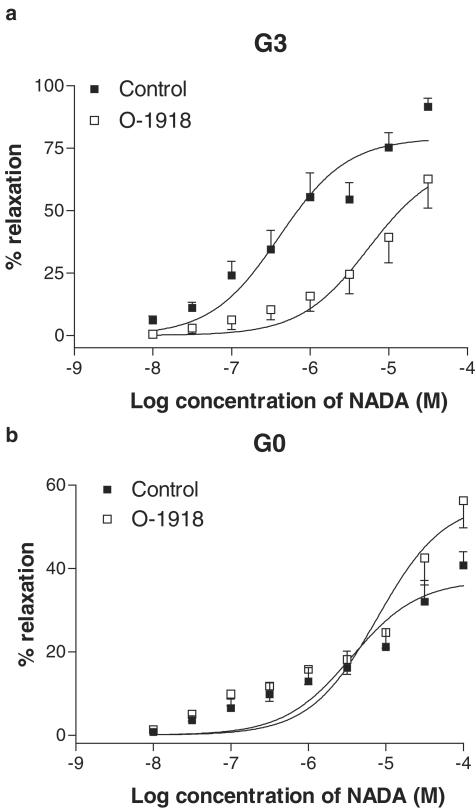
Addition of O-1918 (1 μM) caused a 13.5-fold rightward shift in the concentration–response curve to NADA (Table 2, Figure 9a).
Figure 9.
Effects of O-1918 (1 μM) on vasorelaxation to NADA in small resistance vessels (G3, n=6, a) and in the superior mesenteric artery (G0, n=6, b). Data are given as means, with error bars representing s.e.m.
Superior mesenteric artery (G0)
In the superior mesenteric artery, the presence of L-NAME (300 μM) had a modest inhibitory effect on the maximum vasorelaxant response to NADA (Table 3; Figure 8a). In contrast to the G3 vessels, de-endothelialisation in the superior mesenteric artery (G0) did not reduce vasorelaxation to NADA (Table 3; Figure 8c). Pretreatment with capsaicin substantially reduced the effects of NADA in the superior mesenteric artery (Table 3; Figure 8b), and the inhibitory effect of capsaicin on responses to NADA was not protected by the presence of 300 μM L-NAME (Figure 8c).
Table 3.
Effects of various treatments on the vasorelaxation responses to NADA in the rat superior mesenteric artery (G0)
| pEC50 | Rmax | n | |
|---|---|---|---|
| Control | 5.45±0.15 | 37.2±3.0 | 7 |
| L-NAME | 5.63±0.15 | 23.4±3.0* | 8 |
| Endothelium denuded | 5.29±0.17 | 48.7±5.0 | 7 |
| Capsaicin pretreatment (10 μM) | 3.75±0.92** | 17.6±6.4** | 6 |
| Capsaicin and L-NAME (300 μM) | 5.10±0.74 | 9.7±4.5** | 6 |
| SR141716A (100 nM) | 6.21±0.11 | 16.3±2.3** | 7 |
| AM251 (100 nM) | 6.36±0.51** | 10.7±2.3** | 6 |
| O-1918 (1 μM) | 5.11±0.14 | 56.1±5.1 | 6 |
Data are given as mean±s.e.m.
denotes a significant inhibitory effect compared with control vasorelaxant responses (P<0.05, ** P<0.01.)
Figure 8.
Vasorelaxant responses to NADA in the superior mesenteric artery after nitric oxide synthase inhibition by L-NAME (300 μM, n=8, a), de-endothelisation (n=7, b), depletion of sensory neurotransmitters by capsaicin (10 μM, n=6, c), and after CB1 receptor antagonism with SR141716A (n=7) or AM251 (n=6) at 100 nM (d). Data are given as means with error bars representing s.e.m.
Unlike G3 vessels, pretreatment with SR141716A at 100 nM reduced the maximal vasorelaxant response to NADA (Table 3; Figure 8d). Similarly, AM251 (100 nM) also caused a reduction in the maximal response to NADA (Table 3; Figure 8d). The vasorelaxant responses to NADA were not inhibited by O-1918 in the superior mesentery artery (Table 3; Figure 9b).
Discussion
The aim of the present study was to characterise the vasorelaxant responses of the novel endogenous CB/vanilloid NADA. The results of this study show for the first time that NADA is a potent vasorelaxant in a number of blood vessels. The mechanisms of action of NADA and anandamide appear to be similar in small resistance vessels (G3), involving stimulation of VRs and the novel endothelial receptor, which may be coupled to the release of EDHF. Regional differences in the actions of NADA were also observed, such that, in larger mesenteric vessels (G0), relaxation to NADA was mediated through stimulation of the VR and the CB1 receptor. There was no endothelial component to the responses to NADA in G0, and the novel endothelial receptor antagonised by O-1918 does not appear to be functional in these blood vessels.
It was hypothesised that NADA would have a vasorelaxant effect similar to anandamide, based on its structure and common pharmacology with anandamide (Bisogno et al., 2000; Huang et al., 2002). The initial findings of the present study identified NADA as a vasorelaxant, with similar potency to that of anandamide. More specifically, vasorelaxant activity was found in small resistance and larger conduit vessels, although this was more marked in the small vessels. Little is currently known about the synthesis, source or physiological relevance of NADA, but, given its commonality with anandamide, it might be speculated that NADA is of some pathophysiological importance (Wagner et al., 1997).
To exclude the possibility that NADA acts at dopamine receptors, experiments were carried out in the presence of SCH23390, an antagonist of the D1 receptor, as stimulation of the D1 receptor is known to cause vasodilatation (Goldberg, 1985). It was found that the effects of NADA were not through stimulation of this receptor. This is in agreement with Bisogno et al. (2000), who showed that NADA has little affinity for either D1 or D2 receptors in rat brain membranes.
In order to investigate the mechanisms of vasorelaxation, initial experiments showed that NADA was sensitive to a high potassium depolarising buffer. This is consistent with the notion that CBs may exert their effects through direct activation of potassium channels (Randall & Kendall, 1998) or through mediation via EDHF (Chaytor et al., 1999). Alternatively, the high potassium solution may act on sensory nerves either by rapidly depleting or inhibiting the release of neurotransmitters via a depolarisation-clamp mechanism.
In the present study, the vasorelaxant responses to NADA in G3 vessels were found to be partly endothelium-dependent. This is in agreement with the findings of Chaytor et al. (1999) for anandamide in similar isolated arterial preparations, but contrary to those of White & Hiley (1997). However, it is clear that some other CBs such as abnormal cannabidiol are clearly endothelium-dependent (Járai et al., 1999). This might highlight some potential differences in the actions of various CB molecules. To characterise the endothelium-derived vasorelaxant involved in the NADA response, experiments were performed in the presence of the nitric oxide synthase inhibitor L-NAME, and after potassium channel blockade, to inhibit EDHF activity. It was found that NADA does not act via the release of NO, which is in agreement with some reports on anandamide in isolated mesenteric vessels (White & Hiley, 1997; Chaytor et al., 1999). However, relaxation to NADA was reduced when potassium channels were inhibited, suggesting that release of EDHF contributes to vasorelaxation to NADA, which is in agreement with findings reported for anandamide in rabbit mesenteric vessels (Chaytor et al., 1999). The endothelium-dependent component of vasorelaxation to NADA accounted for about 50% of the response.
In 1999, Zygmunt et al. investigated the possibility that anandamide stimulates VRs on the basis that anandamide is structurally related to capsaicin. They found that, in isolated mesenteric arteries, vasorelaxation to anandamide was sensitive to the VR antagonist capsazepine, capsaicin pretreatment and CGRP receptor antagonism. A role for sensory nerves in vasorelaxation to CBs has been confirmed since then (Ralevic et al., 2000; Harris et al., 2002), although it is not thought that stimulation of the VR accounts for the entire relaxant response to anandamide (White et al., 2001; Harris et al., 2002; Ho & Hiley, 2003). Since NADA has also been found to stimulate VRs (Huang et al., 2002), their potential involvement in the vasorelaxant response to NADA was investigated. It was found that both pretreatment with capsaicin and antagonism of the VR with capsazepine reduced the effects of NADA, confirming the involvement of VRs in vasorelaxation to NADA. This was true for both the resistance mesenteric artery and the superior mesenteric artery. Although it has been previously shown in the whole mesenteric arterial bed that the inhibitory effects of capsaicin on vasorelaxation to anandamide can be prevented by blocking nitric oxide synthesis (Harris et al., 2002), this was not found to be the case in the present study for NADA.
NADA and anandamide have been shown to have similar potency at the CB1 receptor; so, the possibility exists that vasorelaxation to NADA may be mediated, in part, through stimulation of this receptor. The involvement of CB receptors was examined using two different CB1 receptor antagonists, SR141716A and AM251, in endothelium intact preparations. Interestingly, in G3 vessels, SR141716A at 1 μM reduced vasorelaxation to NADA, which may have suggested action via the CB1 receptor, as at 1 μM this antagonist is regarded as being selective (Rinaldi-Carmona et al., 1994). However, in the nanomolar range, SR141716A did not affect responses to NADA, and, furthermore, the structurally similar CB1 antagonist AM251 had no inhibitory effect on the potency of NADA at either 100 nM or 1 μM. This would appear to rule out the involvement of the CB1 receptor in vasorelaxation to NADA in G3 vessels, but does not exclude the possibility of NADA acting at a novel CB receptor sensitive to SR141716A at 1 μM (Járai et al., 1999; Offertáler et al., 2003). Interestingly, these findings are similar to work from Harris et al. (2002), where vasorelaxation to anandamide was inhibited in the whole mesenteric arterial bed in the presence of SR141716A at 3 or 10 μM, but not 1 μM, and was not affected by another CB1 receptor antagonist, LY320135, at 10 μM. Accordingly, it was suggested that SR141716A at 3 μM or 10 μM might be acting at a novel CB receptor with low affinity for SR141716A. However, it cannot be ruled out that SR141617A may be opposing the EDHF-mediated response via inhibition of myoendothelial gap junctions, which has been observed by Chaytor et al. (1999) at a concentration of 10 μM.
After endothelial denudation, SR141716A at 1 μM did not inhibit the vasorelaxant effect of NADA in G3 further than was seen following endothelial denudation alone, suggesting that SR141716A acts at an endothelial site. Járai et al. (1999) reported a similar finding using anandamide, and this group postulated that a novel endothelial receptor exists that is sensitive to SR141716A at 1 μM. To further investigate this, a chemically modified analogue of cannabidiol was used in the present study. This compound binds to the site of action of abnormal cannabidiol, which has been shown to be neither the CB1 nor the CB2 receptor (Járai et al., 1999; Offertáler et al., 2003). In the present study, it was found that O-1918 antagonised the actions of NADA in a similar fashion to SR141716A at 1 μM, confirming that NADA acts at this proposed novel endothelial receptor, and suggesting also that SR141716A at 1 μM antagonises this receptor.
Since the endothelium-derived relaxing factor stimulated by NADA has been shown in the present study to be sensitive to a combination of drugs that are believed to inhibit EDHF activity (Randall & Kendall, 1998; Zygmunt et al., 1997), stimulation of the novel endothelial receptor may be coupled to the release of EDHF. It was also shown in this study that this pathway is independent of stimulation of the VR, since SR141716A at 1 μM in combination with CGRP depletion by capsaicin reduced vasorelaxation to NADA to a greater extent than was seen with either manipulation alone. This is in agreement with previous work, which has shown that vasorelaxation to CBs is not mediated entirely by sensory nerves (Ralevic et al., 2000; Harris et al., 2002; Ho & Hiley, 2003).
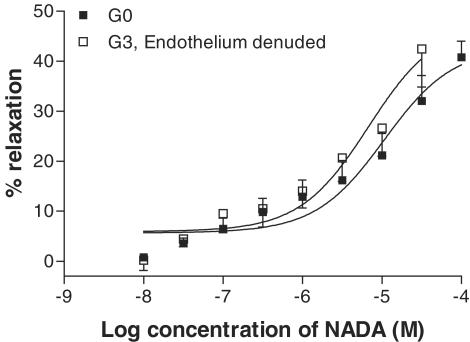
There are different mechanisms of CB-mediated vasorelaxation depending on the vascular bed studied (see Randall et al., 2002). For example, vasorelaxation to anandamide in the rat coronary vessels does not involve sensory nerves (White et al., 2001), and Andersson et al. (2002) showed that, while anandamide is a full agonist of the VR in guinea-pig mesenteric arteries, it is a weak agonist of this receptor in main bronchi. This might suggest that the actions of CBs are dependent on receptor distribution and density, and perivascular nerve density in a given tissue. Indeed, there have been reports of regional differences in anandamide-induced membrane potential changes between the main and daughter branches of the mesenteric artery (Vanheel & van de Voorde, 2001). In the present study, a number of experiments were performed in the superior mesenteric artery (G0) to compare with results found in the small resistance vessels (G3). It was found that there are both differences and similarities between the two vessel types. The main difference is that, in the superior mesenteric artery, the vasorelaxant response to NADA was not sensitive to removal of the endothelium, suggesting that the novel endothelial CB receptor is not expressed in this larger conduit vessel. This is supported by the finding that the cannabidiol analogue O-1918 did not reduce the vasorelaxant response to NADA in the superior mesenteric artery. It is of note that the responses of the endothelium-denuded G3 vessels were identical to those of the control G0 vessels, which could suggest that the endothelial pathway is the only difference between the artery types (see Figure 10).
Figure 10.
Vasorelaxant effects of NADA in the superior mesenteric artery compared with G3 vessels with the endothelium removed.
Unlike G3 vessels, vasorelaxation to NADA in the superior mesenteric artery (G0) was sensitive to both SR141716A and AM 251 at 100 nM, suggesting that stimulation of the CB1 receptor plays a role in the vasorelaxation of larger blood vessels to NADA. Given that both manipulations caused substantial inhibitory effects on vasorelaxation to NADA in G0, this might suggest that there is a degree of synergy between the sensory nerve-mediated vasodilation and the CB1 receptor-mediated effect. Accordingly, in G0, there is clear evidence for participation of both sensory nerves and the CB1 receptor; however, in G3, there is no evidence that CB1 receptors participate in the response to NADA. Indeed, the residual relaxation to NADA in G3 in the presence of SR141716A and following both endothelial denudation and treatment with capsaicin (Figure 7d) points to an additional, unidentified mechanism of action.
In conclusion, the present study has demonstrated and characterised, for the first time, the vasorelaxant properties of the novel endogenous cannabinoid NADA. Its actions appear to be similar to those of the first identified endocannabinoid anandamide. In small resistance arteries, NADA acts at the VR and the novel endothelial CB receptor, which appears to be coupled to EDHF release. In the superior mesenteric artery, NADA acts through stimulation of the vanilloid and CB1 receptors. The difference in efficacy between the small and large vessels may lie solely in the absence of the endothelial pathway in the superior mesenteric artery (see Figure 10).
Acknowledgments
This study was funded by the British Heart Foundation (PG2001/150). The donation of O-1918 by Dr George Kunos is gratefully acknowledged, and we also thank Dr Richard Roberts for the use of a myograph.
Abbreviations
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CB
cannabinoid
- ChTX
charybdotoxin
- EDHF
endothelium-derived hyperpolarising factor
- G3
third-order branch of the superior mesenteric artery
- G0
the superior mesenteric artery
- L-NAME
NG-nitro-L-arginine methyl ester
- NADA
N-arachidonoyl-dopamine
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- VR
vanilloid receptor
References
- ADEAGBO A.S., TRIGGLE C.R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993;21:423–429. [PubMed] [Google Scholar]
- ANDERSSON D.A., ADNER M., HOGESTATT E.D., ZYGMUNT P.M. Mechanisms underlying tissue selectivity of anandamide and other vanilloid receptor agonists. Mol. Pharmacol. 2002;62:705–713. doi: 10.1124/mol.62.3.705. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MELCK D., BOBROV M.Y.U., GRETSKAYA N.M., BEZUGLOV V.V., DE PETROCELLIS L., DI MARZO V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., MARTIN P.E., EVANS W.H., RANDALL M.D., GRIFFITH T.M. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J. Physiol. 1999;520:539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU C.J., HUANG S.M., DE PETROCELLIS L., BISOGNO T., EWING S.A., MILLER J.D., ZIPKIN R.E., DADDARIO N., APPENDINO G., DI MARZO V., WALKER J.M. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.G., KREBSBACH R.J., SCHMID R.J., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Influence of the CB1 receptor antagonist, AM 251, on the regional haemodynamic effects of WIN-55212-2 or HU 210 in conscious rats. Br. J. Pharmacol. 2002;136:581–587. doi: 10.1038/sj.bjp.0704750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG L.I. Dopamine: receptors and clinical applications. Clin. Physiol. Biochem. 1985;3:120–126. [PubMed] [Google Scholar]
- HARRIS D., MCCULLOCH A.I., KENDALL D.A., RANDALL M.D. Characterisation of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J. Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON S., DE PETROCELLIS L., TREVISANI M., BENVENUTI F., BIFULCO M., GEPPETTI P., DI MARZO V. Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. Eur. J. Pharmacol. 2003;475:107–114. doi: 10.1016/s0014-2999(03)02114-9. [DOI] [PubMed] [Google Scholar]
- HO W.S., HILEY C.R. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br. J. Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JÁRAI Z., WAGNER J.A., VARGA K., LAKE K.D., COMPTON D.R., MARTIN B.R., ZIMMER A.M., BONNER T.I., BUCKLEY N.E., MEZEY E., RAZDAN R.K., ZIMMER A., KUNOS G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN S.W., BROADLEY K.J. Atypical antagonism of D1-receptor-mediated vasodilator response in the perfused kidney by SCH23390. Pharmacol. Res. 1995;31:289–297. doi: 10.1016/1043-6618(95)80034-4. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.L. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH A.I., BOTTRILL F.E., RANDALL M.D., HILEY C.R. Characterisation and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br. J. Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- OFFERTÁLER L., MO F.M., BATKAI S., LIU J., BEGG M., RAZDAN R.K., MARTIN B.R., BUKOSKI R.D., KUNOS G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol. Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., ZYGMUNT P.M., MOVAHED P., HÖGESTÄTT E.D. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanandamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br. J. Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., ALEXANDER S.P.H., BENNETT T., BOYD E.A., FRY J.R., GARDINER S.M., KEMP P.A., MCCULLOCH A.I., KENDALL D.A. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem. Biophys. Res. Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., GRIFFITH T.M. Differential effects of L-arginine on the inhibition by NG-nitro-L-arginine methyl ester of basal and agonist-stimulated EDRF activity. Br. J. Pharmacol. 1991;104:743–749. doi: 10.1111/j.1476-5381.1991.tb12498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDALL M.D., HARRIS D., KENDALL D., RALEVIC V. Cardiovascular effects of cannabinoids. Pharm. Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Anandamide and endothelium-derived hyperpolarising factor act via a common vasorelaxant mechanism in rat mesentery. Eur. J. Pharmacol. 1998;346:51–53. doi: 10.1016/s0014-2999(98)00003-x. [DOI] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D., FERRARA P., SOUBRIÉ P., BRELIÉRE J.C., LE FUR G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- TEP-AREENAN P., KENDALL D.A., RANDALL M.D. Mechanisms of vasorelaxation to testosterone in the rat aorta. Eur. J. Pharmacol. 2003;465:125–132. doi: 10.1016/s0014-2999(03)01453-5. [DOI] [PubMed] [Google Scholar]
- VANHEEL B., VAN DE VOORDE J. Regional differences in anandamide- and methanandamide-induced membrane potential changes in rat mesenteric arteries. J. Pharmacol. Exp. Ther. 2001;296:322–328. [PubMed] [Google Scholar]
- WAGNER J.A., VARGA K., ELLIS E.F., RZIGALINSKI B.A., MARTIN B.R., KUNOS G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HO W.S., BOTTRILL F.E., FORD W.R., HILEY C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HOGESTATT E.D., WALDECK K., EDWARDS G., KIRKUP A.J., WESTON A.H. Studies on the effects of anandamide in rat hepatic artery. Br. J. Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H.H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]