Abstract
CGP 12177A mediates cardiostimulation by activation of the ‘putative' β4-adrenoceptor; however, it has recently been reported that disruption of the β1-adrenoceptor gene abolishes this effect. We have adenovirally overexpressed β1-adrenoceptors in isolated, cultured adult rat ventricular cardiomyocytes and observed the inotropic potency of isoprenaline and CGP 12177A (in the presence of 1 μM propranolol).
Isoprenaline was a full inotropic agonist at rat ventricular myocytes (pD2 7.69±0.12). CGP 12177A was a nonconventional partial agonist (pD2 6.34±0.09), increasing inotropy and lusitropy, with an intrinsic activity of 0.34 and antagonised by bupranolol.
β1-adrenoceptor overexpression enhanced the inotropic potency of isoprenaline by 11.7-fold (pD2 8.76±0.14) and CGP 12177A by 5.9-fold (7.11±0.10), respectively. Green fluorescent protein (GFP) overexpression did not alter the potency of isoprenaline or CGP 12177A (pD2 7.41±0.24 and pD2 6.60±0.50, respectively).
The cardiostimulant effects of CGP 12177A were enhanced by IBMX (phosphodiesterase inhibitor) and decreased by Rp-cAMPS (cAMP antagonist). CGP 12177A also increased cAMP levels. CGP 12177A but not isoprenaline initiated arrhythmias at lower concentrations following β1-adrenoceptor overexpression.
125I-Cyanopindolol saturation binding in Adv.β1 myocytes demonstrated ∼18-fold increase in β1-adrenoceptors. 3H-CGP 12177A saturation binding, in the presence of propranolol, increased ∼5-fold following overexpression of β1-adrenoceptors.
This study demonstrates enhanced cardiostimulation by CGP 12177A (in the presence of propranolol) in rat ventricular myocytes overexpressing β1-adrenoceptors, mediated by a Gs/cAMP signalling pathway. ‘Putative' β4-adrenoceptor pharmacology appears to be mediated by activation of a novel affinity state of the β1-adrenoceptor.
Keywords: CGP 12177A, ‘putative' β4-adrenoceptor, β1-adrenoceptors, nonconventional partial agonists, cardiomyocytes
Introduction
Nonconventional partial agonists are β-adrenoceptor antagonists (e.g. pindolol and CGP 12177A) that block the effects of catecholamines in human heart with high affinity at β1- and β2-adrenoceptors, and produce cardiostimulant effects (positive inotropy, lusitropy and chronotropy) at ∼100-fold higher concentrations (Kaumann, 1989; Kaumann & Molenaar, 1997). The cardiostimulant effects of the nonconventional partial agonists are relatively resistant to antagonism by classical β1-adrenoceptor antagonists, including propranolol, but blocked with moderate affinity by bupranolol and carvedilol (Kaumann 1989; 1996; Lowe et al., 2002). A pharmacologically distinct Gs-protein (stimulatory guanine nucleotide binding protein)/Adenylyl cyclase/adenosine 3′,5′-cyclic monophosphate (cAMP)-coupled receptor, the ‘putative' β4-adrenoceptor, was proposed to mediate these effects (Kaumann, 1997; Kaumann & Lynham, 1997). Catecholamines compete stereoselectively at the receptor in radioligand binding studies with low affinity but a profile supporting an adrenoceptor role (Sarsero et al., 1998b). [3H]-CGP 12177A ((−)-[3H]-CGP 12177 (4-(3-t-butylamino-2-hydroxypropoxy) [5,7-3H]benzimidazole-2-one)) has been validated as a radioligand for β-adrenoceptor populations and has been shown to label a site thought to mediate the cardiostimulant effects of CGP 12177A ((±)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazol-2-one) in human (Sarsero et al., 1998a; 2003) and rat (Sarsero et al., 1998b; 1999) atria and ventricles.
Although β4-adrenoceptor pharmacology partly resembles that of the β3-adrenoceptor, it is distinct. The β4-adrenoceptor is not activated by selective β3-adrenoceptor agonists (Kaumann & Molenaar, 1996; Malinowska & Schlicker, 1996; Oostendorp & Kaumann, 2000; Sarsero et al., 1999), SR 59230 (3-(2-Ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate) (selective β3-adrenoceptor antagonist) fails to shift the concentration–response curve to CGP 121777A (Kaumann & Molenaar, 1996) and the cardiostimulant effects of CGP 12177A are still observed in hearts of knockout mice with disruption of the β3-adrenoceptor gene (Kaumann et al., 1998).
Recent evidence suggests that the cardiostimulant or β4-like effects of CGP 12177A may be mediated by a novel state or site of the β1-adrenoceptor with a lower affinity for β-blockers than the high-affinity catecholamine site (Konkar et al., 2000a; Kaumann et al., 2001; Granneman, 2001). CGP 12177A has been observed to upregulate the cAMP response in cell lines overexpressing the β1-adrenoceptor at high density (Pak & Fishman, 1996; Konkar et al., 2000a). In addition, the cardiostimulant effects of CGP 12177A are seen in atria from wild-type and knockout mice with disruption of the β2-adrenoceptor gene, but not in double β1-/β2-adrenoceptor knockout mice despite preservation of cAMP signalling (Kaumann et al., 2001). However, controversy remains over the mechanism of action of CGP 12177A since it has also been shown to upregulate the cAMP response in cell lines overexpressing the β2-adrenoceptor (Pak & Fishman, 1996; Baker & Hill, 2002).
The continued interest in the β4-like effect of CGP 12177A is partly due to its potential role in arrhythmogenesis in man. CGP 12177A produces changes in the cardiac action potential and ventricular extra-systoles in ferret ventricle (Lowe et al., 1998). CGP 12177A also induces arrhythmias in rat ventricular myocytes (Kaumann & Freestone, 1997).
The aim of this study was to examine the cardiostimulant effects of isoprenaline and CGP 12177A (in the presence of propranolol) on isolated and cultured adult rat ventricular cardiomyocytes following adenoviral overexpression of human recombinant β1-adrenoceptors or a reporter gene construct, jelly fish green fluorescent protein (GFP). β1-overexpression increased the arrhythmogenicity of CGP 12177A. A similar magnitude increase in the sensitivity to both isoprenaline and CGP 12177A was observed following overexpression of β1-adrenoceptors but not GFP. An increase in the binding of [3H]-CGP 12177A (in the presence of propranolol) was also observed. Taken together, this suggests that the cardiostimulant effects of CGP 12177A and β4-adrenoceptor pharmacology are mediated by a propranolol-resistant state of the β1-adrenoceptor.
Methods
Cardiomyocyte isolation, culture and adenoviral infection
All studies complied with the United Kingdom Home Office Regulations Governing the Care and Use of Laboratory Animals. Single ventricular myocytes were isolated by enzymatic dissociation as previously described (Vescovo et al., 1989) and were then cultured and infected with adenoviral vector. Adult male Sprague–Dawley rats were heparinised and killed by cervical dislocation, and the heart was rapidly excised and placed in ice-cold Krebs–Henseleit (KH) solution of the following composition (in mM): NaCl 119, KCl 4.7, MgSO4 0.94, KH2PO2 1.2, NaHCO3 25, glucose 11.5 and CaCl2 1; equilibrated to pH 7.4 with 95% O2–5% CO2.
A Langendorff perfusion method similar to that described previously was used (Vescovo et al., 1989). The heart was mounted on a needle canal attached to a Langendorff perfusion apparatus and equilibrated in a retrograde manner for 5 min with KH solution containing 1 mM Ca2+ at 37°C. The perfusate was then changed to a low-Ca2+ solution of the following composition (in mM): NaCl 120, KCl 5.4, MgSO4 5, pyruvate 5, glucose 20, taurine 20, N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid (HEPES) 10 and nitrilotriacetic acid (NTA) 5 and Ca2+ was added to give a final concentration of 12–14 μM as measured with a Ca2+ electrode, also at 37°C and bubbled with 100% O2 at pH 6.95. After 5 min, this was changed to a solution similar to the low-Ca2+ solution but with no NTA and 200 μmol l−1 Ca2+ with 1 mg ml−1 collagenase and 0.6 mg ml−1 hyaluronidase added, for 10 min. The ventricles were then chopped with scissors and the pieces incubated in the same solution twice for 10 min. The medium was shaken gently throughout this incubation at 35°C, and kept under an atmosphere of 100% O2. The dispersed cells were then strained through a 300 μm mesh gauze and the supernatant was centrifuged at 40 × g for 1 min at room temperature. The cells were then washed three times and cultured as previously described (Davia et al., 1999) with plating at 2 × 104 cm−2 in M199 medium with the addition of 0.2% bovine serum albumin, 100 mM ascorbate, 5 mM creatine, 5 mM taurine, 2 mM carnitine, 0.1 μM insulin, 10,000 U ml ml−1 penicillin and 10 mg ml−1 streptomycin. Overexpression of the gene of interest was achieved by addition of the adenovirus containing the sequence for human β1 adrenoceptor (Adv.β1) or jelly fish Green Fluorescent Protein (Adv.GFP) to the medium before plating, at a density of 2 × 106 active particles cm−2, to give a multiplicity of infection (MOI) of 100. All experiments were carried out following 48 h culture.
Measurement of cell contraction
Myocytes in suspension were placed into a 200 μl Perspex bath with a glass floor on the stage of an inverted microscope (Zeiss IM, Germany). After 5 min, to allow cells to spontaneously attach to the floor of the chamber, they were superfused with KH solution adjusted to pH 7.4 by equilibration with 95% O2–5% CO2 at 2 ml min−1. The temperature was maintained at 36±0.5°C by a heater placed just before the chamber inlet and by thermocouples for feedback control of the heater. Cells were paced at 0.5 Hz with the use of a bipolar pulse (width 0.1–0.5 ms) via platinum electrodes along the sides of the bath. Continuous measurement of myocyte contraction amplitude and rates of shortening and relaxation were carried out as previously described (Harding et al., 1988; Gong et al., 2000). A video-motion detection device was used to display cell length and rate of change of cell length continuously to a chart recorder. The scanning rate of the camera was 100 Hz and the spatial resolution was 1–512. Time-to-peak contraction (TTP), time-to-50 and 90% relaxation (R50, R90) were obtained from an average of 6 beats.
The myocytes were selected according to criteria adapted from those described previously (Harding et al., 1988) to ensure that populations of cells with similar functional properties were used for contraction studies. Myocytes used were rod shaped with clear sarcomeres, no blebs or granulations and were quiescent in 1 mM Ca2+. Myocytes were allowed to stabilise for at least 10 min in 1 mM Ca2+ and had to demonstrate stable contraction amplitude and diastolic length at 0.5 Hz pacing prior to drug challenge. Cells that satisfied these criteria were subjected to cumulative concentration–response experiments using isoprenaline (0.1 nM to 3 μM), each concentration of agonist was left in contact for 5 min or until a steady response had been reached. Maximum contraction was judged to have occurred either when there was no increase in contraction amplitude between successive concentrations of isoprenaline or the myocyte developed arrhythmias. All experiments using CGP 12177A were performed in the presence of the nonselective β-adrenergic antagonist propranolol (1μM) unless specifically described in the text. Myocytes were preincubated with propranolol for 20–30 min, and the concentration–response experiments were repeated on the same cell using CGP 12177A (10 nM to 3 μM) in the continued presence of propranolol. To determine whether amplitudes equivalent to those with maximum isoprenaline had been achieved with CGP 12177A, 100 nM and 0.1 mM isoprenaline was then superfused over the myocytes to observe whether maximum contraction had been reached. To observe whether the myocyte would return to baseline contraction amplitude, either CGP 12177A was washed out (in the continued presence of propranolol), or 1 μM bupranolol was added. Spontaneous arrhythmias were defined as myocytes demonstrating wave-like contractions at a rate greater than 2 min−1 in 1 mM Ca2+ when not electrically stimulated.
Measurement of steady-state cAMP accumulation
After isolation, single adult ventricular cardiomyocytes were plated at 2 × 104 cm−2 on 12-well culture plates precoated with laminin (40 μg ml−1) prior to adenoviral infection. Experiments were performed after 48 h culture by washing twice in ES to remove nonviable myocytes. cAMP was measured in the adherent cell cultures using a nonacetylation cAMP enzyme immunoassay system (Amersham Pharmacia biotech, Little Chalfont, U.K.). Cells were incubated in 1 ml low-Ca2+ (KH with no NTA) for 10 min with 1 μM isoprenaline or 3 μM CGP 12177A (a concentration chosen to match the maximum effect observed in contraction experiments), in the presence of 1 μM propranolol at 37°C in a CO2 incubator. Myocyte lysis was performed using 200 μl of lysis reagent (supplied) and confirmed by Trypan blue staining. 100 μl of the supernatant was used in the cAMP assay according to the manufacturer's instructions. Protein concentration was determined by the method of Bradford (Bradford, 1976) with IgG used as the standard (Bio-Rad, Hemel Hempstead, U.K.).
Radioligand receptor binding assay
Following 48 h culture with or without Adv.β1 infection, cardiac myocytes were harvested and stored at −70°C until use. Prior to use, myocytes were thawed, resuspended in incubation buffer (25 mM Tris-HCl (Tris-hydrochloride), pH 7.4) and homogenised on ice with a Polytron homogenizer (3 × 10 s) and further disrupted by repeated passage through a sterile 21-gauge needle. The samples were centrifuged at 1000 × g for 10 min at 4°C to remove unhomogenised debris and the supernatant centrifuged at 40,000 × g for 20 min at 4°C. The pellet was then washed twice in cold incubation buffer (containing 0.32 mM sucrose) and centrifuged at 40,000 × g for 20 min at 4°C. The pellet was resuspended in 2 ml of incubation buffer and filtered through nylon membranes. The binding assays were performed on membrane protein using increasing amounts of the β-adrenoceptor-specific ligands [125I]-cyanopindolol (specific activity 2000 Ci mmol−1, 3.125–100 pM) for β1-adrenoceptors or [3H]-CGP 12177A (specific activity 46 Ci mmol−1, 7.8125–250 nM). Nonspecific binding was defined using 100 μM CGP 12177A. [3H]-CGP 12177A saturation binding was performed in the presence of 500 nM propranolol. Incubation was carried out in 250 μl of incubation buffer at 37°C for 90 min with gentle agitation. The incubation was terminated by rapid vacuum filtration through GF/C glass-fibre filters (Whatman, Clifton, NJ, U.S.A.) with the use of a cell harvester (Brandel, Model M-24). Filters were rapidly washed three times with 5 ml ice-cold incubation buffer and counted in an auto gamma counter for [125I]-cyanopindolol (Packard Instruments, Model 5550, Downers Grove, IL, U.S.A.) at an efficiency of 80% or a Rackbeta Spectral Liquid Scintillation counter for [3H]-CGP 12177A (LKB/Wallac, Model 1219). Protein concentration was determined by the method of Bradford (Bradford, 1976) with IgG used as the standard (Bio-Rad, Hemel Hempstead, U.K.). All assays were performed in duplicate, and receptor density was normalised to mg membrane protein. Kd and the maximal number of binding sites (BMAX) for [125I]-cyanopindolol or [3H]-CGP 12177A were determined by Scatchard analysis of saturation binding curves.
Materials
Pyruvate, taurine, NTA, hyaluronidase (type I-s), adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer (Rp-cAMPS), 3-isobutyl-1-methylxanthine (IBMX), (±)-CGP 12177A and isoprenaline-HCl were obtained from Sigma (Poole, U.K.). Collagenase II (Worthington) was from Cambridge Bioscience (Cambridge, U.K.). All other agents used for KH and low-Ca2+ solutions were obtained from BDH (Poole, U.K.). AristaR-grade KCl and glucose were used for low-Ca2+ solutions. All other reagents were AnalaR grade. AnalaR water (BDH) was used for low-Ca2+ solutions. Milli Q water was used for other solutions. [125I]-cyanopindolol and [3H]-CGP 12177A were purchased from Amersham (Amersham Pharmacia biotech, Little Chalfont, U.K.). Bupranolol was a generous gift of Schwartz-Pharma (Mannheim, Germany). The Adv.GFP virus was a kind gift of Drs Hajjar and del Monte, CVRC, Harvard University, Boston, U.S.A. and the Adv.β1 was a kind gift of Dr W. Koch, Department of Surgery, Duke University Medical Center, Durham, U.S.A.
Data analysis
All data are presented as mean±standard error of the mean (s.e.m.). The mean values for experiments performed on different days and hence in different rat myocyte preparations were used in statistical analysis. Between group comparison was made by unpaired Student's t-test and paired t-test was applied for within-group comparison. Agonist EC50 values were calculated by nonlinear regression analysis using the HYPMIC program or Prism 3.0 software. The n in the text refers to the number of separate myocytes studied. A value of P<0.05 was considered to be statistically significant.
Results
β-adrenoceptor density and [3H]-CGP 12177A binding increases following infection with Adv.β1
Total β-adrenoceptor binding (β1- and β2-adrenoceptors) of [125I]-cyanopindolol to adult rat cardiomyocyte membranes yielded a pKD of 10.7±0.08 and a BMAX of 27.7±3.70 fmol mg protein−1. Following infection with Adv.β1 at an MOI of 100, [125I]-cyanopindolol binding yielded a pKD of 10.6±0.20 and a BMAX of 490±34.0 fmol.mg protein−1, representing an approximate 18-fold increase in the total β-AR myocyte surface receptor population (P<0.001; Figure 1). This MOI of 100, giving a moderate increase in total β-AR receptor number, was used for all further experiments.
Figure 1.
Saturation binding curve showing increase in total β-AR binding (fmol mg protein−1) in membranes from Adv.β1 infected adult rat ventricular cardiomyocytes compared with uninfected myocytes. The values are mean of three experiments, each performed in triplicate.
[3H]-CGP 12177A, in the presence of 1 μM propranolol, appeared to label a significant receptor population that was increased ∼5-fold following overexpression of β1-adrenoceptors (P<0.05 at 250 nM [3H]-CGP 12177A; Figure 2). The pKD in uninfected and Adv.β1 myocytes was 6.78±0.19 and 6.65±0.35, respectively.
Figure 2.
Saturation binding curve showing increase in [3H]-CGP 12177A binding (fmol mg protein−1), in the presence of 500 nM propranolol, in membranes from Adv.β1 infected adult rat ventricular cardiomyocytes compared with uninfected myocytes. The values are mean of six experiments, each performed in duplicate.
Adv.GFP infection does not alter the contractile responses to isoprenaline or CGP 12177A
To establish whether adenoviral transfection of a control gene not involved in cardiomyocyte contraction affects the contractile responses to isoprenaline or CGP 12177A, we used a control virus, Adv.GFP. The successful infection of an individual myocyte with Adv.GFP led to green fluorescence of the myocyte. It was therefore possible to determine that at an MOI of 100, over 75% of myocytes and at an MOI 1000, over 90% of ventricular myocytes were successfully infected and expressing GFP (data not shown). In uninfected cells, isoprenaline produced a concentration-dependent increase in contraction of isolated single adult rat cardiomyocytes, measured as cell shortening (pD2 7.74±0.22). Following infection with Adv.GFP at an MOI of 1000, there was no significant difference in response to isoprenaline (pD2 7.41±0.24; P>0.1; Figure 3a). In the presence of 1 μM propranolol, to block β1- and β2-adrenoceptors, CGP 12177A stimulated a smaller but significant increase in contraction (pD2 6.44±0.26). Following infection with Adv.GFP, there was no significant difference in contraction (pD2 6.60±0.50; P>0.6; Figure 3b).
Figure 3.
Concentration–response curves to isoprenaline (a) and CGP 12177A (b) following 48 h culture in uninfected (n=5) or Adv.GFP (MOI 1000; n=5) infected ventricular myocytes. Contraction amplitude (% shortening) after subtraction of basal amplitude (mean±s.e.m.).
Overexpression of β1-adrenoceptors enhances the contractile responses to both isoprenaline and CGP 12177A
In uninfected cells, isoprenaline produced a concentration-dependent increase in contraction of the single adult rat cardiomyocytes (pD2 7.69±0.12). Following overexpression of β1-adrenoceptors, there was a significant increase in sensitivity to the cardiostimulation induced by isoprenaline with an 11.7-fold left shift in the concentration–response curve (pD2 8.76±0.14; P<0.001; Figure 4a). There was no significant difference in the maximum amplitude (uninfected EMAX 11.2±0.46% shortening; Adv.β1 10.9±2.17% shortening; P>0.57).
Figure 4.
(a) Concentration–response curves to isoprenaline after 48 h culture in uninfected (n=25) or Adv.β1 (MOI 100; n=22) infected ventricular myocytes. (b) Concentration–response curves to CGP 12177A after 48 h culture in uninfected (n=41) or Adv.β1 (MOI 100; n=34) infected ventricular myocytes. Contraction amplitude (% shortening) after subtraction of basal amplitude (mean±s.e.m.).
In uninfected cells, in the presence of 1 μM propranolol, CGP 12177A produced a concentration-dependent increase in contraction of rat cardiomyocytes (pD2 6.34±0.09; 34.4±4.90% of the maximal isoprenaline response). Following overexpression of β1-adrenoceptors, there was also a significant increase in sensitivity to CGP 12177A with a 5.9-fold left shift in the concentration response (pD2 7.11±0.10; P<0.001; 48.5±6.2% of the maximal isoprenaline response; Figure 4b). There was also an increase in the maximum contraction (uninfected EMAX 2.77±0.13% shortening; n=39; Adv.β1-AR 4.82±0.73%; n=15; P<0.005). Examples of traces are shown in Figure 5.
Figure 5.
(a) Sample trace of cumulative concentration–response curve to CGP 12177A, in the presence of 1 μM propranolol, in an uninfected single adult rat ventricular cardiomyocyte. (b) Typical trace following 48 h culture in an Adv.β1 (MOI 100) infected ventricular myocyte.
CGP 12177A enhances diastolic relaxation
CGP 12177A did not significantly alter the TTP in either uninfected or Adv.β1 infected myocytes (Figure 6a). CGP 12177A (1 μM) slightly shortened the R50, but this was not statistically significant. Shortening of relaxation became more marked for the R90 (basal 136.4±14.8 ms; CGP 12177A 94.6±7.4 ms; P<0.05; Figure 6b and c). Following β1-adrenoceptor overexpression, basal R90 was significantly shorter compared with uninfected myocytes (P<0.05). CGP 12177A produced a further significant shortening of R90 in Adv.β1 infected myocytes (basal 105±5.7 ms; CGP 12177A 84.8±4.6 ms; P<0.05). However, the CGP 12177A stimulated R90 was not significantly different between uninfected and Adv.β1 infected myocytes. Similarly, isoprenaline stimulation shortened R90 in uninfected (basal 134±8 ms; isoprenaline 81.3±3.9 ms; P<0.001) and β1-adrenoceptor overexpressing myocytes (basal 116±5.3; isoprenaline 93.3±6.4 ms; P<0.01) to a degree that was not significantly different from shortening of R90 by CGP 12177A.
Figure 6.
Basal and stimulated (1 μM CGP 12177A) values for TTP (a), R50 (b) and R90 (c) after 48 h culture in uninfected or Adv.β1 infected (MOI 100) ventricular myocytes. Number of cells from 24 preparations; uninfected basal n=14, uninfected+CGP 12177A n=13; Adv.β1 basal n=23, Adv.β1+CGP 12177A n=21. **P<0.05 vs uninfected basal; ***P<0.05 vs Adv.β1 basal; ## P<0.01 vs uninfected basal (milliseconds (ms)±s.e.m.).
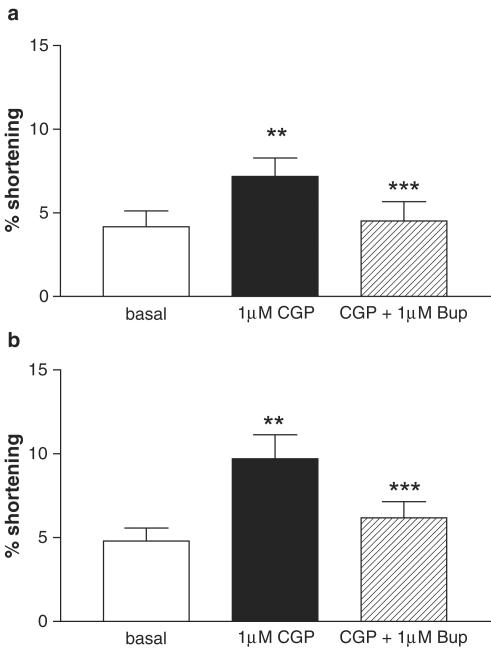
CGP 12177A cardiostimulation was antagonised by bupranolol
In uninfected myocytes, 1 μM propranolol modestly but significantly shifted the concentration–response curve to CGP 12177A rightwards (− propranolol pD2 7.08±0. 17; + propranolol 6.31±0.13; P<0.05; Figure 7a). However, in Adv.β1 infected myocytes, propranolol failed to shift the concentration–response curve to CGP 12177A (− propranolol pD2 7.25±0.07; + propranolol 7.09±0.16; P>0.21; Figure 7b). In both uninfected and Adv.β1 infected myocytes, following stimulation with 1 μM CGP 12177A (in the presence of 1 μM propranolol), contraction amplitude could be returned to baseline by 1 μM bupranolol (uninfected: basal 4.17±1.06% shortening; CGP 12177A 7.18±1.09%; CGP 12177A+bupranolol 4.52±1.15%. Adv.β1-AR; basal 4.79±0.77%; CGP 12177A 9.71±1.43%; CGP 12177A+bupranolol 6.18±0.96%; Figure 8a and b).
Figure 7.
(a) Concentration–response curves to CGP 12177A after 48 h culture in uninfected ventricular myocytes in the presence (n=41) or absence of 1 μM propranolol (n=10). (b) Concentration–response curves to CGP 12177A after 48 h culture in Adv.β1 infected myocytes in the presence (n=34) or absence of 1 μM propranolol (n=8). Contraction amplitude (% shortening) after subtraction of basal amplitude (mean±s.e.m.).
Figure 8.
Antagonist effect of 1 μM bupranolol on maximum contraction with 1 μM CGP 12177A (in the presence of 1 μM propranolol) on uninfected (a) and Adv.β1 infected (MOI 100; b) myocytes. Amplitudes (% shortening, mean±s.e.m.) are shown before and during exposure to CGP 12177A followed by the addition of bupranolol (uninfected n=4; Adv.β1 n=6). **P<0.05 vs basal, ***P<0.05 vs maximum contraction with CGP 12177A, ## P<0.01 vs basal, ###P<0.01 vs maximum contraction with CGP 12177A.
CGP 12177A cardiostimulation increases cAMP
In uninfected cells, in the presence of 1 μM propranolol, nonselective phosphodiesterase inhibition using IBMX increased the sensitivity (Pre-IBMX pD2 5.74±0. 22; Post-IBMX pD2 6.91±0.18; P<0.001) and maximum cardiostimulant effects of CGP 12177A (Pre-IBMX EMAX 4.48±1.04% shortening; Post-IBMX EMAX 9.12±1.21%; P<0.01; Figure 9). Rp-cAMPS (100 μM), a competitive antagonist of cAMP at protein kinase A (PKA), significantly depressed CGP 12177A cardiostimulant effects in both uninfected (P<0.05 at 3 μM CGP 12177A; Figure 10a) and Adv.β1 infected myocytes (P<0.02 at 3 μM CGP 12177A; Figure 10b).
Figure 9.
Concentration–response curves to CGP 12177A after 48 h culture in uninfected ventricular myocytes in the presence (n=41) or absence of 1–10 μM IBMX (n=10). Contraction amplitude (% shortening) after subtraction of basal amplitude (mean±s.e.m.).
Figure 10.
(a) Concentration–response curves to CGP 12177A after 48 h culture in uninfected (n=3) ventricular myocytes in the presence or absence of 100 μM Rp-cAMPS. (b) Concentration–response curves to CGP 12177A after 48 h culture in Adv.β1 (MOI 100; n=9) infected ventricular myocytes in the presence of absence of 100 μM Rp-cAMPS. Contraction amplitude (% shortening) after subtraction of basal amplitude. (mean±s.e.m.).
CGP 12177A stimulated a small steady-state cAMP increase in uninfected myocytes (basal 2.82±0.52 pmol mg protein−1, CGP 4.18±0.56, n=7; P<0.05). This represents approximately 47% of the maximal cAMP following stimulation with isoprenaline (data not shown). Following overexpression with β1-adrenoceptors, there was a much larger steady-state cAMP response to CGP 12177A (basal 3.85±0.75 pmol mg protein−1, CGP 12177A 6.29±0.95; P<0.005; Figure 11). There was no difference between basal cAMP in uninfected and β1-adrenoceptor overexpressing myocytes (P>0.3).
Figure 11.
Steady-state cAMP levels (pmol mg protein−1) after 48 h culture in uninfected or Adv.β1 infected adult rat ventricular myocytes in the absence (clear bars) or presence (shaded bars) of 3 μM CGP 12177A; mean±s.e.m.; MOI 100; n=7 preparations, each in triplicate, with 1 μM propranolol present in all conditions. **P<0.05, ***P<0.005 vs respective basal.
Isoprenaline and CGP 12177A induce arrhythmias in myocytes overexpressing β1-adrenoceptors
In unpaced β1-adrenoceptor overexpressing ventricular myocytes, there was no increase in the number of cells displaying spontaneous arrhythmia compared with uninfected myocytes (1 of 43 uninfected and 1 of 60 Adv.β1 myocytes; P=NS, χ2). Following pacing and stimulation with isoprenaline, eight of 11 uninfected and nine of 14 Adv.β1 myocytes demonstrated spontaneous arrhythmia. There was no change in the concentration of isoprenaline at which arrhythmias first appeared (uninfected mean isoprenaline concentration threshold 0.49 μM, Adv.β1 0.13 μM; P>0.3; Figure 12a). However, when stimulated with CGP 12177A, there was an excess of arrhythmias in overexpressing myocytes compared with uninfected cells (11 of 43 uninfected and 26 of 47 Adv.β1 myoytes; P<0.05, χ2). There was a reduced CGP 12177A concentration threshold at which arrhythmias first occurred following overexpression of β1-adrenoceptors (uninfected mean CGP 12177A concentration threshold 1.19 μM, Adv.β1 0.40 μM; P<0.05; Figure 12b). Adv.β1 infected myocytes demonstrating arrhythmia at 3 μM CGP 12177A were additionally superfused with 1 μM bupranolol, in the continued presence of CGP 12177A. In six of nine cells, the arrhythmia was reversed in addition to a decrease in contraction amplitude.
Figure 12.
(a) Bar chart showing distribution of concentrations at which isoprenaline initiates arrhythmia in uninfected (n=8) and Adv.β1 infected (n=9) ventricular myocytes. (b) Bar chart showing distribution of concentrations at which CGP 12177A initiates arrhythmia in uninfected (n=11) and Adv.β1 infected (n=26) ventricular myocytes.
Discussion
In this study, CGP 12177A was a partial agonist compared with isoprenaline but the cardiostimulation was propranolol resistant and antagonised by bupranolol. Overexpression of β1-adrenoceptors by adenoviral transfection in single adult rat cardiomyocytes increased the inotropic potency and radioligand binding to both isoprenaline and CGP 12177A to a similar extent. The cardiostimulant effects of CGP 12177A were enhanced by IBMX and depressed by Rp-cAMPS consistent with the involvement of a cAMP/PKA second messenger signalling pathway, which was confirmed biochemically. In addition, the ability of isoprenaline and CGP 12177A to initiate arrhythmia appear to differ with increased arrhythmogenic potency of CGP 12177A following overexpression of β1-adrenoceptors.
CGP 12177A cardiostimulation
We have demonstrated that CGP 12177A increased inotropy and lusitropy (significant shortening of R90) of single rat adult cardiomyocytes in a nonconventional partial agonist fashion compared with isoprenaline. This confirms previous reports which have shown that CGP 12177A is a nonconventional partial agonist producing cardiostimulation in rat ventricle (Kaumann, 1989) attributed to activation of a novel β4-adrenoceptor (Kaumann, 1997).
Propranolol has been reported to have a pKB of ∼6.2 as an antagonist of the inotropic responses of CGP 12177A in ferret ventricle (Lowe et al., 2002). A small rightward shift in the concentration–response curve to CGP 12177A was observed in uninfected cells, but the cardiostimulant effect of CGP 12177A in Adv.β1 myocytes was propranolol resistant. Consistent with mediation of the effects of CGP 12177A by a β4-like receptor, 1 μM bupranolol antagonised cardiostimulation.
We have also shown that adenoviral transfection of the control gene GFP into adult rat ventricular cardiomyocytes did not alter contractile responses to either isoprenaline or CGP 12177A. The lack of change in agonist response to Adv.GFP infection at MOI 1000 suggests that at an MOI of only 100 Adv.β1 (used in these experiments), any change in the cardiostimulant effects of CGP 12177A must be due to the increase in β1-adrenoceptors alone.
Cardiostimulation by isoprenaline and CGP 12177A mediated by different states of the β1-adrenoceptor
In uninfected rat ventricular myocytes, the total β-adrenoceptor population (β1- and β2-adrenoceptors) labelled by [125I]-cyanopindolol agrees with previously published data (Sarsero et al., 1999). The ∼18-fold increase in the total β-adrenoceptor population following overexpression of β1-adrenoceptors must be due to increased β1-adrenoceptors, which normally account for ∼90% of the total (Ranu et al., 2000).
We have used overexpression of β1-adrenoceptors in a functional assay and demonstrated a similar magnitude increase in potency to both isoprenaline and CGP 12177A, suggesting that the enhanced cardiostimulant effects of CGP 12177A are due to the increase in β1-adrenoceptors. Since the cardiostimulant effects of CGP 12177A are propranolol resistant at a concentration expected to block completely the action of catecholamines at β1- and β2-adrenoceptors, CGP 12177A must activate a noncatecholamine site or state of the β1-adrenoceptor. The increase in the maximum contraction stimulated by CGP 12177A following β1-adrenoceptor overexpression can be explained by its partial agonist activity.
These findings are in agreement with previous overexpression studies, where CGP 12177A antagonised catecholamines at low concentrations but could increase adenylyl cyclase activity at higher concentrations in cell lines overexpressing β1-adrenoceptors at high (Pak & Fishman, 1996) and physiological density (Konkar et al., 2000a, 2000b). More recent studies suggested that a propranolol-resistant state of the β1-adrenoceptor mediates the stimulant effects of CGP 12177A in brown adipose tissue (Konkar et al., 2000a) and heart in β1-/β2-adrenoceptor double knockout mice (Kaumann et al., 2001).
The increase in [3H]-CGP 12177A binding (in the presence of propranolol masking the β1- and β2-adrenoceptor catecholamine sites) following overexpression of β1-adrenoceptors suggests a corresponding increase in the low-affinity CGP 12177A binding site, previously attributed to a separate β4-adrenoceptor. This is supporting evidence for mediation of the cardiostimulant effects of CGP 12177A by a low-affinity state of the β1-adrenoceptor and not a separate receptor. The BMAX obtained with [3H]-CGP 12177A binding to uninfected rat ventricular myocyte membranes is similar to previously reported values (Sarsero et al., 1999). Following overexpression of β1-adrenoceptors, there is some discrepancy between the increase in β1-adrenoceptors (∼18-fold) and that of the low-affinity site (∼5-fold). However, the low-affinity site in uninfected myocytes is difficult to quantitate due to the low specific activity of [3H]-CGP 12177A and the small amounts of protein available. Kaumann et al. (2001) identified a site labelled by [3H]-CGP 12177A in both wild-type and β1-/β2-adrenoceptor double knockout mice, but this site did not appear to mediate the cardiostimulant effects of CGP 12177A. However, Sarsero et al. (2003) have demonstrated that in atrium from failing heart, there is a similar reduction in the densities of the low- and high-affinity [3H]-CGP 12177A binding sites, together with decreased potency of CGP 12177A. This suggests that the site labelled by [3H]-CGP 12177A, in the presence of propranolol, is the site through which CGP 12177A elicits cardiostimulant effects.
Sarsero et al. (1998b) considered whether the low-affinity binding could be interpreted as β1-adrenoceptor binding shifted rightwards by propranolol by estimating the affinities of nonconventional partial agonists and catecholamines at β1-adrenoceptors. The affinities for all ligands tested were higher at the β1-adrenoceptor than at the lower-affinity site; however, the differences between pKi values at the two sites were not consistent (1.6–3.6 log units). If propranolol had simply right shifted the saturation binding, the difference in pKi values would be identical, regardless of the ligand affinity.
Taken together with the increase in the low-affinity site labelled by [3H]-CGP 12177A in the presence of propranolol masking the catecholamine site, the increased potency of CGP 12177A following β1-adrenoceptor overexpression further suggests that the cardiostimulant effects of CGP 12177A are mediated by a low-affinity state of the β1-adrenoceptor and not a separate receptor.
Understanding the molecular basis of CGP 12177A activation of β1-adrenoceptors
The molecular or structural state of the β1-adrenoceptor mediating the cardiostimulant effects of CGP 12177A has not been elucidated. Receptor theory suggests that agonists produce effects by stabilising an active receptor state with antagonists stabilising an inactive state. However, recent evidence suggests that receptors, including the α1A-adrenoceptor and β2-adrenoceptor, can exist in more than one active state or conformation (Samama et al., 1994; Ford et al., 1997; Seifert et al., 1999). The literature also supports the existence of two active states or sites of the β1-adrenoceptor: a high-affinity ‘classical' catecholamine site stabilised by isoprenaline and a low-affinity site stabilised by CGP 12177A, both in equilibrium with an inactive state (Konkar et al., 2000a, 2000b; Figure 13). It has been suggested that the downstream effects of the low-affinity state of the β1-adrenoceptor could occur by interaction with other accessory proteins, possibly in a similar manner to receptor activity modifying proteins (McLatchie et al., 1998), post-translational modifications or conformational changes (Konkar et al., 2000b).
Figure 13.
Proposed model to explain the interaction of catecholamines and CGP 12177A with the β1-adrenoceptor. Catecholamines bind to and stabilise the R form with high affinity (H) resulting in a shift to the RH*CAT form allowing G protein activation and increased contraction. Standard β-adrenoceptor antagonists (e.g. Propranolol) and CGP 12177A can also bind to the inactive R form with high affinity and shift the equilibrium away from R* to RH*CGP/Prop. CGP 12177A also binds to and stabilise the R form but with low affinity (L), resulting in a shift to the RL*CGP and subsequent G protein activation. The low-affinity state also recognises standard β-adrenoceptor antagonists with but low affinity.
Consideration of the structure–activity relationship of CGP 12177A, propranolol and bupranolol at the low-affinity β1-adrenoceptor site improves our understanding of these interactions. β-Adrenoceptor ligands may occupy either folded or extended conformations, with only the extended conformation mediating receptor activation (Blin et al., 1993). The relatively constrained ligand binding pockets of the catecholamine sites of β1- and β2-adrenoceptors only allows interaction with the folded conformations of β-adrenoceptor ligands, where they behave as antagonists. Konkar et al. (2000b) suggested that the low-affinity site of the β1-adrenoceptor has a less constrained binding pocket like the β3-adrenoceptor, allowing interaction with the extended conformation of aryloxypropanolamines such as CGP 12177A, allowing receptor activation. Propranolol is a high-affinity antagonist at the catecholamine site where it may occupy a folded conformation, but in an extended conformation at the novel site has low affinity. Bupranolol has a chloride substitution in the ortho position of the phenyl ring which has been shown to confer higher-affinity antagonism of the cardiovascular effects of CGP 12177A (Malinowska et al., 2003) compared with the unsubstituted ring of propranolol. Thus, the catecholamine and CGP 12177A sites of the β1-adrenoceptor may allow binding of folded and extended conformations, with the CGP 12177A site allowing activation by extended conformers and relatively low affinity for propranolol but higher affinity for bupranolol.
CGP signals via cAMP–PKA pathway
Signalling through a cAMP/PKA pathway was suggested by the increased sensitivity and maximum contraction of uninfected ventricular myocytes in the presence of phosphodiesterase inhibition by IBMX and the significant depressed response to CGP 12177A in the presence of Rp-cAMPS (a competitive antagonist of cAMP at PKA) in both uninfected and Adv.β1 myocytes. Increased CGP 12177A stimulated lusitropy (shortening of R90) is also consistent with signalling via a cAMP pathway. This was confirmed biochemically, with CGP 12177A increasing steady-state cAMP. This increase was greater following overexpression of β1-adrenoceptors. However, basal unstimulated cAMP was not significantly different between the uninfected and Adv.β1 myocytes, suggesting that raised basal cAMP does not explain the increased potency to isoprenaline and CGP 12177A. This confirms previous findings that cardiostimulation by CGP 12177A activates the cAMP/PKA second messenger pathway (Kaumann & Lynham, 1997; Sarsero et al., 2003).
CGP 12177A elicits arrhythmias through the low-affinity state of the β1-adrenoceptor
A potentially important finding was the selective effect of β1-adrenoceptor overexpression to enhance the arrhythmic actions of CGP 12177A. In Adv.β1 myocytes, CGP 12177A initiated arrhythmias twice as often, while the isoprenaline arrhythmia frequency remained unchanged, compared with uninfected myocytes. In addition, although CGP 12177A had an intrinsic activity of 0.49 compared with isoprenaline, it induced arrhythmias in the same proportion of myocytes. There was also a lower concentration threshold at which CGP 12177A initiated arrhythmias in Adv.β1 myocytes. These data support an increased tendency for arrhythmogenesis through a noncatecholamine site since the arrhythmias were observed in the presence of propranolol. A greater potency of CGP 12177A to induce arrhythmias compared with isoprenaline has been previously documented (Freestone et al., 1999). In mouse ventricular myocytes, CGP 12177A was 40 times more potent than isoprenaline in producing arrhythmic Ca2+ transients, but with a potency suggesting interaction at a high-affinity site of the β1-adrenoceptor. To explain this, Kaumann et al. (2001) have suggested that the arrhythmic effects of CGP 12177A are mediated by a high-affinity site of the β1-adrenoceptor that differs from the low-affinity site mediating the cardiostimulant effects. It is also possible that the low-affinity site has a different coupling profile to intracellular signalling pathways. The selective effect of β1-adrenoceptor overexpression to enhance the arrhythmic actions of CGP 12177A indicates the potential importance of activation of this site in arrhythmogenesis. It is interesting to note bupranolol stopped the CGP 12177A induced arrhythmia in 67% of myocytes, suggesting a possible therapeutic role in the treatment of arrhythmia.
Study limitations
The single cardiomyocyte contraction model provides a useful, natural system to study the effect of drugs on G protein-coupled receptors. However, receptor overexpression may result in β1-adrenoceptors being expressed in cellular locations where they are not normally expressed and alter cellular responses. Additional limitations of overexpressing human sequence β1-adrenoceptors in rat myocytes may include altered relative stoichiometry of receptors to host membrane components or the lack of specific human signal transduction pathway components in rat, which may limit the effects seen by overexpression.
The Arginine 389 variant of the common β1-adrenoceptor Glycine 389 Arginine polymorphism has been shown to couple to adenylyl cyclase more tightly than the Glycine 389 variant when activated by isoprenaline and CGP 12177A (Mason et al., 1999; Joseph et al., 2002). The relatively small increases in cAMP (without phosphodiesterase inhibition) seen following β1-adrenoceptor overexpression may be explained by the Glycine 389 variant overexpressed in this study. The influence of the Arginine 389 variant on the response to CGP 12177A in this system will be important to establish.
Conclusions
We have demonstrated that the nonconventional partial agonist CGP 12177A has cardiostimulant effects in single adult rat ventricular myocytes, mediated by a cAMP/PKA signalling mechanism, which are antagonised by bupranolol. Following overexpression of β1-adrenoceptors, there is increased potency of isoprenaline and CGP 12177A (which is propranolol resistant). Low-affinity [3H]-CGP 12177A binding also increases following overexpression of β1-adrenoceptors. Taken together, this suggests that the cardiostimulant effects of CGP 12177A are mediated by a novel low-affinity site or state of the β1-adrenoceptor, previously attributed to a separate β4-adrenoceptor. The use of beta-blockers in the treatment of ischaemic heart disease and heart failure is widespread. It will be important to determine whether beta-blockers with partial agonist activity at the low-affinity state of the β1-adrenoceptor are implicated in arrhythmogenesis or a lack of benefit in treatment of heart failure.
Acknowledgments
We are grateful to Mr Peter O'Gara for expert technical assistance. CL was supported by a Clinical PhD Studentship from the British Heart Foundation and a Sackler Fellowship. Abstracts of this work have previously been presented to the British Pharmacological Society (Winter meeting 2001) and the American College of Cardiology (March 2002).
Abbreviations
- Adv.β1
adenovirus containing sequence for human β1 adrenoceptor
- Adv.GFP
adenovirus containing sequence for green fluorescent protein
- cAMP
adenosine 3′,5′-cyclic monophosphate
- CGP 12177A
(±)-4-(3-t-butylamino-2-hydroxypropoxy)benzimidazol-2-one
- GFP
jelly fish green fluorescent protein
- Gs
stimulatory guanine nucleotide binding protein
- [3H]-CGP 12177A
(−)-[3H]-CGP 12177 (4-(3-t-butylamino-2-hydroxypropoxy)[5,7-3H]benzimidazole-2-one)
- HEPES
N-2-hydroxyethylpiperazine-N'-2-ethanesulphonic acid
- IBMX
3-isobutyl-1-methylxanthine
- KH
Krebs–Henseleit
- MOI
multiplicity of infection
- NTA
nitrilotriacetic acid
- PKA
protein kinase A
- R50
time taken from peak contraction to 50% relaxation
- R90
Time taken from peak contraction to 90% relaxation
- Rp-cAMPS
adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer
- SR 59230
3-(2-Ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate
- Tris-HCl
Tris-hydrochloride
- TTP
time taken from contraction onset to peak
References
- BAKER J., HILL S. Pharmacological characterization of CGP 12177 at the human beta(2)-adrenoceptor. Br. J. Pharmacol. 2002;137:400–408. doi: 10.1038/sj.bjp.0704855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIN N., CAMOIN L., MAIGRET B., STROSBERG A. Structural and conformational features determining selective signal transduction in the β3-adrenergic receptor. Mol. Pharmacol. 1993;44:1094–1104. [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIA K., HAJJAR R.J., TERRACCIANO C., KENT N., RANU H., O'GARA P., ROSENZWEIG A., HARDING S. Functional alterations in adulate rat myocytes after overexpression of phospholamban using adenovirus. 1999;1:41–50. doi: 10.1152/physiolgenomics.1999.1.2.41. [DOI] [PubMed] [Google Scholar]
- FORD A., DANIELS D., CHANG D., GEVER D., JASPER J., LESNICK D., CLARKE D. Pharmacological ploeiotropism of the human recombinant α1A-adrenoceptor: implication for α1A-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESTONE N., HEUBACH J., WETTWER E., RAVENS U., BROWN D., KAUMANN A. β4-Adrenoceptors are more effective than β1-adrenoceptors in mediating arrhythmic Ca2+ transients in mouse ventricular myocytes. Naunyn–Schmiedeberg's Arch. Pharmacol. 1999;360:445–456. doi: 10.1007/s002109900075. [DOI] [PubMed] [Google Scholar]
- GONG H., ADAMSON D., RANU H., KOCH W., HEUBACH J., RAVENS U., ZOLK I., HARDING S. The effect of Gi-protein inactivation on basal, β1- and β2-adrenoceptor stimulated contraction of myocyte form transgenic mice overexpressing the β2-adrenoceptor. Br. J. Pharmacol. 2000;131:594–600. doi: 10.1038/sj.bjp.0703591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANNEMAN J. The putative β4-adrenergic receptor is a novel state of the β1-adrenergic receptor. Am. J. Physiol. Endocrinol. Metab. 2001;43:E199–E202. doi: 10.1152/ajpendo.2001.280.2.E199. [DOI] [PubMed] [Google Scholar]
- HARDING S., VESCOVO G., KIRBY M., JONES S., GURDEN J., POOLE-WILSON P. Contractile responses of isolated rat and rabbit myocytes to isoproterenol and calcium. J. Mol. Cell. Cardiol. 1988;20:635–637. doi: 10.1016/s0022-2828(88)80121-4. [DOI] [PubMed] [Google Scholar]
- JOSEPH S., LYNHAM J., GRACE A., COLLEDGE W., KAUMANN A. The effects of (−)-isoprenaline but not of (−)- CGP12177 are markedly reduced at Gly-389 β1-adrenoceptors compared to Arg-389 β1-adrenoceptors. Br. J. Pharmacol. 2002;135:336P. doi: 10.1038/sj.bjp.0705753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A. Is there a third heart β-adrenoceptor. Trends Pharm. Sci. 1989;10:316–319. doi: 10.1016/0165-6147(89)90065-5. [DOI] [PubMed] [Google Scholar]
- KAUMANN A. CGP 12177-induced increase of human atrial contraction through a putative third β–adrenoceptor. Br. J. Pharmacol. 1996;117:93–98. doi: 10.1111/j.1476-5381.1996.tb15159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A. Four β-adrenoceptor subtypes in mammalian heart. Trends Pharm. Sci. 1997;18:70–76. doi: 10.1016/s0165-6147(96)01033-4. [DOI] [PubMed] [Google Scholar]
- KAUMANN A., ENGELHARDT S., MOLENAAR P., HEIN L., MOLENAAR P., LOHSE M. (−)-CGP 12177-evoked cardiostimulation in double β1-/β2-adrenoceptor knockout mice. Obligatory role of β–adrenoceptors for putative β4–adrenoceptor pharmacology. Naunyn–Schmiedeberg's Arch. Pharmacol. 2001;363:87–93. doi: 10.1007/s002100000336. [DOI] [PubMed] [Google Scholar]
- KAUMANN A., FREESTONE N.Atypical β-adrenoceptor activation by (−)-CGP 12177 increases cytosolic calcium in rat ventricular myocytes Pharmacologist 19973974(Abstr. 293) [Google Scholar]
- KAUMANN A., LYNHAM J. Stimulation of cyclic AMP-dependent protein kinase in rat atria by (−)-CGP 12177 through an atypical β-adrenoceptor. Br. J. Pharmacol. 1997;120:1187–1189. doi: 10.1038/sj.bjp.0701053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A., MOLENAAR P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn–Schmiedebergs' Arch. Pharmacol. 1997;355:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- KAUMANN A., PREITNER F., SARSERO D., MOLENAAR P., REVELLI J., GIACOBINO J. (−)-CGP 12177 causes cardiostimulation and binds to cardiac putative β4-adrenoceptors in both wild-type and β3-adrenoceptor knockout mice. Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- KONKAR A., ZHAI Y., GRANNEMAN J. β1-adrenergic receptors mediate β3-adrenergic-independent effects of CGP 12177 in brown adipose tissue. Mol. Pharmacol. 2000a;57:252–258. [PubMed] [Google Scholar]
- KONKAR A., ZHU Z., GRANNEMAN J. Aryloxypropanolamine and catecholamine ligand interactions with the β1-adrenergic receptor: evidence for interaction with distinct conformations of β1-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000b;294:923–932. [PubMed] [Google Scholar]
- LOWE M., GRACE A., VANDENBERG J., KAUMANN A. Action potential shortening through the putative β4-adrenoceptor in ferret ventricle: comparison with β1- and β2-adrenoceptor-mediated effects. Br. J. Pharmacol. 1998;124:1341–1344. doi: 10.1038/sj.bjp.0702013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE M., LYNHAM J., GRACE A., KAUMANN A. Comparison of the affinity of beta-blockers for two states of the beta 1-adrenoceptor in ferret ventricular myocardium. Br. J. Pharmacol. 2002;135:451–461. doi: 10.1038/sj.bjp.0704450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., KIEC-KONONOWICZ K., FLAU K., GODLEWSKI G., KOZ H., KATHMANN M., SCHLICKER E. Atypical cardiostimulant β-adrenoceptor in the rat heart: stereoselective antagonism by bupranolol but lack of effect by some bupranolol analogues. Br. J. Pharmacol. 2003;139:1548–1554. doi: 10.1038/sj.bjp.0705390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINOWSKA B., SCHLICKER E. Mediation of the positive chronotropic effect of CGP 12177 and cyanopindolol in the pithed rat by atypical beta-adrenoceptors, different from beta 3-adrenoceptors. Br. J. Pharmacol. 1996;117:943–949. doi: 10.1111/j.1476-5381.1996.tb15285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D., MOORE J., GREEN S., LIGGETT S. A gain-of-function polymorphism in a G-protein coupling domain of the human beta 1-adrenergic receptor. J. Biol. Chem. 1999;274:12670–12674. doi: 10.1074/jbc.274.18.12670. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L., FRASER N., MAIN M., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M., FOORD S. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- OOSTENDORP J., KAUMANN A. Pertussis toxin suppresses carbachol-evoked cardiodepression but does not modify cardiostimulation mediated through beta1- and putative beta4-adrenoceptors in mouse left atria: no evidence for beta2- and beta3-adrenoreceptor function. Naunyn–Schmiedeberg's Arch. Pharmacol. 2000;361:134–145. doi: 10.1007/s002109900156. [DOI] [PubMed] [Google Scholar]
- PAK M., FISHMAN P. Anomalous behaviour of CGP 12177A on beta 1-adrenergic receptors. J. Recept. Signal Transduct. Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- RANU H., MAK J., BARNES P., Harding S. G(i)-dependent suppression of beta(1)-adrenoceptor effects in ventricular myocytes from NE-treated guinea pigs. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H1807–H1814. doi: 10.1152/ajpheart.2000.278.6.H1807. [DOI] [PubMed] [Google Scholar]
- SAMAMA P., PEI G., COSTA T., COTECCHIA S., LEFKOWITZ R. Negative antagonists promote an inactive conformation of the β2-adrenergic receptor. Mol. Pharmacol. 1994;45:390–394. [PubMed] [Google Scholar]
- SARSERO D., MOLENAAR P., KAUMANN A. [3H]CGP 12177 radiolabels β1, β2- and putative β4-adrenoceptors in human atrium and ventricle. Naunyn–Schmiedeberg's Arch. Pharmacol. 1998a;358:R629. [Google Scholar]
- SARSERO D., MOLENAAR P., KAUMANN A. Validity of (−)-[3H]CGP 12177 as a radioligand for the ‘putative β4-adrenoceptor' in rat atrium. Br. J. Pharmacol. 1998b;123:371–380. doi: 10.1038/sj.bjp.0701609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., MOLENAAR P., KAUMANN A., FREESTONE N. Putative β4-adrenoceptors in rat ventricle mediate increases in contractile force and cell Ca2+: comparison with atrial receptors and relationship to (−)-[3H]-CGP 12177 binding. Br. J. Pharmacol. 1999;128:1445–1460. doi: 10.1038/sj.bjp.0702936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., RUSSELL F., LYNHAM J., RABNOTT G., YANG I., Fong K., LI L., KAUMANN A., MOLENAAR P. CGP 12177 increases contractile force and hastens relaxation of human myocardial preparations through a propranolol-resistant state of the beta(1)-adrenoceptor. Naunyn–Schmiedebergs Arch. Pharmacol. 2003;367:10–21. doi: 10.1007/s00210-002-0652-9. [DOI] [PubMed] [Google Scholar]
- SEIFERT R., GETHER U., WENZEL-SEIFERT K., KOBILKA B. Effects of guanine, inosine and xanthine nucleotides on β2-adrenergic receptors/Gs interactions: evidence for multiple receptor conformations. Mol. Pharmacol. 1999;56:348–358. doi: 10.1124/mol.56.2.348. [DOI] [PubMed] [Google Scholar]
- VESCOVO G., JONES S., HARDING S., POOLE-WILSON P. Isoproterenol sensitivity of isolated cardiac myocytes from rats with monocrotaline-induced right-sided hypertrophy and heart failure. J. Mol. Cell Cardiol. 1989;21:1047–1061. doi: 10.1016/0022-2828(89)90803-1. [DOI] [PubMed] [Google Scholar]