Abstract
The mechanisms involved in the vasodilator actions of angiotensin II (Ang II) have not yet been completely elucidated. We investigated the potential mechanisms that seem to be involved in the Ang II vasodilator effect using rat isolated mesenteric vascular bed (MVB).
Under basal conditions, Ang II does not affect the perfusion pressure of MVB. However, in vessels precontracted with norepinephrine, Ang II induces vasodilation followed by vasoconstriction. Vasoconstrictor, but not the vasodilation of Ang II, is inhibited by AT1 antagonist (losartan). The vasodilator effect of Ang II was not inhibited by AT2, angiotensin IV and angiotensin 1–7 receptor antagonists alone (PD 123319, divalinal, A 779, respectively).
The vasodilator effect of Ang II is significantly reduced by endothelial removal (deoxycholic acid), but not by indomethacin. Inhibition of NO-synthase by NG-nitro-L-arginine methyl ester (L-NAME) and guanylyl cyclase by 1H-[1,2,3] oxadiazolo [4,4-a] quinoxalin-1-one (ODQ) reduces the vasodilator effect of Ang II. This effect is also reduced by tetraethylammonium (TEA) or L-NAME, and a combination of L-NAME plus TEA increases the inhibitory effect of the antagonists alone. However, indomethacin does not change the residual vasodilator effect observed in vessels pretreated with L-NAME plus TEA.
In vessels precontracted with norepinephrine and depolarized with KCl 25 mM or treated with Ca2+-dependent K+ channel blockers (charybdotoxin plus apamin), the effect of Ang II was significantly reduced. However, this effect is not affected by ATP and voltage-dependent K+ channel blockers (glybenclamide and 4-aminopyridine).
Inhibition of kininase II with captopril significantly potentiates the vasodilator effect of bradykinin (BK) and Ang II in the rat MVB. The inhibitory effect of the B2 receptor antagonist HOE 140 on the vasodilator effect of Ang II is further enhanced by PD 123319 and/or A 779.
The present findings suggest that BK plays an important role in the endothelium-dependent vasodilator effect of Ang II. Probably, the link between Ang II and BK release is modulated by receptors that bind PD 123319 and A 779.
Keywords: Angiotensin II, endothelium, vasodilation, bradykinin, angiotensin 1–7, HOE 140, captopril, EDHF, L-NAME
Introduction
The renin–angiotensin system modulates cardiovascular function, and angiotensin II (Ang II), the most important autacoid of this control system, induces vasoconstriction, facilitates sympathetic transmission and promotes renal salt and water retention (Chung & Unger, 1999). The effects of Ang II on the cardiovascular system are clearly mediated by the activation of the AT1 receptor. In contrast, the exact significance of AT2 receptors on the action of Ang II is not yet clear. The vasodilator effect of Ang II has been shown to occur not only ‘in vitro' (Arima et al., 1997; Endo et al., 1998; Kimura et al., 2001; Soares de Moura et al., 2001; Katada & Majima, 2002), but also ‘in vivo' (Scheuer & Perrone, 1993; Tsutsumi et al., 1999). We have recently demonstrated (Soares de Moura et al., 2001) that Ang II has no vasoconstrictor effect in the rat mesenteric vascular bed (MVB), but, when these vessels are precontracted with norepinephrine (NE), Ang II induces an endothelium-dependent vasodilation.
In the present study, we investigated the mechanism of the vasodilator effect of Ang II on the MVB of the rat. Ang II induces an endothelium-derived relaxing factor (EDRF)-dependent effect that seems to be modulated by an interaction among Ang II, bradykinin (BK) and angiotensin 1–7 (Ang 1–7). Preliminary results have been presented to the British Pharmacological Society (Soares de Moura et al., 2001).
Methods
All experiments were reviewed and approved by the Ethics Committee of Animal Experiments of the State University of Rio de Janeiro. The rat superior MVB was isolated according to McGregor (1965). Briefly, male Wistar rats were killed with inhaled CO2 and the MVB was cannulated and perfused at a flow rate of 4 ml min−1 with a physiological salt solution (PSS) by a peristaltic pump (Lifecare Model 4, Abbott'Shaw). The PSS had the following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, EDTA 0.02 and glucose 11. All experimental groups were performed in the presence of losartan (30 μmol), with the exception of the losartan group, where the effect of Ang II was obtained before and after losartan. The PSS (37°C) was bubbled with 95% O2/5% CO2. Perfusion pressure (PP) was measured with a transducer connected to a preamplifier and chart recorded. Drugs were either dissolved in PSS and perfused at the desired concentration, or were administered as bolus injections directly into the perfusion stream (volume <300 μl).
The preparations were left to equilibrate for 30 min, and then injections of 120 μmol KCl were administered every 10 min until consistent responses were obtained. The basal pressure after the equilibration period was 23.2±1 mmHg (n=198). Ang II (3–300 nmol) was injected before and after the PP had been elevated (80–110 mm Hg) with NE (6–30 μM) added to the perfusion fluid. The vasodilator effect of all the drugs was expressed as a percent decrease in relation to the pressor effect of NE. When the pressor effect of NE reached a plateau, dose–response curves to bolus injections of Ang II (3–300 nmol), Ang 1–7 (10–300 nmol), angiotensin IV (Ang IV; 10–300 nmol) and CGP 42112 A (10–300 nmol), a putative AT2 agonist, were obtained. Acetylcholine (ACh; 10 pmol), BK (10 pmol) and nitroglycerin (NG; 1 nmol) were injected as single doses.
The vasodilator effects of Ang II were studied before and after perfusion with losartan (30 μM), an inhibitor of the AT1 receptor, PD 123319 (10 μM) a putative inhibitor of the AT2 receptor, A 779 (1 μM), a putative inhibitor of the Ang 1–7 receptor, glybenclamide (1 μM), an inhibitor of the ATP-dependent K+ (KATP) channel, aminopyridine (4-AP; 1 mM), an inhibitor of the voltage-dependent K+ (KV) channel, indomethacin (3 and 10 μM), an inhibitor of cyclooxygenase (COX), divalinal-Ang IV, an inhibitor of the Ang IV receptor PD 123319 (10 μM) plus A 779 (1 μM); HOE 140 (0.01 μM), an antagonist of the BK B2 receptor, HOE 140 (0.01 μM) plus PD 123319 (10 μM), HOE 140 (0.01 μM) plus A 779 (1 μM) and HOE 140 (0.01 μM) plus PD 123319 (10 μM) plus A 779 (1 μM).
The vasodilator effects of Ang II, ACh and NG were studied before and after perfusion with deoxycholic acid (2.5 mM) dissolved in PSS for 3 min to chemically remove the endothelium, NG-nitro-L-arginine methyl ester (L-NAME; 0.3 mM), an inhibitor of nitric oxide (NO) synthase or 1H-[1,2,3] oxadiazolo [4,4-a] quinoxalin-1-one (ODQ; 10 μM), an inhibitor of guanylyl cyclase (GC), tetraethylammonium (TEA), a nonspecific inhibitor of K+ channels, TEA plus L-NAME, TEA plus L-NAME plus indomethacin, charybdotoxin (ChTx) plus apamin inhibitors of Ca2+-dependent K+ (KCa2+) channels or in vessels perfused with high K+ solution (25 mM). In vessels pretreated with deoxycholic acid, L-NAME and indomethacin, the concentration of NE was adjusted in order to maintain the same increase in PP. The vasodilator effects of Ang II, ACh, NG and BK were studied before and after perfusion with HOE 140 (0.2 μM). Dose–response curves were constructed to CGP 42112 A and Ang IV in vessels perfused with PSS, and the vasodilator effects of Ang IV (300 nmol) and Ang II (30 nmol) were studied before and shortly after (1–5 min) injections of Divanilal-Ang IV (300 nmol). The vasodilator effects of Ang II, ACh, NG and BK were studied before and after perfusion with captopril (10 μM), an inhibitor of kininase II. The effects of Ang 1–7 were studied before and after perfusion with A 779 (1 μM).
Statistical analysis
Relaxation is expressed as the percentage decrease in the increase in PP induced by NE. All results are presented as mean±s.e.m. for the number of rats. One-way analysis of variance (ANOVA) plus Bonferroni and Student's unpaired t-test were used for statistical analysis. Values of P<0.05 were considered statistically significant.
Drugs
The following compounds were used: Ang II, Ang 1–7, ACh, BK, deoxycholic acid, L-NAME, indomethacin, ODQ, 4-aminopyridine, ChTx, apamin, glybenclamide, HOE 140 and captopril were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). CGP 42112 A was purchased from Neosystem, U.S.A. PD 123319 was a gift from Parke-Davis, Warner-Lambert (Ann Arbor, MI, U.S.A.); divalinal-Ang IV was obtained from Pacific Northwest Biotechnology (Pullman, WA, U.S.A.); A 779 and Ang IV were obtained from Bachem, St Helens, U.K.; losartan was a gift from Laboratórios Biosintetica Ltda, Brazil; NG was obtained from Innovatec-Divisão Cristalia, Brazil.
Results
Actions of losartan, PD 123319, A 779, glybenclamide, 4-AP and indomethacin on the vasodilator effect of Ang II in the MVB of the rat
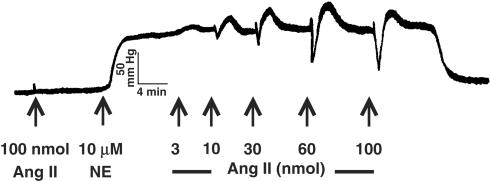
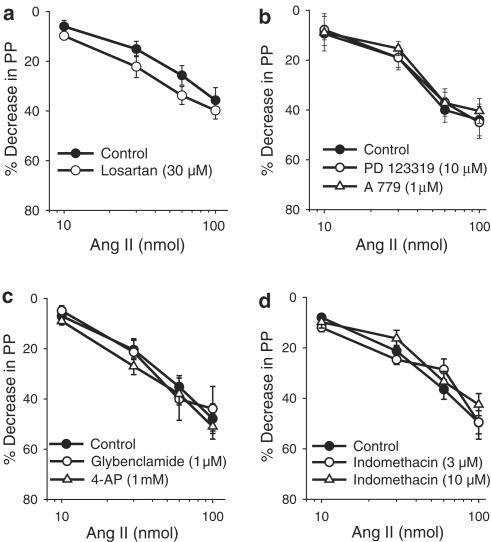
Injections of Ang II (10–100 nmol) did not induce any change in MVB PP. However, when the PP was raised with NE, Ang II injections (10–100 nmol) induced a dual effect, characterized by vasodilation followed by vasoconstriction (Figure 1). The vasoconstrictor (data not shown), but not the vasodilator (Figure 2), effects of Ang II are completely inhibited by 30 μM losartan (n=6). In vessels pretreated with losartan (30 μM) and constricted with NE, the vasodilator effect of Ang II was not inhibited by PD 123319 (10 μM; n=11), A 779 (1 μM; n=7), glybenclamide (1 μM; n=7), 4-AP (1 mM; n=6) or indomethacin (3 μM; n=6 and 10 μM; n=6; Figure 2).
Figure 1.
Representative PP (mmHg) trace showing the effect of Ang II in MVB before and during NE (10 μM) treatment. NE indicates the beginning of NE infusion. Arrows indicate injections of 3, 10, 30, 60 and 100 nmol of Ang II.
Figure 2.
Effects of losartan (a), PD 123319 and A 779 (b), glybenclamide and 4-aminopyrydine (4-AP; c) and indomethacin (d) on Ang II-induced vasodilator effect in the MVB precontracted with NE. In panels (b–d), the vessels were treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: log dose (nmol) of Ang II. Each point is presented as mean±s.e.m. n=7–11 rats/group.
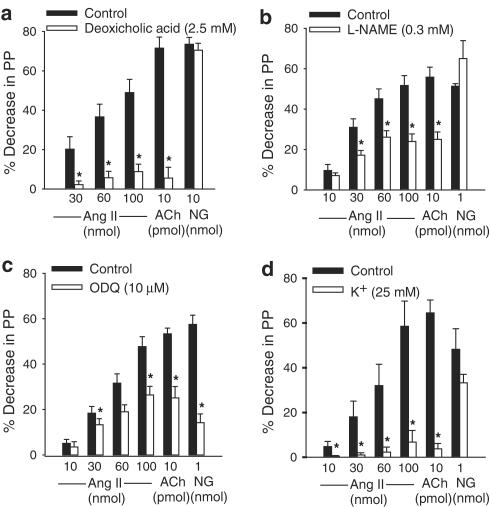
Actions of deoxycholic acid, L-NAME, ODQ and high K+ solution on the vasodilator effect of Ang II in the MVB of the rat
Treatment with deoxycholic acid induced a slight, but not maintained, increase in PP, while L-NAME, ODQ and high K+ solution (25 mM) did not change the basal PP. In vessels pretreated with losartan (30 μM) and constricted with NE, chemical removal of the endothelial cells with deoxycholic acid (2.5 mM, during 3 min; n=9), L-NAME (0.3 mM; n=8), ODQ (10 μM; n=7) or high K+ (25 mM; n=5) significantly reduced the vasodilator effects of Ang II and ACh. However, the vasodilator effect of NG was only inhibited by ODQ (Figure 3).
Figure 3.
Effects of endothelial removal induced by deoxycholic acid (a), L-NAME (b), ODQ (c) and high K+ solution (d) on the vasodilator effects induced by Ang II, ACh or NG in vessels treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: doses of Ang II, ACh or NG. Each bar is presented as mean±s.e.m. n=5–9 rats/group. *Significantly different from the corresponding control group (P<0.05).
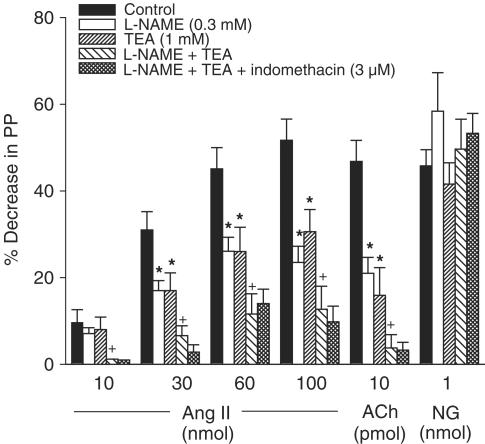
Actions of L-NAME, TEA and indomethacin on the vasodilator effect of Ang II in the MVB of the rat
In vessels pretreated with losartan (30 μM) and constricted with NE, the vasodilator effects of Ang II and ACh, but not NG, were significantly reduced by L-NAME (0.3 mM) or TEA (1 mM). The inhibitory effect of L-NAME or TEA was further enhanced when the two inhibitors were used together; however, indomethacin (3 μM) did not induce any further inhibition on the residual vasodilator effect of Ang II observed in vessels treated with L-NAME plus TEA (Figure 4).
Figure 4.
Vasodilator effects of Ang II, ACh or NG obtained before (control responses) and after treatment with L-NAME, TEA, L-NAME plus TEA and L-NAME plus TEA plus indomethacin in vessels treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: doses of Ang II, ACh or NG. Each bar is presented as mean±s.e.m. n=8 rats/group. *Significantly different from the corresponding control group (P<0.05). +Significantly different from the corresponding L-NAME or TEA-treated group (P<0.05).
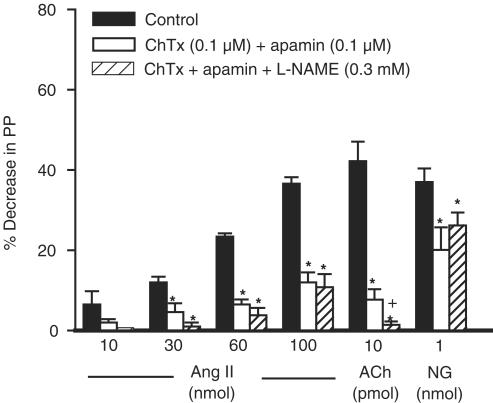
Actions of ChTx plus apamin and L-NAME on the vasodilator effect of Ang II in the MVB of the rat
In vessels pretreated with losartan (30 μM) and constricted with NE, the vasodilator effects of Ang II, ACh and NG were significantly reduced by ChTx (0.1 μM) plus apamin (0.1 μM; n=5). However, addition of L-NAME (0.3 mM) did not further increase the inhibitory effect of ChTx plus apamin (n=5) on the vasodilator effect of Ang II (Figure 5).
Figure 5.
Vasodilator effects of Ang II, ACh or NG obtained before (control responses) and after treatment with ChTx plus apamin and ChTx plus apamin plus L-NAME in vessels treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: doses of Ang II, ACh or NG. Each bar is presented as mean±s.e.m. n=5 rats/group. *Significantly different from the corresponding control group (P<0.05). +Significantly different from the corresponding ChTx plus apamin-treated group (P<0.05).
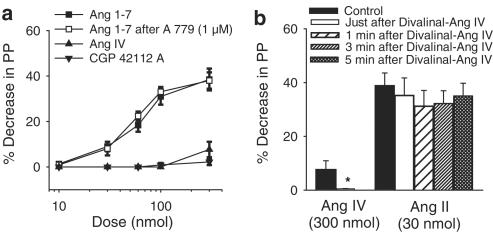
Action of divanilal-Ang IV and A 779 on the effects of Ang IV, Ang II and Ang 1–7, and effect of CGP 42112 A in the MVB of the rat
In vessels pretreated with losartan (30 μM) and constricted with NE, Ang IV (10–300 nmol; n=8) induced a very small vasodilator effect and CGP 42112 A (n=7) was without effect in the rat MVB. Ang 1–7 induced a dose-dependent vasodilator effect in the rat MVB that was not changed by A 779 (1 μM; n=7) (Figure 6a). Divalinal-Ang IV (300 nmol; n=5), injected shortly before Ang IV administration, abolished the small vasodilator effect of Ang IV (300 nmol), but did not change the vasodilator effect of Ang II (30 nmol, n=5) (Figure 6b).
Figure 6.
Vasodilator effects of Ang 1–7 in vessels treated with losartan (30 μM) and precontracted with NE before and after treatment with A 779. Effects of Ang IV and CGP 42112 A in vessels treated with losartan (30 μM) and precontracted with NE. Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: log dose of Ang 1–7; Ang IV and CGP 42112 A. Each point is presented as mean±s.e.m. n=7–8 rats/group (panel (a)). Vasodilator effects of Ang II and Ang IV obtained before (control responses), just after, and 1, 3 and 5 min after injections of Divalinal-Ang IV in vessels treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: doses of Ang IV and Ang II. Each bar is presented as mean±s.e.m. n=5–8 rats (panel (b)). *Significantly different from the corresponding control response (P<0.05).
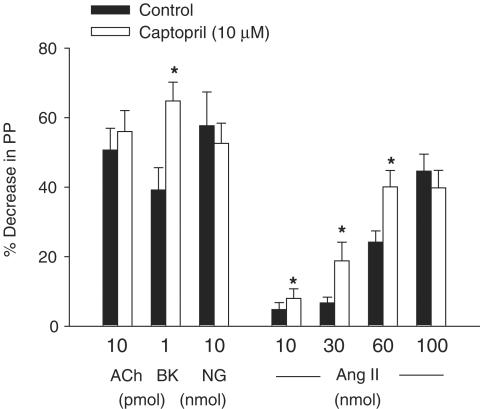
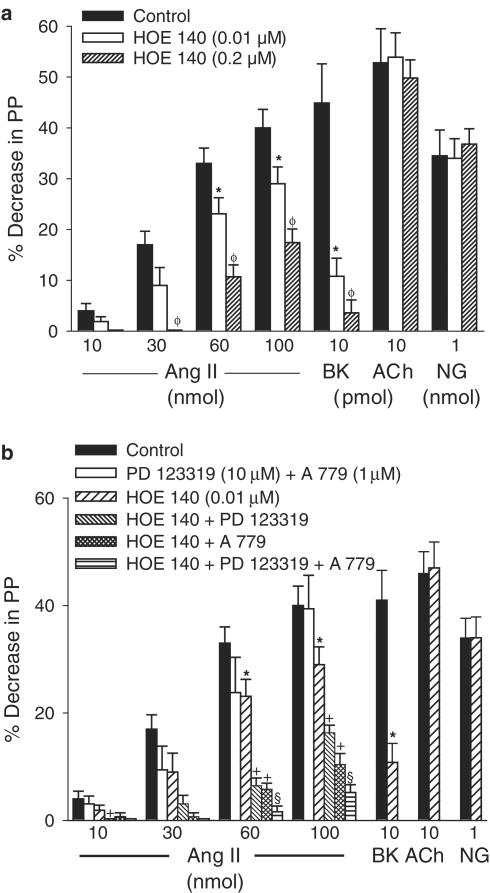
Action of captopril, HOE 140, A 779 and PD 123319 on the vasodilator effects of Ang II
In vessels pretreated with losartan and constricted with NE, the vasodilator effects of Ang II and BK, but not the effects of ACh or NG, were significantly enhanced by captopril (10 μM, n=6; Figure 7). The effect of BK, but not ACh or NG, was significantly reduced and almost abolished by HOE 140 (0.01 and 0.2 μM; n=8 and n=5, respectively). However, the vasodilator effect of Ang II was significantly inhibited by the larger concentration (0.2 μM) of HOE 140, but only slightly (significant only at doses of 60 and 100 nmol) reduced by HOE 140 (0.01 μM) (Figure 8a). The vasodilator effect of Ang II was not changed by PD 123319 (10 μM) plus A 779 (1 μM; n=7). In addition, the slight inhibitory effect of HOE 140 (0.01 μM) on the vasodilator effect of Ang II was enhanced by a combination of HOE 140 plus PD 123319 (n=11) or HOE 140 plus A 779 (n=7), and further enhanced by HOE 140 plus PD 123319 plus A 779 (n=7) (Figure 8b).
Figure 7.
Vasodilator effects of Ang II, ACh, NG or BK obtained before (control responses) and after treatment with captopril in vessels treated with losartan (30 μM). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction induced by NE. Abscissa: doses of Ang II, ACh, NG or BK. Each bar is presented as mean±s.e.m. n=6 rats/group. *Significantly different from the corresponding control group (P<0.05).
Figure 8.
Vasodilator effects of Ang II, BK, ACh, NG in vessels precontracted with NE and treated with losartan (30 μM), before (control responses) and after treatment with HOE 140 (panel (a)). Vasodilator effects of Ang II, BK, ACh, NG in vessels precontracted with NE before (control responses) and after treatment with PD 123319+A 779, HOE 140, HOE 140+PD 123319, HOE 140+A 779 and HOE 140+PD 123319+A 779 (panel (b)). Ordinate: vasodilation (%), expressed as a percentage decrease of the vasoconstriction. Abscissa: doses of Ang II, BK, ACh and NG. Each bar is presented as mean±s.e.m. n=7–11 rats. *Significantly different from the corresponding control response (P<0.05). +Significantly different from the corresponding HOE 140-treated group (P<0.05). §Significantly different from the corresponding HOE 140 plus PD 123319 and HOE 140 plus A 779-treated group (P<0.05).
Discussion
The present results confirm the previous findings of our group, showing that, Ang II in the MVB of the rat, unlike in most vascular beds, does not produce vasoconstriction (Soares de Moura et al., 2001). However, when the tonus of the mesenteric vessels is increased with infusions of NE, Ang II produces an initial vasodilation, then followed by vasoconstriction. The vasoconstrictor, but not the vasodilator effect of Ang II, depends on AT1 receptor activation, because this effect is completely abolished by losartan or irbesartan (Soares de Moura, personal observation). The vasodilator effect of Ang II depends on the endothelium since Ang II- and ACh-induced vasodilation are significantly inhibited in vessels pretreated with deoxycolic acid, while the effect of NG, an endothelium-independent vasodilator, is not affected by endothelium removal. The endothelial-dependent vasodilator effect of Ang II has also been demonstrated in the isolated microperfused rabbit afferent arteriole (Arima et al., 1997) and in perfused rat mesenteric arteries (Katada & Majima, 2002). However, the results obtained by Katada & Majima (2002) differ from our present results, given that the vasodilator effect of Ang II was only observed after the blockade of AT1 receptors by losartan.
The vasodilator effect of Ang II in the rat MVB might be due to release of vasodilator autacoids by the endothelial cells, including prostacyclin, NO and hyperpolarizing factor(s). In this study, the involvement of prostaglandins on the vasodilator effect of Ang II seems improbable, since indomethacin, even at a high concentration (10 μM), does not affect the vasodilator effect of Ang II. Our results are in accordance with previous findings on the microperfused rabbit afferent arteriole (Arima et al., 1997). The lack of involvement of prostaglandins on the vasodilator effect of Ang II in the MVB of the rat is further supported by the present results, which demonstrate that the residual vasodilator effect induced by Ang II in vessels pretreated with TEA plus L-NAME is not reduced by indomethacin. In contrast, our present results differ from those obtained by Katada & Majima (2002), showing that indomethacin (10 μM) modestly inhibits Ang II vasodilation in perfused rat mesenteric arteries. A possible reduction in the responsiveness of vascular preparation in Katada & Majima's (2002) experiments might have been caused by the continuous infusion of Ang II, rather than by the inhibition of vasodilation mediated by indomethacin, since the Ang II vasodilator response decreased as the number of applications increased.
In this study, NO seems to play an important role in the vasodilator effect of Ang II, since L-NAME substantially reduces the vasodilator effect of Ang II in the rat MVB. The involvement of the NO-guanosine 3′5′ cyclic monophosphate (cGMP) pathway in the vasodilator effect of Ang II is further corroborated by our results with ODQ treatment, showing a significant reduction in the vasodilator effect of Ang II. It is conceivable that NO is not the only autacoid released from endothelium in response to Ang II, given that the vasodilator effect of Ang II is not completely inhibited by L-NAME. Since the vasodilator effect of Ang II and ACh, but not NG, is inhibited by ChTx plus apamin, it is possible that KCa2+ channel activation might be involved in the vasodilator effect of Ang II. Probably, KATP and Kv channels are not involved in the Ang II-dependent vasodilation, because this effect is not inhibited by glybenclamide or 4-AP. The inhibitory effect of L-NAME or TEA on the vasodilator effect of Ang II was further enhanced when those two inhibitors were used together, but the inhibitory effect of ChTx plus apamin was not further enhanced by L-NAME, suggesting that Ang II-induced hyperpolarization is a crucial mechanism involved in the vasodilator effect of Ang II in the rat MVB. Ang II could induce hyperpolarization not only by releasing of endothelium-derived hyperpolarizing factor (EDHF), which activates K+ channels inhibited by ChTx plus apamin or TEA, but also by a mechanism modulated by cGMP that induces hyperpolarization by activating a KCa2+ channel inhibited by ChTx (Robertson et al., 1993; Vanhoutte, 1999). This hypothesis is supported by our findings, which show that, in vessels depolarized with high K+ solution and constricted with NE, the vasodilator effect of Ang II is almost completely abolished.
It has been shown that the vasodilator effect of Ang II on the microperfused rabbit afferent arteriole (Arima et al., 1997) and rat mesenteric arterial segments (Katada & Majima, 2002) is inhibited by PD 123319. However, in the current study, the vasodilator effect of Ang II was not inhibited by PD 123319 alone. Furthermore, we failed to demonstrate a vasodilator effect of CGP 42112 A. However, when the B2 receptors are not completely inhibited by HOE 140 (0.01 μM), a combination of HOE 140 plus PD 123319 further enhances the inhibitory effect of HOE 140, suggesting that AT2 receptors could participate in the BK-dependent vasodilation induced by Ang II.
The vasodilator effect of Ang II could be modulated by Ang IV, a vasodilator component of the renin–angiotensin system (Haberl et al., 1991; Coleman et al., 1998; Patel et al., 1998). Alternatively, Ang II could be metabolized to Ang IV by aminopeptidase A and aminopeptidase M. However, our results demonstrate that Ang IV most likely does not participate in the vasodilator effects of Ang II, since its vasodilator effect is much smaller than the vasodilator effect of Ang II. Moreover, divalinal-Ang IV, an antagonist of the Ang IV receptor (Krebs et al., 1996) which reduces the vasodilation effect of Ang IV in the MVB, does not inhibit the vasodilator effect of Ang II.
BK seems to play an important role in the vascular effects of Ang II (Seyedi et al., 1995; Gohlke et al., 1998; Tsutsumi et al., 1999; Sosa-Canache et al., 2000; Katada & Majima, 2002). Probably, the vasodilator effect of Ang II in the rat MVB is modulated by BK mechanisms, since we showed that captopril enhanced the vasodilator effects of BK and Ang II. In the present results, we also showed that HOE 140, at a concentration (0.01 μM) that significantly reduced the vasodilator effect of BK, induced a small but significant reduction in the vasodilator effect induced by high doses of Ang II. However, HOE 140, at a concentration that almost abolished the vasodilator effect of BK, significantly reduced the vasodilator effect of Ang II in all doses studied, confirming that BK must play an important role in the vasodilator effect of Ang II in the rat MVB.
Ang 1–7, a potent vasodilator compound (Meng & Busija, 1993; Osei et al., 1993; Pörsti et al., 1994; Brosnihan et al., 1996; Ren et al., 2002), could be involved in the mechanism of the action of Ang II in the rat MVB. Ang II could be transformed into Ang 1–7 by carboxypeptidase P, or might also stimulate the Ang 1–7 receptor (Ferrario et al., 1990). Our study demonstrates that Ang 1–7 induces a significant vasodilator effect on the MVB in the same dose range of Ang II. Unlike other vascular beds, where A 779, a putative Ang 1–7 antagonist (Santos et al., 1994), inhibits the vasodilator effect of Ang 1–7 (Bayorh et al., 2002; Ren et al., 2002), our results demonstrate that the vasodilator effect of Ang 1–7 on the MVB could involve other receptors, given that A 779 does not inhibit Ang 1–7 vasodilation. BK appears to modulate Ang 1–7-induced vasodilation, since several reports have shown that the vasodilator effect of BK is enhanced by Ang 1–7 and vice versa (Brosnihan et al., 1996; Gorelik et al., 1998; Almeida et al., 2000; Fernandes et al., 2001; Tom et al., 2001). Also, the involvement of BK in the vasodilator effect of Ang 1–7 is suggested by Brosnihan et al. (1996), who demonstrated that the vasodilator effect of Ang 1–7 was inhibited by HOE 140. BK and Ang 1–7 may share a common mechanism involving NO, since the Ang 1–7 potentiation of BK-induced vasodilation in rat mesenteric vessels is abolished by L-NAME (Oliveira et al., 1999). In the present study, the partial inhibition of Ang II vasodilation induced by HOE 140 (0.01 μM) is significantly enhanced by a combination of HOE 140 (0.01 μM) plus A 779, suggesting that Ang 1–7 might participate in the BK-dependent Ang II vasodilation. Therefore, the BK link to the EDRF-dependent vasodilator effect of Ang II probably involves the participation of receptors inhibited by PD 123319 and A 779, since the inhibitory effect of HOE 140 plus PD 123319 or HOE 140 plus A 779 on the vasodilator effect of Ang II is further enhanced by a combination of HOE 140, PD 123379 and A 779.
In conclusion, in the present study, we demonstrated that Ang II induces an EDRF-dependent vasodilator response in the rat MVB, which depends on NO release. Hyperpolarization due to activation of the KCa2+ channel could be involved in the vasodilator effect of Ang II. The results also suggest that BK could play a very important role in the mechanism of the vasodilator effect of Ang II. Probably, the link between Ang II and BK release might be modulated by receptors that bind PD 123319 and A 779. Finally, the Ang II-dependent vasodilation does not appear to be modulated by prostaglandin release, activation of KATP or KV channels and AT1 or Ang IV receptors.
Acknowledgments
This work was supported in part by CNPq and FAPERJ .
Abbreviations
- ACh
acetylcholine
- Ang 1–7
angiotensin 1–7
- Ang II
angiotensin II
- Ang IV
angiotensin IV
- 4-AP
4-aminopyridine
- BK
bradykinin
- cGMP
guanosine 3′5′ cyclic monophosphate
- ChTx
charybdotoxin
- COX
cyclooxygenase
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- GC
guanylyl cyclase
- KATP channel
ATP-dependent K+ channel
- KCa2+ channel
Ca2+-dependent K+ channel
- KV channel
voltage-dependent K+ channel
- L-NAME
NG-nitro-L-arginine methyl ester
- MVB
mesenteric vascular bed
- NE
norepinephrine
- NG
nitroglycerin
- NO
nitric oxide
- ODQ
1H-[1,2,3] oxadiazolo [4,4-a] quinoxalin-1-one
- PP
perfusion pressure
- PSS
physiological salt solution
- TEA
tetraethylammonium
References
- ALMEIDA A.P., FRABREGAS B.C., MADUREIRA M.M., SANTOS R.J., CAMPGNOLE-SANTOS M.J., SANTOS R.A. Angiotensin-(1–7) potentiates the coronary vasodilatatory effect of bradykinin on the isolated rat heart. Braz. J. Med. Biol. Res. 2000;33:709–713. doi: 10.1590/s0100-879x2000000600012. [DOI] [PubMed] [Google Scholar]
- ARIMA S., ENDO Y., YAOITA H., OMATA K., TSUNODA K., TAKEUCHI K., ABE K., ITO S. The possible role of P450 metabolite of arachidonic acid in the vasodilator mechanism of the angiotensin II type 2 receptor in the isolated microperfused rabbit afferent arteriole. J. Clin. Invest. 1997;100:2816–2823. doi: 10.1172/JCI119829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYORH M.A., EATMAN D., WALTON M., SOCCI R.R., THIERRY-PALMER M., EMMETT N. A-779 attenuates the angiotensin-(1–7) depressor response in salt-induced hypertensive rats. Peptides. 2002;23:57–64. doi: 10.1016/s0196-9781(01)00579-4. [DOI] [PubMed] [Google Scholar]
- BROSNIHAN K.B., LI P., FERRARIO C.M. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523–528. doi: 10.1161/01.hyp.27.3.523. [DOI] [PubMed] [Google Scholar]
- CHUNG O., UNGER T. Angiotensin II receptor blockade and end-organ protection. Am. J. Hypertens. 1999;12:150–156. doi: 10.1016/s0895-7061(99)00218-6. [DOI] [PubMed] [Google Scholar]
- COLEMAN J.K., KREBS L.T., HAMILTON T.A., ONG B., LAWRENCE K.A., SARDINIA M.F., HARDING J.W., WRIGHT J.W. The autoradiographic identification of kidney angiotensin IV binding sites and angiotensin IV-induced renal cortical blood flow changes in rats. Peptides. 1998;19:269–277. doi: 10.1016/s0196-9781(97)00291-x. [DOI] [PubMed] [Google Scholar]
- ENDO Y., ARIMA S., YAOITA H., TSUNODA K., OMATA K., ITO S. Vasodilation mediated by the angiotensin II type 2 receptor is impaired in the afferent arterioles of young spontaneously hypertensive rats. J. Vasc. Res. 1998;35:421–427. doi: 10.1159/000025613. [DOI] [PubMed] [Google Scholar]
- FERNANDES L., FORTES Z.B., NIGRO D., TOSTES R.C., SANTOS R.A., CATELLI DE CARVALHO M.H. The potentiation of bradykinin by angiotensin-(1–7) on arterioles of spontaneously hypertensive rats studied in vivo. Hypertension. 2001;37:703–709. doi: 10.1161/01.hyp.37.2.703. [DOI] [PubMed] [Google Scholar]
- FERRARIO C.M., BARNES K.L., BLOCK C.H., BROSNIHAN K.B., DIZ D.I., KHOSLA M.C., SANTOS R.A. Pathways of angiotensin formation and function in the brain. Hypertension. 1990;15:13–19. doi: 10.1161/01.hyp.15.2_suppl.i13. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., PEES C., UNGER T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- GORELIK G., CARBINI L.A., SCICLI A.G. Angiotensin 1–7 induces bradykinin-mediated relaxation in the porcine coronary artery. J. Pharmacol. Exp. Ther. 1998;286:403–407. [PubMed] [Google Scholar]
- HABERL R.L., DECKER P.J., EINHAUPL K.M. Angiotensin degradation products mediate the endothelium-dependent dilation of rabbit brain arterioles. Circ. Res. 1991;68:1621–1627. doi: 10.1161/01.res.68.6.1621. [DOI] [PubMed] [Google Scholar]
- KATADA J., MAJIMA M. AT2 receptor-dependent vasodilation is mediated by the activation of vascular kinin generation under flow conditions. Br. J. Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA T., TODA N., NODA Y., OKAMURA T. Mechanisms of relaxation induced by angiotensin II in isolated canine and human uterine arteries. J. Cardiovasc. Pharmacol. 2001;37:538–595. doi: 10.1097/00005344-200105000-00010. [DOI] [PubMed] [Google Scholar]
- KREBS L.T., KRAMAR E.A., HANESWORTH J.M., SARDINIA M.F., BALL A.E., WRIGHT J.W., HARDING J.W. Characterisation of the binding properties and physiological action of divalinal-angiotensin IV, a putative AT4 receptor antagonist. Regul. Pept. 1996;67:123–130. doi: 10.1016/s0167-0115(96)00121-8. [DOI] [PubMed] [Google Scholar]
- MCGREGOR D.D. The effect of sympathetic nerve stimulation on vasoconstrictor response in the perfused mesenteric blood vessels of the rat. J. Physiol. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENG W., BUSIJA D.W. Comparative effects of angiotensin-(1–7) and angiotensin II on piglet pial arterioles. Stroke. 1993;24:2041–2044. doi: 10.1161/01.str.24.12.2041. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA M.A., FORTES Z.B., SANTOS R.A., KOSLA M.C., DE CARVALHO M.H. Synergistic effect of angiotensin (1–7) on bradykinin arteriolar dilation in vivo. Peptides. 1999;20:1195–1201. doi: 10.1016/s0196-9781(99)00123-0. [DOI] [PubMed] [Google Scholar]
- OSEI S.Y., AHIMA R.S., MINKES R.K., WEAVER J.P., KHOSLA M.C., KADOWITZ P.J. Differential responses to angiotensin-(1–7) in the feline mesenteric and hindquarters vascular beds. Eur. J. Pharmacol. 1993;234:35–42. doi: 10.1016/0014-2999(93)90703-k. [DOI] [PubMed] [Google Scholar]
- PATEL J.M., MARTENS J.R., LI Y.D., GELBAND C.H., RAIZADA M.K., BLOCK E.R. The angiotensin IV receptor-mediated activation of lung endothelial NOS is associated with vasorelaxation. Am. J. Physiol. 1998;275:L1061–L1068. doi: 10.1152/ajplung.1998.275.6.L1061. [DOI] [PubMed] [Google Scholar]
- PÖRSTI I., BARA A.T., BUSSE R., HECKER M. The release of nitric oxide by angiotensin-(1–7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br. J. Pharmacol. 1994;111:652–654. doi: 10.1111/j.1476-5381.1994.tb14787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN Y., GARVIN J.L., CARRETERO O.A. The vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension. 2002;39:799–802. doi: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B.E., SCHUBERT R., HESCHELER J., NELSON M.T.CGMP-dependent protein kinase activities Ca-activated K channels in cerebral artery smooth muscle cells Am. J. Physiol. 1993265C299–C303.(Part 1) [DOI] [PubMed] [Google Scholar]
- SANTOS R.A., CAMPAGNOLE-SANTOS M.J., BARACHO N.C., FONTES M.A., SILVA L.C., NEVES L.A., OLIVEIRA D.R., CALIGIORNE S.M., RODRIGUES A.R., GROPEN JUNIOR C. The characterisation of a new angiotensin antagonist selective for angiotensin-(1–7): evidence that the actions of angiotensin-(1–7) are mediated by specific angiotensins receptors. Brain. Res. Bull. 1994;35:293–298. doi: 10.1016/0361-9230(94)90104-x. [DOI] [PubMed] [Google Scholar]
- SCHEUER D.A., PERRONE M.H. Angiotensin type 2 receptors mediate the depressor phase of biphasic pressure response to angiotensin. Am. J. Physiol. 1993;264:917–923. doi: 10.1152/ajpregu.1993.264.5.R917. [DOI] [PubMed] [Google Scholar]
- SEYEDI N., XU X., NASJLETTI A., HINTZE T.H. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- SOARES DE MOURA R., CARVALHO L.C.R.M., EMILIANO A.F., RESENDE A.C. Vasodilator effect of angiotensin II in the isolated mesenteric vascular bed of the rat (abstract) Br. J. Pharmacol. 2001;133:101. doi: 10.1038/sj.bjp.0705669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOSA-CANACHE B., CIERCO M., GUTIERREZ C.I., ISRAEL A. Role of bradykinins and nitric oxide in AT2 receptor-mediated hypotension. J. Hum. Hypertens. 2000;14:40–46. doi: 10.1038/sj.jhh.1000986. [DOI] [PubMed] [Google Scholar]
- TOM B., DE VRIES R., SAXENA P.R., DANSER A.H.J. Bradykinin by angiotensin-(1–7) and ACE inhibitors correlates with ACE C- and N-domain blockade. Hypertension. 2001;38:95. doi: 10.1161/01.hyp.38.1.95. [DOI] [PubMed] [Google Scholar]
- TSUTSUMI Y., MATSUBARA H., MASAKI H., KURIHARA H., MURASAWA S., TAKAI S., MIYAZAKI M., NAZAWA Y., OZONO R., NAKAGAWA K., MIWA T., KAWADA N., MORI Y., SHIBASAKI Y., TANAKA Y., FUJIYAMA S., KOYAMA Y., FUJIYAMA A., TAKAHASHI H., IWASAKA T. Angiotensin II type 2 receptor over expression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelium Dependent Hyperpolarization 1999Amsterdam, The Netherlands: Harwood Academic Publishers; 433(ed.) [Google Scholar]