Abstract
Recent studies have shown that sodium butyrate and other short-chain fatty acids (SCFAs) can prevent inflammation in colon diseases. Our aim was to elucidate whether sodium butyrate and SCFAs regulate the inflammatory responses in different neural inflammation models in cell cultures.
Inflammatory responses to LPS-induced microglial activation were recorded by the secretion of nitric oxide (NO) and cytokines IL-6 and TNF-α and related to the changes in the DNA-binding activities of NF-κB complex.
We observed that sodium butyrate is strongly anti-inflammatory against LPS-induced responses in rat primary microglia as well as in hippocampal slice cultures and in neural cocultures of microglial cells, astrocytes and cerebellar granule neurons.
In murine N9 microglial cell line, instead, sodium butyrate and other SCFAs (propionate, valerate and caproate) enhanced the LPS-induced inflammatory response.
The pretreatment with butyrate before LPS exposure induced an equal or more enhanced response than simultaneous exposure with butyrate and LPS. This indicates that butyrate induces an adaptative response against microglial activation.
We also observed that butyrate treatment both in transformed N9 cells and in hippocampal slice cultures downregulates the NF-κB-binding capacity induced by LPS stimulation.
Our results show that butyrate is anti-inflammatory in primary, brain-derived microglial cells, as observed recently in colon diseases, but proinflammatory in transformed, proliferating N9 microglial cells, which may be related to the anticancer properties of butyrate observed in tumor cells.
Keywords: Acetylation, Alzheimer, inflammation, innate immunity, neurodegeneration, HDAC, LPS, NF-κB
Introduction
Inflammation and innate immune reactions are involved in the pathology of several neurodegenerative diseases, stroke and traumatic brain injuries (McGeer & McGeer, 1995; Morganti-Kossmann et al., 2002; Wyss-Coray & Mucke, 2002). Activation of microglial cells is a defence reaction of brain against a variety of external and internal insults and hence should be beneficial but as an excessive response may be destructive to neurons. Anti-inflammatory compounds have been studied as a therapy for Alzheimer's disease and other degenerative diseases and injuries of brain (McGeer & McGeer, 1995). The microglial activation process itself as well as the factors that stimulate and inhibit the inflammatory response have been intensively studied and reviewed in detail (Nakamura, 2002).
Recently, short-chain fatty acids (SCFAs) and notably butyrate have received a lot of attention as maintaining bowel homeostasis since they prevent inflammation and induce antiproliferative and apoptotic effects in colon cancer cells (Wachtershauser & Stein, 2000; Yin et al., 2001; Hinnebusch et al., 2002). The anti-inflammatory effect of butyrate is probably mediated by the suppression of NF-κB activation, a well-known inflammatory mediator (Yin et al., 2001). The antiproliferative and apoptotic effects of butyrate and other SCFAs may be linked to histone hyperacetylation (Hinnebusch et al., 2002; Davie, 2003). SCFAs, especially butyrate, nonspecifically inhibit class I and II histone deacetylases and induce histone acetylation (Chen et al., 2003).
Our aim was to elucidate whether sodium butyrate and other SCFAs regulate the inflammatory responses also in different neural inflammation models, as observed in colon diseases (Wachtershauser & Stein, 2000). Interestingly, we observed that sodium butyrate is strongly anti-inflammatory against LPS-induced responses in rat primary microglia as well as in hippocampal slice cultures and in neural cocultures with microglial cells, astrocytes and cerebellar granule neurons. Instead, butyrate and other SCFAs enhanced the LPS-induced inflammatory response in transformed murine N9 microglial cell line. Our results show that the effects of butyrate and SCFAs are different in transformed cells and primary cells in neural inflammatory models.
Methods
Reagents
Sodium butyrate and SCFAs were purchased from Sigma. Lipopolysaccharide used in all experiments was from Escherichia coli 055:B5 lyophilized powder (L 6529 from Sigma, St Louis, U.S.A.).
Murine N9 microglia
Murine N9 microglial cell line was kindly provided by Dr Paola Ricciardi-Castagnoli (University of Milano-Bicocca, Milan, Italy). N9 cells were cultured in Iscove's modified Dulbecco's medium (Gibco) with 5% heat-inactivated FBS (Gibco) on Nunc (Nalgene) dishes and clusters. N9 microglial cells were plated at a density of 4 × 104 cells cm−2 for 24 h to start the experiment.
Rat primary astrocytes, microglia and cerebellar granule cells
Primary astrocytes were isolated from 1- to 2-day-old Wistar rat as described earlier (Kerokoski et al., 2001). The cerebral cortices and midbrain were excised and the tissue without meninges and blood vessels was minced into small pieces. Cells were separated by trypsinization (0.025% trypsin, 15 min, 37°C). After centrifugation and trituration, the tissue was filtered through 230 μm strainer. The cells were cultured in tissue culture flask in DMEM media (Sigma) supplemented with 10% heat-inactivated FBS, 2 mM glutamine (Gibco, Carlsbad, U.S.A.), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Gibco, Carlsbad, U.S.A.). Flasks for pure astrocyte cultures were shaken vigorously before each medium change, which was done first after 2 days and then after every 4 days. The astrocytes were detached by trypsinization after 2 weeks and used for cocultures.
Primary microglial cells were isolated in the same way as astrocytes and they matured on the top of astrocytes. The culture flasks were not shaken until 2 weeks after the isolation, when the floating microglia were harvested from confluent astroglial layers by rotating the flasks on an orbital shaker for a few hours. Microglia were harvested from the same flasks 4–5 times every seventh day. After 1–2 h of plating, the medium was changed to remove nonadherent cells. Cells were used for coculture or pure microglia culture. Experiments were initiated 24 h after plating. The purity of the microglial cultures (over 95%) was confirmed using antisera to CD11b (OX-42, Serotec, Oxford, U.K.).
Cerebellar granule cells were isolated from the cerebella of 7-day-old Wistar rats and cultured as described by Schousboe et al. (1989). After prepairing the cerebella out of brains, the cells were separated by trypsin treatment (0.025% trypsin, 15 min, 37°C) followed by centrifugation and trituration. The cells were plated onto poly-D-lysine-coated (Sigma) plates (2.5 × 105 cells cm−2) in the DMEM media supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin and 25 mM KCl. After 2 days in culture, the medium was supplemented with 10 μM 1-β-D-cytosine-arabinofuranoside (Ara-C, Sigma).
Cells for cocultures were plated onto 24-well plates. At 4 days after isolation of the cerebellar cells, the astrocytes (3 × 104 cells cm−2) were added to wells in DMEM media supplemented with 10% heat-inactivated dialyzed FBS (Sigma), 2 mM glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin and 25 mM KCl without Ara-C. Microglia (5 × 104 cells cm−2) were added 3 days after astrocytes and the experiments were initiated after 24 h of adding microglia.
Hippocampal slice cultures
Organotypic slice cultures from hippocampus were prepared using the modified interface culture method described earlier in detail (Stoppini et al., 1991). Postnatal day 7 (P7) Wistar rat pups were decapitated, the brains rapidly dissected and placed in a Petri dish in ice-cold PBS (Gibco). The hippocampi of both sides were isolated and cut into 400 μm transversal slices using a McIlwain tissue chopper. The slices were then carefully separated and transferred onto porous membrane inserts (one slice per insert) of 12-well culture plates (Transwell, Costar). Culture medium (500 μl), consisting of Neurobasal medium (Gibco) with B27-supplement (Gibco), 1 mM glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin, was added to the lower compartment of each well. For the first culture day, 10% of inactivated FBS was added to the culture medium, and fresh medium without B27-supplement was changed before exposing the cultures to the treatment for 24 h after 4 days in vitro.
EMSA assays
EMSA assays were performed as described earlier in detail (Helenius et al., 2001). Nuclear proteins were isolated according to the modified protocol of Dignam et al. (1983). Double-stranded consensus and mutated oligonucleotides for NF-κB-binding sites were from Santa Cruz Biotechnology. Probes were labelled with T4 polynucleotide kinase (Promega). Unspecific binding was blocked by 2 μg of poly(dI-dC) : poly(dI-dC) (Roche Applied Science) in an assay volume of 20 μl (Helenius et al., 2001). The binding assays were performed as described earlier (Helenius et al., 1996). Bound and free probes were separated in a native 4% polyacrylamide gel. Radioactive bands were visualized with Storm 860 PhosphorImager (Molecular Dynamics) and pixel volumes of specific bands were calculated with ImageQuaNT 4.2a software (Molecular Dynamics).
ELISA, LDH and NO assays
The nitrite concentration in the medium was measured by the Griess reaction, that is into a 100 μl of sample an equal amount of the Griess reagent (1 : 1 of 0.1% naphthylethylene diamide in H2O and 1% sulfanilamide in 5% concentrated H2PO4) was added and the OD was measured at 550 nm using an ELISA microplate reader after 10 min incubation. LDH leakage to the medium was measured with the cytotoxicity kit obtained from Promega. The amount of IL-6 and TNF-α was measured using OptEIA™ kits or sets obtained from Pharmingen.
Exposure of cells to butyrate and other SCFAs
Inflammatory models were optimized before the experiments with respect to LPS, sodium butyrate and SCFA concentrations as well as the timing of inflammatory responses. Experiments were performed either using pretreatment with butyrate followed by washing and then LPS exposure or with the simultaneous exposure with LPS and SCFAs. Primary microglial cells or cocultures were exposed with 5 μg ml−1 of LPS and N9 cells and hippocampal slices with 10 μg ml−1. Exposure times were normally 22–24 or 12 h in NF-κB-binding assay in Figure 4.
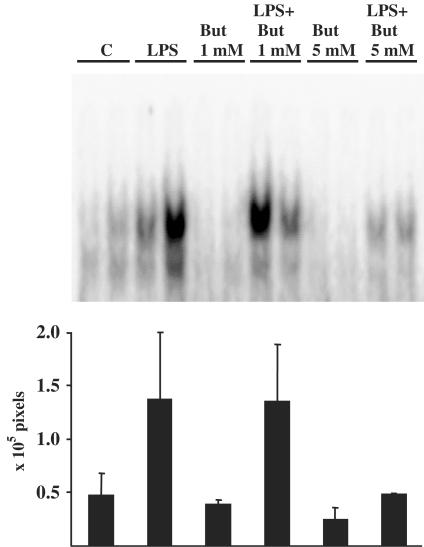
Figure 4.
Butyrate treatment reduced DNA-binding activity of NF-κB complex in LPS-stimulated N9 microglia. Left panel shows specific DNA-binding activity of NF-κB complex in butyrate pretreated (upper part) and in simultaneously treated cells with butyrate and LPS (12 h) (lower part). Right panel shows the pixel values (× 105 pixels) of Storm PhosphorImager of the specific NF-κB band (upper band). Values are means±s.d. (n=4 for each group, *P<0.05 between LPS-only treated and butyrate and LPS-treated groups). Drug concentrations: 0.6 mM for butyrate and 10 μg ml−1 for LPS.
Statistical analysis
All values were expressed as means±s.d. The difference between control and treated groups was analyzed using Mann–Whitney U-test.
Results
Butyrate and other SCFAs potentiate LPS-induced inflammatory response in transformed N9 microglial cells
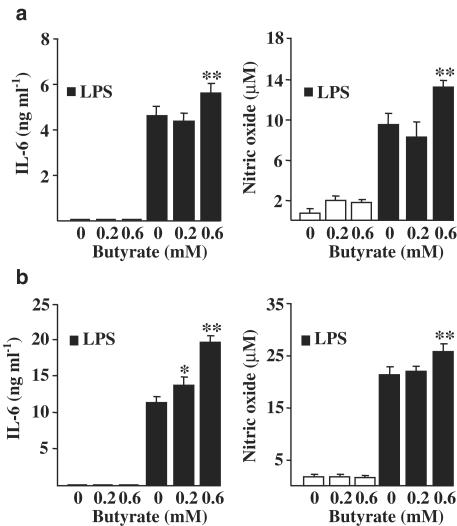
N9 cells are transformed microglial cell line used frequently in inflammation studies after their establishment (Ferrari et al., 1996). LPS treatment induced an inflammatory response in these cells as assayed by the secretion of IL-6 and NO (Figure 1). Sodium butyrate concentration of 0.6 mM but not that of 0.2 mM potentiated the LPS-induced secretion of both IL-6 and NO (Figure 1). Furthermore, our results show that the potentiation was very similar whether cells were pretreated for 17 h with butyrate, which was washed out before LPS exposure for 22 h (Figure 1a), or whether cells were exposed simultaneously to butyrate and LPS treatment for 22 h (Figure 1b).
Figure 1.
Potentiation of LPS-induced inflammatory response in N9 microglial cells by sodium butyrate. LPS-induced IL-6 and NO secretion was studied in butyrate pretreated cultures (17 h pretreatment) (a) or in cultures where both butyrate and LPS were simultaneously present (b). LPS concentration was 10 μg ml−1. Values are means±s.d. (n=4–8 in each group). Statistical significance of the butyrate-induced potentiation (compared to LPS-treated group after 22 h treatment): *P<0.01, **P<0.001.
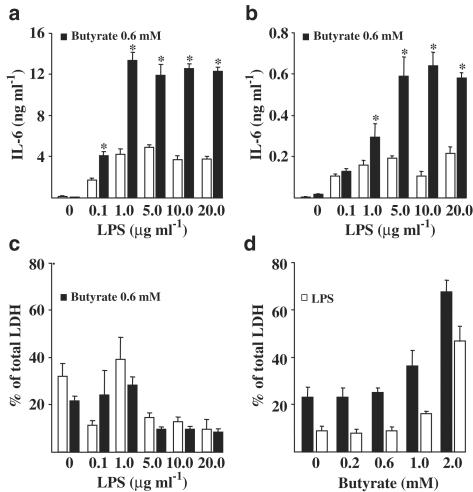
Figure 2 shows that sodium butyrate enhanced both the LPS-induced IL-6 secretion (Figure 2a) and the expression of IL-6 protein in N9 cells (Figure 2b). We also verified that the potentiation was connected to the enhanced expression of IL-6 and iNOS mRNA. Northern blot assay showed that butyrate enhanced the expression of IL-6 and iNOS mRNA in LPS-stimulated N9 cells (data not shown). Figure 2 shows that the butyrate-induced potentiation of LPS response reached the highest level for 22 h incubation when the LPS concentrations was 1 μg ml−1 in the case of secretion and of 5 μg ml−1 in the case of cellular expression. Furthermore, Figure 2c shows that LPS was not toxic to N9 cells up to the concentration of 20 μg ml−1 as measured by LDH release. Sodium butyrate was neither toxic to N9 cells at the concentrations used in this study (Figure 2d). Toxicity assayed by LDH release appears at the 2 mM butyrate level. Interestingly, LPS-induced activation of N9 cells reduced the LDH release at all butyrate concentrations studied (Figure 2d).
Figure 2.
Effect of LPS concentration on butyrate-induced potentiation of inflammatory response both in IL-6 secretion (Figure 2a) and in IL-6 protein expression in N9 cells (Figure 2b). (c,d) LDH release from N9 cells induced by LPS and sodium butyrate treatments. LPS and butyrate were simultaneously added and incubation was for 22 h. LPS concentration was 10 μg ml−1 in (d). Values are means±s.d. (n=4–6 in each group). Statistical significance of the butyrate-induced potentiation (compared to LPS-treated group after 22 h treatment): *P<0.01.
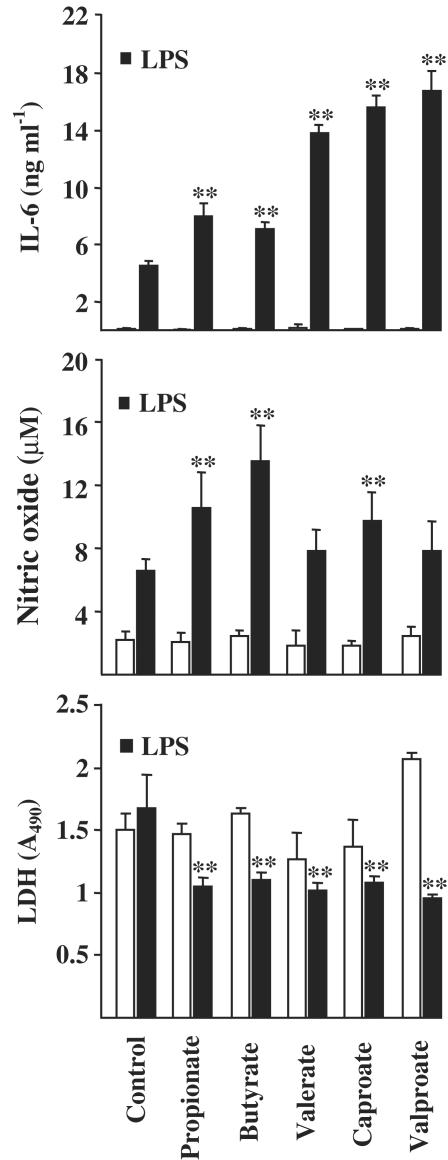
SCFAs, that is propionic, butyric, valeric and caproic acids, have similar effects in protein acetylation (Benjamin & Jost, 2001). Next, we studied whether other SCFAs show the similar response as butyrate in N9 inflammation model. Figure 3 shows that propionate, valerate and caproate induced a similar potentiation as butyrate in the LPS-stimulated secretion of IL-6. Propionate and caproate potentiated also the secretion of NO (Figure 3). Interestingly, valproate (alpha-propyl-valeric acid), an inhibitor of histone deacetylase (Jeong et al., 2003) and antiepileptic drug with mood-stabilizing properties (Harwood & Agam, 2003) enhanced the LPS-stimulated secretion of IL-6 but not that of NO (Figure 3). SCFAs did not increase LDH release but the cotreatment of cells with LPS and SCFAs reduced the LDH release from N9 cells (Figure 3).
Figure 3.
Potentiation of LPS-induced inflammatory response in N9 microglial cells by SCFAs. LPS and SCFAs were added at the same time. LPS concentration was 10 μg ml−1. SCFA concentrations were for butyrate 1 mM, propionate and caproate 3 mM and valerate and valproate 2 mM. LDH release is shown in each group (on bottom). Values are means±s.d. (n=4–15 in each group). Statistical significance of the SCFA-induced potentiation (compared to LPS-treated group after 24 h treatment): **P<0.001. Statistical significance between SCFA-treated and SCFA and LPS-treated groups: **P<0.001.
Studies on colonocytes (Yin et al., 2001) and cancer cells (Luhrs et al., 2001) have shown that butyrate inhibits the activation of NF-κB signaling and the DNA binding of NF-κB complex. We also studied whether butyrate affects the NF-κB activation in transformed N9 microglial cells. Figure 4 shows that both the pretreatment with butyrate followed by LPS treatment without butyrate or the simultaneous exposure with butyrate and LPS for 12 h inhibited the DNA-binding activity of NF-κB complex in N9 microglial cells. Interestingly, the pretreatment of cells with butyrate itself reduced the DNA-binding activity of NF-κB (Figure 4).
Butyrate induces protection against LPS-induced inflammatory response in primary microglia, neural cocultures and hippocampal slice cultures
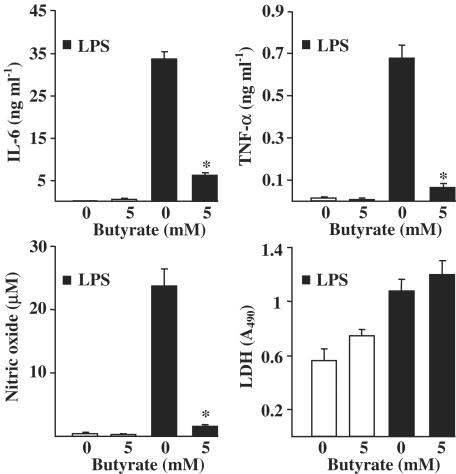
Sodium butyrate is known to prevent the excess inflammation in bowel diseases (Segain et al., 2000; Wachtershauser & Stein, 2000). Next, we studied whether butyrate could induce anti-inflammatory response in our neural inflammation models, which use rat primary untransformed microglial cells in contrast to transformed N9 cells. Interestingly, in rat primary microglial cells, butyrate induced a significant protection against LPS-induced inflammatory response in cells pretreated with butyrate (Figure 5a). However, the simultaneous treatment with butyrate and LPS reduced only the secretion of IL-6 but not that of TNF-α or NO (Figure 5b). This result strongly implies that butyrate pretreatment induces adaptive changes in the microglial activation system. Furthermore, our results show that butyrate responses are opposite in transformed and nontransformed microglial cells.
Figure 5.
Sodium butyrate induced protection against LPS-stimulated inflammatory response in rat primary microglia. LPS-induced IL-6, TNF-α and NO secretion were studied (a) in butyrate-pretreated (for 22 h) cultures or (b) in cultures where butyrate and LPS were present simultaneously (for 24 h). LPS concentration was 5 μg ml−1. Values are means±s.d. (n=6 in each group). Statistical significance of the butyrate-induced changes (compared to LPS-treated group after 24 h treatment): *P<0.01.
Next, we verified the difference in LPS-induced responses between transformed N9 microglial cells and normal brain tissue-derived microglial cells using both cerebellar cocultures with microglia, astrocytes and neurons and hippocampal slice cultures. Figure 6 shows that pretreatment with butyrate induces a significant anti-inflammatory response in cocultures with cerebellar granule neurons, microglia and astrocytes. LPS-induced secretion of cytokines and NO most likely originates in microglial cells because in the same culture conditions, LPS induces only minimal secretion of IL-6, TNF-α or NO from astrocytic cultures (data not shown). The simultaneous exposure of cocultures to butyrate and LPS induced a similar reduction in the secretion of NO, IL-6 and TNF-α as the pretreatment (data not shown).
Figure 6.
Sodium butyrate induced protection against LPS-induced inflammatory response in butyrate pretreated (19 h) cocultures of rat cerebellar granule neurons, astrocytes and microglial cells. LPS treatment was for 24 h with concentration of 5 μg ml−1. Values are means±s.d. (n=6 in each group). Statistical significance of the butyrate-induced changes (compared to LPS-treated group): *P<0.01.
Figure 7 shows that butyrate induced a concentration-dependent anti-inflammatory response in hippocampal slice cultures exposed for 24 h either simultaneously with LPS (Figure 7a) or in pretreatment (data not shown). Butyrate is probably toxic to hippocampal neurons because it increases LDH release, although values are low (Figure 7a). Butyrate is not toxic for primary microglia at the concentration level of 5 mM (data not shown). Figure 7b shows that propionate and caproate did not produce protection against LPS-induced inflammatory response in hippocampal slices. LDH release was also unaffected. It seems that in hippocampal slices, there are differences between effects of different SCFAs in contrast to transformed N9 cells (Figure 3). Butyrate seems to be more potent than other SCFAs in hippocampal slices.
Figure 7.
Effects of butyrate, propionate and caproate on LPS-stimulated inflammatory response in hippocampal slice cultures. (a) Butyrate-treated slices, (b) propionate and caproate-treated samples. LPS and SCFAs were added at the same time and exposed for 24 h. LPS concentration was 10 μg ml−1 and those of propionate and caproate 3 mM. Values are means±s.d. (n=6 in each group). Statistical significance of the butyrate-induced changes (compared to LPS-treated group): *P<0.01.
Next, we studied whether butyrate affects the DNA-binding efficiency of NF-κB transcription factor. Figure 8 shows that the higher concentration of butyrate considerably reduced the binding efficiency of NF-κB factor. Downregulation of NF-κB signaling seems to be the general response to butyrate treatment both in transformed N9 cells (Figure 4) and in hippocampal slice culture samples (Figure 8).
Figure 8.
Effects of butyrate treatment on DNA-binding activity of NF-κB complex in LPS-stimulated cultured hippocampal slices. Upper panel shows specific DNA-binding activities of NF-κB complex in LPS (10 μg ml−1) and butyrate-treated hippocampal slices after 24 h treatment. Three slices were combined together (from the experiment shown in Figure 7a). Lower panel shows the pixel values (× 105 pixels) of Storm PhosphorImager of the specific NF-κB band. Two lanes were shown for each treatment. Samples marked with LPS and butyrate were cotreated samples. Values are means±s.d. (n=2 for each group).
Discussion
Recent studies have shown that sodium butyrate has both anti-inflammatory and cancer chemopreventive properties in colon (Wachtershauser & Stein, 2000; Yin et al., 2001; Hinnebusch et al., 2002). Butyric acid is a SCFA produced in intestine by microbial metabolism, but SCFAs are also transported through blood–brain barrier into brain and used for energy production by short-chain L-3-hydroxyacyl-CoA dehydrogenase (He et al., 1999). Interestingly, this enzyme is known also as ERAB, an endoplasmic reticulum amyloid beta-peptide-binding protein, and as a multifunctional protein can also oxidize 17β-estradiol (He et al., 1999). The anticancer and antiproliferative properties of butyrate in intestine are not related to energy metabolism but probably to the modulation of gene expression (Mariadason et al., 2000) via the inhibition of histone deacetylases (Chen et al., 2003; Davie, 2003) and NF-κB signaling (Chakravortty et al., 2000; Segain et al., 2000; Luhrs et al., 2001; Yin et al., 2001).
All our results showed that pretreatment with butyrate before LPS exposure induced an equal or more enhanced response than simultaneous exposure with LPS. This suggests that butyrate affects gene expression also in microglial cells, as observed, for instance, in colonocytes (Mariadason et al., 2000; Bocker et al., 2003). Adaptive responses in NF-κB signaling induced by butyrate could regulate the responsiveness of microglial cells to LPS. Several studies with colonocytes (Yin et al., 2001), adenocarcinoma cells (Luhrs et al., 2001) and murine macrophages (Chakravortty et al., 2000) have shown that butyrate downregulates NF-κB signaling induced by cytokines or LPS. Interestingly, we observed a similar downregulation in NF-κB-binding efficiency as observed in butyrate-treated colonocytes and murine macrophages. Suppression appeared both in butyrate-treated N9 cells and in hippocampal slice cultures against LPS-induced inflammatory response. Yin et al. (2001) have shown that butyrate inhibits proteasome activity that upregulates the level of inhibitory IκB proteins and hence inhibits cytosolic NF-κB activation. Bocker et al. (2003) have observed that butyrate downregulates the expression of Toll-like receptor 4 (TLR4) that transmits LPS signals to activate NF-κB system. Both of these changes will inhibit NF-κB activation. Future studies will show whether these mechanisms are involved in the inhibition of NF-κB signaling in microglial cells.
Most interestingly, we observed that butyrate treatment induced a proinflammatory response in transformed N9 microglial cell line and anti-inflammatory response in primary, brain-derived microglial cells. Inhibition of NF-κB activation should lead to anti-inflammatory response, as we observed in primary hippocampal slices, but how suppression of NF-κB signaling might induce a potentiation of inflammatory response. This is probably related to the ability of butyrate and other SCFAs to induce protein hyperacetylation by inhibiting histone deacetylases (Chen et al., 2003; Davie, 2003). We have recently observed that trichostatin A, a specific inhibitor of class I and II histone deacetylases, induce a strong potentiation of LPS-induced secretion of IL-6 and NO in N9 cells (Suuronen et al., 2003). The butyrate-induced response observed in this study is much less than that induced by trichostatin A, probably due to the lower level of protein acetylation. Butyrate and other SCFAs are promising anticancer therapeutics (Chen et al., 2003) as well as other histone deacetylase inhibitors (Johnstone, 2002). There may be several mechanisms but one is probably the inhibition of NF-κB since NF-κB signaling regulates several antiapoptotic genes (Wang et al., 1998).
Acknowledgments
This study was supported by grants from the Academy of Finland (AS) and the University of Kuopio, Finland (JH, TS, TN, SK).
Abbreviations
- EMSA
electrophoretic mobility shift assay
- FBS
fetal bovine serum
- HDAC
histone deacetylase
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- NO
nitric oxide
- SCFA
short-chain fatty acid
References
- BENJAMIN D., JOST J.-P. Reversal of methylation-mediated repression with short-chain fatty acids: evidence for an additional mechanism to histone deacetylation. Nucleic Acid. Res. 2001;29:3603–3610. doi: 10.1093/nar/29.17.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCKER U., YEZERSKYY O., FEICK P., MANIGOLD T., PANJA A., KALINA U., HERWECK F., ROSSOL S., SINGER M.V. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with Toll-like receptor 4 but not Toll-like receptor 2 or CD14 expression. Int. J. Colorectal. Dis. 2003;18:25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- CHAKRAVORTTY D., KOIDE N., KATO Y., SUGIYAMA T., MU M.M., YOSHIDA T., YOKOCHI T. The inhibitory action of butyrate on lipopolysaccharide-induced nitric oxide production in RAW 264.7 murine macrophage cells. J. Endotoxin Res. 2000;6:243–247. [PubMed] [Google Scholar]
- CHEN J.S., FALLER D.V., SPANJAARD R.A. Short-chain fatty acid inhibitors of histone deacetylases: promising anticancer therapeutics. Curr. Cancer Drug Targets. 2003;3:219–236. doi: 10.2174/1568009033481994. [DOI] [PubMed] [Google Scholar]
- DAVIE J.R. Inhibition of histone deacetylase activity by butyrate. J. Nutr. 2003;133:2485S–2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- DIGNAM J.D., LEBOVITZ R.M., ROEDER R.G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acid Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI D., VILLALBA M., CHIOZZI P., FALZONI S., RICCIARDI-CASTAGNOLI P., DI VIRGILIO F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J. Immunol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- HARWOOD A.J., AGAM G. Search for a common mechanism of mood stabilizers. Biochem. Pharmacol. 2003;66:179–189. doi: 10.1016/s0006-2952(03)00187-4. [DOI] [PubMed] [Google Scholar]
- HE X.Y., MERZ G., MEHTA P., SCHULZ H., YANG S.Y. Human brain short chain L-3-hydroxyacyl coenzyme A dehydrogenase is a single-domain multifunctional enzyme. Characterization of a novel 17beta-hydroxysteroid dehydrogenase. J. Biol. Chem. 1999;274:15014–15019. doi: 10.1074/jbc.274.21.15014. [DOI] [PubMed] [Google Scholar]
- HELENIUS M., HANNINEN M., LEHTINEN S.K., SALMINEN A. Changes with aging and replicative senescence in the regulation of transcription factor nuclear factor-κB. Biochem. J. 1996;318:603–608. doi: 10.1042/bj3180603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELENIUS M., KYRYLENKO S., VEHVILAINEN P., SALMINEN A. Characterization of aging-associated up-regulation of constitutive nuclear factor-κB binding activity. Antioxid. Redox Signal. 2001;3:147–156. doi: 10.1089/152308601750100669. [DOI] [PubMed] [Google Scholar]
- HINNEBUSCH B.F., MENG S., WU J.T., ARCHER S.Y., HODIN R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002;132:1012–1017. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- JEONG M.R., HASHIMOTO R., SENATOROV V.V., FUJIMAKI K., REN M., LEE M.S., CHUANG D.-M. Valproic acid, a mood stabilizer and anticonvulsant, protects rat cerebral cortical neurons from spontaneous cell death: a role of histone deacetylase inhibition. FEBS Lett. 2003;542:74–78. doi: 10.1016/s0014-5793(03)00350-8. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE R.W. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- KEROKOSKI P., SOININEN H., PIRTTILÄ T. Beta-amyloid (1–42) affects MTT reduction in astrocytes: implications for vesicular trafficking and cell functionality. Neurochem. Int. 2001;38:127–134. doi: 10.1016/s0197-0186(00)00071-1. [DOI] [PubMed] [Google Scholar]
- LUHRS H., GERKE T., SCHAUBER J., DUSEL G., MELCHER R., SCHEPPACH W., MENZEL T. Cytokine-activated degradation of inhibitory κB protein α is inhibited by the short-chain fatty acid butyrate. Int. J. Colorectal Dis. 2001;16:195–201. doi: 10.1007/s003840100295. [DOI] [PubMed] [Google Scholar]
- MARIADASON J.M., CORNER G.A., AUGENLICHT L.H. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–4572. [PubMed] [Google Scholar]
- MCGEER P.L., MCGEER E.G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res. Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- MORGANTI-KOSSMANN M.C., RANCAN M., STAHEL P.F., KOSSMANN T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr. Opin. Crit. Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y. Regulating factors for microglial activation. Biol. Pharm. Bull. 2002;25:945–953. doi: 10.1248/bpb.25.945. [DOI] [PubMed] [Google Scholar]
- SCHOUSBOE A., MEIER E., DREJER J., HERTZ L.Preparation of primary cultures of mouse (rat) cerebellar granule cells A Dissection and Tissue Culture Manual of the Nervous System 1989New York: AR Liss; 203–206.ed. Shahar A., de Vellis J., Vernadalis A. & Haber B. pp [Google Scholar]
- SEGAIN J.P., RAINGEARD DE LA BLETIERE D., BOURREILLE A., LERAY V., GERVOIS N., ROSALES C., FERRIER L., BONNET C., BLOTTIERE H.M., GALMICHE J.P. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOPPINI L., BUCHS P.-A., MULLER D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- SUURONEN T., HUUSKONEN J., PIHLAJA R., KYRYLENKO S., SALMINEN A. Regulation of microglial inflammatory response by histone deacetylase inhibitors. J. Neurochem. 2003;87:407–416. doi: 10.1046/j.1471-4159.2003.02004.x. [DOI] [PubMed] [Google Scholar]
- WACHTERSHAUSER A., STEIN J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur. J. Nutr. 2000;39:164–171. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- WANG C.Y., MAYO M.W., KORNELUK R.G., GOEDDEL D.V., BALDWIN A.S., JR NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- WYSS-CORAY T., MUCKE L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- YIN L., LAEVSKY G., GIARDINA C. Butyrate suppression of colonocyte NF-κB activation and cellular proteosome activity. J. Biol. Chem. 2001;276:44641–44646. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]