Abstract
We tested whether pretreatment of reagents known to induce hypoxia-inducible factor-1 (HIF-1) may confer chemoresistance against cytotoxicity of 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) to rat C6 glioma cells. We also studied which cytotoxic mechanism(s) of chloroethylnitrosoureas could be neutralized by cobalt preconditioning.
Preconditioning of rat C6 glioma cells with cobalt chloride (300 μM, 2 h) induced HIF-1 binding activity based on electrophoretic mobility shift assay (EMSA). Results from Western blotting confirmed a heightened HIF-1α level upon cobalt chloride exposure (300–400 μM, 2 h). Cobalt chloride (300 μM) pretreatment for 2 h substantially neutralized BCNU toxicity, leading to increases in glioma cell survival based on MTT assay. In addition, pre-exposure of C6 cells with desferrioxamine (DFO; 400 μM, 3 h), an iron chelator known to activate HIF-1, also induced HIF-1 binding and rendered the glioma cells resistant to cytotoxicity of BCNU.
Pre-incubation with cobalt chloride abolished the cytotoxicity of several carbamoylating agents including 2-chloroethyl isocyanate and cyclohexyl isocyanate, the respective carbamoylating metabolites of BCNU and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. The protective effect of cobalt exposure, however, was not observed when cells were challenged with alkylating agents including temozolomide.
Cadmium chloride (50 μM) effectively reversed cobalt-induced HIF-1 activation. Correspondingly, cadmium chloride suppressed carbamoylating chemoresistance mediated by cobalt chloride pretreatment. Furthermore, both double-stranded oligodeoxynucleotide (ODN) decoy with HIF-1 cognate sequence and antisense phosphorothioate ODNs against HIF-1α partially abolished the carbamoylating chemoresistance associated with cobalt preconditioning.
Our results suggest that cobalt- or DFO-preconditioning may enhance glioma carbamoylating chemoresistance that is dependent, at least in part, on induction of HIF-1.
Keywords: Alkylation, antisense phosphorothioate oligodeoxynucleotide, BCNU, brain tumor, carbamoylation, chemotherapy, oligodeoxynucleotide decoy
Introduction
Glioblastoma multiforme (GBM) is the most common type of primary brain tumor accounting for more than 40% of neoplasm in the central nervous system (Kleihues et al., 1995). Compared to other cancers, the life expectancy of patients with GBM is relatively short. Combination of surgery, radiotherapy, and chemotherapy results in survival of approximately 14 months (Rajkumar et al., 1999). 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) is the most commonly used adjunct chemotherapy for GBM following surgical resection and radiation therapy, due in part to its lipophilic character that allows better passage across the blood–brain barrier (Paoletti, 1984). BCNU and other related chloroethylnitrosoureas kill tumor cells via multiple cytotoxic actions including carbamoylation and alkylation (Wheeler et al., 1974). Unfortunately, BCNU does not appear to substantially prolong median survival of GBM patients, even though the proportion of patients living more than 18 months increased from 5 to 15% with adjunct BCNU chemotherapy (Walker et al., 1980; Chang et al., 1983; Green et al., 1983; Fine et al., 1993). The reason for the unsatisfactory clinical outcomes following adjunct chemotherapy in GBM remains unclear, but may involve acquired chemoresistance against BCNU. Multidrug resistance genes (Nutt et al., 2000), O6-methylguanine DNA methyltransferase (Rolhion et al., 1999), and glutathione S-transferase have all been shown to be associated with the development of BCNU chemoresistance. Here, we report another mechanism involving activation of hypoxia-inducible factor-1 (HIF-1) that may contribute to BCNU chemoresistance.
HIF-1, a heterodimeric protein complex consisting of alpha (HIF-1α) and beta (HIF-1β or ARNT; aryl hydrocarbon receptor nuclear translocator) subunits, is a key regulator of mammalian oxygen homeostasis. HIF-1α expression is tightly regulated by the cellular oxygen tension (Wang et al., 1995; Jiang et al., 1996), whereas the expression of HIF-1β is oxygen-independent. Hypoxic conditions prevent ubiquitylation and subsequent proteasomal degradation of HIF-1α (Epstein et al., 2001). Thus, the activity of this basic helix–loop–helix transcription factor is increased in most cells in response to low oxygen tension (Semenza & Wang, 1992; Wang & Semenza, 1993a, 1993b). In addition to tissue hypoxia, several reagents including cobalt chloride and iron chelator desferrioxamine (DFO) are also known to induce HIF-1 (Semenza et al., 1994). Rapid tumor growth, invasion, and metastasis resulting in higher metabolic demands are frequently accompanied by hypoxia. Indeed, low oxygen tension is an indicator of tumor malignancy (Dachs & Chaplin, 1998). A growing body of evidence has also shown HIF-1 activation in response to hypoxia in tumors (Semenza, 1998; 2000). HIF-1 appears to play a key role in cancer growth by transactivating genes such as erythropoietin (EPO) (Wang & Semenza, 1993a, 1993b), vascular endothelial growth factor (VEGF) (Kimura et al., 2000) and inducible nitric oxide synthase (iNOS) (Yin et al., 2001) that may confer cytoprotective as well as angiogenic effects. Understanding the pathophysiological role of HIF-1 in tumors may broaden our insight into the development of better therapeutic strategies for GBM.
In the present study, we demonstrate that preconditioning with reagents mimicking hypoxia and hence capable of HIF-1 induction, namely cobalt chloride and DFO, enhanced chemoresistance of C6 glioma cells against chloroethylnitrosoureas such as BCNU, which is the mainstay of chemotherapy in GBM. Tumor-killing actions of chloroethylnitrosoureas include carbamoylating and alkylating reactions (Wheeler et al., 1974). The chemoresistance conferred by such preconditioning is confined to the carbamoylating, but not alkylating action of BCNU. Furthermore, downregulation of cobalt-mediated HIF-1 activation, either by co-incubation with cadmium ions or transfection with HIF-specific oligodeoxynucleotide (ODN) decoy or an antisense phosphorothioate ODN against HIF-1α, abolished at least in part the carbamoylating chemoresistance associated with cobalt preconditioning, suggesting a putative role of HIF-1 implicated in the observed chemoresistance.
Methods
Materials
All the chemicals were purchased from Sigma (St Louis, MO, U.S.A.) unless otherwise specified. BCNU was from Bristol-Myers Squibb Inc. (Princeton, NJ, U.S.A.). Temozolomide was a gift from Dr W. Robert Bishop of Schering-Plough Corporation (Kenilworth, NJ, U.S.A.). 1,2-bis(sulfonyl)hydrazine derivatives including 1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-hydrazine (compound #1) and 1,2-bis(methylsulfonyl)-1-[[(methyl)amino]carbonyl]-hydrazine (compound #5) were generously provided by Dr Alan C. Sartorelli, Department of Pharmacology, Yale University School of Medicine. The synthesis and characterizations of compounds #1 have been previously described in details (Shyam et al., 1996; Penketh et al., 2000). The synthesis of compound #5-related 1,2-bis(methylsulfonyl)hydrazines was also reported by Shyam et al. (1996).
Cell culture and induction of HIF-1α
Rat C6 glioma cells (American Type Culture Collection, Rockland, MD, U.S.A.) were grown as previously described (Yin et al., 2000; 2001). To induce chemical hypoxia, cells were treated with 300 or 400 μM CoCl2 or 400 μM DFO for 2 h. In selected experiments, cells were treated with 300 μM CoCl2 along with 50 μM CdCl2 for 2 h to counteract cobalt induction of HIF-1α. The cobalt or DFO pretreatments were followed by addition of various chemotherapy drugs with selective carbamoylating or alkylating actions as described in figure legends.
Electrophoretic mobility shift assay (EMSA)
The detailed protocols for EMSA to assess HIF-1 DNA-binding activities have been described earlier (Yin et al., 2000). An oligonucleotide probe (5′-agcttGCCCTACGTGCTGTCTCAg-3′ and 5′-aattcTGAGACAGCACGTAGGGCa-3′) corresponding to the hypoxia-response element (HRE) in the EPO gene was used. The oligonucleotides were labeled using [γ32P]-ATP according to the Promega Technical Bulletin number 106 (Promega, Madison, WI, U.S.A.). The binding reaction was performed in a reaction mixture of 20 μl that contained binding buffer (10 mM Tris-HCl, 20 mM NaCl, 1 mM DTT, 1 mM EDTA, and 5% glycerol, pH 7.6), 0.1 ng of labeled probe (>10,000 c.p.m.), 30 μg of nuclear proteins, and 1 μg of poly(dI-dC). After incubation for 20 min at room temperature, the mixture was subjected to gel electrophoresis on a nondenaturing 6% polyacrylamide gel at 180 V for 2 h under a low ionic strength condition. The gel was vacuum dried and subjected to autoradiography. The experimental procedures for HIF-1α antibody binding assay were the same as described above, except that 1 μg of anti-HIF-1α antibody (Novus Biologicals, Littleton, CO, U.S.A.) was added to the samples 1 h prior to the addition of labeled probes.
Cell viability assays
For quantitative assessment of the extent of cell survival following challenges with chemotherapeutic reagents, the MTT assay was performed as previously described (Xu et al., 1998).
Transfection of HIF-specific ODN decoy and antisense phosphorothioate ODN against HIF-1α
ODNs with consensus sequence in the EPO gene promoter were synthesized (sense: 5′-GCCCTACGTGCTGTCTCA-3′; antisense: 5′-TGAGACAGCACGTAGGGC-3′) to serve as a double-stranded ODN decoy for HIF-1. The mutant ODNs (sense: 5′-GCCCTTACAACTGTCTCA-3′; antisense: 5′-TGAGACAGTTGTAAGGGC-3′) were synthesized to serve as a negative control (Wang & Semenza, 1993a, 1993b). To make double-stranded ODN decoy, sense and antisense ODNs (dissolved in 10 mM Tris pH 8.0 with 50 mM NaCl) were mixed at equal molar ratio before heating to 95°C for 5 min and then cooled down slowly to room temperature to permit the annealing of both ODN strands. The ODNs were dispensed into aliquots and stored at −20°C until use. For suppression of HIF-1α expression, phosphorothioate antisense ODNs (5′-CCTCCATGGCGAATCGGTGC-3′) or scrambled ODNs (5′-ACTCGTACCGCGGCAGTTCG-3′) were synthesized for transfection as previously reported by Kakinuma et al. (2001).
Transfection of ODNs into C6 glioma cells was achieved using Effectene reagent (QIAGEN Inc., Valencia, CA, U.S.A.) according to the manufacturers' protocols with modifications. For transfection into each well of a 96-well plate, ODNs (0.10 μg per well for HIF- decoy or mutant ODNs and 0.08 μg per well for antisense or scrambled ODNs) were first diluted in 6 μl buffer EC that was provided in the Effectene reagent kit. This was followed by addition of Enhancer (0.80 μl per well each for HIF decoy or mutant ODNs and 0.64 μl per well each for antisense or scrambled ODNs) into the respective reaction mixture with a brief vortexing. The mixture was then incubated at room temperature for 5 min before addition of 20 μl diluted Effectene reagent (2 μl Effectene stock solution diluted with 18 μl EC buffer) for each well. The mixture of ODN and Effectene in EC buffer was incubated at room temperature for 5 min before addition of 20 μl regular C6 medium at the end of incubation. Transfection of C6 cells with the final ODN mixture in EC buffer was allowed to proceed for 6 h before cobalt pretreatment. To collect proteins for Western analysis, transfection of HIF-1α antisense or scrambled ODNs was performed in six-well culture plates as described above, except that 2.4 μg ODNs were diluted in 120 μl buffer EC with 12.8 μl Enhancer for each well. Diluted Effectene reagent (400 μl; 40 μl Effectene stock solution with 360 μl buffer EC) was added into each well; this was followed by addition of 400 μl regular culture medium and an incubation for 6 h to transfect ODNs into C6 glioma cells. At the end of transfection, cells were treated with 400 μM cobalt chloride for 2 h and proteins collected for Western analysis.
Western blotting
Western blotting for identification of the HIF-1α protein was performed as described previously (Semenza et al., 1994; Yin et al., 2000), using a primary polyclonal rabbit anti-HIF-1α antibody (1 : 600, Novus Biologicals) followed by incubation for with a secondary horseradish peroxidase-conjugated donkey anti-rabbit IgG antibody (1 : 5000, Amersham Biosciences Corp., Piscataway, NJ, U.S.A.). A mouse monoclonal anti-actin antibody was purchased from CHEMICON International, Inc. (Temecula, CA, U.S.A.) and used at 1 : 5000 dilution. Secondary anti-mouse IgG antibody linked to alkaline phosphatase was used at 1 : 7000 dilution (Sigma). Detection of immunoreactive components of HIF-1α on the blot was performed using ECL Plus Western blotting Detection Reagents from Amersham Biosciences Corp. The actin proteins on the blot were detected with BCIP and NBT from Sigma according to the manufacturers' protocols.
Statistical analysis
Results are expressed as means ±s.d. Multiple groups were analyzed by one-way analysis of variance (ANOVA) followed by a post-hoc Student–Newman–Keuls test. Statistical analysis between two groups was performed using Student's unpaired t-test between two experimental groups. A P-value less than 0.05 was considered significant.
Results
Induction of HIF-1 and protective effect against BCNU by cobalt preconditioning
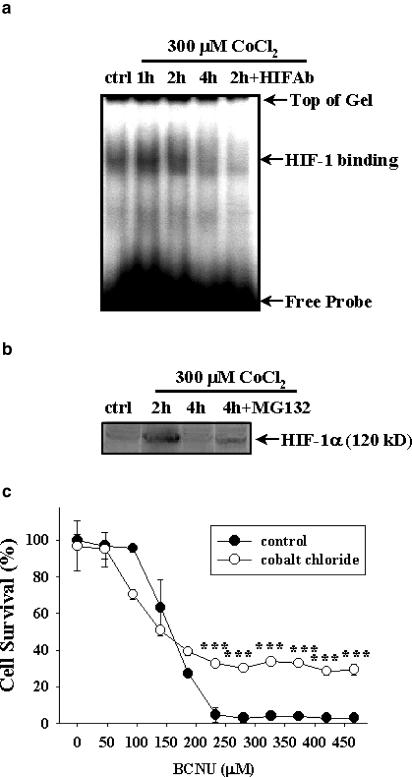
We have previously shown that exposure of C6 glioma cells to 1% oxygen led to an increase in nuclear HIF-1-binding activity (Yin et al., 2000). In the present study, we applied cobalt ions to induce chemical hypoxia (Semenza et al., 1994). At 300 μM, cobalt chloride increased nuclear HIF-1 binding to the oligonucleotide probe with the HRE consensus sequence in the EPO gene based on EMSA (Figure 1a). The HIF-1-binding activity increased at 1 and 2 h following cobalt chloride exposure, but declined thereafter. Co-incubation of nuclear extract with the HIF-1α antibody appeared to interfere with cobalt-induced binding activity, thereby confirming the specific HIF-1 binding (Figure 1a). The same antibody was also used in Western analysis to demonstrate the cobalt-induced HIF-1α accumulation. Consistent with EMSA results, Western blot showed an increase in HIF-1α protein content at 2 h, but not 4 h after cobalt chloride treatment (Figure1b and Yin et al., 2000). MG-132, a proteasome inhibitor known to inhibit ubiquitin-mediated protein degradation (Salceda & Caro, 1997), stabilized the HIF-1α protein induced by cobalt chloride treatment, allowing the detection of HIF-1α protein at 4 h (Figure 1b).
Figure 1.
CoCl2 induction of HIF-1 activation and chemoresistance against BCNU. (a) EMSA showing HIF-1-binding activity in C6 glioma cells treated with 300 μM CoCl2 for 1, 2, or 4 h. 2h+HIFAb: nuclear extracts from cells exposed to CoCl2 for 2 h were co-incubated with HIF-1α antibody for EMSA. ctrl: control without CoCl2 exposure. (b) Western blot showing the induction of the 120 kDa HIF-1α protein following CoCl2 treatment (300 μM, 2 or 4 h). 4h+MG-132: stabilization of HIF-1α by co-treatment of CoCl2 (300 μM) with proteasomal inhibitor MG-132 (20 μM) for 4 h. (c) Cell survival as determined by the MTT assay. C6 glioma cells were pretreated with or without 300 μM CoCl2 for 2 h prior to exposure to BCNU at indicated concentrations for additional 12 h. ***P<0.001 compared to cells treated with the same concentrations of BCNU but without prior exposure to CoCl2. Representative data from three independent experiments of similar results are shown for each panel.
BCNU is a chloroethylnitrosourea that has been the mainstay in adjunct chemotherapy of GBM. Coupled with surgical resection and radiotherapy, BCNU chemotherapy has not been able to provide satisfactory clinical outcome, in part due to acquired chemoresistance. We hypothesized that cobalt chloride pretreatment that mimics a hypoxic microenvironment may lead to activation of HIF-1 binding, thereby compromising the tumoricidal effect of BCNU. Results demonstrated that pre-incubation with cobalt chloride substantially neutralized cytotoxicity of BCNU at concentrations above 200 μM, leading to an increase in cell survival (Figure 1c).
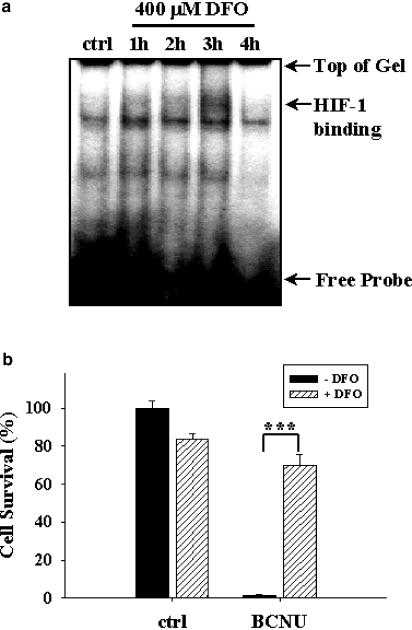
To strengthen the contention that HIF-1 activation may in part confer chemoresistance against BCNU, we test the effect of an iron chelator DFO (Wang & Semenza, 1993a, 1993b) that is also known to activate HIF-1. Results from EMSA indicated that HIF-1 activation began at 1 h following DFO exposure, reached the maximal level at 3 h, and returned to basal level by 4 h (Figure 2a). In consistence with our hypothesis, DFO pretreatment also substantially increased chemoresistance of C6 glioma cells against 470 μM (100 μg ml−1) BCNU (Figure 2b). Results shown in Figures 1 and 2 together demonstrate that preconditioning of rat C6 glioma cells with cobalt chloride and DFO elicits HIF-1 activation and confers chemoresistance against BCNU, thereby providing correlative evidence linking activation of HIF-1 and chemoresistance against BCNU tumoricidal effect.
Figure 2.
DFO effects on HIF-1 activation and chemoresistance against BCNU. (a) EMSA showing HIF-1 activation after pretreatment with 400 μM DFO for indicated times. (b) Cell survival with or without 400 μM DFO pretreatment for 3 h followed by exposure to 470 μM (100 μg ml−1) BCNU for additional 12 h. ***P<0.001. Representative data from three independent experiments of similar results are shown.
Preconditioning-mediated protection against carbamoylating, but not alkylating agents
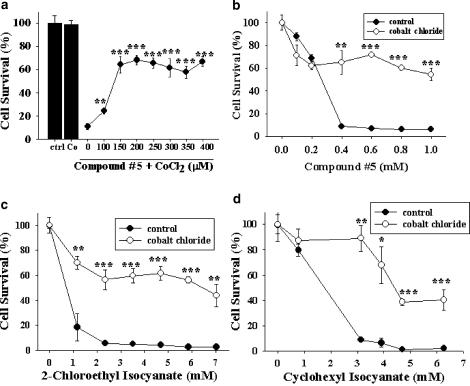
Chloroethylnitrosoureas including BCNU kill tumor cells by alkylating and carbamoylating action (Wheeler et al., 1974). We therefore tested which tumoricidal action of BCNU is affected by this cobalt pretreatment. Previously, by virtue of a panel of compounds each carrying differential alkylating or carbamoylating cytotoxicity, we have been able to demonstrate that iNOS expression selectively neutralized the carbamoylating, but not alkylating, cytotoxicity of BCNU and other chloroethylnitrosoureas (Yin et al., 2001). These chemicals include compound #5 (a selective carbamoylating agent), compound #1, and temozolomide (both are selective alkylating agents), 2-chloroethyl isocyanate, and cyclohexyl isocyanate (the respective carbamoylating metabolite of BCNU and CCNU) (Baril et al., 1975). In the present study, pretreatment with cobalt chloride dose-dependently protected C6 glioma cells against compound #5, a selective carbamoylating agent (Figure 3a). This protection was effective within a wide range of compound #5 concentrations (Figure 3b). In addition, cobalt chloride pretreatment also conferred chemoresistance to C6 glioma cells against 2-chloroethyl isocyanate (Figure 3c) and cyclohexyl isocyanate (Figure 3d), the respective carbamoylating metabolites of BCNU and CCNU. In addition to cobalt preconditioning, pretreatment of C6 glioma cells with 400 μM DFO for 3 h also resulted in remarkable protection against carbamoylating cytotoxicity caused by 0.6 mM of compound #5 (data not shown).
Figure 3.
CoCl2 effects on carbamoylating cytotoxicity. (a) Cells were pre-incubated with CoCl2 at indicated concentrations for 2 h before treatment with compound #5 (0.6 mM) for additional 24 h. ctrl: cells without any treatment. Co: cells pretreated with 300 μM CoCl2 for 2 h only. **P<0.01 and ***P<0.001 compared to the cells exposed to the same concentrations of compound #5 without pretreatment with CoCl2. (b–d) Cells were pretreated without or with 300 μM CoCl2 for 2 h prior to exposure to carbamoylating agents compound #5 (b), 2-chloroethyl isocyanate (c), or cyclohexyl isocyanate (d) at indicated concentrations for additional 24 h. *P<0.05, **P<0.01, and ***P<0.001 compared to cells treated with the same concentrations of carbamoylating agents, but without CoCl2 pretreatment. Representative data from three independent experiments of similar results are shown.
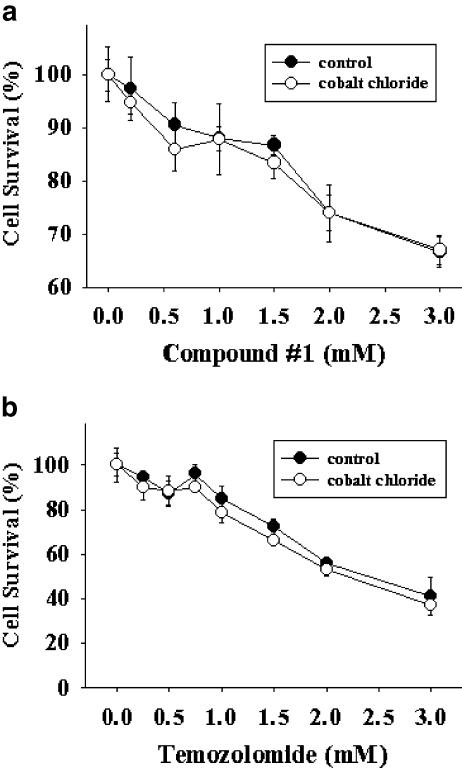
In addition to carbamoylation, BCNU also carries alkylating action that is important for its tumoricidal toxicity. We therefore examined whether alkylating action of BCNU may also be modulated by similar cobalt chloride preconditioning. Compound #1 is a specific reagent with chloroethylating, but not carbamoylating, action (Penketh et al., 2000). Cobalt chloride was ineffective in enhancing chemoresistance against compound #1 (Figure 4a). Temozolomide, recently approved as an adjunct chemotherapeutic agent in GBM, is another selective alkylating (methylating) agent (Denny et al., 1994). Cobalt chloride also failed to induce chemoresistance against temozolomide (Figure 4b). Results in Figures 3 and 4 together reveal that the cobalt-dependent chemoresistance is restricted to the carbamoylating action only; neither chloroethylating nor methylating potential is affected by cobalt chloride preconditioning.
Figure 4.
CoCl2 effect on alkylating cytotoxicity. Cells were pretreated with or without 300 μM CoCl2 for 2 h prior to treatment with compound #1 (a) or temozolomide (b) for an additional 24 h. Note that no statistical significance was observed between cells treated with the same concentrations of alkylating agents with or without CoCl2 exposure. Representative data from three independent experiments of similar results are shown.
Effects of cadmium ions, HIF-1 ODN decoy, and HIF-1α antisense ODN on cobalt-mediated chemoresistance against carbamoylating agents
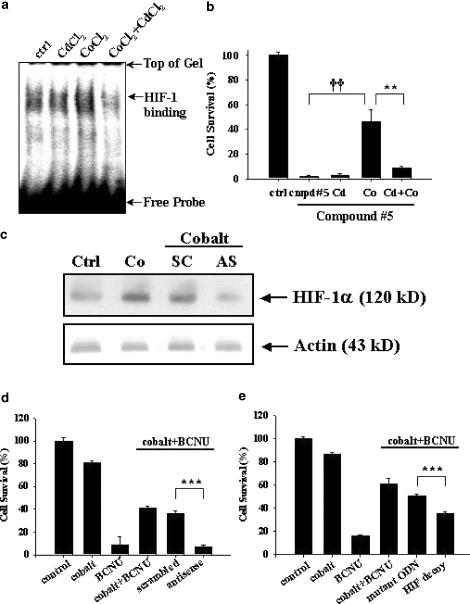
Although cobalt preconditioning induced HIF-1-binding activity as well as carbamoylating chemoresistance in C6 glioma cells, these two events may only be correlative. We therefore further explored the causal relationship of HIF-1 activation in cobalt-induced chemoresistance against carbamoylating agents. Cadmium ion has been shown to abolish HIF-1-binding activity induced by cobalt chloride through its enhancement of proteasome-dependent HIF-1α degradation (Chun et al., 2000). Co-treatment of C6 glioma cells with cobalt chloride and cadmium chloride suppressed cobalt-induced HIF-1 activation (Figure 5a). Cadmium chloride correspondingly neutralized cobalt-induced chemoresistance against compound #5 (Figure 5b).
Figure 5.
Effects of HIF-1 inhibition on cobalt-induced carbamoylating chemoresistance. (a) EMSA showing downregulation of CoCl2-induced HIF-1-binding activity by CdCl2. Cells were pre-exposed to CoCl2 (300 μM) or CdCl2 (50 μM) or both for 2 h before nuclear proteins isolated for EMSA. (b) Cells subjected to pretreatment with CoCl2 or CdCl2 or both for 2 h were exposed to 0.8 mM compound #5 for additional 24 h. **P<0.01 and ††P<0.01. (c) Cells were transfected with 2.4 μg each of the HIF-1α antisense (AS) or scrambled (SC) ODNs, as described in detail in Methods. This was followed by cobalt exposure (400 μM for 2 h) prior to protein collection for Western blotting. Ctrl, cells without any treatments. Co, cells treated with 400 μM CoCl2 for 2 h. The lower part of the same blot was probed with a monoclonal antibody against actin to serve as internal standards. (d) Cells were separately transfected with HIF-1α antisense ODN or scrambled ODN in quadruplicates (0.08 μg per well each in a 96-well plate) for 6 h using Effectene reagent as described in Methods prior to cobalt preconditioning, and subsequently BCNU exposure (470 μM) for an additional 12 h. (e) The experimental conditions were the same as those described in (d), except that HIF-specific decoy ODN or mutant ODN (0.10 μg per well each in a 96-well plate) were transfected. ***P< 0.001. Representative data from three independent experiments of similar results are shown.
Despite its neutralizing effect on HIF-1-binding activity and carbamoylating chemoresistance associated with cobalt preconditioning, cadmium chloride is a pharmacological agent that may exert nonspecific cellular action independent of its inhibitory effect on cobalt-dependent HIF-1α accumulation. To firmly establish the causative role of HIF-1 in cobalt-mediated chemoresistance, two molecular biological approaches were adopted to counteract HIF-1 action as a result of cobalt pretreatment. The first approach was to transfect phosphorothioate antisense ODN against HIF-1α to abolish cobalt-dependent HIF-1α protein accumulation. Results based on Western analysis confirmed a reduction of HIF-1α expression in glioma cells transfected with antisense, but not scrambled, ODN upon cobalt preconditioning (Figure 5c). The same antisense ODN has also been used in cultured cardiomyocytes to inhibit expression of HIF-1 downstream genes (Kakinuma et al., 2001). Results in Figure 5d indicate that, as compared to the scrambled control ODNs, HIF-1α antisense ODN effectively antagonized cobalt-induced chemoresistance against BCNU. We then take advantages of a HIF-specific ODN decoy as a second gene-specific approach to suppress HIF-1 activity (Morishita et al., 1995). This strategy entails a double-stranded ODN decoy containing HIF-specific cognate sequence to, upon delivery into glioma cells, compete for the endogenous HIF-1-binding sites, thus suppressing the preconditioning-induced HIF-1 activation. Similar decoy approach has been proved to successfully suppress hypoxia-mediated induction of HIF-1 downstream genes in a human bladder cancer cell line T24 (Oikawa et al., 2001). Results shown in Figure 5e clearly demonstrate that, as compared to mutant ODNs as negative controls, application of HIF-1 ODN decoy prior to cobalt exposure selectively, albeit partially, abolished cobalt-dependent protection against BCNU. Together, results in Figure 5 support the contention that HIF-1 activation may play a causal role, at least in part, in the observed carbamoylating chemoresistance associated with cobalt chloride preconditioning.
Discussion
The major finding of the present report is that preconditioning with reagents known to induce HIF-1 may render rat C6 glioma cells resistant to cytotoxicity of carbamoylating, but not alkylating, chemotherapeutic reagents. Furthermore, results derived from cadmium chloride, HIF-1α antisense ODN, and HIF-specific ODN decoy together suggest a causative role of HIF-1 involved in these cobalt effects against carbamoylating cytotoxicity.
Chloroethylnitrosoureas, especially BCNU, have been a mainstay in the adjunct chemotherapy of GBM following surgical resection and radiation. Unfortunately, the clinical outcomes with the combination of these three modalities of treatment remain far from satisfactory, due in part to acquired chemoresistance. The underlying mechanisms of chemoresistance against chloroethylnitrosoureas such as BCNU are not fully understood, but may depend on their tumoricidal actions. BCNU kills cells via multiple mechanisms including alkylation and carbamoylation. In this study, we demonstrate that HIF-1 activation often observed in malignant brain tumors may potentially alter glioma resistance to carbamoylating chemotherapeutic reagents including BCNU, at least in vitro. The half-maximal lethal dosage of BCNU effective for killing tumor cells varies from 2 to 60 μg ml−1, depending on the cell types and the duration of BCNU treatment, in most cases 3–6 days (Carmichael et al., 1988; Heim et al., 2000), whereas clinically relevant doses of BCNU is below 100 μM. In this report, preconditioning with cobalt chloride conferred chemoresistance to C6 glioma cells against BCNU toxicity at concentrations from 235 μM (50 μg ml−1) to 470 μM (100 μg ml−1), but not at lower concentrations of BCNU where it failed to cause appreciable cytotoxicity during a 12-h exposure. Thus, the BCNU dosages we selected appear to be higher than those used in vivo so that significant cytotoxicity can be observed. In this regard, we are cautious in extending these in vitro results into in vivo or even clinical settings. Interpretation of our findings presented in this report also requires additional caution because it has been recognized that in vivo pharmacological efficacy of BCNU is supported primarily by its alkylating effect, rather than carbamoylating action. Therefore, the clinical relevance of the present results could potentially be limited. Nevertheless, despite well-established alkylating tumoricidal action of BCNU, carbamoylating activity may still modulate or even exert synergistic effects on additional tumoricidal actions of adjunct chemotherapeutics or radiation therapy. Thus, the synergism between BCNU and radiation in the generation of DNA single-stranded breaks can be potentiated by the carbamoylating action of BCNU (Ali-Osman et al., 1990). Carbamoylation of glutathione reductase by BCNU was also associated with functional inhibition of multi-drug resistance protein (Vanhoefer et al., 1997), which is another candidate gene for acquired chemoresistance. Compromised carbamoylating action may eliminate synergistic tumoricidal effects of BCNU or radiation and restore the multi-drug resistance protein function, contributing to the development of radioresistance or chemoresistance. Furthermore, the present study revealed an in vitro effect of HIF-1 induction in neutralizing the immediate cytotoxic carbamoylation of chloroethylnitrosoureas, but not their alkylating effect. This finding is in itself novel, despite potentially limited clinical implication.
Hypoxia has been shown to increase chemoresistance against BCNU in human glioma cell lines (Liang, 1996). The expression of the drug resistance genes was, however, unchanged under this condition, suggesting alternative mechanisms that may exist in hypoxia-induced chemoresistance. In this study, we provide experimental evidence supporting the contention that HIF-1 induction by cobalt chloride or DFO pretreatment, which mimics a hypoxic microenvironment, may contribute to acquired chemoresistance against BCNU through inhibition of its carbamoylating cytotoxicity. The molecular mechanisms underlying HIF-1-mediated chemoresistance against carbamoylating cytotoxicity of chloroethylnitrosoureas remain unclear, but may involve the transcriptional activation of genes downstream of HIF-1. Genes that are upregulated by microenvironmental hypoxia through activation of HIF include glucose transporters, glycolytic enzymes, and angiogenic growth factors such as VEGF and EPO (Shweiki et al., 1992; Semenza et al., 1994; Ebert et al., 1995). In hypoxic human glioblastomas, at least 10 novel transcripts have been identified that were induced to a greater extent than VEGF in response to hypoxia, based on the results of serial analysis of gene expression (Lal et al., 2001). These genes also responded to hypoxia in breast and colon cancer cells and were activated by HIF-1. Induced genes included stanniocalcin 1 (a calcium homeostasis protein), hexabrachion (an extracellular matrix glycoprotein), and an angiopoietin-related gene. The cytoprotective effects of HIF with resultant expression of its target genes have been documented in a number of experimental paradigms both in vivo and in vitro. For example, preconditioning hypoxia causing sublethal stresses induces tolerance against focal permanent ischemia in adult mice, in part due to the induction of VEGF and EPO (Bernaudin et al., 2002). Furthermore, transactivation of several HIF-1-responsive genes including EPO, glycolytic enzymes, and glucose transporter contributes to HIF-1-dependent protection against glutathione depletion in primary cortical neurons (Zaman et al., 1999). Carbamoylating agents are irreversible inhibitors of the glutathione reductase, which may lead to accumulation of the oxidized form of glutathione. Thus, carbamoylating action constitutes a chemical-induced oxidative stress that may, as shown in the present study, be neutralized in a hypoxic microenvironment with resultant HIF-1 activation. Despite numerous studies suggesting a pivotal role of HIF-1 in antagonizing oxidative stress, fewer reports have offered causative evidence to unambiguously pinpoint the crucial roles of HIF-1. In this report, we provide direct experimental evidence supporting an important role of HIF-1 in the observed preconditioning effects. Whether overexpression of HIF-1 alone is sufficient to mediate carbamoylating chemoresistance remains to be explored.
Overall, HIF-1 activation may represent the first step of hypoxia-induced expression of a panel of genes that together exerts protection against carbamoylating potential of BCNU chemotherapy. Downregulation of HIF-1 likely represents a feasible strategy to develop more effective chemotherapeutic regimens to reduce acquired chemoresistance against carbamoylating cytotoxicity of BCNU, besides disruption of angiogenic potential of HIF-1.
Acknowledgments
We thank Dr Alan C. Sartorelli (Department of Pharmacology, Yale University School of Medicine) for the generous supply of compounds #1 and #5 and Dr W. Robert Bishop (Schering-Plough Corporation) of temozolomide. We also thank Mr Wei-Han Hsu for excellent technical assistance. This work was supported by the National Science Council in Taiwan (NSC90-2314-B-320-012 to Ding-I Yang) and by Washington University School of Medicine-Pharmacia Biomedical Research Support Program and Lowell B. Miller Memorial Research Grant (National Brain Tumor Foundation) to Chung Y. Hsu.
Abbreviations
- ARNT
aryl hydrocarbon receptor nuclear translocator
- BCNU, 1
3-bis(2-chloroethyl)-1-nitrosourea
- CCNU
1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea
- DFO
desferrioxamine
- EMSA
electrophoretic mobility shift assay
- EPO
erythropoietin
- GBM
glioblastoma multiforme
- HIF-1
hypoxia inducible factor-1
- iNOS
inducible nitric oxide synthase
- ODN
oligodeoxynucleotide
- VEGF
vascular endothelial growth factor
References
- ALI-OSMAN F., SRIVENUGOPAL K., BERGER M.S., STEIN D.E. DNA interstrand crosslinking and strand break repair in human glioma cell lines of varying [1,3-bis(2-chloroethyl)-1-nitrosourea] resistance. Anticancer Res. 1990;10:677–682. [PubMed] [Google Scholar]
- BARIL B.B., BARIL E.F., LASZLO J., WHEELER G.P. Inhibition of rat liver DNA polymerase by nitrosoureas and isocyanates. Cancer Res. 1975;35:1–5. [PubMed] [Google Scholar]
- BERNAUDIN M., NEDELEC A.S., DIVOUX D., MACKENZIE E.T., PETIT E., SCHUMANN-BARD P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- CARMICHAEL J., MITCHELL J.B., DEGRAFF W.G., GAMSON J., GAZDAR A.F., JOHNSON B.E., GLATSTEIN E., MINNA J.D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer. 1988;57:540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG C.H., HORTON J., SCHOENFELD D., SALAZER O., PEREZ-TAMAYO R., KRAMER S., WEINSTEIN A., NELSON J.S., TSUKADA Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997–1007. doi: 10.1002/1097-0142(19830915)52:6<997::aid-cncr2820520612>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- CHUN Y.S., CHOI E., KIM G.T., CHOI H., KIM C.H., LEE M.J., KIM M.S., PARK J.W. Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha. Eur. J. Biochem. 2000;267:4198–4204. doi: 10.1046/j.1432-1327.2000.01453.x. [DOI] [PubMed] [Google Scholar]
- DACHS G.U., CHAPLIN D.J. Microenvironmental control of gene expression: implications for tumor angiogenesis, progression, and metastasis. Semin. Radiat. Oncol. 1998;8:208–216. doi: 10.1016/s1053-4296(98)80046-5. [DOI] [PubMed] [Google Scholar]
- DENNY B.J., WHEELHOUSE R.T., STEVENS M.F., TSANG L.L., SLACK J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry. 1994;33:9045–9051. doi: 10.1021/bi00197a003. [DOI] [PubMed] [Google Scholar]
- EBERT B.L., FIRTH J.D., RATCLIFFE P.J. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct cis-acting sequences. J. Biol. Chem. 1995;270:29083–29089. doi: 10.1074/jbc.270.49.29083. [DOI] [PubMed] [Google Scholar]
- EPSTEIN A.C., GLEADLE J.M., MCNEILL L.A., HEWITSON K.S., O'ROURKE J., MOLE D.R., MUKHERJI M., METZEN E., WILSON M.I., DHANDA A., TIAN Y.M., MASSON N., HAMILTON D.L., JAAKKOLA P., BARSTEAD R., HODGKIN J., MAXWELL P.H., PUGH C.W., SCHOFIELD C.J., RATCLIFFE P.J. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- FINE H.A., DEAR K.B., LOEFFLER J.S., BLACK P.M., CANELLOS G.P. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- GREEN S.B., BYAR D.P., WALKER M.D., PISTENMAA D.A., ALEXANDER E.J., BATZDORF U., BROOKS W.H., HUNT W.E., MEALEY J.J., ODOM G.L., PAOLETTI P., RANSOHOFF J.D., ROBERTSON J.T., SELKER R.G., SHAPIRO W.R., SMITH K.R.J., WILSON C.B., STRIKE T.A. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Caner Treat. Rep. 1983;67:121–132. [PubMed] [Google Scholar]
- HEIM M.M., EBERHARDT W., SEEBER S., MULLER M.R. Differential modulation of chemosensitivity to alkylating agents and platinum compounds by DNA repair modulators in human lung cancer cell lines. J. Cancer Res. Clin. Oncol. 2000;126:198–204. doi: 10.1007/s004320050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG B.H., SEMENZA G.L., BAUER C., MARTI H.H. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am. J. Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- KAKINUMA Y., MIYAUCHI T., YUKI K., MURAKOSHI N., GOTO K., YAMAGUCHI I. Novel molecular mechanism of increased myocardial endothelin-1 experssion in the failing heart involving the transcriptional factor hypoxia-inducible factor-1α induced for impaired myocardial energy metabolism. Circulation. 2001;103:2387–2394. doi: 10.1161/01.cir.103.19.2387. [DOI] [PubMed] [Google Scholar]
- KIMURA H., WEISZ A., KURASHIMA Y., HASHIMOTO K., OGURA T., D'ACQUISTO F., ADDEO R., MAKUUCHI M., ESUMI H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- KLEIHUES P., SOYLEMEZOGLU F., SCHAUBLE B., SCHEITHAUER B.W., BURGER P.C. Histopathology, classification and grading of gliomas. Glia. 1995;15:211–221. doi: 10.1002/glia.440150303. [DOI] [PubMed] [Google Scholar]
- LAL A., PETERS H., ST CROIX B., HAROON Z.A., DEWHIRST M.W., STRAUSBERG R.L., KAANDERS J.H., VAN DER KOGEL A.J., RIGGINS G.J. Transcriptional response to hypoxia in human tumors. J. Natl. Cancer Inst. 2001;93:1337–1343. doi: 10.1093/jnci/93.17.1337. [DOI] [PubMed] [Google Scholar]
- LIANG B.C. Effects of hypoxia on drug resistance phenotype and genotype in human glioma cell lines. J. Neurooncol. 1996;29:149–155. doi: 10.1007/BF00182138. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., GIBBONS G.H., HORIUCHI M., ELLISON K.E., NAKAJIMA M., ZHANG L., KANEDA Y., OGIHARA T., DZAU V.J. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUTT C.L., NOBLE M., CHAMBERS A.F., CAIRNCROSS J.G. Differential expression of drug resistance genes and chemosensitivity in glial cell lineages correlate with differential response of oligodendrogliomas and astrocytomas to chemotherapy. Cancer Res. 2000;60:4812–4818. [PubMed] [Google Scholar]
- OIKAWA M., ABE M., KUROSAWA H., HIDA W., SHIRATO K., SATO Y. Hypoxia induces transcription factor ETS-1 via the activity of hypoxia-inducible factor. Biochem. Biophys. Res. Commun. 2001;289:39–43. doi: 10.1006/bbrc.2001.5927. [DOI] [PubMed] [Google Scholar]
- PAOLETTI P. Therapeutic strategy for central nervous system tumors: present status, criticism and potential. J. Neurosurg. Sci. 1984;28:51–60. [PubMed] [Google Scholar]
- PENKETH P.G., SHYAM K., SARTORELLI A.C. Comparison of DNA lesions produced by tumor-inhibitory 1,2-bis(sulfonyl)hydrazines and chloroethylnitrosoureas. Biochem. Pharmacol. 2000;59:283–291. doi: 10.1016/s0006-2952(99)00328-7. [DOI] [PubMed] [Google Scholar]
- RAJKUMAR S.V., BUCKNER J.C., SCHOMBERG P.J., PITOT H.C.T., INGLE J.N., CASCINO T.L. Phase I evaluation of pre-irradiation chemotherapy with carmustine and cisplatin and accelerated radiation therapy in patients with high-grade gliomas. Neurosurgery. 1999;44:67–73. doi: 10.1097/00006123-199901000-00036. [DOI] [PubMed] [Google Scholar]
- ROLHION C., PENAULT-LLORCA F., KEMENY J.L., KWIATKOWSKI F., LEMAIRE J.J., CHOLLET P., FINAT-DUCLOS F., VERRELLE P. O(6)-methylguanine-DNA methyltransferase gene (MGMT) expression in human glioblastomas in relation to patient characteristics and p53 accumulation. Int. J. Cancer. 1999;84:416–420. doi: 10.1002/(sici)1097-0215(19990820)84:4<416::aid-ijc15>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- SALCEDA S., CARO J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem. Pharmacol. 2000;59:47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L., ROTH P.H., FANG H.M., WANG G.L. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- SEMENZA G.L., WANG G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHWEIKI D., ITIN A., SOFFER D., KESHET E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- SHYAM K., PENKETH P.G., LOOMIS R.H., ROSE W.C., SARTORELLI A.C. Antitumor 2-(aminocarbonyl)-1,2-bis(methylsulfonyl)-1-(2-chloroethyl)-hydrazines. J. Med. Chem. 1996;39:796–801. doi: 10.1021/jm9505021. [DOI] [PubMed] [Google Scholar]
- VANHOEFER U., YIN M.B., HARSTRICK A., SEEBER S., RUSTUM Y.M. Carbamoylation of glutathione reductase by N,N-bis(2-chloroethyl)-N-nitrosourea associated with inhibition of multidrug resistance protein (MRP) function. Biochem. Pharmacol. 1997;53:801–809. doi: 10.1016/s0006-2952(97)00010-5. [DOI] [PubMed] [Google Scholar]
- WALKER M.D., GREEN S.B., BYAR D.P., ALEXANDER E.J., BATZDORF U., BROOKS W.H., HUNT W.E., MACCARTY C.S., MAHALEY M.S.J., MEALEY J.J., OWENS G., RANSOHOFF J.D., ROBERTSON J.T., SHAPIRO W.R., SMITH K.R.J., WILSON C.B., STRIKE T.A. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N. Engl. J. Med. 1980;303:1323–1329. doi: 10.1056/NEJM198012043032303. [DOI] [PubMed] [Google Scholar]
- WANG G.L., JIANG B.H., RUE E.A., SEMENZA G.L. Hypoxia-inducible factor 1 is a basic-helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG G.L., SEMENZA G.L. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993a;82:3610–3615. [PubMed] [Google Scholar]
- WANG G.L., SEMENZA G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHEELER G.P., BOWDON B.J., GRIMSLEY J.A., LLOYD H.H. Interrelationships of some chemical, physicochemical, and biological activities of several 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1974;34:194–200. [PubMed] [Google Scholar]
- XU J., YEH C.-H., CHEN S., HE L., SENSE S.L., CANZONIERO L.M.T., CHOI D.W., HSU C.Y. Involvement of de novo ceramide biosynthesis in tumor necrosis factor-α/cycloheximide-induced cerebral endothelial cell death. J. Biol. Chem. 1998;273:16521–16526. doi: 10.1074/jbc.273.26.16521. [DOI] [PubMed] [Google Scholar]
- YIN J.H., YANG D.I, CHOU H., THOMPSON E.M., XU J., HSU C.Y. Inducible nitric oxide synthase neutralizes carbamoylating potential of 1,3-bis(2-chloroethyl)-1-nitrosourea in C6 glioma cells. J. Pharmacol. Exp. Ther. 2001;297:308–315. [PubMed] [Google Scholar]
- YIN J.H., YANG D.I, KU G., HSU C.Y. iNOS expression inhibits hypoxia-inducible factor-1 activity. Biochem. Biophys. Res. Commun. 2000;279:30–34. doi: 10.1006/bbrc.2000.3896. [DOI] [PubMed] [Google Scholar]
- ZAMAN K., RYU H., HALL D., O'DONOVAN K., LIN K.I., MILLER M.P., MARQUIS J.C., BARABAN J.M., SEMENZA G.L., RATAN R.R. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J. Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]