Abstract
The α1-adrenoceptor subtypes involved in responses to exogenous and neurally released noradrenaline in rat femoral resistance arteries were characterised using a small vessel myograph, with antagonists prazosin (nonsubtype selective), 5-methyl-urapidil (α1A-selective), BMY 7378 (α1D-selective) and the alkylating agent chloroethylclonidine (preferential for α1B-).
Prazosin and 5-methyl-urapidil produced rightward shifts of the exogenous noradrenaline concentration – response curve (CRC) with pA2 values of 9.2 and 9.1 respectively, in agreement with the presence of α1A-adrenoceptors. BMY 7378 (1 μM) shifted the noradrenaline CRC with an apparent pKB of 6.7, in agreement with the presence of α1A-, but not α1D-, adrenoceptors. Chloroethylclonidine at 1 μM had no effect and at 10 μM produced only a small reduction (c. 20%) in the maximum response to noradrenaline, indicating little, if any, contribution from α1B-adrenoceptors.
Responses of the rat femoral resistance arteries to electrical field stimulation (EFS) at 5–30 Hz for 10 s and 0.05 ms pulse width were principally due to α1-adrenoceptor stimulation. Prazosin and 5-methyl-urapidil inhibited EFS-mediated responses with pIC50s of 9.3 and 8.2, respectively, consistent with the α1A-adrenoceptor being the predominant subtype. Responses to EFS at 10–30 Hz were relatively insensitive to BMY 7378 (pIC50, 6.5–6.7), while responses to 5 Hz were inhibited with a significantly higher pIC50 of 8.02, suggesting the contribution of α1D-adrenoceptors. Chloroethylclonidine had no effect on responses to EFS, ruling out the contribution of an α1B-subtype.
In the presence of cocaine, the predominant subtype involved in responses to EFS was the α1A-adrenoceptor, with a contribution from α1D-adrenoceptors at low frequency, as seen in the absence of cocaine. However, there was also a significant increase in the sensitivity to BMY 7378 at higher frequencies, suggesting that a further small α1D-adrenoceptor component may be uncovered in the presence of cocaine.
The present study has shown a predominant role of the α1A-adrenoceptor in contractions due to exogenous noradrenaline and to neurally released noradrenaline in rat femoral resistance arteries. α1D-Adrenoceptors are not involved in responses to exogenous noradrenaline but appear to be activated by neurally released noradrenaline at a low frequency of stimulation and at higher frequencies in the presence of neuronal-uptake blockade.
Keywords: α1-Adrenoceptors, α1-adrenoceptor subtypes, α1A-adrenoceptor, α1B-adrenoceptor, α1D-adrenoceptor, noradrenaline, electrical field stimulation, rat femoral artery, resistance artery
Introduction
α1-Adrenoceptors located in vascular smooth muscle play an important role in the regulation of peripheral resistance and systemic arterial blood pressure. It is now well accepted that there are three functional α1-adrenoceptor subtypes, α1A-, α1B- and α1D-, corresponding to the three cloned α1-adrenoceptors, designated α1a-, α1b- and α1d (Hieble et al., 1995; Bylund et al., 1998). These three subtypes display high affinity for prazosin (pKB>9) in functional and radioligand binding experiments and can be designated as α1H-adrenoceptor subtypes. α1-Adrenoceptors with low affinity for prazosin (pA2<9) have also been identified in functional studies and classified as the α1L-subtype (Flavahan & Vanhoutte, 1986; Muramatsu et al., 1990a, 1990b). The α1L-adrenoceptor subtype has not been cloned and evidence suggests that it is not a separate gene product but a low-affinity state of the α1A-adrenoceptor (Ford et al., 1997).
The relative importance of the different subtypes in regulation of peripheral resistance and systemic arterial blood pressure is not clear, as the contribution of different subtypes to vasoconstriction varies with species and vascular bed (Vargas & Gorman, 1995; Guimarães & Moura, 2001). A further factor that may influence the subtypes involved in vasoconstriction physiologically is the manner of receptor activation, that is, whether by circulating catecholamines or by neurally released noradrenaline. Honner & Docherty (1999) have shown that responses of rat vas deferens to exogenous noradrenaline involve α1A-adrenoceptors while responses to nerve stimulation involve non-α1A-adrenoceptors, resembling the α1D-adrenoceptor. Yang & Chiba (2001) have recently shown that in canine splenic artery exogenous noradrenaline produces contraction via α1A-adrenoceptors while noradrenaline released by nerve stimulation produced contraction via α1B- and α1D-adrenoceptors. This may suggest that circulating catecholamines produce vasoconstriction via extrajunctional α1A-adrenoceptors while sympathetic nerve stimulation produces vasoconstriction via junctional α1B- and α1D-adrenoceptors in this artery. In contrast, other studies have shown no difference in the receptor subtype involved in responses to exogenous and endogenous, neurally released noradrenaline (Williams & Clarke, 1995).
The aim of the present study was therefore to compare the α1-adrenoceptor subtypes involved in responses to exogenous and endogenous, neurally released, noradrenaline in rat femoral resistance arteries, as the skeletal muscle vascular bed is a major vascular bed with a large contribution to the peripheral vascular resistance. We previously reported that small branches of the rat femoral artery showed a predominance of the α1A-adrenoceptor in responses to exogenous α1-adrenoceptor agonists (Jarajapu et al., 2001b). Preliminary experiments with these branches showed that they did not all respond to electrical field stimulation. We therefore carried out a direct comparison of responses to exogenous and endogenous noradrenaline, using only branches which responded to both exogenous noradrenaline and to electrical field stimulation.
Preliminary accounts of this work have been presented to the British Pharmacological Society (Zacharia et al., 2002; 2003).
Methods
Male Wistar rats (200–250 g, 10–13 weeks) were killed by stunning and exsanguination. Hindlimbs were removed and transported to the lab in physiological saline solution (PSS) under ice-cold conditions. Second - and third-order femoral arteries were dissected out under a microscope (Zeiss) within an hour. The vessel segments were incubated in PSS of composition (mM): NaCl (119), KCl (4.5), NaHCO3 (25), KH2PO4 (1.2), MgSO4·7H2O (1.2), (+) glucose (11) and CaCl2 (2.5) at 37oC and gassed with carbogen.
Arterial segments of 2 mm length (normalised diameter, IC0.9, 224±5, n=129) were mounted in a four-channel wire myograph (Danish Myotech, Aarhus, Denmark) for isometric tension measurement. The vessels were incubated in the PSS for 1 h after mounting. The vessels were then normalised, that is, the resting tension – internal circumference relation was determined for each vessel segment (Mulvany & Halpern, 1977). The resting tension was set to a normal internal circumference of IC0.9, where IC0.9=0.9 IC100 and IC100 is the internal circumference of the vessel under an effective resting transmural pressure (ERTP) of 100 mmHg (13.3 kPa). ERTP was calculated from the Laplace equation (ERTP=wall tension/(internal circumference/2π)). The Myodaq-Myodata software was used for data acquisition. At 30 min after normalisation, the vessels were exposed to 123 mM potassium solution twice followed by 10 μM noradrenaline in the presence of 123 mM potassium solution. The arteries were considered viable if the ERTP produced by 123 mM potassium was greater than 100 mmHg (13.3 kPa). The presence of functional endothelium was checked with 1 μM carbachol after precontracting with 1 μM noradrenaline. All vessels studied produced greater than 60% relaxation. Vessels were allowed to equilibrate for a further 30 min before beginning experimentation.
Functional studies using exogenous noradrenaline
After equilibration, three to four cumulative concentration – response curves (CRCs) to noradrenaline were obtained in each arterial segment (30 min between each CRC). Preliminary experiments showed that repeated CRCs were reproducible and no correction for time-dependent changes was required. The first CRC was taken as control and subsequent curves were obtained after incubating the vessels with different concentrations of antagonists for 30 min. For chloroethylclonidine treatment, arterial segments were incubated with chloroethylclonidine (10 μM) for 30 min followed by washing for 60 min (each wash every 15 min). RS 79948 (0.1 μM, α2-adrenoceptor blocker), propranolol (1 μM, β-adrenoceptor blocker), cocaine (3 μM, neuronal uptake blocker) and corticosterone (3 μM, non-neuronal uptake blocker) were added to the PSS 30 min before each CRC. EDTA (0.023 mM) and ascorbic acid (0.3 mM) were included in the PSS to prevent oxidation of noradrenaline.
Results are expressed as mean±s.e.m. n being the number of vessels. Agonist potency is expressed as the pEC50 (the negative logarithm of the concentration required to produce 50% of the maximum response, Emax). The EC50 and Emax values were calculated using the Graphpad Prism software program that fits CRCs to the four-parameter logistic equation below:
where X is the logarithm of the molar concentration of agonist, Y is the response and P is the Hill slope.
Antagonist affinity was expressed either as pA2 or pKB values. When three different concentrations of the antagonist were used, pA2 values were obtained from the x-intercept of the plot of log (r-1) vs log(B), where r is the ratio of the agonist EC50 in the presence and absence of antagonist and B is the molar concentration of antagonist (Arunlakshana & Schild, 1959). pKB values were used when one concentration of antagonist was used to obtain the affinity. It was calculated from the Schild (1949) equation:
where KB is the equilibrium dissociation constant.
Electrical field stimulation
For electrical field stimulation (EFS), vessels were placed between platinum electrodes and stimulated every 5 min at 20 V and 0.05 ms pulse width applied for 10 s at frequencies of 5–30 Hz using a Harvard stimulator. Three to four frequency – response curves were obtained in each arterial segment (15 min between each frequency – response curve). After each frequency – response curve the vessels were thoroughly washed with PSS. Preliminary experiments showed that repeated frequency – response curves were reproducible over a period of 2 h and no correction for time – dependent changes was required. The first frequency – response curve was taken as control and subsequent curves were obtained after incubating the vessels with different concentrations of antagonists for 15 min. In experiments with cocaine, vessels were incubated with cocaine (3 μM) for the 15-min period before the frequency – response curve. The effects of cocaine were reproducible over five stimulation periods in the absence of antagonist. Antagonist potencies were expressed as mean pIC50 or pIC30 values (the negative logarithm of the concentration of antagonist producing 50 or 30% inhibition respectively of the prazosin-sensitive component of the response to field stimulation).
Drugs
The following drugs were used: (−)- noradrenaline (arterenol) bitartrate, propranolol hydrochloride, corticosterone acetate, guanethidine mono sulphate, suramin Na salt and prazosin hydrochloride (Sigma, Dorset, U.K.); cocaine HCl (Thornton and Ross, U.K.); (8aR, 12aS, 13aS)-5,8,8a,9,10,11,12,12a,13a-decahydro-3-methoxy-12-(ethylsulphonyl)-6H-isoquino[2,1-g][1,6]- naphthyridine (RS 79948) UK 14304 (brimonidine) and tetrodotoxin (Tocris, Bristol, U.K.); α,β methylene ATP, 5-methyl-urapidil, chloroethylclonidine 2HCl and (8-[2-[4-(2-methoxyphenyl)-1 piperazinyl]ethyl]-8-azaspiro[4.5]decane-7,9-dione (BMY 7378) (RBI, Natick, U.S.A.).
UK 14304 was dissolved in 10% dimethyl sulphoxide and corticosterone in 20% absolute ethanol. Stock solutions of all other drugs were prepared in distilled water. All drug dilutions were made using PSS.
Statistics
Significances of differences were obtained by using paired or unpaired Student's t-test as appropriate to compare two groups and repeated measures one-way analysis of variance (ANOVA) followed by Dunnett's post test for multiple group comparisons.
Results
Vasoconstrictor responses to exogenous noradrenaline
Noradrenaline produced concentration-dependent contractile responses that were unaffected by the α2-adrenoceptor antagonist RS 79948 (0.1 μM) (pEC50s: control, 6.11±0.02 ; RS 79948, 6.05±0.02, n=7, P>0.05). UK 14304 (up to 30 μM) had no contractile effect.
Prazosin (1–100 nM) produced a parallel rightward shift of the noradrenaline CRC (Figure 1a). Maximum responses were unaffected by 1 nM but were significantly reduced by 10 and 100 nM prazosin (Emax %, n=9: control, 100±2; 1 nM prazosin, 95±2, P>0.05; 10 nM prazosin, 89±2, P<0.01; 100 nM prazosin, 82±3, P<0.01). A Schild plot gave a pA2 value of 9.2 with a slope of 1.09 (95% CL: 0.92–1.26), not significantly different from unity.
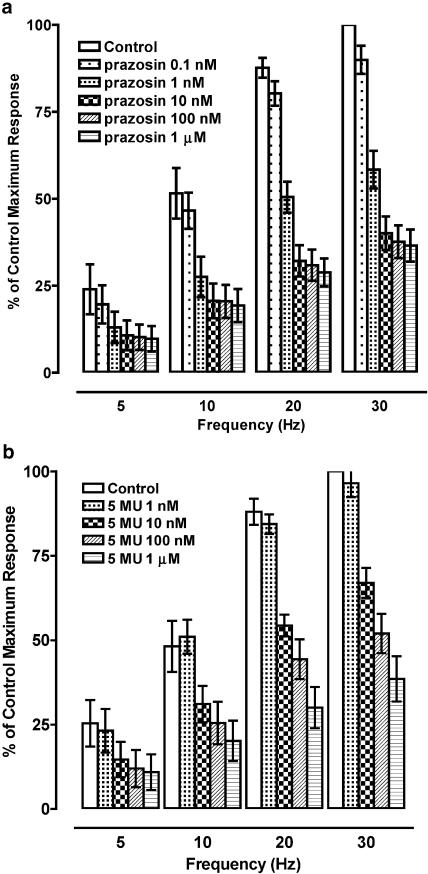
Figure 1.
Effects of antagonists on responses of rat femoral resistance arteries to exogenous noradrenaline; (a) prazosin (n=9); (b) 5-methyl-urapidil (5 MU) (n=6); (c) BMY 7378 (BMY) (n=5); (d) chloroethylclonidine (CEC) (n=4).
5-Methyl-urapidil (1–100 nM) also produced a parallel rightward shift of the noradrenaline CRC (Figure 1b). Maximum responses were unaffected by 1 nM but were significantly reduced by 10 and 100 nM 5-methyl-urapidil (Emax, %, n=6: control, 99±2; 1 nM 5-methyl-urapidil, 98±1, P>0.05); 10 nM 5-methyl-urapidil, 83±2, P<0.01; 100 nM 5-methyl-urapidil, 69±2, P<0.01). The Schild plot gave a pA2 value of 9.1 with a slope of 0.94 (95% CL: 0.71–1.18), not significantly different from unity.
BMY 7378 (10 nM–1 μM) had no significant effect on the noradrenaline CRC at concentrations of 10 and 100 nM (Figure 1c). BMY 7378 (1 μM) produced an eight-fold shift of the noradrenaline CRC, giving an estimated pKB of 6.7. The maximum response to noradrenaline was also significantly reduced (Emax %, n=5: control, 101±4; 1 μM BMY 7378, 85±2 (P<0.05)).
Chloroethylclonidine had no significant effect on the noradrenaline CRC at a concentration of 1 μM (Figure 1d). However, 10 μM chloroethylclonidine shifted the noradrenaline CRC to the right with a reduction in the maximum response (Emax %, n=4: control 101±3%; 10 μM chloroethylclonidine, 80±3% (P<0.01)).
Vasoconstrictor responses to EFS
EFS (5–30 Hz) produced frequency-dependent contractile responses of the femoral resistance arteries. Responses to 30 Hz were approximately 40% of the size of those elicited by 1 μM noradrenaline. Tetrodotoxin (1 μM, Figure 2a) and guanethidine (10 μM, Figure 2b), significantly reduced, but did not completely abolish responses. RS 79488 (0.1 μM) potentiated responses at all frequencies, particularly at 10 Hz (Figure 2c). Prazosin (1 μM) abolished the tetrodotoxin-sensitive contractile responses due to nerve stimulation (Figure 3a). Subsequent addition of 10 μM α, β-methylene ATP produced a small, nonsignificant, additional blockade (Figure 3a). On addition of 10 μM α,β-methylene ATP first, a potentiation of the nerve-induced responses was obtained (Figure 3b). Subsequent addition of prazosin (0.1 μM) reduced the responses to the size of the tetrodotoxin-resistant component (Figure 3b). Suramin (500 μM) also potentiated contractile responses to EFS, although the size of the potentiation was less than that obtained with α,β-methylene ATP (e.g. % potentiation at 30 Hz: α,β-methylene ATP, 40±7, n=4; suramin, 20±6, n=4, P<0.05).
Figure 2.
Effects of drugs on the responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width. (a) Tetrodotoxin (TTX, 1 μM) (n=5); (b) guanethidine (GUAN, 10 μM) (n=4); (c) RS 79488 (0.1 μM) (n=4). Significance of difference from control, *P<0.05, **P<0.01, ***P<0.001 (paired t-test.)
Figure 3.
Effects of prazosin and α, β-methylene ATP on the responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width. (a) Prazosin (0.1 μM) followed by α, β-methylene ATP (10 μM) (n=5); (b) α,β-methylene ATP (10 μM) followed by prazosin (0.1 μM) (n=4). Significance of difference from control, *P<0.05, **P<0.01, ***P<0.001 (paired t-test.)
Prazosin (0.1 nM – 1 μM) produced concentration-dependent inhibition of the responses to field stimulation with no significant differences in the sensitivity to prazosin at different frequencies (Figure 4a, Table 1). 5-Methyl-urapidil also produced a concentration-dependent inhibition of responses to field stimulation, with a potency around ten times less than that of prazosin at all frequencies (Figure 4b, Table 1). BMY 7378 at concentrations of 10 and 100 nM had no effect on responses to field stimulation at 10–30 Hz (Figure 5a). Responses to field stimulation were more sensitive to BMY 7378 at 5 Hz, with significant inhibition by 10 and 100 nM (Figure 5a), giving a higher pIC50 than at higher frequencies (Table 1). Chloroethylclonidine (1 and 10 μM) had no significant effect on responses at any frequency (data not shown).
Figure 4.
(a) Effect of different concentrations of prazosin on responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width. Significances of difference from control have been omitted for clarity. All treatment values were significantly different from control (P<0.001) except for 0.1 nM prazosin (P>0.5) at all frequencies (repeated measures ANOVA with post tests, n=8). (b) Effect of different concentrations of 5-methyl-urapidil (5 MU) on responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width. Significances of difference from control have been omitted for clarity. All treatment values were significantly different from control (P<0.001) except for 1 nM 5 MU (P>0.5) at all frequencies, (repeated measures ANOVA with post-tests, n=5).
Table 1.
Inhibition of responses to electrical field stimulation in rat femoral resistance arteries
| pIC50 values | |||||
|---|---|---|---|---|---|
| n | 5 Hz | 10 Hz | 20 Hz | 30 Hz | |
| Absence of cocaine | |||||
| Prazosin | 8 | 9.30±0.07 | 9.26±0.19 | 9.23±0.13 | 9.26±0.10 |
| 5-MU | 5 | 8.26±0.15 | 8.22±0.10 | 8.17±0.17 | 8.05±0.19 |
| BMY 7378 | 5 | 8.06±0.07 | 6.61±0.05a | 6.50±0.06a | 6.50±0.07a |
| Presence of cocaine | |||||
| Prazosin | 6 | 9.51±0.07 | 9.31±0.15 | 9.32±0.07 | 8.95±0.10 |
| 5-MU | 5 | 8.17±0.15 | 7.96±0.09 | 7.74±0.13 | 7.62±0.14 |
| BMY 7378 | 7 | 7.34±0.07b | 6.85±0.15 | 6.78±0.12 | 6.79±0.19 |
pIC50 represents the negative logarithm of the concentration required to produce 50% inhibition of the noradrenergic response.
Significantly different from value at 5 Hz (P<0.001), repeated measures ANOVA with post test.
Significantly different from value in absence of cocaine (P<0.001), unpaired t-test.
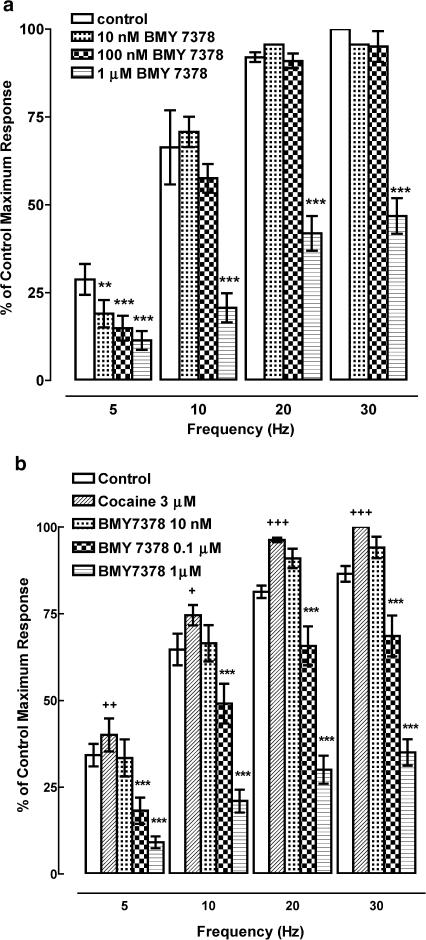
Figure 5.
(a) Effect of BMY 7378 on responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width (n=5). Significance of difference from control, **P<0.01, ***P<0.001 (repeated measures ANOVA followed by post-tests. (b) Effect of BMY 7378 on responses of rat femoral resistance arteries to electrical field stimulation at different frequencies for 10 s and 0.05 ms pulse width in the presence of cocaine, 3 μM (n=7). Significance of difference from control, +P<0.05, ++P<0.01, +++P<0.001 (paired t-test). Significance of difference from cocaine, ***P<0.001 (repeated measures ANOVA with post-test).
Cocaine (3 μM) increased the size of responses to electrical field stimulation (Figure 5b). The potencies of prazosin and 5-methyl-urapidil were unaffected by cocaine (Table 1). Responses at 10–30 Hz were more sensitive to BMY 7378 in the presence of cocaine, with significant inhibition of responses at 100 nM BMY 7378 (Figure 5b). Although this increase in sensitivity to BMY 7378 in the presence of cocaine was not reflected in a significant difference in pIC50 values (Table 1), pIC30 values in the absence and presence of cocaine were significantly different (Table 2). As in the absence of cocaine, the pIC50 of BMY 7378 was significantly higher at 5 Hz than at greater frequencies (Table 1). In the presence of cocaine, chloroethylclonidine had no effect on responses (data not shown), similar to the results obtained in the absence of cocaine.
Table 2.
Inhibition of responses to electrical field stimulation in rat femoral resistance arteries by BMY 7378
| BMY 7378 pIC30 values | ||||
|---|---|---|---|---|
| n | 10 Hz | 20 Hz | 30 Hz | |
| Absence of cocaine | 5 | 6.90±0.08 | 6.63±0.06 | 6.64±0.09 |
| Presence of cocaine | 7 | 7.47±0.13** | 7.40±0.14** | 7.50±0.17** |
The pIC30 represents the negative logarithm of the concentration required to produce 30% inhibition of the noradrenergic response.
Significantly different from value in absence of cocaine (P<0.01), unpaired t-test.
Discussion
Exogenous functional studies
In the present study, responses to exogenous noradrenaline were unaffected by the α2-adrenoceptor antagonist, RS 79948, indicating a lack of contribution of postjunctional α2-adrenoceptors to the responses. This was confirmed by the lack of a contractile response to the α2-adrenoceptor agonist, UK 14304, in agreement with our previous study (Jarajapu et al., 2001b).
Prazosin produced concentration-dependent, parallel rightward shifts in the sensitivity of noradrenaline, consistent with competitive antagonism, although there was a small reduction in the maximum responses at 10 and 100 nM prazosin. An effect of prazosin on maximum responses to noradrenaline has previously been reported in rat aorta (Godfraind & Alosachie, 1988) and in human skeletal muscle resistance arteries (Jarajapu et al., 2001a). The Schild slope, close to unity, is also consistent with reversible competitive antagonism. The pKB value of 9.2 from the Schild plot is consistent with an action of noradrenaline at α1H-adrenoceptor subtypes and rules out the presence of the α1L-subtype (Flavahan & Vanhoutte, 1986; Muramatsu et al., 1990a, 1990b).
5-Methyl-urapidil also produced a parallel rightward displacement of the noradrenaline CRC although there was a reduction in maximum responses at the higher concentrations of 5-methyl-urapidil. A Schild slope of 0.94 was observed, consistent with simple competitive antagonism. The pA2 value of 5-methyl-urapidil of 9.1 is in agreement with the reported affinity at the cloned mammalian α1a- adrenoceptor expressed in rat fibroblasts (Ford et al., 1996).
BMY 7378 produced no significant effect on the noradrenaline CRC at 10 and 100 nM, indicating the absence of a contribution from α1D- adrenoceptors. At 1 μM BMY 7378 there was a significant shift in the sensitivity to noradrenaline giving an estimated pKB value of 6.7, corresponding to the affinity of BMY 7378 for α1A-adrenoceptors (6.6, Goetz et al., 1995).
Chloroethylclonidine (1 μM) had no significant effect on maximum responses or sensitivity of noradrenaline indicating a lack of the α1B-adrenoceptor subtype. Chloroethylclonidine (10 μM) did produce a small reduction in the sensitivity and maximum responses of noradrenaline and this may indicate the presence of a small population of α1B- and/or α1D-adrenoceptors. However, chloroethylclonidine has also been shown to alkylate cloned α1a-adrenoceptors of several species, including rat (Büscher et al., 1996) and native α1A-adrenoceptors of murine tissues (Yang et al., 1998) and therefore its effect may represent alkylation of the predominant α1A- subtype in rat femoral resistance arteries.
Thus, the present study has confirmed our previous functional studies in different branches of the rat femoral artery (Jarajapu et al., 2001b): responses to exogenous noradrenaline are predominantly mediated by the α1A-adrenoceptor, with little or no evidence for the α1B- or α1D- subtypes or for α2-adrenoceptors.
Electrical field stimulation
In the present study, frequencies between 5 and 30 Hz were chosen because these frequencies fall within the physiological range for sympathetic nerve firing activity, based on studies on human skin nerves (Hallin & Torebjörk, 1974). In the presence of tetrodotoxin (1 μM) contractile responses to EFS were greatly reduced but were not completely abolished, suggesting that most of the EFS-induced contraction was due to activation of excitatory nerve fibres. Guanethidine, a sympathetic neurone blocker, inhibited nerve-mediated responses to a similar extent as tetrodotoxin, suggesting that a small amount of direct muscle stimulation may be responsible for the tetrodotoxin-resistant response.
The α2-adrenoceptor antagonist RS 79448 was used to determine the role of α2-adrenoceptors in the response to endogenous noradrenaline. RS 79448 potentiated the EFS responses, therefore indicating the presence of inhibitory prejunctional α2-adrenoceptors. A negative feedback mechanism may play an important role in determining the relative contributions of α1-adrenoceptors, α2-adrenoceptors and purinoceptors in vasoconstriction responses (MacDonald et al., 1992). The experiments do not, however, rule out the activation of postjunctional α2-adrenoceptors by neurally released noradrenaline, as effects of postjunctional α2-adrenoceptors may be masked by the prejunctional effects. However, the experiments with RS 79488 and exogenous noradrenaline and the lack of effect of UK 14304 in this and our previous study (Jarajapu et al., 2001b) suggest a lack of postjunctional α2-adrenoceptors in this preparation.
Characterisation of the responses to field stimulation using prazosin (1 μM) followed by the P2x-purinoceptor-desensitising agent, α,β-methylene ATP (10 μM) (Kasakov & Burnstock, 1983), provided a clear picture that the tetrodotoxin-sensitive responses were due to α1-adrenoceptor activation and that P2X-purinoceptors did not contribute significantly to the response. When the vessels were incubated with α,β-methylene ATP (10 μM) first, responses to EFS were potentiated. As α,β-methylene ATP first activates and later desensitises P2X-purinoceptors (Kasakov & Burnstock, 1983) the mechanism of the potentiating effect has been attributed to residual depolarisation caused by α,β-methylene ATP stimulation of postjunctional P2X-purinoceptors (Neild & Kotecha, 1986). Alternatively, α,β-methylene ATP may be blocking prejunctional inhibitory purinoceptors that inhibit the release of noradrenaline, as shown in rat mesenteric arteries (Shinozuka et al., 2001). The fact that suramin, a competitive P2X-purinoceptor antagonist (Leff et al., 1990), also produced potentiation suggests that potentiation involves blockade of prejunctional P2x-purinoceptors. Suramin produced less potentiation than with α,β-methylene ATP however and this may also suggest that part of the potentiating effect of α,β-methylene ATP is postjunctional.
Prazosin produced concentration-dependent inhibition of nerve-mediated responses to EFS with a pIC50 of around 9.3 at all the frequencies recorded. The pIC50 is not a direct measure of affinity for the α1-adrenoceptors activated by EFS, since equilibrium conditions do not apply, but would be expected to be related to it. In fact, the pIC50 values obtained (∼9.3) are almost identical to the pKB values (9.2) of prazosin obtained in these arteries using exogenous noradrenaline and are consistent with the activation of high-affinity α1H-adrenoceptors (Flavahan & Vanhoutte, 1986). Inhibition of neurogenic contractions by low concentrations of prazosin is in agreement with studies in other nerve-stimulated vascular preparations for example rat tail artery (Papanicolaou & Medgett, 1986), isolated perfused rat mesentery (Williams & Clarke, 1995), rat hepatic mesentery (Phillips et al., 1998).
5-Methyl-urapidil also produced concentration-dependent inhibition of nerve-mediated responses to EFS. A pIC50 of around 8.2 was recorded at all frequencies. In contrast to prazosin where the pIC50 (9.3) and pKB (9.2) values were identical, the pIC50 for 5 MU (8.2) against EFS was around 10 fold smaller that the pKB value (9.1). Thus the prazosin/5-methyl-urapidil potency ratio was approximately 1.0 against exogenous noradrenaline and 10 against endogenous noradrenaline. This may suggest that not all of the prazosin-sensitive α1-adrenoceptors activated by endogenous noradrenaline are of the α1A- subtype since 5-methyl-urapidil is relatively less potent. Both ratios, however, are consistent with the α1A-adrenoceptor being the predominant subtype.
Responses to EFS were not affected by BMY 7378 (10 and 100 nM) at 10–30 Hz showing a lack of contribution of the α1D-adrenoceptor subtype at these frequencies. A higher concentration of BMY 7378 (1 μM) did inhibit responses to EFS at 10–30 Hz, giving pIC50 values of 6.5–6.7, consistent with the affinity of BMY 7378 at the α1A- subtype. At 5 Hz, low concentrations of BMY 7378 (10 and 100 nM) produced concentration-dependent inhibition of responses with a pIC50 of 8.02, suggesting a significant contribution of α1D-adrenoceptors to the response.
Chloroethylclonidine had no significant effect on nerve-mediated contractions making the contribution of an α1B-adrenoceptor subtype unlikely.
Cocaine increased the size of the responses to EFS, as would be expected from inhibition of neuronal uptake of noradrenaline. The predominant subtype involved in responses to EFS in the presence of cocaine was the α1A-adrenoceptor as found in the absence of cocaine. As in the absence of cocaine, the pIC50 of BMY 7378 was higher at the lowest frequency of 5 Hz, suggesting a contribution of α1D-adrenoceptors to the response at this frequency. There was also a significant difference in the sensitivity to BMY 7378 at higher frequencies, suggesting that a further small α1D-adrenoceptor component may be uncovered in the presence of cocaine. This suggests that there are extrajunctional α1D-adrenoceptors which can be activated by neurally released noradrenaline in the presence of cocaine, but surprisingly are not activated by exogenous noradrenaline. The physiological significance of this is not clear and further studies are required to elucidate the role of α1D-adrenoceptors in vasoconstriction.
This study has shown that, in rat femoral resistance arteries, responses to exogenous noradrenaline and to endogenous noradrenaline, released by nerve stimulation, are mediated predominantly by α1A-adrenoceptors. The predominance of the α1A-adrenoceptor in responses to an exogenous α-adrenoceptor agonist is in agreement with several other studies in rat small arteries for example mesenteric (Kong et al., 1994; Williams & Clarke, 1995; Chen et al., 1996; Ipsen et al., 1997), renal (Blue et al., 1995), caudal (Lanchit et al., 1997), femoral (Jarajapu et al., 2001b) and hind limb (Zhu et al., 1997) arteries. In a few studies in rat mesenteric resistance arteries, evidence for an α1L-subtype was presented (Chen et al., 1996; Van der Graaf et al., 1996; Stam et al., 1999). Since it has been proposed that the α1L-adrenoceptor subtype represents a low-affinity state of the α1A-adrenoceptor (Ford et al., 1997), then it is possible that different experimental conditions may account for this discrepancy. α1A-Adrenoceptors have also been shown to be predominant in human skeletal muscle (Jarajapu et al., 2001a) and subcutaneous (Jarajapu et al., 2001c) resistance arteries. Canine subcutaneous resistance arteries also contain an α1A-/α1L- adrenoceptor (Argyle & McGrath, 2000).
There are a few exceptions to the general finding of α1A- or α1L-adrenoceptors mediating contraction to exogenous α1-adrenoceptor agonists in resistance arteries. Leech & Faber (1996) found an ‘α1D-like' adrenoceptor in rat cremaster muscle arterioles, although the low affinity of BMY 7378 (pKB 6.86) casts doubt on this conclusion. One study has suggested that the receptor mediating contraction to phenylephrine in rat mesenteric resistance vessels is the α1B-adrenoceptor (Piascik et al., 1997). This conclusion was based on the indirect evidence of a lack of receptor protection by BMY 7378 (α1D-adrenoceptor ligand) and A-61603 (α1A-adrenoceptor ligand) from inactivation by alkylating agents, phenoxybenzamine and chloroethylclonidine. An α1-adrenoceptor ‘resembling the α1B' has also been reported in rabbit cutaneous resistance arteries (Smith et al., 1997).
The involvement of the α1A-adrenoceptor in responses of resistance arteries to neurally released noradrenaline is also in agreement with other studies in resistance arteries of the rat (Kong et al., 1994; Williams & Clarke, 1995; Phillips et al., 1998). However, the present study also shows a significant contribution from α1D-adrenoceptors to neurally released noradrenaline. A contribution from α1D-adrenoceptors (and α1B-) in responses to nerve stimulation but not to exogenous noradrenaline was also seen in canine splenic arteries (Yang & Chiba, 2001). The significance of this is not clear: the fact that the α1D-adrenoceptor contribution is most apparent at a low frequency of stimulation in the presence of a normal uptake mechanism and at higher frequencies with uptake inhibited and is not seen in responses to exogenous noradrenaline suggests that the explanation is not simply location of the receptors in relation to the neurovascular junction as suggested by Yang & Chiba (2001) and that other factors such as the time of exposure and peak concentration of noradrenaline may also be important. In vivo studies in the rat have shown α1D-adrenoceptors to contribute to vasopressor responses to α1-adrenoceptor agonists (Zhou & Vargas, 1996; Villalobos-Molina et al., 1999) and to nerve stimulation (Castillo et al., 1998), suggesting that the current observations may have relevance to the physiological response in vivo. Studies in α1D- knockout mice have also directly shown that the α1D-adrenoceptor participates in the regulation of systemic blood pressure in this species (Tanoue et al., 2002).
In conclusion, the present study has shown a dominant role of the α1A- adrenoreceptor in contractions due to exogenous noradrenaline and to neurally released noradrenaline in rat femoral resistance arteries. α1D-Adrenoceptors do not appear to be involved in responses to exogenous noradrenaline but are activated by neurally released noradrenaline at a low frequency of stimulation and at higher frequencies of stimulation in the presence of uptake blockade.
Abbreviations
- CRC
concentration – response curve
- EFS
electrical field stimulation
- ERTP
effective resting transmural pressure
- PSS
physiological saline solution
References
- ARGYLE S.A., McGRATH J.C. An α1A/α1L-adrenoceptor mediates contraction of canine subcutaneous resistance arteries. J. Pharmacol. Exp. Ther. 2000;295:627–633. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUE D.R., JR, BONHAUS D.W., FORD A.P.D.W., PFISTER J.R., SHARIF N.A., SHIEH I.A., VIMONT R.L., WILLIAMS T.J., CLARKE D.E. Functional evidence equating the pharmacologically-defined α1A- and cloned α1C-adrenoceptor: studies in the isolated perfused kidney of rat. Br. J. Pharmacol. 1995;115:283–294. doi: 10.1111/j.1476-5381.1995.tb15875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜSCHER R., HEEKS C., TAGUCHI K., MICHEL M.C. Comparison of guinea-pig, bovine and rat α1-adrenoceptor subtypes. Br. J. Pharmacol. 1996;117:703–711. doi: 10.1111/j.1476-5381.1996.tb15247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYLUND D.B., BOND R.A., CLARKE D.E., EIKENBURG J.P., HIEBLE J.P., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., MOLINOFF P.B., RUFFOLO R.R., STROSBERG A.D., TRENDELENBURG U.G. Adrenoceptors, IUPHAR Receptor Compendium: London. 1998.
- CASTILLO E.F., LÓPEZ R.M., RODRÍGUEZ-SILVERIO J., BOBADILLA R.A., CASTILLO O. α1D-Adrenoceptors contribute to the neurogenic vasopressor response in pithed rats. Fund. Clin. Pharmacol. 1998;12:584–589. doi: 10.1111/j.1472-8206.1998.tb00990.x. [DOI] [PubMed] [Google Scholar]
- CHEN H., FETSCHER C., SCHÄFERS R.F., WAMBACH G., PHILIPP T., MICHEL M.C. Effects of noradrenaline and neuropeptide Y on rat mesenteric microvessel contraction. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:314–323. doi: 10.1007/BF00168634. [DOI] [PubMed] [Google Scholar]
- FLAVAHAN N.A., VANHOUTTE P.M. α1-Adrenoceptor subclassification in vascular smooth muscle. Trends Pharmacol. Sci. 1986;7:347–349. [Google Scholar]
- FORD A.P.D.W., ARREDONDO N.F., BLUE D.R., JR, BONHAUS D.W., JASPER J., KAVA M.S., LESNICK J., PFISTER J.R., SHIEH I.A., VIMONT R.L., WILLIAMS T.J., MCNEAL J.E., STAMEY T.A., CLARKE D.E. RS-17053 (N-[2-(2-cyclopropylmethoxyphenoxy)ethyl]-5-chloro-α, α-dimethyl-1H-indole-3-ethanamine hydrochloride), a selective α1A-adrenoceptor antagonist, displays low affinity for functional α1-adrenoceptors in human prostate: implications for adrenoceptor classification. Mol. Pharmacol. 1996;49:209–215. [PubMed] [Google Scholar]
- FORD A.P.D.W., DANIELS D.V., CHANG D.J., GEVER J.R., JASPER J.R., LESNICK J.D., CLARKE D.E. Pharmacological pleiotropism of the human recombinant α1A-adrenoceptor: implications for α1A-adrenoceptor classification. Br. J. Pharmacol. 1997;121:1127–1135. doi: 10.1038/sj.bjp.0701207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODFRAIND T., ALOSACHIE I.Influence of endothelium and cyclic GMP on alpha-adrenoceptors Vasodilatation: Vascular Smooth Muscle, Peptides, Autonomic Nerves and Endothelium 1988New York: Raven Press Ltd; 437–442.ed. Vanhoutte, P.M. pp [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D.C., TRUE T.A., RIMELE T.J., SAUSSY D.L., JR BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GUIMARÃES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HALLIN R.G., TOREBJÖRK E.H. Single unit sympathetic activity in human skin nerves during rest and various manoeuvres. Acta Physiol. Scand. 1974;92:303–317. doi: 10.1111/j.1748-1716.1974.tb05749.x. [DOI] [PubMed] [Google Scholar]
- HIEBLE J.P., BYLUND D.B., CLARKE D.E., EIKENBURG D.C., LANGER S.Z., LEFKOWITZ R.J., MINNEMAN K.P., RUFFOLO R.R. International Union of Pharmacology. X. Recommendations for nomenclature of α1-adrenoceptors: consensus updated. Pharmacol. Rev. 1995;47:267–270. [PubMed] [Google Scholar]
- HONNER V., DOCHERTY J.R. Investigation of the subtype of α1-adrenoceptor mediating contractions of rat vas deferens. Br. J. Pharmacol. 1999;128:1323–1331. doi: 10.1038/sj.bjp.0702913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPSEN M., ZHANG Y., DRAGSTED N., HAN C., MULVANY M.J. The antipsychotic drug sertindole is a specific inhibitor of α1A-adrenoceptors in rat mesenteric small arteries. Eur. J. Pharmacol. 1997;336:29–35. doi: 10.1016/s0014-2999(97)01242-9. [DOI] [PubMed] [Google Scholar]
- JARAJAPU Y.P.R., COATS P., McGRATH J.C., HILLIER C., MACDONALD A. Functional characterization of α1-adrenoceptor subtypes in human skeletal muscle resistance arteries. Br. J. Pharmacol. 2001a;133:679–686. doi: 10.1038/sj.bjp.0704130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARAJAPU Y.P.R., HILLIER C., MACDONALD A. The α1A-adrenoceptor subtype mediates contraction in rat femoral resistance arteries. Eur. J. Pharmacol. 2001b;422:127–135. doi: 10.1016/s0014-2999(01)01051-2. [DOI] [PubMed] [Google Scholar]
- JARAJAPU Y.P.R., JOHNSTON F., BERRY C., RENWICK A., McGRATH J.C., MACDONALD A., HILLIER C. Functional characterisation of α1-adrenoceptor subtypes in human subcutaneous resistance arteries. J. Pharmacol. Exp. Ther. 2001c;299:729–734. [PubMed] [Google Scholar]
- KASAKOV L., BURNSTOCK G. The use of the slowly degradable analog, α,β-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on non-adrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacol. 1983;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- KONG J., TAYLOR D.A., FLEMING W.W. Functional distribution and role of alpha-1 adrenoceptors in the mesenteric vasculature of the rat. J. Pharmacol. Exp. Ther. 1994;268:1153–1159. [PubMed] [Google Scholar]
- LANCHIT W.G., TRAN A.M., CLARKE D.E., FORD A.P.D.W. Pharmacological characterization of an α1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of rat. Br. J. Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEECH C.J., FABER J.E. Different α-adrenoceptor subtypes mediate constriction of arterioles and venules. Am. J. Physiol. 1996;270:H710–H722. doi: 10.1152/ajpheart.1996.270.2.H710. [DOI] [PubMed] [Google Scholar]
- LEFF P., WOOD B.E., O'CONNOR S.E. Suramin is a slowly-equilibrating but competitive antagonist at P2x-receptors in the rabbit isolated ear artery. Br. J. Pharmacol. 1990;101:645–649. doi: 10.1111/j.1476-5381.1990.tb14134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACDONALD A., DALY C.J., BULLOCH J.M., McGRATH J.C. Contributions of α1-adrenoceptors, α2-adrenoceptors and P2X-purinoceptors to neurotransmission in several rabbit isolated blood vessels: role of neuronal uptake and autofeedback. Br. J. Pharmacol. 1992;105:347–354. doi: 10.1111/j.1476-5381.1992.tb14257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile responses of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- MURAMATSU I., KIGOSHI S., OSHITA M. Two distinct α1-adrenoceptor subtypes involved in noradrenaline contraction of rabbit thoracic aorta. Br. J. Pharmacol. 1990a;101:662–666. doi: 10.1111/j.1476-5381.1990.tb14137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURAMATSU I., OHMURA T., KIGOSHI S., HASHIMOTO S., OSHITA M. Pharmacological subclassification of α1-adrenoceptors in vascular smooth muscle. Br. J. Pharmacol. 1990b;99:197–201. doi: 10.1111/j.1476-5381.1990.tb14678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEILD T.O., KOTECHA N. Effects of alpha beta methylene ATP on membrane potential, neuromuscular transmission and smooth muscle contraction in the rat tail artery. Gen. Pharmacol. 1986;17:461–464. doi: 10.1016/0306-3623(86)90193-x. [DOI] [PubMed] [Google Scholar]
- PAPANICOLAOU M., MEDGETT I.C. Effects of blockade of α1- and α2-adrenoceptors on vasoconstrictor responses to single and twin pulse stimulation in rat tail artery. Eur. J. Pharmacol. 1986;131:211–218. doi: 10.1016/0014-2999(86)90574-1. [DOI] [PubMed] [Google Scholar]
- PHILLIPS J.K., MCLEAN A.J., HILL C.E. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br. J. Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIASCIK M.T., HROMETZ S.L., EDELMANN S.E., GUARINO R.D., HADLEY R.W., BROWN R.D. Immunocytochemical localization of the alpha-1B adrenergic receptor and the contribution of this and the other subtypes to vascular smooth muscle contraction: analysis with selective ligands and antisense oligonucleotides. J. Pharmacol. Exp. Ther. 1997;283:854–868. [PubMed] [Google Scholar]
- SCHILD H.O. pAx and competitive drug antagonism. Br. J. Pharmacol. 1949;4:277–280. doi: 10.1111/j.1476-5381.1949.tb00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINOZUKA K., TANIOKA Y., KWON Y.M., TANAKA N., KUBOTA Y., NAKAMURA K., KUNITOMO M. Characterization of prejunctional purinoceptors inhibiting noradrenaline release in rat mesenteric arteries. Jpn. J. Pharmacol. 2001;85:41–46. doi: 10.1254/jjp.85.41. [DOI] [PubMed] [Google Scholar]
- SMITH K.M., MACMILLAN J.B., McGRATH J.C. Investigation of α1-adrenoceptor subtypes mediating vasoconstriction in rabbit cutaneous resistance arteries. Br. J. Pharmacol. 1997;122:825–832. doi: 10.1038/sj.bjp.0701451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAM W.B., VAN DER GRAAF P.H., SAXENA P.R. Analysis of α1L-adrenoceptor pharmacology in rat small mesenteric artery. Br. J. Pharmacol. 1999;127:661–670. doi: 10.1038/sj.bjp.0702598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANOUE A., NASA Y., KOSHIMIZU H., OSHIKAWA S., KAWAI T., SUNADA S., TAKEO S., TSUJIMOTO G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER GRAAF P.H., SHANKLEY N.P., BLACK J.W. Analysis of the effect of α1-adrenoceptor antagonists on noradrenaline-mediated contraction of rat mesenteric artery. Br. J. Pharmacol. 1996;118:1308–1316. doi: 10.1111/j.1476-5381.1996.tb15538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARGAS H.M., GORMAN A.J. Vascular alpha-1 adrenergic receptor subtypes in the regulation of arterial pressure. Life Sci. 1995;57:2291–2308. doi: 10.1016/0024-3205(95)02224-7. [DOI] [PubMed] [Google Scholar]
- VILLALOBOS-MOLINA R., LÓPEZ-GUERRERO J.J., IBARRA M. Functional evidence of α1D-adrenoceptors in vasculature of young and adult spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1534–1536. doi: 10.1038/sj.bjp.0702468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS T.J., CLARKE D.E. Characterization of α1-adrenoceptors mediating vasoconstriction to noradrenaline and nerve stimulation in the isolated perfused mesentery of rat. Br. J. Pharmacol. 1995;114:531–536. doi: 10.1111/j.1476-5381.1995.tb13259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG M., REESE J., COTECCHIA S., MICHEL M.C. Murine alpha1-adrenoceptor subtypes: 1. Radioligand binding studies. J. Pharmacol. Exp. Ther. 1998;286:841–847. [PubMed] [Google Scholar]
- YANG X.P., CHIBA S. Existence of different α1-adrenoceptor subtypes in junctional and extrajunctional neurovascular regions in canine splenic arteries. Br. J. Pharmacol. 2001;132:1852–1858. doi: 10.1038/sj.bjp.0704020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZACHARIA J., HILLIER C., MACDONALD A. Neurally evoked responses of rat femoral small arteries are predominantly mediated by α1A-adrenoceptors. Br. J. Pharmacol. 2002;137:90P. [Google Scholar]
- ZACHARIA J., HILLIER C., MACDONALD A. Effect of cocaine on the α1-adrenoceptor subtypes involved in neurally-evoked contractions of rat femoral resistance arteries. Br. J. Pharmacol. 2003;138:158P. doi: 10.1038/sj.bjp.0705690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU L., VARGAS H.M. Vascular α1D-adrenoceptors have a role in the pressor response to phenylephirine in the pithed rat. Eur. J. Pharmacol. 1996;305:173–176. doi: 10.1016/0014-2999(96)00229-4. [DOI] [PubMed] [Google Scholar]
- ZHU W., ZHANG Y., HAN C. Characterization of subtype of α1-adrenoceptor mediating vasoconstriction in perfused rat hind limb. Eur. J. Pharmacol. 1997;329:55–61. doi: 10.1016/s0014-2999(97)10104-2. [DOI] [PubMed] [Google Scholar]