Abstract
2-p-(2-carboxyethylphenethylamino-5′-ethylcarboxamidoadenosine) (CGS 21680) is considered the reference compound to study adenosine A2A receptors. However, CGS 21680 binding in the cerebral cortex, where adenosine A1 receptors are predominant, displays a mixed A2A/A1 receptor pharmacology. We now use adenosine A1 and A2A receptor knockout mice to investigate the characteristics of cortical [3H]CGS 21680 binding.
[3H]CGS 21680 binding to the cerebral cortex was strongly reduced in adenosine A1 receptor knockout mice, but only slightly reduced in A2A receptor knockout mice compared with the corresponding wild-type littermates.
Another selective A2A receptor ligand, [3H]-5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine ([3H]SCH 58261), displayed a saturable binding to mouse cortical membranes, albeit with a binding density 20 times lower than that of striatal membranes, and this [3H]SCH58261 binding was abolished in both striatal and cortical membranes of A2A receptor knockout mice and unchanged in A1 receptor knockout mice.
The presence of A2A receptors in cortical neurons was further confirmed by Western blot in mouse cortical nerve terminal membranes.
It is concluded that, although A2A receptors are present in the cerebral cortex, the purportedly selective A2A receptor agonist [3H]CGS 21680 binds in the cerebral cortex to an entity that requires the presence of adenosine A1 receptors. Thus, CGS 21680 should be used with care in all preparations where adenosine A1 receptors out-number A2A receptors.
Keywords: CGS 21680, A1 receptors, A2A receptors, cortex, striatum, adenosine, SCH 58261
Introduction
The purine nucleoside adenosine and its various receptor subtypes play multiple roles in the modulation of the central nervous system (Dunwiddie & Masino, 2001). Adenosine exerts its physiological actions through activation of cell surface receptors (Fredholm et al., 2001). Of these, A1 receptors are widespread throughout the brain, whereas adenosine A2A receptors are far more abundant in the basal ganglia (Fastbom et al., 1987; Jarvis & Williams, 1989; Parkinson & Fredholm, 1990). However, there is evidence that adenosine A2A receptors also exist outside the striatum, for example, in the cerebral cortex (reviewed in Cunha, 2001; Fredholm et al., 2003). Although these cortical adenosine A2A receptors are not abundant, they may play a fundamental role in the viability of cortical tissue. For instance, the blockade or genetic disruption of A2A receptors confers a profound neuroprotection in situations of ischemia in adult animals (Monopoli et al., 1998; Chen et al., 1999; Behan & Stone, 2002), whereas gene disruption seems to have the opposite effect in early life (Ådén et al., 2003).
A number of potent and rather selective ligands are available for the study of adenosine A2A receptors (Klotz, 2000). The most widely used adenosine A2A receptor agonist is CGS 21680. It displays both high affinity and selectivity for the rat adenosine A2A receptor (Jarvis et al., 1989). However, the binding of CGS 21680 to cortical or hippocampal regions displays several differences from binding to ‘typical' striatal-like A2A receptors, especially in terms of pharmacology, which is intermediary between that of A1 and A2A receptors (Wan et al., 1990; James et al., 1992; Johansson et al., 1993; Kirk & Richardson, 1995; Cunha et al., 1996; 1997; 1999; Lindström et al., 1996). Given that CGS 21680 remains the drug predominantly used to characterize A2A receptors and to define A2A receptor-mediated effects, it is obviously important to clarify what the ‘atypical' binding profile of CGS 21680 binding in the cerebral cortex actually represents.
The recent introduction of adenosine A1 (Johansson et al., 2001) and A2A (Ledent et al., 1997; Chen et al., 1999) receptor knockout mice has provided a new tool to test the selectivity of adenosine receptor ligands, in particular when ligands are of low affinity or suspected to have poor selectivity between receptor subtypes or when the relative densities of receptor subtypes are considerably different. Thus, instead of carrying out another detailed characterization of CGS 21680 binding, we decided to characterize the impact of the genetic elimination of A1 or A2A receptors on the binding of CGS 21680 to the cerebral cortex.
Methods
Animals
Adenosine A1 receptor knockout mice were on a mixed 129/OlaHsd/C57B6 background generated as described (Johansson et al., 2001). Heterozygous animals of the third generation were bred and all experiments were conducted on littermates genotyped using PCR. Adenosine A2A receptor knockout mice were either on an outbred CD1 background, generated as described (Ledent et al., 1997), or on a pure 129-Steel genetic background, as previously described (Chen et al., 1999). Heterozygous mice were bred and littermates with determined genotype (PCR assay) were used. The following symbols are used throughout the text: (+/+) refers to wildtype; (+/−) to heterozygous and (−/−) to homozygous null-mutant mice. All animals were handled according to the EU guidelines (86/609/EEC).
Drugs and solutions
2-p-(2-Carboxyethyl-[3H]-N-phenethylamino-5′-ethylcarboxamidoadenosine ([3H]CGS 21680, specific activity 41.2 Ci mmol−1) and 8-cyclopentyl(2,3-[3H]N)-1,3-dipropylxanthine ([3H]DPCPX, specific activity 116.0 Ci mmol−1) were from DuPont NEN (Stevenage, U.K.), [3H]-5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine ([3H]SCH 58261, specific activity 77 Ci mmol−1) was prepared by Amersham and was a generous gift of Dr E. Ongini (Shering-Plough, Milan, Italy), (±)N6-R-phenylisopropyladenosine (R-PIA) was from Sigma (Poole Dorset, U.K.), adenosine deaminase (ADA, calf intestine suspension, 200 U mg−1, EC 3.5.4.4) was from Roche (Germany) and 9-chloro-2-(2-furyl)[1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS 15943) and 8-{4-[(2-aminoethyl)amino]carbonylmethyl-oxyphenyl}xanthine (XAC) were from Research Biochemicals International (Natick, MA, U.S.A.). All drugs were diluted daily into the appropriate media from 10 mM stock solutions made up in dimethylsulfoxide stored at 4°C, except DPCPX, which was dissolved in ethanol. [3H]DPCPX, [3H]SCH 58261, [3H]CGS 21680 and adenosine deaminase were prepared directly into the incubation solution each day. All other reagents were of the highest purity available.
Membrane-binding experiments
Membranes were prepared as previously described (Cunha et al., 1996). Briefly, the brains were removed from two to five mice of each genotype and the striata and cortices were dissected out at 4°C in sucrose solution (0.32 M) containing 50 mM Tris-HCl, and 2 mM EGTA, pH 7.6. The tissue was homogenized in a Potter-Elvehjem homogenizer at 4°C. The resulting homogenates were centrifuged at 1000 × g for 10 min at 4°C. The supernatants were re-centrifuged at 14 000 × g for 12 min at 4°C. The pellets were then resuspended in a solution containing 50 mM Tris-HCl (pH 7.4), 2 mM EGTA, 1 mM EDTA, 2 U ml−1 ADA and incubated for 30 min at 37°C to remove endogenous adenosine. After centrifugation at 14 000 × g the pellets were resuspended in the incubation solution (50 mM Tris-HCl and 10 mM MgCl2 (for [3H]CGS 21680 and [3H]SCH 58261 experiments) or 2 mM MgCl2 (for [3H]DPCPX experiments), pH 7.4). Aliquots were then frozen at −80°C.
Samples were incubated with 0.1–20 nM [3H]CGS 21680 for 4 h, with 0.3–10 nM [3H]SCH 58261 for 1 h, or with 0.1–10 nM [3H]DPCPX for 2 h at 23–25°C. This was performed with 192–331 μg ([3H]CGS 21680 and [3H]SCH 58261 binding in the cortex) or 22–57 μg ([3H]DPCPX binding and [3H]CGS 21680 or [3H]SCH 58261 binding in the striatum) of membrane protein in a final volume of 300 μl in an incubation solution containing 50 mM Tris-HCl and 10 mM MgCl2 (pH 7.4) for [3H]CGS 21680 and [3H]SCH 58261 experiments and 50 mM Tris-HCl and 2 mM MgCl2 (pH 7.4) for [3H]DPCPX experiments, both containing 4 U ml−1 ADA. When tissue from A1 receptor (−/−) and A2A receptor (−/−) was tested, the tissue from the corresponding wild type (+/+) (i.e., with the same genetic background) was also run in the same assay.
Nonspecific binding was measured in the presence of the A1/A2A receptor antagonist CGS 15943 (1 μM) for [3H]CGS 21680 experiments, of the A1/A2A receptor antagonist XAC (1 μM) for [3H]SCH 58261 experiments and of the A1 receptor agonist R-PIA (100 μM) in the case of [3H]DPCPX experiments. All binding assays were performed in duplicate. The binding reactions were stopped by vacuum filtration. The washing volume was 5 ml for [3H]DPCPX and [3H]SCH 58261 experiments and 10 ml for [3H]CGS 21680 experiments, with the respective incubation buffer maintained at 4°C. The filters were placed in scintillation liquid (Ready Safe, Wallac, Finland) and radioactivity was determined after at least 12 h. The counting efficiency was 55–60%. The protein concentration was determined using the Bio-Rad protein assay based on Bradford dye-binding procedure.
The specific binding from saturation experiments was fitted by nonlinear regression to a one-site binding equation using the Raphson–Newton method, performed with commercial software (GraphPad, San Diego, CA, U.S.A.). Data are the mean±s.e.m. values or mean (95% confidence interval) of n experiments. In some cases, the significance was calculated with a paired Student's t-test. A value of P<0.05 was considered to represent a significant difference.
Autoradiography experiments
Mice were anesthetized with carbon dioxide and thereafter decapitated. The brain was dissected out, directly frozen on dry ice and stored at −80°C. Sections (14 μm) were cut using a cryostat and mounted on Polysine microslides (Menzel-Gläser, Germany). The autoradiographies were performed essentially as described before (Halldner et al., 2000). In brief, slides were incubated with 0.2–10 nM of [3H] DPCPX, 0.1–10 nM of [3H]SCH 58261, or 0.3–30 nM [3H]CGS 21680. For evaluation of nonspecific binding, the adenosine analogue R-PIA (20 μM) was added to DPCPX experiments, the adenosine analogue NECA (50 μM) was added to the SCH 58261 experiments and the adenosine receptor agonist 2-chloroadenosine (20 μM) was added to the CGS 21680 experiments. After incubation, dried sections were apposed to 3Hyperfilm (Amersham) together with tritium standards. Optical densities were measured by means of the MCID M5 system (Imaging Research, St. Catherine's, Canada). Results are given as fmol mg−1 tissue.
RT–PCR experiments
After brief CO2 anesthesia, A1 (+/+), A1 (−/−), and A2A (−/−) mice were decapitated. The striata and the cortex were dissected out and directly transferred to β-mercaptoethanol-containing RLT buffer (Rneasy, Qiagen). The tissue was homogenized with a syringe and a 30 G needle. Total RNA was extracted by means of Rneasy RNA extraction kit (Qiagen) according to the manufacturer's instructions, and finally dissolved in RNase free water. Total RNA was reverse transcribed (RT) for 55 min at 37°C. Aliquots (1 μl) of cDNA were amplified using the primers 5′-CTC CAC CAT GAT GTA CAC-3′and 5′-CAT GGT TTC GGG AGA TGC AG-3′. PCR conditions consisted of an initial denaturation for 2 min at 94°C followed by 25 cycles of: 94°C, 30 s; 56°C, 60 s; 72°C, 30 s, and then a final 10 min incubation at 72°C. After amplification, the products were electrophoresed through a 0.5 × TBE (Tris-borate/EDTA) 1% agarose gel. Bands were visualized by means of UV light. The A2A (−/−) samples were used as a negative control.
Western blot analysis
The analysis of adenosine A2A receptor immunoreactivity was carried out by Western blot analysis (Rebola et al., 2002) in whole membranes of the striatum and in membranes from a Percoll-purified synaptosomal fraction of the cerebral cortex, prepared as previously described (e.g., Cunha et al., 1996). After the amount of protein had been determined, each sample was diluted with two volumes of a solution containing 8 M urea, 100 mM dithiothreitol, 2% (w v−1) sodium dodecyl sulfate and 375 mM Tris-HCl (pH 6.8) and incubated for 2 h at 37°C. These diluted samples and the prestained molecular weight markers (Amersham) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10% with a 4% concentrating gel) under reducing conditions and electro-transferred to polyvinylidene difluoride membranes (0.45 μm, from Amersham). After blocking for 2 h at room temperature with 5% milk in Tris-buffered saline (pH 7.6) containing 0.1% Tween 20 (TBS-T), the membranes were incubated overnight at 4°C with a goat anti-adenosine A2A receptor antibody (1 : 100 dilution from a 200 μg ml−1 stock from Santa Cruz Biotechnology). After four washing periods of 10 min with TBS-T containing 0.5% milk, the membranes were incubated for 90 min at room temperature with the alkaline phosphatase-conjugated anti-goat secondary antibody (1 : 2000 dilution from Calbiochem) in TBS-T containing 1% milk. After five 10-min washes in TBS-T with 0.5% milk, the membranes were incubated with enhanced chemi-fluorescence (ECF) for 5 min and then analyzed with a Storm (Molecular Devices).
Results
Binding of [3H]DPCPX to the cerebral cortex
The pharmacological characterization of adenosine A1 receptors has mainly been carried out in cerebral cortical preparations. We now confirmed that the selective A1 receptor antagonist [3H]DPCPX displayed a saturable binding profile in the cerebral cortex of wild-type (+/+) mice (Figure 1a and b). In fact, in the autoradiographic experiments summarized in Figure 1, [3H]DPCPX bound with a KD of 0.38 nM (95% confidence interval: 0.25 to 0.52 nM) and a Bmax of 218 (200–236) fmol mg−1 gray matter (n=6), whereas in membrane-binding studies [3H]DPCPX bound with a KD of 2.93 nM and a Bmax of 4196 fmol mg−1 protein (n=1) to cerebral cortical preparations of wild-type (+/+) mice. As illustrated in Figure 1a, the binding of [3H]DPCPX to cortical tissue was decreased by nearly 50% in adenosine A1 (+/−) mice, displaying a Bmax of 109 (100–118) fmol mg−1 gray matter and a KD of 0.35 (0.22–0.49) nM, and the binding disappeared in the A1 receptor knockout (−/−) mice, as previously reported (Johansson et al., 2001). This disappearance of [3H]DPCPX binding to cortical tissue was also found in membrane-binding studies (Figure 1b).
Figure 1.
Saturation-binding curves of the selective adenosine A1 receptor antagonist [3H]DPCPX to the cerebral cortex of adenosine A1 receptor knockout mice and their respective wild-type littermates (a and b) and to the cerebral cortex of adenosine A2A receptor knockout mice and their respective wild-type littermates (c and d) measured by autoradiography (a and c) or membrane binding (b and d).
We then compared the binding of [3H]DPCPX to the cerebral cortex of mice with a partial (+/−) or total disruption (−/−) of the adenosine A2A receptor gene with that in control mice (+/+). As illustrated in Figures 1c and d, the binding of [3H]DPCPX to the cerebral cortex was nearly identical in wild-type (+/+), heterozygote (+/−) and A2A receptor knockout (−/−) mice, as assessed both by autoradiography (Figure 1c) and by membrane-binding studies (Figure 1d). For instance, [3H]DPCPX bound with a KD ranging from 0.25–0.52 nM and Bmax values ranging from 173 to 188 fmol mg−1 gray matter to the striatum of A2A (+/+), A2A (+/−) and A2A (−/−). This indicates that there is no apparent change in the binding properties of cortical adenosine A1 receptors in A2A receptor knockout animals.
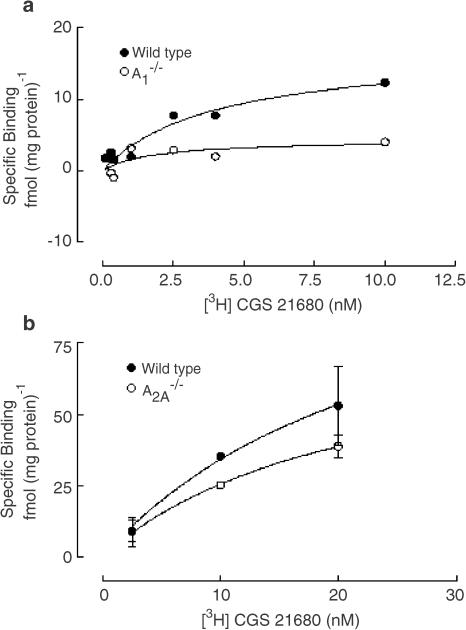
Binding of [3H]CGS 21680 to the basal ganglia
We then wanted to confirm the lack of binding of the prototypical adenosine A2A receptor agonist CGS 21680 to adenosine A2A knockout animals, once again using both autoradiography and membrane binding. For this purpose, we studied the binding of [3H]CGS 21680 to the caudate putamen, which has been the gold standard to characterize adenosine A2A receptor pharmacology. There was a complete lack of [3H]CGS 21680 binding in the caudate putamen of A2A receptor knockout (−/−) mice, while in wild-type (+/+) mice there is an evident saturable curve, as assessed both by autoradiography (not shown) and by membrane-binding studies in striatal membranes (Figure 2a).
Figure 2.
Saturation-binding curves of the adenosine A2A receptor agonist, [3H]CGS 21680, to the basal ganglia of adenosine A2A receptor knockout mice and their wild-type littermates (a) and of adenosine A1 receptor knockout mice and their wild-type littermates (b) measured by membrane binding. In both panels, the ordinates represent the specific binding of [3H]CGS 21680, obtained upon subtraction of the nonspecific binding determined with 1 μM CGS 15943 from total binding. Nonspecific binding amounted to 20–30% of the total binding.
We then compared the binding of [3H]CGS 21680 to the basal ganglia in mice with a disruption (−/−) of the adenosine A1 receptor gene with binding in their controls (+/+). The binding of [3H]CGS 21680 to the striatum was nearly identical in wild-type (+/+) and A1 knockout (−/−) mice, as assessed both by autoradiography in the caudate putamen (not shown) and by membrane-binding studies in the striatum (Figure 2b). This indicates that there is no apparent change in the binding properties of adenosine A2A receptors in A1 receptor knockout animals.
Binding of [3H]CGS 21680 to the cerebral cortex
Since [3H]CGS 21680 binding to the cerebral cortex of rats displays a mixed A1/A2A receptor pharmacology (Wan et al., 1990; James et al., 1992; Johansson et al., 1993; Kirk & Richardson, 1995; Cunha et al., 1996; 1997; 1999; Lindström et al., 1996), we hoped to clarify the adenosine receptor subtype recognized by CGS 21680 in the cerebral cortex with the use of adenosine A1 and A2A receptor knockout mice.
To our surprise, autoradiographic quantitation of the [3H]CGS 21680 (3 and 30 nM) binding showed that adenosine A1 rather than A2A receptors appeared to be involved, since [3H]CGS 21680 binding is abolished in A1 receptor knockout (−/−) mice (Figure 3a) and is essentially preserved in adenosine A2A receptor knockout (−/−) mice (Figure 3b). However, the strength of this conclusion is partially hampered by the small binding densities of [3H]CGS 21680 binding found in these autoradiographic experiments (see Figure 3).
Figure 3.
Panels a and b show autoradiograms of [3H]CGS 21680 (30 nM) binding to wild-type mice in the absence (a) or in the presence of 2-chloroadenosine (20 μM) to define nonspecific binding (b). Panels c and d display the average autoradiographic binding data of [3H]CGS 21680 (3 and 30 nM) to the cerebral cortex of adenosine A1 receptor knockout (−/−), heterozygous (+/−), and their corresponding wildtypes (+/+) (a) and to the cerebral cortex of adenosine A2A receptor knockout (−/−), heterozygous (+/−), and their corresponding wildtypes (+/+) (b). In both panels, the ordinates represent the specific binding of [3H]CGS 21680, obtained upon subtraction of the nonspecific binding determined with 20 μM 2-chloroadenosine from total binding. The data are mean±s.e.m., n=3–6. Note that the binding of [3H]CGS 21680 to the cerebral cortex is essentially preserved in the adenosine A2A receptor knockout mice and is progressively decreased and abolished in the adenosine A1 receptor heterozygous and knockout mice.
Therefore, we repeated the same experiments, but now using membrane-binding studies to be able to increase the protein concentration of cortical membranes in the assay and consequently to amplify the signal, although the binding characteristics of adenosine receptors are expected to be different in autoradiography and membrane-binding experiments (e.g. Fredholm et al., 2003). As shown in Figure 4, membrane-binding studies essentially confirmed the major conclusion drawn from autoradiography experiments. Thus, it was observed that the specific binding of [3H]CGS 21680 to cerebral cortical membranes decreased by nearly 80% in adenosine A1 receptor knockout (−/−) mice (Figure 4a). In contrast, in two experiments, we noted a slight, near 30% decrease (not evaluated statistically) in the binding density of [3H]CGS 21680 in A2A receptor knockout (−/−) mice (Figure 4b) when compared to their wild-type (+/+) littermates.
Figure 4.
Panel a displays saturation-binding curves of [3H]CGS 21680 binding to membranes from the cerebral cortex of adenosine A1 receptor knockout mice (open symbols) and corresponding wildtype (filled symbols). The data were obtained from one experiment performed in duplicate. Panel b displays the saturation-binding curve of [3H]CGS 21680 to membranes from the cerebral cortex of adenosine A2A receptor knockout mice (open symbols) and corresponding wild-type (filled symbols) mice. The data are mean±s.e.m. of two experiments performed in duplicate, which precludes statistical analysis of the results.
Binding of [3H]SCH 58261 to the cerebral cortex
One possible explanation is that there are major changes in A2A receptors when the expression of adenosine A1 receptors in the cerebral cortex is genetically altered. We first confirmed that a low expression (compared to striatum) of A2A receptor mRNA is present in mouse cerebral cortex, as had previously been shown to occur in the rat (Cunha et al., 1994), and that the expression remains in A1 (−/−) (not shown). We next decided to make binding experiments with [3H]SCH 58261, which is described to selectively recognize A2A receptors both in membrane-binding (Zocchi et al., 1996) and autoradiography studies (Fredholm et al, 1998), and not to interfere with ‘atypical' [3H]CGS 21680 binding sites (Lindström et al., 1996). In addition, SCH 58261 discriminates between two binding sites of CGS 21680, recognizing the A2A receptor-like binding site and not interfering with the ‘atypical' one (Lindström et al., 1996).
Using receptor autoradiography, we first characterized [3H]SCH 58261 binding to the caudate putamen of adenosine A1 and A2A receptor knockout (−/−) mice and their corresponding control littermates. As expected for a selective adenosine A2A receptor ligand, [3H]SCH 58261 binding to the caudate putamen was halved in A2A (+/−) (Bmax=134.5 fmol mg−1 gray matter, 95% confidence interval: 107.5–161.5, compared with a Bmax=287.9 fmol mg−1 gray matter, 95% confidence interval: 219.4–356.4, in wild type mice) and abolished in adenosine A2A receptor knockout (−/−) mice (n=5–6), whereas the averaged KD values were similar in A2A (+/+) (0.67 nM, 95% confidence interval: 0.07–1.26 nM) and A2A (+/−) (0.48 nM, 95% confidence interval: 0.09–0.87 nM). Furthermore, [3H]SCH 58261 binding in caudate putamen was nearly unchanged in A1 receptor knockout (−/−) mice, when compared to their corresponding wild-type (+/+) littermates (not shown). However, in these autoradiographic experiments, we observed a very low binding density of [3H]SCH 58261 to the cerebral cortex (lower than 5 fmol mg−1 gray matter) that precluded any reliable analysis of the effect of the different adenosine A1 and A2A receptor genotypes. To overcome this sensitivity problem in the autoradiography experiments, we carried out saturation curves of [3H]SCH 58261 binding in striatal and cortical membranes from adenosine A2A receptor knockout (−/−) mice and their corresponding wild-type littermates.
As illustrated in Figure 5a, in wild-type (+/+) mice, [3H]SCH 58261 bound to striatal membranes with a KD of 1.02 nM (95% confidence interval: 0.41–1.63 nM) and a Bmax of 1079 fmol mg−1 protein (95% confidence interval: 856–1301 fmol mg−1 protein, n=4), whereas the binding density of [3H]SCH 58261 in cortical membranes (Figure 5b) was nearly 20 times lower (Bmax of 56 fmol mg−1, 95% confidence interval: 10–103 fmol mg−1 protein, n=4) with a similar affinity (KD of 1.02 nM, 95% confidence interval: 0.33–1.72 nM, n=4). Interestingly, this saturable binding of [3H]SCH 58261 found in the striatum and cerebral cortex was completely abolished in membranes derived from A2A receptor knockout (−/−) mice (Figure 5). This strongly suggests that there are indeed A2A receptors in the cortex as well as in the striatum. The results also confirm that SCH 58261 is a selective ligand for adenosine A2A receptors both in the striatum as well as in the cerebral cortex.
Figure 5.
Saturation-binding curves of [3H]SCH 58261 to striatal (a) and cortical (b) membranes of adenosine A2A receptor knockout mice (open symbols) and corresponding wildtype (filled symbols). The ordinates represent the specific binding of [3H]SCH 58261, obtained upon subtraction of the nonspecific binding determined with 1 μM XAC from total binding. Nonspecific binding represented 42–67% of the total binding. The data are mean±s.e.m. of three to four experiments. Note that the density of binding of [3H]SCH 58261 to striatal membranes is nearly 20-fold greater than that observed in cortical membranes, but for both brain regions [3H]SCH 58261 binding is abolished in A2A receptor knockout mice.
One key issue to validate the conclusion that [3H]CGS 21680 bound to an entity requiring the expression of adenosine A1 receptors rather than solely to A2A receptors is that the density of A2A receptors in the cerebral cortex is not changed in A1 receptor knockout (−/−) mice. Since we validated [3H]SCH 58261 as a selective A2A receptor ligand, we compared the binding of [3H]SCH 58261 to cortical membranes of A1 receptor knockout (−/−) mice and in their corresponding wild type (+/+) littermates. We found no change in the density of [3H]SCH 58261 binding in cortical tissue from the two groups of mice (Bmax of 45 fmol mg−1 protein, 95% confidence interval: 27–62 and 41 fmol mg−1 protein, 95% confidence interval: 21–60 fmol mg−1 protein in A1 receptor (+/+) and (−/−) mice, respectively, n=3).
Adenosine A2A receptor Western blot analysis in mouse cortical neurons
To further confirm that, even with a low density, adenosine A2A receptors are indeed present in the cortex, we used an immunological approach. We decided to use one of the preparations obtained from brain tissue that displays the lowest level of non-neuronal contaminations, that is, the synaptosomal preparation obtained with the Percoll/sucrose method (Cunha et al., 1992). Thus, we investigated by Western blot analysis if the molecular entities recognized by adenosine A2A receptor antibodies in striatal membranes and in membranes from cortical nerve terminals displayed a similar electrophoretic mobility. Figure 6 shows a Western blot, representative of four similar blots carried out in membranes from different animals. It demonstrates the presence of similar bands with a molecular weight of 42 kDa recognized by adenosine A2A receptor antibodies both in the striatum and in cortical nerve terminal membranes. There is a much lower intensity of immunoreactivity in the cortex (note that the amount of protein used was larger in cortical than striatal lanes of the blot shown in Figure 6), and the band is not present in A2A receptor knockout mice (−/−).
Figure 6.
Western blot identifying adenosine A2A receptors in membranes prepared from Percoll-purified nerve terminal membranes prepared from the rat cerebral cortex and from whole membranes of the striatum. The SDS–PAGE gel was loaded with the amount of protein indicated below each lane, and is representative of four similar separations carried out with membranes prepared from different animals.
Discussion
The major conclusion derived from the present study is that, although adenosine A2A receptors are located also in other brain regions than the striatum, the purportedly selective adenosine A2A receptor agonist CGS 21680 binds primarily not to these extrastriatal adenosine A2A receptors, but mainly to a site associated with adenosine A1 receptors.
The selective adenosine A2A receptor antagonist [3H]SCH 58261 bound to cortical membranes in a manner similar to its binding to striatal membranes, where adenosine A2A receptors were pharmacologically defined (Bruns et al., 1986; Jarvis et al., 1989; Zocchi et al., 1996). Binding of [3H]SCH 58261 was completely abolished in the adenosine A2A receptor knockout mice both in cortical and in striatal membranes, which allows considering SCH 58261 a selective A2A receptor ligand both in the striatum as well as in extrastriatal regions. This confirms previous results showing the existence of adenosine A2A receptor mRNA in cortical tissue both by PCR (Cunha et al., 1994; Dixon et al., 1996) and by Northern blot analysis (Peterfreund et al., 1996), and extend previous observations reporting a specific binding of [3H]SCH 58261 in the cerebral cortex of mice that was attenuated in A2A receptor knockout mice (El Yacoubi et al., 2001).
Whereas these results confirm that adenosine A2A receptors are found in cerebral cortex, they do not tell us which cells express them. The cerebral cortex is richly endowed with blood vessels, and, for example, endothelial cells possess adenosine A2A receptors (Feoktistov et al., 2002). Furthermore, there is evidence that different glial cells express adenosine A2A receptors (Li et al., 2001; Nishizaki et al., 2002). The present finding that it was easier to detect [3H]SCH 58261 in homogenates of mouse cortex than in autoradiographic experiments could indicate that the A2A receptors detected in the latter type of experiments are expressed for example, by blood vessels. We do, however, have data that directly support the presence of adenosine A2A receptors in neurons. The finding that adenosine A2A receptor immunoreactivity could be detected in a synaptosomal preparation and that this immunoreactivity is no longer detected in A2A (−/−) mice does provide good evidence for a neuronal localization of at least some adenosine A2A receptors in neurons outside the striatum. This could explain why adenosine A2A receptor antagonists or disruption of the adenosine A2A receptor gene affords neuroprotection (Gao & Phillis, 1994; Jones et al., 1998; Monopoli et al., 1998; Chen et al., 1999; Behan & Stone, 2002, Petroni et al., 2002). In particular, the presently observed location of adenosine A2A receptors in nerve terminals of the cerebral cortex provides a molecular support for the proposal that A2A receptors may enhance the release of glutamate in the cerebral cortex (O'Reagan et al., 1992; Marchi et al., 2002), one of the possible mechanisms to understand neuroprotection afforded by blunting adenosine A2A receptor function (discussed in Fredholm et al., 2003).
However, it should be pointed out that the density of these cortical adenosine A2A receptors is considerably lower than the density of striatal adenosine A2A receptors. In fact, the binding density of [3H]SCH 58261 to cortical membranes is about 20 times lower than that of striatal membranes and the immunoreactivity found in cortical nerve terminals is clearly fainter than that found in striatal membranes. This considerably lower abundance of adenosine A2A receptors in the cerebral cortex could have consequences for the ability of endogenous adenosine to activate cortical A2A receptors. It is well known that the efficiency of agonists at A2A receptors is dependent on the level of receptor expression (Svenningsson et al., 1999; Arslan et al., 2002). There is evidence that the adenosine A2A receptors on striatopallidal GABAergic neurons are tonically activated by endogenous adenosine in the concentration present already under basal conditions (Svenningsson et al., 1999; Fredholm et al., 2003). By contrast, adenosine A2A receptors in cortex, being much less abundant than in striatum, are likely to be activated only at supra-physiological concentrations, such as those present during hypoxia and ischemia.
The second major conclusion derived from the present study is that CGS 21680, although being a useful adenosine A2A receptor ligand in the striatum, behaves as a nonselective A2A receptor ligand in the cerebral cortex. In fact, in the striatum, the binding of [3H]CGS 21680 is completely abolished in adenosine A2A receptor knockout mice, indicating that in this tissue [3H]CGS 21680 only binds to adenosine A2A receptors. In the cerebral cortex, CGS 21680 also binds to adenosine A2A receptors (see also Cunha et al., 1996), as now tentatively concluded by the slight reduction of [3H]CGS 21680 binding (not evaluated statistically) observed in adenosine A2A receptor knockout mice when compared with their respective wild-type littermates (see Figure 4b). However, in the cerebral cortex, CGS 21680 also binds to an entity that is different from adenosine A2A receptors and is essentially dependent on the presence of adenosine A1 receptors. This is concluded from the marked reduction of [3H]CGS 21680 binding to the cortex of adenosine A1 receptor knockout mice when compared with their wild-type littermates. This is not likely to be due to a loss of adenosine A2A receptors in A1 receptor knockout mice, since both the binding of [3H]CGS 21680 to striatal tissue and the binding of [3H]SCH 58261 to cortical membranes were unaffected in A1 (−/−) mice. Thus, we conclude that [3H]CGS 21680 mostly binds in the cerebral cortex to a molecular entity that requires the presence of adenosine A1 receptors.
It is important to emphasize that the data so far gathered only allow us to state that [3H]CGS 21680 binds to a molecular entity that requires the A1 receptor, and does not necessarily imply that [3H]CGS 21680 binds directly to A1 receptors. In fact, if [3H]CGS 21680 bound to adenosine A1 receptors, one would expect: (1) that CGS 21680 would bind to heterologously expressed adenosine A1 receptors, which was not observed (unpublished observations), and (2) that CGS 21680 would be able to displace the binding of selective adenosine A1 receptor ligands, and this has not been observed either (Cunha et al., 1996; 1997; Lopes et al., 2002). Moreover, since adenosine A1 receptors are also abundant in the striatum (Fastbom et al., 1987), one would expect a residual binding of [3H]CGS 21680 in the striatum of adenosine A2A receptor knockout mice and a reduction in striatal [3H]CGS 21680 binding in A1 receptor knockout mice, neither of which was observed (Figure 2). Thus, one has to conclude that a minor fraction of adenosine A1 receptors in the cerebral cortex, for some unexplained reason, displays pharmacological properties different from adenosine A1 receptors in other brain regions, in particular, being able to bind CGS 21680. In fact, several reports have documented the ability of A1 receptor ligands to displace [3H]CGS 21680 binding to the cerebral cortex (James et al., 1992; Johansson et al., 1993; Johansson & Fredholm, 1995; Kirk & Richardson, 1995; Cunha et al., 1996; Lopes et al., 2002). It has been described that cortical A1 receptors may be found as dimers (Ciruela et al., 1995) or dimerize with other receptors (e.g. P2Y1, see Yoshioka et al., 2001), and this could confer to them the possibility of binding CGS 21680. It should also be pointed out that adenosine A1 receptors possess adaptor proteins that control the strength of their coupling to G proteins (Nanoff et al., 1995) and it is currently unknown if these adaptor proteins modify the binding profile of adenosine A1 receptors. Clearly, further studies need to be carried out to explore these possibilities. This effort might not only be of academic interest but may also shed light on physiological processes that have been described as being mediated by ‘atypical' A2A receptors in the cortex, namely in terms of behavioral activity (El Yacoubi et al., 2000) and in terms of neuroprotection (Latini et al., 1999; Tebano et al., 2002).
In conclusion, the present paper provides evidence for the presence of adenosine A2A receptors in the cerebral cortex and shows that the prototypical adenosine A2A receptor agonist CGS 21680 is not an adequate tool to characterize these cortical A2A receptors. In fact, although [3H]CGS 21680 also binds to cortical A2A receptors, it mostly binds to an entity that requires the presence of adenosine A1 receptors. This casts major doubts on the use of CGS 21680 as a selective adenosine A2A receptor agonist in physiological or pharmacological studies, and the effects of CGS 21680 can only be ascribed as being mediated by A2A receptor if data are independent of stimulation or inhibition of adenosine A1 receptor.
Acknowledgments
We thank Cecilia Lövdahl and Björn Kull for technical assistance and helpful suggestions. We also acknowledge Janet Hólmen for the careful revision of the manuscript and Dr Ennio Ongini for the generous gift of [3H]SCH 58261. This work was supported by Fundação para a Ciência e Tecnologia (POCTI/FCB/44740/2002), the Swedish Science Research council (proj. No 2553), by the European Commission (proj. No QLK1-CT-2000-00069), the Swedish Foundation for Strategic Research and by Karolinska Institutet. LV Lopes received a FEBS Summer Fellowship to carry part of this work at Karolinska Institutet.
Abbreviations
- A1R(−/−)
(adenosine A1 receptor knockout)
- A1R(+/−)
(adenosine A1 receptor heterozygot)
- A1R(+/+)
(adenosine A1 receptor wild-type)
- A2AR(−/−)
(adenosine A2A receptor knock-out)
- A2AR(+/−)
(adenosine A2A receptor heterozygot)
- A2AR(+/+)
(adenosine A2A receptor wild-type)
- ADA
(adenosine deaminase)
- CGS 15943
(9-chloro-2-(2-furanyl)-5,6-dihydro-[1,2,4]-triazolo-[1,5]quinazolin-5-imine monomethanesulfonate)
- CGS 21680
(2-[p-(2-carboxyethyl)phenylethylamino]-5′-N-ethylcarboxamidoadenosine)
- DPCPX
(1,3-dipropyl-8-cyclopentylxanthine)
- NECA
(5′-N-ethylcarboxamidoadenosine)
- R-PIA
((±)N6-R-phenylisopropyladenosine)
- SCH 58261
(5-amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine)
- XAC
(8-{4-[(2-aminoethyl)amino]carbonylmethyl-oxyphenyl}xanthine)
References
- ÅDÉN U., HALLDNER L., LAGERCRANTZ H., DALMAU I., LEDENT C., FREDHOLM B.B. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34:739–744. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- ARSLAN G., KULL B., FREDHOLM B.B. Anoxia redistributes adenosine A2A receptors in PC12 cells and increases receptor-mediated formation of cAMP. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;365:150–157. doi: 10.1007/s002100100456. [DOI] [PubMed] [Google Scholar]
- BEHAN W.M.H., STONE T.W. Enhanced neuronal damage by co-administration of quinolinic acid and free radicals, and protection by adenosine A2A receptor antagonists. Br. J. Pharmacol. 2002;135:1435–1442. doi: 10.1038/sj.bjp.0704613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNS R.F., LU G.H., PUGSLEY T.A. Characterization of the A2 adenosine receptor labelled with [3H]NECA in rat striatal membranes. Mol. Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- CHEN J.F., HUANG Z., MA J., ZHU J., MORATALLA R., STANDAERT D., MOSKOWITZ M.A., FINK J.S., SCHWARZSCHILD M.A. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 1999;19:9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRUELA F., CASADO V., MALLOL J., CANELA E.I., LLUIS C., FRANCO R. Immunological identification of A1 adenosine receptors in brain cortex. J. Neurosci. Res. 1995;42:818–828. doi: 10.1002/jnr.490420610. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., CONSTANTINO M.D., RIBEIRO J.A. ZM241385 is an antagonist of the facilitatory responses produced by the A2A adenosine receptor agonists CGS21680 and HENECA in the rat hippocampus. Br. J. Pharmacol. 1997;122:1279–1284. doi: 10.1038/sj.bjp.0701507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA R.A., CONSTANTINO M.D., RIBEIRO J.A. G-protein coupling of CGS 21680 binding sites in the rat hippocampus and cortex is different from that of adenosine A1 and striatal A2A receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:295–302. doi: 10.1007/pl00005355. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., JOHANSSON B., CONSTANTINO M.D., SEBASTIÃO A.M., FREDHOLM B.B. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H]CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:261–271. doi: 10.1007/BF00168627. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., JOHANSSON B., VAN DER PLOEG I., SEBASTIÃO A.M., RIBEIRO J.A., FREDHOLM B.B. Evidence for functionally important adenosine A2a receptors in the rat hippocampus. Brain Res. 1994;649:208–216. doi: 10.1016/0006-8993(94)91066-9. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., SEBASTIÃO A.M., RIBEIRO J.A. Ecto-5′-nucleotidase is associated with cholinergic nerve terminals in the hippocampus but not in the cerebral cortex of the rat. J. Neurochem. 1992;59:657–666. doi: 10.1111/j.1471-4159.1992.tb09420.x. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., GUBITZ A.K., SIRINATHSINGHJI D.J.S., RICHARDSON P.J., FREEMAN T.C. Tissue distribution of adenosine receptor mRNA in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNWIDDIE T.V., MASINO S.A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- EL YACOUBI M., LEDENT C., PARMENTIER M., COSTENTIN J., VAUGEOIS J. SCH 58261 and ZM 241385 differentially prevent the motor effects of CGS 21680 in mice: evidence for a functional ‘atypical' adenosine A2A receptor. Eur. J. Pharmacol. 2000;401:63–77. doi: 10.1016/s0014-2999(00)00399-x. [DOI] [PubMed] [Google Scholar]
- EL YACOUBI M., LEDENT C., PARMENTIER M., ONGINI E., COSTENTIN J., VAUGEOIS J.M. In vivo labelling of the adenosine A2A receptor in mouse brain using the selective antagonist [3H]SCH 58261. Eur. J. Neurosci. 2001;14:1567–1570. doi: 10.1046/j.0953-816x.2001.01771.x. [DOI] [PubMed] [Google Scholar]
- FASTBOM J., PAZOS A., PALACIOS J.M. The distribution of adenosine A1 receptors and 5′-nucleotidase in the brain of some commonly used experimental animals. Neuroscience. 1987;22:813–826. doi: 10.1016/0306-4522(87)92961-7. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., GOLDSTEIN A.E., RYZHOV S., ZENG D., BELARDINELLI L., VOYNO-YASENETSKAYA T., BIAGGIONI I. Differential expression of adenosine receptors in human endothelial cells. Role of A2B receptors in angiogenic factor regulation. Circ. Res. 2002;90:531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., CUNHA R.A., SVENNINGSSON P. Pharmacology of adenosine A2A receptors and therapeutic applications. Curr. Top. Med. Chem. 2003;3:413–426. doi: 10.2174/1568026033392200. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., KLOTZ K.N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., LINDSTRÖM K., DIONISOTTI S., ONGINI E. [3H]SCH 58261, a selective adenosine A2A receptor antagonist, is a useful ligand in autoradiographic studies. J. Neurochem. 1998;70:1210–1216. doi: 10.1046/j.1471-4159.1998.70031210.x. [DOI] [PubMed] [Google Scholar]
- GAO Y., PHILLIS J.W. CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the mongolian gerbil. Life Sci. 1994;55:61–65. doi: 10.1016/0024-3205(94)00889-2. [DOI] [PubMed] [Google Scholar]
- HALLDNER L., LOZZA G., LINDSTRÖM K., FREDHOLM B.B. Lack of tolerance to motor stimulant effects of a selective adenosine A2A receptor antagonist. Eur. J. Pharmacol. 2000;406:345–354. doi: 10.1016/s0014-2999(00)00682-8. [DOI] [PubMed] [Google Scholar]
- JAMES S., XUEREB J.H., ASKALAN R., RICHARDSON P.J. Adenosine receptors in post-mortem human brain. Br. J. Pharmacol. 1992;105:238–244. doi: 10.1111/j.1476-5381.1992.tb14240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.F., SCHULZ R., HUTCHISON A.J., DO U.H., SILLS M.A., WILLIAMS M. [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J. Pharmacol. Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- JARVIS M.F., WILLIAMS M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur. J. Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- JOHANSSON B., FREDHOLM B.B. Further characterization of the binding of the adenosine receptor agonist [3H]CGS 21680 to rat brain using autoradiography. Neuropharmacology. 1995;34:393–403. doi: 10.1016/0028-3908(95)00009-u. [DOI] [PubMed] [Google Scholar]
- JOHANSSON B., GEORGIEV V., PARKINSON F.E., FREDHOLM B.B. The binding of the adenosine A2 receptor selective agonist [3H]CGS 21680 to rat cortex differs from its binding to rat striatum. Eur. J. Pharmacol. 1993;247:103–110. doi: 10.1016/0922-4106(93)90066-i. [DOI] [PubMed] [Google Scholar]
- JOHANSSON B., HALLDNER L., DUNWIDDIE T.V., MASINO S.A., POELCHEN W., GIMENEZ-LLORT L., ESCORIHUELA R.M., FERNANDEZ-TERUEL A., WIESENFELD-HALLIN Z., XU X.J., HARDEMARK A., BETSHOLTZ C., HERLENIUS E., FREDHOLM B.B. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc. Natl. Acad. Sci. USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES P.A., SMITH R.A., STONE T.W. Protection against hippocampal kainate excitotoxicity by intracerebral administration of an adenosine A2A receptor antagonist. Brain Res. 1998;800:328–335. doi: 10.1016/s0006-8993(98)00540-x. [DOI] [PubMed] [Google Scholar]
- KIRK I.P., RICHARDSON P.J. Further characterization of [3H]-CGS 21680 binding sites in the rat striatum and cortex. Br. J. Pharmacol. 1995;114:537–543. doi: 10.1111/j.1476-5381.1995.tb13260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOTZ K.N. Adenosine receptors and their ligands. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:382–391. doi: 10.1007/s002100000315. [DOI] [PubMed] [Google Scholar]
- LATINI S., BORDONI F., CORRADETTI R., PEPEU G., PEDATA F. Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices. Br. J. Pharmacol. 1999;128:1035–1044. doi: 10.1038/sj.bjp.0702888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDENT C., VAUGEOIS J.M., SCHIFFMANN S.N., PEDRAZZINI T., EL YACOUBI M., VANDERHAEGHEN J.J., COSTENTIN J., HEATH J.K., VASSART G., PARMENTIER M. Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature. 1997;388:674–678. doi: 10.1038/41771. [DOI] [PubMed] [Google Scholar]
- LI X.X., NOMURA T., AIHARA H., NISHIZAKI T. Adenosine enhances glial glutamate efflux via A2a adenosine receptors. Life Sci. 2001;68:1343–1350. doi: 10.1016/s0024-3205(00)01036-5. [DOI] [PubMed] [Google Scholar]
- LINDSTRÖM K., ONGINI E., FREDHOLM B.B. The selective adenosine A2A receptor antagonist SCH 58261 discriminates between two different binding sites for [3H]-CGS 21680 in the rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:539–541. doi: 10.1007/BF00168448. [DOI] [PubMed] [Google Scholar]
- LOPES L.V., CUNHA R.A., KULL B., FREDHOLM B.B., RIBEIRO J.A. Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience. 2002;112:319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- MARCHI M., RAITERI L., RISSO F., VALLARINO A., BONFANTI A., MONOPOLI A., ONGINI E., RAITERI M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br. J. Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOPOLI A., LOZZA G., FORLANI A., MATTAVELLI A., ONGINI E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. NeuroReport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- NANOFF C., MITTERAEUR T., ROKA F., HOHENEGGER M., FREISSMUTH M. Species differences in A1 adenosine receptor/G protein coupling: identification of a membrane protein that stabilizes the association of the receptor/G protein complex. Mol. Pharmacol. 1995;48:806–817. [PubMed] [Google Scholar]
- NISHIZAKI T., NAGAI K., NOMURA T., TADA H., KANNO T., TOZAKI H., LI X.X., KONDOH T., KODAMA N., TAKAHASHI E., SAKAI N., TANAKA K., DAITO N. A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. Glia. 2002;39:133–147. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- O'REAGAN M.H., SIMPSON R.E., PERKINS L.M., PHILLIS J.W. Adenosine receptor agonists inhibit the release of γ-aminobutyric acid (GABA) from the ischemic rat cerebral cortex. Brain Res. 1992;582:22–26. doi: 10.1016/0006-8993(92)90312-w. [DOI] [PubMed] [Google Scholar]
- PARKINSON F.E., FREDHOLM B.B. Autoradiographic evidence for G-protein coupled A2-receptors in rat neostriatum using [3H]-CGS 21680 as a ligand. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:85–91. doi: 10.1007/BF00178977. [DOI] [PubMed] [Google Scholar]
- PETERFREUND R.A., MACCOLLIN M., GUSELLA J., FINK J.S. Characterization and expression of the human A2a adenosine receptor gene. J. Neurochem. 1996;66:362–368. doi: 10.1046/j.1471-4159.1996.66010362.x. [DOI] [PubMed] [Google Scholar]
- PETRONI A., PAPINI N., BLASEVICH M., GALLI C. Blockade of A2A adenosine receptor leads to c-fos inhibition in a rat model of brain ischemia. Pharmacol. Res. 2002;45:125–128. doi: 10.1006/phrs.2001.0918. [DOI] [PubMed] [Google Scholar]
- REBOLA N., OLIVEIRA C.R., CUNHA R.A. Transducing system operated by adenosine A2A receptors to facilitate acetylcholine release in the rat hippocampus. Eur. J. Pharmacol. 2002;454:31–38. doi: 10.1016/s0014-2999(02)02475-5. [DOI] [PubMed] [Google Scholar]
- SVENNINGSSON P., LE MOINE C., FISONE G., FREDHOLM B.B. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog. Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- TEBANO M.T., DOMENICI M.R., POPOLI P. SCH 58261 differentially influences quinolinic acid-induced effects in striatal and in hippocampal slices. Eur. J. Pharmacol. 2002;450:253–257. doi: 10.1016/s0014-2999(02)02148-9. [DOI] [PubMed] [Google Scholar]
- WAN W., SUTHERLAND G.R., GEIGER J.D. Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human and rat brain: evidence of multiple affinity sites. J. Neurochem. 1990;55:1763–1771. doi: 10.1111/j.1471-4159.1990.tb04967.x. [DOI] [PubMed] [Google Scholar]
- YOSHIOKA K., SAITOH O., NAKATA H. Heteromeric association creates a P2Y-like adenosine receptor. Proc. Natl. Acad. Sci. USA. 2001;98:7617–7622. doi: 10.1073/pnas.121587098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOCCHI C., ONGINI E., FERRARA S., BARALDI P.G., DIONISOTTI S. Binding of the radioligand [3H]-SCH 58261, a new non-xanthine A2A adenosine receptor antagonist, to rat striatal membranes. Br. J. Pharmacol. 1996;117:1381–1386. doi: 10.1111/j.1476-5381.1996.tb15296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]