Abstract
Binding of the novel radioligand 3H-2-(2-dimethylaminomethyl-phenylsulphanyl)-5-methyl-phenylamine (3H-MADAM) to the serotonin transporter (SERT) was used to characterise a range of selective serotonin re-uptake inhibitors (SSRIs) in vitro and in vivo.
3H-MADAM bound with high affinity in a saturable manner to both human SERT expressed in CHO cells (Kd=0.20 nM (pKd=9.74±0.12), Bmax=35±4 fmol mg−1 protein) and mouse cerebral cortex membranes (Kd=0.21 nM (pKd=9.66±0.10), Bmax=50±24 fmol mg−1 protein).
Binding of 3H-MADAM was highly selective for SERT in vitro as demonstrated by the in vitro profile of MADAM tested at 75 different receptors, ion channels and transporters. This was further substantiated by the pharmacological profile of the binding. Hence, the binding of 3H-MADAM was potently inhibited by SSRIs but not by selective inhibitors of noradrenaline transport and dopamine transport. Likewise, a 5-HT2A/2C receptor antagonist did not inhibit 3H-MADAM binding.
3H-MADAM binding in vivo was inhibited only by compounds which also inhibited the binding of 3H-MADAM in vitro (the SSRIs, mixed SERT/noradrenaline transport inhibitors and clomipramine), confirming the selectivity of 3H-MADAM for SERT also in vivo. Moreover, compounds effective in inhibiting 3H-MADAM binding were the only ones found to be active in the mouse 5-HTP potentiation test confirming the model as a behavioural correlate to in vivo 5-HT uptake.
Finally, it was found that a SERT occupancy of 85–95% was necessary to produce 50% of the maximum behavioural response (ED50).
Keywords: Escitalopram, SERT, radioligand, occupancy, SSRI, in vivo binding, 5-HTP potentiation, depression
Introduction
Enhancement of serotonin (5-HT) neurotransmission by inhibition of the serotonin transporter (SERT) has been suggested as the primary mechanism of action for a class of antidepressants called selective serotonin re-uptake inhibitors (SSRIs; Suehiro et al., 1991; Blier & de Montigny, 1994; Artigas et al., 1996). This hypothesis has been supported by a number of microdialysis studies in rats showing an increase of brain serotonin following the administration of a SSRI (Fuller, 1994; Hjorth & Auerbach, 1994; Bosker et al., 1995; Gartside et al., 1995; Mørk et al., 2003). Likewise, SSRIs are effective in behavioural animal models predictive of antidepressant activity (Mitchell, 1994; Sanchez & Meier, 1997). In humans, inhibition of 5-HT synthesis, and thus a decrease of 5-HT levels, results in depression relapse in subjects who have recovered from depression and are drug free (Smith et al., 1997).
Although many groups have attempted to make a positron emission tomography (PET) SERT ligand, the success has been limited. Hence, the tricyclic compounds imipramine, clomipramine, and cyanoimipramine have all been tested as PET ligands but displayed high nonspecific binding in the brain (Hashimoto et al., 1987; Nakamura et al., 1989). Additionally, the tricyclic compounds are not highly selective for the SERT relative to the noradrenaline transporter (Hyttel, 1982). The phenyl nortropane series shows high SERT affinity and selectivity but poor signal-to-noise ratios (Nielsen et al., 1989; Bosker et al., 1995; Blough et al., 1997; Helfenbein et al., 1999a, 1999b), making them unsuitable for in vivo quantification of the SERT. Equally, sertraline, paroxetine, fluoxetine, and citalopram have been labelled with 11C but, in spite of good in vitro binding properties, these ligands also showed poor signal-to-noise ratios in vivo (Hashimoto & Goromaru, 1990; Suehiro et al., 1991; Hume et al., 1992; Gartside et al., 1995; Shiue et al., 1995; Choi et al., 2000; Emond et al., 2002).
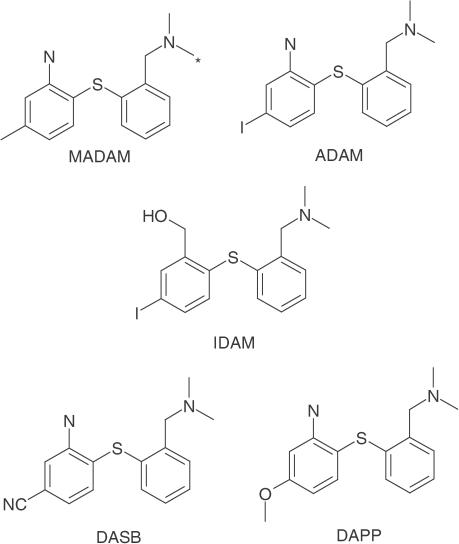
Substituted diphenyl sulphides represent a new class of potent and selective ligands for the SERT (Emond et al., 2002). Four derivatives of this class of compounds, IDAM, ADAM, DASB and DAPP (Figure 1), have been found to have nanomolar to picomolar affinities for the SERT (Oya et al., 1999; Choi et al., 2000; Wilson et al., 2000). All compounds show selectivity for the SERT over the dopamine transporter and noradrenaline transporter in vitro (Oya et al., 1999; Choi et al., 2000; Wilson et al., 2000). DASB and DAPP were tested as PET ligands in man, with DASB showing a better brain penetration than DAPP (Houle et al., 2000). DASB was used for a small PET study, revealing a SERT occupancy for citalopram and paroxetine of about 80% in depressed patients (Meyer et al., 2001). However, the study failed to link the occupancy to the final Hamilton depression scale score after 6 weeks of treatment (Meyer et al., 2001). Interestingly, a methylated derivative, 2-(2-dimethylaminomethyl-phenylsulphanyl)-5-methyl-phenylamine (MADAM, Figure 1), retains the high affinity for the SERT (Ki=1.65 nM) as well as the high selectivity over the noradrenaline transporter (Ki=683 nM) and the dopamine transporter (Ki>1000 nM, Tarkiainen et al., 2001; Emond et al., 2002). Moreover, MADAM can be labelled with 3H, making it suitable for in vitro studies. Here we report a comprehensive in vitro binding profile of MADAM, an in vitro 3H-MADAM-binding method and a method of measuring the SERT occupancy by in vivo binding experiments using 3H-MADAM as the radioligand. Moreover, we profile a number of SSRIs using this in vivo binding method and relate the calculated SERT occupancies to behavioural responses in the mouse 5-hydroxytryptophan (5-HTP) potentiation model, which is frequently used as a functional measure of 5-HT reuptake inhibition in vivo (Ortmann et al., 1980).
Figure 1.
Structures of the substituted diphenyl sulphides MADAM, ADAM, IDAM, DASB, and DAPP. The radiolabelled position on MADAM is marked by an asterisk.
Methods
Materials
Male NMRI/BOM mice (18–25 g; Bomholtgaard, Denmark) were used for in vivo binding and 5-HTP potentiation experiments. They were housed in plastic cages (35 × 30 × 12 cm3) in groups of five and habituated to the animal facilities for at least a week before testing. The room temperature (21±2°C), relative humidity (55±5%), and air exchange (16 times per h) were automatically controlled. The animals received food and water ad libitum. Ethical permissions for the studies were granted by the animal welfare committee, appointed by the Danish Ministry of Justice, and all animal procedures were carried out in compliance with the EC Directive 86/609/EEC and with the Danish law regulating experiments on animals.
Drugs
Escitalopram oxalate, fluoxetine hydrochloride, duloxetine hydrochloride, and sertraline hydrochloride were synthesised at H. Lundbeck A/S, Valby, Denmark. Paroxetine hydrochloride, venlafaxine, and reboxetine fumarate were extracted from commercially available tablets. Wallac OptiPhase Super Mix was purchased from NEN, Boston, MA, U.S.A. The BCA kit was from Pierce, Rockford, IL, U.S.A. All other chemicals were purchased from Sigma, Copenhagen, Denmark.
3H-MADAM (56–65 Ci mmol−1) was synthesised and radiolabelled at the Karolinska Institute, Stockholm. 3H-5-HT (25–27 Ci mmol−1) was purchased from Amersham Biosciences, Cardiff, U.K.
All test compounds were dissolved in DMSO for the in vitro studies (maximum final DMSO concentration 0.5%) and saline for in vivo experiments. In vivo results are given as mg base kg−1 body weight.
Cell line generation
A PCR fragment encoding the human SERT was amplified from cDNA reverse transcribed from human whole brain RNA (Clonetech) using standard methods. The fragment was inserted into a pCIneo (Promega) vector using XhoI/XbaI restriction sites included in the amplification primer and sequenced. The construct pCISERTneo was transfected into CHO cells using Lipofectamine (Invitrogen). The cells were trypsinised 2 days after transfection and diluted in Dulbecco's modified Eagle medium (DMEM) containing 10% foetal calf serum, Glutamax (1X), and 1 mg ml−1 G-418 and grown for 12–18 days. Cells were isolated using cloning rings from clonal cell clusters. The clones were tested for 3H-5-HT uptake activity (see below) and the best clones selected and subcloned.
3H-5-HT uptake activity was measured in HBS (150 mM NaCl, 1 mM CaCl2, 2.5 mM KCl, 2.5 mM MgSO4, 10 mM glucose, 10 mM HEPES, pH 7.4) containing 1 μCi 3H-5-HT and 2 μM 5-HT. The background was defined as uptake in the presence of 5 μM citalopram. The cells were incubated for 15 min at 37°C, transferred to ice and washed with ice-cold HBS. The cells were lysed with 0.5 ml 0.2 M NaOH, scintillation cocktail (Optima Gold, Parkard Instruments B.V., Groningen, The Netherlands) was added and the samples were counted in a scintillation counter (Packard Topcounter).
In vitro binding experiments
In vitro binding was carried out on mouse brain synaptosomes and membranes from a recombinant cell line expressing the human SERT (see above). For the synaptosome preparation, male NMRI mice were decapitated and the brains quickly removed. Cerebral cortex was dissected and homogenised in ice-cold buffer (50 mM TRIS, 120 mM NaCl, 5 mM KCl, pH 7.5) using an UltraTurrax homogeniser. The homogenate was centrifuged at 30,000 × g for 15 min at 4°C. The supernatant was discarded and the pellet re-suspended in buffer to a final protein concentration of 80 μg protein per well.
For the cell line preparation, cells were harvested in phosphate-buffered saline and centrifuged for 3 min at 1500 × g. The pellet was resuspended in centrifugation buffer (15 mM Tris, 2 mM MgCl2, 0.3 mM EDTA, 1 mM EGTA, pH 7.5) and homogenised in a glass-teflon homogeniser. After a centrifugation at 2000 × g for 10 min, the supernatant was centrifugated at 40,000 × g for 25 min, the pellet washed and centrifugated again at 40,000 × g for 25 min. The final pellet was resuspended in freezing buffer (7.5 mM Tris, 12.5 mM MgCl2, 0.3 mM EDTA, 1 mM EGTA, 250 mM sucrose, pH 7.5) and kept at −80°C.
The test compound, 3H-MADAM (0.5 nM) and tissue suspension were mixed and incubated for 60 min at 37°C. Increasing concentrations of 3H-MADAM (0.006–2.6 nM) were used for saturation experiments. The incubation was terminated by rapid filtration through UniFilter GF/C on a semi-automated Tomtec harvester (Mach IIIM) rinsing the filters three times with ice-cold buffer. After drying, the filters were dissolved in 35 μl Packard OptiPhase and counted on a MicroBeta TriLux scintillation counter. Binding in the absence of test compound defined the total binding, whereas binding in the presence of 10 μM fluoxetine defined the nonspecific binding. Inhibition curves were analysed using XLfit (IDBS, Guildford, U.K.) one-site competition curve (y=A+(B–A)/(1+(C/x)D), where A=minimum y; B=maximum y; C=log EC50 and D=slope factor). Kd and Bmax values were calculated using Prism (GraphPad, San Diego, CA, U.S.A.) one binding site hyberbola (y=(Bmaxx)/(Kd+x)).
In vivo binding experiments
In vivo binding experiments were essentially carried out as described by Andersen et al. (1987), with a few modifications.
All test compounds were dissolved in 0.9% NaCl and administered s.c. to male NMRI mice 30 min prior to treatment with 3H-MADAM. Citalopram (30 mg kg−1) defined the nonspecific binding and was given s.c. 30 min before 3H-MADAM administration in all experiments. Total binding was measured using saline-treated mice. The test compounds were administered at varying doses below and over the ED50 value.
Animals received 4 μCi (2.9–5.4 μCi) 3H-MADAM i.v. The mice were killed by cervical dislocation after 15 min, the brain quickly removed and cerebral cortex was dissected and homogenised in ice-cold buffer (50 mM TRIS-HCl, 120 mM NaCl, 5 mM KCl, pH 7.5). A volume of 0.5 ml of the homogenate was filtered on Whatmans GF/C filters (soaked in 0.1% PEI) and washed twice with 5 ml ice-cold buffer. This was always completed in less than 60 s subsequent to the cervical dislocation. Aliquots of homogenate (25 μl) were saved for protein determination and for counting and ‘normalisation' of filter samples (0.5 ml). The samples and filters were counted in a Packard Tricarb scintillation counter using Wallac OptiPhase Super Mix as scintillation liquid. The BCA protein determination assay was used for protein determination (Smith et al., 1985). In time experiments, the mice were killed 5, 15, 30, 60, and 120 min after receiving 4 μCi 3H-MADAM i.v.
ED50 values were calculated using XLfit as described for the in vitro binding experiments. Occupancy at a given dose was calculated as % occupancy=100%·dose at behaviour ED50/((ED50 (binding)) · dose.
TLC analysis
3H-MADAM (12 μCi ) was injected i.v. into male NMRI mice. After 15 min, the mice were killed, the brain quickly removed, the cerebral cortex dissected and homogenised in ethanol. The homogenate was centrifuged at 40,000 × g for 60 min at 4°C and the supernatant was analysed on TLC plates (silica gel 60, F254, Merck) using 2 mM MADAM as reference. The TLC plates were run in 60% heptane, 35% ethylacetate, and 5% triethylamine, dried and cut into 1 cm broad strips. Each strip was counted in a Packard Tricarb scintillation counter using 4 ml Wallac OptiPhase Super Mix as scintillation liquid.
Potentiation of 5-HTP-induced behavioural changes
The test was carried out as described in detail by Hyttel et al. (1992). In brief, 30 min after s.c. administration of test compound, mice were given 5-HTP (100 mg kg−1, i.v.). Thereafter, the animals were evaluated in their home cage during a 15-min observation period with respect to stereotypy (lateral head movements), tremor, and hind limb abduction. The behavioural changes were scored as 0=not present, 1=present in mild-to-moderate degree, 2=present in a marked degree. A total of 5–10 mice were used per dose. The drug response was calculated as mean (% of maximum score)±s.e.m. score. ED50 values were calculated by means of log-probit analyses.
Results
In vitro binding
The Hill coefficients were close to one, indicating a single binding site for 3H-MADAM in both synaptosomes from murine cerebral cortex and the CHO cell line. To confirm this, the curves were fitted to a one-site and a two-site competition model using Prism (GraphPad, San Diego, CA, U.S.A.). All data presented best fit to a one-site binding model (P< 0.05). The specific binding of 3H-MADAM was 70% for the CHO cell line preparation and 50% for the murine cerebral cortex preperation.
The affinity for 3H-MADAM was similar in both preparations with Kd values of 0.21 nM (pKd=9.66±0.10, n=3) and of 0.20 nM (pKd=9.74±0.12, n=3) for the synaptosomal and the CHO cell line preparations, respectively. Bmax values were 50±24 fmol mg−1 protein (n=3) and 35±4 fmol mg−1 protein (n=3) for mouse cerebral cortex synaptosomes and the CHO cell line, respectively.
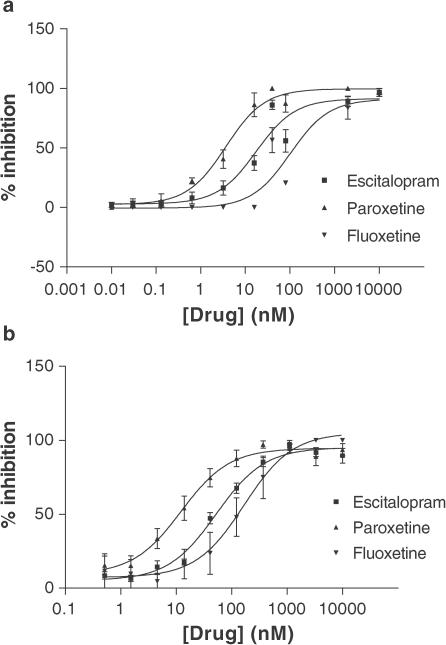
Results of the pharmacological profiling of the 3H-MADAM in vitro binding in murine synaptosomes and membranes from hSERT-expressing CHO cells are shown in Table 1 and Figure 2. SSRIs displaced 3H-MADAM with Ki values in the nM range (0.4–65), with paroxetine showing the highest affinity followed by escitalopram and fluoxetine. Cocaine, nisoxetine, desipramine, GBR12909, and mirtazepine did not displace 3H-MADAM from mouse synaptosomes. Nisoxetine and desipramine displayed low affinities (600–1500 nM) for the hSERT, as measured by inhibition of 3H-MADAM binding.
Table 1.
Using 3H-MADAM as radioligand, a range of SSRIs (escitalopram, fluoxetine, paroxetine, sertraline, and fluvoxamine), two mixed SERT/ noradrenaline transport inhibitors (duloxetine and venlafaxine), a nonselective monoamine transport inhibitor (cocaine), two selective noradrenaline noradrenaline re-uptake inhibitors (desipramine and nisoxetine), a dopamine transport inhibitor (GBR-12909), a tricyclic antidepressant (clomipramine), and a 5-HT2A/2C antagonist (mirtazepine) were tested in vitro on murine synaptosomes (second column) and a stable hSERT-expressing CHO cell line (third column) and in in vivo binding (fourth column). Additionally, the compounds were tested in vivo in 5-HTP potentiation (fifth column) and using the in vivo binding ED50, the occupancy at the ED50 from the 5-HTP potentiation experiments was calculated (sixth column). The in vitro binding data are mean Ki and numbers in paragraphs are mean pKi±s.e.m. for at least three experiments. For the in vivo data, please see the 95% confidence interval in the table. Larger than (>) signifies that there was less than 30% response at the given concentration or dose of drug. The > values represent the maximum dose of drug examined and was selected so that known behavioural effects (e.g. effects on locomotor activity) would not obscure the behavioural scoring in the 5-HTP potentiation test. ED50 values are the mean of at least three experiments unless otherwise stated in the table.
| Compound | In vitro Ki (nM) murine synaptosomes (pKi±s.e.m.) | In vitro Ki (nM) hSERT in CHO cell line (pKi±s.e.m.) | ED50 in vivo binding (mg kg−1) 95% confidence interval | ED50 5-HTP potentiation (mg/kg−1) 95% confidence interval | Occupancy (%) at the ED50 of 5-HTP potentiation |
|---|---|---|---|---|---|
| Escitalopram | 8 | 11 | 0.07 | 0.36 | 84 |
| (8.12±0.09) | (7.97±0.07) | 0.04–0.14 | 0.20–0.65 | ||
| Fluoxetine | 33 | 64 | 2.00 | 30 | 94 |
| (7.48±0.10) | (7.19±0.10) | 1.26–3.25 | 18–51 | ||
| Paroxetine | 0.6 | 2.7 | 0.05 | 1.2 | 96 |
| (9.24±0.14) | (8.57±0.14) | 0.029–0.100 | 0.86–1.7 | ||
| Sertraline | 5 | 18 | 0.25 | 3.3 | 93 |
| (8.29±0.09) | (7.75±0.11) | 0.17–0.36 | 1.8–5.9 | ||
| Fluvoxamine | 20 | 51 | 0.45 | 3.2 | 93 |
| (7.70±0.11) | (7.29±0.09) | 0.33–0.61 | 2.1–4.8 | ||
| Duloxetine | 5 | 10 | 0.25 | 1.1 | 81 |
| (8.34±0.12) | (8.02±0.07) | 0.17–0.35 | 0.85–1.4 | ||
| Venlafaxine | 300 | 150 | 0.83 | 3.6 | 81 |
| (6.52±0.14) | (6.82±0.12) | 0.53–1.29 | 2.6–5.0 | ||
| Cocaine | >10,000 | >1000 | 7.1 | >4.5 | |
| 5.4–9.3 | |||||
| Desipramine | >10,000 | 597 | >10 | >35 | |
| (6.22±0.10) | (n=2) | ||||
| Nisoxetine | >10,000 | 800 | >30 | >20 | |
| (6.10±0.16) | (n=2) | ||||
| GBR-12909 | >10,000 | >10,000 | >30 (n=2) | >20 | |
| Clomipramine | 19 | 4.7 | 0.60 | 7.9 | 93 |
| (7.73±0.09) | (8.33±0.10) | 0.44–0.80 | 5.6–11 | ||
| Mirtazepine | >10,000 | >10,000 | >30 (n=2) | >10 | |
| Reboxetine | >10,000 | 416 | >1.25 | >11 | |
| (6.38±0.11) | (n=2) |
Figure 2.
Inhibition of specific 3H-MADAM in vitro binding by escitalopram, fluoxetine, and paroxetine, n⩾3 for each curve. Top: The inhibition experiments were carried out on synaptosomes from murine cerebral cortex. Bottom: binding carried out on hSERT expressed in CHO cells. Increasing concentrations of drug (x-axis) were added to the tissue preparation, incubated for 60 min at 37°C and harvested by rapid filtration over vacuum.
In order to further explore the selectivity of MADAM in vitro, the compound was profiled in a commercial screen (Cerep, France) for activity at 75 different receptors, ion channels, and transporters using a test concentration of 1 μM. The compound was tested in two independent experiments at all targets. Briefly, MADAM had low affinity for noradrenaline uptake (IC50=450 nM, rat hypothalamus), histamine H2 (Ki=600 nM, pKi=6.22±0.01 guinea-pig striatum), serotonin 5-HT7 (Ki=1100 nM, pKi=5.97±0.03, human recombinant) and the NPY receptor Y2 (Ki=600 nM, pKi=6.22±0.07 human neuroblastoma cells).
MADAM displayed no affinity, that is, less than 30% inhibition at the 1 μM test concentration, at the following targets: adenosine (A1–A3), α1- and α2-adrenergic, β1- and β2-adrenergic, angiotensin AT1 and AT2, atrial natriuretic peptide, central and peripheral benzodiazepine receptors, bombesin, bradykinin B2, calcitonin gene-related peptide, cannabinoid CB1 and CB2, cholecystokinin A and B, dopamine (D1, D2, D3, D4, and D5), dopamine uptake, endothelin A and B, GABA (nonselective), galanin, platelet-derived growth factor, interleukin 8B, tumour necrosis factor α, chemokine receptor 1, histamine H1, melatonin ML1, muscarinic (M1–M5), tachykinin NK1–NK3, neuropeptide Y1, neurotensin (NTS1), δ-, κ-, and μ-opioid receptors, orphanin ORL1, PACAP PAC1, phencyclidine, prostanoid TXA2/ PGH2 and PGI2, purinergic P2X and P2Y, serotonin receptors (5-HT1A, 5-HT1B, 5-HT2A, 5-HT2C, 5-HT3, 5-HT5A, 5-HT6), sigma (rat brain) somatostatin, vasoactive intestinal peptide VIP1, vasopressin V1a, calcium channel (L-type), voltage-dependent potassium channels or sodium (site 2), and chloride channels.
In vivo binding
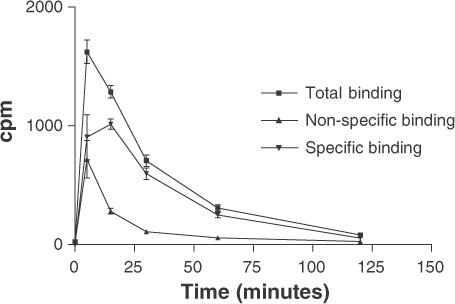
The specific 3H-MADAM binding in vivo reached a maximum after 15 min. Consequently, this time point was chosen as the radioligand pretreatment time for future experiments (Figure 3).
Figure 3.
Kinetic profile of 3H-MADAM binding to murine cerebral cortex in vivo. The radioligand (4 μCi in 0.2 ml 0.9% NaCl) was injected i.v. in the tail after 5–120 min (x-axis, n=3). Subsequently, the radioactivity in 0.5 ml cerebral cortex homogenate was measured (y-axis) and is expressed on the graph as counts per minute (c.p.m.). The specific binding reached a maximum after 15 min. Citalopram (30 mg kg−1) defined the nonspecific binding and was given s.c. 30 min before administration of 3H-MADAM.
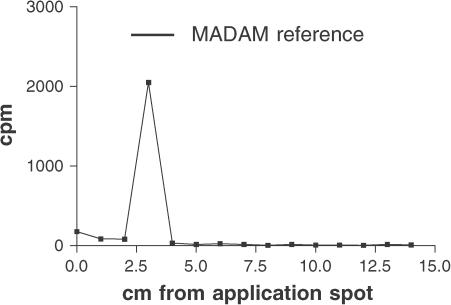
To verify that the radioactivity measured in the in vivo experiments was nonmetabolised 3H-MADAM, a TLC analysis was carried out on the murine cerebral cortex homogenate. TLC analysis of 3H-MADAM extracted with ethanol from murine cerebral cortex homogenate showed that 15 min after i.v. injection the vast majority of the radioactivity was found in a band corresponding to pure reference MADAM (Figure 4).
Figure 4.
TLC analysis of 3H-MADAM extracted with ethanol from murine cerebral cortex homogenate 15 min after injection (i.v.). TLC plates were run in 60% heptane, 35% ethylacetate, and 5% triethylamine, dried and cut into 1-cm strips as expressed on the x-axis. The radioactivity accumulated on the strips was measured and is expressed in c.p.m. on the graph. The vast majority of the radioactivity was localised on the plate corresponding to reference MADAM when examined under UV light.
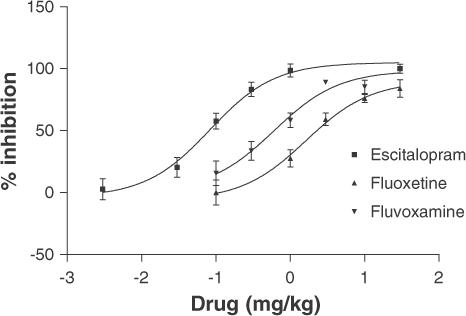
To confirm that 3H-MADAM also bound to the SERT in vivo, a range of compounds were tested in the in vivo binding assay (Figure 5 and Table 1). The SSRIs, escitalopram, paroxetine, fluoxetine, fluvoxamine and sertraline, and the mixed SERT/noradrenaline transporter inhibitors, venlafaxine and duloxetine inhibited 3H-MADAM binding with ED50 values between 0.05 (paroxetine) and 2.00 (fluoxetine) mg kg−1. Cocaine had an ED50 value of 7.1 mg kg−1, whereas nisoxetine, desipramine, GBR12909, and mirtazepine had no effect at doses which are effective in in vivo models of noradrenaline or dopamine transport inhibition or inhibition of 5-HT2A/2C. The Hill coefficients of in vivo inhibition curves were close to one, indicating a single binding site as shown in the in vitro experiments. This was confirmed by fitting the curves to a one-site and a two-site competition model using Prism (GraphPad, San Diego, CA). Once again, all data presented best fit to a one-site binding model (P<0.05).
Figure 5.
Pharmacological profile of specific 3H-MADAM binding in vivo. Test compounds or vehicle were administered s.c. 30 min before 3H-MADAM and 3H-MADAM was administered 15 min before the mice were killed by cervical dislocation. Cerebral cortex homogenate was homogenised by an Ultra Turrax and filtered over vacuum. Each point represents an average from nine mice in three separate experiments.
5-HTP potentiation
In the 5-HTP potentiation model, only compounds with nM affinity for the SERT showed an effect confirming that this animal model reflects SERT activity. Hence, the SSRIs, the mixed SERT/noradrenaline transport inhibitors, and the tricyclic antidepressant clomipramine potentiated the behavioural effects of 5-HTP in mice, whereas cocaine and the selective noradrenaline and dopamine transport inhibitors had no or very limited effect (Table 1).
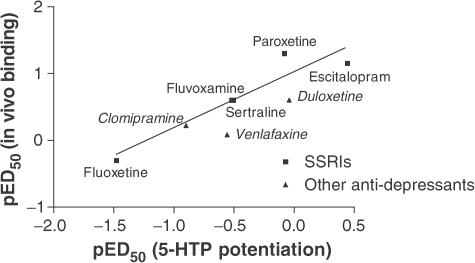
The in vivo binding data exhibited a significant correlation (r2=0.86) with data from the behavioural studies as shown in Figure 6.
Figure 6.
Correlation between in vivo binding pED50 and pED50 obtained from behavioural 5-HTP experiments. The slope of the graph was 0.82 and r2=0.86, indicating a good correlation between the in vivo binding and the 5-HTP behavioural model of SERT activation. The correlation was based only on the data from the SSRIs, but duloxetine, venlafaxine, and clomipramine were included on the graph to illustrate that they follow the same trend as the SSRIs.
Using the ED50 values obtained from the 5-HTP potentiation experiments and the ED50 values and compound concentrations from the in vivo binding experiments, we calculated SERT occupancies as described (Methods section, In vivo binding experiments). SERT occupancies varied from around 80% for escitalopram and the mixed SERT/noradrenaline transport inhibitors to more than 90% for the classical SSRIs.
Discussion
The present study demonstrates that 3H-MADAM specifically binds to SERT in vitro as well as in vivo. Additionally, all in vitro and in vivo data were fitted to a one-site and a two-site competition model, and best fit was found for the one site model. The synaptosome preparation and the CHO cell line showed equal affinity for 3H-MADAM and the two preparations contained approximately the same amount of protein.
In vitro, the selectivity of MADAM was tested against 75 different receptors, ionchannels and transporters, demonstrating an excellent specificity for the SERT with a factor of more than 1000 against NA uptake inhibition, affinity for the histamine H2, 5-HT7, and neuropeptide Y2 receptors. In addition, a range of compounds were tested on murine synaptosomes and a stable CHO cell line expressing hSERT, confirming that 3H-MADAM was displaced by compounds known to have nM affinity for the SERT. Compounds selective for the noradrenaline and dopamine transport inhibited specific 3H-MADAM binding with weak or no affinity. This is in accordance with a preliminary in vitro study reported by Chalon et al. (2003).
In vivo, 3H-MADAM penetrated the brain rapidly with the specific binding reaching a maximum after 15 min. Specific binding of 3H-MADAM was potently inhibited by SSRIs, that is, escitalopram, paroxetine, fluoxetine, fluvoxamine and sertraline, as well as by the nonselective SERT/noradrenaline transport blockers venlafaxine and duloxetine. The stimulant drug cocaine inhibited 3H-MADAM binding with an ED50 100-fold higher than that of escitalopram. Moreover, the selective noradrenaline transport inhibitors nisoxetine and desipramine, the selective DA uptake inhibitor GBR12909, and the 5-HT2A/2C antagonist mirtazepine did not affect 3H-MADAM binding in vivo. Since the rank order of potency of the SSRIs was similar in vitro and in vivo and since the selective noradrenaline re-uptake inhibitors, a dopamine re-uptake inhibitor, and a 5-HT2A/2C antagonist did not displace 3H-MADAM in vitro or in vivo, we conclude that MADAM binds selectively to the SERT in vitro and in vivo and that 3H-MADAM can be used as a radioligand for SERT also in in vivo binding studies. Since the binding observed in murine cerebral cortex in vivo showed a good signal-to-noise ratio (total: nonspecific binding=4), it is likely that MADAM could also be used as a PET ligand. However, this may also depend on the pharmacokinetic properties of MADAM in human.
Occupancy measured by in vivo binding has previously been reported for a number of receptors and ion channels. Thus, reports of in vivo binding to the G-protein-coupled receptors dopamine D1 and D2 (Nielsen et al., 1989), 5-HT1A (Laporte et al., 1994), and metabotropic glutamate mGluR5 (Anderson et al., 2002) receptors have been published. In addition, methods for quantifying ion-channel-coupled NMDA (Murray et al., 2000) and benzodiazepine (Goeders & Kuhar, 1985) receptor occupancy are known. However, this report is the first description of a radioligand suitable for in vivo binding to the SERT and, hence, measurements of SERT occupancy.
In order to compare data from the mouse 5-HTP potentiation test, a behavioural correlate of in vivo 5-HT uptake inhibition, and the in vivo binding studies, the route of administration for the drugs (s.c.) and pretreatment periods (30 min) were kept identical. Thus, factors such as metabolism, blood–brain barrier penetration, and plasma protein binding did not differ between the models, and thus influenced the data sets to the same extent.
The rank order of potency achieved in the mouse 5-HTP potentiation model was consistent with the results achieved from the 3H-MADAM binding both in vitro and in vivo. The good correlation between the ED50 values of the in vivo binding and behavioural data supports the usefulness of animal models such as 5-HTP potentiation for characterisation of putative SERT inhibitors in vivo.
SERT occupancy calculations revealed that the classical SSRIs like sertraline, paroxetine, fluoxetine, and fluovoxamine, displayed a 93–96% occupancy of the SERT at the dose, yielding 50% of the maximal behavioural response. The mixed SERT/noradrenaline transport inhibitors produced the same behavioural response at only 81% SERT occupancy. However, a recently published microdialysis study demonstrated that venlafaxine and duloxetine produced increases of extracellular 5-HT brain levels that were similar to those of paroxetine, citalopram, and fluoxetine (Felton et al., 2003). Although the exact molecular mechanism for the low SERT occupancy of duloxetine and venlafaxine in the present animal model is unclear, it could be speculated that the presumably extremely high concentrations of brain 5-HT in the 5-HTP potentiation model results in interactions with aspects of the noradrenergic system (i.e. release). However, a thorough discussion of these complex interactions falls outside the scope of this paper.
The tricyclic antidepressant clomipramine demonstrated the same SERT occupancy (93%) in the behavioural model as the four classical SSRIs. Clomipramine has a mixed target profile with nanomolar inhibition of the SERT among other targets, but the exact molecular mechanism of action of clomipramine in this animal model remains obscure.
Dopamine transport inhibition by GBR-12909 and 5-HT2A/2C antagonism by mirtazepine did not affect the 5-HTP potentiation. This demonstrates that the 5-HTP potentiation model is not influenced by dopamine transport inhibition and 5-HT2A/2C receptor inhibition, since this would have altered the SERT occupancy. Noradrenaline transport inhibition appears to be involved although to a fairly small degree.
A SERT occupancy of 93–96% was required to produce 50% of the maximum behavioural response for four classical SSRIs. Escitalopram, on the other hand, was significantly more potent in the 5-HTP potentiation model and displayed an occupancy of only 84% at the dose required to induce 50% of the maximal response. However, this was not significantly different from the four classical SSRIs, presumably due to the large variation in the in vivo binding model, resulting in a relatively large accumulated variation in the calculated occupancies.
The dose levels used in this study were clinically relevant, since the ED50 value of escitalopram in the 5-HTP potentiation model corresponds to a plasma concentration of approximately 50 ng ml−1 (unpublished observation). This concentration is very similar to the plasma concentrations measured in humans treated with clinically active doses of escitalopram (Gutierrez & Mengel, 2002). Moreover, a small PET study in humans showed that citalopram and paroxetine produced an occupancy of about 80% at clinically relevant doses (Meyer et al., 2001) and this correlates well with the results in this study.
Clinically, escitalopram has been shown to have superior efficacy compared to paroxetine in a recent study in patients with social anxiety disorder (Montgomery et al., 2003). Additionally, escitalopram has consistently shown superior efficacy over citalopram in the three double-blind randomised clinical trials conducted in patients with major depressive disorder (Burke et al., 2002; Colonna et al., 2002; Lepola et al., 2003). However, although escitalopram displayed a lower SERT occupancy, the clinical superiority would not have been predicted from the present study.
In conclusion, this study supports the usefulness of in vivo binding to the SERT for predicting the in vivo level of SERT occupancy necessary for keeping depressed patients free from symptoms. In addition, the close correlation between the pED50 values of the in vivo binding and behavioural data supports the value of animal models such as 5-HTP potentiation for characterisation of putative SERT inhibitors in vivo and strongly suggests that the doses used in the behavioural models are clinically relevant. Escitalopram showed a tendency towards a lower SERT occupancy at active pharmacological doses as compared to the classical SSRIs. Although nonsignificant, it is tempting to suggest that this finding could support that escitalopram is a more efficacious SSRI.
Acknowledgments
We thank Nina Guldhammer, Tina Kimer, Berit Rasmussen, Lisbeth Petri, Kamilla Rønne, Dorit Skov, and Kirsten Assing for their excellent technical assistance.
Abbreviations
- ADAM
2-([2-([dimethylamino]methyl)phenyl]thio)-5-[(123)I]iodophenylamine
- CHO
chinese hamster ovary
- DAPP
(N,N-dimethyl-2-(2-amino-4-methoxyphenylthio)benzylamine
- DASB
(N,N-dimethyl-2-(2-amino-4-cyanophenylthio)benzylamine
- 5-HT
5-hydroxytryptamine
- 5-HTP
5-hydroxytryptophan
- IDAM
5-iodo-2-((2-((dimethylamino)methyl)phenyl)thio)benzyl alcohol
- i.v.
intravenous
- MADAM
2-(2-dimethylaminomethyl-phenylsulphanyl)-5-methyl-phenylamine
- PET
positron emission tomography
- s.c.
subcutaneous
- SERT
serotonin transporter
- SSRI
selective serotonin re-uptake inhibitor
- u.v.
ultraviolet
References
- ANDERSEN P.H., JANSEN J.A., NIELSEN E.B. [3H]GBR 12935 binding in vivo in mouse brain: labelling of a piperazine acceptor site. Eur. J. Pharmacol. 1987;144:1–6. doi: 10.1016/0014-2999(87)90002-1. [DOI] [PubMed] [Google Scholar]
- ANDERSON J.J., RAO S.P., ROWE B., GIRACELLO D.R., HOLTZ G., CHAPMAN D.F., TEHRANI L., BRADBURY M.J., COSFORD N.D., VARNEY M.A. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J. Pharmacol. Exp. Ther. 2002;303:1044–1051. doi: 10.1124/jpet.102.040618. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BLOUGH B.E., ABRAHAM P., MILLS A.C., LEWIN A.H., BOJA J.W., SCHEFFEL U., KUHAR M.J., CARROLL F.I. 3 Beta-(4-ethyl-3-iodophenyl)nortropane-2 beta-carboxylic acid methyl ester as a high-affinity selective ligand for the serotonin transporter. J. Med. Chem. 1997;40:3861–3864. doi: 10.1021/jm970492z. [DOI] [PubMed] [Google Scholar]
- BOSKER F.J., KLOMPMAKERS A.A., WESTENBERG H.G. Effects of single and repeated oral administration of fluvoxamine on extracellular serotonin in the median raphe nucleus and dorsal hippocampus of the rat. Neuropharmacology. 1995;34:501–508. doi: 10.1016/0028-3908(95)00023-y. [DOI] [PubMed] [Google Scholar]
- BURKE W.J., GERGEL I., BOSE A. Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. J. Clin. Psychiatry. 2002;63:331–336. doi: 10.4088/jcp.v63n0410. [DOI] [PubMed] [Google Scholar]
- CHALON S., TARKIAINEN J., GARREAU L., HALL H., EMOND P., VERCOUILLIE J., FARDE L., DASSE P., VARNAS K., BESNARD J.C., HALLDIN C., GUILLOTEAU D. Pharmacological characterization of N,N-dimethyl-2-(2-amino-4-methylphenyl thio)benzylamine as a ligand of the serotonin transporter with high affinity and selectivity. J. Pharmacol. Exp. Ther. 2003;304:81–87. doi: 10.1124/jpet.102.042226. [DOI] [PubMed] [Google Scholar]
- CHOI S.R., HOU C., OYA S., MU M., KUNG M.P., SICILIANO M., ACTON P.D., KUNG H.F. Selective in vitro and in vivo binding of [(125)I]ADAM to serotonin transporters in rat brain. Synapse. 2000;38:403–412. doi: 10.1002/1098-2396(20001215)38:4<403::AID-SYN5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- COLONNA L., REINES E.H., ANDERSEN H.F. Escitalopram is well tolerated and more efficacious than citalopram in long-term treatment of moderately depressed patients. Int. J. Psych. Clin. Prac. 2002;6:243–244. [Google Scholar]
- EMOND P., VERCOUILLIE J., INNIS R., CHALON S., MAVEL S., FRANGIN Y., HALLDIN C., BESNARD J.C., GUILLOTEAU D. Substituted diphenyl sulphides as selective serotonin transporter ligands: synthesis and in vitro evaluation. J. Med. Chem. 2002;45:1253–1258. doi: 10.1021/jm010939a. [DOI] [PubMed] [Google Scholar]
- FELTON T.M., KANG T.B., HJORTH S., AUERBACH S.B. Effects of selective serotonin and serotonin/noradrenaline reuptake inhibitors on extracellular serotonin in rat diencephalon and frontal cortex. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:297–305. doi: 10.1007/s00210-002-0688-x. [DOI] [PubMed] [Google Scholar]
- FULLER R.W. Uptake inhibitors increase extracellular serotonin concentration measured by brain microdialysis. Life Sci. 1994;55:163–167. doi: 10.1016/0024-3205(94)00876-0. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJOS M., SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEDERS N.E., KUHAR M.J. Benzodiazepine receptor binding in vivo with [3H]-Ro 15-1788. Life Sci. 1985;37:345–355. doi: 10.1016/0024-3205(85)90505-3. [DOI] [PubMed] [Google Scholar]
- GUTIERREZ M., MENGEL H. Pharmacokinetics of escitalopram. Poster Presented at the 42nd Annual New Clinical Drug Evaluation Unit Meeting June 10–13, Boca Raton, FL, U.S.A. 2002.
- HASHIMOTO K., GOROMARU T. Evaluation of 3H-paroxetine as a radioligand for in vivo study of 5-hydroxytryptamine uptake sites in mouse brain. Radioisotopes. 1990;39:335–341. doi: 10.3769/radioisotopes.39.8_335. [DOI] [PubMed] [Google Scholar]
- HASHIMOTO K., INOUE O., SUZUKI K., YAMASAKI T., KOJIMA M. Synthesis and evaluation of [11C]cyanoimipramine. Int. J. Rad. Appl. Instrum. B. 1987;14:587–592. doi: 10.1016/0883-2897(87)90030-4. [DOI] [PubMed] [Google Scholar]
- HELFENBEIN J., EMOND P., SANDELL J., HALLDIN C., PEREYRE S., FRANGIN Y., GARREAU L., BESNARD J.C., GUILLOTEAU D., CHALON S. Synthesis and radiolabelling of 2-beta-carboxymethoxy-3 beta-(3′-iodo-4′-isopropylphenyl) nortropane as a radioligand for the exploration of the serotonin transporter by spet. J. Labelled Cpd. Radiopharm. 1999a;42:337–347. [Google Scholar]
- HELFENBEIN J., LOCH C., BOTTLAENDER M., EMOND P., COULON C., OTTAVIANI M., FUSEAU C., CHALON S., GUENTHER I., BESNARD J.C., FRANGIN Y., GUILLOTEAU D., MAZIERE B. A selective radiobrominated cocaine analogue for imaging of dopamine uptake sites: pharmacological evaluation and PET experiments. Life Sci. 1999b;65:2715–2726. doi: 10.1016/s0024-3205(99)00540-8. [DOI] [PubMed] [Google Scholar]
- HJORTH S., AUERBACH S.B. Lack of 5-HT1A autoreceptor desensitization following chronic citalopram treatment, as determined by in vivo microdialysis. Neuropharmacology. 1994;33:331–334. doi: 10.1016/0028-3908(94)90062-0. [DOI] [PubMed] [Google Scholar]
- HOULE S., GINOVART N., HUSSEY D., MEYER J.H., WILSON A.A. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur. J. Nucl. Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- HUME S.P., LAMMERTSMA A.A., BENCH C.J., PIKE V.W., PASCALI C., CREMER J.E., DOLAN R.J. Evaluation of S-[11C]citalopram as a radioligand for in vivo labelling of 5-hydroxytryptamine uptake sites. Int. J. Radiat. Appl. Instrum. B. 1992;19:851–855. doi: 10.1016/0883-2897(92)90171-t. [DOI] [PubMed] [Google Scholar]
- HYTTEL J. Citalopram – pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1982;6:277–295. doi: 10.1016/s0278-5846(82)80179-6. [DOI] [PubMed] [Google Scholar]
- HYTTEL J., BØGESØ K.P., PERREGAARD J., SÁNCHEZ C. The pharmacological effect of citalopram residues in the (S)-(+)-enantiomer. J. Neural. Transm. Gen. Sect. 1992;88:157–160. doi: 10.1007/BF01244820. [DOI] [PubMed] [Google Scholar]
- LAPORTE A.M., LIMA L., GOZLAN H., HAMON M. Selective in vivo labelling of brain 5-HT1A receptors by [3H]WAY 100635 in the mouse. Eur. J. Pharmacol. 1994;271:505–514. doi: 10.1016/0014-2999(94)90812-5. [DOI] [PubMed] [Google Scholar]
- LEPOLA U.M., LOFT H., REINES E.H. Escitalopram (10–20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. Int. Clin. Psychopharmacol. 2003;18:211–217. doi: 10.1097/00004850-200307000-00003. [DOI] [PubMed] [Google Scholar]
- MEYER J.H., WILSON A.A., GINOVART N., GOULDING V., HUSSEY D., HOOD K., HOULE S. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: a [(11)C]DASB PET imaging study. Am. J. Psychiatry. 2001;158:1843–1849. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- MITCHELL P.J.Prediction of antidepressant activity from ethiological analysis of agonistic behaviour in rats Ethology and Psychopharmacolgy 1994Chichester: John Wiley & Sons Ltd; 85–109.ed. Cooper S.J. & Hendrie C.A. pp [Google Scholar]
- MONTGOMERY S.A., LADER M., NIL R. Escitalopram and paroxetine in fixed doses for the treatment of social anxiety disorder (SAD) Nord. J. Psychiatry. 2003;57:103. [Google Scholar]
- MURRAY F., KNNEDY J., HUTSON P.H., ELLIOT J., HUSCROFT I., MOHNEN K., RUSSELL M.G., GRIMWOOD S. Modulation of [3H]MK-801 binding to NMDA receptors in vivo and in vitro. Eur. J. Pharmacol. 2000;397:263–270. doi: 10.1016/s0014-2999(00)00263-6. [DOI] [PubMed] [Google Scholar]
- MØRK A., KREILGAARD M., SANCHEZ C. The R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharmacology. 2003;45:167–173. doi: 10.1016/s0028-3908(03)00138-2. [DOI] [PubMed] [Google Scholar]
- NAKAMURA H., EDO K., HISHINUMA T., TAKAHASHI T., IDO T., MIZUGAKI M. Synthesis of C-labeled imipramine and its biodistribution in mice: a potential tracer for positron emission tomography. Chem. Pharm. Bull. (Tokyo) 1989;37:3376–3379. doi: 10.1248/cpb.37.3376. [DOI] [PubMed] [Google Scholar]
- NIELSEN E.B., RANDRUP K., ANDERSEN P.H. Amphetamine discrimination: effects of dopamine receptor agonists. Eur. J. Pharmacol. 1989;160:253–262. doi: 10.1016/0014-2999(89)90498-6. [DOI] [PubMed] [Google Scholar]
- ORTMANN R., WALDMEIER P.C., RADEKE E., FELNER A., DELINI-STULA A. The effects of 5-HT uptake- and MAO-inhibitors on L-5-HTP-induced excitation in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1980;311:185–192. doi: 10.1007/BF00510258. [DOI] [PubMed] [Google Scholar]
- OYA S., KUNG M.P., ACTON P.D., MU M., HOU C., KUNG H.F. A new single-photon emission computed tomography imaging agent for serotonin transporters: [123I]IDAM, 5-iodo-2-((2-((dimethylamino)methyl)phenyl)thio)benzyl alcohol. J. Med. Chem. 1999;42:333–335. doi: 10.1021/jm9806751. [DOI] [PubMed] [Google Scholar]
- SANCHEZ C., MEIER E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike. Psychopharmacology (Berl.) 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- SHIUE C.Y., SHIUE G.G., CORNISH K.G., O'ROURKE M.F. PET study of the distribution of [11C]fluoxetine in a monkey brain. Nucl. Med. Biol. 1995;22:613–616. doi: 10.1016/0969-8051(94)00146-b. [DOI] [PubMed] [Google Scholar]
- SMITH K.A., FAIRBURN C.G., COWEN P.J. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- SMITH P.K., KROHN R.I., HERMANSON G.T., MALLIA A.K., GARTNER F.H., PROVENZANO M.D., FUJIMOTO E.K., GOEKE N.M., OLSON B.J., KLENK D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- SUEHIRO M., WILSON A.A., SCHEFFEL U., DANNALS R.F., RAVERT H.T., WAGNER H.N. Radiosynthesis and evaluation of N-(3-[18F]fluoropropyl)paroxetine as a radiotracer for in vivo labeling of serotonin uptake sites by PET. Int. J. Radiat. Appl. Instrum. B. 1991;18:791–796. doi: 10.1016/0883-2897(91)90019-h. [DOI] [PubMed] [Google Scholar]
- TARKIAINEN J., VERCOUILLIE J., EMOND P., SANDELL J., HILTUNEN J., FRANGIN Y., GUILLOTEAU D., HALLDIN C. Carbon-11 labelling of MADAM in two different positions: a highly selective PET radioligand for the serotonin transporter. J. Labelled Cpd. Radiopharm. 2001;44:1013–1023. [Google Scholar]
- WILSON A.A., GINOVART N., SCHMIDT M., MEYER J.H., THRELKELD P.G., HOULE S. Novel radiotracers for imaging the serotonin transporter by positron emission tomography: synthesis, radiosynthesis, and in vitro and ex vivo evaluation of (11)C-labeled 2-(phenylthio)araalkylamines. J. Med. Chem. 2000;43:3103–3110. doi: 10.1021/jm000079i. [DOI] [PubMed] [Google Scholar]