Abstract
In the present study we determined whether angiotensin II (Ang II) could increase cyclic GMP levels in two blood vessels that exhibit markedly different angiotensin II receptor subtype expression: rat uterine artery (UA; AT2 receptor-predominant) and aorta (AT1 receptor-predominant), and investigated the receptor subtype(s) and intracellular pathways involved.
UA and aorta were treated with Ang II in the absence and presence of losartan (AT1 antagonist; 0.1 μM), PD 123319 (AT2 antagonist; 1 μM), NOLA (NOS inhibitor; 30 μM), and HOE 140 (B2 antagonist; 0.1 μM), or in combination.
Ang II (10 nM) induced a 60% increase in UA cyclic GMP content; an effect that was augmented with PD 123319 and HOE 140 pretreatment, and abolished by cotreatment with losartan, as well as by NOLA.
In aorta, Ang II produced concentration-dependent increases in cyclic GMP levels. Unlike effects in UA, these responses were abolished by PD 123319 and by NOLA, whereas losartan and HOE 140 caused partial inhibition.
Thus, in rat UA, Ang II stimulates cyclic GMP production through AT1 and, to a less extent, AT2 receptors. In rat aorta, the Ang II-mediated increase in cyclic GMP production is predominantly AT2 receptor-mediated. In both preparations, NO plays a critical role in mediating the effect of Ang II, whereas bradykinin has differential roles in the two vessels. In UA, B2 receptor blockade may result in a compensatory increase in cyclic GMP production, whilst in aorta, bradykinin accounts for approximately half of the cyclic GMP produced in response to Ang II.
Keywords: AT2 receptor, AT1 receptor, angiotensin II, cyclic GMP, rat uterine artery, rat aorta, bradykinin, nitric oxide, signal transduction
Introduction
While the ‘classical' cardiovascular effects of angiotensin II (Ang II) are known to be mediated by the AT1 receptor (Timmermans et al., 1993; Griendling et al., 1996), the function of the AT2 receptor has remained incompletely characterised. However, research regarding the physiological role of the AT2 receptor has intensified in the past decade, and there now exists an established body of evidence to suggest that AT2 receptors may oppose a range of actions mediated by AT1 receptors (de Gasparo & Siragy, 1999; Horiuchi et al., 1999; Gallinat et al., 2000; Widdop et al., 2003).
AT2 receptor activation has been shown not only to counteract Ang II-mediated vasoconstriction, but to actively induce vasodilation in its own right (Barber et al., 1999; Matrougui et al., 1999; Dimitropoulou et al., 2001; Katada & Majima, 2002; Widdop et al., 2002). In addition, we have previously demonstrated a functional interaction between AT1 and AT2 receptors in modulating vascular tone in vitro (Zwart et al., 1998; Hannan et al., 2003). In isolated, endothelium-intact uterine arteries (UA) from rats, the selective AT2 receptor antagonist PD 123319 potentiated the contractile response to increasing concentrations of Ang II, as did the bradykinin B2 receptor antagonist, HOE 140 and the NOS inhibitor, NOLA (Hannan et al., 2003). Given that the effects of these antagonists were not additive when given in combination, it would appear that the modulatory influence of AT2 receptors on Ang II-induced contractions involves a bradykinin–NO vasodilator cascade. In this context, we also found in preliminary studies that Ang II stimulated cyclic GMP production in rat UA (Hannan et al., 2003), which is consistent with a number of studies implicating endothelial-derived NO and bradykinin release in the mechanisms underlying AT2 receptor activation (Wiemer et al., 1993; Siragy & Carey, 1996; 1997; 1999; Gohlke et al., 1998; Tsutsumi et al., 1999).
Having already established a functional interaction between AT1 and AT2 receptors in the rat UA in vitro (Zwart et al., 1998; Hannan et al., 2003), the primary aim of the present study was to extend our preliminary observation that Ang II stimulated cyclic GMP production in rat UA. It is worth noting that AT1 receptor activation has also been associated with endothelial NO synthesis in a number of isolated blood vessel preparations (Caputo et al., 1995; Thorup et al., 1998). Thus, the second aim of the study was to determine the receptor subtype(s) responsible for mediating the effect of Ang II, and to examine the contributions of NO and bradykinin to the observed response. Additionally, there is a marked difference in AT2 receptor expression between rat UA and aorta. The abundance of AT2 receptors present in the UA (Cox et al., 1996a, 1996b; Burrell & Lumbers, 1997) makes this an ideal preparation in which to study vascular AT2 receptor function, and stands in contrast to the relatively low AT2 expression levels present in adult rat aorta (Viswanathan et al., 1991). Therefore, the third aim of this study was to compare the large conduit vessel with the relatively smaller, resistance-like UA, in relation to cyclic GMP content. We hypothesised that, given the greater level of AT2 receptor expression in UA than aorta, and the strong link between AT2 receptor activation and cyclic GMP production, the effects of Ang II-mediated cyclic GMP production would be more apparent in UA compared with the aorta.
Methods
General procedures
Female virgin Sprague-Dawley rats (8–10 weeks of age, 200–250 g) were housed in standard rat cages at 21±3°C, with a 12 h light/dark cycle. Food and water were provided ad libitum. Animals were killed by stunning and exsanguination. Aorta and both uterine horns, together with their vasculature, were removed en bloc and placed immediately in ice cold physiological salt solution (PSS, composition in mM: NaCl 119, KCl 4.7, CaCl2·2H2O 2.5, MgSO4·7H2O 1.17, NaHCO3 25, KH2PO4 1.18, ethylene-diaminetetraacetic acid (EDTA) 0.027 and glucose 5.5) gassed with carbogen (95% O2: 5% CO2). The entire length of main uterine artery was dissected free from the horn and surrounding tissue under a binocular dissection light microscope (Olympus SZ40) and cut into segments approximately 2 mm in length. Endothelial integrity was initially confirmed functionally in a sample of UA segments, which were mounted in small vessel wire myographs in the same manner as described previously (Zwart et al., 1998; Hannan et al., 2003), and a relaxation response to acetylcholine (ACh; 10 μM) of phenylephrine-precontracted tissues of at least 70% was then established.
Experimental protocol
Tissues were incubated in either Eppendorf vials or polypropylene tubes containing carbogenated PSS, at 37°C, for an equilibration period of 30 min prior to the addition of any treatment. A subgroup of tissues were treated with the positive controls, ACh (10 μM) (n=8–10) and sodium nitroprusside (SNP; 10 μM) (n=5), for 1 min, to assess whether or not an increase in cyclic GMP content could be observed in response to agonists known to mediate their vasodilator effects via this pathway. Additionally, stimulatory effects of ACh provided further confirmation of endothelial integrity in the two vascular preparations.
UA tissues were incubated with a single concentration of Ang II (10 nM) (n=9) for 4 min. In preliminary experiments, this concentration and duration of treatment was optimal for the induction of submaximal contractions of isolated UA. The small amount of rat UA harvested precluded full concentration–response (CR) analysis. However, CR curves to Ang II were performed in aorta, whereby segments from the one rat were exposed to Ang II (1, 10 or 100 nM) (n=8–10) for 4 min.
In both UA (n=12) and aorta (n=15), exposure to Ang II occurred in the absence of any ligand, or after 30 min' incubation with either the AT1 receptor antagonist, losartan (0.1 μM) (n=7–8), the AT2 receptor antagonist, PD 123319 (1 μM) (n=5–8), the NOS inhibitor, NOLA (30 μM) (n=4–6) or the bradykinin B2 receptor antagonist, HOE 140 (0.1 μM) (n=6–8). In some cases, these inhibitors were used in combination (n=5). Additionally, the effects of losartan (n=6–7) and PD 123319 (n=7–8) on basal cyclic GMP content were investigated.
The rationale for the chosen Ang II receptor antagonist concentrations was based on the relative Ki values of losartan and PD 123319 for AT1 and AT2 receptors, respectively (Brechler et al., 1993; Macari et al., 1993). In addition, PD 123319, at a concentration of 1 μM, has previously been shown to inhibit Ang II-induced increases in aortic cyclic GMP content (Tsutsumi et al., 1999), and to selectively inhibit AT2 receptor function in the rat UA without affecting responses to other contractile agents (Zwart et al., 1998; Hannan et al., 2003). The concentration of HOE 140 (0.1 μM) was chosen on the basis that this same dose exhibits effective and selective inhibition of bradykinin-mediated increases in cyclic GMP levels in isolated blood vessels (Hecker et al., 1993; Holzmann et al., 1994). The nonselective NOS inhibitor, Nω-nitro-L-arginine (NOLA) was used at a concentration of 30 μM, which abolishes endothelium-dependent relaxation to ACh in the rat isolated UA (Hannan et al., 2003).
At the conclusion of the treatment period, tissues were snap-frozen in liquid nitrogen and stored at −80°C until cyclic GMP content was determined.
Determination of cyclic GMP content
Segments of UA from different rats assigned to a given treatment group were pooled so as to yield at least 5 mg of tissue frozen weight, the minimum tissue sample weight required for cyclic GMP detection. Thus frozen samples of pooled UA tissue (≈5–10 mg) and individual aortic segments (≈20 mg each) were weighed and homogenised in ice-cold 6% trichloroacetic acid. Following centrifugation, the samples were then processed according to published methods (Caputo et al., 1995; Rosenkranz et al., 2002). Cyclic GMP content was determined using an RIA kit (NEN-Life Science, NEX I-33), and expressed as fmol mg−1 tissue frozen weight.
Statistical analysis
Differences in cyclic GMP content between the various treatment groups were analysed using a one-way analysis of variance (ANOVA) unless otherwise stated. Post hoc Bonferroni's multiple comparison tests were employed where appropriate (Graphpad Prism Software, San Diego, CA, U.S.A.) Values shown are expressed as mean±s.e.m. In all cases, statistical significance was accepted where P<0.05.
Drugs
Drugs and their sources were: acetylcholine HCl (Sigma, St Louis, MO, U.S.A.), angiotensin II (American Peptide Co., CA, U.S.A.), HOE 140 (Sigma), losartan (DuPont-Merck Sharp & Dohme, NJ, U.S.A.), Nω-nitro-L arginine (NOLA; Sigma, St Louis, MO, U.S.A.), PD 123319 (Parke Davis, MI, U.S.A.), phenylephrine HCl (Sigma, St Louis, MO, U.S.A.) and sodium nitroprusside (SNP; Sigma, St Louis, MO, U.S.A.). Stock solutions of all drugs were made up in distilled water, with the exception of NOLA, which was made up in 10% NaHCO3, and phenylephrine, which was made up in catecholamine diluent. All drugs were diluted in distilled water on the day of experimentation.
Results
Basal cyclic GMP content
Basal cyclic GMP content was significantly higher in rat UA than in aorta (P<0.05, Table 1). In UA, ACh (10 μM) and SNP (10 μM) stimulated an increase in cyclic GMP content, of 50% (P<0.05) and 120% (P<0.01), respectively, relative to control. In aorta, ACh and SNP produced five-fold and 6.5-fold increases in cyclic GMP, respectively (P<0.001). Thus, ACh-induced increases in cyclic GMP content indicated the presence of intact endothelium in both tissues. Neither losartan (0.1 μM) nor PD 123319 (1 μM) significantly affected basal cyclic GMP content in either vascular preparation (Table 1).
Table 1.
Effects of acetylcholine (ACh), sodium nitroprusside (SNP), losartan and PD 123319 (PD) on cyclic GMP levels (fmol mg−1 tissue) of rat isolated uterine artery (UA) and aorta
| Vessel | Basal | ACh (10 μM) | SNP (10 μM) | Losartan (0.1 μM) | PD (1 μM) |
|---|---|---|---|---|---|
| UA | 4.4±0.7 (12) | 6.5±0.9*(8) | 9.7±1.0**(5) | 5.7±0.6 (6) | 4.8±1.2 (8) |
| Aorta | 2.4±0.4† (15) | 12.4±2.2***(10) | 15.6±2.5***(5) | 1.5±0.8 (7) | 3.3±1.1 (7) |
Values shown are mean ± s.e.m., n-Values appear in parentheses.
P<0.05
P<0.01
P<0.001 versus basal levels (same vessel, one-way ANOVA).
P<0.05 versus UA basal levels (Student's unpaired t-test).
Uterine artery cyclic GMP
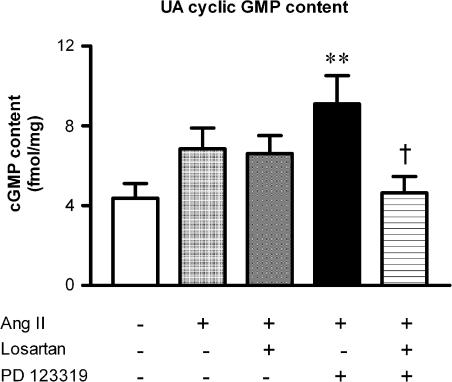
Ang II (10 nM) increased UA cyclic GMP content by 60% above basal levels (Figure 1), although this trend was not significant. The response to Ang II was unaffected by losartan (0.1 μM). However, a significant two-fold increase relative to control values was unmasked in the presence of PD 123319 (1 μM) (P<0.01, Figure 1), and this effect was abolished by the addition of losartan (Figure 1). Therefore, we chose to examine the signalling pathways of AT1 receptor-mediated (PD123319-resistant) increases in cyclic GMP production in UA in subsequent studies.
Figure 1.
Effect of Ang II (10 nM) (n=9) on UA cyclic GMP content relative to control (n=12), and the effect of pretreatment with either losartan (0.1 μM) (n=8), PD 123319 (1 μM) (n=8) or the combination of both (n=8). **P<0.01 versus control; †P<0.05 versus PD 123319 plus Ang II (one-way ANOVA).
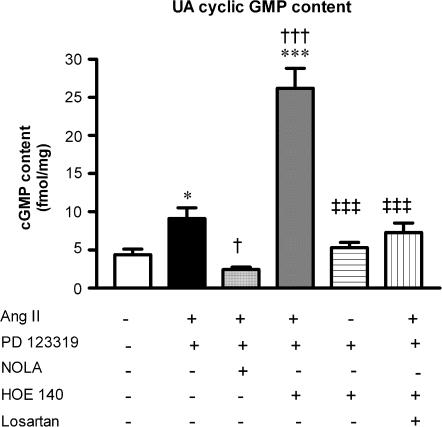
The Ang II-induced increase in UA cyclic GMP content in the presence of AT2 receptor blockade was abolished by NOLA (30 μM) (Figure 2). In contrast, HOE 140 (0.1 μM) did not inhibit the AT1 receptor-mediated increase in cyclic GMP production, but rather significantly potentiated it, such that levels were six-fold greater than that seen under basal conditions (P<0.001), and were approximately three-fold greater than cyclic GMP levels in response to PD 123319 plus Ang II (P<0.001, Figure 2). This striking increase in cyclic GMP content was not the result of a spurious effect of the antagonists on basal levels, as the combination of HOE 140 plus PD 123319 had no effect on basal cyclic GMP production. However, cotreatment with losartan abolished this enhanced effect of HOE 140 (Figure 2), confirming an involvement of AT1 receptors in mediating the response to Ang II.
Figure 2.
AT1 receptor-mediated increases in UA cyclic GMP content, demonstrated by the combination of PD 123319 (1 μM) plus Ang II (10 nmol/l) (n=8). Effect of pretreatment with either NOLA (30 μM) (n=6), HOE 140 (0.1 μM) (n=6) or the combination of HOE 140 (0.1 μM) plus losartan (0.1 μM) (n=5), and the effect of HOE 140 plus PD 123319 on basal UA cyclic GMP levels (n=5). *P<0.05, ***P<0.001 versus control; †P<0.05, †††P<0.001 versus PD 123319 plus Ang II; ‡‡‡P<0.001 versus HOE 140 plus PD 123319 plus Ang II (one-way ANOVA).
Aortic cyclic GMP
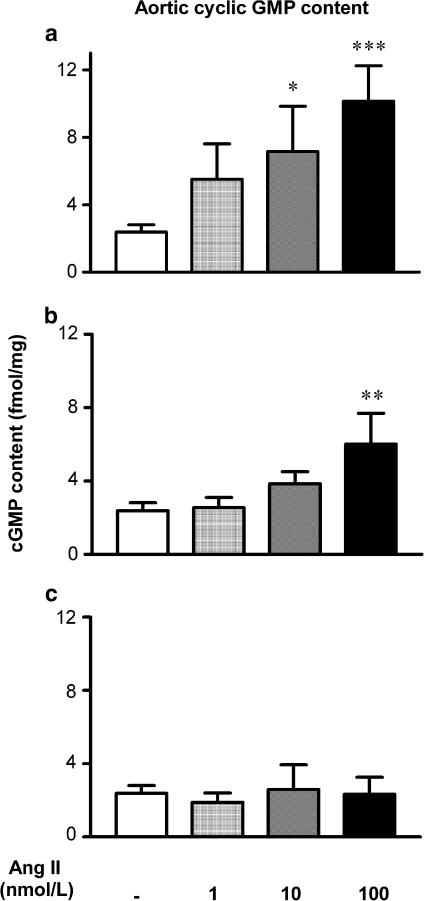
Ang II elicited concentration-dependent increases in aortic cyclic GMP content (P<0.05, P<0.01 versus control, Figure 3a). In the presence of losartan (0.1 μM), Ang II stimulated cyclic GMP accumulation, but only at a maximal concentration of 100 nM, where a 2.5-fold increase was seen (P<0.01, Figure 3b). In contrast, pretreatment with PD 123319 (1 μM) completely abolished the stimulatory effect of Ang II, such that even a maximal concentration of Ang II failed to elevate cyclic GMP production above basal levels (Figure 3c).
Figure 3.
Concentration-dependent effects of Ang II (1–100 nM) (n=8–10) on aortic cyclic GMP content relative to control (n=15) (a), and the effect of pretreatment with either (b) losartan (0.1 μM) (n=7) or (c) PD 123319 (1 μM) (n=5). *P<0.05, **P<0.01, ***P<0.001 versus respective group control (one-way ANOVA).
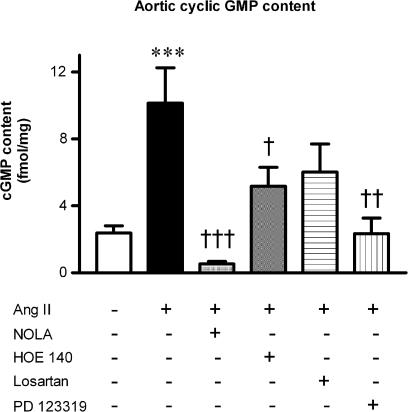
A single concentration of Ang II was subsequently used to examine signalling pathways. While the increased cyclic GMP production caused by the two higher concentrations of Ang II were abolished by PD 123319, we chose to use the highest concentration of Ang II (100 nM) for further analysis since this concentration conferred the greater degree of AT2 receptor selectivity (PD 123319-sensitive and losartan-resistant) that, interestingly, contrasted with the AT1 receptor stimulation in UA. Thus, Ang II was tested in the presence of NOLA (30 μM) and HOE 140 (0.1 μM) (Figure 4). Like PD 123319, NOLA abolished the four-fold increase in aortic cyclic GMP content (Figure 4), whilst HOE 140 significantly attenuated the Ang II-induced increase in cyclic GMP content by 50% (P<0.05, Figure 4), in a similar manner to losartan (Figure 4).
Figure 4.
Maximal Ang II (100 nM)-induced increases in aortic cyclic GMP content (n=8). Effect of pretreatment with either NOLA (30 μM) (n=4), HOE 140 (0.1 μM) (n=8), losartan (0.1 μM) (n=7) or PD 123319 (1 μM) (n=5). ***P<0.001 versus control; †P<0.05, ††P<0.01, †††P<0.001 versus Ang II (one-way ANOVA).
Discussion
A novel and significant finding to emerge from the present study was that Ang II stimulates cyclic GMP production in isolated UA of rats, through the activation of both AT1 and AT2 receptors, but principally via the AT1 receptor. In contrast, the stimulatory effect of Ang II on cyclic GMP production in rat aorta appears to be predominantly AT2 receptor-mediated. Therefore, at the Ang II concentrations used in the present study, our original hypothesis that greater AT2 receptor-mediated cyclic GMP signalling would occur in response to Ang II in UA (because of greater AT2 receptor expression than aorta) could not be supported. These results extend those of our preliminary observations regarding Ang II-induced increases in UA cyclic GMP content, and our previous functional experiments whereby AT2 receptor blockade enhanced Ang II-induced contractions in UA mediated by the AT1 receptor, which suggested that AT2 receptors may modulate vascular tone in vitro (Zwart et al., 1998; Hannan et al., 2003). However, while Ang II increased UA cyclic GMP content, the over riding effect in this vessel is AT1 receptor-mediated contraction (Zwart et al., 1998).
It is becoming more widely accepted that, in relation to cardiovascular status, the AT2 receptor may play a regulatory role in opposing various AT1 receptor-mediated pressor and trophic effects (Matsubara, 1998; de Gasparo & Siragy, 1999; Gallinat et al., 2000; Widdop et al., 2003). In angiotensin II-dependent (Siragy et al., 2000) and renal wrap models of hypertension in rats (Siragy & Carey, 1999), for example, selective stimulation of unopposed AT2 receptors by circulating angiotensin II is thought to contribute to the antihypertensive effect of AT1 receptor antagonist treatment. Interestingly, cardiac hypertrophy in rats is associated with increased AT2 receptor expression (Lopez et al., 1994), and under such pathological conditions, AT2 receptors have been implicated in reducing Ang II-induced left ventricular growth and increasing cardiac cyclic GMP content (Bartunek et al., 1999). These studies demonstrate a potentially beneficial role of AT2 receptors in certain cardiovascular disease states, and highlight the need to isolate the precise mechanisms underlying AT2 receptor activation, under both normal and pathophysiological conditions.
We found that in rat isolated UA, Ang II, at a concentration that produces submaximal contractions in vitro (10 nM), caused a 60% increase in cyclic GMP content. These results are consistent with the Ang II-induced increase in UA cyclic GMP synthesis seen in sheep (Magness et al., 1996). However, in that study, the Ang II receptor subtype responsible for mediating this increase was not investigated.
The AT2 receptor antagonist PD 123319 not only failed to inhibit the effect of Ang II, but unmasked a significant two-fold elevation in cyclic GMP levels; an effect that was abolished by cotreatment with losartan. Thus, a greater role for AT1 receptors appears likely in mediating Ang II-evoked increases in cyclic GMP content in the rat UA. These results are somewhat unexpected, not least because on an expression level, AT2 receptors are abundant in human and ovine UA, constituting up to 85% of the Ang II receptor population (Cox et al., 1996a, 1996b; Burrell & Lumbers, 1997), and are expressed in rat UA (St-Louis et al., 2001; Zwart et al., unpublished data). However, a contribution of AT2 receptors cannot be ruled out altogether, as the 60% increase produced by Ang II alone was losartan-insensitive. Moreover, in preliminary studies, the AT2 receptor agonist CGP 42112 also increased cyclic GMP production, implying an AT2 receptor involvement using this compound (Hannan et al., 2003). Thus, it is possible that a greater involvement of AT2 receptors in mediating the effects of Ang II may be seen with maximal Ang II concentrations, as used in previous studies (Tsutsumi et al., 1999). However, in the present study, we chose to examine the different Ang II receptor subtype effects in the two vessels, since this was a novel and unexpected finding.
Previously, Ang II has been found to stimulate an increase in cellular NO levels or cyclic GMP content in cultured aortic endothelial cells (Wiemer et al., 1993) and a number of isolated blood vessels, including rat carotid artery (Caputo et al., 1995), aorta (Gohlke et al., 1998), mouse aorta (Tsutsumi et al., 1999) and ovine UA (Magness et al., 1996). Furthermore, the predominant involvement of AT1 receptors in mediating the effect of Ang II on UA cyclic GMP content is supported by previous studies in rat vasculature (Caputo et al., 1995). Ang II, via the AT1 receptor, has also been shown to stimulate NO release directly in bovine aortic endothelial cells, as measured by nitrate/nitrite production (Saito et al., 1996), and in isolated perfused renal arteries, as determined by a NO-sensitive probe (Thorup et al., 1998). In the latter study, losartan significantly reduced Ang II-stimulated NO release without abolishing the response altogether, and the authors concluded that the residual, losartan-insensitive increase in NO production may be mediated by an AT2 receptor mechanism (Thorup et al., 1998).
Other studies have supported the notion that both AT1 and AT2 receptors may participate in mediating activation of the NO-cyclic GMP pathway, as evidenced by the abolishment of Ang II-induced nitrite release in dog coronary microvessels by either losartan or PD 123319 (Seyedi et al., 1995). Interestingly, the results of the present study suggest that in rat UA, the form of crosstalk that occurs between AT1 and AT2 receptors is such that Ang II receptor subtype-mediated stimulation of cyclic GMP production is a mutually exclusive event. Thus blockade of one Ang II receptor subtype results in activation, by Ang II, of the other Ang II receptor subtype-mediated cyclic GMP signalling pathway. Collectively, the aforementioned results (Seyedi et al., 1995; Thorup et al., 1998), together with those of the present study, suggest that AT1 and AT2 receptors may not always act in direct opposition to each other, at least at the level of cyclic GMP production.
Having unmasked a significant role of the AT1 receptor in the stimulation of UA cyclic GMP synthesis (i.e. with the combination of PD 123319 plus Ang II), we examined the mechanisms underlying this response. Consistent with previous studies in which there was an AT1 receptor-mediated increase in cyclic GMP production in rat carotid arteries (Caputo et al., 1995), NOLA completely abolished the AT1 receptor-mediated effect, confirming that the activation of cyclic GMP did occur via a NO (and presumably soluble guanylate cyclase)-dependent pathway. By contrast, HOE 140 potentiated this effect, which raises the possibility that bradykinin B2 receptors counteract AT1 receptor-mediated cyclic GMP synthesis. However, this hypothesis is counter intuitive, as bradykinin is a recognised endothelium-dependent vasodilator and is known to stimulate, not inhibit, NO (and hence cyclic GMP) production in isolated blood vessels (Mombouli et al., 1992; Gohlke et al., 1993; Hecker et al., 1993). In support of our observations, an enhancement of Ang II-induced cyclic GMP production by HOE 140, and an abolishment of the Ang II effects by NOS inhibition, has recently been reported in PC12W cells (which are of neuronal not vascular origin), albeit via an AT2 receptor-dependent mechanism (Zhao et al., 2003). In any event, these studies demonstrate that, in both neuronal cells and vascular preparations, HOE 140 may potentiate Ang II-induced increases in cyclic GMP synthesis, irrespective of the Ang II receptor subtype involved.
Interestingly, in the present study, the potentiating effect of HOE 140 was abolished by cotreatment with losartan, confirming an involvement of AT1 receptors in mediating the response to Ang II. While our interpretation of this finding is purely speculative at this stage, we suggest that the significant AT1 receptor component of the UA cyclic GMP response to Ang II may be upregulated in a compensatory manner, in conditions where the bradykinin–(NO)-cyclic GMP pathway is impaired (e.g. in the presence of HOE 140). Thus, although the mechanisms underlying the augmented cyclic GMP response to Ang II in the presence of bradykinin B2 receptor blockade remain unclear, it appears that bradykinin may play a role, albeit a nonconventional one, in modulating the effects of Ang II, via the AT1 receptor, on UA cyclic GMP production. The current results highlight the marked heterogeneity that exists between Ang II receptor subtypes within the vasculature, in relation to mechanisms underlying Ang II-mediated activation of the NO-cyclic GMP pathway, and in particular, the involvement of bradykinin in this cascade (Gohlke et al., 1998).
We also demonstrated that Ang II evokes concentration-dependent increases in aortic cyclic GMP content, with a maximal, four-fold increase observed in response to Ang II (100 nM), which is consistent with previous studies using aortic explants from stroke-prone spontaneously hypertensive rats (Gohlke et al., 1998). Losartan tended to impair the concentration-dependent response to Ang II, suggesting a potential contribution of AT1 receptors to Ang II-aortic cyclic GMP signalling, although at 100 nM, Ang II was still capable of inducing a 2.5-fold increase in cyclic GMP above basal levels. This suggested a greater involvement of AT2 receptors in mediating the response. Indeed, PD 123319 abolished the effect of Ang II at all concentrations tested, which is quite remarkable, given that the AT2 : AT1 receptor expression ratio in rat aorta is quite low (approximately 30%:70%) (Viswanathan et al., 1991), and almost inversely proportional to that seen in UA. This finding contrasts the results of the in vivo protocol employed by Pees et al. (2003), wherein Ang II did not stimulate cyclic GMP production in aorta of WKY rats. Thus, to our knowledge, this is the first demonstration of Ang II-induced, AT2 receptor-cyclic GMP signalling in aorta of normotensive rats. Moreover, the abolishment by PD 123319 of the aortic response to all concentrations of Ang II tested completely contrasts the Ang II-induced increase in cyclic GMP that was actually unmasked by PD 123319 in the rat UA, and highlights regional vascular heterogeneity with respect to the relative roles of AT1 and AT2 receptors in mediating the cyclic GMP response to Ang II.
The four-fold increase in aortic cyclic GMP content in response to Ang II (100 nM) was also abolished by NOLA in addition to PD 123319, whereas HOE 140 caused a 50% decrease. Thus, in rat aorta, NO accounts for all Ang II-induced increases in cyclic GMP content, and it appears that a proportion of this NO production (approximately 50%) is mediated by bradykinin B2 receptors. This is in agreement with a number of studies that have implicated endothelial-derived NO and bradykinin release in the mechanisms underlying AT2 receptor activation, in relation to cyclic GMP synthesis (Wiemer et al., 1993; Gohlke et al., 1998; Siragy & Carey, 1999; Tsutsumi et al., 1999). In stroke-prone spontaneously hypertensive rats, Ang II infusion produced a 60% increase in the cyclic GMP content of aortic explants, which was abolished by PD 123319, HOE 140 and L-NAME (Gohlke et al., 1998). These compounds also reversed the Ang II-induced increase in aortic cyclic GMP content that was unmasked by targeted AT2 receptor over expression in transgenic mice (Tsutsumi et al., 1999).
Although it was not a focal point to investigate the effects of all inhibitors on basal cyclic GMP content, the present study established that losartan and PD 123319 did not affect basal cyclic GMP levels, in either rat UA or aorta. Moreover, the combination of HOE 140 plus PD 123319 did not significantly alter UA cyclic GMP. Therefore, this would suggest that HOE 140 alone does not affect basal levels, in agreement with previous findings (Gohlke et al., 1993). By contrast, NOS inhibition has been shown to reduce basal cyclic GMP content in rat carotid artery (Caputo et al., 1995). Importantly though, in that study, basal levels in the presence of L-NAME were not significantly different to those in the presence of L-NAME plus Ang II. In any case, the present study has demonstrated that Ang II stimulation of cyclic GMP production in both preparations is indeed sensitive to inhibition by NOLA.
In conclusion, although the AT2 receptor is the predominant subtype in rat UA, the present study indicates that cyclic GMP production stimulated by AT1 receptors and, to a less extent, AT2 receptors, serves to modulate vascular tone in the rat UA, whereas AT2 receptor signalling is more apparent in the rat aorta. Despite the relatively low AT2 receptor expression levels seen in rat aorta, the small proportion that are present appear largely responsible for mediating the effect of Ang II on aortic cyclic GMP content. In both preparations, Ang II-induced increases occur via a NO-dependent pathway, while bradykinin has differential roles in the two vessels examined. In UA, bradykinin B2 receptor blockade may result in a compensatory increase in cyclic GMP production, mediated by an AT1 receptor-dependent pathway, whilst in aorta, bradykinin accounts for approximately half of the cyclic GMP produced in response to Ang II. This provides an indication of the potential importance of the aortic AT2 receptor-bradykinin–NO-cyclic GMP vasodilator cascade under normal conditions, in addition to the hypertensive states previously studied (Gohlke et al., 1998). While there was a greater Ang II-mediated increase in cyclic GMP production in aorta than in UA, the effects of Ang II in UA were markedly potentiated with the PD 123319 plus HOE 140 combination, as mentioned previously. Thus, Ang II is a potent stimulant of cyclic GMP production in both vessels, although in UA, this effect is most pronounced after blockade of AT2 receptors and bradykinin B2 receptors, highlighting the complex and unique interplay of the various cyclic GMP-generating pathways within rat UA. These multiple mechanisms for raising cyclic GMP levels in rat UA may be related to the vessel's physiological function in vivo, to maintain uteroplacental blood flow. This may be of particular importance during pregnancy where changes in Ang II receptor subtypes and cyclic GMP production are known to occur (Magness et al., 1996; Burrell & Lumbers, 1997). Clearly, further studies examining Ang II receptor subtype signalling during pregnancy are required.
Acknowledgments
We thank Iresha Welungoda for her assistance in the dissection of uterine arteries. These studies were supported in part by the National Health and Medical Research Council of Australia (REW).
Abbreviations
- ACh
acetylcholine
- Ang II
angiotensin II
- CR
concentration–response
- EDTA
ethylene-diaminetetracetic acid
- NO
nitric oxide
- NOLA
Nω-nitro-L arginine
- NOS
nitric oxide synthase
- PSS
physiological salt solution
- RAS
renin–angiotensin system
- SNP
sodium nitroprusside
- UA
uterine artery
References
- BARBER M.N., SAMPEY D.B., WIDDOP R.E. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- BARTUNEK J., WEINBERG E.O., TAJIMA M., ROHRBACH S., LORELL B.H. Angiotensin II type 2 receptor blockade amplifies the early signals of cardiac growth response to angiotensin II in hypertrophied hearts. Circulation. 1999;99:22–25. doi: 10.1161/01.cir.99.1.22. [DOI] [PubMed] [Google Scholar]
- BRECHLER V., JONES P.W., LEVENS N.R., DE GASPARO M., BOTTARI S.P. Agonistic and antagonistic properties of angiotensin analogs at the AT2 receptor in PC12W cells. Regul. Pept. 1993;44:207–213. doi: 10.1016/0167-0115(93)90244-3. [DOI] [PubMed] [Google Scholar]
- BURRELL J.H., LUMBERS E.R. Angiotensin receptor subtypes in the uterine artery during ovine pregnancy. Eur. J. Pharmacol. 1997;330:257–267. doi: 10.1016/s0014-2999(97)00167-2. [DOI] [PubMed] [Google Scholar]
- CAPUTO L., BENESSIANO J., BOULANGER C.M., LEVY B.I. Angiotensin II increases cGMP content via endothelial angiotensin II AT1 subtype receptors in the rat carotid artery. Arteriosc. Thromb. Vasc. Biol. 1995;15:1646–1651. doi: 10.1161/01.atv.15.10.1646. [DOI] [PubMed] [Google Scholar]
- COX B.E., ROSENFELD C.R., KALINYAK J.E., MAGNESS R.R., SHAUL P.W. Tissue specific expression of vascular smooth muscle angiotensin II receptor subtypes during ovine pregnancy. Am. J. Physiol. 1996a;271:H212–H221. doi: 10.1152/ajpheart.1996.271.1.H212. [DOI] [PubMed] [Google Scholar]
- COX B.E., WORD R.A., ROSENFELD C.R. Angiotensin II receptor characteristics and subtype expression in uterine arteries and myometrium during pregnancy. J. Clin. Endocrinol. Metab. 1996b;81:49–58. doi: 10.1210/jcem.81.1.8550793. [DOI] [PubMed] [Google Scholar]
- DE GASPARO M., SIRAGY H.M. The AT2 receptor: fact, fancy and fantasy. Regul. Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- DIMITROPOULOU C., WHITE R.E., FUCHS L., ZHANG H., CATRAVAS J.D., CARRIER G.O. Angiotensin II relaxes microvessels via the AT(2) receptor and Ca(2+)-activated K(+) (BK(Ca)) channels. Hypertension. 2001;37:301–307. doi: 10.1161/01.hyp.37.2.301. [DOI] [PubMed] [Google Scholar]
- GALLINAT S., BUSCHE S., RAIZADA M.K., SUMNERS C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am. J. Physiol. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., LAMBERTY V., KUWER I., BARTENBACH S., SCHNELL A., LINZ W., SCHOLKENS B.A., WIEMER G., UNGER T. Long-term low-dose angiotensin converting enzyme inhibitor treatment increases vascular cyclic guanosine 3′,5′-monophosphate. Hypertension. 1993;22:682–687. doi: 10.1161/01.hyp.22.5.682. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., PEES C., UNGER T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., LASSEGUE B., ALEXANDER R.W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- HANNAN R.E., DAVIS E.A., WIDDOP R.E. Functional role of angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. Br. J. Pharmacol. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECKER M., BARA A.T., BUSSE R. Relaxation of isolated coronary arteries by angiotensin-converting enzyme inhibitors: role of endothelium-derived kinins. J. Vasc. Res. 1993;30:257–262. doi: 10.1159/000159004. [DOI] [PubMed] [Google Scholar]
- HOLZMANN S., KUKOVETZ W.R., WINDISCHHOFER W., PASCHKE E., GRAIER W.F. Pharmacologic differentiation between endothelium-dependent relaxations sensitive and resistant to nitro-L-arginine in coronary arteries. J. Cardiovasc. Pharmacol. 1994;23:747–756. doi: 10.1097/00005344-199405000-00009. [DOI] [PubMed] [Google Scholar]
- HORIUCHI M., AKISHITA M., DZAU V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- KATADA J., MAJIMA M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br. J. Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ J.J., LORELL B.H., INGELFINGER J.R., WEINBERG E.O., SCHUNKERT H., DIAMANT D., TANG S.S. Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am. J. Physiol. 1994;267:H844–H852. doi: 10.1152/ajpheart.1994.267.2.H844. [DOI] [PubMed] [Google Scholar]
- MACARI D., BOTTARI S., WHITEBREAD S., DE GASPARO M., LEVENS N. Renal actions of the selective angiotensin AT2 receptor ligands CGP 42112B and PD 123319 in the sodium-depleted rat. Eur. J. Pharmacol. 1993;249:85–93. doi: 10.1016/0014-2999(93)90665-5. [DOI] [PubMed] [Google Scholar]
- MAGNESS R.R., ROSENFELD C.R., HASSAN A., SHAUL P.W. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI 2 and NO in pregnancy. Am. J. Physiol. 1996;270:H1914–H1923. doi: 10.1152/ajpheart.1996.270.6.H1914. [DOI] [PubMed] [Google Scholar]
- MATROUGUI K., LOUFRANI L., HEYMES C., LEVY B.I., HENRION D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- MATSUBARA H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ. Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., ILLIANO S., NAGAO T., SCOTT-BURDEN T., VANHOUTTE P.M. Potentiation of endothelium-dependent relaxations to bradykinin by angiotensin I converting enzyme inhibitors in canine coronary artery involves both endothelium-derived relaxing and hyperpolarizing factors. Circ. Res. 1992;71:137–144. doi: 10.1161/01.res.71.1.137. [DOI] [PubMed] [Google Scholar]
- PEES C., UNGER T., GOHLKE P. Effect of angiotensin AT2 receptor stimulation on vascular cyclic GMP production in normotensive Wistar Kyoto rats. Int. J. Biochem. Cell Biol. 2003;35:963–972. doi: 10.1016/s1357-2725(02)00265-0. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ A.C., HOOD S.G., WOODS R.L., DUSTING G.J., RITCHIE R.H. Acute antihypertrophic actions of bradykinin in the rat heart: importance of cyclic GMP. Hypertension. 2002;40:498–503. doi: 10.1161/01.hyp.0000032854.74042.cf. [DOI] [PubMed] [Google Scholar]
- SAITO S., HIRATA Y., EMORI T., IMAI T., MARUMO F. Angiotensin II activates endothelial constitutive nitric oxide synthase via AT1 receptors. Hypertens. Res. 1996;19:201–206. doi: 10.1291/hypres.19.201. [DOI] [PubMed] [Google Scholar]
- SEYEDI N., XU X., NASJLETTI A., HINTZE T.H. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′,5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J. Clin. Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J. Clin. Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- SIRAGY H.M., DE GASPARO M., CAREY R.M. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- ST-LOUIS J., SICOTTE B., BEDARD S., BROCHU M. Blockade of angiotensin receptor subtypes in arcuate uterine artery of pregnant and postpartum rats. Hypertension. 2001;38:1017–1023. doi: 10.1161/hy1101.095008. [DOI] [PubMed] [Google Scholar]
- THORUP C., KORNFELD M., WINAVER J.M., GOLIGORSKY M.S., MOORE L.C. Angiotensin-II stimulates nitric oxide release in isolated perfused renal resistance arteries. Pflügers Arch. 1998;435:432–434. doi: 10.1007/s004240050535. [DOI] [PubMed] [Google Scholar]
- TIMMERMANS P.B., WONG P.C., CHIU A.T., HERBLIN W.F., BENFIELD P., CARINI D.J., LEE R.J., WEXLER R.R., SAYE J.A., SMITH R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- TSUTSUMI Y., MATSUBARA H., MASAKI H., KURIHARA H., MURASAWA S., TAKAI S., MIYAZAKI M., NOZAWA Y., OZONO R., NAKAGAWA K., MIWA T., KAWADA N., MORI Y., SHIBASAKI Y., TANAKA Y., FUJIYAMA S., KOYAMA Y., FUJIYAMA A., TAKAHASHI H., IWASAKA T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISWANATHAN M., TSUTSUMI K., CORREA F.M., SAAVEDRA J.M. Changes in expression of angiotensin receptor subtypes in the rat aorta during development. Biochem. Biophys. Res. Commun. 1991;179:1361–1367. doi: 10.1016/0006-291x(91)91723-p. [DOI] [PubMed] [Google Scholar]
- WIDDOP R.E., JONES E.S., HANNAN R.E., GASPARI T.A. Angiotensin AT2 receptors: cardiovascular hope or hype. Br. J. Pharmacol. 2003;140:809–824. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDOP R.E., MATROUGUI K., LEVY B.I., HENRION D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- WIEMER G., SCHOLKENS B.A., BUSSE R., WAGNER A., HEITSCH H., LINZ W. The functional role of angiotensin II–subtype AT2–receptors in endothelial cells and isolated ischemic rat hearts. Pharm. Pharmacol. Lett. 1993;3:24–27. [PubMed] [Google Scholar]
- ZHAO Y., BIERMANN T., LUTHER C., UNGER T., CULMAN J., GOHLKE P. Contribution of bradykinin and nitric oxide to AT2 receptor-mediated differentiation in PC12 W cells. J. Neurochem. 2003;85:759–767. doi: 10.1046/j.1471-4159.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- ZWART A.S., DAVIS E.A., WIDDOP R.E. Modulation of AT1 receptor-mediated contraction of rat uterine artery by AT2 receptors. Br. J. Pharmacol. 1998;125:1429–1436. doi: 10.1038/sj.bjp.0702210. [DOI] [PMC free article] [PubMed] [Google Scholar]