Abstract
The coexistence of both inhibitory A1 and facilitatory A2 adenosine receptors in the rat myenteric plexus prompted the question of how adenosine activates each receptor subtype to regulate cholinergic neurotransmission.
Exogenously applied adenosine (0.3–300 μM) decreased electrically evoked [3H]acetylcholine ([3H]ACh) release. Blocking A1 receptors with 1,3-dipropyl-8-cyclopentylxanthine (10 nM) transformed the inhibitory action of adenosine into a facilitatory effect. Adenosine-induced inhibition was mimicked by the A1 receptor agonist R-N6-phenylisopropyladenosine (0.3 μM), but the A2A agonist CGS 21680C (0.003 μM) produced a contrasting facilitatory effect.
Increasing endogenous adenosine levels, by the addition of (1) the adenosine precursor AMP (30–100 μM), (2) the adenosine kinase inhibitor 5′-iodotubercidin (10 μM) or (3) inhibitors of adenosine uptake (dipyridamole, 0.5 μM) and of deamination (erythro-9(2-hydroxy-3-nonyl)adenine, 50 μM), enhanced electrically evoked [3H]ACh release (5 Hz for 40 s). Release facilitation was prevented by adenosine deaminase (ADA, 0.5 U ml−1) and by the A2A receptor antagonist ZM 241385 (50 nM); these compounds decreased [3H]ACh release by 31±6% (n=7) and 37±10% (n=6), respectively.
Although inhibition of ecto-5′-nucleotidase by α,β-methylene ADP (200 μM) or by concanavalin A (0.1 mg ml−1) attenuated endogenous adenosine formation from AMP, analysed by HPLC, the corresponding reduction in [3H]ACh release only became evident when stimulation of the myenteric plexus was prolonged to over 250 s.
In summary, we found that endogenously generated adenosine plays a predominantly tonic facilitatory effect mediated by prejunctional A2A receptors. Extracellular deamination and cellular uptake may restrict endogenous adenosine actions to the neuro-effector region near the release/production sites.
Keywords: Rat myenteric plexus, ecto-nucleotidases, ecto-adenosine deaminase, adenosine uptake, adenosine kinase, adenosine A1 receptor, adenosine A2A receptor, acetylcholine release
Introduction
Adenosine, which is neither stored nor released as a classical neurotransmitter, is a recognised neuromodulator in the peripheral and central nervous systems. This nucleoside controls the release of neurotransmitters by activating inhibitory A1 and facilitatory A2A adenosine receptors, which may coexist in the same nerve terminal (Correia-de-Sá et al., 1991; for a review, see Sebastião & Ribeiro, 2000). Besides the well-characterised inhibitory effect of adenosine in the gastrointestinal tract mediated by neuronal A1 receptors (Gustaffson et al., 1985; Broad et al., 1992; Nitahara et al., 1995; Barajas-López et al., 1996; De Man et al., 2003), the involvement of A2 receptors in the excitation of myenteric neurons has also been reported (Christofi et al., 1994; Tomaru et al., 1995). The coexistence of both subtypes of high-affinity adenosine receptors in cholinergic neurons prompted the questions of how and under what conditions adenosine discriminates between A1 or A2 receptors. It appears that the way in which adenosine is able to achieve a balance in the control of neuronal communication depends on the extracellular concentration of the nucleoside, which is achieved by balancing extracellular adenosine generation and inactivation (via cellular uptake and/or extracellular deamination) (Gonçalves & Queiroz, 1993; Correia-de-Sá & Ribeiro, 1996).
Extracellular adenosine can be generated via nonconcentrative bidirectional nucleoside transport (Cass et al., 1998), in parallel with the formation of adenosine from the hydrolysis of released ATP through the ecto-nucleotidase pathway (Cunha et al., 1996; for a review, see Zimmermann, 2000). In the myenteric plexus, adenosine is released per se in response to electrical stimulation (see e.g. Begg et al., 2002), while ATP mainly originates from smooth muscle cells in response to activation of muscarinic M3 receptors by endogenous acetylcholine (ACh) (Nitahara et al., 1995), although it may also be released from myenteric neurons (White & Leslie, 1982). Histochemical studies have shown that the myenteric plexus contains the enzymes responsible for the catabolism of ATP into adenosine (Nitahara et al., 1995). The existence of topographically distinct sources of extracellular adenosine, two nucleoside inactivation mechanisms and two types of adenosine receptors with opposite physiological functions, raise the important question of how these systems interact to control endogenous adenosine actions.
Since ACh is regarded as the major excitatory neurotransmitter in the myenteric plexus and the prime regulator of gastrointestinal motility (Costa et al., 1996), the present study was undertaken to investigate the kinetics and pharmacology of adenosine generation in order to probe its role in controlling electrically evoked [3H]ACh release from isolated myenteric plexus–longitudinal muscle of the rat ileum. To characterise the enzymatic pathways responsible for the extracellular formation and removal of adenosine leading to differential activation of inhibitory A1 and excitatory A2A receptors in the rat ileum, we used several pharmacological inhibitors designed to either limit or increase the availability of endogenous adenosine. The results obtained by manipulating the endogenous levels of adenosine were compared with the effects of the nucleoside itself, of the adenosine precursor AMP, and of stable adenosine analogues displaying a high degree of selectivity for A1 or A2A receptors.
Methods
Experimental preparation
Rats (Wistar, 150–200 g) of either sex (Charles River, Barcelona, Spain) were kept at a constant temperature (21°C) and a regular light (06.30–19.30 h) and dark (19.30–06.30 h) cycle, with food and water ad libitum. The animals were killed after stunning followed by exsanguination. Animal handling and experiments followed the guidelines of the International Council for Laboratory Animal Science (ICLAS). A section of the rat ileum not including the terminal 5 cm was removed and the longitudinal muscle strip with the myenteric plexus attached was prepared according to Paton & Vizi (1969). The experiments were performed at 37°C in myenteric plexus–longitudinal muscle preparations superfused with gassed (95% O2 and 5% CO2) Tyrode's solution containing (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1, NaH2PO4 0.4, NaHCO3 11.9, glucose 11.2 and choline 0.001.
Kinetic experiments and high-performance liquid chromatography (HPLC) analysis
For the kinetic experiments on nucleotide or nucleoside catabolism, strips of the longitudinal muscle with the myenteric plexus attached (26.7±1.6 mg, n=19) were mounted in a 3 ml organ bath. After a 30 min equilibration period, the preparations were incubated with 30 μM AMP or adenosine (zero time). Samples of 75 μl were collected from the organ bath at different times up to 45 min for HPLC analysis of the change in substrate disappearance and product formation (see Cunha & Sebastião, 1991). The concentrations of the substrate and products were plotted as a function of time (progress curves). When the modification of extracellular catabolism of an initial substrate by an inhibitor was tested, the preparations were preincubated in the presence of the modifiers for at least 15 min before starting the kinetic experiment with the modifier still present. The only product that was spontaneously released from the preparation in concentrations that did not exceed 2 μM was IMP. The spontaneous degradation of AMP and adenosine at 37°C under control conditions was negligible (0–5%) over 45 min. At the end of the experiments, the remaining incubation medium was collected and used to quantify lactate dehydrogenase (E.C. 1.1.1.27) activity (Keiding et al., 1974). The negligible level (0.12±0.01 U ml−1, n=20) of lactate dehydrogenase activity in bath samples collected at the end of the experiments is an indication that the integrity of the cells was maintained during the experimental procedure.
[3H]ACh release experiments
The procedures used for labelling the preparations and measuring evoked [3H]ACh release were as previously described (e.g. Correia-de-Sá et al., 1991), with minor modifications. Longitudinal muscle–myenteric plexus strips were mounted in 3 ml capacity, and vertical perfusion chambers heated to 37°C. Nerve terminals were labelled for 40 min with 1 μM [3H]choline (specific activity 2.5 μCi nmol−1) under electrical stimulation at a frequency of 1 Hz, using two platinum electrodes placed above and below the suspended muscle strip (longitudinal field stimulation). Washout of the preparations was performed for 60 min, by superfusion (15 ml min−1) with Tyrode solution supplemented with the choline uptake inhibitor hemicholinium-3 (10 μM). Tritium outflow was evaluated by liquid scintillation spectrometry (% counting efficiency: 40±2%) after appropriate background subtraction, using 2 ml bath samples collected automatically every 3 min. After the loading and washout periods, preparations contained (10,648±324) × 103 d.p.m. g−1 wet weight of tissue and the resting release was (115±18) × 103 d.p.m. g−1 (n=8). The fractional release was calculated to be 1.08±0.14% of the radioactivity present in the tissue in the first collected sample.
[3H]ACh release was evoked by two periods of electrical field stimulation, starting in the 12th (S1) and 39th (S2) minutes after the end of washout (zero time), each consisting of 200, 750 or 1350 square wave pulses of 1 ms duration delivered at a frequency of 5 Hz. Other investigators have used this stimulation pattern to study ileum contractility in rodents (see e.g. De Man et al., 2003). Prevention of the evoked tritium outflow in the absence of external calcium (0 Ca2+ + EGTA, 1 mM) and in the presence of 1 μM tetrodotoxin (Duarte-Araújo et al., 2000, personal communication) indicates that [3H]ACh release results from vesicle exocytosis of depolarised nerve terminals. Therefore, the evoked [3H]ACh release was calculated by subtracting the basal tritium outflow from the total tritium outflow during the stimulation period (cf. Correia-de-Sá et al., 1991).
Test drugs were added 15 min before S2 and were present up to the end of the experiments (see e.g. Figure 3). The percentage change in the ratio between the evoked [3H]ACh release during the two stimulation periods (S2/S1) relative to that observed in control situations (in the absence of test drugs) was taken as a measure of the effect of the tested drugs. Positive and negative values represent facilitation and inhibition of evoked [3H]ACh release, respectively. When we evaluated changes in the effect of tested drugs induced by a modifier, the modifier was applied 15 min before starting sample collection and hence was present during S1 and S2. When the same drug was present in S1 and S2, the S2/S1 ratios were not significantly (P>0.05) different from those obtained in control conditions, that is, without addition of drugs (see Table 1). None of the drugs significantly (P>0.05) changed basal tritium outflow (see e.g. Figure 3).
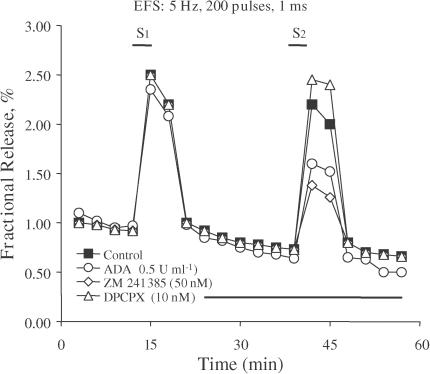
Figure 3.
Effects of ADA (circles), ZM 241385 (lozenges) and DPCPX (triangles) on [3H]ACh release from myenteric neurons. The graph shows the time course of tritium outflow from the longitudinal muscle–myenteric plexus of the rat ileum taken from typical experiments. Tritium outflow (ordinates) is expressed as a percentage of the total radioactivity present in the tissue at the beginning of the collection period. The abscissa indicates the times at which the samples were collected. The release of [3H]ACh in response to electrical field stimulation (200 pulses of 1 ms duration delivered at a 5 Hz frequency) was elicited twice during the periods indicated (S1 and S2). ADA (0.5 U ml−1), ZM 241385 (50 nM) and DPCPX (10 nM) were applied 15 min before S2, as represented by the horizontal bar. The time course of tritium outflow in control conditions (in the absence of test drugs) is also shown for comparison (filled squares). None of the drugs used modified basal tritium outflow.
Table 1.
Influence of the number of pulses (train length) on the tonic effect of adenosine on electrically evoked [3H]ACh release from myenteric motoneurons of the rat ileum
| Number of pulses | Control | Drugs in S2 (% of control) | |||
|---|---|---|---|---|---|
| S1 (103 dpm g−1) | S2/S1 | ADA (0.5 U ml−1) | Con A (0.1 mg ml−1) | AOPCP (200 μM) | |
| 200 pulses | 44±5 (15) | 0.83±0.11 (4) | −31±6% (7)* | +4±8% (4) | −1±9% (4) |
| 750 pulses | 94±11 (18) | 0.84±0.07 (4) | −32±9% (4)* | −13±4% (4) | +2±9% (5) |
| 1350 pulses | 220±14 (8) | 0.85±0.05 (4) | −38±3% (4)* | −41±4% (4)* | −36±5% (3)* |
Evoked [3H]ACh release was elicited by two trains (S1 and S2) of electrical field stimulation consisting of 200, 750 or 1350 pulses delivered at a 5 Hz frequency (1 ms pulse duration). Values for S1 and S2/S1 are means±s.e.m. ADA (0.5 U ml−1), Con A (0.1 mg ml−1) and AOPCP (200 μM) were applied 15 min before S2. The effects of the drugs on the stimulation (S2)-evoked release of ACh were measured and are expressed as percentage changes (± s.e.m.) in S2/S1 ratios as compared to controls. The number of experiments is between parentheses.
P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences from the control.
Materials and solutions
Adenosine, adenosine deaminase (ADA) (type VI, 1803 U ml−1, EC 3.5. 44), AMP, choline chloride, concanavalin A (Con A), IMP, inosine (INO), hemicholinium-3, hypoxanthine, α,β-methylene ADP (AOPCP), S-(p-nitrobenzyl)-6-thioinosine (NBTI) and R-N6-phenylisopropyl adenosine (R-PIA) were from Sigma (St Louis, MO, U.S.A.); dipyridamole was from Boehringer Ingelheim (Germany); 2-[4-(2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamido adenosine (CGS 21680C), 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), erythro-9(2-hydroxy-3-nonyl) adenine (EHNA) and 5′-iodotubercidin (ITU) were from Research Biochemicals (Natick, MA, U.S.A.); (4-(2-[7-amino-2-(2-furyl{1,2,4}-triazolo{2,3-a{1,3,5}triazin-5-yl-aminoethyl)phenol (ZM 241385) was from Tocris Cookson Inc., (U.K.); [methyl-3H]choline chloride (ethanol solution, 80 Ci mmol−1) was from Amersham (U.K.). EHNA was made up in a 5 mM stock solution in ethanol. DPCPX was made up in a 5 mM stock solution in 99% dimethylsulphoxide (DMSO) + 1% NaOH 1 M (v v−1). ZM 241385, NBTI and R-PIA were made up in 5, 10 and 50 mM stock solutions in DMSO, respectively. ITU was made up in a 5 mM stock solution in DMSO, which was kept in the dark to prevent photodecomposition. All stock solutions were stored as frozen aliquots at −20°C. Dilutions of these stock solutions were made daily and appropriate solvent controls were performed. No statistically significant differences between control experiments, made in the absence or presence of the solvents at the maximal concentrations used (0.5% v v−1), were observed. The pH of the superfusion solution did not change following addition of the drugs at the maximum concentrations applied to the preparations.
Statistics
The data are expressed as mean±s.e.m., with n indicating the number of experiments. Statistical analysis of data was carried out using paired or unpaired Student's t-tests or one-way analysis of variance (ANOVA) followed by Dunnett's modified t-test. P-values at <0.05 were considered to represent significant differences.
Results
Myenteric motoneurons possess both A1 inhibitory and A2A facilitatory adenosine receptors modulating the evoked [3H]ACh release
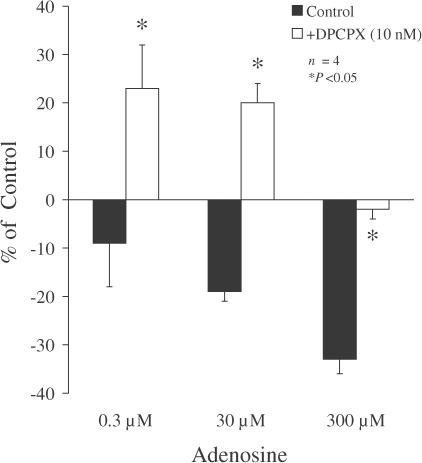
Exogenously applied adenosine (0.3–300 μM) decreased electrically evoked [3H]ACh release from myenteric motoneurons of the rat ileum in a concentration-dependent manner (Figure 1). In the presence of the A1 receptor antagonist DPCPX (10 nM), adenosine (0.3 and 30 μM) significantly (P<0.05) increased, rather than decreased, tritium outflow. When adenosine was applied at a higher concentration (300 μM), inhibition of [3H]ACh release was completely prevented by DPCPX (10 nM), but the facilitatory effect was no longer observed.
Figure 1.
Effect of exogenously added adenosine on [3H]ACh release from myenteric motoneurons stimulated electrically in the absence (filled bars) and presence (open bars) of the A1 receptor antagonist DPCPX (10 nM). Adenosine was added 15 min before S2; DPCPX (10 nM) was added to the incubation media at the beginning of the release period (time zero), and was present throughout the assay, including S1 and S2. The ordinates are percentage changes in S2/S1 ratios compared to controls. The average S2/S1 ratio in the presence of DPCPX (10 nM, 0.89±0.07, n=6) was not significantly (P>0.05) different from the control value (0.83±0.11, n=4) (see Table 1). The data are means ± s.e.m. of four experiments. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences compared with the effect of the same concentration of adenosine in the absence of DPCPX.
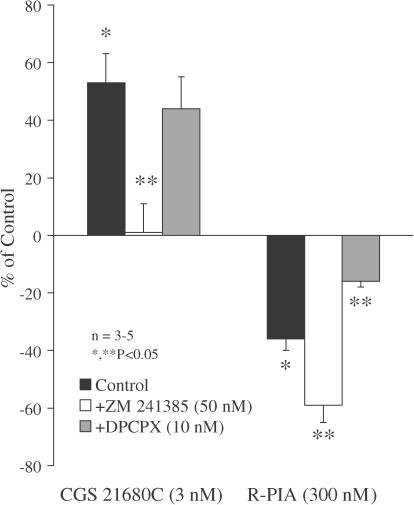
The selective adenosine A1 receptor agonist R-PIA (100–300 nM) significantly (P<0.05) decreased the evoked tritium outflow in a concentration-dependent manner. Inhibition of transmitter release by R-PIA (300 nM) was attenuated in the presence of the A1 receptor antagonist DPCPX (10 nM), but it was enhanced upon blocking A2A receptors with ZM 241385 (50 nM) (Figure 2). In contrast, activation of adenosine A2A receptors with CGS 21680C (1–3 nM) facilitated the evoked release of [3H]ACh in a concentration-dependent manner. A higher concentration (10 nM) of CGS 21680C also increased evoked tritium outflow (P<0.05), but the increase was smaller (+22±4%, n=3) than that observed with 3 nM CGS 21680C. The facilitatory effect of CGS 21680C (3 nM) was abolished in the presence of ZM 241385 (50 nM), but was virtually unaffected by DPCPX (10 nM) (Figure 2).
Figure 2.
Actions of selective adenosine A1 and A2A receptor antagonists on the effects of two stable adenosine analogues, CGS 21680C and R-PIA, on [3H]ACh release from myenteric motoneurons stimulated electrically. CGS 21680C (3 nM) and R-PIA (300 nM) were applied 15 min before S2. The adenosine receptor antagonists ZM 241385 (50 nM) and DPCPX (10 nM) were added to the incubation media at the beginning of the release period (time zero) and were present throughout the assay, including S1 and S2. The ordinates are percentage changes in S2/S1 ratios compared to controls. The average S2/S1 ratio in the presence of DPCPX (10 nM, 0.89±0.07, n=6) and ZM 241385 (50 nM, 0.89±0.03, n=5) were not significantly (P>0.05) different from the control value (0.83±0.11, n=4) (see Table 1). The data are means±s.e.m. of three to five individual experiments. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences from the control; **P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences compared with the effect of CGS 21680C or R-PIA in the absence of the adenosine receptor antagonists.
Endogenous adenosine preferentially activates facilitatory A2A receptors on myenteric motoneurons
To study the net tonic action of endogenous adenosine on electrically evoked [3H]ACh release from myenteric motoneurons, we compared the effect of ADA (EC 3.5. 44), the enzyme that inactivates adenosine by converting it into ION (Arch & Newsholme, 1978), with the effects resulting from the blockade of adenosine A1 and A2A receptors with DPCPX and ZM 241385, respectively (Figure 3). ADA (0.5 U ml−1) inhibited evoked [3H]ACh release by 31±6% (n=7), indicating that endogenous adenosine produces a predominantly tonic facilitatory action on neurotransmitter release in the rat myenteric plexus (Figure 3). DPCPX (10 nM) slightly increased the release of [3H]ACh by 17±4% (n=4) (Figure 3). In contrast, ZM 241385 (50 nM) maximally decreased the evoked tritium outflow by 37±10% (n=6) (Figure 3), an effect that was not statistically (P>0.05) different from the inhibition caused by ADA (0.5 U ml−1). The concentrations of DPCPX (10 nM) and ZM 241385 (50 nM) used in the present study are within the range usually required to selectively block adenosine A1 and A2A receptors, respectively, in functional studies (see e.g. Cunha et al., 2001; Oliveira et al., 2002). Higher concentrations of these antagonists did not further enhance their actions, that is, 50 nM DPCPX facilitated the release of [3H]ACh by 21±8% (n=4), while 100 nM ZM 241385 inhibited the release by 37±6% (n=4). The results indicate that endogenous adenosine exerts a predominantly facilitatory action by activating A2A receptors on myenteric neurons.
Involvement of ADA and adenosine uptake in the regulation of the extracellular concentration of adenosine in the rat myenteric plexus
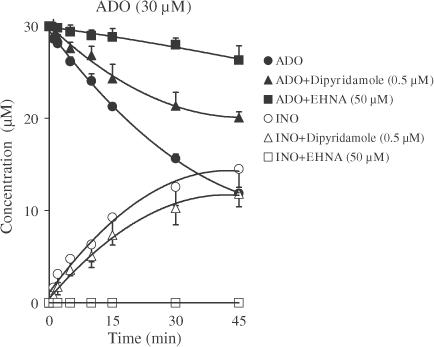
As illustrated in the progress curve of extracellular adenosine disappearance in the rat myenteric plexus (Figure 4), the bath concentration of adenosine (30 μM) decreased with time. The only metabolites detected in the bath were INO and hypoxanthine. The INO concentration increased continuously over 45 min, reaching a value of 14.51±1.98 μM (n=4). The absence of AMP formation from adenosine suggests that no extracellular adenosine kinase (AK) (E.C. 2.7.1.20) activity was present in this preparation. When INO (30 μM) was added to the preparation, it disappeared slowly and only small amounts of hypoxanthine (1.94±0.29 μM) appeared after 45 min (n=2) (data not shown).
Figure 4.
Effects of dipyridamole and EHNA on adenosine (ADO, filled symbols) disappearance and inosine (INO, open symbols) formation in the myenteric plexus of the rat ileum. Adenosine (30 μM) was incubated at zero time with longitudinal muscle–myenteric plexus preparations in the absence (circles) and presence of either dipyridamole (0.5 μM, triangles) or EHNA (50 μM, squares). Each sample collected was analysed by HPLC to separate and quantify adenosine and its metabolites. Symbols represent average results obtained in three to four experiments; the vertical bars represent the s.e.m. and are shown when they exceed the symbols in size. The progress curves using each adenosine inactivation modifier were obtained from the same preparation. Hypoxanthine at concentrations ranging from 0.48–1.99 μM was also detected in the bath after 15 min incubation, but is not represented for the sake of clarity; the concentration of hypoxanthine was unaltered in the presence of dipyridamole (0.5 μM), but it was virtually undetectable during incubation with EHNA (50 μM).
In the presence of the nucleoside transport inhibitor dipyridamole (0.5 μM) (for a review, see e.g. Griffith & Jarvis, 1996), the disappearance of adenosine (30 μM) from the bath was slower while the formation of INO remained virtually unchanged (Figure 4). The average half-disappearance time of adenosine increased from 34.10±1.30 min in control conditions to 75.6±9.4 min in the presence of dipyridamole (0.5 μM, n=3); these values were estimated from polynomial fitting of semilogarithmic progress curves of adenosine catabolism in the absence and presence of dipyridamole (0.5 μM). Increasing the concentration of dipyridamole to 2 μM did not further increase the amount of adenosine remaining in the bath or prolong its time course. No inhibition of the enzymatic activity of ADA (0.0005–0.5 U ml−1) was revealed in the presence of dipyridamole (2 μM) (data not shown), ruling out the possibility that dipyridamole inhibited adenosine deamination (Deuticke & Gerlach, 1966; but see e.g. Hopkins & Goldie, 1971). S-(p-nitrobenzyl)-6-thioinosine (NBTI, 30 μM, n=4), another compound designed to inhibit equilibrative nucleoside transporters (NTS) (for a review see e.g. Griffith & Jarvis, 1996), did not alter either the rate of adenosine disappearance (33.70±0.40 min) or the rate of INO generation, indicating that the adenosine transporter present at the rat myenteric plexus is insensitive to NBTI (ei). Concentrative nucleoside transport is found in the gastrointestinal system of a wide variety of species. Since ouabain (2 mM, n=2) did not modify the kinetics of adenosine disappearance and INO appearance in the bath, it is highly improbable that a sodium-dependent concentrative nucleoside transport system is responsible for adenosine uptake in the rat myenteric plexus.
Interestingly, in the presence of the ADA inhibitor EHNA (50 μM, n=4) (Agarwal et al., 1977), adenosine disappearance from the bath was reduced by more than 90% at 45 min and the extracellular formation of INO was virtually abolished (Figure 4). These results suggest that, in addition to a dipyridamole-sensitive equilibrative nucleoside uptake system, the longitudinal muscle–myenteric plexus of the rat ileum displays a very high level of ecto-adenosine deaminase activity, and that both mechanisms may act cooperatively to control extracellular adenosine accumulation.
Synaptic adenosine accumulation through the blockade of extracellular inactivation (uptake or deamination) or the inhibition of intracellular phosphorylation enhances the A2A facilitatory tonus
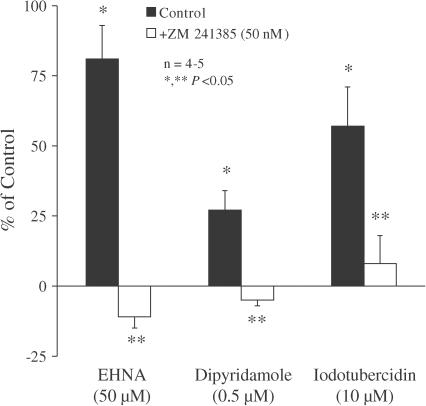
Extracellular adenosine accumulation following application of dipyridamole (0.5 μM) and EHNA (50 μM) induced a significant (P<0.05) increase in evoked [3H]ACh release (Figure 5). When used at a higher concentration, dipyridamole (2 μM) did not further facilitate the release of transmitter, in agreement with the findings from the kinetic studies suggesting that adenosine uptake was maximally inhibited with 0.5 μM dipyridamole. Since the maximal facilitatory effect of EHNA was greater than that of dipyridamole, it seems likely that deamination is relatively more efficient than uptake in regulating extracellular adenosine levels in the rat myenteric plexus.
Figure 5.
Action of the selective adenosine A2A receptor antagonist ZM 241385 on the facilitatory effects induced by EHNA, dipyridamole and ITU on [3H]ACh release from myenteric motoneurons stimulated electrically. EHNA (50 μM), dipyridamole (0.5 μM) and ITU (10 μM) were applied 15 min before S2. The adenosine A2A receptor antagonist ZM 241385 (50 nM) was added to the incubation media at the beginning of the release period (time zero) and was present throughout the assay, including S1 and S2. The ordinates are percentage changes in S2/S1 ratios compared to controls. The average S2/S1 ratio in the presence of ZM 241385 (50 nM, 0.89±0.03, n=5) was not significantly (P>0.05) different from the control value (0.83±0.11, n=4) (see Table 1). The data are means±s.e.m. of four to five individual experiments. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences from the control; **P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences compared with the effect of EHNA, dipyridamole or ITU in the absence of ZM 241385.
It has been reported that intracellular metabolism of adenosine may directly affect the extracellular concentration of adenosine through the nucleoside facilitated transport system (Geiger & Fyda, 1991). Of the enzymes that catabolise adenosine, AK has the highest affinity (Arch & Newsholme, 1978) and thus, under baseline conditions, is most likely to affect the rate of intracellular adenosine catabolism (Lloyd & Fredholm, 1995). Application of the AK inhibitor 5′-iodotubercidin (ITU, 10 μM) increased the release of [3H]ACh induced by electrical field stimulation of the myenteric plexus. The facilitatory effect of ITU (10 μM) was twice that of supramaximal dipyridamole (0.5 μM) (Figure 5), ruling out the possibility that ITU might be facilitating transmitter release mainly because of its ability to inhibit adenosine uptake (see e.g. Lloyd & Fredholm, 1995). Furthermore, pretreatment with dipyridamole (0.5 μM, applied throughout the assay, including S1 and S2) prevented the facilitation caused by ITU (10 μM, −4±10%, n=3) (data not shown). Therefore, the facilitation of transmitter release caused by inhibition of intracellular AK is probably due to outward membrane transport of adenosine via a dipyridamole-sensitive mechanism that is also present in the central nervous system (Pak et al., 1994; Lloyd & Fredholm, 1995; Golembiowska et al., 1996).
Like the excitation caused by CGS 21680C (see Figure 2), the facilitatory effects of dipyridamole (0.5 μM), EHNA (50 μM) and ITU (10 μM) were also prevented by ZM 241385 (50 nM) (Figure 5), confirming the assumption that extracellular adenosine accumulation facilitates the release of [3H]ACh through the activation of presynaptic A2A receptors.
Ecto-adenosine deaminase controls delivery of exogenous adenosine to prejunctional facilitatory A2A receptors
Extracellular inactivation may account for the failure of exogenous adenosine to reach receptors located on highly protected regions, like most neuro-effector synapses (see e.g. Dowdall, 1978; Daly, 1982). Pretreatment with EHNA, applied throughout the assay at a concentration (50 μM) that virtually blocked extracellular adenosine deamination (see above), transformed the effect of adenosine (0.3 μM) from inhibition to consistent facilitation (+34±8%, n=4) (data not shown), consistent with the effect obtained after blocking the A1 receptors with DPCPX (10 nM) (see Figure 1). Thus, blockade of ecto-adenosine deaminase activity around cholinergic nerve terminals allows the exogenously applied nucleoside to reach facilitatory A2A receptors in concentrations high enough to overcome inhibition of transmitter release by A1 receptors.
Contribution of the ecto-5′-nucleotidase pathway to adenosine modulation of [3H]ACh release from myenteric motoneurons
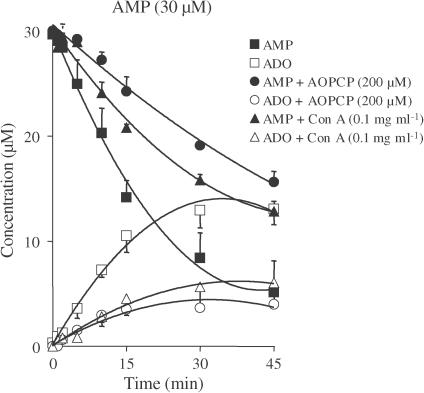
As illustrated in Figure 6, the bath concentration of AMP (30 μM) decreased continuously with time during 45 min of incubation with the longitudinal muscle–myenteric plexus of the rat ileum. The average half-degradation time of exogenously added AMP was 15.04±2.42 min. The AMP metabolites detected in the bath were adenosine, INO and hypoxanthine, whose concentrations in the bathing fluid increased progressively, reaching maximum values of 12.95±2.21 μM at 30 min, 7.37±0.96 μM at 45 min and 1.67±0.92 μM at 45 min, respectively. Exposure of the myenteric plexus to a higher concentration of AMP (100 μM) did not alter its rate of hydrolysis or the pattern of appearance of its metabolites (data not shown). Figure 6 also shows the time course of AMP degradation in the presence of two chemically distinct ecto-5′-nucleotidase inhibitors, the ADP analogue α,β-methylene ADP (AOPCP, 200 μM) (Naito & Lowenstein, 1985) and the non-nucleoside lectin Con A (0.1 mg ml−1) (Riordan & Slavik, 1974; Stefanovic et al., 1975). These two membrane-impermeable inhibitors proportionally reduced AMP degradation and adenosine formation by about 60%. In the presence of AOPCP (200 μM) and Con A (0.1 mg ml−1), the average half-degradation times of exogenously added AMP increased to 45.82±2.79 min (n=5) and 34.15±1.23 min (n=3), respectively. Increasing the concentration of Con A to 0.2 mg ml−1 did not significantly (P>0.05) increase the amount of AMP remaining in the bath or prolong its degradation time course.
Figure 6.
Inhibitory effects of α,β-methylene ADP (AOPCP) and concanavalin A (Con A) on the catabolism of AMP (filled symbols) and formation of adenosine (ADO, open symbols) in the myenteric plexus of the rat ileum. AMP (30 μM) was incubated at zero time with longitudinal muscle–myenteric plexus preparations in the absence (squares) and presence of AOPCP (200 μM, circles) or Con A (0.1 mg ml−1, triangles). Both progress curves were obtained from the same preparation. Samples (75 μl) were collected at the times indicated on the abscissa and analysed by HPLC. Symbols represent average results obtained in three to five experiments; the vertical bars represent the s.e.m. and are shown when they exceed the symbols in size. INO formation was detected in the bath from the second minute at concentrations ranging from 0.39–7.37 μM. Hypoxanthine was formed linearly from the 15th minute (0.23–1.67 μM) of incubation. Neither of these compounds is represented for the sake of clarity. In the presence of AOPCP (200 μM) and Con A (0.1 mg ml−1), INO formation was only detected in the bath from the 10th minute at concentrations ranging from 0.07–2.23 μM, while hypoxanthine was virtually undetectable.
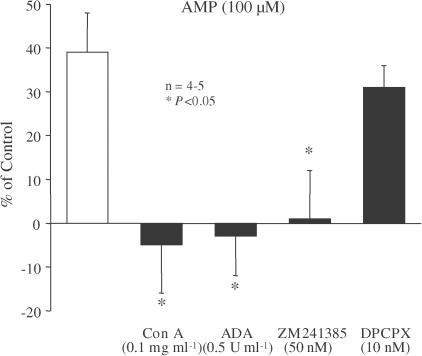
At 30 and 100 μM, AMP facilitated the electrically evoked release of [3H]ACh from myenteric motoneurons by 21±8% (n=3) and 39±9% (n=5), respectively. As shown in Figure 7, the facilitatory effect of 100 μM AMP was prevented by both ADA (0.5 U ml−1) and Con A (0.1 mg ml−1). These observations suggest that AMP has to be catabolised extracellularly into adenosine to facilitate transmitter release. Activation of adenosine A2A receptors is probably responsible for the facilitatory effect of AMP (100 μM), since AMP-induced facilitation was prevented by the A2A receptor antagonist ZM 241385 (50 nM), but virtually unaffected by the A1 antagonist DPCPX (10 nM) (Figure 7).
Figure 7.
The facilitatory effect of AMP on evoked [3H]ACh release from myenteric motoneurons depends on the activation of presynaptic A2A receptors by adenosine generated extracellularly. AMP (100 μM) was applied 15 min before S2. Con A (0.1 mg ml−1), ADA (0.5 U ml−1), ZM 241385 (50 nM) and DPCPX (10 nM) were present throughout the assay, including S1 and S2. The ordinates are percentage changes in S2/S1 ratios compared to controls. The average S2/S1 ratios in the presence of Con A (0.1 mg ml−1), ADA (0.5 U ml−1), ZM 241385 (50 nM) or DPCPX (10 nM) were not statistically (P>0.05) different from the control value (see Table 1). The data are means±s.e.m. of four to five individual experiments. *P<0.05 (one-way ANOVA followed by Dunnett's modified t-test), significant differences compared with the facilitatory effect of AMP alone.
In view of the potential role of the ecto-nucleotidase pathway in regulating extracellular adenosine accumulation, we decided to investigate the effect of endogenous adenosine formed from released adenine nucleotides on the activation of nearby adenosine A2A receptors facilitating the release of [3H]ACh. For this purpose, we compared the influence of the stimulation train length on the inhibitory effect of ADA, which removes all the endogenous extracellular adenosine (see Figure 3), with the effects of AOPCP and Con A, which only prevent the formation of adenosine from extracellular catabolism of released adenine nucleotides (Table 1). In contrast to ADA (0.5 U ml−1), which consistently inhibited evoked [3H]ACh release by 30–40%, AOPCP (200 μM) and Con A (0.1 mg ml−1) failed to modify the release of [3H]ACh when the stimulation train length was shorter than 2.5 min (Table 1). However, upon increasing the stimulation train length to 4.5 min (while keeping the stimulation frequency at 5 Hz), both Con A (0.1 mg ml−1) and AOPCP (200 μM) significantly reduced the release of [3H]ACh (Table 1). Under these stimulation conditions, A2A receptor blockade with ZM 241385 (50 nM) prevented the inhibitory effects of Con A (0.1 mg ml−1, −8±1%, n=3) and AOPCP (200 μM, −3±2%, n=4).
Discussion
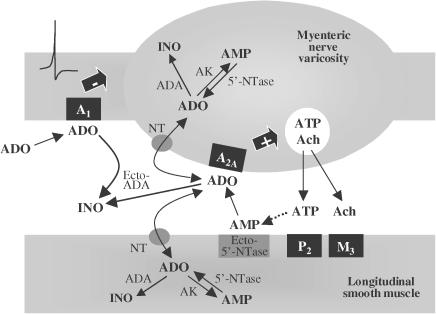
In this study, we provide evidence indicating that synaptic accumulation of endogenous adenosine has a predominantly tonic facilitatory effect on ACh release from electrically stimulated myenteric motoneurons, via the activation of adenosine A2A receptors. Although adenosine may be formed by the extracellular catabolism of adenine nucleotides released via the ecto-nucleotidase pathway, this might not represent the major source of endogenous adenosine modulating short-term cholinergic neurotransmission in the rat myenteric plexus. Another major strength of the present work is the demonstration that extracellular deamination is the most efficient mechanism regulating synaptic adenosine levels and, consequently, tonic activation of facilitatory A2A receptors on myenteric nerve terminals. Besides the very high level of ecto-adenosine deaminase activity in this tissue, a less-efficient (NBTI-insensitive, ei) nucleoside transport system may also contribute to the inactivation of extracellular adenosine. Owing to the effectiveness of both inactivation mechanisms, endogenous adenosine actions may be restricted to the release/production region at the myenteric cholinergic synapse, while exogenously added adenosine seems to activate preferentially extrajunctional inhibitory A1 receptors (Figure 8). While the distribution and efficacy of the different elements involved in the extracellular metabolism and action of adenosine must be considered as factors in its functional selectivity, the lack of biochemical and morphological studies preclude further interpretation.
Figure 8.
Schematic representation of the neuromodulatory action of adenosine in the myenteric plexus of rat ileum. In response to stimulation, extracellular adenosine (ADO) can be originated directly via bi-directional NT from both nerve and muscle cells. In addition, released ATP may be sequentially dephosphorylated by extracellular nucleotidases to form endogenous adenosine. Ecto-5′-nucleotidase (Ecto-5′-NTase), the limiting enzyme of the ectonucleotidase pathway, plays an important role in regulating the rate of local adenosine production from adenine nucleotides. Endogenously generated adenosine can interact with facilitatory A2A receptors located on myenteric nerve varicosities to stimulate the release of ACh. Adenosine signalling is tightly regulated by the nucleoside inactivation mechanisms. Deamination to form INO by ADA existing extracellularly (Ecto-ADA) represents the most efficient mechanism regulating synaptic adenosine levels. Adenosine uptake into cells via facilitated NTS may also contribute and serve to restrict adenosine actions to the release/production region. Note that while the facilitatory adenosine A2A receptor seems to be localised at the neuro-effector region, the inhibitory A1 receptor may be located further away from the sites of adenosine formation and removal and hence may be more accessible to exogenous adenosine. For the sake of clarity, prejunctional muscarinic and P2 receptors are omitted.
In addition to the role of inhibitory adenosine A1 receptors expressed on both cholinergic and tachykinergic myenteric neurons (see e.g. Gustaffson et al., 1985; Broad et al., 1992; Christofi & Wood, 1994), activation of A2A receptors may facilitate depolarisation of enteric neurons and increase ileal contractions elicited by electrical field stimulation (Christofi et al., 1994; Tomaru et al., 1995). In the present work, we showed that exogenously applied adenosine consistently inhibited electrically evoked [3H]ACh release. The finding that DPCPX (10 nM) not only counteracted the inhibitory effect of adenosine on [3H]ACh release from myenteric motoneurons but also converted it into a facilitatory effect (cf. Correia-de-Sá et al., 1991; Tomaru et al., 1995) indicates that exogenous adenosine may activate facilitatory receptors providing that coexistent inhibitory A1 receptors are blocked. Using two stable adenosine analogues displaying high subtype selectivity for adenosine receptors, R-PIA (100–300 nM) and CGS 21680C (1–10 nM), we clearly demonstrated the coexistence of inhibitory A1 and facilitatory A2A receptors modulating [3H]ACh release from rat myenteric motoneurons. The presence of two adenosine receptors, A1 and A2A, raised the questions of how endogenous adenosine interacts with each receptor subtype, and which response prevails. Tonic adenosinergic regulation in the ileum has been investigated before, with conflicting results. Using the guinea-pig ileum as a model, most studies provided indications that adenosine-induced inhibitory tonus is mediated by A1 receptors located on myenteric motoneurons (Christofi & Wood, 1993; Nitahara et al., 1995; Lee et al., 2001). This was observed in spite of the fact that in some of the studies the A1 antagonist DPCPX failed to affect the contractile responses to electrical stimulation (Nitahara et al., 1995; Tomaru et al., 1995). Differences in the frequency of stimulation may also be significant, as most authors used lower frequencies (0.1–2 Hz) than that used here (5 Hz) (see also e.g. De Man et al., 2003), which may therefore have been insufficient to cause a significant release/accumulation of extracellular adenosine. Overall, these discrepancies indicate that the mechanism of adenosine-induced modulation of cholinergic transmission in the myenteric plexus may differ between species and with the conditions under which signals are being recorded (cf. Correia-de-Sá et al., 1996). The present results showed that selective blockade of A2A receptors (with ZM 241385) inhibited evoked [3H]ACh release, while antagonism of A1 receptors (with DPCPX) produced a small but consistent increase in transmitter release. It has been shown that pretreatment with DPCPX shifted the balance between A1 and A2A receptor activation towards activation of A2A receptors (Correia-de-Sá & Ribeiro, 1996). The fact that the action of ZM 241385 mimicked the inhibition caused by removing extracellular adenosine with ADA (0.5 U ml−1) is consistent with the idea that endogenous adenosine exerts a predominantly tonic facilitatory effect (of about 30%) on cholinergic neurotransmission. Furthermore, increasing the extracellular adenosine levels using inhibitors of ADA (EHNA, 50 μM), nucleoside transport (dipyridamole, 0.5 μM), or AK (ITU, 10 μM) facilitated ACh release in a manner sensitive to A2A receptor blockade. To our knowledge, this is the first report showing that endogenous adenosine tonically facilitates the release of ACh from enteric motoneurons through the activation of adenosine A2A receptors.
Regardless of the fact that adenosine is derived from ATP released either from activated smooth muscle cells (Vizi et al., 1992; Katsuragi et al., 1993) and/or from autonomic nerve terminals (White & Leslie, 1982), our data suggest that the release of adenosine per se induced by electrical stimulation (Begg et al., 2002) plays a major role in regulating myenteric excitability. This was inferred because inhibition of endogenous AMP breakdown to give adenosine failed to modify the release of [3H]ACh from stimulated (200 pulses delivered at a frequency of 5 Hz) myenteric motoneurons, whereas removal of all extracellular adenosine by bath application of ADA (0.5 U ml−1) caused a consistent inhibitory effect. Similar results were obtained in isolated strips of the guinea-pig ileum, where inhibition of ecto-5′-nucleotidase with AOPCP yielded either no effect or a weak inhibitory effect on cholinergic neurotransmission (e.g. Wiklund & Gustafsson, 1986; Katsuragi et al., 1993). Since P2 purinoceptor activation could complicate the interpretation of data obtained with the ADP analogue AOPCP (200 μM) (Naito & Lowenstein, 1985), we tested the non-nucleoside inhibitor of ecto-5′-nucleotidase, Con A (0.1 mg ml−1) (Riordan & Slavik, 1974; Stefanovic et al., 1975), which was also ineffective. Adenosine originating from the catabolism of released nucleotides only became functionally relevant after the stimulation period was prolonged to 4.5 min (see Table 1). The sensitivity of the AOPCP (200 μM)- and Con A (0.1 mg ml−1)-induced inhibitory effects to blockade by ZM 241385 reveals that adenosine produced via the ecto-nucleotidase pathway activates facilitatory A2A receptors in a time-dependent manner. The failure of ecto-5′-nucleotidase inhibitors to modify [3H]ACh release during brief stimulation trains contrasts with the facilitatory effect of the exogenously added adenosine precursor AMP. These findings indicate that the amounts of adenosine generated from released adenine nucleotides are probably insufficient to activate prejunctional facilitatory A2A receptors, which may be the result of insufficient release of adenine nucleotides. Alternatively, the postsynaptic localization of ecto-5′-nucleotidase (Nitahara et al., 1995) may lead to a delay in the accumulation of adenosine that is produced some distance away from the adenosine receptor sites on the myenteric nerve terminals.
We are uncertain whether the extracellular levels of adenosine achieved in the current study are physiologically relevant. However, both in vivo and in vitro models suggest that the balance between inhibitory adenosine A1 and facilitatory A2A receptors may be important in regulating intestinal motility. This has been confirmed because administration of DPCPX, which reveals A2A receptor-mediated effects (Correia-de-Sá et al., 1991; Tomaru et al., 1995), promoted faecal expulsion (Tomaru et al., 1994) and may reverse postoperative ileus (Kadowaki et al., 2003) in rats. In addition, Milusheva et al. (1990) demonstrated that hypoxia inhibited the release of ACh from the myenteric plexus, through the elevation of endogenous adenosine. Recently, De Man et al. (2003) reported that chronic intestinal inflammation enhanced enteric contractile activity in part due to a loss of the cholinergic neuromodulatory role of adenosine mediated by A1 receptors. Desensitisation of purinoceptors on enteric nerves may occur during chronic inflammation, because purines such as adenosine and ATP are released from mast cells (Marquardt et al., 1984) located in the vicinity of myenteric neurons in several species including man (Stead et al., 1989; Bogers et al., 2000). It is worth noting that human post-ganglionic myenteric neurons coexpress adenosine A1 and A2A receptors, which exhibit a heterogeneous regional distribution (Christofi et al., 2001). Therefore, there is a considerable interest in the neuroprotective effects exerted by adenosine during ischaemic and inflammatory insults, and it is conceivable that adenosine contributes to an overall homeostatic effect on enteric excitability during pathological conditions when adenosine levels become elevated. Although the release of adenosine per se may be the main source of extracellular adenosine in most stressed cells (for a review, see Cunha, 2001), the pathophysiological implications of the production of adenosine directly, from neighbouring neurogenic, myogenic, vascular and inflammatory sources, or indirectly, as an ATP breakdown product, remain to be elucidated. In the light of the present data, it is tempting to speculate that adenosine generated away from the active zones is more prone to inactivation by uptake and deamination during diffusion towards the synaptic region, and this favours the activation of neuroprotective inhibitory adenosine A1 receptors located in the soma or in the axons of myenteric neurons (cf. Barajas-López et al., 1996). In contrast, adenosine formed at myenteric neuro-effector junctions might be a major contributor to the maintainance of cholinergic neurotransmission through the activation of prejunctional facilitatory A2A receptors.
Acknowledgments
This research was partially supported by FCT projects (POCTI/FCB/36545/2000, POCTI/FCB/45549/2002 and UMIB-215/94) with the participation of FEDER funding. We also thank Mrs. M. Helena Costa e Silva, Suzete Liça and Belmira Silva for their technical assistance.
Abbreviations
- ACh
acetylcholine
- ADA
adenosine deaminase
- ADO
adenosine
- AK
adenosine kinase
- AOPCP
α, β-methylene ADP
- CGS 21680C
2-[4-(2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamido adenosine
- Con A
concanavalin A
- DMSO
dimethylsulphoxide
- DPCPX
1, 3-dipropyl-8-cyclopentyl xanthine
- EHNA
erythro-9(2-hydroxy-3-nonyl) adenine
- INO
inosine
- ITU
5′-iodotubercidin
- NBTI
S-(p-nitrobenzyl)-6-thioinosine
- NT
nucleoside transporter
- 5′-NTase
5′-nucleotidase
- R-PIA
R-N6-phenylisopropyl adenosine
- ZM 241385
(4-(2-[7-amino-2-(2-furyl{1,2,4}-triazolo{2,3-a{1,3, 5}triazin-5-yl-aminoethyl)phenol.
References
- AGARWAL R.P., SPECTOR T., PARKS R.E., JR Tight-binding inhibitors. IV. Inhibition of adenosine deaminases by various inhibitors. Biochem. Pharmacol. 1977;26:359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- ARCH J.R.S., NEWSHOLME E.A.The control of metabolism and the hormonal role of adenosine Essays in Biochemistry 1978New York: Academic Press; 82–123.ed. Campbell, P.N. & Aldridge, W.N. pp [PubMed] [Google Scholar]
- BARAJAS-LÓPEZ C., PERES A.L., ESPINOSA-LUNA R. Cellular mechanisms underlying adenosine actions on cholinergic transmission in enteric neurons. Am. J. Physiol. (Cell Physiol.) 1996;271:C264–C275. doi: 10.1152/ajpcell.1996.271.1.C264. [DOI] [PubMed] [Google Scholar]
- BEGG M., DALE N., LLAUDET E., MOLLEMAN A., PARSONS M.E. Modulation of the release of endogenous adenosine by cannabinoids in the myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum. Br. J. Pharmacol. 2002;137:1298–1304. doi: 10.1038/sj.bjp.0704985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGERS J., MOREELS T., DE MAN J.G., VROLIX G., JACOBS W., PELKMANS P.A., VAN MARCK E.A. Schistosoma mansoni infection causing diffuse enteric inflammation and damage of the enteric nervous system in the mouse small intestine. Neurogastroenterol. Motil. 2000;12:431–440. doi: 10.1046/j.1365-2982.2000.00219.x. [DOI] [PubMed] [Google Scholar]
- BROAD R.M., MCDONALD T.J., BRODIN E., COOK M.A. Adenosine A1 receptors mediate inhibition of tachykinin release from perifused enteric nerve endings. Am. J. Physiol. (Gastrointest. Liver Physiol.) 1992;262:G525–G531. doi: 10.1152/ajpgi.1992.262.3.G525. [DOI] [PubMed] [Google Scholar]
- CASS C.E., YOUNG J.D., BALDWIN S.A. Recent advances in the molecular biology of nucleoside transporters of mammalian cells. Biochem. Cell Biol. 1998;76:761–770. doi: 10.1139/bcb-76-5-761. [DOI] [PubMed] [Google Scholar]
- CHRISTOFI F.L., BAIDAN L.V., FERTEL R.H., WOOD J.D. Adenosine A2 receptor-mediated excitation of a subset of AH/type 2 neurons and elevation of cAMP levels in myenteric ganglia of guinea-pig ileum. Neurogastroenterol. Mot. 1994;6:67–78. [Google Scholar]
- CHRISTOFI F.L., WOOD J.D. Presynaptic inhibition by adenosine A1 receptors on guinea-pig small intestinal myenteric neurons. Gastroenterology. 1993;104:1420–1429. doi: 10.1016/0016-5085(93)90351-c. [DOI] [PubMed] [Google Scholar]
- CHRISTOFI F.L., WOOD J.D. Electrophysiological subtypes of inhibitory P1 purinoceptors on myenteric neurons of guinea-pig small bowel. Br. J. Pharmacol. 1994;113:703–710. doi: 10.1111/j.1476-5381.1994.tb17050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOFI F.L., ZHANG H., YU J.-G., GUZMAN J., XUE J., KIM M., WANG Y.-Z., COOK H.J. Differential gene expression of adenosine A1, A2A, A2B and A3 receptors in the human enteric nervous system. J. Comp. Neurol. 2001;439:46–64. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- CORREIA-DE-SÁ P., RIBEIRO J.A. Adenosine uptake and deamination regulate tonic A2A receptor facilitation of evoked [3H]acetylcholine release from the rat motor nerve terminals. Neuroscience. 1996;73:85–92. doi: 10.1016/0306-4522(96)00028-0. [DOI] [PubMed] [Google Scholar]
- CORREIA-DE-SÁ P., SEBASTIÃO A.M., RIBEIRO J.A. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve endings of the rat. Br. J. Pharmacol. 1991;103:1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORREIA-DE-SÁ P., TIMÓTEO M.A., RIBEIRO J.A. Presynaptic A1 inhibitory/A2A facilitatory adenosine receptor activation balance depends on motor nerve stimulation paradigm at the rat hemidiaphragm. J. Neurophysiol. 1996;76:3910–3919. doi: 10.1152/jn.1996.76.6.3910. [DOI] [PubMed] [Google Scholar]
- COSTA M., BROOKES S.J.H., STEELE P.A., GIBBINS I., BURCHER E., KANDIAH C.J. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem. Int. 2001;38:107–125. doi: 10.1016/s0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., ALMEIDA T., RIBEIRO J.A. Parallel modification of adenosine extracellular metabolism and modulatory action in the hippocampus of aged rats. J. Neurochem. 2001;76:372–382. doi: 10.1046/j.1471-4159.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- CUNHA R.A., CORREIA-DE-SÁ P., SEBASTIÃO A.M., RIBEIRO J.A. Preferential activation of excitatory adenosine receptors at the rat hippocampal and neuromuscular synapses by adenosine formed from released adenine nucleotides. Br. J. Pharmacol. 1996;119:253–260. doi: 10.1111/j.1476-5381.1996.tb15979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA R.A., SEBASTIÃO A.M. Extracellular metabolism of adenine nucleotides and adenosine in the innervated skeletal muscle of the frog. Eur. J. Pharmacol. 1991;197:83–92. doi: 10.1016/0014-2999(91)90368-z. [DOI] [PubMed] [Google Scholar]
- DALY J.W. Adenosine receptors: targets for future drugs. J. Med. Chem. 1982;25:197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- DE MAN J.G., SEERDEN T.C., DE WINTER B.Y., VAN MARCK E.A., HERMAN A.G., PELKMANS P.A. Alteration of the purinergic modulation of enteric neurotransmission in the mouse ileum during chronic intestinal inflammation. Br. J. Pharmacol. 2003;139:172–184. doi: 10.1038/sj.bjp.0705218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTICKE B., GERLACH E. Kompetitive hemung der adenosin-desaminase als mögliche ursache der coronardilatierenden wirkung einer pyrimido-pyrimidin-verbinding. Naunyn-Schmiedeberg's Arch. Pharmakol. Exp. Path. 1966;255:107–119. [PubMed] [Google Scholar]
- DOWDALL M.J. Adenine nucleotides in cholinergic transmission: presynaptic aspects. J. Physiol. (Paris) 1978;74:497–501. [PubMed] [Google Scholar]
- DUARTE-ARAÚJO M., TIMÓTEO M.A., CORREIA DE-SÁ P.Neuromodulatory role of endogenous adenosine at the myenteric plexus of the rat ileum XXXI Meeting of the Portuguese Pharmacological Society 2000. Porto
- GEIGER J.D., FYDA D.Adenosine transport in CNS Adenosine in the Nervous System 1991London: Academic Press; 1–23.ed. Stone, T.W. pp [Google Scholar]
- GOLEMBIOWSKA K., WHITE T.D., SAWYNOK J. Adenosine kinase inhibitors augment release of adenosine from spinal cord slices. Eur. J. Pharmacol. 1996;307:157–162. doi: 10.1016/0014-2999(96)00248-8. [DOI] [PubMed] [Google Scholar]
- GONÇALVES J., QUEIROZ G. Facilitatory and inhibitory modulation by endogenous adenosine of noradrenaline release in the epididymal portion of rat vas deferens. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;348:367–371. doi: 10.1007/BF00171335. [DOI] [PubMed] [Google Scholar]
- GRIFFITH D.A., JARVIS S.M. Nucleoside and nucleobase transport systems of mammalian cells. Biochim. Biophys. Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- GUSTAFFSON L.E., WILKUND N.P., LUNDIN J., HEDQVIST P. Characterization of pre- and post-junctional adenosine receptors in guinea-pig ileum. Acta Physiol. Scand. 1985;123:195–203. doi: 10.1111/j.1748-1716.1985.tb07578.x. [DOI] [PubMed] [Google Scholar]
- HOPKINS S.V., GOLDIE R.G. A species difference in the uptake of adenosine by heart. Biochem. Pharmacol. 1971;20:3359–3365. doi: 10.1016/0006-2952(71)90440-0. [DOI] [PubMed] [Google Scholar]
- KADOWAKI M., NAGAKURA Y., TOKITA K., HANAOKA K., TOMOI M. Adenosine A1 receptor blockade reverses experimental postoperative ileus in rat colon. Eur. J. Pharmacol. 2003;458:197–200. doi: 10.1016/s0014-2999(02)02766-8. [DOI] [PubMed] [Google Scholar]
- KATSURAGI T., SHIRAKABE K., SOEJIMA O., TOKUNAGA T., MATSUO K., SATO C., FURUKAWA T. Possible transynaptic cholinergic neuromodulation by ATP released from ileal longitudinal muscles of guinea pigs. Life Sci. 1993;53:911–918. doi: 10.1016/0024-3205(93)90443-7. [DOI] [PubMed] [Google Scholar]
- KEIDING R., HORDER M., GERHARDT W., PITKANEN E., TENHUNEN R., STROMME J.H., THEORDERSEN L., WALDENSTROM J., TRYDING N., WESTLUND L. Recommended methods for the determination of four enzymes in the blood. Scand. J. Clin. Lab. Invest. 1974;33:291–306. [Google Scholar]
- LEE J.J., TALUBMOOK C., PARSONS M.E. Activation of presynaptic A1-receptors by endogenous adenosine inhibits actylcholine release in the guinea-pig ileum. J. Auton. Pharmacol. 2001;21:29–38. doi: 10.1046/j.1365-2680.2001.00201.x. [DOI] [PubMed] [Google Scholar]
- LLOYD H.G., FREDHOLM B.B. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem. Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- MARQUARDT D.L., GRUBER H.E., WASSERMAN S.I. Adenosine release from stimulated mast cells. Proc. Natl. Acad. Sci. U.S.A. 1984;81:6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILUSHEVA E., SPERLÁGH B., KISS J., SZPORNY L., PASTZTOV E., PAPASOVA M., VIZI E.S. Inhibitory effect of hypoxic condition on acetylcholine release is partly due to the effect of adenosine released from the tissue. Brain Res. Bull. 1990;24:369–373. doi: 10.1016/0361-9230(90)90091-d. [DOI] [PubMed] [Google Scholar]
- NAITO Y., LOWENSTEIN J.M. 5′-Nucleotidase from rat heart membranes. Inhibition by adenine nucleotides and related compounds. Biochem. J. 1985;226:645–651. doi: 10.1042/bj2260645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NITAHARA K., KITTEL A., LIANG S.D., VIZI E.S. A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5′-nucleotidase. Neuroscience. 1995;67:159–168. doi: 10.1016/0306-4522(94)00585-s. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA L., TIMÓTEO M.A., CORREIA-DE-SÁ P. Modulation by adenosine of both muscarinic M1-facilitation and M2-inhibition of [3H]-acetylcholine release from the rat motor nerve terminals. Eur. J. Neurosci. 2002;15:1728–1736. doi: 10.1046/j.1460-9568.2002.02020.x. [DOI] [PubMed] [Google Scholar]
- PAK M.A., HAAS H.L., DECKING U.K., SCHRADER J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacology. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- PATON W.D.M., VIZI E.S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea pig-ileum longitudinal muscle strip. Br. J. Pharmacol. 1969;35:10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIORDAN J.R., SLAVIK M. Interactions of lectins with membrane glycoproteins effects of concanavalin A on 5′-nucleotidase. Biochim. Biophys. Acta. 1974;373:356–360. doi: 10.1016/0005-2736(74)90015-7. [DOI] [PubMed] [Google Scholar]
- SEBASTIÃO A.M., RIBEIRO J.A. Fine-tuning neuromodulation by adenosine. Trends Pharmacol. Sci. 2000;21:341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- STEAD R.H., DIXON M.F., BRAMWELL N.H., RIDDELL R.H., BIENNENSTOCK J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- STEFANOVIC V., MANDEL P., ROSENBERG A. Concanavalin A inhibition of ecto-5′-nucleotidase of intact cultured C6 glioma cells. J. Biol. Chem. 1975;250:7081–7083. [PubMed] [Google Scholar]
- TOMARU A., INA Y., KISHIBAYASHI N., KARASAWA A. Excitation and inhibition via adenosine receptors of the twitch response to electrical stimulation in isolated guinea pig ileum. Jpn. J. Pharmacol. 1995;69:429–433. doi: 10.1254/jjp.69.429. [DOI] [PubMed] [Google Scholar]
- TOMARU A., ISHII A., KISHIBAYASHI N., SHIMADA J., SUSUKI F., KARASAWA A. Possible physiological role of endogenous adenosine in defecation in rats. Eur. J. Pharmacol. 1994;264:91–94. doi: 10.1016/0014-2999(94)90641-6. [DOI] [PubMed] [Google Scholar]
- VIZI E.S., SPERLAGH B., BARANYI M. Evidence that ATP released from the postsynaptic site by noradrenaline, is involved in mechanical responses of guinea-pig vas deferens: cascade transmission. Neuroscience. 1992;50:455–465. doi: 10.1016/0306-4522(92)90437-7. [DOI] [PubMed] [Google Scholar]
- WHITE T.D., LESLIE R.A. Depolarization-induced release of adenosine 5′-triphosphate from isolated varicosities derived from the myenteric plexus of the guinea-pig small intestine. J. Neurosci. 1982;2:206–215. doi: 10.1523/JNEUROSCI.02-02-00206.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIKLUND N.P., GUSTAFSSON L.E. Neuromodulation by adenine nucleotides as indicated by experiments with inhibitors of nucleotide inactivation. Acta Physiol. Scand. 1986;126:217–223. doi: 10.1111/j.1748-1716.1986.tb07808.x. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]