Abstract
Fast-desensitizing P2X3 receptors of nociceptive dorsol root ganglion (DRG) neurons are thought to mediate pain sensation. Since P2X3 receptor efficiency is powerfully modulated by desensitization, its underlying properties were studied with patch-clamp recording.
On rat cultured DRG neurons, 2 s application of ATP (EC50=1.52 μM), ADP (EC50=1.1 μM) or α,β-meATP (EC50=1.78 μM) produced similar inward currents that fully desensitized, at the same rate, back to baseline. Recovery from desensitization was much slower after ATP and ADP than after α,β-meATP and, in all cases, it had sigmoidal time course.
By alternating the application of ATP and α,β-meATP, we observed complete cross-desensitization indicating that these agonists activated the same receptors. This notion was confirmed by the similar antagonism induced by 2′, 3′-O-(2,4,6,trinitrophenyl)-adenosine triphosphate (TNP-ATP).
Recovery from desensitization elicited by ATP was unexpectedly shaped by transient application of α,β-methylene-adenosine triphosphate (α,β-meATP), and vice versa. Thus, short-lasting, full desensitization produced by α,β-meATP protected receptors from long-lasting desensitization induced by subsequent ATP applications. ATP and ADP had similar properties of recovery from desensitization.
Low nM concentrations of α,β-meATP (unable to evoke membrane currents) could speed up recovery from ATP-induced desensitization, while low nM concentrations of ATP enhanced it. Ambient ATP levels were found to be in the pM range (52±3 pM).
The phenomenon of cross-desensitization and protection was reproduced by rP2X3 receptors expressed by rat osteoblastic cell 17/2.8 or human embryonic kidney cell 293 cells, indicating P2X3 receptor specificity.
It is suggested that transient application of an agonist that generates rapid recovery from desensitization, is a novel, powerful tool to modulate P2X3 receptor responsiveness to the natural agonist ATP.
Keywords: DRG, purinergic, ATP, nociception, desensitization, ROS cells
Introduction
ATP is suggested to be an important messenger to generate pain signals in sensory neurons (Burnstock, 2001; Cook & McCleskey, 2002; North, 2004). On nociceptive dorsal root ganglion (DRG) neurons, ATP operates mainly via rapidly desensitizing ionotropic P2X3 receptors (Chen et al., 1995; Lewis et al., 1995; for reviews, see Khakh et al., 2001; North, 2002). The outstanding feature of such receptors is not only fast development of their desensitization, but also the exceptionally slow recovery process taking up to 6–20 min after a single, short (1–2 s) ATP application (Cook et al., 1998; Rae et al., 1998; Sokolova et al., 2001). When DRG neurons express heteromeric P2X2/3 receptors, their pharmacological properties, including desensitization, are changed (Liu et al., 2001).
Since the ambient ATP concentration ranges from pM to nM (Lazarowski et al., 2003), it is, in theory, possible that a fraction of P2X3 receptors is constitutively desensitized by ambient ATP (see analogy with P2X1 receptors; Rettinger & Schmalzing, 2003). Desensitization might then be an intrinsic mechanism to control P2X3 receptor function in response to phasic application of ATP.
Although the mechanism of P2X3 receptor desensitization is poorly understood, in contrast for example to P2X1 (Rettinger & Schmalzing, 2003) or P2X2 receptors (Ding & Sachs, 1999; Skorinkin et al., 2003), it may also represent an important target for regulating receptor activity by extracellular divalent cations (Cook et al., 1998; Giniatullin et al., 2003) or inflammatory mediators (Paukert et al., 2001). The extracellular loop of P2X3 receptors appears to contain structural determinants essential for ligand selectivity and recovery from desensitization (Koshimizu et al., 2002).
The present study on native P2X3 receptors of rat DRG neurons and on wild-type P2X3 receptors expressed in rat osteoblastic cells (ROS) sought to understand if agonist efficacy and certain properties of desensitization were tightly related. We observed that for analogous, complete desensitization, recovery from it was dependent on the nature of the agonist used, namely ATP or α,β-methylene-ATP (α,β-meATP). Owing to the very large difference in recovery rate depending on the choice of agonist, short application of a certain agonist enabled to transiently rescue P2X3 receptors from their profound desensitization or to deepen their desensitization phase.
Methods
DRG cell culture
Rat DRG neurons in culture were prepared as previously described (Sokolova et al., 2001). Rats (2–3 week old) of both sexes were deeply anesthetized with ether and killed by decapitation, a procedure (including animal handling and care) in accordance with the Italian Animal Welfare Act and approved by the Local Authority Veterinary Service. Such a procedure accords with the European Communities Council Directive (24 November 1986; 86/609/EEC). DRG neurons were plated on poly-L-lysine (5 mg ml−1)-coated Petri dishes and cultured for 1–2 days under an atmosphere containing 5% CO2. Nerve growth factor (2.5S NGF; 50 ng ml−1) was added at the time when DRG neurons were attached to poly-L-lysine. Cells were used within 2 days of plating when they lacked processes.
Cell culture and stable transfection
The rat osteoblastic cell line ROS 17/2.8 (ROS) was kindly provided by Professor R. Civitelli (Washington University, School of Medicine, St Louis, MO, U.S.A.). Human embryonic kidney 293 (HEK) were supplied by our in-house cell bank. Cells were grown in MEM supplemented with 10% heat-inactivated calf serum, nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine and penicillin/streptomycin. ROS or HEK cells expressing the rat P2X3 purinergic receptor were produced by transfection with a rat P2X3 gene using the pcDNA3 mammalian expression vector, generously provided by Professor A. North (Sheffield University, U.K.). Cells were transfected with calcium phosphate precipitation and selected in 400 μg ml−1 Geneticin (G418). G418-resistant cells were assayed for rat P2X3 protein expression with immunofluorescence and Western immunoblotting. All cells were grown in a humidified atmosphere containing 5% CO2.
Immunofluorescence and Western immunoblotting
For immunofluorescence experiments, transfected or untransfected ROS (or HEK) cells were plated on glass coverslips. Cells were washed with phosphate buffer solution, fixed in 4% paraformaldehyde for 10 min at room temperature and permeabilized in ice-cold acetone for 5 min at −20°. Coverslips were incubated for 2 h with a rabbit anti-P2X3 polyclonal antibody (Chemicon, 1 : 100). The immunoreaction was detected using an RITC-conjugated anti-rabbit secondary antibody (Sigma, 1 : 200). Controls for signal specificity were performed by incubating the transfected cells with the secondary antibody only.
For Western immunoblots of transfected or untransfected ROS (or HEK) cells, these were lysed using a buffer containing 25 mM Tris-HCl (pH 7.5), 1 mM EDTA, Triton X-100 (1%), 1 mM spermidin, 1 mM iodoacetamide and protease inhibitors, and separated on 10% polyacrylamide gel. Proteins transferred to the nitrocellulose membrane were blocked at room temperature for at least 5 h in TBS buffer (Tris 25 mM, pH 7.5, NaCl 150 mM, 5% dry milk and 2% foetal calf serum) and then incubated overnight at 4°C with antibodies against P2X3 (Neuromics; 1:1000). Immunocomplexes were detected with a peroxidase-conjugated secondary antibody (Sigma, Milan, Italy; 1 : 2000) for 2 h and a chemiluminescence ECL kit (Amersham).
Measurement of ATP concentration
DRG neurons plated on 35 mm Petri dishes were washed continuously with standard basic solution for 10 min. A sample of 1 ml of the solution was collected from each dish after the washing period and placed immediately into ice for biochemical assay of ATP with a bioluminescence method (ENLITEN®, Promega, Milan, Italy). In particular, the RLuciferase/Luciferin reagent was reconstituted with reconstitution buffer 1 h before ATP concentration measurements to allow the reagent to rehydrate at room temperature. An ATP standard calibration curve was first constructed with fresh aliquots of ATP concentrations, then assays of test samples were performed. The samples and ATP standard curve were run at least in duplicate for assay reliability.
Electrophysiological recording
We selected IB4-sensitive, small and medium (15–30 μm diameter) neurons as previously described (Giniatullin et al., 2003) for recording membrane currents in the whole cell configuration, while cells were continuously superfused with control solution containing (in mM): NaCl 152, KCl 5, MgCl2 1, CaCl2 2, glucose 10, HEPES 10; pH was adjusted to 7.4 with NaOH and osmolarity was adjusted to 320 mOsm with glucose. Patch pipettes had a resistance of 3–4 MΩ when filled (in mM) with CsCl 130; HEPES 20; MgCl2 1, magnesium ATP 3; EGTA 5; pH was adjusted to 7.2 with CsOH. Osmolarity of the pipette solution was adjusted to 290 mOsm with sucrose. In most cells, series resistance was compensated by 80%. Cells were voltage-clamped at −70 mV. Currents were filtered at 1 kHz and acquired on IBM PC by means of pCLAMP 7.0 software (Axon Instruments, Foster City, CA, U.S.A.).
Drugs and their application
Agonists and antagonists were applied by a rapid superfusion system (Rapid Solution Changer RSC-200, BioLogic Science Instruments, Grenoble, France) placed 100–150 μm near the cell. Time for the solution exchange across the cell was about 30 ms. Cells were accepted for experimental testing if they generated, at the beginning of the protocol, at least three successive equiamplitude responses to ATP or related agonists applied at 6 min interval without run up or down of current amplitude. ATP, α,β-meATP or ADP applications were 2 s long unless otherwise indicated. For construction of dose–response plots, agonist concentrations were applied sequentially every 6 min for doses up to 10 μM. For 100 μM concentrations, intervals were ⩾10 min to avoid interactions with subsequent responses. There was no difference in dose–response curves obtained by either alternating agonists at each concentration or applying the full range of concentrations of the same agonist before testing the next agonist.
All chemicals, including enzymes for cell culture, were from Sigma (Milan, Italy); culture mediums were obtained from Gibco BRL (Life Technologies, Milan, Italy), the antibody against P2X3 was from Chemicon (Milan, Italy), while the secondary anti-rabbit antibody was from Sigma (Milan, Italy). G418 was from Invitrogen (San Giuliano Milanese, Italy). The purity of ADP stated by Sigma on the basis of HPLC assay was 98%.
Data analysis
All data are presented as mean±s.e.m. (n=number of cells) with statistical significance assessed with paired t-test (for parametric data) or Mann–Whitney rank sum test (for nonparametric data). Best fits of data with a sigmoid function (obtained with Origin software; version 6.0) were compared with respective control fits using SigmaStat (Jandel Scientific; version 2.0). A value of P<0.05 was accepted as indicative of statistically significant difference.
The fitting function for recovery (R) as a function of time (t) was in the form of the Boltzmann equation:
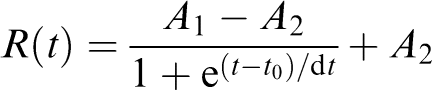 |
where A1, A2, are the start and finish levels.
Results
Distinct rates of recovery from desensitization produced by ATP or α,β-meATP
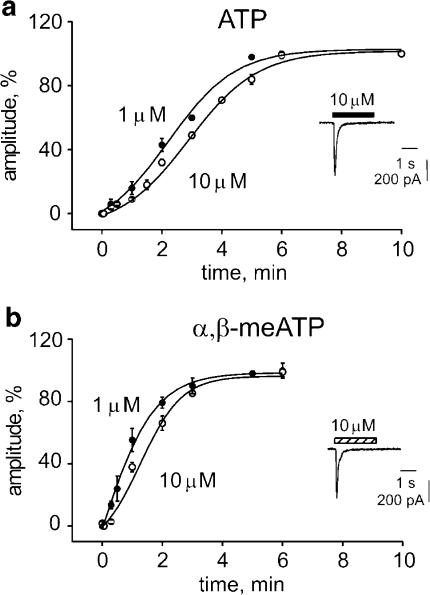
The 2 s application of 10 μM ATP to DRG neurons activated an inward current that peaked and decayed back to baseline, indicating complete receptor desensitization (see example in the inset to Figure 1a). The current decay, which represents the onset of desensitization, was biexponential (on average, τ1=61±8 and τ2=421±88 ms; n=14). Recovery from desensitization (expressed as t1/2, namely the time to regain 50% of control peak amplitude) was very slow and was best fitted by a sigmoidal function (Figure 1a). The recovery process was dependent on agonist concentration (1 μM ATP t1/2=2.42±0.1 min and 10 μM ATP t1/2=3.04±0.6 min, n=5 or 15, respectively), although desensitization was complete in either case.
Figure 1.
Sigmoidal time course of recovery from desensitization of P2X3 receptors of DRG neurons. (a) Responses are elicited by 2 s application of 1 (filled circles) or 10 (open circles) μM application of ATP (see inset for representative inward current induced by 10 μM ATP). Recovery is plotted as % peak amplitude of control response. Data points are from 5 to 15 cells; error bars are omitted when smaller than symbols. Curves are fitted as described in the Methods. (b) Recovery plots for responses induced by 1 or 10 μM α,β-meATP. Data points are from 5 to 6 cells. For further details see (a).
The P2X3 receptor agonist α,β-meATP (10 μM) elicited inward currents as large as those induced by 10 μM ATP (compare Figure 1b inset with Figure 1a inset, data from the same cell) and that fully desensitized with biexponential time course (τ1=67±7 ms; τ2=450±95 ms; n=14). Desensitization recovery was faster in the case of α,β-meATP than that of ATP (compare Figure 1b with Figure 1a), although it still depended on agonist concentration (1 μM α,β-meATP t1/2=0.99±0.3 min, n=5; 10 μM α,β-meATP t1/2=1.51±0.87 min, n=6). This difference was significant even when the recovery rate with 10 μM α,β-meATP was compared to the one with 1 μM ATP (compare Figure 1b, open circles, with Figure 1a, closed circles; P<0.05). Thus, recovery from α,β-meATP-induced desensitization was significantly faster than the recovery from ATP-induced desensitization despite similar rate of desensitization onset.
Agonist dose–response curve and inhibitory action of 2′, 3′-O-(2,4,6,trinitrophenyl)-adenosine triphosphate (TNP-ATP)
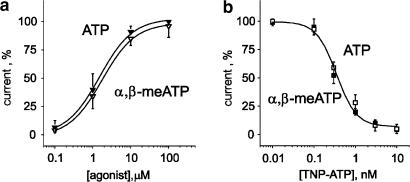
One important question was whether, under the present experimental conditions, ATP and α,β-meATP behaved both as full agonists with similar efficacy and potency. Figure 2a shows almost identical dose–response curves for ATP and α,β-meATP (ATP EC50=1.52 μM; α,β-meATP EC50=1.78 μM) with the same maximum response.
Figure 2.
Efficacy and antagonism sensitivity of ATP- and α,β-meATP-induced currents of DRG neurons. (a) Plots of response amplitude (as % of maximum) versus log agonist concentrations. Responses to ATP or α,β-meATP were very similar, indicating analogous efficacy and potency. Data were normalized with respect to the response evoked by 100 μM ATP. Data are from 5 to 8 cells. (b) Plot of response amplitude against log concentrations of the antagonist TNP-ATP. Open squares indicate 10 μM ATP responses while filled squares are for 10 μM α,β-meATP responses. Note similar antagonism. Data are from five paired cells.
TNP-ATP, which is a very potent and selective antagonist of fast desensitizing P2X receptors and P2X2/3 receptors (Liu et al., 2001; North, 2002), was equieffective (IC50=350 pM, n=5) in blocking membrane currents activated by 10 μM ATP or 10 μM α,β-meATP (Figure 2b). Thus, analogous pharmacological antagonism of ATP or α,β-meATP-mediated responses was consistent with activation of the same homogeneous receptor class by either agonist. Hence, distinct rates of recovery for ATP or α,β-meATP desensitization were not seemingly due to activation of different ATP receptors.
α,β-meATP could protect receptors from ATP-induced desensitization
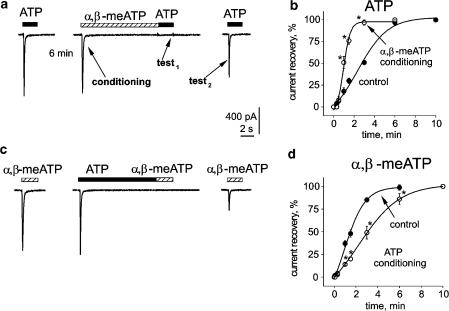
As P2X3 receptors showed distinct, agonist-specific recovery from desensitization, it might be interesting to exploit this property to speed up or retard recovery of receptor function. Thus, we explored if the transient application of one agonist could shape the process of recovery tested with the other agonist. For this purpose, the standard protocol indicated in Figure 3a was employed. After obtaining three stable responses to applications of 10 μM ATP (6 min interval) to ensure full recovery from desensitization (see Figure 1a), receptors were completely desensitized with 10 μM α,β-meATP (applied for 10 s; conditioning response), so that the next application of 10 μM ATP, at the end of α,β-meATP administration, produced little or no response (test1 response). After a variable interval (1.5 min in the example of Figure 3a), 10 μM ATP was again applied to produce test2 response that was measured as % of the standard ATP response before the protocol had started. Note that in this example the test2 response had largely recovered after α,β-meATP conditioning (Figure 3a). Average data obtained by spacing the test2 response at different time after test1 response are plotted in Figure 3b (open circles) and compared with the recovery curve obtained in the absence of α,β-meATP conditioning (filled circles). Thus, after conditioning, a 1.5 min interval between test1 and test2 responses restored the current amplitude to a level which otherwise would have taken 4.5 min to achieve. With this protocol, the recovery rate from ATP desensitization had therefore acquired the property of that of α,β-meATP. Figure 3c shows that, on the same cell shown in Figure 3a, the mirror situation developed when conditioning was done with 10 s application of 10 μM ATP and α,β-meATP was the test agent (average data are plotted in Figure 3d).
Figure 3.
Agonist-dependent modulation of P2X3 receptor desensitization of DRG neurons. (a) Protocol to study interaction between agonists during the process of recovery from desensitization. In particular, after a response to ATP (10 μM) that peaked and fully desensitized, 6 min rest was allowed for full recovery and 10 μM α,β-meATP was applied for 10 s (conditioning) to produce a peak current that fully decayed to baseline. After α,β-meATP washout, the subsequent ATP application (test1) elicited no response. In this example, after a 1.5 min interval, the next ATP application (test2) produced a much larger current than in the standard recovery protocol. (b) plots of desensitization recovery. Time is either the interval between ATP applications (10 μM) in control conditions (filled circles), or the time interval between test1 and test2 ATP responses after 10 μM α,β-meATP conditioning (open circles). n=3–6 cells, statistically significant differences (P<0.05) are indicated with asterisks. Note strong acceleration of recovery after α,β-meATP conditioning. (c) Protocol similar to the one shown in (a) (same cell) to explore 10 μM ATP conditioning effect on responses evoked by 10 μM α,β-meATP. ATP conditioning largely delayed recovery from desensitization. (d) Plots of recovery of α,β-meATP responses in control conditions or after 10 μM ATP conditioning. For further details see (b) Note that recovery becomes significantly slower after ATP conditioning (n=4–6; *P<0.05).
In summary, α,β-meATP induced complete receptor desensitization and, at the same time, imparted its own property of desensitization recovery to subsequent tests done with ATP. This finding outlined the possibility that the efficiency of receptor signalling to the natural transmitter ATP could be rescued by transient application of α,β-meATP.
Activation and desensitization of P2X3 receptors by ADP
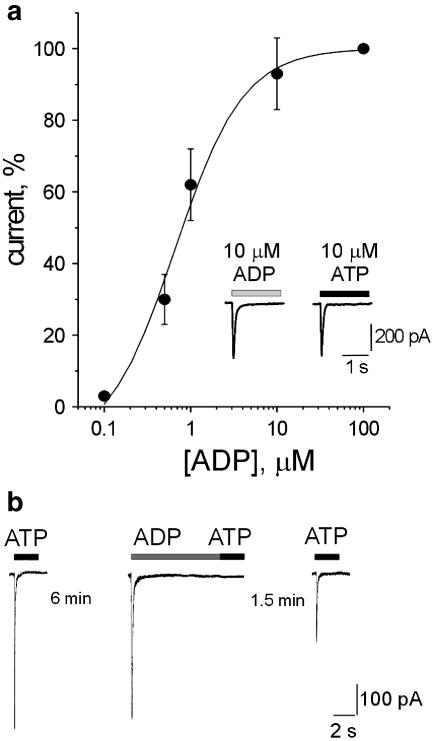
It should be interesting to compare the effects of ATP with the ones induced by its natural breakdown product ADP that is reported to show agonist properties on P2X3 receptors (North & Surprenant 2000). In the present study, inward currents evoked by 10 μM ADP or 10 μM ATP were similar in amplitude and fully desensitized during 2 s application as exemplified in the inset to Figure 4a (records from the same DRG neuron). On average, ADP (10 μM) was equiactive as ATP (10 μM) on the same cells (−420±52 and −463±78 pA, respectively, n=7; P>0.05) and induced full current desensitization (τ1 and τ2 values were 57±4 and 606±150 ms, respectively; n=9). Figure 4a shows the concentration response curve for ADP that was similar to those generated by ATP or α,β-meATP (see Figure 2a). The ADP EC50 value was 1.1 μM (n=4), which is analogous to the one of ATP or α,β-meATP. Time for 50% recovery from 10 μM ADP-evoked desensitization (2.6±0.6 min; n=3) was also the same as the one observed with ATP itself (see Figure 1a), indicating that, unlike α,β-meATP, this ATP metabolite had similar properties like the parent compound including recovery from desensitization.
Figure 4.
Effect of ADP on DRG neurons. (a) Concentration response curve for ADP (data are from four cells). Inset shows example of fast inward currents induced on the same neuron by 10 μM ADP or 10 μM ATP with full desensitization. (b) Cross desensitization between ATP and ADP studied with the same protocol shown in Figure 3. In particular, after a response to ATP (10 μM) that peaked and fully desensitized, 6 min rest was allowed for full recovery and then 10 μM ADP was applied for 10 s to produce a peak current that fully decayed to baseline. After ADP washout, the subsequent ATP application elicited no response. In this example, after a 1.5 min interval, the next ATP application produced a current that was as large as in the standard recovery protocol (see Figure 1).
We next investigated cross-desensitization between ATP and ADP using the protocol previously reported (see Figure 3a,c). Figure 4b shows that, when conditioning was carried out with 10 μM ADP for 10 s, the first response (test1) to 10 μM ATP was fully blocked, indicating complete cross-desensitization between these compounds (n=6). After 1.5 min, the test2 response to ATP was 28±4%,which is as large as the one following conditioning with 10 μM ATP itself (28±3%). Likewise, conditioning with 10 μM ATP (10 s) completely suppressed the test1 response to 10 μM ADP and generated a test2 response to ADP, after 1.5 min, which was 27±5% of control (n=4). Parallel tests indicated that, at the same time point, for 10 μM ADP applications, the standard response desensitization was 30±2 % (n=4). These observations are consistent with equal rates of recovery from desensitization for both ATP and ADP.
Desensitization of P2X3 receptors by low concentrations of agonists
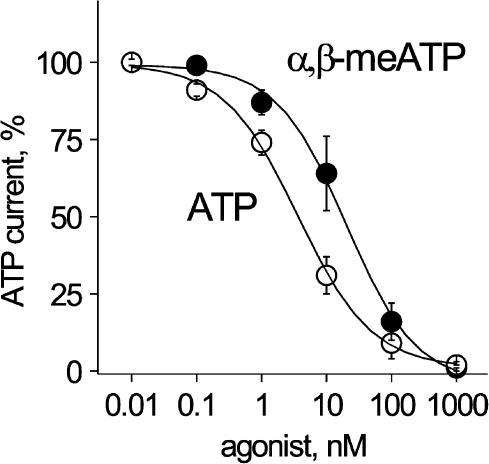
We wondered whether desensitization could also be observed with low doses of one agonist unable to induce macroscopic receptor activation and could impart agonist-specific recovery from desensitization. To this end, we bath-applied extremely low doses of one agonist (below current response threshold) for 90 s and measured the current induced by 10 μM ATP applied at a 6 min interval to minimize desensitization. Figure 5 shows the extent of reduction in ATP current amplitude by varying concentrations of the conditioning agent (ATP or α,β-meATP). It is apparent that, within a range of very low (100 pM–100 nM) concentrations, ATP was a 10-fold more potent desensitizing agent than α,β-meATP (IC50 values were 2 and 20 nM, respectively), even though both agonists had virtually the same EC50 value for receptor activation (see Figure 2a).
Figure 5.
Bath-applied, low agonist concentrations strongly depress responses of DRG neurons to ATP. Plot of % amplitude of response induced by 10 μM ATP versus conditioning agonist log concentration always applied for 1.5 min. Note that ATP (open circles) is more powerful than α,β-meATP (filled circles) to inhibit ATP response. Data are from 4 to 6 cells.
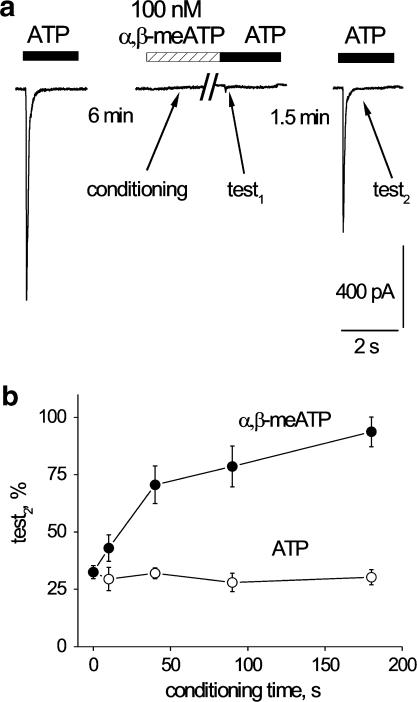
Since low concentrations of ATP or α,β-meATP were so potent to desensitize ATP responses, we explored whether they could also modulate recovery from desensitization. A protocol based on varying low agonist concentrations was, however, time consuming and complex because of the slow recovery phase. Hence, we adopted the same scheme shown in Figure 3a in which, after the conditioning application of either 100 nM ATP or 100 nM α,β-meATP, the amplitude of 10 μM ATP test1 and test2 responses was recorded while eliciting test2 at the fixed interval of 1.5 min from test1 (see example of this protocol in Figure 6a).
Figure 6.
Duration of application of a small concentration of agonist shapes recovery of response to ATP of DRG neurons. (a) Example of protocol to test low concentration of agonist (100 nM α,β-meATP) on ATP responses. Conditioning agent, which does not induce any measurable membrane current, is switched off immediately before applying ATP and yet it fully desensitizes the subsequent test1 response. Test2 response is generated at a fixed 1.5 min interval after test1 to probe recovery from desensitization. (b) Length of conditioning application of 100 nM ATP (open circles) or 100 nM α,β-meATP (filled circles) versus amplitude of test2 responses evoked by 10 μM ATP. Note that the amplitude of test2 responses is uniformly depressed by ATP conditioning (n=5–7 cells). Conversely, conditioning with 100 nM α,β-meATP largely facilitates recovery of test2 responses, a phenomenon dependent on the length of α,β-meATP conditioning (n=4–6 cells).
Figure 6b shows that the amplitude of the test2 response was independent of the duration of conditioning with 100 nM ATP (open circles). However, when the conditioning agonist was 100 nM α,β-meATP, the ATP test2 response became dependent on the duration of α,β-meATP application (Figure 6b, filled circles). In particular, taking as an example test2 responses measured after 90 s conditioning with ATP or α,β-meATP, despite virtually identical degree of suppression of test1 responses (see also Figure 5), there was a dramatic difference in recovery depending on the conditioning agent.
These results demonstrate that agonist-specific recovery could occur even when the conditioning agent did not evoke macroscopic receptor activation.
Desensitization induced by low doses of α,β-meATP could be used to potentiate ATP-induced currents
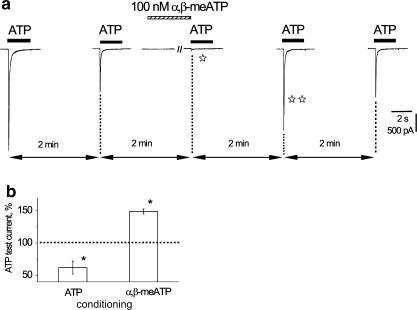
The unusual recovery process conferred by α,β-meATP could actually be exploited to transiently rescue ATP receptors from long lasting desensitization (Figure 7). Hence, repeated application of 10 μM ATP (2 s) every 2 min elicited reproducibly smaller currents (41% of nondesensitized response control; Figure 7a). Application of 100 nM α,β-meATP (1.5 min) induced two effects: (a) almost complete desensitization to the next ATP application (open star) that was carried out immediately after turning off α,β-meATP; (b) strong potentiation of the subsequent ATP current (double star). With this scheme, the histograms of Figure 7b show that ATP currents were on average significantly potentiated by α,β-meATP with respect to their stable responses, whereas, when the similar protocol relied on 100 nM ATP (1.5 min) there was, instead, augmentation of desensitization and, thus, smaller current amplitude.
Figure 7.
Conditioning with low dose of α,β-meATP can induce strong potentiation of ATP current of DRG neuron. (a) After a control response to 10 μM ATP (first trace on the left), subsequent response (2 min later) has smaller amplitude. Conditioning (1.5 min) with 100 nM α,β-meATP evokes no membrane current but it strongly depresses subsequent response to ATP (open star) applied immediately after terminating the application of α,β-meATP. The next ATP response (double star) is, however, largely enhanced. This enhancement is transient because the subsequent ATP application induces an ATP current amplitude like the one observed for the second response in the series. All ATP applications are spaced at 2 min interval. (b) Histograms showing average facilitation of 10 μM ATP test response by 100 nM α,β-meATP (n=5) and intensification of current depression by 100 nM ATP (n=6). The reproducible response amplitude is 100% (dashed line) before conditioning with either α,β-meATP or ATP. Asterisks indicate P<0.05.
Ambient ATP level
Since extracellular concentration of ATP under resting conditions is tissue dependent (see review by Lazarowski et al., 2003), ambient ATP levels might have been high enough to influence P2X3 receptor responses recorded from DRG cells in culture. Background concentrations of ATP in the present conditions were therefore measured with the luciferase/luciferin method (see Methods). Samples of effluents collected from DRG culture dishes (n=4) contained 52±3 pM ATP. Although these measurements cannot directly reflect ATP concentrations at membrane level, it seems likely that this ATP concentration reflects a dynamic state between endogenous ATP and its hydrolysis (Lazarowski et al., 2003).
Recombinant P2X3 receptors confirmed protective phenomenon
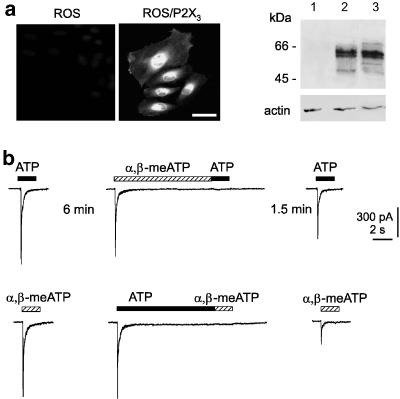
It was necessary to rule out the possible contribution by heterogeneous P2X receptor classes and P2Y receptors (Khakh et al., 2001; North, 2004) especially because ATP can coactivate P2X and P2Y receptors, while α,β-meATP acts on P2X receptors only (Khakh et al., 2001; North, 2002). Hence, experiments were carried out with ROS cells used to express the rat P2X3 gene (ROS/P2X3), taking advantage of the fact that they lack native P2Y receptors (Jorgensen et al., 1997; Pines et al., 2003). Immunofluorescence experiments using an anti-P2X3 antibody confirmed correct P2X3 expression by such cells (Figure 8a). To evaluate the size and mobility of the recombinant P2X3 protein in ROS/P2X3 cells, we performed Western immunoblot analysis that demonstrated bands from 55 to 60 kDa (Figure 8a, lane 2) in accordance with previous results (Nicke et al., 1998; Vulchanova et al., 1998). Such bands were absent in untransfected ROS cells (lane 1). Transfected ROS cell extracts were comparable with the pattern obtained from rat DRG tissue lysates (Figure 8a, right; lane 3).
Figure 8.
P2X3 receptors expressed by ROS cells show same interaction between ATP and α,β-meATP responses as observed with native DRG receptors. (a) Immunofluorescence-based detection of P2X3 receptor expression using an anti-P2X3 antibody. Transfected cells are immunopositive (middle panel), while untransfected cells are not stained (left). Calibration bar=50 μM. Right, Western immunoblot analysis of transfected ROS cells demonstrates bands from 55 to 60 kDa in accordance with previous results (Nicke et al., 1998; Vulchanova et al., 1998). Transfected ROS cell extracts (lane 2) are comparable with the pattern obtained from rat DRG tissue lysates (lane 3), while untransfected ROS cell extracts give negative result (lane 1). (b) Top records: on transfected ROS cell, 10 μM α,β-meATP (10 s) generates an inward current that fully decays to baseline and suppresses subsequent response to 10 μM ATP. Response to ATP evoked 1.5 min later has, however, regained larger amplitude. Bottom records: 10 μM ATP (10 s) induces an inward current that fully desensitizes and prevents subsequent response to α,β-meATP (10 μM). After a 1.5 min interval, current evoked by α,β-meATP remains substantially depressed. All traces are from the same cell. Note that effects recorded from ROS cell are the same as observed on DRG neurons (Figure 3).
On ROS/P2X3 cells, ATP (10 μM) and α,β-meATP (10 μM) induced inward currents of similar amplitude (−228±80 pA, n=10, and −214±78 pA, n=10, respectively) and rapid decline (Figure 8b) from which full recovery was observed after a 6 min wash. No current responses to ATP or α,β-meATP were detected in untransfected cells. Table 1 lists the main properties of desensitization of ROS/P2X3 receptors compared with those of native receptors of DRG cells.
Table 1.
Comparison of onset of P2X3 receptor desensitization and recovery between DRG neurons and ROS cells
| Agonist | DRG cells | ROS/rP2X3 | ||||
|---|---|---|---|---|---|---|
| τ1 | τ2 | 1.5 min recovery | τ1 | τ2 | 1.5 min recovery | |
| ATP (10 μM) | 61±8 ms (n=14) | 421±88 ms (n=14) | 30±3% (n=12) | 88±24 ms (n=9) | 893±232 ms (n=9) | 29±6% (n=6) |
| αβ-meATP (10 μM) | 67±7 ms (n=14) | 450±95 ms (n=14) | 48±4% (n=10) | 94±36 ms (n=12) | 874±182 ms (n=12) | 74±5% (n=6) |
τ1 and τ2 correspond to the biexponential decline of membrane currents indicative of desensitization onset.
Figure 8b shows that conditioning with 10 μM α,β-meATP (for 10 s) fully suppressed the test1 response to 10 μM ATP. After a 1.5 min interval, the ATP test2 response had strongly recovered in analogy with the phenomenon observed with native P2X3 receptors of DRG neurons. Thus, the amplitude of ATP-induced test2 response after α,β-meATP conditioning was 63±5% of the nondesensitized one (n=6), while it was 29±6% without conditioning (n=6). A similar protocol based on conditioning with 10 μM ATP (Figure 8b) led to full suppression of the test1 response to α,β-meATP and poor recovery of α,β-meATP test2 response after 1.5 min (16±4% of nondesensitized current, n=6, versus 74±5% without conditioning, n=6). Thus, P2X3 receptors expressed by ROS cells replicated agonist-specific desensitization.
Studies were also carried out on HEK cells that have been extensively used to express P2X receptors (Cook et al., 1998; Thomas et al., 1998; Fischer et al., 2003). HEK/P2X3 receptors displayed sigmoidal recovery from desensitization slower with 10 μM ATP (t1/2=2.37±0.96 min, n=7) than with 10 μM α,β-meATP (t1/2=0.94±0.56 min; n=6). Conditioning with 10 μM α,β-meATP accelerated recovery of the test2 ATP responses (66±5%, n=7 versus 38±5% control, n=6; P<0.05) 1.5 min after the test1 response. Conversely, conditioning with 10 μM ATP significantly retarded recovery of the test2 response to α,β-meATP (35±5%, n=5 versus 70±5% control, n=5, P<0.05). Hence, the phenomenon of agonist-specific desensitization was also present in P2X3 receptors expressed by HEK cells. Collectively, the current results provided the important validation that the properties of desensitization and recovery observed with native DRG receptors were not due to coactivation of other classes of P2X receptors, lacking from ROS and HEK cells, or to P2Y receptors, which (although expressed constitutively by HEK cells; Schachter et al., 1997; Fischer et al., 2003) are not found in ROS cells (Jorgensen et al., 1997; Pines et al., 2003).
Discussion
The principal findings of the present study are the demonstration of the complex recovery process of P2X3 receptors from desensitization, and the unusual agonist dependence of such a recovery which could be exploited to provide either pharmacological protection of these receptors from desensitization or prolongation of desensitization. In view of the role of P2X3 receptors in pain signalling, the present data can help to understand certain molecular mechanisms underlying nociception and devise novel strategies to control it.
Recovery from P2X3 receptor desensitization has complex time course with agonist and concentration dependence
Among the large family of P2X receptors, some are particularly prone to rapid desensitization, which is manifested as loss of response despite continuous presence of the agonist (North, 2002). In this category, P2X1 and P2X3 receptors are traditionally considered as fast desensitizing ones with slow recovery (Cook et al., 1998; Giniatullin et al., 2003; Rettinger & Schmalzing, 2003). As far as primary afferent neurons like DRG ones are concerned, P2X1 receptors are expressed only by a minority of cell bodies of rat DRG neurons (Dunn et al., 2001) and appear to be present mainly in a discrete plexus of dorsal horn fibres (Vulchanova et al., 1996). The overwhelming majority of IB4-sensitive, small-size DRG neurons used for the present study strongly express P2X3 receptors (Chen et al., 1995; Lewis et al., 1995; Vulchanova et al., 1998). This consideration suggests that responses under investigation were primarily mediated by P2X3 receptors, a notion fully supported by analogous observations with recombinant P2X3 receptors.
In keeping with this notion, the present study showed that inward currents generated by near-maximal concentrations of ATP or α,β-meATP rapidly faded back to baseline indicating fast and complete desensitization (see also Krishtal et al., 1988; Burgard et al., 1999; Grubb & Evans, 1999) with slow recovery (Cook et al., 1998; Giniatullin et al., 2003). One novel feature of desensitization recovery was its sigmoidal time course to indicate complexity in this phenomenon. This is a relatively unusual property because the standard theory for desensitization derived from studies of muscle-type nicotinic receptors (Katz & Thesleff, 1957) predicts monoexponential time course of recovery. It should, however, be noted that sigmoidal time course has recently been observed for neuronal α7 nicotinic receptors (Mike et al., 2000) and AMPA receptors (Robert & Howe, 2003). Another remarkable characteristic of the recovery process of fully desensitized P2X3 receptors was its dependence on the nature of the agonist (recovery after α,β-meATP was much faster than after ATP or ADP) and on the agonist concentration (responses to smaller doses required less time for recovery). Again, these features are at variance with Katz & Thesleff's (1957) theory that stated recovery to be agonist and concentration independent. The present observations with P2X3 receptors thus raised a number of issues concerning the mechanisms responsible for these phenomena. It seemed important to explore them because understanding the strong desensitization of such receptors may help to clarify certain molecular properties underlying signalling in nociceptive neurons.
ATP and α,β-meATP operate through the same ATP receptors
One obvious possibility to account for agonist-based diversity in the P2X3 receptor recovery process is that the action of ATP and α,β-meATP was mediated by heterogeneous receptors. Our dose–response studies indicated that ATP and α,β-meATP were both full agonist of equivalent potency (see also review by North, 2002) acting on receptors equisensitive to the antagonist TNP-ATP. Note that the EC50 values observed in the present study for DRG P2X3 receptors are close to those previously reported (North, 2002). Furthermore, the full cross-desensitization between the two agonists, and, in particular, the reproducibility of the phenomenon of agonist-dependent recovery for P2X3 receptors expressed in heterologous systems clearly argue for ATP and α,β-meATP acting via the same P2X3 receptors. The sharp difference in recovery rates between ATP and α,β-meATP helps to exclude another possibility, namely that α,β-meATP inhibited the ATP-hydrolytic enzyme ecto-ATPase and therefore increased ambient level ATP (Beigi & Dubyak, 2000). Should this have been the case, one would expect to observe delayed recovery from desensitization with α,β-meATP, contrary to the present findings.
ATP and ADP have analogous activity on P2X3 receptors of DRG neurons
The possibility that ATP might have been rapidly converted into its active breakdown metabolite ADP prompted our study of the action of ADP on DRG neurons. Previous work on recombinant P2X3 receptors has shown ADP to be a much weaker agonist than ATP itself (reviewed by North & Surprenant, 2000). On native receptors expressed by nociceptive DRG neurons, ADP had analogous properties like ATP in terms of receptor activation potency, desensitization and recovery. Although this finding is surprisingly different from previous studies of recombinant receptors, the similar EC50 value for ADP and ATP indicates that the effectiveness of ADP could not be explained by its small contamination by ATP as, instead, suggested in a report about P2X1 receptors (Mahaut-Smith et al., 2000).
The equivalent receptor activation and recovery rate for ATP and ADP suggest that, under the present conditions of fast superfusion, either agonist was rapidly removed from the receptor area and that the unduly long recovery from ATP was not generated by its local biotransformation into the agonist ADP in the receptor microenvironment.
In the low-density, continuously superfused neuronal cultures used in the present study, we found low pM levels of ambient ATP, below threshold (1 nM) to inhibit P2X3 receptors. Even if one hypothesized that the local concentration of ATP at membrane level was higher than in the bulk solution, this should not have been in excess of 0.1 nM, because applying this ATP concentration to DRGs had minimal (about 10%) effect on membrane currents elicited by ATP (Figure 5).
Agonist conditioning is a highly sensitive method to modulate desensitization recovery
As desensitization recovery is such a prominent component in the operation of P2X3 receptors, perturbing this process might be expected to generate either sustained receptor inactivity or, conversely, rapid restoration of function. Indeed, using a protocol based on conditioning receptors into a fully desensitized state by a certain agonist, two opposite conditions could be demonstrated: (a) improved recovery for ATP responses after conditioning with α,β-meATP; (b) retarded recovery of α,β-meATP responses after conditioning with ATP. Thus, receptor function was dictated by the conditioning agent even after its full washout.
A subtler method to modulate recovery from desensitization would be to condition receptors with agonist concentrations per se subthreshold to elicit detectable currents. Such approach is interesting because sustained block of P2X3 receptors might be achieved by using a subthreshold dose of an agonist rather than an antagonist.
The present observations showed strong desensitization by low nM concentrations of ATP, demonstrating high-affinity desensitization of rat P2X3 receptors previously reported for hP2X3 receptors in expression systems tested with Ca2+ imaging (McDonald et al., 2002). High-affinity desensitization was not associated with apparent changes in membrane currents in analogy with studies of nicotinic receptors (Wooltorton et al., 2003), and serotonin 5-HT3 receptors (Bartrup & Newberry, 1996) exposed to very small agonist doses.
High-affinity desensitization achieved by conditioning with α,β-meATP aided the recovery process of P2X3 receptors, demonstrating that agonist-dependent recovery from desensitization was also present with agonist subthreshold doses. However, in the low dose range, ATP was distinctly more effective as an inhibitor than α,β-meATP. The simplest interpretation is that certain nonconducting receptor states would have higher affinity for ATP than for α,β-meATP, although both agonists induce similar receptor activation.
A unified hypothesis for the differential desensitiziting properties of ATP and α,β-meATP
Suprathreshold concentrations of ATP or α,β-meATP induced very similar, full desensitization of P2X3 receptors, although with different recovery rates. Subthreshold concentrations of ATP were, however, more potent than those of α,β-meATP to induce desensitization. Although this type of high-affinity desensitization is different from the one shown by P2X1 receptors, which always require measurable activation before desensitization (Rettinger & Schmalzing, 2003), it does possess striking similarities to desensitization of neuronal nicotinic receptors induced by low concentrations of nicotine or ACh with no apparent macroscopic response (Paradiso & Steinbach, 2003). Furthermore, nicotine (like ATP in the present study) produces longer lasting desensitization than ACh (which behaves like α,β-meATP). Our results might therefore be interpreted by using a scheme similar to the one proposed for nicotinic receptors (Paradiso & Steinbach, 2003) derived from Cachelin & Colquhoun (1988) who suggested the existence of multiple desensitized states. Within this framework, P2X3 receptors (which need binding three agonist molecules for their full activation; Jiang et al., 2003) are postulated to undergo various transitions to reach an open state from which desensitization rapidly develops: this phenomenon would be the same for applications of either ATP or α,β-meATP. The difference in recovery between ATP and α,β-meATP would arise from the fact that ATP is thought to be much more potent than α,β-meATP at facilitating entry of the P2X3 receptor into a slowly recovering state. This notion is not incompatible with the analogous recovery from desensitization observed with ATP and ADP because it is feasible that, despite lack of detailed data on this issue, these two agents have similar dissociation rate from the P2X3 receptor of DRG neurons. Hence, it is interesting to note how onset of desensitization was virtually the same for the three agonists ATP, ADP and α,β-meATP, while recovery was distinctly faster for α,β-meATP. The very similar receptor activating and desensitiziting properties of these substances indicate that it may not be necessary to assume special properties for α,β-meATP like the possibility that this synthetic agonist recognizes the receptor channel differently from the others. Nevertheless, a systematic study of P2X3 receptor recovery after testing a wide range of ATP agonists is required to clarify this issue.
The theory by Paradiso & Steinbach (2003), based on a cyclic kinetic scheme, includes the possibility of multiple transitions from agonist-bound/closed states to agonist-bound/desensitized states without the need of receptor activation. This notion implies that even mono- or biliganded closed receptors would rapidly desensitize as suggested by the observation of desensitization evoked by low doses of ATP or α,β-meATP. In this sense, subthreshold doses of ATP were more potent than those of α,β-meATP at inducing desensitization probably because ATP had higher affinity for the mono- and biliganded desensitized states like nicotine has versus ACh on neuronal nicotinic receptors (Paradiso & Steinbach, 2003).
Physiological implications
The level of endogenous ATP in the extracellular space is tissue dependent at rest and is influenced by experimental conditions (Lazarowski et al., 2003). In some tissues, it appears to exceed the IC50 for high-affinity desensitization reported in the current study, while in others it is reportedly lower. Of course, bulk solution assays cannot disclose the effective concentration of ATP at membrane level near its release sites nor its time profile which depends on a variety of factors like rate of release, extracellular volume, rate of hydrolysis, etc. It seems, however, likely that any rise in concentration of ATP would be a transient phenomenon and that under resting conditions ambient ATP levels reflect the steady-state equilibrium between its release and inactivation. Under in vivo conditions, removal of ATP from the P2X3 receptor area may rely on its metabolic transformation into ADP (and other nucleotides) rather than simple diffusion. Although ADP is a weak agonist on recombinant P2X3 receptors (North & Surprenant, 2000), this agent was observed to be an efficient agonist on native P2X3 receptors of DRG cells. In this case, any ADP generated by ATP breakdown would contribute to the desensitization phase.
Hence, P2X3 receptors in vivo may or may not be constitutively desensitized depending on local conditions. When desensitization at rest is small, such receptors would be strongly responsive to abrupt changes in ATP concentration produced by tissue damage or injury (Cook & McCleskey, 2002). The present study suggests that the extent of constitutive desensitization might determine the effectiveness of agonists like α,β-meATP in restoring P2X3 receptor signalling.
The present data seem to be applicable to understanding the function of homomeric P2X3 receptors which are expressed by small nociceptive neurons (Chen et al., 1995; Lewis et al., 1995) projecting to superficial layers of the spinal cord and primarily involved in mediating inflammatory pain (Cockayne et al., 2000; Souslova et al., 2000). The same P2X3 subunit can also participate in heteromeric P2X2/3 receptors (North, 2002) expressed by larger nociceptive neurons projecting to deeper spinal laminae and apparently responsible for neuropathic pain (Tsuda et al., 2000). Future work should clarify if heteromeric P2X2/3 receptors of such neurons also present the property of agonist-dependent desensitization.
Acknowledgments
We are especially grateful to Professor R. Civitelli (Washington University, School of Medicine, St Louis, MO, U.S.A.) for supplying the ROS cell line, to Professor R.A. North (University of Sheffield, U.K.) for generously donating the rat P2X3 receptor plasmid, to Professor P. D'Andrea (University of Trieste, Italy) for much advice concerning the ROS cell culture and Dr M. Righi (SISSA, Trieste) for his support with cell cultures. We also thank Dr D. Smirnov (Minnesota University, U.S.A.) for providing preliminary material for molecular biology experiments. This work was supported by cofinanced grants from MIUR (FIRB), Regione Friuli Venezia Giulia, INFM, RFBR (Russia) and Italian Ministry for Foreign Affairs through a cultural and scientific cooperation agreement between Italy and Russia.
Abbreviations
- α,β-meATP, α
β-methylene-adenosine triphosphate
- DRG
dorsal root ganglion
- HEK
human embryonic kidney cell
- P2X
subclass of ATP-sensitive ionotropic receptors
- P2Y
subclass of ATP-sensitive metabotropic receptors
- ROS
rat osteoblastic cells
- TNP-ATP
2′, 3′-O-(2,4,6, trinitrophenyl)-adenosine triphosphate
References
- BARTRUP J.T., NEWBERRY N.R. Electrophysiological consequences of ligand binding to the desensitized 5-HT3 receptor in mammalian NG108-15 cells. J. Physiol. 1996;490:679–690. doi: 10.1113/jphysiol.1996.sp021177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEIGI R.D., DUBYAK G.R. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J. Immunol. 2000;165:7189–7198. doi: 10.4049/jimmunol.165.12.7189. [DOI] [PubMed] [Google Scholar]
- BURGARD E.C., NIFORATOS W., VAN BEISSEN T., LYNCH K.J., TOUMA E., METZGER R.E., KOWALUK E.A., JARVIS M.F. P2X receptor-mediated ionic currents in dorsal root ganglion neurons. J. Neurophysiol. 1999;82:1590–1598. doi: 10.1152/jn.1999.82.3.1590. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- CACHELIN A.B., COLQUHOUN D. Desensitization of the acetylcholine receptor of frog end-plates measured in a vaseline-gap voltage clamp. J. Physiol. 1988;415:159–188. doi: 10.1113/jphysiol.1989.sp017717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C.C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G, ZHU Q.M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:951–952. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- COOK S.P., MCCLESKEY E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- COOK S.P., RODLAND K.D., MCCLESKEY E.W. A memory for extracellular Ca2+ by speeding recovery of P2X receptors from desensitization. J. Neurosci. 1998;18:9238–9244. doi: 10.1523/JNEUROSCI.18-22-09238.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING S., SACHS F. Single channel properties of P2X2 purinoceptors. J. Gen. Physiol. 1999;113:695–720. doi: 10.1085/jgp.113.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN P.M., ZHONG Y., BURNSTOCK G. P2X receptors in peripheral neurons. Prog. Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- FISCHER W., WIRKNER K., WEBER M., EBERTS C., KOLES L., REINHARDT R., FRANKE H., ALLGAIER C., GILLEN C., ILLES P. Characterization of P2X3, P2Y1 and P2Y4 receptors in cultured HEK293-hP2X3 cells and their inhibition by ethanol and trichloroethanol. J. Neurochem. 2003;85:779–790. doi: 10.1046/j.1471-4159.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- GINIATULLIN R., SOKOLOVA E., NISTRI A. Modulation of P2X3 receptors by Mg2+ on rat DRG neurons in culture. Neuropharmacology. 2003;44:132–140. doi: 10.1016/s0028-3908(02)00338-6. [DOI] [PubMed] [Google Scholar]
- GRUBB B.D., EVANS R.J. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur. J. Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- JIANG L.H., KIM M., SPELTA V., BO X., SURPRENANT A., NORTH R.A. Subunit arrangement in P2X receptors. J. Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORGENSEN N.R., GEIST S.T., CIVITELLI R., STEINBERG T.H. ATP and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J. Cell Biol. 1997;139:497–506. doi: 10.1083/jcb.139.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J. Physiol. 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SEGUELA P., VOIGT M., HUMPHREY P.P. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KOSHIMIZU T.A., UENO S., TANOUE A., YANAGIHARA N., STOJILKOVIC S.S., TSUJIMOTO G. Heteromultimerization modulates P2X receptor functions through participating extracellular and C-terminal subdomains. J. Biol. Chem. 2002;277:46891–46899. doi: 10.1074/jbc.M205274200. [DOI] [PubMed] [Google Scholar]
- KRISHTAL O.A., MARCHENKO S.M., OBUKHOV A.G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988;27:995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., BOUCHER R.C., HARDEN T.K. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- LIU M., KING B.F., DUNN P.M., RONG W., TOWNSEND-NICHOLSON A., BURNSTOCK G. Coexpression of P2X3 and P2X2 receptor subunits in varying amounts generates heterogeneous populations of P2X receptors that evoke a spectrum of agonist responses comparable to that seen in sensory neurons. J. Pharmacol. Exp. Ther. 2001;296:1043–1050. [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., ENNION S.J., ROLF M.G., EVANS R.J. ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br. J. Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD H.A., CHU K.L., BIANCHI B.R., MCKENNA D.G., BRIGGS C.A., BURGARD E.C., LYNCH K.J., FALTYNEK C., CARTMELL J., JARVIS M.F. Potent desensitization of human P2X3 receptors by diadenosine polyphosphates. Eur. J. Pharmacol. 2002;435:135–142. doi: 10.1016/s0014-2999(01)01568-0. [DOI] [PubMed] [Google Scholar]
- MIKE A., CASTRO N.G., ALBUQUERQUE E.X. Choline and acetylcholine have similar kinetic properties of activation and desensitization on the alpha7 nicotinic receptors in rat hippocampal neurons. Brain Res. 2000;882:155–168. doi: 10.1016/s0006-8993(00)02863-8. [DOI] [PubMed] [Google Scholar]
- NICKE A., BAUMERT H.G., RETTINGER J., EICHELE A., LAMBRECHT G., MUTSCHLER E., SCHMALZING G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. P2X3 receptors and peripheral pain mechanisms. J. Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A., SURPRENANT A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- PARADISO K.G., STEINBACH J.H. Nicotine is highly effective at producing desensitization of α4β2 neuronal nicotinic receptors. J. Physiol. 2003;553:857–871. doi: 10.1113/jphysiol.2003.053447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUKERT M., OSTEROTH R., GEISLER H.S., BRANDLE U., GLOWATZKI E., RUPPERSBERG J.P., GRUNDER S. Inflammatory mediators potentiate ATP-gated channels through the P2X3 subunit. J. Biol. Chem. 2001;276:21077–21082. doi: 10.1074/jbc.M101465200. [DOI] [PubMed] [Google Scholar]
- PINES A., ROMANELLO M., CESARATTO L., DAMANTE G., MORO L., D'ANDREA P., TELL G. Extracellular ATP stimulates the early growth response protein 1 (Egr-1) through a PKC dependent pathway in the human osteoblastic HOBIT cell line. Biochem. J. 2003;373:815–824. doi: 10.1042/BJ20030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAE M.G., ROWAN E.G., KENNEDY C. Pharmacological properties of P2X3-receptors present in neurones of the rat dorsal root ganglia. Br. J. Pharmacol. 1998;124:176–180. doi: 10.1038/sj.bjp.0701803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RETTINGER J., SCHMALZING G. Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J. Gen. Physiol. 2003;121:451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERT A., HOWE J.R. How AMPA receptor desensitization depends on receptor occupancy. J. Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHTER J.B., SROMEK S.M., NICHOLAS R.A., HARDEN T.K. HEK293 human embryonic kidney cells endogenously express the P2Y1 and P2Y2 receptors. Neuropharmacology. 1997;36:1181–1187. doi: 10.1016/s0028-3908(97)00138-x. [DOI] [PubMed] [Google Scholar]
- SKORINKIN A., NISTRI A., GINIATULLIN R. Bimodal action of protons on ATP currents of rat PC12 cells. J. Gen. Physiol. 2003;122:33–44. doi: 10.1085/jgp.200308825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKOLOVA E., NISTRI A., GINIATULLIN R. Negative cross-talk between anionic GABAA and cationic P2X ionotropic receptors of rat dorsal root ganglion neurons. J. Neurosci. 2001;21:4958–4968. doi: 10.1523/JNEUROSCI.21-14-04958.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUSLOVA V., CESARE P., DING Y., AKOPIAN A.N., STANFA L., SUZUKI R., CARPENTER K., DICKENSON A., BOYCE S., HILL R., NEBENUIS-OSTHUIZEN D., SMITH A.J., KIDD E.J., WOOD J.N. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- THOMAS S., VIRGINIO C., NORTH R.A., SURPRENANT A. The antagonist trinitrophenyl-ATP reveals co-existence of distinct P2X receptor channels in rat nodose neurones. J. Physiol. 1998;509:411–447. doi: 10.1111/j.1469-7793.1998.411bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M., KOIZUMI S., KITA A., SHIGEMOTO Y., UENO S., INOUE K.Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons J. Neurosci. 200020RC90, 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., RIEDL M.S., SHUSTER S.J., STONE L.S., HARGREAVES K.M., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur. J. Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- WOOLTORTON J.R., PIDOPLICHKO V.I., BROIDE R.S., DANI J.A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]